Abstract
Epidemiological studies have shown a link between carbapenem use and resistance; however, the clinical relationship between antibiotic consumption and the epidemiology of carbapenem-intermediate or -resistant Enterobacteriaceae (CIRE) remains unclear. This study sought to analyze temporal antibiotic consumption trends for relationships with incident CIRE. In total, 310,892 days of therapy and 55 deduplicated CIRE were analyzed. When conservative corrections were applied for multiple comparisons, carbapenem class use and piperacillin-tazobactam use retained significant positive and negative relationships with the incidence of CIRE, respectively.
TEXT
Carbapenem-intermediate or -resistant Enterobacteriaceae (CIRE) are of increasing concern and have rapidly spread globally (1–3) and in the United States (4–6). The midwestern United States has not been spared from the emergence of CIRE. The first reported isolates of CIRE were Klebsiella pneumoniae strains, and mobile genetic plasmids have subsequently spread to other Gram-negative rods (7). While epidemiological studies have shown a link between carbapenems and resistance in nonfermentative Gram-negative organisms (8–12), the contribution of antibiotic consumption to CIRE incidence remains unclear. As CIRE are incident in the midwestern United States, we used this optimal opportunity to analyze temporal antibiotic consumption trends for relationships with incident CIRE.
This study was performed at Northwestern Memorial Hospital (NMH), an 897-bed, tertiary-care academic medical center located in Chicago, IL. Broad-spectrum Gram-negative antibiotics with a potential for influencing CIRE and in use at NMH were included in the analysis. Consumption was measured as numbers of days of therapy (DOT) per 1,000 patient days (13). DOT were aggregated quarterly from 1 January 2006 through 31 December 2010. Quarterly compilation allowed capture of immediate and time-delayed effects of antimicrobial use on resistance (14, 15). These antibiotics included β-lactams (aztreonam, cefepime, ceftazidime, ertapenem, imipenem-cilastatin, meropenem, piperacillin-tazobactam), fluoroquinolones (ciprofloxacin, moxifloxacin), glycylcyclines (tigecycline), and polymyxins (colistin methanesulfonate). Only antibiotics achieving relevant systemic concentrations were captured; any antibiotic prescribed as an ophthalmic solution, ointment, or enema was excluded from the study. Antibiotics with formulary status throughout the time period were analyzed individually. Since the preferred carbapenem(s) changed at our institution during the study period, carbapenems were analyzed as a combinatorial class.
All isolates were obtained from clinical cultures from 1 January 2006 through 31 December 2010. Only the first isolate per patient per quarter was included in the study, and incidence rate was calculated by standardizing the at-risk population to 1,000 patient hospitalization days. Susceptibility testing for all isolates was performed with the Vitek 2 system using the AST-GN25 (22230) card by following the manufacturer's instructions (bioMérieux, St. Louis, MO). CIRE were defined as isolates for which the carbapenem MIC was ≥2 mg/liter.
Antibiotic use and CIRE incidence were assessed for trends by utilizing the chi-square test for trend with Epi Info version 3.5.3 (Centers for Disease Control and Prevention, Atlanta, GA). Correlations between CIRE incidence and antibiotic consumption were assessed with Spearman's correlations using Intercooled Stata version 11.1 (StataCorp, College Station, TX). Bonferroni corrections were applied to correct for multiple comparisons, with a P of <0.0056 (i.e., P = 0.05, with 9 comparisons) being considered significant. Significant relationships were further assessed using the Marquardt-Levenberg algorithm (SigmaPlot 12; Systat Software Inc., San Jose, CA).
During the study period, a total of 310,892 DOT and 6,753 DOT/1,000 patient days were analyzed. Antibiotic use showed increasing trends during the analysis period for the carbapenem class (P < 0.001). Aztreonam (P < 0.001), cefepime (P = 0.003), ceftazidime (P < 0.001), moxifloxacin (P < 0.001), and piperacillin-tazobactam (P < 0.001) had decreasing trends over the study analysis period. Ciprofloxacin, colistin, and tigecycline usage were found to be consistent throughout the study period (P > 0.05 for all).
A total of 27,818 isolates were identified as Enterobacteriaceae, out of which there were 55 deduplicated CIRE. The following organism types were identified as CIRE: Klebsiella pneumoniae (n = 47 [85.5%]), Escherichia coli (n = 4 [7.3%]), Enterobacter cloacae (n = 2 [3.6%]), Enterobacter aerogenes (n = 1 [1.8%]), and Klebsiella oxytoca (n = 1 [1.8%]). The number of CIRE isolates/1,000 patient days increased significantly as a function of time (P < 0.001) per the chi-square test for trend.
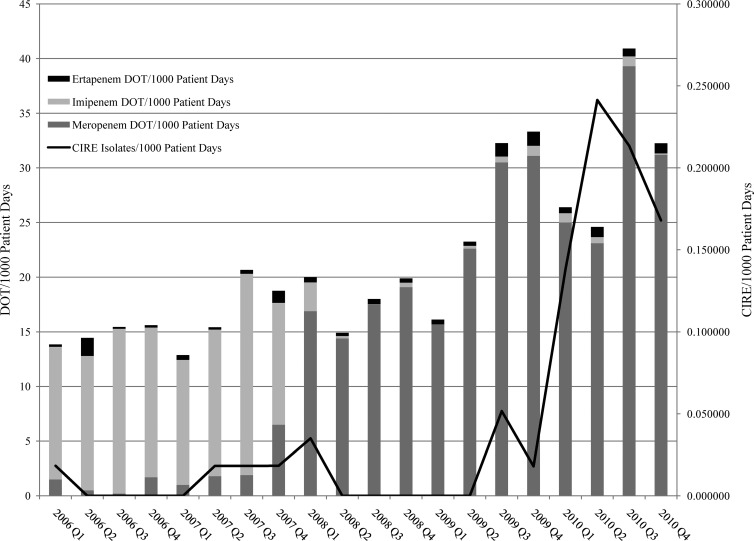
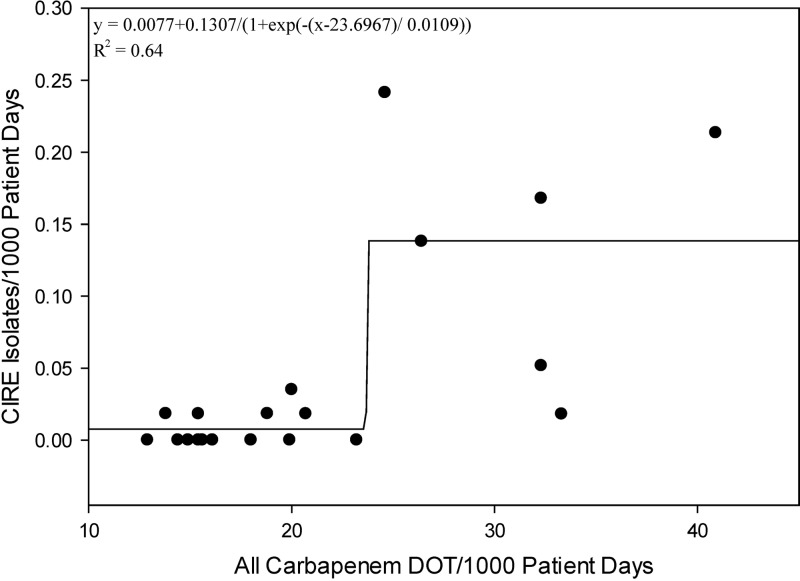
There was a positive correlation between CIRE incidence and the carbapenem class (r = 0.62, P = 0.004) (Fig. 1); the association remained significant when the analysis was corrected for multiple comparisons (i.e., significance at the level of ≤0.0056). The number of CIRE isolates/1,000 patient days increased as a mathematical function of all days of carbapenem therapy/1,000 patient days (y = 0.0077 + 0.1307/{1 + exp[−(x − 23.6967)/0.0109]}; r2 = 0.64) (Fig. 2). This equation identified a sharp increase in the number of CIRE/1,000 patient days after the number of days of carbapenem therapy exceeded 22/1,000 patient days.
Fig 1.
All days of carbapenem therapy/1,000 patient days and numbers of CIRE/1,000 patient days. DOT, days of therapy; CIRE, carbapenem-intermediate or -resistant Enterobacteriaceae; Q1 to -4, first to fourth quarters.
Fig 2.
Numbers of CIRE isolates/1,000 patient days versus all days of carbapenem therapy/1,000 patient days.
There was a negative correlation with ceftazidime (r = −0.52, P = 0.018), piperacillin-tazobactam (r = −0.64, P = 0.0021), and moxifloxacin (r = −0.51, P = 0.022) usage. When the analysis was corrected for multiple comparisons among these negative correlations (i.e., significance at the level of ≤0.0056), only the correlation with piperacillin-tazobactam remained significant.
We found that increasing carbapenem use was correlated with increasing resistance in Enterobacteriaceae. Previous epidemiological studies with select Gram-negative organisms support our findings. One study analyzed carbapenem use and found that carbapenem use and carbapenem-resistant A. baumannii increased simultaneously (12). The correlation of carbapenem exposure and antimicrobial resistance has also been reported in case-control studies (16, 17). These analyses found that progression to CIRE is likely the result of a multifactorial process with respect to antibiotic exposure; our study corroborates these results and may indicate that replacing several antibiotics with the class of carbapenems is potentially problematic.
Limitations to our study should be noted. First, we excluded organisms tested only by disk diffusion because MICs were not available for analysis. Thus, these numbers may slightly underreport CIRE incidence. Second, these data are epidemiologic in nature and cannot confirm or disprove causality. However, the statistical power gained with epidemiologic analyses allowed us to stratify and analyze individual antibiotic associations. Third, carbapenems were analyzed as a class due to a change in formulary from imipenem-cilastatin to meropenem in mid-2007, though CIRE incidence occurred largely in the presence of meropenem use. This modification should improve validity, since the use of each individual carbapenem varied depending on formulary status. Fourth, our institution actively screens for nosocomial organism outbreaks. There was one outbreak of carbapenem-resistant Enterobacteriaceae involving three patients; however, this represented a very small portion (5.5%) of isolates studied. Thus, our results are unlikely biased by horizontal organism transfer. Lastly, this study was conducted at a single large institution in Chicago, IL. Results may not apply to areas where different pathogens predominate ecologically.
The incidence of CIRE is increasing at our institution and was associated with increasing carbapenem class use and decreasing piperacillin-tazobactam use. Further research is needed to elucidate whether knowledge of associations between antibiotic consumption and CIRE may be used to design interventions that slow CIRE spread.
(Portions of this research were presented at the 51st Interscience Conference on Antimicrobial Agents and Chemotherapy [Chicago, IL, 19 September 2011].)
ACKNOWLEDGMENTS
This work was supported in part by a Midwestern University Student Research Grant.
Midwestern University had no part in the design or conduct of the study, in the collection, management, analysis, or interpretation of the data, or in the preparation, review, or approval of the manuscript. No authors have relevant conflicts of interest or financial conflicts to disclose.
Footnotes
Published ahead of print 8 July 2013
REFERENCES
- 1.Cuzon G, Naas T, Truong H, Villegas MV, Wisell KT, Carmeli Y, Gales AC, Venezia SN, Quinn JP, Nordmann P. 2010. Worldwide diversity of Klebsiella pneumoniae that produce beta-lactamase blaKPC-2 gene. Emerg. Infect. Dis. 16:1349–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goren MG, Navon-Venezia S, Chmelnitsky I, Carmeli Y. 2010. Carbapenem-resistant KPC-2-producing Escherichia coli in a Tel Aviv Medical Center, 2005 to 2008. Antimicrob. Agents Chemother. 54:2687–2691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wendt C, Schutt S, Dalpke AH, Konrad M, Mieth M, Trierweiler-Hauke B, Weigand MA, Zimmermann S, Biehler K, Jonas D. 2010. First outbreak of Klebsiella pneumoniae carbapenemase (KPC)-producing K. pneumoniae in Germany. Eur. J. Clin. Microbiol. Infect. Dis. 29:563–570 [DOI] [PubMed] [Google Scholar]
- 4.Bratu S, Landman D, Alam M, Tolentino E, Quale J. 2005. Detection of KPC carbapenem-hydrolyzing enzymes in Enterobacter spp. from Brooklyn, New York. Antimicrob. Agents Chemother. 49:776–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bratu S, Landman D, Haag R, Recco R, Eramo A, Alam M, Quale J. 2005. Rapid spread of carbapenem-resistant Klebsiella pneumoniae in New York City: a new threat to our antibiotic armamentarium. Arch. Intern. Med. 165:1430–1435 [DOI] [PubMed] [Google Scholar]
- 6.Bratu S, Tolaney P, Karumudi U, Quale J, Mooty M, Nichani S, Landman D. 2005. Carbapenemase-producing Klebsiella pneumoniae in Brooklyn, NY: molecular epidemiology and in vitro activity of polymyxin B and other agents. J. Antimicrob. Chemother. 56:128–132 [DOI] [PubMed] [Google Scholar]
- 7.Nordmann P, Dortet L, Poirel L. 2012. Carbapenem resistance in Enterobacteriaceae: here is the storm! Trends Mol. Med. 18:263–272 [DOI] [PubMed] [Google Scholar]
- 8.Cao J, Song W, Gu B, Mei YN, Tang JP, Meng L, Yang CQ, Wang H, Zhou H. 2 February 2012. Correlation between carbapenem consumption and antimicrobial resistance rates of Acinetobacter baumannii in a university-affiliated hospital in China. J. Clin. Pharmacol. 10.1177/0091270011435988 [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 9.Goel N, Wattal C, Oberoi JK, Raveendran R, Datta S, Prasad KJ. 2011. Trend analysis of antimicrobial consumption and development of resistance in non-fermenters in a tertiary care hospital in Delhi, India. J. Antimicrob. Chemother. 66:1625–1630 [DOI] [PubMed] [Google Scholar]
- 10.Iosifidis E, Antachopoulos C, Tsivitanidou M, Katragkou A, Farmaki E, Tsiakou M, Kyriazi T, Sofianou D, Roilides E. 2008. Differential correlation between rates of antimicrobial drug consumption and prevalence of antimicrobial resistance in a tertiary care hospital in Greece. Infect. Control Hosp. Epidemiol. 29:615–622 [DOI] [PubMed] [Google Scholar]
- 11.Su CH, Wang JT, Hsiung CA, Chien LJ, Chi CL, Yu HT, Chang FY, Chang SC. 2012. Increase of carbapenem-resistant Acinetobacter baumannii infection in acute care hospitals in Taiwan: association with hospital antimicrobial usage. PLoS One 7:e37788. 10.1371/journal.pone.0037788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meyer E, Schwab F, Schroeren-Boersch B, Gastmeier P. 2010. Dramatic increase of third-generation cephalosporin-resistant E. coli in German intensive care units: secular trends in antibiotic drug use and bacterial resistance, 2001 to 2008. Crit. Care. 14:R113. 10.1186/cc9062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Polk RE, Fox C, Mahoney A, Letcavage J, MacDougall C. 2007. Measurement of adult antibacterial drug use in 130 US hospitals: comparison of defined daily dose and days of therapy. Clin. Infect. Dis. 44:664–670 [DOI] [PubMed] [Google Scholar]
- 14.Goossens H, Ghysels G, Van Laethem Y, De Wit S, Levy J, De Mol P, Clumeck N, Butzler JP, Wenzel RP. 1986. Predicting gentamicin resistance from annual usage in hospital. Lancet ii:804–805 [DOI] [PubMed] [Google Scholar]
- 15.Sun L, Klein EY, Laxminarayan R. 2012. Seasonality and temporal correlation between community antibiotic use and resistance in the United States. Clin. Infect. Dis. 55:687–694 [DOI] [PubMed] [Google Scholar]
- 16.Marchaim D, Chopra T, Bhargava A, Bogan C, Dhar S, Hayakawa K, Pogue JM, Bheemreddy S, Blunden C, Shango M, Swan J, Lephart PR, Perez F, Bonomo RA, Kaye KS. 2012. Recent exposure to antimicrobials and carbapenem-resistant Enterobacteriaceae: the role of antimicrobial stewardship. Infect. Control Hosp. Epidemiol. 33:817–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel N, Harrington S, Dihmess A, Woo B, Masoud R, Martis P, Fiorenza M, Graffunder E, Evans A, McNutt LA, Lodise TP. 2011. Clinical epidemiology of carbapenem-intermediate or -resistant Enterobacteriaceae. J. Antimicrob. Chemother. 66:1600–1608 [DOI] [PubMed] [Google Scholar]