Abstract
Ex vivo antimalarial sensitivity testing in human malaria parasites has largely depended on microscopic determination of schizont maturation. While this microscopic method is sensitive, it suffers from poor precision and is laborious. The recent development of portable, low-cost cytometers has allowed us to develop and validate a simple, field-optimized protocol using SYBR green and dihydroethidium for the accurate and objective determination of antimalarial drug sensitivity in freshly isolated Plasmodium vivax and Plasmodium falciparum.
TEXT
Microscopic examination of ex vivo matured malaria parasites remains the gold standard method used to determine the intrinsic sensitivity of fresh Plasmodium vivax and Plasmodium falciparum isolates to antimalarial drugs (1–8). Ex vivo studies involve the manipulation of primary clinical samples of Plasmodium spp. in an artificial environment for no longer than 48 h. The modified WHO microtest assay is sensitive, relatively simple, and inexpensive and continues to be applied to a range of studies (9–17), especially those seeking novel antimalarial therapeutics against drug-resistant malaria (18–21). However, the microscopic examination of Giemsa-stained thick films central to this method is tedious and time-consuming and requires skilled microscopists. Moreover, large inter- and intraobserver variations of parasite staging are frequently observed (7). Attempts to find an alternative ex vivo method suitable for both P. vivax and P. falciparum have been largely unsuccessful due to the high background noise present in clinical isolates (caused by a number of factors, including leukocytes, red blood cell autofluorescence, gametocytes, and contaminating protein signatures in host plasma) compared with the low target signal of the maturing parasite (clinical isolates frequently have parasitemias of <0.1%) (22–24). Perhaps the most objective and direct method to determine schizont maturation is the use of flow cytometry (25–28). However, the high expense and fragility of most flow cytometers significantly limit their use in field laboratories. Fortunately, the recent development of relatively cheap, portable 2-laser flow cytometers (such as the Accuri C6; Becton, Dickinson) for the first time allows flow cytometric evaluation of ex vivo susceptibility assays in areas where malaria is endemic (29). Capitalizing on this new capability, we have developed a precise, accurate, fast, and simple flow cytometry (FC) method to conduct ex vivo drug sensitivity assays of P. vivax and P. falciparum under field conditions using only 2 colors.
Forty-eight isolates of P. vivax and 15 isolates of P. falciparum with parasitemias of between 0.02% and 0.5%, predominantly at the early ring stage (>80% of the total stages present), were collected from patients attending clinics at the Thai-Myanmar border (collected under the approved ethics protocol FMT-019-10 [Mahidol University, Faculty of Tropical Medicine Internal Review Board]). The isolates were transported to the Shoklo Malaria Research Unit (SMRU) field laboratory within 6 h of collection; the stages of parasitemia were assessed, and samples were then depleted of white blood cells (WBCs) by cellulose medium fiber (Sigma catalogue no. C6288) filtration as previously described (30) and cultured in the presence of 8 to 514 ng/ml of chloroquine diphosphate (molecular weight [MW], 515.9) (CQ) or 0.3 to 19 ng/ml sodium artesunate (MW, 406.4) (AS) using the protocol described by Russell et al. (8). At harvest (∼42 h postculture), the 200 μl of blood medium in each well was mixed, and 20 μl from each well was dispensed into a small curved-bottom tube (Micronic) and stained with 2 μl of dihydroethidium (Sigma) and 5 μl of SYBR green (made up with 63 μl of phosphate-buffered saline [PBS]) (Sigma) and incubated for 20 min at room temperature. During the staining time, thick films (3 μl packed red blood cells [RBCs]) were made from each of the wells for Giemsa staining and microscopic examination. The fluorescent staining reaction was stopped after 20 min with the addition of 400 μl of PBS, and the reaction products were stored on an ice brick until FC analysis. The FC analysis was conducted using an Accuri C6 (Becton, Dickinson), and the gating strategy was per the method of Malleret et al. (29) (see Fig. 1A in the supplemental material). However, two special modifications were made to this protocol. First, only 60,000 events rather than 300,000 events were counted (reducing the count time per well from ∼1.2 min to ∼15 s). Note that for parasitemias less than 0.1%, we suggest using 100,000 events (see Fig. 1B and C in the supplemental material). Second, no CD45 staining was necessary, as >98% of the WBCs were removed from the isolates by cellulose. Slide counts for the microscopy were conducted as described by Russell et al. (8). The proportion of events in the target gate (for cytometry) or the mature schizonts (for microscopy) at each of the treatments was normalized to that in the drug-free control. The proportion of schizont maturation at each corresponding drug concentration was then entered into the online ICEstimator (http://www.antimalarial-icestimator.net/MethodIntro.htm), and the 50% inhibitory concentration (IC50) was calculated by nonlinear regression analysis (31, 32).
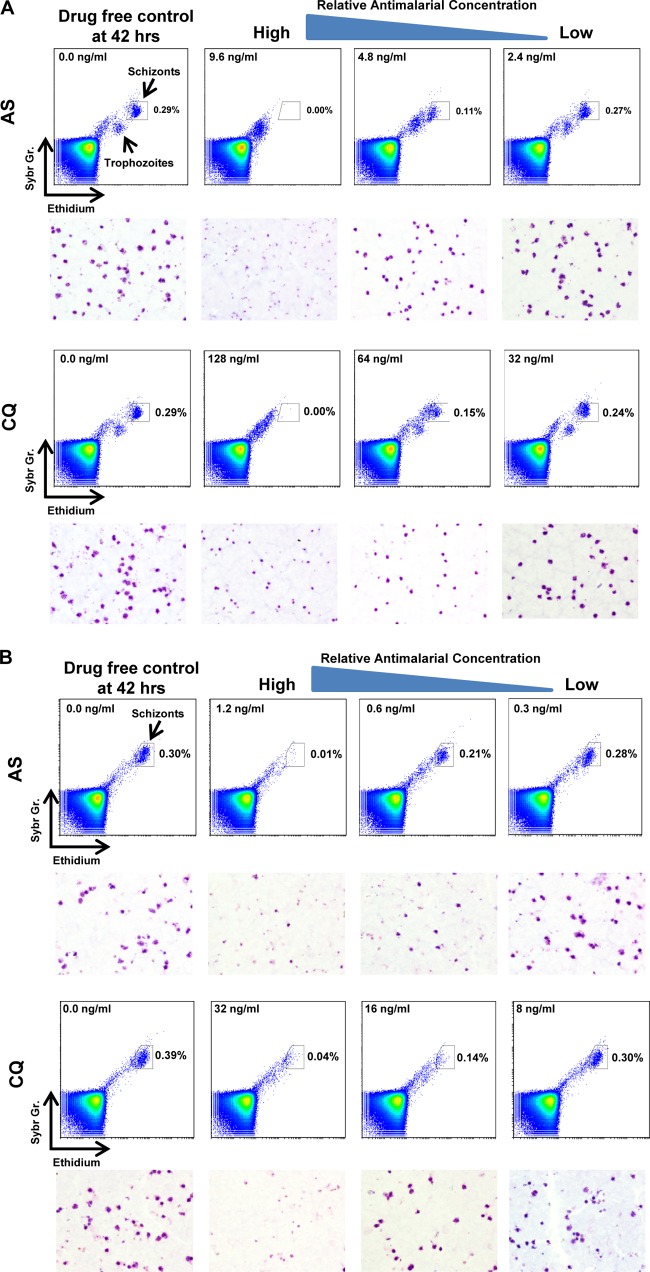
Fig 1.
Representative flow cytometry plot outputs from chloroquine (CQ) and artesunate (AS) sensitivity assays conducted on P. falciparum (A) and P. vivax (B). The target gate representing schizont development events is indicated on each plot. Underneath the plots are the corresponding micrographs of Giemsa-stained thick films collected from the same culture wells.
After 42 h of culture in the drug-free controls, the schizont “target gate” on the cytometer plot corresponding to the cluster of events with the highest levels of DNA (SYBR green, y axis) and RNA (dihydroethidium, x axis) can be clearly discerned for both species (Fig. 1), with the number of events in the FC plot target gate corresponding to the presence or absence of schizonts in the thick films (Fig. 1). As it is important to ensure that later-stage parasites and gametocytes initially present in the precultured isolate (time 0) do not confound the events in the schizont gate postculture, we ran an FC analysis on these initial samples. Any background events present near the target gate were then later subtracted from the events present at gates of the control and treatments postculture.
The culture success rate for both species was good, with 95.8% (46/50) and 86.7% (13/15) of P. vivax and P. falciparum samples, respectively, reaching at least 60% schizonts in the drug-free control after at least 42 h culture. Of the 46 successful P. vivax cultures, we were unable to model the IC50 data for one of the CQ assays.
The geometric mean IC50s of P. vivax CQ and AS determined by microscopy and FC were 17.93 ng/ml (95% CI, 16.2 to 19.84; n = 45) versus 17.20 ng/ml (95% CI, 15.52 to 19.07; n = 46) and 0.57 ng/ml (95% CI, 0.45 to 0.72; n = 45) versus 0.66 ng/ml (95% CI, 0.51 to 0.86; n = 45), respectively (Fig. 2C). For P. falciparum, the geometric mean IC50s of CQ and AS determined by microscopy and FC were 45.82 ng/ml (95% CI, 24.22 to 84.2; n = 13) versus 46.22 ng/ml (95% CI, 24.22 to 88.2; n = 13) and 3.47 ng/ml (95% CI, 2.38 to 5.1; n = 13) versus 3.97 ng/ml (95% CI, 2.78 to 5.67; n = 13) (Fig. 2A). Paired t test analysis showed that the only comparison where there was a significant difference was the sensitivity of P. vivax to AS (Fig. 1C) (P < 0.01). It should be noted that the interspecies differences between the IC50s for CQ and AS are expected and already noted in numerous studies; however, the mechanism behind this still remains unknown.
Fig 2.
Ex vivo sensitivities of Plasmodium falciparum (A) and Plasmodium vivax (C) to chloroquine (CQ) and artesunate (AS), compared using microscopy and flow cytometry (Accuri C6). Solid horizontal lines and associated values are the geometric mean IC50 (ng/ml). A paired t test showed that there was a significant difference (P < 0.01) between the AS and FC IC50s, as calculated by microscopy. Bland-Altman comparisons of IC50s for P. falciparum (B) and P. vivax (D) (AS and CQ combined) were determined by microscopy and flow cytometry. The upper and lower 95% limits of agreement are denoted by the dotted lines.
Bland-Altman analysis indicated good agreement between the methodologies (independent of drug type used) (Fig. 2B and D). There was a slight bias toward higher IC50s with the flow cytometry method for both P. falciparum (−0.03 log10 units) and P. vivax (−0.025 log10 units).
In summary, the antimalarial sensitivity data for the new FC assay matched those of the traditional microscopy very closely. In the one case where there was a significant difference between the IC50 analysis of FC and microscopy, the actual mean difference in AS IC50 for P. vivax was less than 0.1 ng/ml, which is unlikely to be of biological significance. This 0.1-ng/ml disparity should also be put in the context of interreader variability between microscopists, which in our experience can be an order of magnitude greater. It should also be noted that the time to acquire data from the FC method is only ∼2 min per drug (8 wells), compared to 18 min by microscopy. While assay described here used an extended exposure of AS (42 h), we have also used a more physiological 2-h “pulse exposure” of AS at the beginning of the FC assay to mimic the <1-h half-life pharmacokinetic profile of this drug in vivo (this results in an ∼10-fold increase in the AS IC50[data not presented]). In conclusion, our data support the use of this simple FC protocol as a precise and more objective alternative to the microscopic determination of antimalarial drug sensitivity in fresh isolates of P. vivax and P. falciparum. Further studies involving a wider range of drugs are planned.
Supplementary Material
ACKNOWLEDGMENTS
We thank all of the patients and staff of SMRU for their contribution to this study.
This study was supported by the National University Health System (Singapore), Singapore Immunology Network and Horizontal Programme on Infectious Diseases under Agency Science Technology and Research (Singapore), University of Malaya, High Impact Research Fund (E000051-20001) (Malaysia) and the Wellcome Trust (United Kingdom). SMRU is sponsored by The Wellcome Trust of Great Britain as part of the Oxford Tropical Medicine Research Programme of Wellcome Trust-Mahidol University.
Footnotes
Published ahead of print 22 July 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.00682-13.
REFERENCES
- 1.Rieckmann KH, Campbell GH, Sax LJ, Mrema JE. 1978. Drug sensitivity of plasmodium falciparum. An in-vitro microtechnique. Lancet i:22–23 [DOI] [PubMed] [Google Scholar]
- 2.Gajanana A, Raichowdhuri AN. 1984. Plasmodium vivax: micro in vitro test for assaying chloroquine susceptibility. Trans. R. Soc. Trop. Med. Hyg. 78:416–417 [DOI] [PubMed] [Google Scholar]
- 3.Renapurkar DM, Pradhan VR, Sutar NK, Deshmukh RA, Pandit CH, Marathe SN. 1989. Micro test for assaying sensitivity of Plasmodium vivax in vitro. Chemotherapy 35:160–163 [DOI] [PubMed] [Google Scholar]
- 4.Basco LK, Le Bras J. 1994. Short-term in vitro culture of Plasmodium vivax and P. ovale for drug-susceptibility testing. Parasitol. Res. 80:262–264 [DOI] [PubMed] [Google Scholar]
- 5.Tasanor O, Noedl H, Na-Bangchang K, Congpuong K, Sirichaisinthop J, Wernsdorfer WH. 2002. An in vitro system for assessing the sensitivity of Plasmodium vivax to chloroquine. Acta Trop. 83:49–61 [DOI] [PubMed] [Google Scholar]
- 6.Russell BM, Udomsangpetch R, Rieckmann KH, Kotecka BA, Coleman RE, Sattabongkot J. 2003. Simple in vitro assay for determining the sensitivity of Plasmodium vivax isolates from fresh human blood to antimalarials in areas where P. vivax is endemic. Antimicrob. Agents Chemother. 47:170–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Russell B, Suwanarusk R, Malleret B, Costa FT, Snounou G, Baird JK, Nosten F, Renia L. 2012. Human ex vivo studies on asexual Plasmodium vivax: the best way forward. Int. J. Parasitol. 42:1063–1070 [DOI] [PubMed] [Google Scholar]
- 8.Russell B, Chalfein F, Prasetyorini B, Kenangalem E, Piera K, Suwanarusk R, Brockman A, Prayoga P, Sugiarto P, Cheng Q, Tjitra E, Anstey NM, Price RN. 2008. Determinants of in vitro drug susceptibility testing of Plasmodium vivax. Antimicrob. Agents Chemother. 52:1040–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chotivanich K, Udomsangpetch R, Chierakul W, Newton PN, Ruangveerayuth R, Pukrittayakamee S, Looareesuwan S, White NJ. 2004. In vitro efficacy of antimalarial drugs against Plasmodium vivax on the western border of Thailand. Am. J. Trop. Med. Hyg. 70:395–397 [PubMed] [Google Scholar]
- 10.Auliff A, Wilson DW, Russell B, Gao Q, Chen N, Anh LN, Maguire J, Bell D, O'Neil MT, Cheng Q. 2006. Amino acid mutations in Plasmodium vivax DHFR and DHPS from several geographical regions and susceptibility to antifolate drugs. Am. J. Trop. Med. Hyg. 75:617–621 [PubMed] [Google Scholar]
- 11.Suwanarusk R, Russell B, Chavchich M, Chalfein F, Kenangalem E, Kosaisavee V, Prasetyorini B, Piera KA, Barends M, Brockman A, Lek-Uthai U, Anstey NM, Tjitra E, Nosten F, Cheng Q, Price RN. 2007. Chloroquine resistant Plasmodium vivax: in vitro characterisation and association with molecular polymorphisms. PLoS One 2:e1089. 10.1371/journal.pone.0001089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharrock WW, Suwanarusk R, Lek-Uthai U, Edstein MD, Kosaisavee V, Travers T, Jaidee A, Sriprawat K, Price RN, Nosten F, Russell B. 2008. Plasmodium vivax trophozoites insensitive to chloroquine. Malar. J. 7:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suwanarusk R, Chavchich M, Russell B, Jaidee A, Chalfein F, Barends M, Prasetyorini B, Kenangalem E, Piera KA, Lek-Uthai U, Anstey NM, Tjitra E, Nosten F, Cheng Q, Price RN. 2008. Amplification of pvmdr1 associated with multidrug-resistant Plasmodium vivax. J. Infect. Dis. 198:1558–1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hasugian AR, Tjitra E, Ratcliff A, Siswantoro H, Kenangalem E, Wuwung RM, Purba HL, Piera KA, Chalfien F, Marfurt J, Penttinen PM, Russell B, Anstey NM, Price RN. 2009. In vivo and in vitro efficacy of amodiaquine monotherapy for treatment of infection by chloroquine-resistant Plasmodium vivax. Antimicrob. Agents Chemother. 53:1094–1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imwong M, Russell B, Suwanarusk R, Nzila A, Leimanis ML, Sriprawat K, Kaewpongsri S, Phyo AP, Snounou G, Nosten F, Renia L. 2011. Methotrexate is highly potent against pyrimethamine-resistant Plasmodium vivax. J. Infect. Dis. 203:207–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rijken MJ, Boel ME, Russell B, Imwong M, Leimanis ML, Phyo AP, Muehlenbachs A, Lindegardh N, McGready R, Renia L, Snounou G, Singhasivanon P, Nosten F. 2011. Chloroquine resistant vivax malaria in a pregnant woman on the western border of Thailand. Malar. J. 10:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaewpongsri S, Sriprawat K, Suwanarusk R, Kyle DE, Lek-Uthai U, Leimanis M, Lwin KM, Phyo AP, Zwang J, Russell B, Nosten F, Renia L. 2011. The presence of leukocytes in ex vivo assays significantly increases the 50-percent inhibitory concentrations of artesunate and chloroquine against Plasmodium vivax and Plasmodium falciparum. Antimicrob. Agents Chemother. 55:1300–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leimanis ML, Jaidee A, Sriprawat K, Kaewpongsri S, Suwanarusk R, Barends M, Phyo AP, Russell B, Renia L, Nosten F. 2010. Plasmodium vivax susceptibility to ferroquine. Antimicrob. Agents Chemother. 54:2228–2230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rottmann M, McNamara C, Yeung BK, Lee MC, Zou B, Russell B, Seitz P, Plouffe DM, Dharia NV, Tan J, Cohen SB, Spencer KR, Gonzalez-Paez GE, Lakshminarayana SB, Goh A, Suwanarusk R, Jegla T, Schmitt EK, Beck HP, Brun R, Nosten F, Renia L, Dartois V, Keller TH, Fidock DA, Winzeler EA, Diagana TT. 2010. Spiroindolones, a potent compound class for the treatment of malaria. Science 329:1175–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marfurt J, Chalfein F, Prayoga P, Wabiser F, Kenangalem E, Piera KA, Machunter B, Tjitra E, Anstey NM, Price RN. 2011. Ex vivo drug susceptibility of ferroquine against chloroquine-resistant isolates of Plasmodium falciparum and P. vivax. Antimicrob. Agents Chemother. 55:4461–4464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Price RN, Marfurt J, Chalfein F, Kenangalem E, Piera KA, Tjitra E, Anstey NM, Russell B. 2010. In vitro activity of pyronaridine against multidrug-resistant Plasmodium falciparum and Plasmodium vivax. Antimicrob. Agents Chemother. 54:5146–5150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Basco LK, Marquet F, Makler MM, Le Bras J. 1995. Plasmodium falciparum and Plasmodium vivax: lactate dehydrogenase activity and its application for in vitro drug susceptibility assay. Exp. Parasitol. 80:260–271 [DOI] [PubMed] [Google Scholar]
- 23.Kosaisavee V, Suwanarusk R, Nosten F, Kyle DE, Barrends M, Jones J, Price R, Russell B, Lek-Uthai U. 2006. Plasmodium vivax: isotopic, PicoGreen, and microscopic assays for measuring chloroquine sensitivity in fresh and cryopreserved isolates. Exp. Parasitol. 114:34–39 [DOI] [PubMed] [Google Scholar]
- 24.Druilhe P, Brasseur P, Blanc C, Makler M. 2007. Improved assessment of Plasmodium vivax response to antimalarial drugs by a colorimetric double-site plasmodium lactate dehydrogenase antigen capture enzyme-linked immunosorbent assay. Antimicrob. Agents Chemother. 51:2112–2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whaun JM, Rittershaus C, Ip SH. 1983. Rapid identification and detection of parasitized human red cells by automated flow cytometry. Cytometry 4:117–122 [DOI] [PubMed] [Google Scholar]
- 26.Janse CJ, van Vianen PH, Tanke HJ, Mons B, Ponnudurai T, Overdulve JP. 1987. Plasmodium species: flow cytometry and microfluorometry assessments of DNA content and synthesis. Exp. Parasitol. 64:88–94 [DOI] [PubMed] [Google Scholar]
- 27.Pattanapanyasat K, Thaithong S, Kyle DE, Udomsangpetch R, Yongvanitchit K, Hider RC, Webster HK. 1997. Flow cytometric assessment of hydroxypyridinone iron chelators on in vitro growth of drug-resistant malaria. Cytometry 27:84–91 [DOI] [PubMed] [Google Scholar]
- 28.Grimberg BT, Erickson JJ, Sramkoski RM, Jacobberger JW, Zimmerman PA. 2008. Monitoring Plasmodium falciparum growth and development by UV flow cytometry using an optimized Hoechst-thiazole orange staining strategy. Cytometry A 73:546–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malleret B, Claser C, Ong AS, Suwanarusk R, Sriprawat K, Howland SW, Russell B, Nosten F, Renia L. 2011. A rapid and robust tri-color flow cytometry assay for monitoring malaria parasite development. Sci. Rep. 1:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sriprawat K, Kaewpongsri S, Suwanarusk R, Leimanis ML, Lek-Uthai U, Phyo AP, Snounou G, Russell B, Renia L, Nosten F. 2009. Effective and cheap removal of leukocytes and platelets from Plasmodium vivax infected blood. Malar. J. 8:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaddouri H, Nakache S, Houze S, Mentre F, Le Bras J. 2006. Assessment of the drug susceptibility of Plasmodium falciparum clinical isolates from Africa by using a Plasmodium lactate dehydrogenase immunodetection assay and an inhibitory maximum effect model for precise measurement of the 50-percent inhibitory concentration. Antimicrob. Agents Chemother. 50:3343–3349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Le Nagard H, Vincent C, Mentre F, Le Bras J. 2011. Online analysis of in vitro resistance to antimalarial drugs through nonlinear regression. Comput. Methods Programs Biomed. 104:10–18 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.