Abstract
Thioviridamide is a unique peptide antibiotic containing five thioamide bonds from Streptomyces olivoviridis. Draft genome sequencing revealed a gene (the tvaA gene) encoding the thioviridamide precursor peptide. The thioviridamide biosynthesis gene cluster was identified by heterologous production of thioviridamide in Streptomyces lividans.
TEXT
Thioviridamide is an N-acylated undecapeptide antibiotic which induces apoptosis selectively in E1A-transformed cells (1). The most unique structural feature of thioviridamide is the presence of five thioamide bonds formed between the amino acids (Fig. 1) (2). Only five thioamide compounds have been found in natural sources. Among them, thioviridamide, apo-methanobactin, and closthioamide are of bacterial origin, isolated from Streptomyces olivoviridis, Methylosinus trichosporium, and Clostridium cellulolyticum, respectively (3). Apo-methanobactin is a copper chelator containing two thioamide-bearing oxazolone rings and belongs to the ribosomally synthesized and posttranslationally modified peptide (RiPP) family. Genomic analysis of M. trichosporium has identified a gene encoding the apo-methanobactin precursor peptide containing a LCGSCYPCSCM sequence (underlining indicates the thioamide precursor). It is presumed that the sulfur atoms in the two thioamide groups could be supplied from the adjacent cysteine residues (the precursors of the oxazolone rings) (4). Closthioamide is a polythioamide antibiotic isolated from a strictly anaerobic bacterium (5). Although the closthioamide producer genome has been sequenced, its biosynthesis genes have remained elusive (6). In this study, we identified the thioviridamide biosynthesis gene cluster of S. olivoviridis NA05001 and demonstrated heterologous production of thioviridamide in Streptomyces lividans TK23.
Fig 1.
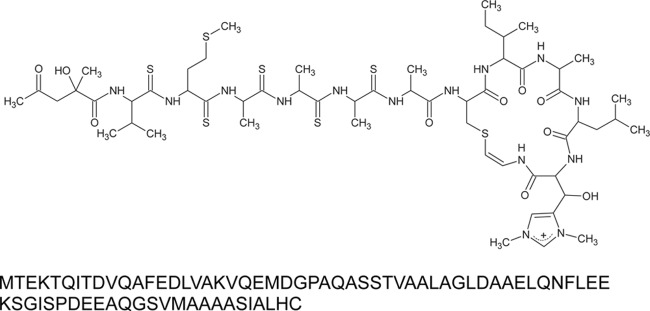
Structure of thioviridamide (top) and amino acid sequence of TvaA (bottom).
Although most peptide antibiotics are biosynthesized by nonribosomal peptide synthetases (NRPSs) (7), thioviridamide contains an S-(2-aminovinyl)cysteine (AviCys) residue, which has been found in the linaridin family of RiPPs (8). Epidermin (9), microbisporicin (10), and cypemycin (11) are known to be AviCys-containing linaridins. AviCys is formed by cyclization between a serine/cysteine-derived dehydroalanine and a C-terminal cysteine via oxidative decarboxylation (11, 12). Thus, thioviridamide is presumed to be biosynthesized by posttranslational modification of a ribosomal precursor peptide containing a VMAAAASIALHC or VMAAAACIALHC sequence.
Genomic DNA isolated from S. olivoviridis NA05001 was sequenced using an Illumina Hiseq 2000 platform at TaKaRa Bio Inc. (Otsu, Japan) after purification with a Genomic-tip 20/G kit (Qiagen). The sequence reads were assembled using the Edena assembler (version 3) (13) into 205 contigs totaling 7,712,087 bp. A sequence search identified the tvaA gene, encoding 75 amino acids containing VMAAAASIALHC at the C-terminal end (Fig. 1). FramePlot analysis (14) of 7.5 kbp from the 5′ end and 23 kbp from the 3′ end of the tvaA gene showed the presence of a putative thioviridamide biosynthesis gene cluster (Fig. 2).
Fig 2.
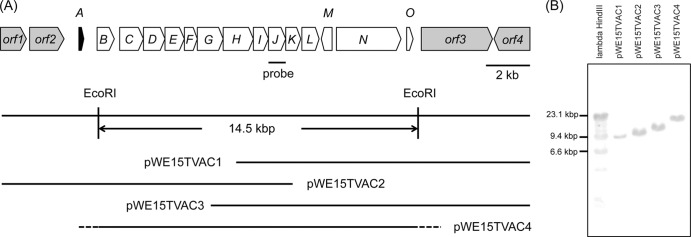
Thioviridamide biosynthesis gene cluster from Streptomyces olivoviridis NA05001. (A) The tva gene cluster from Streptomyces olivoviridis NA05001 and four overlapping cosmids, pWE15TVAC1 to pWE15TVAC4. (B) Southern analysis of the EcoRI-digested cosmids.
The cluster contained 10 genes arranged as an operon (tvaC-tvaL) downstream of a putative regulatory gene (the tvaB gene). Two presumably cotranscribed genes (orf1 and orf2) upstream of the tvaA gene were excluded from the cluster based on high homology to a DNA polymerase and a protease, respectively (Table 1). A putative DNA polymerase gene (orf4) was also excluded, although assignments of four genes between the tvaL and orf4 genes were unclear. To identify genes required for thioviridamide biosynthesis and the biosynthesis gene cluster, the candidate genes were cloned and expressed in Streptomyces lividans to produce thioviridamide.
Table 1.
ORFs in the thioviridamide biosynthetic gene clustera
| Protein | Size (aa) | Homologous protein (origin) | Identity/similarity (%) | GenBank or NCBI accession no. | Deduced function |
|---|---|---|---|---|---|
| Orf1 | 401 | DNA polymerase III subunit delta′ (S. davawensis) | 90/93 | CCK28488 | |
| Orf2 | 529 | Protease (S. avermitilis) | 83/87 | NP_825801 | |
| TvaA | 75 | ABC transporter (Penicillium griseofulvum) | 46/62 | ACR02669 | Precursor peptide |
| TvaB | 275 | SARP family regulator (S. lavendulae) | 35/55 | BAG74713 | Regulator |
| TvaC | 377 | Phosphotransferase family protein (Nostoc sp.) | 29/45 | YP_007077015 | Unknown |
| TvaD | 328 | Hypothetical protein (Calothrix sp.) | 25/41 | YP_007137464 | Unknown |
| TvaE | 308 | Aminoglycoside phosphotransferase (Frankia sp.) | 48/65 | EHI90509.1 | Unknown |
| TvaF | 197 | Phosphopantothenoylcysteine decarboxylase (Exiguobacterium sp.) | 39/60 | YP_002887224 | Oxidative decarboxylase |
| TvaG | 407 | Type 12 methyltransferase (Cyanothece sp.) | 43/61 | YP_002481771 | Methyltransferase |
| TvaH | 452 | Methanogenesis marker protein 1 (Methanolinea tarda) | 36/53 | EHF09819.1 | Unknown |
| TvaI | 218 | TfuA-like core domain-containing protein (M. tarda) | 44/63 | EHF09820.1 | Regulator |
| TvaJ | 280 | Phytanoyl-CoA dioxygenase (Roseobacter litoralis) | 29/44 | YP_004691882 | Oxygenase |
| TvaK | 220 | Papain family cysteine protease (Tannerella forsythia) | 27/39 | YP_005014309 | Protease |
| TvaL | 282 | Integral membrane protein (S. pristinaespiralis) | 31/49 | EDY64257.2 | Unknown |
| TvaM | 160 | Histidine kinase (S. somaliensis) | 55/66 | WP_010468209.1 | Regulator |
| TvaN | 1,003 | Large transcriptional regulator (S. pristinaespiralis) | 67/75 | EDY63194.2 | Regulator |
| TvaO | 94 | Hypothetical protein (S. viridochromogenes) | 54/68 | ELS50220.1 | Unknown |
| Orf3 | 1,089 | Transcriptional regulator (S. avermitilis) | 63/69 | NP_825812 | |
| Orf4 | 561 | DNA polymerase I (S. griseoflavus) | 87/92 | EFL40026.1 |
S., Streptomyces; SARP, Streptomyces antibiotic regulatory protein; CoA, coenzyme A.
A cosmid library was constructed in pWE15 (Agilent Technologies) from Sau3AI-digested genomic DNA of S. olivoviridis NA05001. Cosmid clones containing the tva gene cluster were detected by colony hybridization using a 0.8-kbp tvaJ gene fragment. The probe was labeled with an AlkPhos direct labeling kit (GE Healthcare) after the fragment was amplified and cloned from the genomic DNA with primers containing additional HindIII sites (5′-TAAAGCTTTCACGCGTCATCAGCCGGCCCGA-3′ and 5′-TCAAGCTTATGACGGCTGCGCGGAAGGGATT-3′). The cosmid clones were digested with EcoRI and subsequently analyzed by Southern hybridization using the same probe. A cosmid clone (pWE15TVAC4) contained a 14.5-kbp fragment from the tvaB gene to the tvaO gene (Fig. 2). A 2.0-kbp tvaA gene fragment containing the terminal EcoRI site was amplified from S. olivoviridis NA05001 genomic DNA by KOD-Plus (Toyobo) DNA polymerase using primers with an additional XbaI site (5′-CCTTCAGACGGAATTCATCGGCGAACGGC-3′ and 5′-GAGGGCGTCTAGAGAGCACCCCCGGAAAC-3′). The fragment was digested with EcoRI/XbaI and cloned into pBluescript II SK to construct pBS-TVA1. A 14.5-kbp EcoRI fragment of pWE15TVAC4 was ligated into EcoRI-digested pBS-TVA1. Resulting clones were sequenced to select one with the insert in the correct orientation. The plasmid was digested with XbaI/HindIII and ligated into XbaI/HindIII sites of the Escherichia coli/Streptomyces shuttle vector pWHM3 (15) to construct pWHM3-TVA. The expression plasmid passaged through E. coli JM110 was introduced into S. lividans TK23.
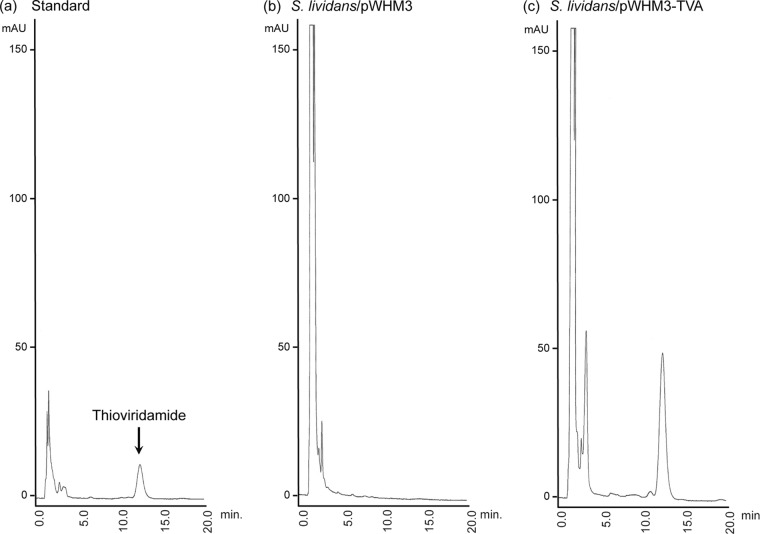
The transformant was cultivated in Erlenmeyer flasks containing a medium consisting of 2.5% glucose, 1.5% soybean meal, 0.2% dry yeast, 0.4% CaCO3, and 10 μg/ml thiostrepton (pH 6.2) on a rotary shaker at 27°C for 4 days. The mycelial acetone extract was evaporated and then extracted with ethyl acetate (EtOAc) at pH 3. The extract was analyzed by high-pressure liquid chromatography (HPLC) using a YMC-Pack R-ODS-7 column with 80% MeOH–0.2% H3PO4 (2 ml/min). A UV absorption peak for thioviridamide at 274 nm was detected at a retention time of 12.4 min (Fig. 3). The production of thioviridamide was confirmed by the 1H nuclear magnetic resonance (NMR) spectrum of the purified sample (see Fig. S2 in the supplemental material), thereby showing that the tva gene cluster (tvaA to tvaO) is responsible for thioviridamide production.
Fig 3.
HPLC analysis of the mycelial extract of S. lividans expressing the tva gene cluster. HPLC profiles of (a) standard thioviridamide and the culture extract of S. lividans carrying (b) a control plasmid (pWHM3) or (c) a tva gene cluster-containing plasmid (pWHM3-TVA).
Among the tva gene products, a putative decarboxylase, TvaF, exhibits homology to EpiD (25% identity, 49% similarity) (9), MibD (39% identity, 57% similarity) (10), and CypD (27% identity, 49% similarity) (11), which are involved in AviCys formation. TvaK is a putative cysteine protease that likely cleaves the leader peptide from TvaA. Thioviridamide contains a unique amino acid, β-hydroxy-N1,N3-dimethylhistidinium, which has not been isolated from other organisms. Nevertheless, N1-methylhistidine, N3-methylhistidine, and β-hydroxyhistidine are commonly found in natural sources and known to be produced by posttranslational modification enzymes, including histidine protein methyltransferase Hpm1 (16), carnosine N-methyltransferase (17), and the bifunctional lysine-specific demethylase and histidyl-hydroxylase, NO66 (18). A putative methyltransferase, TvaG, and a putative dioxygenase, TvaJ, are presumably required for the formation of the β-hydroxy-N1,N3-dimethylhistidinium residue. These proteins, however, reveal no homology to known histidine-modifying enzymes. TvaB, TvaM, and TvaN are categorized as regulatory proteins based on sequence similarities. TvaI is also assignable as a regulator, because a homologous protein, TfuA, can regulate the production of trifolitoxin, a RiPP family antibiotic (19).
The remaining tva gene products might be involved in self-resistance, N-terminal acylation, or thioamide formation. TvaC and TvaE share similarity to aminoglycoside phosphotransferase. Since this enzyme is known to be responsible for resistance to aminoglycoside antibiotics, TvaC and/or TvaE are predicted to confer self-resistance. TvaL is a putative membrane protein with four transmembrane helices and might function as a transporter, because topologically related proteins are found in several RiPP biosynthesis clusters (20, 21). N-acylated RiPPs reported are few and include polytheonamides and epilancins. The N-terminal 5-dimethyl-2-oxohexanoate of polytheonamides A and B and the N-terminal lactate of epilancin 15X have been reported to be derived from threonine and serine, respectively (21, 22). The N-acyl group of thioviridamide appears to be serine-derived from the precursor peptide sequence. Although posttranslational modification genes in the polytheonamide and epilancin producers have been identified, no related gene is found in the tva gene cluster.
The tva gene products share no significant similarity to any proteins of the closthioamide producer, C. cellulolyticum H10, suggesting that the two thioamide producers adopt different mechanisms for thioamide formation. TvaH is a possible candidate for a thioamide-forming enzyme and shows homology to a YcaO domain of GodD (24% identity, 40% similarity) in the biosynthesis gene cluster of goadsporin, a RiPP containing thiazole and oxazole rings (23). A YcaO domain-containing protein, BalhD, has been shown to activate the amide backbone of the precursor peptide using ATP in the biosynthesis of RiPPs (24). The activated amide carbon is proposed to react with the SH group of a cysteine residue as a possible mechanism of thiazole formation in azole-containing RiPPs. TvaH might catalyze the conversion of amide bonds into thioamide bonds in the presence of a sulfur donor. Our heterologous expression system is expected to be useful for identification of the gene functions.
In conclusion, this study has identified the biosynthesis gene cluster of thioviridamide from S. olivoviridis NA05001 and demonstrated heterologous production of thioviridamide in S. lividans TK23. Thioviridamide is confirmed to be derived from a ribosomally synthesized prepeptide.
Nucleotide sequence accession number.
The sequence of the thioviridamide biosynthesis gene cluster was deposited in DDBJ under accession number AB819757.
Supplementary Material
ACKNOWLEDGMENT
This work was supported in part by a Grant-in-Aid for Scientific Research, The Ministry of Education, Science, Sports and Culture, Japan.
Footnotes
Published ahead of print 30 August 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.01978-13.
REFERENCES
- 1.Hayakawa Y, Sasaki K, Adachi H, Furihata K, Nagai K, Shin-ya K. 2006. Thioviridamide, a novel apoptosis inducer in transformed cells from Streptomyces olivoviridis. J. Antibiot. (Tokyo) 59:1–5 [DOI] [PubMed] [Google Scholar]
- 2.Hayakawa Y, Sasaki K, Nagai K, Shin-ya K, Furihata K. 2006. Structure of thioviridamide, a novel apoptosis inducer from Streptomyces olivoviridis. J. Antibiot. (Tokyo) 59:6–10 [DOI] [PubMed] [Google Scholar]
- 3.Banala S, Sussmuth RD. 2010. Thioamides in nature: in search of secondary metabolites in anaerobic microorganisms. Chembiochem 11:1335–1337 [DOI] [PubMed] [Google Scholar]
- 4.Krentz BD, Mulheron HJ, Semrau JD, Dispirito AA, Bandow NL, Haft DH, Vuilleumier S, Murrell JC, McEllistrem MT, Hartsel SC, Gallagher WH. 2010. A comparison of methanobactins from Methylosinus trichosporium OB3b and Methylocystis strain Sb2 predicts methanobactins are synthesized from diverse peptide precursors modified to create a common core for binding and reducing copper ions. Biochemistry 49:10117–10130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lincke T, Behnken S, Ishida K, Roth M, Hertweck C. 2010. Closthioamide: an unprecedented polythioamide antibiotic from the strictly anaerobic bacterium Clostridium cellulolyticum. Angew. Chem. Int. Ed. Engl. 49:2011–2013 [DOI] [PubMed] [Google Scholar]
- 6.Behnken S, Hertweck C. 2012. Anaerobic bacteria as producers of antibiotics. Appl. Microbiol. Biotechnol. 96:61–67 [DOI] [PubMed] [Google Scholar]
- 7.Schwarzer D, Finking R, Marahiel MA. 2003. Nonribosomal peptides: from genes to products. Nat. Prod. Rep. 20:275–287 [DOI] [PubMed] [Google Scholar]
- 8.Arnison PG, Bibb MJ, Bierbaum G, Bowers AA, Bugni TS, Bulaj G, Camarero JA, Campopiano DJ, Challis GL, Clardy J, Cotter PD, Craik DJ, Dawson M, Dittmann E, Donadio S, Dorrestein PC, Entian KD, Fischbach MA, Garavelli JS, Goransson U, Gruber CW, Haft DH, Hemscheidt TK, Hertweck C, Hill C, Horswill AR, Jaspars M, Kelly WL, Klinman JP, Kuipers OP, Link AJ, Liu W, Marahiel MA, Mitchell DA, Moll GN, Moore BS, Muller R, Nair SK, Nes IF, Norris GE, Olivera BM, Onaka H, Patchett ML, Piel J, Reaney MJ, Rebuffat S, Ross RP, Sahl HG, Schmidt EW, Selsted ME, Severinov K, Shen B, Sivonen K, Smith L, Stein T, Sussmuth RD, Tagg JR, Tang GL, Truman AW, Vederas JC, Walsh CT, Walton JD, Wenzel SC, Willey JM, van der Donk WA. 2013. Ribosomally synthesized and post-translationally modified peptide natural products: overview and recommendations for a universal nomenclature. Nat. Prod. Rep. 30:108–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bierbaum G, Gotz F, Peschel A, Kupke T, van de Kamp M, Sahl HG. 1996. The biosynthesis of the lantibiotics epidermin, gallidermin, Pep5 and epilancin K7. Antonie Van Leeuwenhoek 69:119–127 [DOI] [PubMed] [Google Scholar]
- 10.Foulston LC, Bibb MJ. 2010. Microbisporicin gene cluster reveals unusual features of lantibiotic biosynthesis in actinomycetes. Proc. Natl. Acad. Sci. U. S. A. 107:13461–13466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Claesen J, Bibb M. 2010. Genome mining and genetic analysis of cypemycin biosynthesis reveal an unusual class of posttranslationally modified peptides. Proc. Natl. Acad. Sci. U. S. A. 107:16297–16302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McIntosh JA, Donia MS, Schmidt EW. 2009. Ribosomal peptide natural products: bridging the ribosomal and nonribosomal worlds. Nat. Prod Rep. 26:537–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hernandez D, Francois P, Farinelli L, Osteras M, Schrenzel J. 2008. De novo bacterial genome sequencing: millions of very short reads assembled on a desktop computer. Genome Res. 18:802–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishikawa J, Hotta K. 1999. FramePlot: a new implementation of the frame analysis for predicting protein-coding regions in bacterial DNA with a high G + C content. FEMS Microbiol. Lett. 174:251–253 [DOI] [PubMed] [Google Scholar]
- 15.Vara J, Lewandowska-Skarbek M, Wang YG, Donadio S, Hutchinson CR. 1989. Cloning of genes governing the deoxysugar portion of the erythromycin biosynthesis pathway in Saccharopolyspora erythraea (Streptomyces erythreus). J. Bacteriol. 171:5872–5881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Webb KJ, Zurita-Lopez CI, Al-Hadid Q, Laganowsky A, Young BD, Lipson RS, Souda P, Faull KF, Whitelegge JP, Clarke SG. 2010. A novel 3-methylhistidine modification of yeast ribosomal protein Rpl3 is dependent upon the YIL110W methyltransferase. J. Biol. Chem. 285:37598–37606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drozak J, Chrobok L, Poleszak O, Jagielski AK, Derlacz R. 2013. Molecular identification of carnosine N-methyltransferase as chicken histamine N-methyltransferase-like protein (hnmt-like). PLoS One 8:e64805. 10.1371/journal.pone.0064805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ge W, Wolf A, Feng T, Ho CH, Sekirnik R, Zayer A, Granatino N, Cockman ME, Loenarz C, Loik ND, Hardy AP, Claridge TD, Hamed RB, Chowdhury R, Gong L, Robinson CV, Trudgian DC, Jiang M, Mackeen MM, McCullagh JS, Gordiyenko Y, Thalhammer A, Yamamoto A, Yang M, Liu-Yi P, Zhang Z, Schmidt-Zachmann M, Kessler BM, Ratcliffe PJ, Preston GM, Coleman ML, Schofield CJ. 2012. Oxygenase-catalyzed ribosome hydroxylation occurs in prokaryotes and humans. Nat. Chem. Biol. 8:960–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Breil B, Borneman J, Triplett EW. 1996. A newly discovered gene, tfuA, involved in the production of the ribosomally synthesized peptide antibiotic trifolitoxin. J. Bacteriol. 178:4150–4156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoffmann A, Schneider T, Pag U, Sahl HG. 2004. Localization and functional analysis of PepI, the immunity peptide of Pep5-producing Staphylococcus epidermidis strain 5. Appl. Environ. Microbiol. 70:3263–3271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Velásquez JE, Zhang X, van der Donk WA. 2011. Biosynthesis of the antimicrobial peptide epilancin 15X and its N-terminal lactate. Chem. Biol. 18:857–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freeman MF, Gurgui C, Helf MJ, Morinaka BI, Uria AR, Oldham NJ, Sahl HG, Matsunaga S, Piel J. 2012. Metagenome mining reveals polytheonamides as posttranslationally modified ribosomal peptides. Science 338:387–390 [DOI] [PubMed] [Google Scholar]
- 23.Onaka H, Nakaho M, Hayashi K, Igarashi Y, Furumai T. 2005. Cloning and characterization of the goadsporin biosynthetic gene cluster from Streptomyces sp. TP-A0584. Microbiology 151:3923–3933 [DOI] [PubMed] [Google Scholar]
- 24.Dunbar KL, Melby JO, Mitchell DA. 2012. YcaO domains use ATP to activate amide backbones during peptide cyclodehydrations. Nat. Chem. Biol. 8:569–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.