Abstract
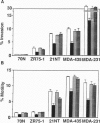
Maspin, a novel serine protease inhibitor (serpin), inhibits tumor invasion and metastasis of mammary carcinoma. We show here that recombinant maspin protein blocks the motility of these carcinoma cells in culture over 12 h, as demonstrated by time-lapse video microscopy. Lamellopodia are withdrawn but ruffling continues. Both exogenous recombinant maspin and maspin expressed by tumor transfectants exhibit inhibitory effects on cell motility and cell invasion as shown in modified Boyden chamber assays. In addition, three prostatic cancer cell lines treated with recombinant maspin exhibited similar inhibition of both invasion and motility, suggesting a similar mode of maspin action in these two glandular epithelial cancers. When mammary carcinoma cells were treated with recombinant maspin, the protein was shown by immunostaining to bind specifically to the cell surface, suggesting that maspin activity is membrane associated. When pretreated with antimaspin antibody, maspin loses its inhibitory effects on both invasion and motility. However, when maspin is added to these cells preceding antibody treatment, the activity of maspin is no longer inhibited by subsequent addition of the antibody. It is concluded therefore that the inhibition of invasion and motility by maspin is initially localized to the cell surface.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aertgeerts K., De Bondt H. L., De Ranter C. J., Declerck P. J. Mechanisms contributing to the conformational and functional flexibility of plasminogen activator inhibitor-1. Nat Struct Biol. 1995 Oct;2(10):891–897. doi: 10.1038/nsb1095-891. [DOI] [PubMed] [Google Scholar]
- Band V., Sager R. Distinctive traits of normal and tumor-derived human mammary epithelial cells expressed in a medium that supports long-term growth of both cell types. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1249–1253. doi: 10.1073/pnas.86.4.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Band V., Zajchowski D., Swisshelm K., Trask D., Kulesa V., Cohen C., Connolly J., Sager R. Tumor progression in four mammary epithelial cell lines derived from the same patient. Cancer Res. 1990 Nov 15;50(22):7351–7357. [PubMed] [Google Scholar]
- Behrendt N., Rønne E., Ploug M., Petri T., Løber D., Nielsen L. S., Schleuning W. D., Blasi F., Appella E., Danø K. The human receptor for urokinase plasminogen activator. NH2-terminal amino acid sequence and glycosylation variants. J Biol Chem. 1990 Apr 15;265(11):6453–6460. [PubMed] [Google Scholar]
- Busso N., Masur S. K., Lazega D., Waxman S., Ossowski L. Induction of cell migration by pro-urokinase binding to its receptor: possible mechanism for signal transduction in human epithelial cells. J Cell Biol. 1994 Jul;126(1):259–270. doi: 10.1083/jcb.126.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cajot J. F., Bamat J., Bergonzelli G. E., Kruithof E. K., Medcalf R. L., Testuz J., Sordat B. Plasminogen-activator inhibitor type 1 is a potent natural inhibitor of extracellular matrix degradation by fibrosarcoma and colon carcinoma cells. Proc Natl Acad Sci U S A. 1990 Sep;87(18):6939–6943. doi: 10.1073/pnas.87.18.6939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrell R. W., Stein P. E., Fermi G., Wardell M. R. Biological implications of a 3 A structure of dimeric antithrombin. Structure. 1994 Apr 15;2(4):257–270. doi: 10.1016/s0969-2126(00)00028-9. [DOI] [PubMed] [Google Scholar]
- Chu Y. W., Runyan R. B., Oshima R. G., Hendrix M. J. Expression of complete keratin filaments in mouse L cells augments cell migration and invasion. Proc Natl Acad Sci U S A. 1993 May 1;90(9):4261–4265. doi: 10.1073/pnas.90.9.4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conese M., Nykjaer A., Petersen C. M., Cremona O., Pardi R., Andreasen P. A., Gliemann J., Christensen E. I., Blasi F. alpha-2 Macroglobulin receptor/Ldl receptor-related protein(Lrp)-dependent internalization of the urokinase receptor. J Cell Biol. 1995 Dec;131(6 Pt 1):1609–1622. doi: 10.1083/jcb.131.6.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer L. P., Mitchison T. J., Theriot J. A. Actin-dependent motile forces and cell motility. Curr Opin Cell Biol. 1994 Feb;6(1):82–86. doi: 10.1016/0955-0674(94)90120-1. [DOI] [PubMed] [Google Scholar]
- Ellis V., Wun T. C., Behrendt N., Rønne E., Danø K. Inhibition of receptor-bound urokinase by plasminogen-activator inhibitors. J Biol Chem. 1990 Jun 15;265(17):9904–9908. [PubMed] [Google Scholar]
- Fazioli F., Blasi F. Urokinase-type plasminogen activator and its receptor: new targets for anti-metastatic therapy? Trends Pharmacol Sci. 1994 Jan;15(1):25–29. doi: 10.1016/0165-6147(94)90130-9. [DOI] [PubMed] [Google Scholar]
- Foekens J. A., Buessecker F., Peters H. A., Krainick U., van Putten W. L., Look M. P., Klijn J. G., Kramer M. D. Plasminogen activator inhibitor-2: prognostic relevance in 1012 patients with primary breast cancer. Cancer Res. 1995 Apr 1;55(7):1423–1427. [PubMed] [Google Scholar]
- Foekens J. A., Schmitt M., van Putten W. L., Peters H. A., Bontenbal M., Jänicke F., Klijn J. G. Prognostic value of urokinase-type plasminogen activator in 671 primary breast cancer patients. Cancer Res. 1992 Nov 1;52(21):6101–6105. [PubMed] [Google Scholar]
- Gettins P., Patston P. A., Schapira M. The role of conformational change in serpin structure and function. Bioessays. 1993 Jul;15(7):461–467. doi: 10.1002/bies.950150705. [DOI] [PubMed] [Google Scholar]
- Grøndahl-Hansen J., Christensen I. J., Rosenquist C., Brünner N., Mouridsen H. T., Danø K., Blichert-Toft M. High levels of urokinase-type plasminogen activator and its inhibitor PAI-1 in cytosolic extracts of breast carcinomas are associated with poor prognosis. Cancer Res. 1993 Jun 1;53(11):2513–2521. [PubMed] [Google Scholar]
- Hopkins P. C., Whisstock J. Function of maspin. Science. 1994 Sep 23;265(5180):1893–1894. [PubMed] [Google Scholar]
- Jensen P. H., Lorand L., Ebbesen P., Gliemann J. Type-2 plasminogen-activator inhibitor is a substrate for trophoblast transglutaminase and factor XIIIa. Transglutaminase-catalyzed cross-linking to cellular and extracellular structures. Eur J Biochem. 1993 May 15;214(1):141–146. doi: 10.1111/j.1432-1033.1993.tb17906.x. [DOI] [PubMed] [Google Scholar]
- Joneson T., White M. A., Wigler M. H., Bar-Sagi D. Stimulation of membrane ruffling and MAP kinase activation by distinct effectors of RAS. Science. 1996 Feb 9;271(5250):810–812. doi: 10.1126/science.271.5250.810. [DOI] [PubMed] [Google Scholar]
- Karikó K., Kuo A., Boyd D., Okada S. S., Cines D. B., Barnathan E. S. Overexpression of urokinase receptor increases matrix invasion without altering cell migration in a human osteosarcoma cell line. Cancer Res. 1993 Jul 1;53(13):3109–3117. [PubMed] [Google Scholar]
- Laug W. E., Cao X. R., Yu Y. B., Shimada H., Kruithof E. K. Inhibition of invasion of HT1080 sarcoma cells expressing recombinant plasminogen activator inhibitor 2. Cancer Res. 1993 Dec 15;53(24):6051–6057. [PubMed] [Google Scholar]
- Lingappa V. R., Lingappa J. R., Blobel G. Chicken ovalbumin contains an internal signal sequence. Nature. 1979 Sep 13;281(5727):117–121. doi: 10.1038/281117a0. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Mandler R., Murano G., Katz D. A., Gordon R. K., Chiang P. K., Schiffmann E. Tumor cell autocrine motility factor. Proc Natl Acad Sci U S A. 1986 May;83(10):3302–3306. doi: 10.1073/pnas.83.10.3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotan R., Amos B., Watanabe H., Raz A. Suppression of melanoma cell motility factor receptor expression by retinoic acid. Cancer Res. 1992 Sep 15;52(18):4878–4884. [PubMed] [Google Scholar]
- Meek R. L., Walsh K. A., Palmiter R. D. The signal sequence of ovalbumin is located near the NH2 terminus. J Biol Chem. 1982 Oct 25;257(20):12245–12251. [PubMed] [Google Scholar]
- Mueller B. M., Yu Y. B., Laug W. E. Overexpression of plasminogen activator inhibitor 2 in human melanoma cells inhibits spontaneous metastasis in scid/scid mice. Proc Natl Acad Sci U S A. 1995 Jan 3;92(1):205–209. doi: 10.1073/pnas.92.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naldini L., Tamagnone L., Vigna E., Sachs M., Hartmann G., Birchmeier W., Daikuhara Y., Tsubouchi H., Blasi F., Comoglio P. M. Extracellular proteolytic cleavage by urokinase is required for activation of hepatocyte growth factor/scatter factor. EMBO J. 1992 Dec;11(13):4825–4833. doi: 10.1002/j.1460-2075.1992.tb05588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida T., Nakamura M., Mishima H., Otori T. Interleukin 6 promotes epithelial migration by a fibronectin-dependent mechanism. J Cell Physiol. 1992 Oct;153(1):1–5. doi: 10.1002/jcp.1041530102. [DOI] [PubMed] [Google Scholar]
- Nobes C. D., Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995 Apr 7;81(1):53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- Odekon L. E., Blasi F., Rifkin D. B. Requirement for receptor-bound urokinase in plasmin-dependent cellular conversion of latent TGF-beta to TGF-beta. J Cell Physiol. 1994 Mar;158(3):398–407. doi: 10.1002/jcp.1041580303. [DOI] [PubMed] [Google Scholar]
- Odekon L. E., Sato Y., Rifkin D. B. Urokinase-type plasminogen activator mediates basic fibroblast growth factor-induced bovine endothelial cell migration independent of its proteolytic activity. J Cell Physiol. 1992 Feb;150(2):258–263. doi: 10.1002/jcp.1041500206. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Gagnon J., Walsh K. A. Ovalbumin: a secreted protein without a transient hydrophobic leader sequence. Proc Natl Acad Sci U S A. 1978 Jan;75(1):94–98. doi: 10.1073/pnas.75.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasqualini R., Hemler M. E. Contrasting roles for integrin beta 1 and beta 5 cytoplasmic domains in subcellular localization, cell proliferation, and cell migration. J Cell Biol. 1994 Apr;125(2):447–460. doi: 10.1083/jcb.125.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson H. F., Self A. J., Garrett M. D., Just I., Aktories K., Hall A. Microinjection of recombinant p21rho induces rapid changes in cell morphology. J Cell Biol. 1990 Sep;111(3):1001–1007. doi: 10.1083/jcb.111.3.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pemberton P. A., Wong D. T., Gibson H. L., Kiefer M. C., Fitzpatrick P. A., Sager R., Barr P. J. The tumor suppressor maspin does not undergo the stressed to relaxed transition or inhibit trypsin-like serine proteases. Evidence that maspin is not a protease inhibitory serpin. J Biol Chem. 1995 Jun 30;270(26):15832–15837. doi: 10.1074/jbc.270.26.15832. [DOI] [PubMed] [Google Scholar]
- Ridley A. J., Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992 Aug 7;70(3):389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Ridley A. J., Paterson H. F., Johnston C. L., Diekmann D., Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992 Aug 7;70(3):401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- Roldan A. L., Cubellis M. V., Masucci M. T., Behrendt N., Lund L. R., Danø K., Appella E., Blasi F. Cloning and expression of the receptor for human urokinase plasminogen activator, a central molecule in cell surface, plasmin dependent proteolysis. EMBO J. 1990 Feb;9(2):467–474. doi: 10.1002/j.1460-2075.1990.tb08132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen E. M., Meromsky L., Setter E., Vinter D. W., Goldberg I. D. Purified scatter factor stimulates epithelial and vascular endothelial cell migration. Proc Soc Exp Biol Med. 1990 Oct;195(1):34–43. doi: 10.3181/00379727-195-43115. [DOI] [PubMed] [Google Scholar]
- Sager R., Sheng S., Anisowicz A., Sotiropoulou G., Zou Z., Stenman G., Swisshelm K., Chen Z., Hendrix M. J., Pemberton P. RNA genetics of breast cancer: maspin as paradigm. Cold Spring Harb Symp Quant Biol. 1994;59:537–546. doi: 10.1101/sqb.1994.059.01.060. [DOI] [PubMed] [Google Scholar]
- Sager R. Tumor suppressor genes: the puzzle and the promise. Science. 1989 Dec 15;246(4936):1406–1412. doi: 10.1126/science.2574499. [DOI] [PubMed] [Google Scholar]
- Saksela O., Moscatelli D., Rifkin D. B. The opposing effects of basic fibroblast growth factor and transforming growth factor beta on the regulation of plasminogen activator activity in capillary endothelial cells. J Cell Biol. 1987 Aug;105(2):957–963. doi: 10.1083/jcb.105.2.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salonen E. M., Vaheri A., Pöllänen J., Stephens R., Andreasen P., Mayer M., Danø K., Gailit J., Ruoslahti E. Interaction of plasminogen activator inhibitor (PAI-1) with vitronectin. J Biol Chem. 1989 Apr 15;264(11):6339–6343. [PubMed] [Google Scholar]
- Sekine A., Fujiwara M., Narumiya S. Asparagine residue in the rho gene product is the modification site for botulinum ADP-ribosyltransferase. J Biol Chem. 1989 May 25;264(15):8602–8605. [PubMed] [Google Scholar]
- Sheng S., Pemberton P. A., Sager R. Production, purification, and characterization of recombinant maspin proteins. J Biol Chem. 1994 Dec 9;269(49):30988–30993. [PubMed] [Google Scholar]
- Sommers C. L., Thompson E. W., Torri J. A., Kemler R., Gelmann E. P., Byers S. W. Cell adhesion molecule uvomorulin expression in human breast cancer cell lines: relationship to morphology and invasive capacities. Cell Growth Differ. 1991 Aug;2(8):365–372. [PubMed] [Google Scholar]
- Stein P. E., Carrell R. W. What do dysfunctional serpins tell us about molecular mobility and disease? Nat Struct Biol. 1995 Feb;2(2):96–113. doi: 10.1038/nsb0295-96. [DOI] [PubMed] [Google Scholar]
- Stetler-Stevenson W. G., Aznavoorian S., Liotta L. A. Tumor cell interactions with the extracellular matrix during invasion and metastasis. Annu Rev Cell Biol. 1993;9:541–573. doi: 10.1146/annurev.cb.09.110193.002545. [DOI] [PubMed] [Google Scholar]
- Stoker M. Effect of scatter factor on motility of epithelial cells and fibroblasts. J Cell Physiol. 1989 Jun;139(3):565–569. doi: 10.1002/jcp.1041390316. [DOI] [PubMed] [Google Scholar]
- Stracke M. L., Krutzsch H. C., Unsworth E. J., Arestad A., Cioce V., Schiffmann E., Liotta L. A. Identification, purification, and partial sequence analysis of autotaxin, a novel motility-stimulating protein. J Biol Chem. 1992 Feb 5;267(4):2524–2529. [PubMed] [Google Scholar]
- Taylor W. R., Greenberg A. H., Turley E. A., Wright J. A. Cell motility, invasion, and malignancy induced by overexpression of K-FGF or bFGF. Exp Cell Res. 1993 Feb;204(2):295–301. doi: 10.1006/excr.1993.1036. [DOI] [PubMed] [Google Scholar]
- Thompson E. W., Paik S., Brünner N., Sommers C. L., Zugmaier G., Clarke R., Shima T. B., Torri J., Donahue S., Lippman M. E. Association of increased basement membrane invasiveness with absence of estrogen receptor and expression of vimentin in human breast cancer cell lines. J Cell Physiol. 1992 Mar;150(3):534–544. doi: 10.1002/jcp.1041500314. [DOI] [PubMed] [Google Scholar]
- Tooney P. A., Agrez M. V., Burns G. F. A re-examination of the molecular basis of cell movement. Immunol Cell Biol. 1993 Apr;71(Pt 2):131–139. doi: 10.1038/icb.1993.14. [DOI] [PubMed] [Google Scholar]
- Wei A., Rubin H., Cooperman B. S., Christianson D. W. Crystal structure of an uncleaved serpin reveals the conformation of an inhibitory reactive loop. Nat Struct Biol. 1994 Apr;1(4):251–258. doi: 10.1038/nsb0494-251. [DOI] [PubMed] [Google Scholar]
- Zou Z., Anisowicz A., Hendrix M. J., Thor A., Neveu M., Sheng S., Rafidi K., Seftor E., Sager R. Maspin, a serpin with tumor-suppressing activity in human mammary epithelial cells. Science. 1994 Jan 28;263(5146):526–529. doi: 10.1126/science.8290962. [DOI] [PubMed] [Google Scholar]