Abstract
Over 1,400 water samples were collected biweekly over 6 years from an intermittent stream protected and unprotected from pasturing cattle. The samples were monitored for host-specific Bacteroidales markers, Cryptosporidium species/genotypes, viruses and coliphages associated with humans or animals, and bacterial zoonotic pathogens. Ruminant Bacteroidales markers did not increase within the restricted cattle access reach of the stream, whereas the ruminant Bacteroidales marker increased significantly in the unrestricted cattle access reach. Human Bacteroidales markers significantly increased downstream of homes where septic issues were documented. Wildlife Bacteroidales markers were detected downstream of the cattle exclusion practice where stream and riparian habitat was protected, but detections decreased after the unrestricted pasture, where the stream and riparian zone was unprotected from livestock. Detection of a large number of human viruses was shown to increase downstream of homes, and similar trends were observed for the human Bacteroidales marker. There was considerable interplay among biomarkers with stream flow, season, and the cattle exclusion practices. There were no to very weak associations with Bacteroidales markers and bacterial, viral, and parasitic pathogens. Overall, discrete sample-by-sample coherence among the different microbial source tracking markers that expressed a similar microbial source was minimal, but spatial trends were physically meaningful in terms of land use (e.g., beneficial management practice) effects on sources of fecal pollution.
INTRODUCTION
Microbial source tracking (MST) can help reveal the sources of fecal contamination in water resources (1–5). There are a suite of MST tools that have been used in this capacity (6); some of these include the antimicrobial resistance patterns of target bacteria (7, 8), bacterial markers (9), such as Bacteroidales markers (2), host specificity associated with parasitic organisms like Cryptosporidium (10, 11), mitochondrial DNA methods (12, 13), and virus host specificity (14–16). There is potential for these tools to help identify how different land uses can impact the sources of fecal pollution in open-surface-water systems like agricultural watersheds, systems typically prone to variable inputs of human, livestock, and wildlife feces. While these tools have potential utility for such assessments, they are different in their limits of detection, source discrimination power, spatial and temporal stability, persistence in different environmental matrices, and what they express biophysically in water samples; and as such, results from one method may not necessarily be coherent with those of another method (5, 17, 18). Under some circumstances, however, coherency, or a lack of it, can reveal what biomarkers are appropriate for the use at hand, and MST coherence with the occurrence of other fecal indicator organisms and pathogens can be viewed as an important factor for helping to identify mitigation measures that will reduce public health risks (19–21, 59).
Many agricultural beneficial management practices (BMPs) are designed to be protective of water quality, yet such interventions in open systems like watersheds could variably impact the degree and source of fecal contamination in a receiving water body (22, 23). In this context, MST information could provide a more accurate assessment of how fecal pollution mechanisms associated with the target organism of the BMP (e.g., livestock) truly respond to that BMP (e.g., cattle exclusion fencing along a stream). Clearly, for systems impacted by multiple sources of fecal contamination, the mitigation benefits of a BMP could potentially be offset or clouded by other fecal pollution sources.
The objectives of this research were to (i) examine the spatial and temporal consistency among a broad suite of different MST markers (specifically aimed at human, ruminant, rodent, avian, pig) detected along an intermittent stream variably affected by humans, wildlife, and pasturing cattle (23, 59), (ii) examine relationships among MST marker detections and the occurrence of pathogens, and (iii) determine MST signature differences and/or changes that result from a BMP that excludes or does not exclude pasturing cattle from the stream and riparian zone.
MATERIALS AND METHODS
General study site description.
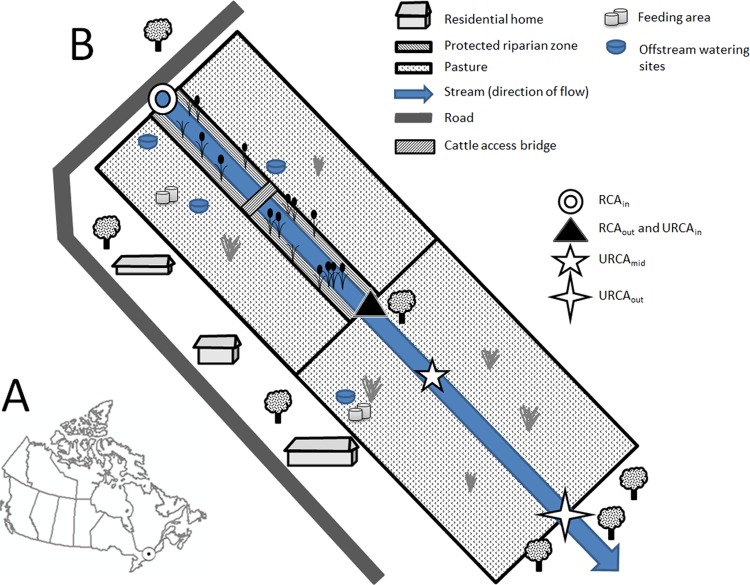
The study site has been described in detail by Sunohara et al. (23) and Wilkes et al. (59). Briefly, the experimental site is located on a small intermittent stream located in the South Nation River Basin in eastern Ontario, Canada (Fig. 1). The stream runs through a pasture where cattle are restricted from the water course by exclusion fencing (∼3- to 5-m buffer from the stream channel; restricted-cattle-access [RCA] experimental area) and a pasture where cattle have unrestricted access to the water course (unrestricted-cattle-access [URCA] experimental area). Land use immediately surrounding the pastures consists of cultivated cropland and several residential homes located in close proximity to the stream system. There is also a home located upstream of the RCA experimental area. The experimental site was designed following an upstream-downstream methodology (24). Livestock (Holstein cows) densities were maintained at 2.5 animals ha−1 for both the RCA and URCA experimental areas starting from 2005 forward. Livestock pastured from May or June to typically November, after which they were trucked off site.
Fig 1.
(A) General location of study site in Canada; (B) simplified graphical depiction of the layout of the experimental site setup and design (the graphic is not to scale and is simplified for clarity; see the work of Sunohara et al. [23] for a detailed map and study site description). The RCA area is 1.8 ha with a flow length of ∼356 m and a gradient of 0.002 m m−1. The URCA area is 2.2 ha of bounded land with a flow length of 348 m and a gradient of 0.004 m m−1.
Stream monitoring and microbiological analysis.
Over 1,400 water samples were collected by hand using sterile gloves and sterile water collection implements from April to May to the time of stream freeze up (from about mid-November to late November) between 2004 and 2010. Samples were collected at a location serving as a baseline of water quality input into the restricted-cattle-access experimental region (RCAin), at a location representing the water quality output from the RCA experimental area and also serving as a baseline of water quality input into the unrestricted-cattle-access area (RCAout\URCAin), at a location representing the midpoint of the URCA area (URCAmid), and finally, at the output of the URCA (URCAout) (Fig. 1). The URCAmid site was introduced into the study in late 2005. Water quality data collected at these sample sites were divided into two groups: (i) temporally concurrent data (TCD) and (ii) all available data (AAD). TCD were data collected concurrently (synchronously) from 2005 to 2010 at RCAin, RCAout\URCAin, and URCAout, and parasite data were collected concurrently from 2005 to 2010 at RCAin and RCAout. TCD were used exclusively for evaluating pasture treatment effects because of equal data support between the upstream and downstream monitoring sites. AAD were all data collected at each monitoring site, including URCAmid, from 2004 to 2010, irrespective of the temporal concurrence of sampling (i.e., one sample site may have more data support than another).
Sampled water was shipped to Agriculture and Agri-Food Canada (AAFC; London, ON, Canada) for Bacteroidales analysis (21), the Alberta Provincial Laboratory for Public Health (Calgary, AB, Canada) for Cryptosporidium oocyst and Giardia cyst quantification and genotyping (25), the Public Health Agency of Canada's (PHAC's) Laboratory for Food-Borne Zoonoses (Lethbridge, AB, Canada) for detection of pathogenic bacteria (26), AAFC (Lacombe, AB) for F-specific RNA (F-RNA) and F-specific DNA (F-DNA) coliphage detection, quantification, and source attribution (described below and by Sunohara et al. [23] and Wilkes et al. [59]), and AAFC (Saint-Hyacinthe, QC, Canada) for pathogen and host marker virus detection and attribution (described by Wilkes et al. [59] and below).
For Bacteroidales, quantitative PCR (qPCR) analyses were performed as follows. Between 25 and 300 ml of water was filtered through 0.45-μm-pore-size Nuclepore membrane filters (Whatman, Thermo Fisher Scientific, Ottawa, ON, Canada). Filters were placed in a 15-ml Falcon tube containing 0.5 ml of GITC buffer (5 M guanidine isothiocyanate, 100 mM EDTA [pH 8.0], 0.5% Sarkosyl) and frozen at −80°C until extraction. DNA was extracted following the manufacturer's instructions using a DNeasy tissue kit (Qiagen, Mississauga, ON, Canada), except that the proteinase K step was omitted. The elution volume was 100 μl. PCR amplification was performed using a Bio-Rad CFX96 qPCR instrument with Bio-Rad CFX Manager software, version 2.0. The primer and probe sequences used in this study are presented by Marti et al. (21) (see Table S1 in the supplemental material) and targeted total Bacteroidales and markers HF183 (human), BacR (ruminant), Pig-2-Bac (pig), and CGOF1-Bac (Canada goose). The marker MuBa (muskrat) is presented by Marti et al. (2). TaqMan chemistry was used for all markers, except for the human marker, for which SYBR green chemistry was used. All primers and probes were synthesized by Sigma-Aldrich (Toronto, ON, Canada). The mix reaction was performed with the Brilliant II QPCR master mix (Agilent, Toronto, ON, Canada) for the TaqMan PCR and the Brilliant II SYBR green Low ROX qPCR master mix (Agilent) for the SYBR green PCR. Two microliters of template DNA was added, and deionized water was used to reach a final volume of 25 μl. Negative controls (no template DNA) were performed in triplicate for each run. Each reaction was run in triplicate with the following cycle conditions: 1 cycle at 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and the annealing temperature for 40 s. For the SYBR green assay, a melting curve step was added in order to check the purity of the PCR product. This step consisted of a ramp temperature from 65 to 95°C at an increment of 0.5°C per step and a hold for 5 s for each step. The presence/absence of PCR inhibitors was verified on a 10× template DNA dilution by using a TaqMan exogenous internal-positive-control kit (Applied Biosystems, Toronto, ON, Canada) following the manufacturer's instructions. If inhibitors were present, a 100× dilution of the DNA template proceeded.
Serotyping of all Salmonella isolates was performed by the PHAC Laboratory for Food-Borne Zoonoses at the Office International des Épizooties Salmonella Reference Laboratory in Guelph, ON, Canada. Somatic and flagellar antigens of all Salmonella isolates were determined using slide agglutination (27) and test tube precipitation with microtiter plates (28). The antigenic formulae of Grimont and Weill were used to identify and name the serovars (29).
For virus analysis, 500-ml samples were processed in accordance with the OPFLP 04 standard method for the recovery and concentration of viruses in artificially and naturally contaminated water in Health Canada's compendium of analytical methods (30, 31). Negative controls were processed in tandem with the water sample. Viral nucleic acids were extracted using a QIAamp viral RNA minikit (Qiagen, Mississauga, ON, Canada). Real-time TaqMan reverse transcription (RT)-PCR and PCR assays for detection of the viruses (some of which are host-attributed viruses) norovirus genogroup I (GI), GII, GIII, and GIV, hepatitis A virus, hepatitis E virus, adenovirus type 40/41, human adenoviruses, astrovirus, Sapovirus, torque teno virus (TTV), torque teno sus virus, rotavirus, and feline calicivirus (as a sample process control) were performed in 25-μl reaction mixtures with the 1-step Brilliant II quantitative RT-PCR (qRT-PCR) core reagent kit for RNA viruses and with the Brilliant I qPCR core kit for DNA viruses (Agilent Technologies Canada, Mississauga, ON, Canada) (32–42). Standard curves were generated using 10-fold serial dilutions of standard cDNA plasmids containing the PCR product of each virus (108 to 100 genomic equivalents) for viral copy quantification (detection limits of assays, between 1 ×101 and 1 × 100 genomic equivalent copies). The RNA viruses hepatitis A virus, astrovirus, norovirus GI and GIV, and Sapovirus are commonly associated with human hosts, while norovirus GIII is commonly associated with bovine hosts. The DNA viruses adenoviruses (general and types 40/41) and torque teno virus are associated with human hosts (43), while torque teno sus virus is associated with swine.
The RNA from confirmed F-RNA coliphage isolates (isolated as described by Sunohara et al. [23]) was obtained from F-RNA coliphage suspensions that were thawed and diluted 1:50 in DNase- and RNase-free water, boiled for 5 min, held on ice for 2 min, and then centrifuged at 14,000 × g for 10 s. The RNA extracts of F-RNA coliphages were genotyped into genogroups I to IV by real-time RT-PCR. Real time RT-PCRs were carried out with a QuantiTect multiplex NoROX RT-PCR kit (Qiagen Inc., Mississauga, ON, Canada) on a Stratagene MX3005P qPCR thermocycler (Agilent) using the conditions described by Jones et al. (44). Genogroups I and IV were detected by a duplex assay using LV1 and GIV primers and probes as described by Jones et al. (44), and genogroups II and III were detected in individual reactions using the primers and probes described by Wolf et al. (45). Each 25-μl reaction mixture contained 200 nM each forward and reverse primers and probe, 0.25 μl of QuantiTect multiplex RT mix, 0.03 μM carboxy-X-rhodamine (ROX) reference dye, and 2.5 μl of RNA extract in 1× QuantiTect multiplex RT-PCR master mix. RNA extracts that were positive for GI or GIV were assigned to F-RNA coliphage of animal origin, and those that were positive for GII or GIII were assigned to F-RNA coliphage of human origin. The detection limit for viable phages was 5 liter−1, and the detection limit for genome copies determined by RT-PCR was 120 liter−1.
Cryptosporidium strains were sequenced and genotyped by phylogenetic analyses and assigned to broad known host classes as described by Ruecker et al. (25) and Wilkes et al. (11).
Statistical analyses.
Site comparisons for Bacteroidales and source-specific pathogen occurrence (MST endpoints) among the sites, among the sites seasonally, and under differing flow regimes (high, low, and no flow) were made using Fisher's exact tests. The Bacteroidales source marker (number of copies per day−1, determined by multiplying daily stream flow by Bacteroidales copies vol−1 of the sampled water) distribution among the sites, among the sites seasonally, and among the sites under different flow regimes were examined using the Kruskal-Wallis and Mann-Whitney U tests. Both the Kruskal-Wallis and Mann-Whitney U test methods rank continuous data. For Mann-Whitney U test results, we calculated the mean rank sum to show the higher-value direction between groups. The previous site comparisons were independently performed with both TCD and AAD. The detection and nondetection of host-specific markers in pathogen-positive samples versus pathogen-negative samples and in marker-positive samples versus marker-negative samples were examined with Fisher's exact tests and odds ratio (OR) estimates using AAD combined. Qualitative occurrences (within 10-day windows) of biomarkers were also examined using a heat map. Lastly, interactions among site, season, and flow (independent variables) in terms of MST target presence/absence (dependent variables) were examined using classification and regression tree analysis (CART; version 6.6; Salford Systems, CA) by the method of Wilkes et al. (59).
RESULTS
Microbial source tracking markers by cattle exclusion BMP, season, and flow.
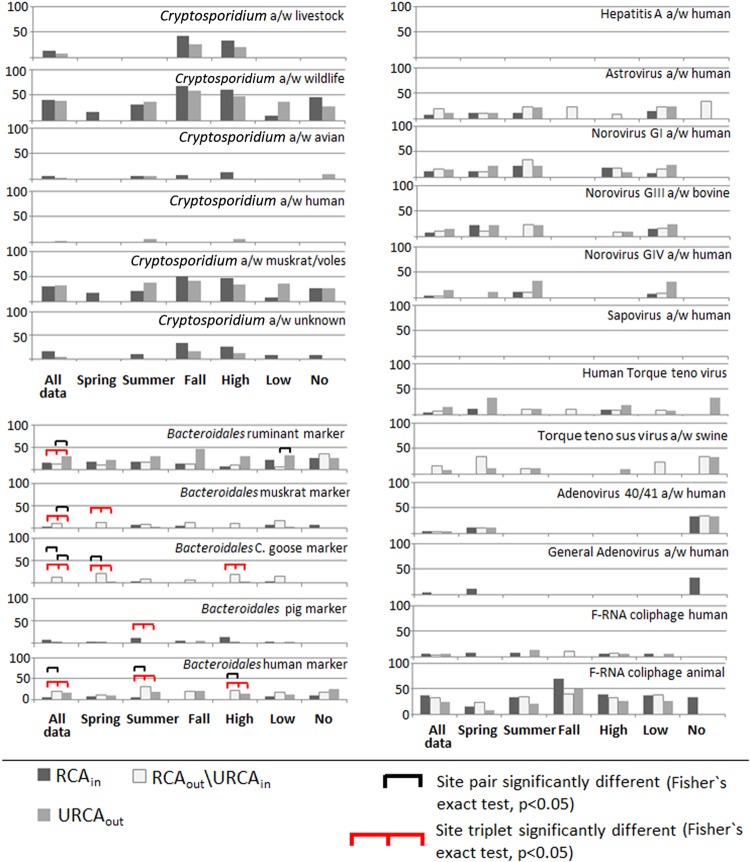
Ruminant Bacteroidales marker detection rates for TCD (Fig. 2) decreased by 3% from the RCAin to RCAout\URCAin sites and increased by 17% (P < 0.05) from the RCAout\URCAin to URCAout sites. At low flows, the URCAout site had significantly higher detections (33%) than the RCAout\URCAin site (7%), suggesting the impact of cattle access to the stream. In summer, the prevalence of the pig Bacteroidales marker was significantly higher at the RCAin site than at the RCAout\URCAin and URCAout sites, which had no detections (Fig. 2). This was likely a result of agricultural drainage from fields that had received swine manure applications (highly transient drainage that occurred from fields out of the watershed proper was channeled via a roadside ditch that drained into the stream locally near the RCAin site).
Fig 2.
Percentage of samples positive for microbial source tracking markers by specific site, season, and flow conditions. TCD were used for the RCAin, RCAout\URCAin, and URCAout sites for source markers and source viruses, and the Cryptosporidium-associated source tracking markers at the RCAin and URCAout sites are summarized in a temporally concurrent manner. See Fig. 3 for parasite source occurrence at the RCAout\URCAin site. High flow, ≥0.018 m3 s−1; low flow, ≥0.002 m3 s−1 and <0.018 m3 s−1; and no flow, <0.002 m3 s−1. a/w, associated with.
Regarding wildlife generalized source classes, detection of muskrat Bacteroidales markers was significantly higher at the RCAout\URCAin site (12%) than at the RCAin site (4%) and the URCAout site (1%) (Fig. 2). This suggests an impact of muskrat habitat protection in the RCA experimental area. This trend also held true in spring, where the rate of muskrat Bacteroidales marker detection was 14% at the RCAout\URCAin site, but there were no detections at the RCAin and URCAout sites (Fig. 2). For all Canada goose Bacteroidales marker data, detection trends were similar to those for the muskrat Bacteroidales marker, where the highest Canada goose marker detections were observed at the RCAout\URCAin site (13%) in relation to 1% at both the RCAin and URCAout sites (Fig. 2). Similar statistically significant trends for Canada goose Bacteroidales marker data were observed for the high-flow and spring data sets.
The rate of detection of the human Bacteroidales marker was significantly higher at the RCAout\URCAin site (19%) than at the RCAin site (4%) for all data (Fig. 2). Similar, statistically significant trends in the human marker were observed in summer and at high flows as well. Support for the trends observed in Fig. 2 to 3 is given in Table S2A and B in the supplemental material. The site trends are believed to correspond to the downstream impact from homes located immediately adjacent to the pasture treatments.
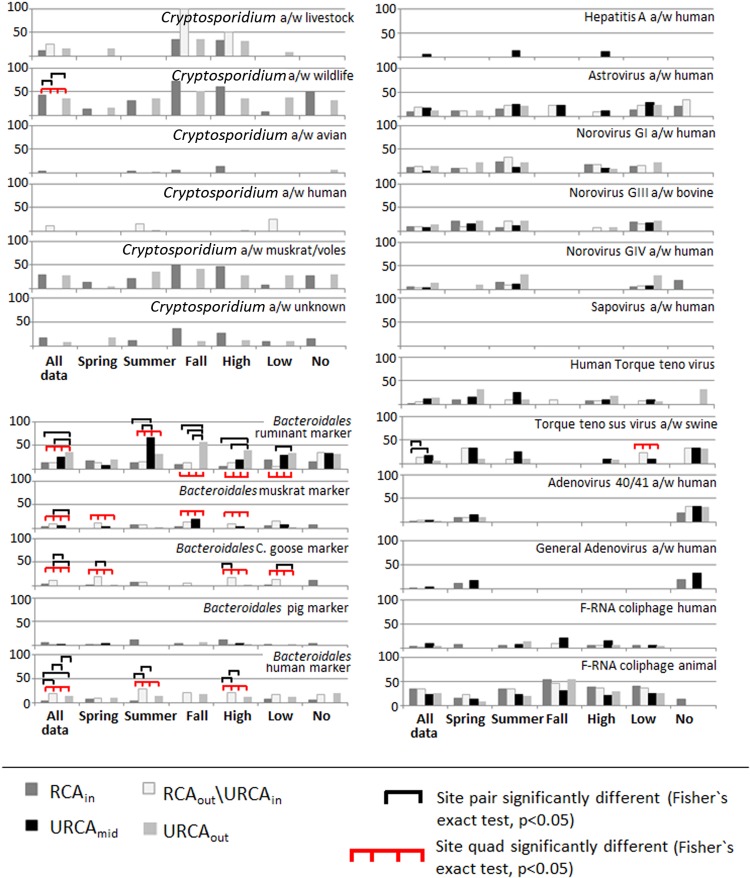
Fig 3.
Percentage of samples positive for microbial source tracking markers by specific site, season, and flow conditions. Data represented here were based on AAD. See Fig. 2 for flow regime limits. a/w, associated with.
To assess the impact of the BMP on marker trends, taking into account small differences in stream flow at each sample site that could impact dilution and mass transport downstream along the pasture system, the numbers of Bacteroidales copies day−1 were compared. Differences in the distribution of the numbers of Bacteroidales copies day−1 in stream water for the ruminant Bacteroidales marker were observed between the RCAout\URCAin and URCAout sites for TCD (Table 1), where lower average rank sums were observed for the RCAout\URCAin site. At low flow, average rank sums of the ruminant Bacteroidales marker were lower for the RCAout\URCAin site than for the URCAout site (TCD). Additionally, ruminant Bacteroidales marker means (number of copies day−1) were consistently higher for the URCAout site than for the RCAout\URCAin site (Table 2); similar trends were observed for AAD (Tables 3 and 4). Significant differences in the distribution of wildlife-related Bacteroidales markers (number of copies day−1), namely, the muskrat marker, were observed between the RCAout\URCAin and the URCAout sites (Tables 1 and 3). Average rank sum values for the muskrat Bacteroidales marker for TCD were higher for the RCAout\URCAin site than for the URCAout site overall, and for spring data, average rank sums showed similar trends. Average rank sums of the muskrat Bacteroidales marker for TCD were greater for the RCAout\URCAin site than for the RCAin site in spring. Muskrat Bacteroidales marker means (number of copies day−1) were higher for the RCAout\URCAin site than for the URCAout site (Table 4) for AAD. Average rank sums of the Canada goose Bacteroidales marker increased from the RCAin site to the RCAout\URCAin site for TCD (all data), complementing the muskrat marker wildlife trends. All average rank sums for the number of copies day−1 for human markers that were significantly different (Table 1) in the TCD were higher in the downstream direction (rank sums were higher for the RCAout\URCAin and URCAout sites than the RCAin site). The results for the number of copies day−1 presented above mimicked very closely the trends in Bacteroidales marker number of copies 100 ml−1 data in Tables S2A and S3B in the supplemental material, due to the relatively short distance between sampling sites and the limited hydrologic contributing area.
Table 1.
Median and mean absolute deviation of source Bacteroidales for TCD by season and flow condition
| Bacteroidales MST marker endpoint | Site (site no.) | Source Bacteroidales (no. of copies day−1)a |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All data |
Spring |
Summer |
Fall |
High flow |
Low flow |
No flow |
|||||||||
| MAD | ME | MAD | ME | MAD | ME | MAD | ME | MAD | ME | MAD | ME | MAD | ME | ||
| Ruminant | RCAin (18) | 3.37E+10 | 0KW(15W) | 6.82E+10 | 0 | 1.23E+10 | 0 | 6.31E+09 | 0KW(15W) | 7.62E+10 | 0 | 6.85E+09 | 0 | 7.78E+08 | 0 |
| Ruminant | RCAout\URCAin (22) | 1.79E+10 | 0KW(15W) | 9.85E+09 | 0 | 3.28E+10 | 0 | 9.25E+09 | 0KW(15W) | 9.25E+09 | 0 | 3.23E+09 | 0(15W) | 2.06E+09 | 0 |
| Ruminant | URCAout (15) | 1.35E+11 | 0KW(18W, 22W) | 3.49E+10 | 0 | 3.13E+11 | 0 | 4.52E+10 | 0KW(18W, 22W) | 6.28E+10 | 0 | 2.57E+11 | 0(22W) | 1.66E+10 | 0 |
| Muskrat | RCAin (18) | 1.62E+08 | 0KW | 0 | 0KW(22W) | 2.38E+08 | 0 | 3.33E+08 | 0 | 0 | 0KW | 3.67E+08 | 0 | 2.06E+07 | 0 |
| Muskrat | RCAout\URCAin (22) | 9.60E+09 | 0KW(15W) | 2.95E+09 | 0KW(18W, 15W) | 1.61E+10 | 0 | 1.26E+10 | 0 | 1.86E+10 | 0KW | 4.64E+09 | 0 | 0 | 0 |
| Muskrat | URCAout (15) | 9.63E+08 | 0KW(22W) | 0 | 0KW(22W) | 2.72E+09 | 0 | 0 | 0 | 0 | 0KW | 2.26E+09 | 0 | 0 | 0 |
| C. goose | RCAin (18) | 2.97E+09 | 0KW(22W) | 0 | 0KW(22W) | 8.40E+09 | 0 | 0 | 0 | 0 | 0KW(22W) | 6.95E+09 | 0 | 0 | 0 |
| C. goose | RCAout\URCAin (22) | 1.37E+11 | 0KW(18W, 15W) | 2.79E+11 | 0KW(18W) | 1.81E+10 | 0 | 2.11E+10 | 0 | 2.93E+11 | 0KW(18W) | 3.38E+10 | 0(15W) | 0 | 0 |
| C. goose | URCAout (15) | 7.20E+10 | 0KW(22W) | 1.52E+11 | 0KW | 0 | 0 | 0 | 0 | 1.62E+11 | 0KW | 0 | 0(22W) | 0 | 0 |
| Pig | RCAin (18) | 7.93E+10 | 0 | 9.01E+09 | 0 | 1.65E+11 | 0KW | 7.54E+10 | 0 | 1.78E+11 | 0(15W) | 9.60E+08 | 0 | 0 | 0 |
| Pig | RCAout\URCAin (22) | 3.29E+10 | 0 | 7.46E+10 | 0 | 0 | 0KW | 0 | 0 | 7.99E+10 | 0 | 0 | 0 | 0 | 0 |
| Pig | URCAout (15) | 2.12E+09 | 0 | 0 | 0 | 0 | 0KW | 8.99E+09 | 0 | 0 | 0(18W) | 4.98E+09 | 0 | 0 | 0 |
| Human | RCAin (18) | 4.37E+09 | 0KW(22W, 15W) | 9.69E+09 | 0 | 0 | 0KW(22W, 15W) | 0 | 0 | 0 | 0KW(22W, 15W) | 1.00E+10 | 0 | 0 | 0 |
| Human | RCAout\URCAin (22) | 1.30E+12 | 0KW(18W) | 1.20E+12 | 0 | 9.56E+11 | 0KW(18W) | 1.96E+12 | 0 | 1.75E+12 | 0KW(18W) | 1.27E+12 | 0 | 2.29E+09 | 0 |
| Human | URCAout (15) | 6.40E+11 | 0KW(18W) | 1.29E+12 | 0 | 2.88E+10 | 0KW(18W) | 2.87E+11 | 0 | 1.48E+12 | 0KW(18W) | 3.85E+10 | 0 | 1.20E+10 | 0 |
Significant differences between sites (identified by site number) are indicated in the median column and are in bold (P < 0.05). High flow, ≥0.018 m3 s−1; low flow, ≥0.002 m3 s−1 and <0.018 m3 s−1; no flow, <0.002 m3 s−1. Accompanying significant Kruskal-Wallis (KW) and Mann-Whitney U (W) test results among sites and site pairs, respectively, are also presented. ME, median; MAD mean absolute deviation. C. goose, Canada goose.
Table 2.
Mean source Bacteroidales, number of samples, and standard deviation for TCD by season and flow conditiona
| Bacteroidales MST marker endpoint | Site | All data |
Spring |
Summer |
Fall |
High flow |
Low flow |
No flow |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Source Bacteroidales (no. of copies day−1) |
N | Source Bacteroidales (no. of copies day−1) |
N | Source Bacteroidales (no. of copies day−1) |
N | Source Bacteroidales (no. of copies day−1) |
N | Source Bacteroidales (no. of copies day−1) |
N | Source Bacteroidales (no. of copies day−1) |
N | Source Bacteroidales (no. of copies day−1) |
|||||||||
| M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | |||||||||
| Ruminant | RCAin | 64 | 1.87E+10 | 1.17E+11 | 26 | 3.79E+10 | 1.81E+11 | 23 | 6.87E+09 | 3.08E+10 | 15 | 3.64E+09 | 1.21E+10 | 26 | 4.13E+10 | 1.83E+11 | 27 | 4.41E+09 | 1.10E+10 | 11 | 4.79E+08 | 1.26E+09 |
| Ruminant | RCAout\URCAin | 64 | 9.97E+09 | 5.17E+10 | 26 | 5.51E+09 | 2.53E+10 | 23 | 1.80E+10 | 8.17E+10 | 15 | 5.34E+09 | 1.50E+10 | 26 | 2.21E+10 | 8.02E+10 | 27 | 1.75E+09 | 6.33E+09 | 11 | 1.44E+09 | 3.11E+09 |
| Ruminant | URCAout | 64 | 8.30E+10 | 4.40E+11 | 26 | 2.02E+10 | 6.69E+10 | 23 | 1.84E+11 | 7.28E+11 | 15 | 3.76E+10 | 6.19E+10 | 26 | 4.48E+10 | 1.07E+11 | 27 | 1.50E+11 | 6.70E+11 | 11 | 9.28E+09 | 3.03E+10 |
| Muskrat | RCAin | 67 | 8.46E+07 | 4.75E+08 | 29 | 0 | 0 | 23 | 1.30E+08 | 5.95E+08 | 15 | 1.78E+08 | 6.91E+08 | 27 | 0 | 0 | 28 | 1.98E+08 | 7.26E+08 | 12 | 1.12E+07 | 3.90E+07 |
| Muskrat | RCAout\URCAin | 67 | 5.40E+09 | 2.35E+10 | 29 | 1.71E+09 | 5.23E+09 | 23 | 8.84E+09 | 3.66E+10 | 15 | 7.24E+09 | 1.96E+10 | 27 | 1.05E+10 | 3.60E+10 | 28 | 2.81E+09 | 7.36E+09 | 12 | 0 | 0 |
| Muskrat | URCAout | 67 | 4.89E+08 | 4.00E+09 | 29 | 0 | 0 | 23 | 1.42E+09 | 6.83E+09 | 15 | 0 | 0 | 27 | 0 | 0 | 28 | 1.17E+09 | 6.19E+09 | 12 | 0 | 0 |
| C. goose | RCAin | 67 | 1.51E+09 | 1.23E+10 | 29 | 0 | 0 | 23 | 4.39E+09 | 2.11E+10 | 15 | 0 | 0 | 27 | 0 | 0 | 28 | 3.61E+09 | 1.91E+10 | 12 | 0 | 0 |
| C. goose | RCAout\URCAin | 67 | 7.88E+10 | 4.26E+11 | 29 | 1.68E+11 | 6.42E+11 | 23 | 9.89E+09 | 3.46E+10 | 15 | 1.13E+10 | 4.38E+10 | 27 | 1.75E+11 | 6.65E+11 | 28 | 1.97E+10 | 5.50E+10 | 12 | 0 | 0 |
| C. goose | URCAout | 67 | 3.81E+10 | 2.94E+11 | 29 | 7.85E+10 | 4.23E+11 | 23 | 0 | 0 | 15 | 0 | 0 | 27 | 8.44E+10 | 4.38E+11 | 28 | 0 | 0 | 12 | 0 | 0 |
| Pig | RCAin | 67 | 4.24E+10 | 1.95E+11 | 29 | 4.67E+09 | 2.51E+10 | 23 | 9.13E+10 | 3.05E+11 | 15 | 4.04E+10 | 1.56E+11 | 27 | 1.05E+11 | 2.99E+11 | 28 | 4.98E+08 | 2.63E+09 | 12 | 0 | 0 |
| Pig | RCAout\URCAin | 67 | 1.67E+10 | 1.37E+11 | 29 | 3.86E+10 | 2.08E+11 | 23 | 0 | 0 | 15 | 0 | 0 | 27 | 4.15E+10 | 2.16E+11 | 28 | 0 | 0 | 12 | 0 | 0 |
| Pig | URCAout | 67 | 1.08E+09 | 8.83E+09 | 29 | 0 | 0 | 23 | 0 | 0 | 15 | 4.82E+09 | 1.87E+10 | 27 | 0 | 0 | 28 | 2.58E+09 | 1.37E+10 | 12 | 0 | 0 |
| Human | RCAin | 67 | 2.25E+09 | 1.30E+10 | 29 | 5.20E+09 | 1.95E+10 | 23 | 0 | 0 | 15 | 0 | 0 | 27 | 0 | 0 | 28 | 5.39E+09 | 1.98E+10 | 12 | 0 | 0 |
| Human | RCAout\URCAin | 67 | 7.53E+11 | 2.46E+12 | 29 | 6.43E+11 | 2.69E+12 | 23 | 5.83E+11 | 1.56E+12 | 15 | 1.22E+12 | 3.16E+12 | 27 | 1.13E+12 | 2.65E+12 | 28 | 7.14E+11 | 2.77E+12 | 12 | 1.25E+09 | 4.33E+09 |
| Human | URCAout | 67 | 3.48E+11 | 2.20E+12 | 29 | 7.04E+11 | 3.31E+12 | 23 | 1.65E+10 | 7.10E+10 | 15 | 1.66E+11 | 5.96E+11 | 27 | 8.37E+11 | 3.44E+12 | 28 | 2.10E+10 | 7.74E+10 | 12 | 8.01E+09 | 1.90E+10 |
See Table 1 for flow regime definitions. M, mean; SD, standard deviation; N, number of samples; C. goose, Canada goose.
Table 3.
Median and mean absolute deviation of source Bacteroidales for AAD by season and flow condition
| Bacteroidales MST marker endpoint | Site (site no.) | Source Bacteroidales (no. of copies day−1)a |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All data |
Spring |
Summer |
Fall |
High |
Low |
No flow |
|||||||||
| MAD | ME | MAD | ME | MAD | ME | MAD | ME | MAD | ME | MAD | ME | MAD | ME | ||
| Ruminant | RCAin (18) | 2.65E+10 | 0KW(15W) | 6.14E+10 | 0 | 8.44E+09 | 0KW(23W, 15W) | 4.91E+09 | 0KW(15W) | 6.68E+10 | 0KW(15W) | 6.51E+09 | 0KW | 4.75E+08 | 0 |
| Ruminant | RCAout\URCAin (22) | 1.76E+10 | 0KW(15W) | 1.12E+10 | 0 | 3.04E+10 | 0KW(23W) | 9.25E+09 | 0KW(15W) | 3.75E+10 | 0KW(15W) | 3.03E+09 | 0KW(23W, 15W) | 2.06E+09 | 0 |
| Ruminant | URCAmid (23) | 1.64E+11 | 0KW | 4.03E+10 | 0 | 3.73E+11 | 0KW(18W, 22W, 15W) | 0 | 0KW(15W) | 2.35E+11 | 0KW | 1.15E+11 | 0KW(22W) | 5.39E+09 | 0 |
| Ruminant | URCAout (15) | 1.27E+11 | 0KW(18W, 22W) | 3.18E+10 | 0 | 2.76E+11 | 0KW(18W, 23W) | 6.01E+10 | 0KW(18W, 22W, 23W) | 9.40E+10 | 0KW(18W, 22W) | 2.05E+11 | 0KW(22W) | 1.60E+10 | 0 |
| Muskrat | RCAin (18) | 1.27E+08 | 0KW(22W) | 0 | 0KW(22W) | 1.66E+08 | 0 | 2.54E+08 | 0 | 0 | 0 | 3.44E+08 | 0 | 1.04E+07 | 0 |
| Muskrat | RCAout\URCAin (22) | 9.12E+09 | 0KW(18W, 15W) | 2.79E+09 | 0KW(18W, 15W) | 1.50E+10 | 0 | 1.26E+10 | 0 | 1.75E+10 | 0 | 4.38E+09 | 0 | 0 | 0 |
| Muskrat | URCAmid (23) | 6.02E+09 | 0KW | 1.55E+09 | 0KW | 0 | 0 | 2.17E+10 | 0(15W) | 1.00E+10 | 0 | 3.34E+09 | 0 | 0 | 0 |
| Muskrat | URCAout (15) | 7.80E+08 | 0KW(22W) | 0 | 0KW(22W) | 2.11E+09 | 0 | 0 | 0(23W) | 0 | 0 | 1.82E+09 | 0 | 0 | 0 |
| C. goose | RCAin (18) | 2.32E+09 | 0KW(22W) | 3.68E+07 | 0KW(22W) | 5.76E+09 | 0 | 0 | 0 | 0 | 0KW(22W) | 6.51E+09 | 0KW | 4.67E+07 | 0 |
| C. goose | RCAout\URCAin (22) | 1.30E+11 | 0KW(18W, 23W, 15W) | 2.64E+11 | 0KW(18W, 23W) | 1.67E+10 | 0 | 2.11E+10 | 0 | 2.76E+11 | 0KW(18W, 23W) | 3.19E+10 | 0KW(15W) | 0 | 0 |
| C. goose | URCAmid (23) | 0 | 0KW(22W) | 0 | 0KW(22W) | 0 | 0 | 0 | 0 | 0 | 0KW(22W) | 0 | 0KW | 0 | 0 |
| C. goose | URCAout (15) | 5.42E+10 | 0KW(22W) | 1.52E+11 | 0KW | 0 | 0 | 0 | 0 | 1.34E+11 | 0KW | 0 | 0KW(22W) | 0 | 0 |
| Pig | RCAin (18) | 6.27E+10 | 0 | 8.19E+09 | 0 | 1.15E+11 | 0 | 5.75E+10 | 0 | 1.59E+11 | 0 | 8.98E+08 | 0 | 0 | 0 |
| Pig | RCAout\URCAin (22) | 3.11E+10 | 0 | 6.99E+10 | 0 | 0 | 0 | 0 | 0 | 7.46E+10 | 0 | 0 | 0 | 0 | 0 |
| Pig | URCAmid (23) | 4.67E+09 | 0 | 9.51E+09 | 0 | 0 | 0 | 0 | 0 | 1.04E+10 | 0 | 0 | 0 | 0 | 0 |
| Pig | URCAout (15) | 4.63E+09 | 0 | 0 | 0 | 0 | 0 | 1.51E+10 | 0 | 7.34E+09 | 0 | 4.01E+09 | 0 | 0 | 0 |
| Human | RCAin (18) | 3.43E+09 | 0KW(22W, 15W) | 8.84E+09 | 0 | 0 | 0KW(22W, 15W) | 0 | 0(22W) | 0 | 0KW(22W, 15W) | 9.39E+09 | 0 | 0 | 0(22W, 15W) |
| Human | RCAout\URCAin (22) | 1.24E+12 | 0KW(18W, 23W) | 1.13E+12 | 0 | 8.95E+11 | 0KW(18W, 23W) | 1.96E+12 | 0(18W) | 1.66E+12 | 0KW(18W, 23W) | 1.19E+12 | 0(23W) | 2.29E+09 | 0(18W) |
| Human | URCAmid (23) | 0 | 0KW(22W, 15W) | 0 | 0 | 0 | 0KW(22W) | 0 | 0 | 0 | 0KW(22W) | 0 | 0(22W) | 0 | 0 |
| Human | URCAout (15) | 5.25E+11 | 0KW(18W, 23W) | 1.29E+12 | 0 | 2.26E+10 | 0KW(18W) | 2.04E+11 | 0 | 1.24E+12 | 0KW(18W) | 4.93E+10 | 0 | 1.03E+10 | 0(18W) |
Significant differences between sites (identified by associated sample site number given in second column) are indicated in the median column and are in bold (P < 0.05). Accompanying significant Kruskal-Wallis (KW) and Mann-Whitney U (W) test results among sites and site pairs, respectively, are also presented. See Table 1 for flow regime definitions. ME, median; MAD, mean absolute deviation; C. goose, Canada goose.
Table 4.
Mean source Bacteroidales and number of samples and standard deviation for AAD under season and flow conditionsa
| Bacteroidales MST marker endpoint | Site | All data |
Spring |
Summer |
Fall |
High flow |
Low flow |
No flow |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Source Bacteroidales (no. of copies day−1) |
N | Source Bacteroidales (no. of copies day−1) |
N | Source Bacteroidales (no. of copies day−1) |
N | Source Bacteroidales (no. of copies day−1) |
N | Source Bacteroidales (no. of copies day−1) |
N | Source Bacteroidales (no. of copies day−1) |
N | Source Bacteroidales (no. of copies day−1) |
|||||||||
| M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | |||||||||
| Ruminant | RCAin | 83 | 1.44E+10 | 1.03E+11 | 29 | 3.39E+10 | 1.71E+11 | 34 | 4.69E+09 | 2.53E+10 | 20 | 2.73E+09 | 1.05E+10 | 30 | 3.58E+10 | 1.70E+11 | 29 | 4.10E+09 | 1.07E+10 | 24 | 2.73E+08 | 8.93E+08 |
| Ruminant | RCAout\URCAin | 68 | 9.93E+09 | 5.03E+10 | 28 | 6.44E+09 | 2.51E+10 | 25 | 1.66E+10 | 7.84E+10 | 15 | 5.34E+09 | 1.50E+10 | 28 | 2.19E+10 | 7.73E+10 | 29 | 1.63E+09 | 6.12E+09 | 11 | 1.44E+09 | 3.11E+09 |
| Ruminant | URCAmid | 48 | 9.89E+10 | 2.87E+11 | 23 | 2.21E+10 | 8.14E+10 | 15 | 2.83E+11 | 4.61E+11 | 10 | 0 | 0 | 21 | 1.45E+11 | 3.75E+11 | 24 | 7.02E+10 | 2.04E+11 | 3 | 4.04E+09 | 7.00E+09 |
| Ruminant | URCAout | 83 | 8.29E+10 | 3.93E+11 | 29 | 1.81E+10 | 6.35E+10 | 30 | 1.64E+11 | 6.45E+11 | 24 | 5.93E+10 | 7.42E+10 | 33 | 7.14E+10 | 1.48E+11 | 35 | 1.25E+11 | 5.89E+11 | 15 | 1.01E+10 | 2.66E+10 |
| Muskrat | RCAin | 86 | 6.59E+07 | 4.20E+08 | 32 | 0 | 0 | 34 | 8.80E+07 | 4.90E+08 | 20 | 1.34E+08 | 5.99E+08 | 31 | 0 | 0 | 30 | 1.84E+08 | 7.02E+08 | 25 | 5.40E+06 | 2.70E+07 |
| Muskrat | RCAout\URCAin | 71 | 5.09E+09 | 2.28E+10 | 31 | 1.60E+09 | 5.07E+09 | 25 | 8.14E+09 | 3.51E+10 | 15 | 7.24E+09 | 1.96E+10 | 29 | 9.76E+09 | 3.48E+10 | 30 | 2.62E+09 | 7.14E+09 | 12 | 0 | 0 |
| Muskrat | URCAmid | 48 | 3.21E+09 | 1.64E+10 | 23 | 8.09E+08 | 3.88E+09 | 15 | 0 | 0 | 10 | 1.36E+10 | 3.49E+10 | 21 | 5.26E+09 | 2.41E+10 | 24 | 1.82E+09 | 6.25E+09 | 3 | 0 | 0 |
| Muskrat | URCAout | 83 | 3.95E+08 | 3.60E+09 | 29 | 0 | 0 | 30 | 1.09E+09 | 5.98E+09 | 24 | 0 | 0 | 33 | 0 | 0 | 35 | 9.36E+08 | 5.54E+09 | 15 | 0 | 0 |
| C. goose | RCAin | 86 | 1.18E+09 | 1.09E+10 | 32 | 1.90E+07 | 1.07E+08 | 34 | 2.97E+09 | 1.73E+10 | 20 | 0 | 0 | 31 | 0 | 0 | 30 | 3.37E+09 | 1.84E+10 | 25 | 2.44E+07 | 1.22E+08 |
| C. goose | RCAout\URCAin | 71 | 7.44E+10 | 4.14E+11 | 31 | 1.58E+11 | 6.21E+11 | 25 | 9.10E+09 | 3.33E+10 | 15 | 1.13E+10 | 4.38E+10 | 29 | 1.63E+11 | 6.42E+11 | 30 | 1.84E+10 | 5.33E+10 | 12 | 0 | 0 |
| C. goose | URCAmid | 48 | 0 | 0 | 23 | 0 | 0 | 15 | 0 | 0 | 10 | 0 | 0 | 21 | 0 | 0 | 24 | 0 | 0 | 3 | 0 | 0 |
| C. goose | URCAout | 83 | 2.74E+10 | 2.50E+11 | 29 | 7.85E+10 | 4.23E+11 | 30 | 0 | 0 | 24 | 0 | 0 | 33 | 6.90E+10 | 3.97E+11 | 35 | 0 | 0 | 15 | 0 | 0 |
| Pig | RCAin | 86 | 3.30E+10 | 1.72E+11 | 32 | 4.23E+09 | 2.39E+10 | 34 | 6.17E+10 | 2.52E+11 | 20 | 3.03E+10 | 1.35E+11 | 31 | 9.12E+10 | 2.80E+11 | 30 | 4.64E+08 | 2.54E+09 | 25 | 0 | 0 |
| Pig | RCAout\URCAin | 71 | 1.58E+10 | 1.33E+11 | 31 | 3.61E+10 | 2.01E+11 | 25 | 0 | 0 | 15 | 0 | 0 | 29 | 3.86E+10 | 2.08E+11 | 30 | 0 | 0 | 12 | 0 | 0 |
| Pig | URCAmid | 48 | 2.38E+09 | 1.65E+10 | 23 | 4.97E+09 | 2.39E+10 | 15 | 0 | 0 | 10 | 0 | 0 | 21 | 5.45E+09 | 2.50E+10 | 24 | 0 | 0 | 3 | 0 | 0 |
| Pig | URCAout | 83 | 2.37E+09 | 1.57E+10 | 29 | 0 | 0 | 30 | 0 | 0 | 24 | 8.21E+09 | 2.89E+10 | 33 | 3.78E+09 | 2.17E+10 | 35 | 2.06E+09 | 1.22E+10 | 15 | 0 | 0 |
| Human | RCAin | 86 | 1.75E+09 | 1.15E+10 | 32 | 4.72E+09 | 1.86E+10 | 34 | 0 | 0 | 20 | 0 | 0 | 31 | 0 | 0 | 30 | 5.03E+09 | 1.92E+10 | 25 | 0 | 0 |
| Human | RCAout\URCAin | 71 | 7.10E+11 | 2.40E+12 | 31 | 6.01E+11 | 2.61E+12 | 25 | 5.37E+11 | 1.50E+12 | 15 | 1.22E+12 | 3.16E+12 | 29 | 1.05E+12 | 2.57E+12 | 30 | 6.66E+11 | 2.68E+12 | 12 | 0 | 4.33E+09 |
| Human | URCAmid | 48 | 0 | 0 | 23 | 0 | 0 | 15 | 0 | 0 | 10 | 0 | 0 | 21 | 0 | 0 | 24 | 0 | 0 | 3 | 0 | 0 |
| Human | URCAout | 83 | 2.85E+11 | 1.98E+12 | 29 | 7.04E+11 | 3.31E+12 | 30 | 1.26E+10 | 6.23E+10 | 24 | 1.19E+11 | 4.75E+11 | 33 | 6.85E+11 | 3.12E+12 | 35 | 2.71E+10 | 9.07E+10 | 15 | 6.41E+09 | 1.72E+10 |
See Table 1 for flow regime definitions. M, mean; SD, standard deviation; N, number of samples; C. goose, Canada goose.
The most commonly occurring serovars of Salmonella were Salmonella serovar I:4,5,12:b:− (n = 22), followed by Salmonella serovars Kentucky (n = 8), Mbandaka (n = 3), and Bovismorbificans (n = 3) (see Table S1C in the supplemental material). Salmonella serovar I:4,5,12:b:− was found most often at the RCAout\URCAin (n = 9), URCAmid (n = 5), and URCAout (n = 8) sites.
Of note, for AAD (Fig. 3), all animal TTV detections were significantly different among some sites and at low flows, where detections were significantly higher at the RCAout\URCAin (15%) and URCAmid (17%) sites than at the RCAin (0%) site (Fig. 3). At low flows, animal TTV virus detections were higher at the RCAout\URCAin site (23%) than at the RCAin (0%), URCAmid (11%), and URCAout (0%) sites.
Cattle exclusion BMP, season, and flow interactions among Bacteroidales source markers and pathogens.
The CART analyses revealed that higher flow in fall was a condition that most strongly delineated occurrences of Cryptosporidium isolates of livestock origin from those of wildlife origin (see Table S4 in the supplemental material). However, the greatest number of occurrences of Cryptosporidium associated with the muskrat genotype (consisting of muskrat genotypes I and II) and of Cryptosporidium linked to unknown sources were delineated respectively in summer and fall and in fall only (see Table S4 in the supplemental material).
In contrast to the Cryptosporidium data, the effects of site (cattle exclusion BMP) and flow regime were more notable for the ruminant and muskrat Bacteroidales source markers. For Canada goose and pig markers, sample site and, hence, cattle exclusion practices were found to be the most important for delineating marker occurrences when CART was used, whereas for the human markers, seasonal and sample site interactions were critical for delineating marker occurrences when CART was used (see Table S4 in the supplemental material). Using CART, it was determined that norovirus GI (human-associated) and GIII (bovine-associated) occurrences were most strongly delineated on the basis of seasonal and flow variable interactions. Cattle exclusion effects were important independent criteria for defining human and animal torque tenovirus observations. For delineating F-RNA coliphages associated with animals, seasonal attributes were found via CART to be of utmost importance.
Co-occurrence of host-specific markers and viruses.
All sites were examined qualitatively through time (10-day windows throughout the course of the study) for the detection of host-specific Bacteroidales markers, generalized host classes of Cryptosporidium, and host-specific viruses using the heat map approach (see Fig. S1 in the supplemental material). For the human markers and pathogens, there were no co-occurrences between human Cryptosporidium and human Bacteroidales marker. There were a total of 53 human virus observations. This generated 23 human virus events within the heat map and 9 co-occurrences of the human Bacteroidales marker (39%) for these 23 events (see Fig. S1 in the supplemental material).
For the ruminants (livestock), there were 4 out of 20 (20%) occurrences of livestock-associated Cryptosporidium (C. andersoni, C. parvum, or C. ubiquitum) when the ruminant Bacteroidales marker was detected (OR, 1.7; 95% confidence interval [CI], 0.3 to 7.6), 2 out of 9 (22%) occurrences of the ruminant Bacteroidales marker when norovirus GIII associated with bovine was present (OR, 0.9; 95% CI, 0.1 to 5), and 9 out of 41 (∼23%) occurrences of the ruminant Bacteroidales marker when F-RNA coliphage (animal) was present (OR, 0.8; 95% CI, 0.3 to 2.1) in samples collected at the same site and time (see Fig. S1 and Table S5A and B in the supplemental material), but here, there were no significant results (see Tables S5B and C in the supplemental material). For pig, there were a total of 10 occurrences of animal torque teno virus associated with swine but no co-occurrences of the pig Bacteroidales marker in these samples.
There were 2 observations of avian-associated Cryptosporidium and no co-occurrence with the Canada goose Bacteroidales marker (see Table S5A in the supplemental material). Of some 42 F-RNA coliphage animal detections, there were only 3 concurrent Canada goose Bacteroidales marker observations (7%). For muskrat/vole-associated Cryptosporidium (muskrat genotypes I and II), there were 17 observations and no co-occurrence with the muskrat Bacteroidales marker. Of some 41 F-RNA coliphage animal observations, there were only 4 co-occurrences of the muskrat marker (10%).
The cross tabulations and contingency table analyses (see Tables 5A to 5C in the supplemental material), performed to assess the overall co-occurrence of host-specific markers quantitatively with Fisher's exact tests and odds ratio analyses using AAD, indicated the following significant findings: (i) positive associations among Cryptosporidium (unknown) and livestock, muskrat genotypes, and wildlife generalized Cryptosporidium host classes, (ii) negative associations among Canada goose Bacteroidales marker and the ruminant Bacteroidales markers, (iii) norovirus GIV associated with humans and astrovirus associated with humans exhibited a positive relationship, (iv) norovirus GIV associated with humans and norovirus GIII associated with bovines showed a positive relationship, and (v) F-RNA coliphages associated with animals and norovirus GIII associated with bovines also exhibited a positive relationship.
Associations among pathogens, Bacteroidales, and other source tracking markers.
There were two notable significant associations between the markers and pathogens, and these were between the ruminant Bacteroidales marker and Cryptosporidium (see Fig. S2 in the supplemental material), where there were significantly higher rates of detection of the ruminant marker in the presence of Cryptosporidium, with an associated OR estimate of ∼4.7 and a CI above unity, and between the Canada goose marker and norovirus GII (OR estimate of 0.00 and a CI below unity indicating negative associations) (Table 5).
Table 5.
Odds ratios (OR) and upper and lower confidence intervals (CI) for MST endpoints versus selected pathogens in water using AADa
| Value for indicated pathogen(s) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| MST endpoint | Estimate | Campylobacter spp. | Salmonella spp. | E. coli O157:H7 | Cryptosporidium | Giardia | Hepatitis E | Norovirus GII | Rotavirus |
| Cryptosporidium a/w livestock | Lower 95% CI OR estimate | 0.25 | 0.29 | 0.00 | 6.76 | 0.24 | 0.00 | 0.00 | 0.00 |
| OR estimate | 1.27 | 3.62 | 0.00 | ∞ | 1.13 | 0.00 | 0.00 | 0.00 | |
| Upper 95% CI OR estimate | 5.35 | 29.35 | ∞ | ∞ | 4.25 | ∞ | 11.81 | ∞ | |
| Cryptosporidium a/w wildlife | Lower 95% CI OR estimate | 0.82 | 0.28 | 0.00 | 17.73 | 0.11 | 0.00 | 0.00 | 0.00 |
| OR estimate | 2.35 | 2.21 | 0.00 | ∞ | 0.42 | 0.00 | 0.00 | 0.00 | |
| Upper 95% CI OR estimate | 6.77 | 17.62 | ∞ | ∞ | 1.34 | ∞ | 77.91 | ∞ | |
| Cryptosporidium a/w avians | Lower 95% CI OR estimate | 0.06 | 0.00 | 0.00 | 0.32 | 0.03 | 0.00 | 0.00 | 0.00 |
| OR estimate | ∞ | 0.00 | 0.00 | ∞ | 1.83 | 0.00 | 0.00 | 0.00 | |
| Upper 95% CI OR estimate | ∞ | 541.39 | ∞ | ∞ | 36.67 | ∞ | ∞ | ∞ | |
| Cryptosporidium a/w humans | Lower 95% CI OR estimate | 0.00 | 0.00 | 0.00 | 0.19 | 0.09 | 0.00 | 0.00 | 0.00 |
| OR estimate | 0.00 | 0.00 | 0.00 | ∞ | 7.18 | 0.00 | 0.00 | 0.00 | |
| Upper 95% CI OR estimate | 13.21 | 79.75 | ∞ | ∞ | 588.77 | ∞ | ∞ | ∞ | |
| Cryptosporidium a/w muskrats/voles | Lower 95% CI OR estimate | 0.65 | 0.42 | 0.00 | 9.75 | 0.18 | 0.00 | 0.00 | 0.00 |
| OR estimate | 2.06 | 3.36 | 0.00 | ∞ | 0.69 | 0.00 | 0.00 | 0.00 | |
| Upper 95% CI OR estimate | 6.35 | 27.26 | ∞ | ∞ | 2.21 | ∞ | ∞ | ∞ | |
| Cryptosporidium a/w unknown sources | Lower 95% CI OR estimate | 0.25 | 0.29 | 0.00 | 2.46 | 0.07 | 0.00 | 0.00 | 0.00 |
| OR estimate | 1.27 | 3.62 | 0.00 | ∞ | 0.70 | 0.00 | 0.00 | 0.00 | |
| Upper 95% CI OR estimate | 5.35 | 29.35 | ∞ | ∞ | 3.66 | ∞ | 77.91 | ∞ | |
| Bacteroidales ruminant marker | Lower 95% CI OR estimate | 0.28 | 0.01 | 0.33 | 1.33 | 0.41 | 0.08 | 0.67 | 0.03 |
| OR estimate | 0.59 | 0.39 | 6.50 | 4.74 | 1.79 | ∞ | 2.68 | 1.54 | |
| Upper 95% CI OR estimate | 1.19 | 3.00 | 389.00 | 21.66 | 6.89 | ∞ | 10.17 | 30.79 | |
| Bacteroidales muskrat marker | Lower 95% CI OR estimate | 0.12 | 0.00 | 0.00 | 0.01 | 0.04 | 0.00 | 0.02 | 0.00 |
| OR estimate | 0.55 | 0.00 | 0.00 | 0.42 | 2.36 | 0.00 | 0.88 | 0.00 | |
| Upper 95% CI OR estimate | 1.97 | 7.85 | 36.75 | 8.46 | 48.35 | 450.19 | 7.85 | 19.46 | |
| Bacteroidales Canada goose marker | Lower 95% CI OR estimate | 0.32 | 0.00 | 0.00 | 0.09 | 0.04 | 0.00 | 0.00 | 0.00 |
| OR estimate | 1.43 | 0.00 | 0.00 | 1.76 | 2.36 | 0.00 | 0.00 | 0.00 | |
| Upper 95% CI OR estimate | 6.43 | ∞ | 53.70 | 106.91 | 48.35 | 1249.15 | 0.83 | 71.37 | |
| Bacteroidales pig marker | Lower 95% CI OR estimate | 0.10 | 0.00 | 0.00 | 0.21 | 0.34 | 0.00 | 0.00 | 0.00 |
| OR estimate | 1.42 | 0.00 | 0.00 | 2.68 | 5.01 | 0.00 | 0.00 | 0.00 | |
| Upper 95% CI OR estimate | 19.90 | 38.60 | 157.86 | 146.06 | 74.80 | 3676.05 | 241.45 | 931.99 | |
| Bacteroidales human marker | Lower 95% CI OR estimate | 0.19 | 0.00 | 0.00 | 0.09 | 0.10 | 0.00 | 0.00 | 0.04 |
| OR estimate | 0.59 | 0.00 | 0.00 | 0.45 | 1.03 | 0.00 | 0.00 | 2.13 | |
| Upper 95% CI OR estimate | 1.59 | 4.38 | 20.93 | 1.95 | 5.87 | 241.45 | 1.81 | 29.00 | |
| Hepatitis A virus a/w humans | Lower 95% CI OR estimate | 0.02 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| OR estimate | ∞ | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| Upper 95% CI OR estimate | ∞ | 483.82 | ∞ | ∞ | ∞ | 3917.62 | 240.89 | 998.20 | |
| Astrovirus a/w humans | Lower 95% CI OR estimate | 0.41 | 0.02 | 0.00 | 0.01 | 0.00 | 0.00 | 0.27 | 0.00 |
| OR estimate | 1.44 | 0.88 | 0.00 | ∞ | 0.00 | 0.00 | 1.68 | 0.00 | |
| Upper 95% CI OR estimate | 5.81 | 7.76 | ∞ | ∞ | ∞ | 240.89 | 7.61 | 9.77 | |
| Norovirus GI a/w humans | Lower 95% CI OR estimate | 0.60 | 0.24 | 0.00 | 0.01 | 0.00 | 0.00 | 0.32 | 0.00 |
| OR estimate | 2.51 | 2.66 | 0.00 | ∞ | 0.00 | 0.00 | 2.06 | 0.00 | |
| Upper 95% CI OR estimate | 15.10 | 17.53 | ∞ | ∞ | ∞ | 283.74 | 9.69 | 11.65 | |
| Norovirus GIII a/w bovines | Lower 95% CI OR estimate | 0.76 | 0.00 | 0.00 | 0.00 | 0.00 | 0.21 | 0.12 | 0.05 |
| OR estimate | 3.85 | 0.00 | 0.00 | 0.00 | 0.00 | ∞ | 1.27 | 2.78 | |
| Upper 95% CI OR estimate | 38.00 | 4.93 | ∞ | ∞ | ∞ | ∞ | 7.07 | 38.29 | |
| Norovirus GIV a/w humans | Lower 95% CI OR estimate | 0.36 | 0.00 | 0.00 | 0.00 | 0.00 | 0.32 | 0.20 | 0.00 |
| OR estimate | 2.16 | 0.00 | 0.00 | 0.00 | 0.00 | ∞ | 2.21 | 0.00 | |
| Upper 95% CI OR estimate | 22.89 | 8.21 | ∞ | ∞ | ∞ | ∞ | 14.21 | 20.95 | |
| Sapovirus a/w humans | Lower 95% CI OR estimate | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| OR estimate | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| Upper 95% CI OR estimate | ∞ | ∞ | ∞ | ∞ | ∞ | ∞ | ∞ | ∞ | |
| Human torque teno virus | Lower 95% CI OR estimate | 0.55 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| OR estimate | 2.97 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| Upper 95% CI OR estimate | 30.17 | 6.19 | ∞ | ∞ | ∞ | 379.94 | 2.79 | 15.99 | |
| Torque teno sus virus a/w swine | Lower 95% CI OR estimate | 0.55 | 0.03 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 |
| OR estimate | 2.97 | 1.44 | 0.00 | 0.00 | 0.00 | 0.00 | 0.67 | 0.00 | |
| Upper 95% CI OR estimate | 30.17 | 13.55 | ∞ | ∞ | ∞ | 379.94 | 5.54 | 15.99 | |
| Adenovirus 40/41 a/w humans | Lower 95% CI OR estimate | 0.16 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| OR estimate | 2.10 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| Upper 95% CI OR estimate | 113.49 | 20.95 | ∞ | ∞ | ∞ | 998.20 | 9.77 | 50.58 | |
| General adenovirus a/w humans | Lower 95% CI OR estimate | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| OR estimate | 0.69 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| Upper 95% CI OR estimate | 54.79 | 69.91 | ∞ | ∞ | ∞ | 2002.36 | 33.77 | 155.20 | |
| F-RNA coliphage a/w human origin | Lower 95% CI OR estimate | 0.17 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.22 | 0.00 |
| OR estimate | 1.03 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 2.52 | 0.00 | |
| Upper 95% CI OR estimate | 7.27 | 11.70 | 52.81 | ∞ | ∞ | 530.43 | 17.55 | 23.41 | |
| F-RNA coliphage a/w animal origin | Lower 95% CI OR estimate | 0.91 | 0.00 | 0.00 | 0.03 | 0.00 | 0.04 | 0.18 | 0.01 |
| OR estimate | 2.01 | 0.00 | 0.00 | 0.73 | 0.00 | ∞ | 0.72 | 0.49 | |
| Upper 95% CI OR estimate | 4.62 | 1.12 | 5.57 | 48.21 | 11.80 | ∞ | 2.57 | 6.41 | |
Significant results are in bold. a/w, associated with.
DISCUSSION
Comparison of ruminant Bacteroidales marker occurrence, the Bacteroidales number of copies day−1 (load), and the number of marker copies 100 ml−1 in stream water within the fenced and unfenced cattle pastures indicates that the reach of the stream protected from pasturing cattle did not increase ruminant Bacteroidales marker occurrences and loading from upstream monitoring sites, but as expected, where cattle were allowed to interact with the stream, ruminant marker occurrence and the number of copies day−1 increased significantly. This was as a consequence of increased direct fecal release in and around the stream and a lack of filtering vegetation in the riparian zone to buffer contaminated drainage from adjacent land where cattle pastured. While there were no significant associations of the ruminant Bacteroidales marker with the suite of pathogens examined here (Campylobacter spp., Salmonella spp., Escherichia coli O157:H7, Cryptosporidium, Giardia, hepatitis E virus, norovirus GII, rotavirus), except for Cryptosporidium spp. (of which 10% of these samples had the human-pathogenic C. parvum), the marker data point to the potential for an increased risk of cattle fecal contamination for stream/riparian systems that are not protected from pasturing cattle, even for those with modest pasturing densities. Wilkes et al. (11, 59) found that the occurrence of E. coli O157:H7 was significantly higher in the unrestricted cattle access area than in other surface water quality monitoring sites in the region, including the RCA sampling sites. Wilkes et al. (59) also found that Salmonella spp. occurred more often in the URCA region; here, we observed a variety of Salmonella serovars in the pasturing corridor, including Salmonella serovar I:4,5,12:b:−, which predominated in the livestock-impacted site (61% of the overall serovars observed). Hence, in the assessment of BMP interventions that are subjected to multiple sources of fecal contamination, Bacteroidales source data appear to be useful for gauging BMP effectiveness and/or the impact of land use change on the sources of fecal inputs. Whether or not Bacteroidales source markers have more utility in this capacity than, for example, fecal indicator bacteria (26) will be the product of future research.
Protecting the riparian zone is certainly important for many reasons, including enhancing wildlife habitat and reducing surface water pollution (46). In many agriculturally intensive watersheds, wildlife habitat is becoming increasingly constrained, and riparian corridors are important areas to maintain and conserve for purposes of biodiversity, as well as for reducing water pollution and enhancing ecological goods and services (47, 48). Sunohara et al. found that cattle exclusion fencing promoted greater numbers and types of plant species and notably greater degrees of wildlife (e.g., muskrats, voles, turtles, birds) in the study site described here (23). The wildlife Bacteroidales markers utilized in this study, the muskrat and Canada goose markers, demonstrated that protecting habitat could indeed significantly increase inputs of wildlife fecal material, yet where cattle have open access to a stream (where they eat plants, trample soil and plants, etc.), the wildlife Bacteroidales markers were significantly reduced in relation to their numbers in protected upstream sites. While there were increases in these wildlife markers in the protected riparian zone, there were no significant increases in pathogen occurrence (59). In fact, Wilkes et al. found that occurrences of Campylobacter spp. significantly decreased from the RCAin site to the URCAout site (59). The wildlife (muskrat, Canada goose)-associated Bacteroidales markers served as excellent indicators of the impact of land use change on wildlife activity, such as how pasturing livestock in and around the stream and riparian zone can disrupt wildlife habitat and, in so doing, reduce the wildlife fecal signature in such systems. Green et al. (49) observed that wildlife markers (i.e., markers targeting avians) were distributed in the surface waters of a geographically wide range of watersheds, and so, too, have Fremaux et al. (50), who observed a large proportion of wildlife (i.e., Canada goose) detections in surface water.
The signature of human Bacteroidales markers significantly increased downstream of the RCAin site at monitoring sites immediately downstream of homes where some small septic leaks were documented. These trends were significantly more pronounced at high flows and in summer as a result of intense rain events promoting drainage to the adjacent stream. Although the trends were not statistically significant, there were noteworthy spatial trends that were similar to those for human Bacteroidales markers among astrovirus associated with humans, norovirus GI associated with humans, human TTV, and Cryptosporidium associated with humans. These trends were all promising in terms of identifying how human sources of fecal pollution behave in a system designed to promote reductions in livestock fecal pollution (open systems). McQuaig et al. have consistently observed the concurrence of human markers (human polyomaviruses and Bacteroides human markers) in septic waste (51), and Peed et al. (52) have observed human markers and septic system contamination of a low-order stream during rainfall events, trends similar to those observed in this work.
Overall, there was promising consistency in the downstream occurrence of the human Bacteroidales markers and human viruses among the water sampling sites, even over the relatively small reach of this intermittent stream. All markers behaved in accordance with expected hypothetical spatial trends, and therefore, Bacteroidales are an enormously powerful tool with which to gauge land use and land use change effects on fecal pollution (19–21, 52, 53). This can have significant potential when assessing how a BMP behaves or does not behave in open watershed systems where multiple sources of fecal inputs occur (22, 54, 55).
While the overall spatial trends described above made sense from a biophysical standpoint, there was no significant systematic sample-by-sample- or temporal window-based coherence among similar source tracking endpoints, nor were there any strong associated links with pathogens. Fremaux et al. (1) and Marti et al. (21) did not find strong associations between Bacteroidales markers and selected waterborne pathogens either. The reason for a generalized lack of coherence among similar marker endpoints (and markers and pathogens) is no doubt multifactorial; however, some factors could be related to (i) the fact that not all water samples that had Bacteroidales source markers (differences in the densities of various markers and pathogens) contained pathogens (of those monitored), (ii) variable dissipation among the Bacteroidales source markers and the pathogens and host-specific pathogens (1, 21, 53, 54), (iii) the size of the organism or microbial source tracking endpoint, resulting in differential transport behavior (56, 57), (iv) detection limits and analytical constraints, such as those discussed by Marti et al. (21), including extraction of DNA/RNA (or nucleic acids) from larger water volumes and the impact of PCR inhibitors (58), and (v) variability in the degree of host specificity among the source tracking endpoints.
Several noteworthy deductions can be drawn from this study: (i) changes in the prevalence of Bacteroidales markers can occur over short distances as a result of land use changes (such as interventions consisting of cattle exclusion BMPs that protect the stream and riparian zone). The ruminant Bacteroidales and the bovine-associated virus markers did not increase significantly in the stream protected from pasturing cattle, suggesting a positive BMP impact, yet ruminant Bacteroidales markers did increase significantly from the output of the restricted area to areas where cattle access to the stream was unrestricted.
(ii) Pig Bacteroidales markers were observed most markedly in close proximity to drainage inputs from fields associated with land applications of swine manure.
(iii) Select wildlife (e.g., muskrat and Canada goose) Bacteroidales marker detections were observed more frequently downstream of the protected stream/riparian zone. Wildlife markers were significantly reduced where cattle had access to the stream as a result of ruminant activity (23). There were no significant positive associations between pathogens and detection of these wildlife Bacteroidales markers, and Wilkes et al. (59) did not observe a significant increase in pathogen detection in the stream associated with the cattle exclusion practice.
(iv) Human Bacteroidales markers, found to be significantly higher downstream of homes located along the experimental pastures, likely occurred as a result of septic system leakages. There were no significant associations with this marker and other pathogens.
(v) There was considerable interplay in MST marker results with stream flow, season, and localized upstream land uses (including cattle exclusion BMP). Interactions were highly variable, depending on the marker.
(vi) Cryptosporidium exhibited consistent seasonal loading over all sites for livestock and wildlife at high flow (with the overall greatest occurrences in the fall perhaps resulting from fall flow events flushing oocysts that had accumulated in the system over spring and summer downstream). There were no significant associations between Cryptosporidium generalized host classes and other MST markers.
(vii) Few viruses and coliphages attributable to a particular source demonstrated significant site-specific affinities. However, a vast majority of human viruses were shown to increase from the stream input into the experimental area to sampling sites downstream, as per human Bacteroidales marker data.
(viii) Positive associations among the MST markers and pathogens were minimal; one exception was that between the ruminant Bacteroidales marker and Cryptosporidium. This suggests that livestock exclusion BMPs could help reduce pathogen exposure risks (11).
(ix) Overall, discrete sample-by-sample (or sampling window) coherence among the different MST markers that expressed a similar microbial source or source class was minimal. The reasons for this lack of coherence are, no doubt, multifactorial, but persistence, variable density factors, and the transport disposition of the various microorganisms would at least seem to be contributing factors.
Supplementary Material
ACKNOWLEDGMENTS
This work was funded in part by the National Water Quality Surveillance Research Program through an agreement between Agriculture and Agri-Food Canada (AAFC) and Health Canada, AAFC's Sustainable Agriculture Environmental Systems (SAGES) program, the Watershed Evaluation of Beneficial Management Practice (WEBs) program, and the Alberta Water Research Institute.
We also thank South Nation Conservation for assistance.
Footnotes
Published ahead of print 2 August 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.01626-13.
REFERENCES
- 1.Fremaux B, Gritzfeld J, Boa T, Yost CK. 2009. Evaluation of host-specific Bacteroidales 16S rRNA gene markers as a complementary tool for detecting fecal pollution in a prairie watershed. Water Res. 43:4838–4849 [DOI] [PubMed] [Google Scholar]
- 2.Marti R, Zhang Y, Lapen DR, Topp E. 2011. Development and validation of a microbial source tracking marker for the detection of fecal pollution by muskrats. J. Microbiol. Methods 87:82–88 [DOI] [PubMed] [Google Scholar]
- 3.Gentry RW, Layton AC, McKay LD, McCarthy JF, Williams DE, Koirala SR, Sayler GS. 2007. Efficacy of Bacteroides measurements for reducing the statistical uncertainty associated with hydrologic flow and fecal loads in a mixed use watershed. J. Environ. Qual. 36:1324–1330 [DOI] [PubMed] [Google Scholar]
- 4.Drozd M, Merrick NN, Sanad YM, Dick LK, Dick WA, Rajashekara G. 2013. Evaluating the occurrence of host-specific Bacteroidales, general fecal indicators, and bacterial pathogens in a mixed-use watershed. J. Environ. Qual. 42:713–725 [DOI] [PubMed] [Google Scholar]
- 5.Boehm AB, Van De Werfhorst LC, Griffith JF, Holden PA, Jay JA, Shanks OC, Wang D, Weisberg SB.Performance of forty-one microbial source tracking methods: a twenty-seven lab evaluation study. J. Water Res. [Epub ahead of print.] Jul 5, 2013. http://dx.doi.org/10.1016/j.watres.2012.12.046. [DOI] [PubMed]
- 6.Santo Domingo JW, Bambic DG, Edge TA, Wuertz S. 2007. Quo vadis source tracking? Towards a strategic framework for environmental monitoring of fecal pollution. Water Res. 41:3539–3552 [DOI] [PubMed] [Google Scholar]
- 7.Harwood VJ, Whitlock J, Withington V. 2000. Classification of antibiotic resistance patterns of indicator bacteria by discriminant analysis: use in predicting the source of fecal contamination in subtropical waters. Appl. Environ. Microbiol. 66:3698–3704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graves A, Hagedorn C, Teetor A, Mahal M, Booth AM, Reneau RB. 2002. Antibiotic resistance profiles to determine sources of fecal contamination in a rural Virginia watershed. J. Environ. Qual. 31:1300–1308 [DOI] [PubMed] [Google Scholar]
- 9.Anderson KL, Whitlock JE, Harwood VJ. 2005. Persistence and differential survival of fecal indicator bacteria in subtropical waters and sediments. Appl. Environ. Microbiol. 71:3041–3048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiao L, Fayer R, Ryan U, Upton SJ. 2004. Cryptosporidium taxonomy: recent advances and implications for public health. Clin. Microbiol. Rev. 17:72–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilkes G, Ruecker NJ, Neumann NF, Gannon VPJ, Jokinen C, Sunohara M, Topp E, Pintar KDM, Edge T, Lapen DR. 2013. A spatiotemporal analysis of Cryptosporidium species/genotypes and relationships with other zoonotic pathogens in surface water from mixed use watersheds. Appl. Environ. Microbiol. 79:434–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caldwell JM, Raley ME, Levine JF. 2007. Mitochondrial multiplex real-time PCR as a source tracking method in fecal-contaminated effluents. Environ. Sci. Technol. 41:3277–3283 [DOI] [PubMed] [Google Scholar]
- 13.Vuong N-M, Villemur R, Payment P, Brousseau R, Topp E, Masson L. 2012. Fecal source tracking in water using a mitochondrial DNA microarray. Water Res. 47:16–30 [DOI] [PubMed] [Google Scholar]
- 14.Noble RT, Allen SM, Blackwood AD, Chu W, Jiang SC, Lovelace GL, Sobsey MD, Stewart JR, Wait DA. 2003. Use of viral pathogens and indicators to differentiate between human and non-human fecal contamination in a microbial source tracking comparison study. J. Water Health 1:195–207 [PubMed] [Google Scholar]
- 15.Hundesa A, Maluquer de Motes C, Bofill-Mas S, Albinana-Gimenez N, Girones R. Identification of human and animal adenoviruses and polyomaviruses for determination of sources of faecal contamination in the environment. Appl. Environ. Microbiol. 72:7886–7893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirs M, Smith DC. 2007. Multiplex quantitative real-time reverse transcriptase PCR for F+-specific RNA coliphages: a method for use in microbial source tracking. Appl. Environ. Microbiol. 73:808–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stoeckel DM, Harwood V. 2007. Performance, design, and analysis in microbial source tracking studies. Appl. Environ. Microbiol. 73:2405–2415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.US Environmental Protection Agency 2011. Region 10. Using microbial source tracking to support TMDL development and implementation. US Environmental Protection Agency, Washington, DC: http://www.epa.gov/region10/pdf/tmdl/mst_for_tmdls_guide_04_22_11.pdfAccessed 4 June 2012 [Google Scholar]
- 19.Walters SP, Gannon VP, Field KG. 2007. Detection of Bacteroidales fecal indicators and the zoonotic pathogens E. coli O157:H7, Salmonella, and Campylobacter in river water. Environ. Sci. Technol. 41:1856–1862 [DOI] [PubMed] [Google Scholar]
- 20.Tambalo DD, Fremaux B, Boa T, Yost CK. 2012. Persistence of host-associated Bacteroidales gene markers and their quantitative detection in an urban and agricultural mixed prairie watershed. Water Res. 46:2891–2904 [DOI] [PubMed] [Google Scholar]
- 21.Marti R, Gannon VPJ, Jokinen C, Lanthier M, Lapen DR, Neumann NF, Ruecker NJ, Scott A, Wilkes G, Zhang Y, Topp E. 2013. Quantitative multi-year elucidation of fecal sources of waterborne pathogen contamination in the South Nation River basin using Bacteroidales microbial source tracking markers. Water Res. 47:2315–2324 [DOI] [PubMed] [Google Scholar]
- 22.Vogel J, Stoeckel DM, Lamendella R, Zelt RB, Santo Domingo JW, Walker SR, Oerther DB. 2007. Identifying fecal sources in a selected catchment reach using multiple source-tracking tools. J. Environ. Qual. 36:718–729 [DOI] [PubMed] [Google Scholar]
- 23.Sunohara MD, Topp E, Wilkes G, Gottschall N, Neumann N, Ruecker N, Jones TH, Edge TA, Marti R, Lapen DR. 2012. Impact of riparian zone protection from cattle on nutrient, bacteria, F-coliphage, and loading of an intermittent stream. J. Environ. Qual. 41:1301–1314 [DOI] [PubMed] [Google Scholar]
- 24.US Department of Agriculture 1996. National handbook of water quality monitoring. US Department of Agriculture, Washington, DC [Google Scholar]
- 25.Ruecker N, Matsune JC, Wilkes G, Lapen DR, Topp E, Edge T, Sensen CW, Xiao L, Neumann NF. 2012. Molecular and phylogenetic approaches for assessing sources of Cryptosporidium contamination in raw water. Water Res. 46:5135–5150 [DOI] [PubMed] [Google Scholar]
- 26.Jokinen C, Edge TA, Ho S, Koning W, Laing C, Mauro W, Medeiros D, Miller J, Robertson W, Taboada E, Thomas JE, Topp E, Ziebell K, Gannon VPJ. 2011. Molecular subtypes of Campylobacter spp., Salmonella enterica, and Escherichia coli O157:H7 isolated from faecal and surface water samples in the Oldman River watershed, Alberta, Canada. Water Res. 45:1247–1257 [DOI] [PubMed] [Google Scholar]
- 27.Ewing WH. 1986. Edwards and Ewing's identification of Enterobacteriaceae, 4th ed. Elsevier Science Publishing Co, Inc, New York, NY [Google Scholar]
- 28.Shipp CR, Rowe BA. 1980. Mechanised microtechnique for Salmonella serotyping. J. Clin. Pathol. 33:595–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grimont PA, Weill FX. 2007. Antigenic formulae of the Salmonella serovars, 9th ed. WHO Collaborating Centre for Reference and Research on Salmonella, Institut Pasteur, Paris, France [Google Scholar]
- 30.Brassard J, Seyer K, Houde A, Simard C, Trottier YL. 2005. Concentration and detection of hepatitis A virus and rotavirus in spring water samples by reverse transcription-PCR. J. Virol. Methods 123:163–169 [DOI] [PubMed] [Google Scholar]
- 31.Brassard J, Simard C, Müller P, Houde A, Trottier YL. 2007. Concentration of hepatitis A virus and rotavirus in spring or mineral bottled water samples and their detection by the reverse-transcription polymerase chain reaction. Health Canada, Ottawa, ON, Canada: http://www.hc-sc.gc.ca/fn-an/res-rech/analy-meth/microbio/volume5/opflp_04-eng.php Accessed 1 October 2008 [Google Scholar]
- 32.Tokita H, Murai S, Kamitsukasa H, Yagura M, Harada H, Takahashi M, Okamoto H. 2002. High TT virus load as an independent factor associated with the occurrence of hepatocellular carcinoma among patients with hepatitis C virus-related chronic liver disease. J. Med. Virol. 67:501–509 [DOI] [PubMed] [Google Scholar]
- 33.Kageyama T, Kojima S, Shinohara M, Uchida K, Fukushi S, Hoshino FB, Takeda N, Katayama K. 2003. Broadly reactive and highly sensitive assay for Norwalk-like viruses based on real-time quantitative reverse transcription-PCR. J. Clin. Microbiol. 41:1548–1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Le Cann P, Ranarijaona S, Manpoeho S, Le Guyader F, Ferré V. 2004. Quantification of human astroviruses in sewage using real-time RT-PCR. Res. Microbiol. 155:11–15 [DOI] [PubMed] [Google Scholar]
- 35.Jothikumar N, Cromeans TL, Hill VR, Lu X, Sobsey MD, Erdman DD. 2005. Quantitative real-time PCR assays for detection of human adenoviruses and identification of serotypes 40 and 41. Appl. Environ. Microbiol. 71:3131–3136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ko G, Jothikumar N, Hill VR, Sobsey MD. 2005. Rapid detection of infectious adenoviruses by mRNA real-time RT-PCR. J. Virol. Methods 127:148–153 [DOI] [PubMed] [Google Scholar]
- 37.Oka T, Katayama K, Hansman GS, Kageyama T, Ogawa S, Wu F-T, Takeda N. 2006. Detection of human Sapovirus by real-time reverse transcription-polymerase chain reaction. J. Med. Virol. 78:1347–1353 [DOI] [PubMed] [Google Scholar]
- 38.Houde A, Guévremont E, Poitras E, Leblanc D, Ward P, Simard C, Trottier YL. 2007. Comparative evaluation of new TaqMan real-time assays for the detection of hepatitis A virus. J. Virol. Methods 140:80–89 [DOI] [PubMed] [Google Scholar]
- 39.Logan C, O'Leary JJ, O'Sullivan N. 2007. Real-time reverse transcription PCR detection of norovirus, Sapovirus and astrovirus as causative agents of acute viral gastroenteritis. J. Virol. Methods 146:36–44 [DOI] [PubMed] [Google Scholar]
- 40.Wolf S, Williamson WS, Hewitt J, Rivera-Aban M, Lin S, Ball A, Scholes P, Greenong GE. 2007. Sensitive multiplex real-time reverse transcription-PCR assay for the detection of human and animal noroviruses in clinical and environmental samples. Appl. Environ. Microbiol. 73:5464–5470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mattison K, Brassard J, Gagné MJ, Ward P, Houde A, Lessard L, Simard C, Shukla A, Pagotto F, Jones TH, Trottier YL. 2009. The feline calicivirus as a sample process control for the detection of food and waterborne RNA viruses. Int. J. Food Microbiol. 132:73–77 [DOI] [PubMed] [Google Scholar]
- 42.Brassard J, Gagné MJ, Houde A, Poitras E, Ward P. 2010. Development of a real-time TaqMan PCR assay for the detection of porcine and bovine torque teno virus. J. Appl. Microbiol. 108:2191–2198 [DOI] [PubMed] [Google Scholar]
- 43.De Jong JC, Bijlsma L, Wermenbol AG, Verweij-Uijterwaal MW, Van der Avoort AM, Wood D, Bailey AS, Osterhaus AD. 1993. Detection, typing, and subtyping of enteric adenoviruses 40 and 41 from fecal samples and observation of changing incidences of infections with these types and subtypes. J. Clin. Microbiol. 31:1562–1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jones TH, Houde A, Poitras E, Ward P, Johns MW. 2009. Development and evaluation of a multiplexed real-time TaqMan RT-PCR assay with a sample process control for the detection of F-specific RNA coliphage genogroups I and IV. Food Environ. Virol. 1:57–65 [Google Scholar]
- 45.Wolf S, Hewitt J, Rivera-Aban M, Greening GE. 2008. Detection and characterization of F+RNA bacteriophages in water and shellfish: application of a multiplex real-time reverse transcription PCR. J. Virol. Methods 149:123–128 [DOI] [PubMed] [Google Scholar]
- 46.Miller JJ, Chanasyk DS, Curtis T, Entz T, Willms WD. 2010. Influence of streambank fencing with a cattle crossing on riparian health and water quality of the Lower Little Bow River in southern Alberta, Canada. Agric. Water Manage. 97:247–258 [Google Scholar]
- 47.Maissonneuve C, Rioux S. 2001. Importance of riparian habitats for small mammal and herpetofaunal communities in agricultural landscapes of southern Québec. Agric. Ecosyst. Environ. 1-2:165–175 [Google Scholar]
- 48.Sarr DA. 2002. Riparian livestock exclosure research in the western United States: a critique and some recommendations. Environ. Manage. 30:516–526 [DOI] [PubMed] [Google Scholar]
- 49.Green HC, Dick LK, Gilpin B, Samadpour M, Field KG. 2012. Genetic markers for rapid PCR-based identification of gull, Canada goose, duck, and chicken fecal contamination in water. Appl. Environ. Microbiol. 78:503–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fremaux B, Boa T, Yost CK. 2010. Quantitative real-time PCR assays for sensitive detection of Canada goose-specific fecal pollution in water sources. Appl. Environ. Microbiol. 76:4886–4889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McQuaig SM, Scott TM, Harwood VJ, Farrah SM, Lukasik JO. 2006. Detection of human-derived fecal pollution in environmental waters by use of a PCR-based human polyomavirus assay. Appl. Environ. Microbiol. 72:7567–7574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peed LA, Nietch CT, Kelty CA, Meckes M, Mooney T, Sivaganesan M, Shanks OC. 2011. Combining land use information and small stream sampling with PCR-based methods for better characterization of diffuse sources of human fecal pollution. Environ. Sci. Technol. 45:5652–5659 [DOI] [PubMed] [Google Scholar]
- 53.Schulz C, Childers GW. 2011. Fecal Bacteroidales diversity and decay in response to variations in temperature and salinity. Appl. Environ. Microbiol. 77:2563–2572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liang Z, He Z, Zhou X, Powell C, Yang Y, He LM, Stoffella PJ. 2013. Impact of mixed land-use practices on the microbial water quality in a subtropical coastal watershed. Sci. Total Environ. 449:426–433 [DOI] [PubMed] [Google Scholar]
- 55.Toledo-Hernandez C, Ryu H, Gonzalez-Nieves J, Huertas E, Toranzos GA, Santo Domingo JW. 2013. Tracking the primary sources of fecal pollution in a tropical watershed in a one-year study. Appl. Environ. Microbiol. 79:1689–1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Payment P, Locas A. 2011. Pathogens in water: value and limits of correlation with microbial indicators. Ground Water 49:4–11 [DOI] [PubMed] [Google Scholar]
- 57.Sokolova E, Aström J, Pettersson TJR, Bergstedt O, Hermansson M. 2012. Decay of Bacteroidales genetic markers in relation to traditional fecal indicators for water quality modeling of drinking water sources. Environ. Sci. Technol. 46:892–900 [DOI] [PubMed] [Google Scholar]
- 58.Schriewer A, Wehlmann A, Wuertz S. 2011. Improving qPCR efficiency in environmental samples by selective removal of humic acids with DAX-8. J. Microbiol. Methods 85:16–21 [DOI] [PubMed] [Google Scholar]
- 59.Wilkes GA, Brassard J, Edge TA, Gannon VPJ, Jokinen CC, Jones TH, Neumann NF, Pintar KDM, Ruecker NJ, Schmidt PJ, Sunohara M, Lapen DR. 2013. Bacteria, viruses, and parasites in an intermittent stream protected from and exposed to pasturing cattle: prevalence, densities, and quantitative microbial risk assessment. Water Res. 10.1016/j.watres.2013.07.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.