Abstract
SUMMARY
The most common prokaryotic signal transduction mechanisms are the one-component systems in which a single polypeptide contains both a sensory domain and a DNA-binding domain. Among the >20 classes of one-component systems, the TetR family of regulators (TFRs) are widely associated with antibiotic resistance and the regulation of genes encoding small-molecule exporters. However, TFRs play a much broader role, controlling genes involved in metabolism, antibiotic production, quorum sensing, and many other aspects of prokaryotic physiology. There are several well-established model systems for understanding these important proteins, and structural studies have begun to unveil the mechanisms by which they bind DNA and recognize small-molecule ligands. The sequences for more than 200,000 TFRs are available in the public databases, and genomics studies are identifying their target genes. Three-dimensional structures have been solved for close to 200 TFRs. Comparison of these structures reveals a common overall architecture of nine conserved α helices. The most important open question concerning TFR biology is the nature and diversity of their ligands and how these relate to the biochemical processes under their control.
INTRODUCTION
Prokaryotes use signal transduction systems to sense alterations in the environment and respond accordingly. These signal transduction systems can be broadly divided into two categories: one-component systems and two-component systems (1, 2). In one-component systems, the sensory and output functions are located on the same polypeptide, while in two-component systems, the sensory and output functions are located on separate polypeptides. While the term two-component system is better known, one-component systems are actually much more abundant in prokaryotes (2). There are at least 20 families of prokaryotic one-component systems that can be defined by amino acid conservation in their DNA-binding domains and are defined by different conserved motifs (e.g., pfam and Interpro) (Table 1). The majority of one-component systems employ a helix-turn-helix DNA-binding domain, the exception being transcription factors of the MetJ family, which instead contain a ribbon-helix-helix domain (3). The DNA-binding domains are typically located at either the N- or C-terminal end of the polypeptide, depending on the particular family, although a few instances where the DNA-binding domain has a more central location are apparent. It has been suggested that there is a correlation between the location of the DNA-binding domain and repressor and activator activity. The suggestion was that repressors generally contain an N-terminal DNA-binding domain, while activators generally contain a C-terminal DNA-binding domain (4, 5). While this may hold true for many transcription factors, we would advise caution because there are well-documented exceptions to this rule (6).
Table 1.
Major families of one-component signal transduction systems
| One-component system | Defining features | Reference(s) |
|---|---|---|
| AraC/XlyS | Involved in regulating pathways for the catabolism of various sugars, primarily transcriptional activators, C-terminal DNA-binding domain | 196 |
| ArgR | Involved in regulating amino acid metabolism, typically function as transcriptional repressors, N-terminal DNA-binding domain | 197 |
| ArsR/SmtB | Involved in regulating metal homeostasis, primarily transcriptional repressors, DNA-binding domain located near the center of the protein | 198 |
| AsnC/Lrp | Involved in regulating amino acid metabolism, function as both transcriptional activators and repressors, N-terminal DNA-binding domain | 199 |
| Crp/Fnr | Involved in regulating many cellular processes, may function as activators and repressors, C-terminal DNA-binding domain | 200 |
| DeoR | Involved in regulating sugar metabolism, typically function as repressors, N-terminal DNA-binding domain | 201 |
| DtxR | Involved in regulating metal homeostasis, primarily transcriptional repressors, N-terminal DNA-binding domain | 202 |
| Fur | Involved in regulating metal homeostasis, primarily transcriptional repressors, N-terminal DNA-binding domain | 202 |
| GntR | Involved in regulating numerous cellular processes, typically function as transcriptional repressors, N-terminal DNA-binding domain | 203 |
| IclR | Involved in regulating carbon metabolism, function as both transcriptional activators and repressors, N-terminal DNA-binding domain | 204 |
| LacI | Involved in regulating carbon metabolism, typically function as transcriptional repressors, N-terminal DNA-binding domain | 205 |
| LuxR | Involved in regulating quorum sensing, typically function as activators, C-terminal DNA-binding domain | 206 |
| LysR | Involved in regulating many cellular processes, function as both activators and repressors, N-terminal DNA-binding domain | 207 |
| MarR | Involved in regulating antibiotic resistance, typically function as transcriptional repressors, DNA-binding domain located near the center of the protein | 208 |
| MerR | Involved in regulating metal homeostasis, typically function as transcriptional repressors, N-terminal DNA-binding domain | 209 |
| MetJ | Involved in regulating many cellular processes, typically function as transcriptional repressors, N-terminal DNA-binding domain | 3 |
| ModE | Involved in regulating metal homeostasis, function as both transcriptional activators and repressors, N-terminal DNA-binding domain | 210 |
| PadR | Poorly characterized family, N-terminal DNA-binding domain | 211 |
| TetR | Involved in regulating antibiotic resistance, typically function as repressors, N-terminal DNA-binding domain | 14 |
| Xre | Involved in regulating various cellular processes, typically function as transcriptional repressors, N-terminal DNA-binding domain | 212, 213 |
The naming of protein families is characterized by a founder effect of sorts, where the family name is derived from the first characterized member. One-component systems are no exception. This can be misleading, however, as not every member of a particular family is likely to be involved in regulating the same basic process as the founder. For example, many regulators in the AraC family are known for their role in sugar metabolism as AraC itself regulates genes required for arabinose catabolism (7). However, some members of the family recognize small molecules other than sugars and play a role in the regulation of virulence, morphological development and antibiotic production (8–10). In fact, some AraC family regulators (e.g., MarA and SoxS) are believed to lack a ligand-binding domain and may not serve as one-component signaling systems at all. Similar to the case for AraC family regulators, not all ArsR or MerR homologs bind metals like the founding member of the family. ArsR homologs have been identified as part of toxin-antitoxin systems (11), and MerR homologs are now known to respond to various chemical stressors (12).
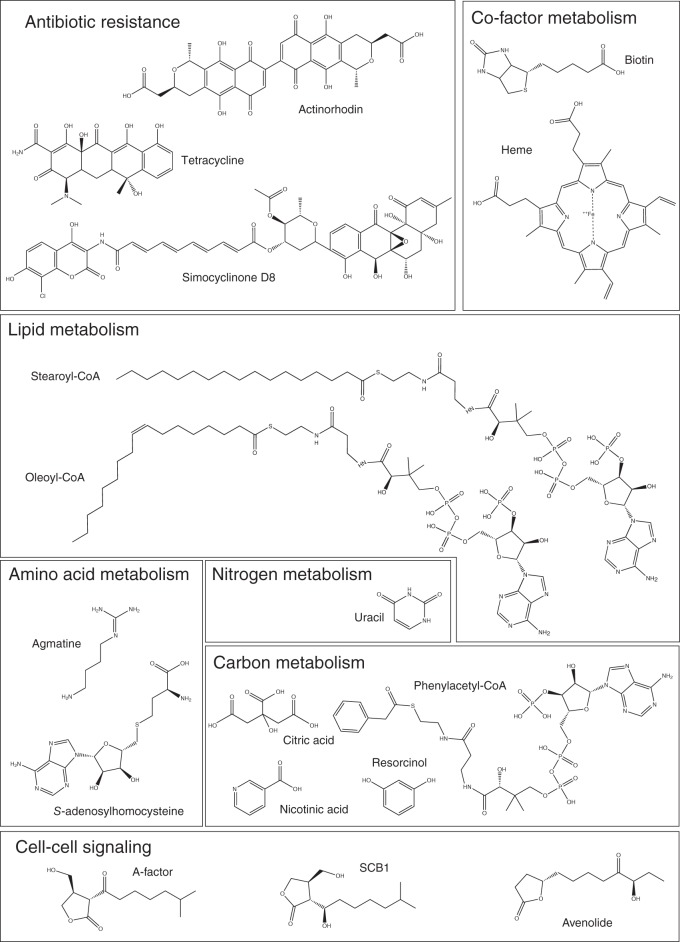
The TetR family of regulators (TFRs) is a large and important family of one-component signal transduction systems (13, 14). While members of this family are best known for their roles as regulators of antibiotic efflux pumps, this in fact describes a minority of their functional roles. Indeed, characterized members are known to regulate numerous aspects of bacterial physiology and to interact with a vast array of ligands (Fig. 1).
Fig 1.
TFRs are known to interact with an exceptionally diverse set of small molecules, including antibiotics, metabolites, and cell-cell signaling molecules.
TetR FAMILY REGULATORS
All TetR family regulators (TFRs) consist of an N-terminal DNA-binding domain and a larger C-terminal domain. The proteins are almost exclusively α helical and function as dimers. In most cases the C-terminal domains interact with one or more ligands, in turn altering the regulator's ability to bind DNA. The exceptional diversity of these ligands is a chief source of interest in these regulators and is a central focus in this review. The name “TFR” is derived from the TetR protein, which was the first family member to be discovered and characterized in detail. Like TetR, many TFRs are repressors; however, there are important exceptions that are activators or that have roles unrelated to transcription.
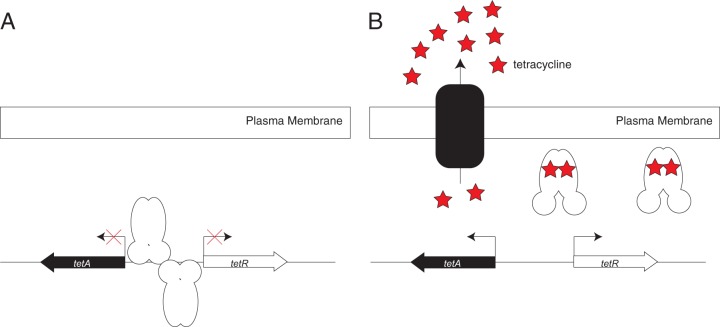
The inducible nature of tetracycline resistance in Escherichia coli was recognized in the mid-1960s (15). The protein factor responsible for the regulation and induction of tetracycline resistance, which we now know as TetR, was partially purified a decade later (16). The sequence of tetR and many of the molecular details surrounding the regulation of tetracycline resistance were unraveled in the 1980s (17–21). We now know that TetR is the repressor of the tetracycline efflux pump encoded by tetA (Fig. 2). In the absence of tetracycline, a pair of TetR dimers bind to overlapping operator sequences in the intergenic region between the divergently transcribed tetR and tetA genes. When tetracycline is present, it binds directly to TetR, trapping it in a conformation that is incompatible with DNA binding (22). This allows transcription of both tetR and tetA.
Fig 2.
TetR regulates the expression of the tetracycline resistance determinant encoded by tetA. (A) In the absence of tetracycline, a pair of TetR dimers bind to repeated palindromic sequences in the intergenic region between tetR and tetA. (B) When present, tetracycline is bound by TetR, causing a conformational change such that TetR can no longer bind DNA. This allows for expression of the tetracycline efflux pump encoded by tetA.
More than 240 TFRs have been at least partially characterized (Table 2), and while TetR remains one of the central models for the family, it is clear that TetR does not represent the enormous diversity seen in the family. Its well-documented role as a regulator of antibiotic efflux is shared by at most 25% of the TFR family members (23). We know that other TFRs function as both repressors and activators (e.g., DhaS), serve as local or global regulators (e.g., AmtR), and can interact with small-molecule or protein ligands (e.g., SlmA). TFRs can be autoregulatory, can be under the control of other transcription factors (e.g., AtrA), or may undergo posttranscriptional regulation (e.g., HapR). In spite of many years of investigation, central questions remain unanswered. For example, while the repressing (i.e., DNA-bound) and induced (i.e., ligand-bound) conformations of TetR have been described in detail, the manner in which the protein converts from one form to the other has not. Furthermore, it is unlikely that the conformational transitions of TetR describe those of all other TFRs, and indeed, the structure of TetR is atypical for the family as a whole (24). It is unclear whether there are distinct conformational subgroups within the family or whether each protein is in fact unique. More globally, in the vast majority of cases, the ligand(s) bound by TFRs have yet to be identified. In this review we discuss what we can learn about TFRs from genomics and structural studies and how this informs, and is informed by, the roles attributed to TFRs in bacterial physiology through more detail-oriented molecular genetic investigation. We incorporate phylogenomics as a new means of organizing TFRs.
Table 2.
TFRs of known function
| TFR | Organism | Descriptiona | Known ligand(s) | PDB ID | Reference(s) |
|---|---|---|---|---|---|
| AbyC | Verrucosispora maris AB-18-032 | Located in the abyssomicin biosynthesis cluster; predicted to regulate abyD encoding a MFS export pump; mutation decreases abyssomicin synthesis | 214 | ||
| AcmG5 | Streptomyces iakyrus | Located in the actinomycin G biosynthesis cluster | 215 | ||
| AcmP | Streptomyces chrysomallus ATCC 11523 | Located in the actinomycin D biosynthesis cluster | 94 | ||
| AcmU | Streptomyces chrysomallus ATCC 11523 | Located in the actinomycin D biosynthesis cluster | 94 | ||
| AcnR | Corynebacterium glutamicum | Regulates the aconitase (acn) gene | 4AC6, 4ACI, 4AF5 | 145 | |
| AcrR | Escherichia coli | Regulator of the AcrAB multidrug efflux pump | Rhodamine 6G, ethidium, proflavine | 3BCG, 3LHQ, 2QOP | 112, 116 |
| AcrR-like | Escherichia coli, Streptococcus uberis | Putative regulator of rdmC and mph(B) genes involved in spiramycin and tylosin resistance | 103, 216 | ||
| ActR (SCO5082) | Streptomyces coelicolor | Located in the actinorhodin biosynthesis cluster; regulates expression of the ActA and ActB efflux pumps | Actinorhodin, (S)-DNPA | 2OPT, 3B6C, 3B6A | 79 |
| AcuR | Alcaligenes faecalis | Putative repressor for genes involved in dimethylsulfoniopropionate and acrylate catabolism | 217 | ||
| AcuR | Rhodobacter sphaeroides | Regulates expression of AcuI and DddL involved in dimethylsulfoniopropionate and acrylate catabolism | Acrylate | 218 | |
| AdeN | Acinetobacter baumannii | Regulator of the AdeIJK efflux pump | 219 | ||
| AefR | Pseudomonas syringae | Regulates AHL production and is required for plant colonization | 3CDL | 40 | |
| AguR | Pseudomonas aeruginosa PAO1 | Regulates AguBA required for agmatine utilization | Agmatine | 181 | |
| AlnR2 | Streptomyces sp. strain CM020 | Located in the alnumycin biosynthesis cluster | 220 | ||
| AlpW | Streptomyces ambofaciens | Located in the alpomycin biosynthesis cluster and involved in the regulation of kinamycin biosynthesis; similar to gamma-butyrolactone receptors | 221 | ||
| AlpZ | Streptomyces ambofaciens | Located in the alpomycin biosynthesis cluster; similar to gamma-butyrolactone receptors | 222 | ||
| AmeR | Agrobacterium tumefaciens | Regulates the tripartite RND exporter AmeABC | 223 | ||
| AmiP | Streptomyces vinaceus-drappus | Located in the amicetin biosynthesis cluster | 95 | ||
| AmtR | Corynebacterium glutamicum | Global regulator of nitrogen control metabolism | GlnK | 37 | |
| Ang8 | Streptomyces sp. strain W007 | Located in an angucyclinone biosynthesis cluster | 224 | ||
| ArpA | Streptomyces griseus | Involved in the regulation of antibiotic production and sporulation | A-factor (GBL) | 225 | |
| ArpR | Pseudomonas putida S12 | Regulates the ArpABC efflux pump involved in the export of multiple antibiotics | 226 | ||
| Asm2 | Actinosynnema pretiosum | Located in the ansamitocin biosynthesis cluster and involved in the regulation of ansamitocin biosynthesis | 227 | ||
| Asm29 | Actinosynnema pretiosum | Located in the ansamitocin biosynthesis cluster and involved in the regulation of ansamitocin biosynthesis | 227 | ||
| AtrA | Streptomyces coelicolor | Pleiotropic regulator of antibiotic biosynthesis | 6 | ||
| AtuR | Pseudomonas aeruginosa | Regulates genes required for acyclic terpene utilization | 174 | ||
| Aur1B | Streptomyces aureofaciens CCM 3239 | Located in the auricin biosynthesis cluster | 228 | ||
| Aur1R | Streptomyces aureofaciens CCM 3239 | Located in the auricin biosynthesis cluster; similar to gamma-butyrolactone receptors | 229 | ||
| AvaR1 | Streptomyces avermitilis | Regulator of avermectin biosynthesis; similar to gamma-butyrolactone receptors | Avenolide | 127 | |
| AvaR2 | Streptomyces avermitilis | Similar to gamma-butyrolactone receptors | 230 | ||
| AvaR3 | Streptomyces avermitilis | Pleiotropic regulator of antibiotic production; similar to gamma-butyrolactone receptors | 230 | ||
| AveI | Streptomyces avermitilis | Ortholog of AtrA; regulator of antibiotic production | 231 | ||
| Azi42 | Streptomyces sahachiroi | Located adjacent to the azinomycin B biosynthetic gene cluster; thought to be beyond the boundaries of the cluster | 232 | ||
| BarA | Streptomyces virginiae | Involved in the regulation of virginiamycin; similar to gamma-butyrolactone-binding proteins | Virginiae butanolide (GBL) | 233 | |
| BarB | Streptomyces virginiae | Involved in the regulation of virginiamycin; similar to gamma-butyrolactone-binding proteins | 234 | ||
| BarZ | Streptomyces virginiae | Located in the virginiamycin biosynthesis cluster; similar to gamma-butyrolactone-binding proteins | 235 | ||
| BdcR (YjgJ) | Escherichia coli | Regulator of BdcA expression | 28 | ||
| BecM | Streptomyces sp. strain DSM 21069 | Located in the biosynthesis cluster for macrolactam BE-14106 biosynthesis | 236 | ||
| BepR | Brucella suis | Regulator of the BepDE efflux pump | Deoxycholate | 237 | |
| BetI | Escherichia coli | Regulates expression of BetT, BetA, and BetB required for the synthesis of glycine betaine from choline | Choline | 238 | |
| BioQ | Corynebacterium glutamicum ATCC 13032 | Regulates biotin biosynthesis and import | 189 | ||
| BpeR | Burkholderia pseudomallei | Regulates the BpeAB-OprB multidrug efflux pump | 239 | ||
| BreR | Vibrio cholerae | Regulates the BreAB efflux pump in response to bile | Deoxycholate | 39 | |
| Brp | Streptomyces clavuligerus | Gamma-butyrolactone receptor involved in the regulation of clavulanic acid and cephamycin C biosynthesis | 240 | ||
| BrtA | Listeria monocytogenes | Regulator of the MdrT efflux pump | Cholate | 241 | |
| BspR | Burkholderia pseudomallei | Involved in regulating type III secretion systems | 242 | ||
| BtrR1 | Bacillus circulans | Located in the butirosin biosynthesis cluster and involved in regulation | 243 | ||
| CalR1 | Micromonospora echinospora | Located in the calicheamicin biosynthesis cluster | 244 | ||
| CampR | Rhodococcus sp. strain NCIMB 9784 | Divergent to camK (6-oxocamphor hydrolase) | 177 | ||
| CamR | Pseudomonas putida | Regulator of camphor degradation | 245 | ||
| CasR | Rhizobium etli | Regulator of CasA required for colonization and infection of the host | 246 | ||
| CgmR (cg2894, Cgl2612) | Corynebacterium glutamicum | Multidrug resistance-related transcription factor | Ethidium bromide, malachite green | 2ZOY, 2ZOZ, 2YVH, 2YVE | 43, 247 |
| ChlF1 | Streptomyces antibioticus | Located in the chlorothricin biosynthetic gene cluster | 248 | ||
| ChryX5 | Streptomyces albaduncus | Located in the chrysomycin biosynthesis cluster; a homolog is not present in the cluster for the related molecule ravidomycin | 249 | ||
| CifR | Pseudomonas aeruginosa | Regulator of the Cif toxin | Epibromohydrin | 250 | |
| CmeR | Campylobacter jejuni | Regulator of the CmeABC efflux pump | Taurocholate, cholate, salicylate | 2QCO, 3QPS, 3QQA | 56 |
| CmtI | Pseudomonas putida | Putative regulator of operons required for p-cymene/p-cumate degradation | 175 | ||
| CmtR | Pseudomonas putida | Putative regulator of operons required for p-cymene/p-cumate degradation | 178 | ||
| ComR | Escherichia coli | Regulator of ComC involved in copper permeability | Copper | 72 | |
| CprA | Streptomyces coelicolor | Similar to gamma-butyrolactone receptors; involved in regulating sporulation and antibiotic production | 134 | ||
| CprB | Streptomyces coelicolor | Similar to gamma-butyrolactone receptors; involved in regulating sporulation and antibiotic production | 1IU5, 1IU6 | 134 | |
| CprS | Streptomyces coelicolor | Similar to gamma-butyrolactone receptors | 251 | ||
| CymR | Pseudomonas putida | Regulator of the cym and cmt operons required for p-cymene and p-cumate degradation | p-Cumate | 176 | |
| DarR (MSMEG_5346) | Mycobacterium smegmatis | First cyclic-di-AMP-responsive transcription factor to be identified in bacteria | Cyclic-di-AMP | 142 | |
| DddH | Halomonas sp. strain HTNK1 | Putative regulator of genes required for dimethylsulfoniopropionate and acrylate catabolism | 252 | ||
| DesT | Pseudomonas aeruginosa | Regulates the expression of the DesCB acyl-CoA desaturase operon | Oleate (corepressor), stearate (inducer) | 3LSJ, 3LSR, 3LSP | 166 |
| DhaR | Rhodococcus rhodochrous | Regulator of haloalkane dehalogenase (DhaA) | 143 | ||
| DhaS | Lactococcus lactis | Regulator of the dha operon; functions as a transcriptional activator | DhaQ-dihydroxyacetone complex | 2IU5 | 69 |
| EbrR | Streptomyces lividans | Regulator of the EbrA efflux pump | 3HTJ, 3HTI, 3HTH, 3HTA | 253 | |
| EbrS | Streptomyces lividans | Regulator of the EbrC efflux pump | 254 | ||
| Ecm10 | Streptomyces lasaliensis | Located in the echinomycin biosynthesis cluster | 255 | ||
| EmhR | Pseudomonas fluorescens | Regulates the EmhABC efflux pump that influences production of 2,4-diacetylphloroglucinol and is required for phenanthrene, anthracene, and fluoranthene efflux | 256, 257 | ||
| EncS | Streptomyces maritimus | Located in the enterocin biosynthesis gene cluster | 258 | ||
| EnvR (AcrS) | Escherichia coli | Divergent to the AcrEF efflux pump; may function as a switch for the alternative expression of AcrAB and AcrEF efflux pumps | 259 | ||
| EpeR | Streptomyces clavuligerus | Controls expression of the EpeA efflux pump | 260 | ||
| EsmT4 | Streptomyces antibioticus Tu 2706 | Located in the esmeraldin biosynthesis cluster | 261 | ||
| EthR | Mycobacterium tuberculosis | Regulator of ethA encoding a monooxygenase required for the activation of ethionamide | Hexadecyl octanoate | 1T56 | 58 |
| FabR | Escherichia coli | Regulator of genes required for unsaturated fatty acid synthesis | Unsaturated thioesters | 165 | |
| Fad35R (Rv2506) | Mycobacterium tuberculosis | Regulator of Fad35 acyl-CoA synthetase | Palmitoyl-CoA | 162 | |
| FadR (YsiA) | Bacillus subtilis | Regulator of fatty acid catabolism | Long-chain acyl-CoAs | 1VIO | 161 |
| FadR | Pseudonocardia autotrophica | Regulates fad genes required for fatty acid degradation | 158 | ||
| FadR | Thermus thermophilus | Regulator of genes required for fatty acid degradation | Medium to long (C10 to C18) straight-chain fatty acyl-CoAs | 3ANG, 3ANP | 150 |
| FarA | Streptomyces sp. strain FRI-5 | Gamma-butyrolactone autoregulator that controls antibiotic production | IM-2 (GBL) | 262 | |
| FasR | Corynebacterium glutamicum | Regulator of accD1 and fasA expression required for lipid synthesis | 157 | ||
| FrrA | Bradyrhizobium japonicum | Regulator of the FreABC efflux pump | Genistein, daidzein | 263 | |
| HapR | Vibrio cholerae | Master quorum-sensing regulator | 2PBX | 264 | |
| HemR | Propionibacterium freudenreichii | Possible regulator of hem gene expression required for the conversion of glutamate to protoheme | 190 | ||
| HlyIIR | Bacillus cereus | Regulator of hemolysin II expression | 265 | ||
| HnoR (HdnoR) | Arthrobacter nicotinovorans | Repressor of 6-hydroxy-d-nicotine oxidase | 6-Hydroxy-d- and 6-hydroxy-l-nicotine | 266 | |
| HrtR | Lactococcus lactis | Regulator of the HrtB-HtrA transporter | Heme | 3VP5, 3VP5, 3VOX | 191, 46 |
| IcaR | Staphylococcus epidermidis | Regulator of the ica operon required for biofilm formation | 2ZCM, 2ZCN | 267 | |
| IfeR | Agrobacterium tumefaciens | Regulator of the IfeAB efflux pump | 268 | ||
| JadR* | Streptomyces venezuelae | Located in the jadomycin biosynthesis cluster | 269 | ||
| JadR2 | Streptomyces venezuelae | Similar to gamma-butyrolactone receptors; involved in the regulation of jadomycin biosynthesis | Jadomycin and chloramphenicol | 133, 270 | |
| KanG | Streptomyces kanamyceticus | Located near the kanamycin biosynthesis cluster but probably beyond cluster boundaries | 271 | ||
| KijA8 | Actinomadura kijaniata | Located in the kijanimicin biosynthesis cluster | Kijanimicin | 272 | |
| KijC5 | Actinomadura kijaniata | Located in the kijanimicin biosynthesis cluster | 272 | ||
| KijR | Streptomyces coelicolor | Regulator of KijX expression and kijanimicin resistance | Kijanimicin, saccharocarcins A and B | 25 | |
| KinR | Streptomyces murayamaensis | Located in the kinamycin biosynthesis cluster | 273 | ||
| KirRII | Streptomyces collinus | Located in the kirromycin biosynthesis cluster | 274 | ||
| KsbA | Kitasatospora setae | Gamma-butyrolactone receptor protein; involved in regulating bafilomycin biosynthesis | GBLs | 275 | |
| KstR | Mycobacterium tuberculosis | Regulator of lipid metabolism | 3MNL | 169 | |
| KstR2 | Mycobacterium tuberculosis | Regulator of cholesterol metabolism | 170 | ||
| LanK | Streptomyces cyanogenus | Located in the landomycin biosynthetic pathway | Landomycin A and intermediates | 78 | |
| Lct13 | Streptomyces rishiriensis | Putative gamma-butyrolactone receptor protein; located in the lactonamycin biosynthesis cluster | 276 | ||
| Lct14 | Streptomyces rishiriensis | Putative gamma-butyrolactone receptor protein; located in the lactonamycin biosynthesis cluster | 276 | ||
| LfrR | Mycobacterium smegmatis | Regulator of LfrA multidrug efflux pump | Proflavine | 2WGB, 2V57 | 55 |
| LitR | Vibrio fischeri | Involved in regulating luminescence and symbiotic light organ colonization | 277 | ||
| LiuQ (Bamb_4589) | Burkholderia ambifaria AMMD | Regulator of branched-chain amino acid degradation | 183 | ||
| LmrA | Bacillus subtilis | Regulator of the LmrB efflux pump | Flavonoids (quercetin, fisetin, galangin, catechin, coumestrol, genistein) | 104 | |
| LplR | Rhodococcus erythropolis | Regulator of l-pantoyl lactone dehydrogenase gene expression | 278 | ||
| LuxR | Vibrio harveyi | Global regulator | 279 | ||
| LuxT | Vibrio harveyi | Global regulator | 280 | ||
| McbR | Corynebacterium glutamicum | Global regulator of l-methionine and l-cysteine biosynthesis | S-Adenosylhomocysteine | 185 | |
| Mce3R | Mycobacterium tuberculosis | Putative regulator of lipid metabolism | 281 | ||
| MdoR | Mycobacterium sp. strain JC1 | Regulator of genes required for methanol oxidation | 147 | ||
| MedORF28 | Streptomyces sp. strain AM-7161 | Located in the medermycin biosynthesis cluster | 282 | ||
| MepR | Pseudomonas putida | Regulates efflux pump involved in toluene resistance | 283 | ||
| MerO | Streptomyces sp. strain NRRL 30748 | Located in the meridamycin biosynthesis cluster | 284 | ||
| MexL | Pseudomonas aeruginosa | Regulator of the MexJK efflux pump | 285 | ||
| MexZ (AmrR) | Pseudomonas aeruginosa | Regulates the MexXY (AmrAB) exporter involved in aminoglycoside resistance | 2WUI | 286 | |
| MlaM | Streptomyces sp. strain MP39-85 | Located in the biosynthetic gene cluster for the macrocyclic lactam ML-449 | 92 | ||
| MmfR | Streptomyces coelicolor | Gamma-butyrolactone-like receptor involved in regulating methylenomycin production | 128, 287 | ||
| MmyR | Streptomyces coelicolor | Gamma-butyrolactone-like receptor involved in regulating methylenomycin production | 128, 287 | ||
| MmyR | Streptomyces violaceoruber | Located in the methylenomycin biosynthesis cluster | 288 | ||
| MnbR | Comamonas sp. strain JS46 | Putative regulator of mnb operon required for 3-nitrobenzoate oxidation | 144 | ||
| MonRII | Streptomyces cinnamonensis | Located in the monensin biosynthesis locus | 289 | ||
| MphR | Escherichia coli | Regulator of macrolide resistance | 14-membered macrolides (erythromycin, oleandomycin) | 3G56, 3FRQ | 101 |
| MSMEG_6564 | Mycobacterium smegmatis | Global regulator of DNA repair genes | 290 | ||
| MtrR | Neisseria gonorrhoeae | Regulator of the mtr efflux pump | 3VIB | 291 | |
| NalC | Pseudomonas aeruginosa | Indirect regulator of the MexAB-OprM efflux pump through regulation of ArmR expression | Chlorinated phenols | 292, 293, 294, 295 | |
| NalD | Pseudomonas aeruginosa | Regulator of the MexAB-OprM efflux pump | 296 | ||
| NapR3 | Streptomyces aculeolatus | Located in the napyradiomycin biosynthesis cluster | 297 | ||
| NapR7 | Streptomyces aculeolatus | Located in the napyradiomycin biosynthesis cluster | 297 | ||
| NcsR2 | Streptomyces carzinostaticus | Gamma-butyrolactone receptor located in the neocarzinostatin biosynthesis cluster | 298 | ||
| NcsR3 | Streptomyces carzinostaticus | Gamma-butyrolactone receptor located in the neocarzinostatin biosynthesis cluster | 298 | ||
| NcsR4 | Streptomyces carzinostaticus | Located in the neocarzinostatin biosynthesis cluster | 298 | ||
| NemR (YdhM) | Escherichia coli | Regulator of N-ethylmaleimide reductase | N-Ethylmaleimide and other Cys modification reagents | 299 | |
| NfxB | Pseudomonas aeruginosa | Regulator of the MexCD-OprJ efflux pump | 300 | ||
| NicS | Pseudomonas putida | Regulator of genes required for nicotinic acid degradation | Nicotinic acid and hydroxynicotinic acid | 148 | |
| NonG | Streptomyces griseus | Located near the nonactin biosynthesis cluster but probably beyond cluster boundaries | 301 | ||
| OpaR | Vibrio parahaemolyticus | Global regulator | 301 | ||
| ORF20p | Streptomyces hygroscopicus | Located in the geldanamycin biosynthesis locus | |||
| OrfH2 | Streptomyces griseoruber | Located in the hedamycin biosynthesis locus | 302 | ||
| OvmY | Streptomyces antibioticus | Located in the oviedomycin biosynthesis cluster | 303 | ||
| PaaR | Azoarcus evansii | Regulator of genes required for phenyl acetic acid degradation | 304 | ||
| PaaR | Thermus thermophilus | Regulator of genes required for phenyl acetic acid degradation | Phenylacetyl coenzyme A | 150 | |
| PapR3 | Streptomyces pristinaespiralis | Located in the pristinamycin biosynthesis cluster; similar to gamma-butyrolactone receptors | 305 | ||
| PapR5 | Streptomyces pristinaespiralis | Located in the pristinamycin biosynthesis cluster; similar to gamma-butyrolactone receptors | 305 | ||
| PG1181 | Porphyromonas gingivalis | Expressed in response to NO stress | 306 | ||
| PgaY | Streptomyces sp. strain PGA64 | Located in the pga angucyclinone biosynthesis cluster | 307 | ||
| PhaD | Pseudomonas putida | Regulator of genes required for polyhydroxyalkanoate metabolism | 167 | ||
| PhlF | Pseudomonas fluorescens | Located in the 2,4-diacetylphloroglucinol biosynthesis cluster | 2,4-Diacetylphloroglucinol (inducer), salicylate (corepressor) | 75 | |
| PhlH | Pseudomonas fluorescens | Located in the 2,4-diacetylphloroglucinol biosynthesis cluster | 308 | ||
| PigZ | Serratia sp. strain ATCC 39006 | Regulator of the ZrpADBC efflux pump | 309 | ||
| Pip (SCO4025) | Streptomyces coelicolor | Regulator of the Pep efflux pump | Pristinamycin I | 100 | |
| PksA | Bacillus subtilis | Located in the bacillaene biosynthesis cluster | 310 | ||
| PlaR2 | Streptomyces sp. strain Tü6071 | Located in the phenalinolactone biosynthesis cluster | 311 | ||
| PltZ | Pseudomonas sp. strain M18 | Located in the pyoluteorin biosynthesis cluster | 312 | ||
| PmeR (PSPTO_4302) | Pseudomonas syringae | Regulator of MexAB-OprM | Flavonoids | 313 | |
| PqrA (SCO1568) | Streptomyces coelicolor | Regulator of the PqrB efflux pump | 314 | ||
| PsbI | Rhodopseudomonas palustris | Regulator of p-cumate catabolism | p-Cumate | 179 | |
| PsrA | Pseudomonas aeruginosa | Regulator of the β-oxidation operon | Long-chain fatty acids | 2FBQ | 163 |
| PydR | Pseudomonas putida KT2440 | Regulator of pyrimidine reductive catabolic pathway | 154 | ||
| Pyr27 | Actinosporangium vitaminophilum | Located in the pyrrolomycin biosynthesis cluster | 315 | ||
| Pyr3 | Actinosporangium vitaminophilum | Located in the pyrrolomycin biosynthesis cluster | 315 | ||
| PyrO | Streptomyces pyridomyceticus | Located in the pyridomycin biosynthesis cluster; similar to gamma-butyrolactone receptors | 316 | ||
| QacR | Staphylococcus aureus | Regulator of the QacA efflux pump | Rhodamine 6G, dequalinium, crystal violet, berberine, DiOC3, methyl green, benzalkonium, tetraphenylarsonium, nitidine, palmatine | 1JTX, 1JT6, 1JTY, 1JUM, 1JUP, 1JUS, 1JTO, 1QVT, 1QVU | 60, 53 |
| QdoR (YxaF) | Bacillus subtilis | Regulator of quercetin dioxygenase QdoI (YxaG) | Flavonoids (quercetin, fisetin, tamarixetin, galangin, genistein, coumestrol) | 317 | |
| RamR (STM0580) | Salmonella enterica serovar Typhimurium | Regulator of the RamA efflux pump; mutations in the RamR binding site result in a multidrug resistance phenotype | 318 | ||
| RefZ (YttP) | Bacillus subtilis | Involved in the switch from medial to polar cell division | 195 | ||
| RegE | Actinoplanes friuliensis | Located in (or adjacent to) the friulimicin biosynthesis cluster | 319 | ||
| RemM | Streptomyces resistomycificus | Located in the resistomycin biosynthesis cluster | 320 | ||
| RemQ | Streptomyces resistomycificus | Located in the resistomycin biosynthesis cluster | 320 | ||
| RifQ | Amycolatopsis mediterranei | Located in the rifamycin biosynthesis cluster | 91 | ||
| RkI | Streptomyces strain sp. 88-682 | Located in the RK-682 biosynthesis cluster | 321 | ||
| RmiR | Rhizobium etli | Regulator of NodTch | 322 | ||
| RmrR | Rhizobium etli | Regulator of the RmrAB efflux pump | 323 | ||
| RolR | Corynebacterium glutamicum | Regulator of resorcinol degradation | Resorcinol | 3AQS, 3AQT | 49 |
| RphA3 | Streptomyces griseoviridis | Located in the prodigiosin biosynthesis cluster | 324 | ||
| RrdA (SCO1104) | Streptomyces coelicolor | Regulator of antibiotic production | 325 | ||
| RutR (YcdC) | Escherichia coli | Regulator of pyrimidine synthesis | Uracil | 326 | |
| Rv3066 | Mycobacterium tuberculosis | Regulator of Mmr multidrug efflux pump | Ethidium | 3V6G, 3V78 | 327 |
| SaaR | Streptomyces ambofaciens | Gamma-butyrolactone receptor involved in regulating spiramycin production | 328 | ||
| SabR | Streptomyces ansochromogenes | Gamma-butyrolactone receptor involved in regulating nikkomycin production | 329 | ||
| SabR | Streptomyces acidiscabies | Gamma-butyrolactone receptor involved in regulating WS5995B production | 330 | ||
| SabS | Streptomyces acidiscabies | Gamma-butyrolactone receptor involved in regulating WS5995B production | 330 | ||
| SACE_7040 | Saccharopolyspora erythraea | Regulator of morphological differentiation | 331 | ||
| SaqK | Micromonospora sp. strain Tu 6368 | Located in the saquayamycin Z biosynthesis cluster | 83 | ||
| SAV3818 | Streptomyces avermitilis | Global upregulator of antibiotic production in Streptomyces species | 332 | ||
| SbtR | Thermus thermophilus HB8 | Contains an intermolecular disulfide bridge that may be involved in ligand affinity | 3VUQ | 333 | |
| SCAB1401 | Streptomyces scabies | Located in the pyochelin biosynthesis cluster | 334 | ||
| ScbR | Streptomyces coelicolor | Gamma-butyrolactone-binding protein; pleiotropic regulator of antibiotic production | SCB1 | 251 | |
| ScbR2 | Streptomyces coelicolor | Similar to gamma-butyrolactone-binding proteins; regulator of Cpk polyketide production and gamma-butyrolactone biosynthesis | Actinorhodin and undecylprodigiosin | 131, 132, 133 | |
| SchA21 | Streptomyces sp. strain SCC-2136 | Located in the biosynthesis cluster for the angucyclinones Sch 47554 and Sch 47555 | 335 | ||
| SchA4 | Streptomyces sp. strain SCC-2136 | Located in the biosynthesis cluster for the angucyclinones Sch 47554 and Sch 47555 | 335 | ||
| SchR3 | Streptomyces chartreusis | Located in the biosynthesis cluster for calcimycin (A23187) | 93 | ||
| SCO0253 | Streptomyces coelicolor | Regulator of SCO0252 | Tetracycline | 3FIW | 336 |
| SCO0332 | Streptomyces coelicolor | Regulator of SCO0330 | 2ZB9 | 337 | |
| SCO1712 | Streptomyces coelicolor | Regulator of antibiotic production | 3BNI | 338, 160 | |
| SCO3201 | Streptomyces coelicolor | Regulator of antibiotic production | 339 | ||
| SczA | Streptococcus pneumoniae | Regulator of metal ion homeostasis | Zn2+ | 71 | |
| SfmR1 | Streptomyces lavendulae | Located in the saframycin A biosynthesis cluster | 340 | ||
| SimR | Streptomyces antibioticus | Located in the simocyclinone D8 biosynthesis cluster | Simocyclinones D8 and C4 | 2Y2Z, 2Y30, 2Y31 | 76 |
| SlgR1 | Streptomyces lydicus | Located in the streptolydigin biosynthesis cluster | 341 | ||
| SlmA | Escherichia coli | Nucleoid occlusion factor | FtsZ | 3NXC | 192, 193 |
| SmcR | Vibrio vulnificus | Global regulator | 3KZ9 | 342, 343 | |
| SmeT | Stenotrophomonas maltophilia | Regulator of the SmeDEF efflux pump | Triclosan | 2W53 | 52, 61, 344 |
| SMU_1349 | Streptococcus mutans | Regulator of the TnSmu2 operon, which contains a secondary metabolite biosynthesis gene cluster | 345, 346 | ||
| SngR | Streptomyces natalensis | Gamma-butyrolactone receptor protein involved in regulating natamycin biosynthesis and sporulation | 347 | ||
| SocA3 | Myxococcus xanthus | Involved in regulating morphological development | 348 | ||
| SpbR | Streptomyces pristinaespiralis | Gamma-butyrolactone receptor protein involved in regulating pristinamycin biosynthesis and sporulation | 349, 305 | ||
| SrpR | Pseudomonas putida | Regulator of the SrpABC efflux pump | SrpS | 350, 351 | |
| SrrA | Streptomyces rochei | Gamma-butyrolactone receptor protein involved in regulating lankacidin and lankamycin biosynthesis and sporulation | 352, 353 | ||
| SrrB | Streptomyces rochei | Gamma-butyrolactone receptor protein involved in regulating lankacidin and lankamycin biosynthesis and sporulation | 352 | ||
| SrrC | Streptomyces rochei | Gamma-butyrolactone receptor protein involved in regulating lankacidin and lankamycin biosynthesis and sporulation | 352 | ||
| SscR | Streptomyces scabies | Gamma-butyrolactone receptor protein involved in regulating secondary metabolism | GBLs | 354 | |
| SsfT2 | Streptomyces sp. strain SF2575 | Located in the biosynthesis cluster for the polyketide SF2575 | 99 | ||
| Strop_2766 | Salinispora tropica | Located in the salinilactam biosynthesis cluster | 355 | ||
| TamK | Streptomyces sp. strain 307-9 | Located in the tirandamycin biosynthesis cluster | 356 | ||
| SwrT | Vibrio parahaemolyticus | Ortholog of V. harveyi LuxT; regulator of swarming motility | 357 | ||
| TarA | Streptomyces tendae | Gamma-butyrolactone receptor protein involved in regulating nikkomycin production | 358 | ||
| TcaR2 | Micromonospora chalcea | Located in the tetrocarcin A biosynthesis cluster | 359 | ||
| TcmR | Streptomyces glaucescens | Located in the tetracenomycin C biosynthesis cluster | 360 | ||
| Tei8 | Actinoplanes teichomyceticus | Located in the teicoplanin biosynthesis cluster | 361 | ||
| TetR | Escherichia coli | Regulator of tetracycline resistance | Tetracycline | 2TCT, 1QPI | 362 |
| TetR | Arthrobacter oxydans | Putative regulator of genes required for phenyl acetic acid degradation | 363 | ||
| TetR | Streptomyces toxytricini | Putative regulator of the propionyl-CoA carboxylase complex | 364 | ||
| Tmn21 | Streptomyces sp. strain NRRL 11266 | Located in the tetronomycin biosynthesis cluster | 365 | ||
| TR | Mycobacterium peregrinum | Putative regulator of macrolide resistance | 366 | ||
| TrdK | Streptomyces sp. strain SCSIO1666 | Located in the tirandamycin biosynthesis cluster | 367 | ||
| Tsn22 | Streptomyces longisporoflavus | Located in the tetronasin biosynthesis cluster | GenBank accession no. FJ462704 | ||
| TtgR | Pseudomonas putida | Regulator of the TtgABC efflux pump | Phloretin, naringenin, chloramphenicol, tetracycline, quercetin, luteolin | 2UXP, 2UXI, 2UXH, 2UXU, 2UXO | 51, 368, 369 |
| TtgW | Pseudomonas putida | Divergent to the TtgGHI efflux pump but does not play a major role in regulation | 370 | ||
| TvrR | Pseudomonas syringae | Required for pathogenesis | 371 | ||
| TylP | Streptomyces fradiae | Gamma-butyrolactone receptor protein involved in regulating tylosin production and sporulation | 372, 373 | ||
| TylQ | Streptomyces fradiae | Gamma-butyrolactone receptor protein involved in regulating tylosin production | 373 | ||
| UidR | Escherichia coli | Regulator of the d-glucuronidase UidA | 374 | ||
| UrdK | Streptomyces fradiae | Located in the urdamycin biosynthesis cluster | 84 | ||
| VanT | Vibrio (Listonella) anguillarum | Global regulator | 375 | ||
| VarR | Streptomyces virginiae | Located in the virginiamycin biosynthesis cluster | Virginiamycin S | 77 | |
| VceR | Vibrio cholerae | Regulator of VceCAB efflux pump | Carbonyl cyanide m-chlorophenyl hydrazone | 376 | |
| VexR | Vibrio cholerae | Regulates the VexAB efflux pump which is expressed in response to bile, sodium dodecyl sulfate, or novobiocin | 39 | ||
| VlmE | Streptomyces viridifaciens | Located in the valanimycin biosynthesis cluster | 377 | ||
| VtpR | Vibrio tubiashii | Global regulator of virulence factors | 378 | ||
| XdhR (SCO1135) | Streptomyces coelicolor | Regulator of xanthine dehydrogenase | 156 |
MFS, major facilitator superfamily; AHL, acyl-homoserine lactone.
GENOMICS OF TFRs
A text-based search for TetR in the NCBI protein database gives well over 200,000 hits (as of 7 March 2013), and this number will continue to grow due to the explosion of whole-genome sequences available. The N-terminal DNA-binding domain of TFR family members is represented by conserved motifs or profiles in the public databases (e.g., IPR001647, PS50977, and pfam00440) and has been defined in previous reviews (14), aiding in the identification of TFRs from whole-genome sequences. While the vast majority of these TFRs have not been characterized, the availability of genome sequences allows us to examine different aspects of the genomics of TFRs.
Distribution of TFRs in Bacterial Genomes
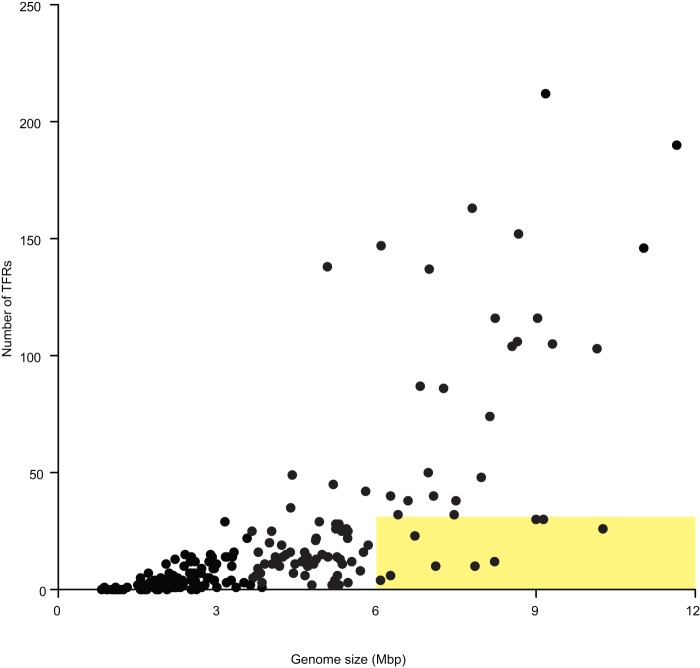
Most sequenced bacterial genomes encode at least one TFR (14, 25). In the over 200 genomes that we examined, 23, from 8 genera, did not encode TFRs. TFRs were not found in at least some representatives from Borrelia, Chlamydia, Chlamydophila, Francisella, Helicobacter, Mycoplasma, Prosthecochloris, and Treponema. These are predominantly pathogens with genomes under 2 Mbp in size. In contrast, the Actinobacteria, along with other soil-dwelling isolates such as Burkholderia, Pseudomonas, and Rhizobium strains, encode the highest numbers of TFRs. Amycolatopsis (formerly Streptomyces) sp. strain AA4 encodes the greatest number of TFRs of the genomes we examined, at 212. Bacteria with large genomes tend to encode more TFRs (Fig. 3) (25). While in some instances this may be a function of the fact that bacteria with large genomes tend to encode a higher number of regulatory proteins, in other instances the situation may be more complex and indicate a preference for TFRs over other families of regulators. For example, Streptomyces coelicolor encodes 965 regulatory proteins in its approximately 8.7-Mbp genome (26). Of these regulators, 153 (15.8%) are TFRs, while only 34 (3.5%) are AraC family regulators and 40 (4.1%) are LysR family regulators (L. Cuthbertson and J. R. Nodwell, unpublished data). E. coli encodes 261 DNA-binding transcription factors in its 4.6-Mbp genome, of which 13 (5.0%) are TFRs, 28 (10.7%) are AraC family regulators, and 46 (17.6%) are LysR family regulators (27). Exceptions where bacteria with large genomes encode a relatively small number of TFRs include some deltaproteobacteria (e.g., Myxococcus and Stigmatella) and members of the phyla Planctomycetes and Verrucomicrobia. The evolutionary significance of this, if there is any, is not clear.
Fig 3.
Distribution of TFRs in sequenced genomes. Large genomes with a low number of TFRs are highlighted with a yellow box.
In some genera we observed a wide range in the number of TFRs in different species. For example, among the Mycobacterium spp., the pathogenic M. tuberculosis encodes 49 TFRs, M. leprae, known which is to have a reduced genome, encodes only 10, and the environmental isolates M. abscessus and M. smegmatis encode 138 and 137 TFRs, respectively. These data indicate a general trend that the number of TFRs encoded by an organism may reflect the diversity of environmental conditions that the organism encounters. Bacteria that grow in changeable niches, in particular the soil, are often enriched for TFRs while those that grow in close association with a host organism are not.
Conservation of TFRs
The availability of genome sequences allows us to examine the conservation of TFRs between strains and species. These comparisons may help to reveal TFRs associated with virulence traits or to distinguish newly acquired TFRs involved in specific adaptive responses from conserved TFRs more likely to be involved in regulating basic physiological processes. For example, a comparison of the TFRs in E. coli K-12 MG1655 and E. coli O157 EDL933 reveals that the two strains share 12 TFRs and that E. coli K-12 MG1655 encodes a single additional TFR not present in E. coli O157 EDL933. In E. coli O157 EDL933, one TFR, BdcR (formerly YjgJ), is truncated and lacks the DNA-binding domain. Further analysis indicates that this truncation is conserved in other O157 genomes as well as the genomes of some Shigella species. BdcR is a regulator of BdcA, a novel c-di-GMP-binding protein involved in biofilm dispersal (28). BdcR expression is thought to be regulated by NsrR, a protein that is involved in sensing nitric oxide (29) and that is also known to regulate other genes required for motility and biofilm development. While data on BdcR function are scant, the conserved deletion in E. coli O157 indicates that it may play a role in regulating an aspect of virulence.
A comparison of the TFRs in Pseudomonas aeruginosa PAO1 and the multidrug-resistant taxonomic outlier PA7 reveals that they have 36 TFRs in common and reveals TFRs unique to each strain that may play a role in the differences in virulence observed between strains. PAO1 encodes five TFRs absent in PA7 (PA1241, PA1290, PA2020, PA2766, and PA2931), while PA7 encodes two TFRs absent from PAO1 (PSPA7_2630 and PSPA7_4004). The PA7-specific TFRs are encoded within genomic islands of this isolate (30). PA2020, MexZ (also see TFRs and Antibiotic Resistance below), encodes a known regulator of the MexXY antibiotic resistance efflux pump (31). Mutations in MexZ are associated with isolates from chronic infections and small-colony variants (32, 33). In PA7, MexZ is truncated, lacking the DNA binding-domain, which leads to overexpression of MexXY and increased aminoglycoside resistance in this isolate (34).
Analyses of TFR conservation can be expanded to include many different species of the same genus. Conservation at the genus level may help to distinguish TFRs more likely to be involved in regulating basic cellular processes (e.g., fatty acid metabolism) as opposed to adaptive functions (e.g., resistance to specific antibiotics) and may point to more recently acquired traits. Our analysis of TFRs from members of the genus Streptomyces, the majority of which encode over 100 TFRs, reveals five TFRs that are conserved in all of the close to 70 strains sequenced as of 26 April 2013, with another seven TFRs highly conserved and missing in only one strain. One of these TFRs is more broadly conserved in Actinobacteria, while another two have been implicated in the regulation of antibiotic production in members of the genus (6, 35). We surmise that all 10 of these TFRs play an important role in regulating general processes important to antibiotic production and development in Streptomyces, while less conserved TFRs are more likely to play a role in regulating specific adaptive functions such as the catabolism of a specific carbon source or resistance to a specific antibiotic. It is interesting to note that four of the five conserved TFRs are type III TFRs (see “Predicting Target Genes” below) and that the regulatory targets cannot be predicted based on genomic orientation.
Predicting Operator Sites
Many TFRs bind palindromic, and often repeated, DNA operator sequences. Informatics approaches to identifying TFR operator sequences have been applied to small numbers of TFRs with success (24). In our experience, however, operator sites for TFRs of unknown function are often difficult to reliably predict. In many cases there is no obvious palindrome, and in others there are palindromes upstream of genes encoding TFRs or predicted targets that do not interact with the cognate TFR. In some cases, these may represent binding sites for other transcription factors. Ramos et al. (14) made use of protein-DNA crystals for QacR and TetR to identify amino acid positions that may generally be important in protein-DNA interactions and give specificity for a particular TFR for its operator sequence. It would be interesting to evaluate this approach to validate potential operator sequences identified through palindrome analysis or to perhaps predict the operator DNA sequence that is recognized by a TFR. Additional information such as DNase I footprinting can aid in the prediction of TFR operator sites from DNA sequence information (23).
Predicting Target Genes
TFRs can be classified into three types based on the orientation and proximity of their structural gene relative to adjacent genes on the chromosome (Fig. 4), and these relationships can be used to predict the regulatory target gene(s) of the TFR (23). The majority of TFRs are classified as type I: their genes show a divergent orientation to one of the adjacent genes, as is the case for tetR and tetA. This relationship is very predictive of a regulatory relationship in those cases where the intergenic region between the two genes is less than ∼200 bp. A longer intergenic region does not rule out a possible regulatory relationship; however, it is more rare in these cases. Type II TFRs are predicted to be cotranscribed with one or more adjacent genes based on orientation and a short distance (less than 35 bp) between genes. The majority of characterized TFRs are known or believed to be autoregulatory, and therefore type II TFRs would also be predicted to regulate the expression of cotranscribed genes. It should be noted, however, that an extensive investigation into autoregulation by TFRs is lacking, and certainly exceptions have been identified (e.g., AmtR [36–38]). In some cases, autoregulation is assumed based on other data (e.g., DNase I foot printing analysis for ActR [23]) but direct evidence is not available. The genes encoding type III TFRs show neither of these relationships with their neighboring genes. In these cases, putative regulatory relationships with neighbors, while they may exist, cannot be predicted by genomic orientation.
Fig 4.
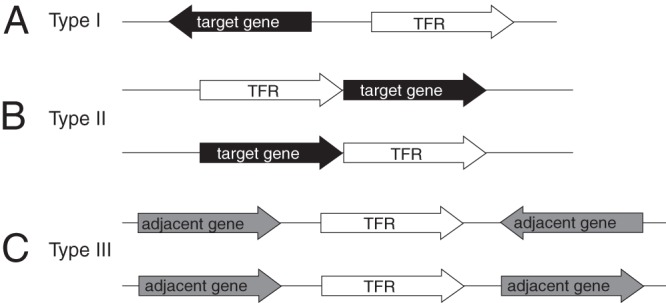
Classification of TFRs based on the orientation and proximity of adjacent genes. (A) Type I TFRs are transcribed divergently from an adjacent gene. A regulatory relationship is predicted when this intergenic region is less than 200 bp. (B) Type II TFRs are predicted to be cotranscribed with and to regulate an adjacent gene based on a distance of less than 35 bp between genes. (C) Type III TFRs show neither of the above-described relationships with adjacent genes, and a regulatory relationship with the adjacent genes cannot be predicted.
Using this classification for TFRs, we can begin to take an inventory of the types of gene products regulated by TFRs (23). This inventory reveals that while the best-characterized TFRs do indeed regulate the expression of efflux pumps like the founding member of the family TetR, a large majority of TFRs actually regulate genes encoding cytoplasmic proteins. These proteins are almost exclusively predicted to be enzymes, and the diversity is extraordinary and includes all of the known functional classes (23). The biochemical functions of most of these enzymes are unknown.
Predicting Ligands
At this time, inducing ligands are known for 61 TFRs but remain unidentified for the vast majority of TFRs, including many of those that have been at least partly characterized. We have employed phylogenomics as a tool to predict ligands for TFRs of unknown function (25). Using this approach, we successfully identified the antibiotic kijanimicin as the inducing ligand for a previously uncharacterized TFR, KijR from Streptomyces coelicolor. Identifying the inducing ligand for KijR provided crucial insight into the function of its target gene, kijX, which acts as a kijanimicin deglycosylase. As discussed above, the majority of TFRs regulate enzymes of unknown function, and methods to identify the small-molecule ligands for TFRs will prove invaluable in determining the substrates and enzymatic functions carried out by the enzymes they regulate.
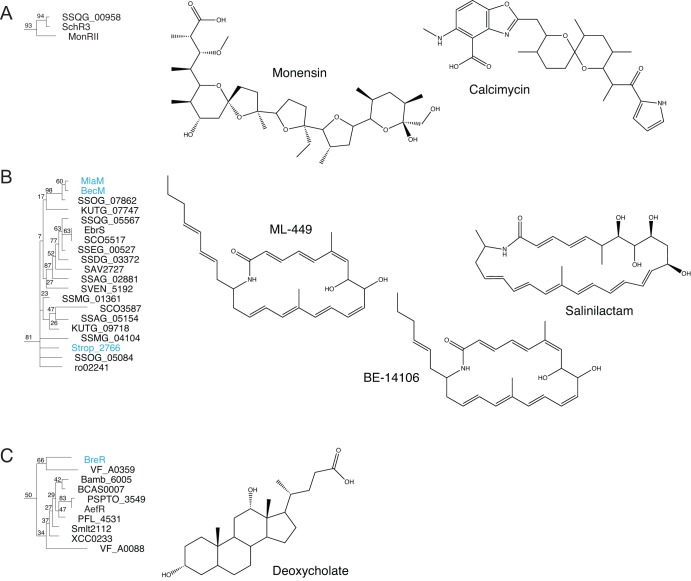
TFRs encoded in antibiotic biosynthesis clusters are known to interact with the products of those clusters (see TFRs and Antibiotic Resistance below) and can help us make predictions for ligands bound by TFRs of unknown function. For example, TFRs in the biosynthesis clusters for two structurally related polyether ionophores, calcimycin and monensin (TFRs SchR3 and MonRII, respectively), form a group in our phylogenetic analysis with the TFR of unknown function SSQG_00958 (Fig. 5A). Based on this clustering, we predict that SSQG_00958 binds a similar polyether ionophore and is involved in regulating resistance to the same molecule. SSQG_00958 is transcribed divergently from a putative exporter encoded by SSQG_00957. In another example, the gene encoding MlaM is located in the biosynthesis cluster for a macrolactam antibiotic and in our phylogenetic analysis falls into a larger group with two other TFRs, BecM and Strop_2766, located in the biosynthesis clusters for structurally related molecules (Fig. 5B). This cluster also contains numerous other TFRs of unknown function which we predict bind similar macrolactam antibiotics.
Fig 5.
Phylogenomics can be used to predict small-molecule ligands for TFRs of unknown function. (A) The TFR of unknown function SSQG_00958 is predicted to bind a polyether ionophore based on grouping with MonRII and SchR3. (B) TFRs encoded in the biosynthesis clusters for macrolactam antibiotics cluster together, leading to the prediction that all of the TFRs in this group interact with macrolactam antibiotics. (C) AefR may recognize a phytosterol based on clustering with BreR. (Adapted from reference 25.)
Ligand predictions based on phylogenomics are not limited to antibiotics. For example, BreR binds bile acids and is thought to be important to the survival of Vibrio cholerae in the intestinal tract (39). BreR and AefR share 30% identity (67% similarity) and grouped together in our analysis (Fig. 5C). AefR is involved in regulating quorum sensing and epiphytic fitness in the plant pathogen Pseudomonas syringae, but its inducing ligand is unknown (40). Given the similarities between BreR and AefR, we predict that the AefR-inducing ligand may be a phytosterol. Phytosterols share structural similarities with bile acids, and some (e.g., tomatidine) are known to have antimicrobial activity (41).
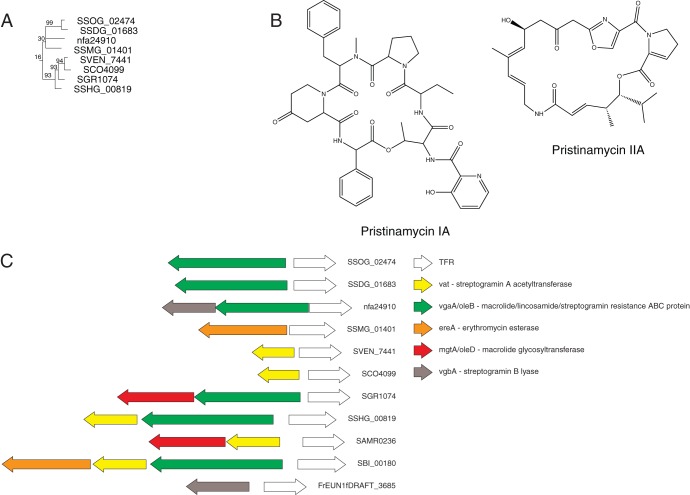
Combining information on TFRs from both phylogenomics and genomic context can also provide a powerful tool for predicting small-molecule ligands for TFRs. As the majority of TFRs are transcribed divergently from their target genes, in cases where the function of the target gene is known, this organization can lead to a prediction of a possible TFR ligand. For example SCO4099 from S. coelicolor is transcribed divergently from SCO4098, which encodes a putative streptogramin A acetyltransferase (vat) homolog. Our phylogenomics analyses coupled with additional database searches identify numerous TFRs sharing high similarity to SCO4099 in other actinomycetes; however, no ligands have been identified for any of them (Fig. 6) (25). These homologs are transcribed divergently from additional gene products implicated in resistance to streptogramin antibiotics (e.g., vgaA and vgbA) as well as gene products known to be involved in antibiotic resistance but not specifically in streptogramin resistance (e.g., mgtA/oleD and ereA). Using a combination of genomics approaches, we can predict that SCO4099 and related TFRs may bind a streptogramin antibiotic and that the genes regulated by these TFRs include both known and potentially novel streptogramin resistance genes.
Fig 6.
Combining information from genomic context with phylogenomics can also lead to ligand predictions for TFRs. (A and C) All of the TFRs in the group shown (A) (data are from reference 25) are type I TFRs predicted to regulate genes involved in streptogramin resistance (C). (B) Structure of the streptogramin antibiotic pristinamycin.
TFR STRUCTURAL BIOLOGY
General Structure of TFRs
X-ray crystal structures are currently available for close to 200 TFRs. Despite the vast sequence divergence seen in TFRs, structural data reveal that all family members share common structural features both in the DNA-binding domains (which are conserved in terms of primary sequence) and also in the ligand-binding domains (which are not) (24) (Fig. 7). The overall conserved structure of TFRs consists of nine α helices. The DNA-binding domain is composed of helices 1 to 3. Helices 2 and 3 form a helix-turn-helix motif, with helix 3 serving as the recognition helix that fits into the major groove upon DNA binding. The length of helix 1 is variable and can range from 12 to 23 residues (24). In many TFRs, helix 1 is preceded by a positively charged region responsible for making contacts with the DNA minor groove (see below) (42).
Fig 7.
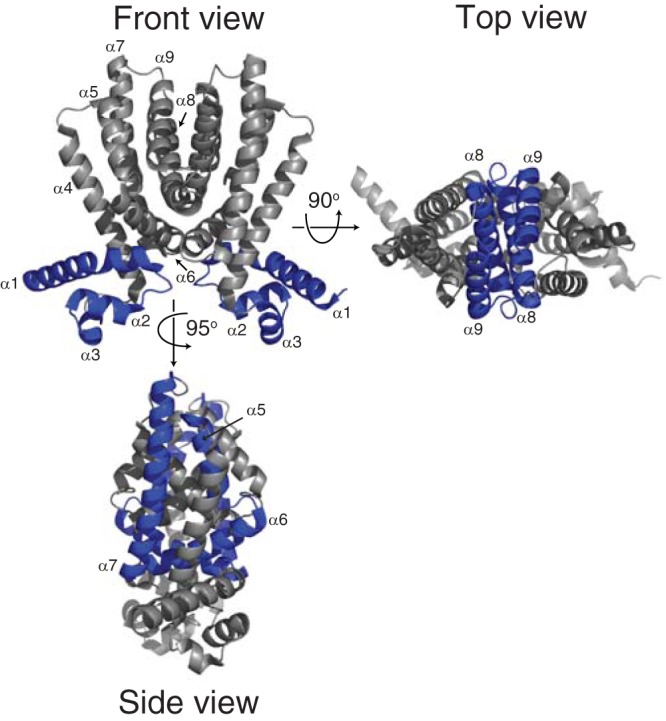
TFRs share nine conserved α helices. In the front view, the DNA-binding domain is made up of helices 1 to 3. In the side view, helices 5 to 7 in the ligand-binding domain form a central triangle. In the top view, helices 8 and 9 from each monomer form a four-helical bundle that makes up the dimer interface. The structure of Rha06780 (PDB ID 2NX4) is shown, as it shows a structure typical of TFRs (24).
The ligand-binding domain is formed by conserved helices 4 to 9. Contacts between helix 1 of the DNA-binding domain and helices 4 and 6 of the ligand-binding domain link the two domains and are responsible for transmitting structural changes between the two domains upon ligand binding (see below). The ligand-binding domain can be divided into two structural subdomains. Helices 5 to 7 form a central triangle, while helices 8 and 9 make up the dimerization interface, forming a four-helix bundle with the same helices from the other monomer. In addition to the nine conserved helices, some TFRs, including TetR itself, contain a long insertion between helices 8 and 9 that may be involved in additional contacts to make up the dimer interface. It has been noted that while TetR serves as an important model for the family, its structure, along with that of another model TFR, QacR, is actually atypical compared to the majority of TFRs of known structure (24).
Interactions of TFRs with DNA
As of February 2013, structures have been solved for seven TFR-DNA complexes: CgmR, DesT, HrtR, QacR, SimR, TetR, and TM1030 (42–47). Based on the TFR-DNA structures currently available, it is clear that while TFRs share structurally similar DNA-binding domains, the mechanisms involved in DNA binding differ in significant ways between proteins. As discussed above, the DNA-binding domain is composed of helices 1 to 3, with helix 3 being responsible for the majority of DNA contacts. Helices 3 and 3′ recognize adjacent major grooves; thus, the spacing between these two helices in the TFR dimer is crucial for structural compatibility with stable DNA binding. In all cases investigated to date, this spacing is the target of conformational changes associated with ligand binding (see below). In general, TFR binding seems to induce a bend in the DNA, although at present there is no sequence or structural explanation for what determines either the direction of bending (toward or away from the TFR) or the degree of bending (43, 44, 47).
For some TFRs (e.g., TetR and QacR) the majority of TFR-DNA contacts are base specific, while for others (e.g., CgmR, DesT, HrtR, and SimR) the majority of TFR-DNA contacts are with the phosphate backbone. In the TetR-DNA complex, Lys48, located C-terminal to the DNA-binding domain, also makes an important DNA contact. The equivalent residue in SimR, Lys71, makes a similar contact, but this contact is absent from other TFR-DNA structures, including DesT and QacR. In SimR, additional DNA contacts are made between the N-terminal “arm” of SimR and the DNA minor groove. Positively charged arginine residues in the arm of SimR mediate these contacts. Sequence alignments and structural predictions reveal that a similar arm may be found in the majority of TFRs (42).
The QacR-DNA complex is distinct from that of other TFRs in that two QacR dimers bind cooperatively. Unlike many other transcription factors (e.g., the lambda phage repressor cI), where this cooperativity is due to protein-protein interactions between adjacent dimers (48), in QacR, cooperative binding is brought about by an alteration in the structure of DNA. Specifically, the interaction of QacR with DNA causes local underwinding that increases the distance between adjacent major grooves, and it is this conformation that most favorably forms the repressed complex with two QacR dimers. A slight widening of the major groove was also seen in the structure of DesT in complex with oleoyl coenzyme A (oleoyl-CoA) and DNA, indicating that this structural change is not limited to the QacR-DNA complex.
TFR-Ligand Interactions
At this time, ligands have been identified for 61 TFRs and X-ray crystal structures solved for 21 TFR-ligand complexes (Table 2). This information allows us to begin comparing the types of ligands recognized by TFRs and the mechanisms of ligand recognition. The known TFR ligands are extraordinarily diverse and include antibiotics, bile acids and other toxic molecules, cell-cell signaling molecules, carbon sources, proteins, fatty acids and fatty acid derivatives, and metal ions (Fig. 1, 5, and 6). This diversity supports a role for TFRs in regulating an equally diverse array of cellular processes from basic carbon and nitrogen metabolism to quorum sensing and antibiotic resistance. Structures are available for TFRs in complex with simple ligands such as citrate and resorcinol (49) to very complex molecules such as acyl-CoA derivatives (44) and antibiotics with multiple functional groups such as simocyclinone (50).
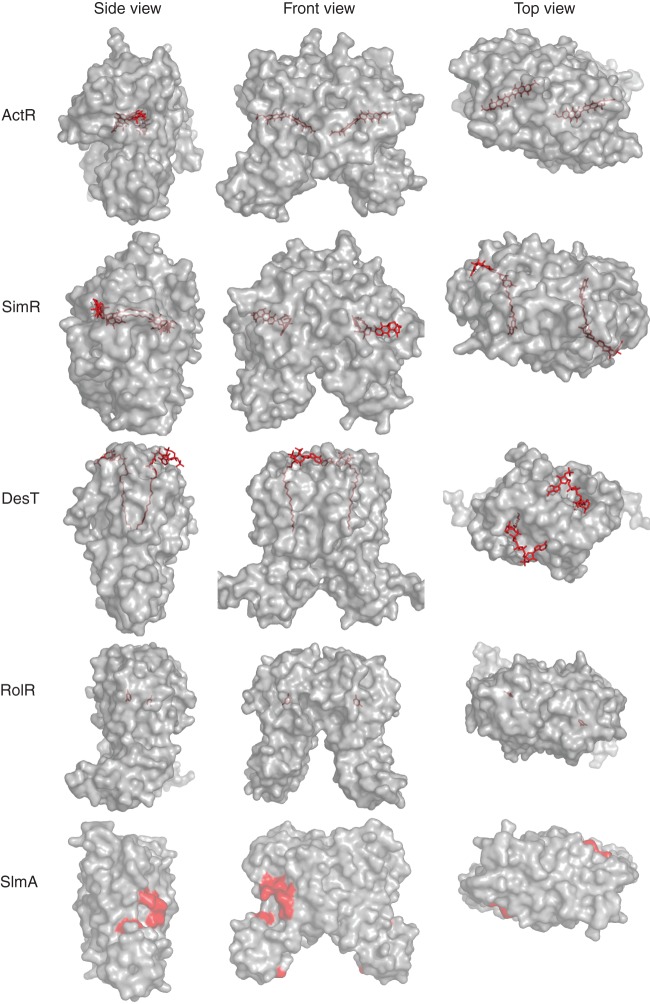
There are many ways that TFRs can interact with ligands. Structural data suggest that there are at least three different points at which ligands can enter a TFR ligand-binding site (Fig. 8). For example, ActR, QacR, SmeT, TetR, and TtgR all have a “side entry” opening distal to the dimerization interface that is believed to be the site of access for different ligands (22, 51–54). Ligands appear to enter CmeR, CgmR, HrtR, LfrR, and SimR via an entry point closer to the “front” of the protein (43, 46, 50, 55, 56). Finally, DesT, EthR, and FadR exhibit a relative “top entry” (44, 57, 58). It is unclear what, if anything, these differing mechanisms of ligand entry mean in terms of the type of ligand bound or the structural influence of ligand binding. For RolR and RutR, which bind resorcinol and uracil, respectively, there is no obvious entrance to the ligand-binding pocket (49). Rather, the ligand is trapped inside an otherwise inaccessible proteinaceous cage (Fig. 8).
Fig 8.
TFRs display different ligand entry points. Based on current TFR-ligand structures, the ligand-binding cavity may be accessible from the side (e.g., ActR), front (e.g., SimR), or top (e.g., DesT) of the TFR. In some structures (e.g., RolR), the ligand is not accessible to the external environment and the entry point cannot be determined. SlmA interacts with a protein rather than a small-molecule ligand. Residues involved in protein-protein interactions are colored in red.
Each tetracycline-binding pocket in TetR is composed primarily, but not exclusively, of residues from an individual monomer (22). This is also seen for the ligand-binding pockets of the majority of TFRs (e.g., ActR, CmeR, and QacR [53, 54, 56]). In contrast, the SimR ligand-binding cavity is composed of residues from both monomers such that each binds either the aminocoumarin or the angucyclinone moiety of the simocyclinone ligand (50).
Two molecules of Mg2+-tetracycline are bound by each dimer of TetR (22). This is also the case for many TFRs (e.g., SimR, CmeR, and MphR [50, 56, 59]), but different drug-binding stoichiometries are seen in some others. In the case of ActR, each ActR dimer is capable of binding either two molecules of actinorhodin or four molecules of (S)-2,4-dinitrophenyl acetate [(S)-DNPA] (54). In contrast, each dimer of LfrR binds only a single molecule of proflavine (55). Like LfrR, the majority of QacR-ligand structures show a single ligand within one monomer of each dimer. However, one structure of QacR in complex with two different ligands, ethidium and proflavine, within the same monomer has been solved (60). For CgmR, different binding stoichiometries are seen for different drugs, and the size of the drug is thought to play a role in the number of molecules required for CgmR derepression (43). TtgR also shows an interesting stoichiometry of binding to the plant antimicrobial phloretin, binding two molecules within one monomer and one in the other (51), while in SmeT, two molecules of triclosan were seen within a single monomer, while none were observed in the other (61).
Structures are available for four TFRs (CgmR, EbrR, QacR, and TtgR) in complex with different drugs, and analysis of these structures may shed light on how a single TFR may recognize a diverse set of ligands (43, 51, 53, 60). Based on the structures currently available, different drugs appear to be accommodated by different drug-binding sites within a single binding cavity. The structure of QacR has been solved in complex with six different cationic drugs. It shows a large binding pocket (1,100 Å3) lined with negatively charged residues that form several separate drug-binding sites. In CgmR, ethidium bromide and methylene blue were found in the same inducer-binding pocket but were bound by different networks of hydrogen bonds. Structures are available for TtgR in complex with five different ligands, two antibiotics and three plant antimicrobials. TtgR contains a large, mainly hydrophobic, binding pocket with two distinct drug-binding sites: a high-affinity site and a general binding site. The high-affinity site is smaller and was occupied by only one of the five TtgR ligands. The general binding site is broader and was found to be occupied by all five ligands.
The first two reported structures of EthR were solved in conjunction with a fortuitous ligand, in once case hexadecyl octanoate (58) and in the other two cases uncharacterized molecules consisting of a six-membered ring (62). These structures have been critical in the design of synthetic EthR ligands (see below), and subsequent structures of EthR have been solved in complex with a number of these molecules (63–65). In one study, two related analogs were found to bind EthR with different orientations, supporting the extremely plastic nature of the EthR ligand-binding pocket (63). While EthR is known to recognize a large variety of ligands, only a small number of residues were found to be in contact with all ligands (66).
Similar to the case of EthR, the structure of CmeR shows how two structurally similar molecules can fit very differently into the same binding pocket (56). The structure of CmeR has been solved in complex with two structurally similar bile acids, taurocholate and cholate. Despite the similarity of these molecules, they were found within the same binding pocket but in opposite orientations, lying antiparallel to each other. Not only is DesT able to recognize different ligands, both saturated and unsaturated fatty acids, but its ability to do so is crucial to its function (44). Binding of the unsaturated oleoyl-CoA increases DNA-binding affinity, while the saturated palmitoyl-CoA serves as the inducer. DesT activity is regulated by the ratio of the two different ligands rather than through a single ligand. A Phe-rich cluster in DesT senses which ligand is bound. This changes the hydrophobic core to create a binding cavity tailored to each particular ligand.
Crystal structures are also available for seven uncharacterized TFRs in complex with bound ligands (3EGQ, 3CJD, 3KKD, 2QIB, 2D6Y, 4ICH, and 2IEK). It is difficult to determine if these TFR-ligand interactions are biologically relevant, but in at least one case, the genes adjacent to the TFR on the chromosome, and hence the predicted regulated genes (23) (see Genomics of TFRs above), indicate a potentially relevant relationship. The TFR Jann_2994 from the alphaproteobacterium Jannaschia sp. strain CCS1 was crystalized with stearic acid (PDB ID 3CJD). Jann_2994 is adjacent to a putative PspA homolog, which is potentially involved in regulating cytoplasmic membrane integrity as well as a putative fatty acid desaturase.
At least three TFRs, AmtR, DhaS, and SlmA, are known to interact with proteins rather than small-molecule ligands (GlnK, DhaQ, and FtsZ, respectively) (67–69). Residues of SlmA involved in interactions with the cell division protein FtsZ have recently been identified (70). These residues form an active site on the ligand-binding domain that in the crystal structure of SlmA is partially blocked by the DNA-binding domain (Fig. 8). The authors proposed that in the DNA-bound form of SlmA, the entire FtsZ interaction interface would be exposed, with implications for SlmA function (see TFRs and Cell Division below) (70).
Two TFRs, SczA and ComR, bind metals, but the molecular details of these interactions are not known (71, 72). Further structural studies will provide clues as to the mechanisms surrounding how TFRs specifically recognize metal ions.
Mechanism of Induction by Ligands
Structures are available for six TFRs (TetR, QacR, HrtR, DesT, SimR, and CgmR) in both DNA-bound and ligand-bound conformations, providing insight into the structural mechanism of derepression (22, 42–47, 50, 53). It has been noted that in most apo-protein structures, the TFR most closely resembles the ligand-bound or induced form rather than the DNA-bound form. It is therefore unlikely that the comparison of apo and ligand-bound structures provides a meaningful insight into induction and that it is necessary to compare the ligand-bound and DNA-bound forms.
In all cases, ligand binding is associated with increased separation of the DNA-binding domains of the two TFR monomers relative to the DNA-bound form. This may be accompanied by further conformational changes involving helix 4 and helix 6, which are in direct contact with the DNA-binding domain. In TetR, ligand binding causes a shift in helix 6 resulting in the pendulum-like motion of helix 4. Using equilibrium protein-unfolding experiments, Reichheld et al. (73) provided evidence that TetR does not occupy two distinct folded states (i.e., DNA bound and tetracycline bound) but rather that ligand binding increases the folding cooperativity between the N- and C-terminal domains. It was suggested by Reichheld et al. (73) that this increases the stability of a conformation in which the DNA-binding domains are too far apart to support binding to adjacent major grooves in the DNA.
Similar to the case for TetR, a pendulum-like motion was noted in helix 4 of both QacR and CgmR, but in these cases, ligand binding caused a coil-to-helix transition in helix 5 and a relocation of helix 6 in QacR. In CgmR, a widening of the inducer-binding pocket and shift in helix 6 was observed. In HrtR, heme binding was shown to cause a coil-to-helix transition in helix 4, resulting in a rigid-body motion of the DNA-binding domain to an orientation not compatible with DNA binding. The case of DesT is perhaps not as simple, as DNA-binding and induced forms of the protein are both bound to ligands, albeit different ones. In the DNA-free form (bound to the inducing ligand palmitoyl-CoA as opposed to oleoyl-CoA), a helix-to-coil transition of helix 4 is seen along with an ordering of the L8-L9 loop and movement of helix 6 and helix 7. These changes in DesT again result in a widening of the distance between the DNA-binding domains. SimR represents yet another variation, where there is no reorientation between the DNA-binding and ligand-binding domains but rather a rigid-body motion of the two SimR monomers relative to each other that results in a widening between the two DNA-binding domains.
It is difficult to posit a universal structural model for the transition between the repressing and induced conformations for TFRs, and indeed, it is unclear whether there are true commonalities throughout the family. Certainly key structural elements, including the conserved helices of the DNA-binding domain and the conserved helix 5 to 7 triangle and four-helix dimerization interface, are relevant. While at first glance it may be difficult to directly apply the Reichheld model for allosteric regulation of TetR (73) to SimR given that there is no reorientation between the DNA-binding and ligand-binding domains in the case of SimR, structural flexibility along the monomer interface may be important in this case. The ligand-binding cavity of SimR is composed of residues from both monomers, and as a result ligand binding will undoubtedly decrease the flexibility between them. Recent work has challenged the Reichheld model (74); however, this work was based primarily on X-ray crystallographic analysis of the protein bound to artificial peptide inducers and therefore should be interpreted with caution. Our view is that nuclear magnetic resonance (NMR) analysis of one or more TFRs, preferably for those where there are X-ray data on both the ligand- and DNA-bound forms (e.g., CgmR, DesT, HrtR, QacR, SimR, or TetR), in which the structural transitions that occur upon ligand binding are monitored would be an ideal means of settling debate in this area.
TFRs AND ANTIBIOTIC RESISTANCE
There are numerous TFRs involved in regulating resistance to antibiotics and other toxic compounds. These TFRs can be divided into three categories: (i) TFRs regulating self-resistance in antibiotic-producing organisms, (ii) TFRs regulating specific antibiotic resistance in nonproducing organisms, and (iii) TFRs regulating multidrug resistance.
TFRs Regulating Self-Resistance in Antibiotic-Producing Organisms
Numerous TFRs have been identified in the biosynthesis clusters for antibiotics and other secondary metabolites in species of Streptomyces and related actinobacteria. Of these, six TFRs, i.e., ActR, KijA8, LanK, PhlF, SimR, and VarR, have been shown to bind the products of the biosynthetic pathways in which they are encoded (25, 75–79). These TFRs primarily regulate the expression of efflux pumps required for antibiotic export but may also regulate the expression of late-stage biosynthetic genes.
Actinorhodin is a benzoisochromanequinone antibiotic produced by S. coelicolor. The biosynthetic pathway for this compound is encoded in a 22-kb region that includes the actR gene and its target operon actAB, which encodes two efflux pumps believed to export actinorhodin from the cell. The biosynthesis of actinorhodin involves a typical type II polyketide synthase that first generates an 18-carbon octaketide (80). This molecule is tailored into a 3-ring intermediate, and, late in the pathway, two of these intermediates are covalently joined to generate the mature six-ring compound. ActR binds both the final biosynthetic product actinorhodin and three-ring biosynthetic intermediates, including (S)-DNPA (79). Genetic evidence suggests that in actinorhodin-producing cells (S)-DNPA and/or other 3-ring intermediates serve to activate the expression of efflux genes, the only known self-resistance mechanism, before the final product is synthesized (81). Furthermore, there are now several reports that the export proteins are required for efficient, high-yield biosynthesis of actinorhodin (81, 82). The biochemical basis for reduced actinorhodin biosynthesis in cells defective in the actAB operon is not well understood, but it has been interpreted as evidence that initial activation of the actinorhodin export genes is primarily dependent on intermediates. However, it is also clear that sustained expression of the actinorhodin efflux pumps throughout the culture (i.e., including cells that produce actinorhodin and those that do not) requires the actinorhodin final product (81). Thus, actinorhodin is believed to act as a cell-cell signal to trigger export and resistance in nonproducing cells.
Like ActR, LanK and SimR are also able to bind both the final products of the biosynthetic pathways in which they are encoded and biosynthetic intermediates. LanK from Streptomyces cyanogenus S136 is located in the biosynthesis cluster for the glycosylated angucyclic polyketide antibiotic landomycin A. LanK regulates both the landomycin A efflux pump encoded by lanJ and the downstream gene lanZ1 (78). LanZ1 is an epimerase required for synthesis of sugar residues required for later-stage landomycin biosynthesis. Thus, as is the case for ActR, at least one step in the induction of the LanK target operon involves the interaction of the repressor with an immature landomycin intermediate. TFRs are also located in the biosynthesis clusters for the related angucyclinone antibiotics urdamycin and saquayamycin, but the role of these TFRs in regulating antibiotic biosynthesis and export has not been investigated (83, 84).
SimR is located in the biosynthesis cluster for simocyclinone D8 in Streptomyces antibioticus Tü 6040 (85, 86). Simocyclinone D8 is a structurally complex inhibitor of DNA gyrase (87, 88). The final molecule is composed of four parts: an angucyclic polyketide, a d-olivose sugar, a tetraene linker, and an aminocoumarin moiety. SimR regulates expression of the simocyclinone efflux pump encoded by simX and is induced by both simocyclinone D8 and the intermediate simocyclinone C4, which lacks the aminocoumarin functional group (76). It is not clear, however, that the interaction of SimR with the C4 intermediate is biologically relevant. Unlike the ActR case, where intermediates are bound more tightly than the finished product, or the LanK case, where induction is required for the completion of biosynthesis, the C4 intermediate binds more weakly than the mature D8 molecule, and there are no known biosynthetic steps that depend on the SimX export protein.
TFRs are encoded in many of the antibiotic biosynthesis gene clusters found in actinomycetes; however, they are also associated with the biosynthesis of other classes of compounds in a great many organisms. For example, PhlF is located in the 2,4-diacetylphloroglucinol biosynthesis cluster of Pseudomonas fluorescens (89). Biosynthesis of 2,4-diacetylphloroglucinol is of interest, as it occurs via a type III polyketide synthase (PKS) thought to be rare in bacteria (90). PhlF binds to the intergenic region between phlF and phlA, repressing expression of the phlABCD operon (75). DNA binding is enhanced in the presence of salicylate and disrupted by the biosynthetic product of the cluster 2,4-diacetylphloroglucinol.
TFRs are also present in the biosynthesis clusters for diverse polyketides, including ansamycins (e.g., rifQ in the rifamycin cluster [91]), macrolactams (e.g., mlaM in the ML-449 cluster [92]), and polyether ionophores (e.g., schR3 in the calcimycin cluster [93]). TFRs are not limited to polyketide biosynthesis clusters but are found in biosynthesis clusters for nonribosomal peptides (e.g., acmP and acmU in the actinomycin cluster [94]) and nucleoside antibiotics (e.g., amiP in the amicetin cluster [95]).
KijR and Pip from S. coelicolor are involved in regulating antibiotic resistance in a nonproducing organism (see below) and are closely related to KijA8 and VarR, respectively (25) (Fig. 9), raising the possibility that KijR and Pip were acquired by horizontal gene transfer from a producing organism. KijA8 regulates expression of the putative kijanimicin efflux pump encoded by kijA5 in response to kijanimicin in the producing organism Actinomadura kijaniata (25). Similarly, VarR regulates expression of the virginiamycin efflux pump in Streptomyces virginiae in response to virginiamycin S (77). Environmental microbes are highly resistant to antibiotics (96) and provide a reservoir for resistance elements in other environmental microbes as well as in clinically relevant pathogens (97, 98). This raises questions as to the possible origins of other TFRs, for example, TetR and MphR of E. coli, in producing organisms. No TFR has been identified in the biosynthesis clusters for oxytetracycline or chlorotetracycline, but a TFR is present in the cluster for the glycosylated anticancer tetracycline SF2575, although our analysis does not show a close relationship to TetR from E. coli (25, 99). The closest homolog of MphR is, however, found in the environmental microbe Myxococcus xanthus (25, 59), revealing possible origins for MphR in the environment.
Fig 9.
Grouping of TFRs involved in antibiotic resistance. (A and B) KijA8 and KijR (A) and VarR and Pip (B) group together in phylogenomics analysis, indicating that KijR and Pip may have been horizontally acquired from an antibiotic-producing organism. (C) Many TFRs controlling the expression of multidrug efflux pumps cluster together in phylogenomics analysis. (Adapted from reference 25.)
TFRs Regulating Specific Antibiotic Resistance in Nonproducing Organisms
The first characterized TFR, TetR, the founding member of the family, is the regulator of tetracycline resistance. Despite this, only a limited number of TFRs have been implicated in specific antibiotic resistance in nonproducing organisms: TetR, KijR, MphR, and Pip and the paralogous TFRs LmrA and QdoR.
Like for TetR, the target of Pip in S. coelicolor (SCO4025) is efflux pump gene of the major facilitator superfamily, pep (SCO4024) (100). Unlike tetR and tetA, pip and pep are cotranscribed. As discussed above, Pip shares a high degree of similarity with VarR in the viginiamycin biosynthesis cluster.
KijR and MphR regulate enzymes involved in antibiotic inactivation (25, 101). KijR regulates the expression of kijX, which encodes a novel antibiotic deglycosylase, and shares similarity with kijA8 in the kijanimicin biosynthesis cluster (see below) (25). MphR regulates expression of mphA, encoding a macrolide phosphotransferase, and mrx, encoding a membrane protein required for high-level resistance (101, 102). Another, unnamed TFR is found upstream of genes encoding a macrolide phosphotransferase (mphB) and a putative methyl esterase (rdmC-like) required for high-level macrolide resistance in some strains of E. coli as well as Streptococcus uberis (103). Despite the fact that they both regulate macrolide resistance genes, this unnamed TFR and MphR were found in separate groups in our analysis (25).
LmrA and QdoR are paralogous TFRs in Bacillus subtilis that bind plant flavonoids (104). LmrA and QdoR regulate expression of their own genes as well as those for LmrB, QdoI, and YxaH. The LmrA/QdoR regulon is organized into two operons: lmrA-lmrB and qdoR-qdoI-yxaH. LmrB is an efflux pump of the major facilitator superfamily. QdoI is a quercetin dioxygenase, responsible for flavonoid inactivation. YxaH is a membrane protein of unknown function.
Rather than regulating a specific antibiotic resistance mechanism, EthR from M. tuberculosis regulates the expression of EthA, an enzyme required for activation of the antibiotic ethionamide (105–108). While EthA is active against a broad range of substrates, including two other tuberculosis prodrugs, isoxyl and thiacetazone (106, 109, 110), the natural substrate for EthA is an unknown molecule believed to be distinct from ethionamide which is not an inducer of ethA expression. Due to its toxicity, ethionamide is currently used as a second-line drug primarily in the treatment of drug-resistant strains of tuberculosis. Activators of EthR are of interest for use in conjunction with ethionamide, as they would increase EthA expression, and therefore activation of ethionamide, allowing for lower ethionamide concentrations to be used (64).
TFRs Involved in Regulating Multidrug Resistance
TFRs are also involved in regulating a number of multidrug resistance pumps, including AcrAB in E. coli, which is regulated by AcrR, and MexXY from Pseudomonas aeruginosa, which is regulated by MexZ (111, 112). The AcrAB efflux pump in E. coli is under the control of several global regulators, including MarA, Rob, SoxS, and SdiA (113, 114). AcrR is thought to play a role in fine-tuning the expression of acrAB rather than serving as an on-off switch (112). Nevertheless, mutations in acrR alone result in increased expression of acrAB and are associated with antibiotic-resistant clinical isolates (115). AcrR has been shown to interact with various synthetic compounds, including ethidium, proflavine, and rhodamine 6G (116); however, the physiological relevance of these ligands for AcrR and other TFRs regulating multidrug resistance pumps such as QacR may be questionable. Clinically, the so-called multidrug resistance pumps, particularly those of the RND family, are a major source of antibiotic resistance in Gram-negative bacteria (117). However, multidrug resistance is typically the result of mutations in the regulators (118) of these pumps, indicating that multidrug resistance is not the native function of these pumps and that they serve other natural functions (119). Identifying bona fide interacting partners for the regulators of these pumps, whether they are small-molecule or protein ligands, will help to elucidate their roles under physiological conditions. A role for AcrAB in removing toxic metabolites has been suggested (120), and it would be interesting to test these putative acrAB inducers as ligands for AcrR.
The MexXY transporter of P. aeruginosa is expressed under conditions of ribosome stress, including the presence of antibiotics that target the ribosome (121). Expression of mexXY is controlled by the TFR MexZ and requires ArmZ (PA5471) (122), which interacts with MexZ (31, 123). armZ encodes a homolog of RtcB, an RNA ligase involved in recovery from stress-induced RNA damage (124), and is cotranscribed with PA5470, which encodes a homolog of PrfH. PfrH is thought to function as a peptide release factor that recognizes mRNA signals other than normal stop codons, possibly signals that result from RNA damage (125). While antibiotics that target the ribosome induce MexZ expression, MexZ does not appear to interact directly with these antibiotics but rather responds to effects downstream of ribosome disruption. Similarly, while the MexXY efflux pump functions as a multidrug efflux pump, its native function is not antibiotic efflux per se but rather its increased expression is a response to ribosome stress. Our phylogenetic analysis reveals a group containing many TFRs regulating putative multidrug efflux pumps (Fig. 9). This group includes, for example, AcrR and EnvR of E. coli, MexZ and NalD of P. aeruginosa, and MtrR of Neisseria gonorrhoeae. Further studies will be required to determine whether this shared grouping is indicative of a common interacting partner (small molecule or protein) for these TFRs and a common function for the efflux pumps that they regulate.
TFRs AND CELL-CELL SIGNALING
GBL Signaling
Gamma-butyrolactone (GBL) signaling molecules are involved in the regulation of antibiotic production and morphological development in Streptomyces and other actinomycetes and are the most well characterized signaling molecules in these species. A-factor from Streptomyces griseus was the first GBL to be characterized, and its identification predates that of the acyl-homoserine lactone quorum-sensing molecules of Gram-negative bacteria (126). The TFR ArpA is the A-factor receptor in S. griseus and is part of a large group of closely related TFRs (Fig. 10) that bind GBLs and related signaling molecules such as avenolide from Streptomyces avermitilis (127) and the methylenomycin furans from S. coelicolor (128). In some cases, such as that of ArpA in S. griseus, GBL signaling plays a major role in both antibiotic production and morphological development (9). In other cases, such as that of ScbR and the GBL SCB1 in S. coelicolor, some global effects have been noted; however, the predominant role of GBL signaling is in the regulation of a single antibiotic gene cluster (129, 130).
Fig 10.
All known TFRs involved in gamma-butyrolactone (GBL) signaling form a single group (data are from reference 25). Within the GBL group, a subclade of TFRs known as the “pseudo”-GBL receptors are highlighted with a yellow box.
The clustering of all known and predicted GBL receptors in our analysis shows the separate clustering of the so called “pseudo”-GBL receptors and helps to identify putative receptors not associated with GBL biosynthetic enzymes (Fig. 10). Pseudo-GBL receptors such as JadR2 from S. venezuelae and ScbR2 from S. coelicolor are reported to play a role in the GBL signaling alongside their cognate GBL receptor (i.e., JadR3 [SVEN_5968] and ScbR) by regulating expression of GBL biosynthesis enzymes (131, 132). GBL signaling systems regulate antibiotic biosynthesis, and one report suggests that pseudo-GBL receptors may interact with the final antibiotic product being regulated (133). While the biological relevance of these data is questionable due to the high concentration of antibiotic used in these studies, the idea that pseudo-GBL receptors play a role in GBL signaling pathways is an interesting one.
While the majority of GBL receptors and pseudoreceptors are associated with GBL biosynthetic enzymes, a number of orphan receptors, not associated with biosynthetic gene clusters or resistance genes, have also been identified. Our data provide support for previous reports concerning the role of some of these proteins, namely, CprA and CprB from S. coelicolor, in regulating secondary metabolite biosynthesis and morphological differentiation in Streptomyces (130, 134). Some bacteria are known to recognize and even metabolize the quorum-sensing signals produced by other bacteria (135). For example, E. coli and Salmonella enterica do not produce acyl-homoserine lactones but are able to sense them through the receptor SdiA (136). It is tempting to speculate that the role of orphan GBL receptors (e.g., CprA and CprB) and GBL receptors in bacteria not known to produce GBLs (e.g., MSMEG_2193 and MSMEG_2195) may be to recognize GBLs produced by other microbes.
Quorum Sensing
In Vibrio cholerae, the TFR HapR plays a major role in quorum-sensing regulation at high cell density (137). HapR orthologs in other species of Vibrio include LuxR of V. harveyi (not to be confused with transcription factors of the LuxR family such as LuxR of V. fischeri) and LitR of V. fischeri. The crystal structures of HapR as well as the orthologous SmcR are available (138, 139), and while they both show a putative ligand-binding cavity, none of the known quorum-sensing molecules have been reported to bind these proteins. Instead, expression of HapR is regulated at the posttranscriptional level through interactions of hapR mRNA with a number of small RNAs (sRNAs) (140). Recent data indicate an integration of cell density with nutrient availability in quorum sensing (141), raising the possibility that another type of small-molecule signal may serve as a ligand for HapR. The TFR DarR from M. smegmatis has been shown to interact with cyclic-di-AMP (142), providing a precedent for interactions between TFRs and second messengers.
TFRs AND CARBON METABOLISM
TFRs have been implicated in both central pathways for carbon metabolism as well as peripheral pathways for the catabolism of specific carbon sources, including the degradation of pollutants and other waste products (e.g., DhaR and MnbR [143, 144]). AcnR in Corynebacterium glutamicum controls the expression of the aconitase gene, acn. Aconitase is a tricarboxylic acid cycle enzyme that converts citrate to isocitrate and is thought to be an important control point in tricarboxylic acid cycle activity in Corynebacterium (145). Structures are available for AcnR (Protein Data Bank [PDB] ID 4AC6, 4ACI, and 4AF5) and show a bound molecule of citrate with evidence for another putative ligand-binding pocket (146). Further studies will be required to determine if AcnR indeed binds multiple small-molecule ligands.
Numerous TFRs have been identified as regulators of the expression of catabolic pathways for different carbon sources. For example, in Lactococcus lactis, DhaS regulates the expression of dihydroxyacetone kinase, which is required for glycerol catabolism (69). Unlike the majority of TFRs, DhaS interacts with a protein rather than a small molecule and acts as a transcriptional activator. MdoR from Mycobacterium sp. strain JC1 also acts as a transcriptional activator (147). MdoR regulates expression of the mdo gene, which is required for oxidation of methanol. The TFRs NicS, PaaR, and RolR are involved in the regulation of metabolism pathways for nicotinic acid, phenyl acetic acid, and resorcinol, respectively (148–150). In each case, the TFR has been shown to interact with the molecule being degraded or a catabolic intermediate.
TFRs AND NITROGEN METABOLISM
AmtR is a master regulator of nitrogen metabolism in Corynebacterium (37). The AmtR regulon is composed of at least 33 genes. These encode proteins that import and metabolize different nitrogen sources as well as other regulators of nitrogen metabolism. Unlike most of the characterized TFRs, AmtR interacts with a protein rather than a small-molecule ligand. Consistent with its role in controlling nitrogen assimilation, AmtR interacts with the adenylylated form of GlnK, which accumulates under conditions of nitrogen limitation (67). To date, residues important for this interaction have not been characterized. AmtR homologs are found in Actinobacteria, including some species of Mycobacterium, Nocardia, Rhodococcus, and Streptomyces (25, 151). In Streptomyces, two OmpR-like regulators, GlnR and GlnRII, are the master regulators of nitrogen metabolism (152). Although not present in Corynebacterium, GlnR homologs are more conserved in actinobacteria than AmtR homologs; however, there are a few species that encode both (151). Mycobacterium abscessus, Nocardia farcinica, Rhodococcus jostii, Streptomyces avermitilis, and Streptomyces scabies all encode both AmtR and GlnR homologs. In these species, the genes for the AmtR homologs are divergent to genes involved in the import and degradation of urea (Fig. 11A), suggesting that like AmtR, they may regulate nitrogen metabolism. The gene for another AmtR homolog in Mycobacterium smegmatis is located in an operon with genes for a putative enoyl-CoA hydratase and a putative fatty acid-CoA ligase, which do not play an obvious role in nitrogen metabolism. Bioinformatic analysis suggests that mycobacteria contain putative GlnR-binding sites throughout their chromosomes, while AmtR-binding sites in strains encoding AmtR homologs were not identified (151). It is tempting to speculate that in strains encoding both GlnR and AmtR homologs, AmtR may function as a local rather than a global repressor.
Fig 11.
TFRs involved in nitrogen metabolism. (A) Homologs of AmtR, a global regulator of nitrogen metabolism in Corynebacterium, may act as local regulators in related organisms. (B) RutR and PydR homologs from separate clades within a larger group of TFRs predicted to be involved in nucleotide metabolism. (C) Homologs of XdhR may be involved in purine metabolism. (Adapted from reference 25.)
In E. coli, RutR is the master regulator of pyrimidine metabolism. RutR regulates transcription of the divergently transcribed rut operon, encoding gene products involved in the degradation of pyrimidines for use as a nitrogen source. RutR also regulates a number of other targets located elsewhere on the chromosome, including the carAB, gadAX, gadBC, ygiF-glnE, and gcl-hyi-glxR operons, which are involved in various aspects of pyrimidine and glutamate metabolism (153). A homolog of RutR, PydR, has been identified as the regulator of genes required for pyrimidine degradation via the reductive pathway in Pseudomonas putida (154). A crystal structure is available for RutR in complex with its inducing ligand uracil (155). Residues involved in uracil binding are conserved in PydR, indicating that PydR may also be a uracil-responsive transcription factor (154). Our analysis shows separate RutR and PydR subclades within a larger group of TFRs probably involved in the metabolism of pyrimidines as well as possibly purines (Fig. 11B). P. aeruginosa encodes four RutR-PydR homologs, and based on genomic context, all four homologs are likely to play a role in nucleotide metabolism. This RutR-PydR group serves as an example of how very similar TFRs may regulate different pathways involved in the same overall physiological process, in this case nucleotide metabolism. Another example of this is the involvement of FabR and DesT in regulating fatty acid saturation in E. coli and P. aeruginosa, respectively (see TFRs and Lipid Metabolism below).
XdhR from S. coelicolor regulates the expression of the divergently transcribed four-gene operon encoding the subunits of, and a maturation factor for, xanthine dehydrogenase. Xanthine dehydrogenase activity is responsible for the conversion of xanthine to uric acid, which can be broken down and used as a nitrogen source. XdhR may therefore provide a link between primary metabolism, morphological development, and antibiotic production in Streptomyces (156), and our analysis shows that XdhR is indeed well conserved in this genus. The potato pathogen Streptomyces scabies carries two xdhR homologs (Fig. 11C). SCAB82081 encodes an ortholog of XdhR, while the role of SCAB83171 is unclear. We have also identified XdhR homologs in a number of Gram-negative bacteria (e.g., Pseudomonas putida and Rahnella). These XdhR homologs are predicted to regulate a putative short-chain dehydrogenase of unknown function. Given the phylogenetic grouping, these XdhR homologs and the short-chain dehydrogenases they regulate should be investigated for a role in purine metabolism.
TFRs AND LIPID METABOLISM
There are numerous parallels between the biosyntheses of polyketide antibiotics and fatty acids. We have noted above the involvement of TFRs in regulating resistance to numerous polyketide antibiotics, including tetracycline. TFRs also play a major role in regulating fatty acid metabolism as well as the metabolism of other lipid compounds, including sterols.
Fatty Acid Biosynthesis and Degradation
FasR from C. glutamicum is a regulator of lipid biosynthesis. In a fasR mutant, 17 genes were differentially expressed, including fasA, fasB, accB, accC, and accD1 (157). In addition, two other TFRs were found to be differentially expressed in the fasR mutant, one of which, Clg1640, may also play a role in fatty acid metabolism. Clg1640 is found in a group with FadR from Pseudonocardia autotropica (Fig. 12A). In P. autotropica, FadR controls an operon involved in fatty acid degradation (158). Also located in this group are the AtrA homologs from Streptomyces. AtrA is a pleiotropic regulator of antibiotic production in Streptomyces (6). The inducing ligand for AtrA is unknown, but given its grouping with two TFRs involved in fatty acid metabolism, a fatty acid derivative should be investigated. Transcriptomics studies support a role for AveI, the AtrA ortholog of Streptomyces avermitilis, in the regulation of carbon flux toward antibiotic production. Indeed, genes involved in fatty acid metabolism were downregulated in an aveI mutant (159). FasR itself is located in a group with another known regulator of antibiotic production in S. coelicolor, SCO1712 (160) (Fig. 12B), further highlighting a connection between fatty acid metabolism and antibiotic production. It is worth highlighting that AtrA acts as a transcriptional activator, in contrast to the role of most TFRs as repressors (6).
Fig 12.
TFRs involved in lipid metabolism. TFRs involved in lipid metabolism are found in many groups. (A to C) TFRs involved in fatty acid biosynthesis and degradation. (D) TFRs regulating fatty acid saturation. (E) TFRs involved in the synthesis and degradation of storage polymers. (F and G) TFRs involved in terpene utilization. (Adapted from reference 25.)
While FasR is currently the only known TFR involved in fatty acid biosynthesis, numerous TFRs are known to play a role in fatty acid degradation. In addition to FadR from P. autotropica (see above), two other TFRs have been called FadR. FadR from B. subtilis regulates five operons required for fatty acid degradation and recognizes long-chain fatty acyl-CoAs (161). FadR from Thermus thermophilus controls the expression of numerous genes implicated in fatty acid degradation (57). Although there are currently three TFRs bearing the name FadR, none grouped together in our analysis (25). While the available data support a role for all three TFRs in fatty acid degradation, the above proteins are clearly not orthologous.
In M. tuberculosis, at least two TFRs are known to play a role in lipid metabolism, Fad35R and Mce3R. Fad35R controls the expression of an acyl-CoA synthetase encoded by Fad35 in response to fatty acid derivatives (162). Mce3R represses the transcription of the virulence-related mce3 locus as well as other genes required for fatty acid degradation. Mce3R is among a group of TFRs containing duplicated TFR domains within a single peptide. Our analysis has shown that the N- and C-terminal TFR domains form separate clusters within the same group (Fig. 12C). This indicates that the N-terminal domains are more similar to each other than they are to the C-terminal domains, and this group of TFRs may be the result of a single duplication and gene fusion event. Although the inducing ligand for Mce3R has not been identified, it is located within a larger group with Fad35R (Fig. 12C), indicating that it may likewise be induced by a fatty acid derivative.
PsrA from P. aeruginosa responds to long-chain fatty acids to control expression of fad genes (163). In addition, PsrA plays a role in resistance to cationic antimicrobial peptides, antibiotic production, quorum sensing, and virulence, indicating that PsrA and long-chain fatty acids play an important role in the physiology of this important opportunistic pathogen.
Lipid Saturation
As mentioned above, FabR from E. coli and DesT from P. aeruginosa regulate different pathways involved in the same overall physiological process, in this case fatty acid saturation. Our phylogenetic analysis shows that they are located in the same group (25) (Fig. 12D). FabR regulates the expression of fabA and fabB, which are required for the synthesis of unsaturated fatty acids (164). The genes encoding FabA, FabB, and FabR are all located in different areas of the chromosome, and unlike most TFRs, FabR is not autoregulatory (165). While not essential for DNA binding by FabR, unsaturated thioesters (i.e., acyl-ACP or acyl-CoA) were found to enhance binding, while the FabR-DNA interaction was disrupted in the presence of saturated thioesters (165). DesT shows a similar pattern of ligand binding, where DNA binding is enhanced by unsaturated acyl-CoAs and disrupted by saturated acyl-CoAs (166). DesT regulates the expression of desC and desB, which are divergently transcribed from desT (166). The desC and desB genes encode a reductase and an acyl-CoA desaturase, respectively. Whereas FabR regulates the biosynthesis of unsaturated fatty acids, DesT regulates gene products required for the desaturation of preformed acyl chains.
Synthesis and Degradation of Storage Polymers
In Pseudomonas putida, PhaD controls the expression of genes involved in polyhydroxyalkanoate (PHA) metabolism (167). PHAs are produced as carbon storage granules and are being investigated for their potential as alternative plastics (168). PHA polymers are synthesized from (R)-3-hydroxyacyl-CoA, which can be produced from various intermediates of fatty acid degradation. Although not experimentally demonstrated, PhaD is thought to bind a fatty acyl-CoA intermediate of β oxidation (167). Interestingly, PhaD is located in a larger group with NGO0393 and NMB0810 from Neisseria gonorrhoeae and Neisseria meningitidis, respectively (Fig. 12E). The NGO0393 and NMB0810 orthologs are one of only two TFRs encoded by each species. The roles of NGO0393 and NMB0810 in the metabolism of storage polymers have not been investigated.
Terpene Utilization
Terpenes, including cholesterol, are an important class of natural product built from isoprene units. Two TFRs, KstR and KstR2, control cholesterol degradation in M. tuberculosis (169–172). The genes in the KstR and KstR2 regulons are known to be upregulated in vivo and are important for virulence of M. tuberculosis (173). Specific inducing ligands for KstR and KstR2 have not been identified. Although KstR and KstR2 are located in separate groups (Fig. 12F and G), other TFRs in both groups further suggest a role in terpene metabolism. For example, Rv0767c is in the same group as KstR and is located in an operon with Rv0764c, encoding a putative steroid demethylase. KstR2 is in a group with AtuR from P. aeruginosa. AtuR controls the expression of genes required for acyclic terpene utilization (174). A number of TFRs (CampR, CmtI, CmtR, CymR, and PsbI) that control the utilization of cyclic terpenes such as camphor and p-cymene have also been identified (175–179).
TFRs AND AMINO ACID METABOLISM
Three TFRs, AguR, LiuQ, and McbR, are involved in regulating amino acid metabolism. While AguR and LiuQ act as local regulators controlling the expression of adjacent genes involved in amino acid degradation, McbR acts as a global regulator for sulfur metabolism.
Agmatine is an intermediate in the arginine decarboxylase (ADC) pathway for arginine degradation (180). In P. aeruginosa PAO1, the TFR AguR controls expression of the aguAB operon, involved in agmatine utilization, and is induced by agmatine (181). AguR is conserved in many members of the genus Pseudomonas but is absent from P. aeruginosa PA14, where an alternative operon for agmatine metabolism also plays a role in biofilm formation (182).
The TFR LiuQ has been identified using comparative genomics as the regulator of the liuABCD genes, required for the degradation of branched-chain amino acids in Burkholderiales (183). Although DNA binding was not experimentally tested, a putative LiuQ binding site was identified.
McbR from C. glutamicum is a global regulator of sulfur metabolism, including the genes required for the biosynthesis of sulfur-containing amino acids (184, 185). DNA binding by McbR is modulated by the small molecule S-adenosylhomocysteine, a by-product of methylation reactions (185). Deletion of mcbR causes numerous pleiotropic effects on additional aspects of growth and metabolism, indicating that McbR plays a central role in regulating numerous physiological processes in C. glutamicum (186).
TFRs AND COFACTOR METABOLISM
Biotin
In many bacteria, BirA controls the expression of biotin biosynthesis genes (187). BirA is a bifunctional protein acting as the biotin-protein ligase as well as a transcriptional regulator. In some organisms, BirA lacks the transcriptional regulatory domain and the biotin biosynthesis genes are controlled by other transcription factors. In alphaproteobacteria, the GntR family regulator BioR controls the expression of biotin biosynthesis genes (188), while in Corynebacterium and certain related actinobacteria, the TFR BioQ is the regulator of biotin biosynthesis (189). BioQ interacts with a 13-bp palindromic region upstream of a number of biotin biosynthesis genes. Biotin itself was not found to disrupt the BioQ-DNA interaction in vitro, but this does not rule out the possibility of a biotin intermediate serving as a ligand for BioQ.
Heme
Two TFRs, HrtR from Lactococcus lactis and HemR from Propionibacterium freudenreichii, have been implicated in heme homeostasis. HemR is a putative regulator of genes required for the conversion of glutamate to protoheme in P. freudenreichii. It is transcribed divergently from hemX, encoding a putative heme transporter. The details of HemR DNA binding and ligand binding have not been investigated (190). L. lactis does not synthesize heme. HrtR senses intracellular heme and regulates expression of a heme exporter encoded by HrtBA (191).
TFRs AND CELL DIVISION
SlmA from E. coli binds and antagonizes polymerization of the bacterial tubulin homolog FtsZ, preventing cell division from occurring over the chromosome, in a process known as nucleoid occlusion (68, 192, 193). SlmA is not known to interact with a small molecule but rather interacts directly with the tubulin-like cell division protein FtsZ. This interaction is believed to be important for preventing the formation of cell division septa around unsegregated chromosomes. In one model for SlmA function (70), the FtsZ interaction interface on SlmA is completely exposed only when SlmA is bound to DNA. Hence SlmA affect FtsZ polymerization only in areas where DNA is present.
Based on our analysis (25), SlmA homologs are found in most members of the gamma- and betaproteobacteria. Exceptions in which this mechanism appears to be absent include Acinetobacter, Francisella, Legionella, Pseudomonas, Stenotrophomonas, Xanthomonas, and Neisseria. A possible SlmA ortholog in Bordetella parapertussis was found to be the product of a pseudogene. In E. coli, the absence of both the Min system, also involved in regulating FtsZ function, and SlmA results in a synthetic lethal phenotype (68). How FtsZ ring placement is regulated in Gram-negative bacteria such as Myxococcus, Campylobacter, and Bacteroides where both the Min system and SlmA are absent is unknown, but evidence suggests that there are as yet-unidentified factors involved in nucleoid occlusion (194).
In Bacillus, Noc, a protein unrelated to SlmA, controls nucleoid occlusion. Noc is a homolog of ParB chromosome-partitioning proteins. The TFR RefZ, however, is involved in regulating the transition from medial to polar cell division during sporulation, possibly as a direct effector of FtsZ polymerization (195). Our analysis does not indicate a close relationship between SlmA and RefZ.
FUTURE DIRECTIONS AND CHALLENGES
TFRs play an important role in regulating numerous aspects of bacterial physiology. Through genomics and structural studies, we have learned a great deal regarding the types of gene products regulated by TFRs and the mechanisms by which TFRs interact with both DNA and small molecules. Although genomics allows us to predict the target genes for the majority of TFRs, this cannot be done for type III TFRs, and other methodologies must be employed. Different models still exist as to the structural changes that TFRs undergo upon ligand binding and the precise molecular mechanisms behind derepression, and future NMR studies may help to resolve discrepancies in the current data. Determination of the identities of the small-molecule ligands, or other interacting partners, bound by the more than 200,000 TFRs in the public databases probably represents the most understudied and challenging area of TFR biology, and future work will be required to identify these ligands.
ACKNOWLEDGMENTS
L.C. was the recipient of a postdoctoral fellowship from the Natural Science and Engineering Research Council. TFR research in the authors' lab is funded by a grant to J.R.N. from the Canadian Institutes of Health Research (MOP 97729).
We thank Alan Davidson, Mark Buttner, and Tung Le for their critical comments on the manuscript.
Biographies

Leslie Cuthbertson received her B.Sc. degree in molecular biology and genetics from the University of Guelph. She continued on to Ph.D. studies in microbiology with Chris Whitfield, where her studies were focused on the export of polysaccharides in Gram-negative bacteria. Upon completing her Ph.D., Dr. Cuthbertson began postdoctoral studies at McMaster University with Justin Nodwell, where she became interested in microbial chemical biology and more specifically how bacteria use transcription factors to recognize small-molecule signals. She believes that understanding how bacteria “see” their environment will be crucial in combatting pathogens while encouraging the growth of beneficial microflora.

Justin Nodwell is a Professor and Chair in the Department of Biochemistry at the University of Toronto. He trained in molecular biology and bacterial genetics with Jack Greenblatt and Richard Losick and worked from 1998 to 2013 at McMaster University, Department of Biochemistry and Biomedical Sciences and Michael DeGroote Institute for Infectious Diseases Research. His research involves the responses of bacterial cells to biologically active small molecules and the regulatory mechanisms that control secondary metabolism. Goals of this work include understanding antibiotic resistance and identifying new antimicrobial compounds.
REFERENCES
- 1. Laub MT, Goulian M. 2007. Specificity in two-component signal transduction pathways. Annu. Rev. Genet. 41:121–145 [DOI] [PubMed] [Google Scholar]
- 2. Ulrich LE, Koonin EV, Zhulin IB. 2005. One-component systems dominate signal transduction in prokaryotes. Trends Microbiol. 13:52–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schreiter ER, Drennan CL. 2007. Ribbon-helix-helix transcription factors: variations on a theme. Nat. Rev. Microbiol. 5:710–720 [DOI] [PubMed] [Google Scholar]
- 4. Pérez-Rueda E, Collado-Vides J. 2000. The repertoire of DNA-binding transcriptional regulators in Escherichia coli K-12. Nucleic Acids Res. 28:1838–1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pérez-Rueda E, Collado-Vides J. 2001. Common history at the origin of the position-function correlation in transcriptional regulators in archaea and bacteria. J. Mol. Evol. 53:172–179 [DOI] [PubMed] [Google Scholar]
- 6. Uguru GC, Stephens KE, Stead JA, Towle JE, Baumberg S, McDowall KJ. 2005. Transcriptional activation of the pathway-specific regulator of the actinorhodin biosynthetic genes in Streptomyces coelicolor. Mol. Microbiol. 58:131–150 [DOI] [PubMed] [Google Scholar]
- 7. Schleif R. 2010. AraC protein, regulation of the l-arabinose operon in Escherichia coli, and the light switch mechanism of AraC action. FEMS Microbiol. Rev. 34:779–796 [DOI] [PubMed] [Google Scholar]
- 8. Joshi MV, Bignell DR, Johnson EG, Sparks JP, Gibson DM, Loria R. 2007. The AraC/XylS regulator TxtR modulates thaxtomin biosynthesis and virulence in Streptomyces scabies. Mol. Microbiol. 66:633–642 [DOI] [PubMed] [Google Scholar]
- 9. McCormick JR, Flärdh K. 2012. Signals and regulators that govern Streptomyces development. FEMS Microbiol. Rev. 36:206–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yang J, Tauschek M, Robins-Browne RM. 2011. Control of bacterial virulence by AraC-like regulators that respond to chemical signals. Trends Microbiol. 19:128–135 [DOI] [PubMed] [Google Scholar]
- 11. Makarova KS, Wolf YI, Koonin EV. 2009. Comprehensive comparative-genomic analysis of type 2 toxin-antitoxin systems and related mobile stress response systems in prokaryotes. Biol. Direct 4:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McEwan AG, Djoko KY, Chen NH, Couñago RL, Kidd SP, Potter AJ, Jennings MP. 2011. Novel bacterial MerR-like regulators their role in the response to carbonyl and nitrosative stress. Adv. Microb. Physiol. 58:1–22 [DOI] [PubMed] [Google Scholar]
- 13. Deng W, Li C, Xie J. 2013. The underling mechanism of bacterial TetR/AcrR family transcriptional repressors. Cell Signal. 25:1608–1613 [DOI] [PubMed] [Google Scholar]
- 14. Ramos JL, Martínez-Bueno M, Molina-Henares AJ, Terán W, Watanabe K, Zhang X, Gallegos MT, Brennan R, Tobes R. 2005. The TetR family of transcriptional repressors. Microbiol. Mol. Biol. Rev. 69:326–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Izaki K, Kiuchi K, Arima K. 1966. Specificity and mechanism of tetracycline resistance in a multiple drug resistant strain of Escherichia coli. J. Bacteriol. 91:628–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yang HL, Zubay G, Levy SB. 1976. Synthesis of an R plasmid protein associated with tetracycline resistance is negatively regulated. Proc. Natl. Acad. Sci. U. S. A. 73:1509–1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Beck CF, Mutzel R, Barbé J, Müller W. 1982. A multifunctional gene (tetR) controls Tn10-encoded tetracycline resistance. J. Bacteriol. 150:633–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bertrand KP, Postle K, Wray LV, Reznikoff WS. 1983. Overlapping divergent promoters control expression of Tn10 tetracycline resistance. Gene 23:149–156 [DOI] [PubMed] [Google Scholar]
- 19. Postle K, Nguyen TT, Bertrand KP. 1984. Nucleotide sequence of the repressor gene of the Tn10 tetracycline resistance determinant. Nucleic Acids Res. 12:4849–4863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wray LV, Jorgensen RA, Reznikoff WS. 1981. Identification of the tetracycline resistance promoter and repressor in transposon Tn10. J. Bacteriol. 147:297–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zupancic TJ, King SR, Pogue-Geile KL, Jaskunas SR. 1980. Identification of a second tetracycline-inducible polypeptide encoded by Tn10. J. Bacteriol. 144:346–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kisker C, Hinrichs W, Tovar K, Hillen W, Saenger W. 1995. The complex formed between Tet repressor and tetracycline-Mg2+ reveals mechanism of antibiotic resistance. J. Mol. Biol. 247:260–280 [DOI] [PubMed] [Google Scholar]
- 23. Ahn SK, Cuthbertson L, Nodwell JR. 2012. Genome context as a predictive tool for identifying regulatory targets of the TetR family transcriptional regulators. PLoS One 7:e50562. 10.1371/journal.pone.0050562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yu Z, Reichheld SE, Savchenko A, Parkinson J, Davidson AR. 2010. A comprehensive analysis of structural and sequence conservation in the TetR family transcriptional regulators. J. Mol. Biol. 400:847–864 [DOI] [PubMed] [Google Scholar]
- 25. Cuthbertson L, Ahn SK, Nodwell JR. 2013. Deglycosylation as a mechanism of inducible antibiotic resistance revealed using a global relational tree for one-component regulators. Chem. Biol. 20:232–240 [DOI] [PubMed] [Google Scholar]
- 26. Bentley SD, Chater KF, Cerdeño-Tárraga AM, Challis GL, Thomson NR, James KD, Harris DE, Quail MA, Kieser H, Harper D, Bateman A, Brown S, Chandra G, Chen CW, Collins M, Cronin A, Fraser A, Goble A, Hidalgo J, Hornsby T, Howarth S, Huang CH, Kieser T, Larke L, Murphy L, Oliver K, O'Neil S, Rabbinowitsch E, Rajandream MA, Rutherford K, Rutter S, Seeger K, Saunders D, Sharp S, Squares R, Squares S, Taylor K, Warren T, Wietzorrek A, Woodward J, Barrell BG, Parkhill J, Hopwood DA. 2002. Complete genome sequence of the model actinomycete Streptomyces coelicolor A3(2). Nature 417:141–147 [DOI] [PubMed] [Google Scholar]
- 27. Ishihama A. 2010. Prokaryotic genome regulation: multifactor promoters, multitarget regulators and hierarchic networks. FEMS Microbiol. Rev. 34:628–645 [DOI] [PubMed] [Google Scholar]
- 28. Ma Q, Yang Z, Pu M, Peti W, Wood TK. 2011. Engineering a novel c-di-GMP-binding protein for biofilm dispersal. Environ. Microbiol. 13:631–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Partridge JD, Bodenmiller DM, Humphrys MS, Spiro S. 2009. NsrR targets in the Escherichia coli genome: new insights into DNA sequence requirements for binding and a role for NsrR in the regulation of motility. Mol. Microbiol. 73:680–694 [DOI] [PubMed] [Google Scholar]
- 30. Roy PH, Tetu SG, Larouche A, Elbourne L, Tremblay S, Ren Q, Dodson R, Harkins D, Shay R, Watkins K, Mahamoud Y, Paulsen IT. 2010. Complete genome sequence of the multiresistant taxonomic outlier Pseudomonas aeruginosa PA7. PLoS One 5:e8842. 10.1371/journal.pone.0008842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yamamoto M, Ueda A, Kudo M, Matsuo Y, Fukushima J, Nakae T, Kaneko T, Ishigatsubo Y. 2009. Role of MexZ and PA5471 in transcriptional regulation of mexXY in Pseudomonas aeruginosa. Microbiology 155:3312–3321 [DOI] [PubMed] [Google Scholar]
- 32. Feliziani S, Luján AM, Moyano AJ, Sola C, Bocco JL, Montanaro P, Canigia LF, Argaraña CE, Smania AM. 2010. Mucoidy, quorum sensing, mismatch repair and antibiotic resistance in Pseudomonas aeruginosa from cystic fibrosis chronic airways infections. PLoS One 5:e12669. 10.1371/journal.pone.0012669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wei Q, Tarighi S, A Dötsch, S Häussler, M Müsken, Wright VJ, M Cámara, Williams P, Haenen S, Boerjan B, Bogaerts A, Vierstraete E, Verleyen P, Schoofs L, Willaert R, De Groote VN, Michiels J, Vercammen K, Crabbé A, Cornelis P. 2011. Phenotypic and genome-wide analysis of an antibiotic-resistant small colony variant (SCV) of Pseudomonas aeruginosa. PLoS One 6:e29276. 10.1371/journal.pone.0029276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Morita Y, Cao L, Gould VC, Avison MB, Poole K. 2006. nalD encodes a second repressor of the mexAB-oprM multidrug efflux operon of Pseudomonas aeruginosa. J. Bacteriol. 188:8649–8654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yang YH, Kim JN, Song E, Kim E, Oh MK, Kim BG. 2008. Finding new pathway-specific regulators by clustering method using threshold standard deviation based on DNA chip data of Streptomyces coelicolor. Appl. Microbiol. Biotechnol. 80:709–717 [DOI] [PubMed] [Google Scholar]
- 36. Hasselt K, Rankl S, Worsch S, Burkovski A. 2011. Adaptation of AmtR-controlled gene expression by modulation of AmtR binding activity in Corynebacterium glutamicum. J. Biotechnol. 154:156–162 [DOI] [PubMed] [Google Scholar]
- 37. Jakoby M, Nolden L, Meier-Wagner J, Krämer R, Burkovski A. 2000. AmtR, a global repressor in the nitrogen regulation system of Corynebacterium glutamicum. Mol. Microbiol. 37:964–977 [DOI] [PubMed] [Google Scholar]
- 38. Muhl D, Jessberger N, Hasselt K, Jardin C, Sticht H, Burkovski A. 2009. DNA binding by Corynebacterium glutamicum TetR-type transcription regulator AmtR. BMC Mol. Biol. 10:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cerda-Maira FA, Ringelberg CS, Taylor RK. 2008. The bile response repressor BreR regulates expression of the Vibrio cholerae breAB efflux system operon. J. Bacteriol. 190:7441–7452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Quiñones B, Pujol CJ, Lindow SE. 2004. Regulation of AHL production and its contribution to epiphytic fitness in Pseudomonas syringae. Mol. Plant Microbe Interact. 17:521–531 [DOI] [PubMed] [Google Scholar]
- 41. Mitchell G, Gattuso M, Grondin G, Marsault É Bouarab K, Malouin F. 2011. Tomatidine inhibits replication of Staphylococcus aureus small-colony variants in cystic fibrosis airway epithelial cells. Antimicrob. Agents Chemother. 55:1937–1945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Le TB, Stevenson CE, Fiedler HP, Maxwell A, Lawson DM, Buttner MJ. 2011. Structures of the TetR-like simocyclinone efflux pump repressor, SimR, and the mechanism of ligand-mediated derepression. J. Mol. Biol. 408:40–56 [DOI] [PubMed] [Google Scholar]
- 43. Itou H, Okada U, Suzuki H, Yao M, Wachi M, Watanabe N, Tanaka I. 2005. The CGL2612 protein from Corynebacterium glutamicum is a drug resistance-related transcriptional repressor: structural and functional analysis of a newly identified transcription factor from genomic DNA analysis. J. Biol. Chem. 280:38711–38719 [DOI] [PubMed] [Google Scholar]
- 44. Miller DJ, Zhang YM, Subramanian C, Rock CO, White SW. 2010. Structural basis for the transcriptional regulation of membrane lipid homeostasis. Nat. Struct. Mol. Biol. 17:971–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Orth P, Schnappinger D, Hillen W, Saenger W, Hinrichs W. 2000. Structural basis of gene regulation by the tetracycline inducible Tet repressor-operator system. Nat. Struct. Biol. 7:215–219 [DOI] [PubMed] [Google Scholar]
- 46. Sawai H, Yamanaka M, Sugimoto H, Shiro Y, Aono S. 2012. Structural basis for the transcriptional regulation of heme homeostasis in Lactococcus lactis. J. Biol. Chem. 287:30755–30768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schumacher MA, Miller MC, Grkovic S, Brown MH, Skurray RA, Brennan RG. 2002. Structural basis for cooperative DNA binding by two dimers of the multidrug-binding protein QacR. EMBO. J. 21:1210–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hochschild A, Lewis M. 2009. The bacteriophage lambda CI protein finds an asymmetric solution. Curr. Opin. Struct. Biol. 19:79–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Li DF, Zhang N, Hou YJ, Huang Y, Hu Y, Zhang Y, Liu SJ, Wang DC. 2011. Crystal structures of the transcriptional repressor RolR reveals a novel recognition mechanism between inducer and regulator. PLoS One 6:e19529. 10.1371/journal.pone.0019529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Le TB, Fiedler HP, den Hengst CD, Ahn SK, Maxwell A, Buttner MJ. 2009. Coupling of the biosynthesis and export of the DNA gyrase inhibitor simocyclinone in Streptomyces antibioticus. Mol. Microbiol. 72:1462–1474 [DOI] [PubMed] [Google Scholar]
- 51. Alguel Y, Meng C, Terán W, Krell T, Ramos JL, Gallegos MT, Zhang X. 2007. Crystal structures of multidrug binding protein TtgR in complex with antibiotics and plant antimicrobials. J. Mol. Biol. 369:829–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hernández A, Maté MJ, Sánchez-Díaz PC, Romero A, Rojo F, Martínez JL. 2009. Structural and functional analysis of SmeT, the repressor of the Stenotrophomonas maltophilia multidrug efflux pump SmeDEF. J. Biol. Chem. 284:14428–14438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Schumacher MA, Miller MC, Grkovic S, Brown MH, Skurray RA, Brennan RG. 2001. Structural mechanisms of QacR induction and multidrug recognition. Science 294:2158–2163 [DOI] [PubMed] [Google Scholar]
- 54. Willems AR, Tahlan K, Taguchi T, Zhang K, Lee ZZ, Ichinose K, Junop MS, Nodwell JR. 2008. Crystal structures of the Streptomyces coelicolor TetR-like protein ActR alone and in complex with actinorhodin or the actinorhodin biosynthetic precursor (S)-DNPA. J. Mol. Biol. 376:1377–1387 [DOI] [PubMed] [Google Scholar]
- 55. Bellinzoni M, Buroni S, Schaeffer F, Riccardi G, De Rossi E, Alzari PM. 2009. Structural plasticity and distinct drug-binding modes of LfrR, a mycobacterial efflux pump regulator. J. Bacteriol. 191:7531–7537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lei HT, Shen Z, Surana P, Routh MD, Su CC, Zhang Q, Yu EW. 2011. Crystal structures of CmeR-bile acid complexes from Campylobacter jejuni. Protein Sci. 20:712–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Agari Y, Agari K, Sakamoto K, Kuramitsu S, Shinkai A. 2011. TetR-family transcriptional repressor Thermus thermophilus FadR controls fatty acid degradation. Microbiology 157:1589–1601 [DOI] [PubMed] [Google Scholar]
- 58. Frénois F, Engohang-Ndong J, Locht C, Baulard AR, Villeret V. 2004. Structure of EthR in a ligand bound conformation reveals therapeutic perspectives against tuberculosis. Mol. Cell 16:301–307 [DOI] [PubMed] [Google Scholar]
- 59. Zheng J, Sagar V, Smolinsky A, Bourke C, LaRonde-LeBlanc N, Cropp TA. 2009. Structure and function of the macrolide biosensor protein, MphR(A), with and without erythromycin. J. Mol. Biol. 387:1250–1260 [DOI] [PubMed] [Google Scholar]
- 60. Grkovic S, Hardie KM, Brown MH, Skurray RA. 2003. Interactions of the QacR multidrug-binding protein with structurally diverse ligands: implications for the evolution of the binding pocket. Biochemistry 42:15226–15236 [DOI] [PubMed] [Google Scholar]
- 61. Hernández A, Ruiz FM, Romero A, Martínez JL. 2011. The binding of triclosan to SmeT, the repressor of the multidrug efflux pump SmeDEF, induces antibiotic resistance in Stenotrophomonas maltophilia. PLoS Pathog. 7:e1002103. 10.1371/journal.ppat.1002103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Dover LG, Corsino PE, Daniels IR, Cocklin SL, Tatituri V, Besra GS, Fütterer K. 2004. Crystal structure of the TetR/CamR family repressor Mycobacterium tuberculosis EthR implicated in ethionamide resistance. J. Mol. Biol. 340:1095–1105 [DOI] [PubMed] [Google Scholar]
- 63. Flipo M, Willand N, Lecat-Guillet N, Hounsou C, Desroses M, Leroux F, Lens Z, Villeret V, Wohlkönig A, Wintjens R, Christophe T, Kyoung Jeon H, Locht C, Brodin P, Baulard AR, Déprez B. 2012. Discovery of novel N-phenylphenoxyacetamide derivatives as EthR inhibitors and ethionamide boosters by combining high-throughput screening and synthesis. J. Med. Chem. 55:6391–6402 [DOI] [PubMed] [Google Scholar]
- 64. Willand N, Dirié B, Carette X, Bifani P, Singhal A, Desroses M, Leroux F, Willery E, Mathys V, R Déprez-Poulain, Delcroix G, Frénois F, Aumercier M, Locht C, Villeret V, B Déprez, Baulard AR. 2009. Synthetic EthR inhibitors boost antituberculous activity of ethionamide. Nat. Med. 15:537–544 [DOI] [PubMed] [Google Scholar]
- 65. Willand N, Desroses M, Toto P, Dirié B, Lens Z, Villeret V, Rucktooa P, Locht C, Baulard A, Deprez B. 2010. Exploring drug target flexibility using in situ click chemistry: application to a mycobacterial transcriptional regulator. ACS Chem. Biol. 5:1007–1013 [DOI] [PubMed] [Google Scholar]
- 66. Carette X, Blondiaux N, Willery E, Hoos S, Lecat-Guillet N, Lens Z, Wohlkönig A, Wintjens R, Soror SH, Frénois F, Dirié B, Villeret V, England P, Lippens G, Deprez B, Locht C, Willand N, Baulard AR. 2012. Structural activation of the transcriptional repressor EthR from Mycobacterium tuberculosis by single amino acid change mimicking natural and synthetic ligands. Nucleic Acids Res. 40:3018–3030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Beckers G, Strösser J, Hildebrandt U, Kalinowski J, Farwick M, Krämer R, Burkovski A. 2005. Regulation of AmtR-controlled gene expression in Corynebacterium glutamicum: mechanism and characterization of the AmtR regulon. Mol. Microbiol. 58:580–595 [DOI] [PubMed] [Google Scholar]
- 68. Bernhardt TG, de Boer PA. 2005. SlmA, a nucleoid-associated, FtsZ binding protein required for blocking septal ring assembly over chromosomes in E. coli. Mol. Cell 18:555–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Christen S, Srinivas A, Bähler P, Zeller A, Pridmore D, Bieniossek C, Baumann U, Erni B. 2006. Regulation of the dha operon of Lactococcus lactis: a deviation from the rule followed by the TetR family of transcription regulators. J. Biol. Chem. 281:23129–23137 [DOI] [PubMed] [Google Scholar]
- 70. Cho H, Bernhardt TG. 2013. Identification of the SlmA active site responsible for blocking bacterial cytokinetic ring assembly over the chromosome. PLoS Genet. 9:e1003304. 10.1371/journal.pgen.1003304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kloosterman TG, van der Kooi-Pol MM, Bijlsma JJ, Kuipers OP. 2007. The novel transcriptional regulator SczA mediates protection against Zn2+ stress by activation of the Zn2+-resistance gene czcD in Streptococcus pneumoniae. Mol. Microbiol. 65:1049–1063 [DOI] [PubMed] [Google Scholar]
- 72. Mermod M, Magnani D, Solioz M, Stoyanov JV. 2012. The copper-inducible ComR (YcfQ) repressor regulates expression of ComC (YcfR), which affects copper permeability of the outer membrane of Escherichia coli. Biometals. 25:33–43 [DOI] [PubMed] [Google Scholar]
- 73. Reichheld SE, Yu Z, Davidson AR. 2009. The induction of folding cooperativity by ligand binding drives the allosteric response of tetracycline repressor. Proc. Natl. Acad. Sci. U. S. A. 106:22263–22268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Sevvana M, Goetz C, Goeke D, Wimmer C, Berens C, Hillen W, Muller YA. 2012. An exclusive α/β code directs allostery in TetR-peptide complexes. J. Mol. Biol. 416:46–56 [DOI] [PubMed] [Google Scholar]
- 75. Abbas A, Morrissey P, Marquez C, Sheehan M, Delany R, O'Gara F. 2002. Characterization of interactions between the transcriptional repressor PhlF and its binding Site at the phlA promoter in Pseudomonas fluorescens F113. J. Bacteriol. 184:3008–3016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Le TB, Schumacher MA, Lawson DM, Brennan RG, Buttner MJ. 2011. The crystal structure of the TetR family transcriptional repressor SimR bound to DNA and the role of a flexible N-terminal extension in minor groove binding. Nucleic Acids Res. 39:9433–9447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Namwat W, Lee CK, Kinoshita H, Yamada Y, Nihira T. 2001. Identification of the varR gene as a transcriptional regulator of virginiamycin S resistance in Streptomyces virginiae. J. Bacteriol. 183:2025–2031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ostash I, Ostash B, Luzhetskyy A, Bechthold A, Walker S, Fedorenko V. 2008. Coordination of export and glycosylation of landomycins in Streptomyces cyanogenus S136. FEMS Microbiol. Lett. 285:195–202 [DOI] [PubMed] [Google Scholar]
- 79. Tahlan K, Ahn SK, Sing A, Bodnaruk TD, Willems AR, Davidson AR, Nodwell JR. 2007. Initiation of actinorhodin export in Streptomyces coelicolor. Mol. Microbiol. 63:951–961 [DOI] [PubMed] [Google Scholar]
- 80. Das A, Khosla C. 2009. Biosynthesis of aromatic polyketides in bacteria. Acc. Chem. Res. 42:631–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Xu Y, Willems A, Au-Yeung C, Tahlan K, Nodwell JR. 2012. A two-step mechanism for the activation of actinorhodin export and resistance in Streptomyces coelicolor. mBio 3(5):e00191–12. 10.1128/mBio.00191-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Bystrykh LV, Fernández-Moreno MA, Herrema JK, Malpartida F, Hopwood DA, Dijkhuizen L. 1996. Production of actinorhodin-related “blue pigments” by Streptomyces coelicolor A3(2). J. Bacteriol. 178:2238–2244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Erb A, Luzhetskyy A, Hardter U, Bechthold A. 2009. Cloning and sequencing of the biosynthetic gene cluster for saquayamycin Z and galtamycin B and the elucidation of the assembly of their saccharide chains. Chembiochem 10:1392–1401 [DOI] [PubMed] [Google Scholar]
- 84. Faust B, Hoffmeister D, Weitnauer G, Westrich L, Haag S, Schneider P, Decker H, Künzel E, Rohr J, Bechthold A. 2000. Two new tailoring enzymes, a glycosyltransferase and an oxygenase, involved in biosynthesis of the angucycline antibiotic urdamycin A in Streptomyces fradiae Tü2717. Microbiology 146:147–154 [DOI] [PubMed] [Google Scholar]
- 85. Galm U, Schimana J, Fiedler HP, Schmidt J, Li SM, Heide L. 2002. Cloning and analysis of the simocyclinone biosynthetic gene cluster of Streptomyces antibioticus Tü 6040. Arch. Microbiol. 178:102–114 [DOI] [PubMed] [Google Scholar]
- 86. Trefzer A, Pelzer S, Schimana J, Stockert S, Bihlmaier C, Fiedler HP, Welzel K, Vente A, Bechthold A. 2002. Biosynthetic gene cluster of simocyclinone, a natural multihybrid antibiotic. Antimicrob. Agents. Chemother. 46:1174–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Edwards MJ, Flatman RH, Mitchenall LA, Stevenson CE, Le TB, Clarke TA, McKay AR, Fiedler HP, Buttner MJ, Lawson DM, Maxwell A. 2009. A crystal structure of the bifunctional antibiotic simocyclinone D8, bound to DNA gyrase. Science 326:1415–1418 [DOI] [PubMed] [Google Scholar]
- 88. Flatman RH, Howells AJ, Heide L, Fiedler HP, Maxwell A. 2005. Simocyclinone D8, an inhibitor of DNA gyrase with a novel mode of action. Antimicrob. Agents Chemother. 49:1093–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Bangera MG, Thomashow LS. 1999. Identification and characterization of a gene cluster for synthesis of the polyketide antibiotic 2,4-diacetylphloroglucinol from Pseudomonas fluorescens Q2-87. J. Bacteriol. 181:3155–3163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Gross H, Loper JE. 2009. Genomics of secondary metabolite production by Pseudomonas spp. Nat. Prod. Rep. 26:1408–1446 [DOI] [PubMed] [Google Scholar]
- 91. August PR, Tang L, Yoon YJ, Ning S, Müller R, Yu TW, Taylor M, Hoffmann D, Kim CG, Zhang X, Hutchinson CR, Floss HG. 1998. Biosynthesis of the ansamycin antibiotic rifamycin: deductions from the molecular analysis of the rif biosynthetic gene cluster of Amycolatopsis mediterranei S699. Chem. Biol. 5:69–79 [DOI] [PubMed] [Google Scholar]
- 92. Jørgensen H, Degnes Dikiy KF, Fjaervik A, Klinkenberg E, Gand Zotchev SB. 2010. Insights into the evolution of macrolactam biosynthesis through cloning and comparative analysis of the biosynthetic gene cluster for a novel macrocyclic lactam, ML-449. Appl. Environ. Microbiol. 76:283–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Wu Q, Liang J, Lin S, Zhou X, Bai L, Deng Z, Wang Z. 2011. Characterization of the biosynthesis gene cluster for the pyrrole polyether antibiotic calcimycin (A23187) in Streptomyces chartreusis NRRL 3882. Antimicrob. Agents. Chemother. 55:974–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Keller U, Lang M, Crnovcic I, Pfennig F, Schauwecker F. 2010. The actinomycin biosynthetic gene cluster of Streptomyces chrysomallus: a genetic hall of mirrors for synthesis of a molecule with mirror symmetry. J. Bacteriol. 192:2583–2595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Zhang G, Zhang H, Li S, Xiao J, Zhang G, Zhu Y, Niu S, Ju J, Zhang C. 2012. Characterization of the amicetin biosynthesis gene cluster from Streptomyces vinaceusdrappus NRRL 2363 implicates two alternative strategies for amide bond formation. Appl. Environ. Microbiol. 78:2393–2401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. D'Costa VM, McGrann KM, Hughes DW, Wright GD. 2006. Sampling the antibiotic resistome. Science 311:374–377 [DOI] [PubMed] [Google Scholar]
- 97. Benveniste R, Davies J. 1973. Aminoglycoside antibiotic-inactivating enzymes in actinomycetes similar to those present in clinical isolates of antibiotic-resistant bacteria. Proc. Natl. Acad. Sci. U. S. A. 70:2276–2280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Wright GD. 2010. Antibiotic resistance in the environment: a link to the clinic? Curr. Opin. Microbiol. 13:589–594 [DOI] [PubMed] [Google Scholar]
- 99. Pickens LB, Kim W, Wang P, Zhou H, Watanabe K, Gomi S, Tang Y. 2009. Biochemical analysis of the biosynthetic pathway of an anticancer tetracycline SF2575. J. Am. Chem. Soc. 131:17677–17689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Folcher M, Morris RP, Dale G, Salah-Bey-Hocini K, Viollier PH, Thompson CJ. 2001. A transcriptional regulator of a pristinamycin resistance gene in Streptomyces coelicolor. J. Biol. Chem. 276:1479–1485 [DOI] [PubMed] [Google Scholar]
- 101. Noguchi N, Takada K, Katayama J, Emura A, Sasatsu M. 2000. Regulation of transcription of the mph(A) gene for macrolide 2′-phosphotransferase I in Escherichia coli: characterization of the regulatory gene mphR(A). J. Bacteriol. 182:5052–5058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Szczepanowski R, Krahn I, Bohn N, A Pühler, Schlüter A. 2007. Novel macrolide resistance module carried by the IncP-1beta resistance plasmid pRSB111, isolated from a wastewater treatment plant. Antimicrob. Agents Chemother. 51:673–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Achard A, Guérin-Faublée V, Pichereau V, Villers C, Leclercq R. 2008. Emergence of macrolide resistance gene mph(B) in Streptococcus uberis and cooperative effects with rdmC-like gene. Antimicrob. Agents Chemother. 52:2767–2770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Hirooka K, Kunikane S, Matsuoka H, Yoshida K, Kumamoto K, Tojo S, Fujita Y. 2007. Dual regulation of the Bacillus subtilis regulon comprising the lmrAB and yxaGH operons and yxaF gene by two transcriptional repressors, LmrA and YxaF, in response to flavonoids. J. Bacteriol. 189:5170–5182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Baulard AR, Betts JC, Engohang-Ndong J, Quan S, McAdam RA, Brennan PJ, Locht C, Besra GS. 2000. Activation of the pro-drug ethionamide is regulated in mycobacteria. J. Biol. Chem. 275:28326–28331 [DOI] [PubMed] [Google Scholar]
- 106. DeBarber AE, Mdluli K, Bosman M, Bekker LG, Barry CE. 2000. Ethionamide activation and sensitivity in multidrug-resistant Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. U. S. A. 97:9677–9682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Fraaije MW, Kamerbeek NM, Heidekamp AJ, Fortin R, Janssen DB. 2004. The prodrug activator EtaA from Mycobacterium tuberculosis is a Baeyer-Villiger monooxygenase. J. Biol. Chem. 279:3354–3360 [DOI] [PubMed] [Google Scholar]
- 108. Vannelli TA, Dykman A, Ortiz de Montellano PR. 2002. The antituberculosis drug ethionamide is activated by a flavoprotein monooxygenase. J. Biol. Chem. 277:12824–12829 [DOI] [PubMed] [Google Scholar]
- 109. Dover LG, Alahari A, Gratraud P, Gomes JM, Bhowruth V, Reynolds RC, Besra GS, Kremer L. 2007. EthA, a common activator of thiocarbamide-containing drugs acting on different mycobacterial targets. Antimicrob. Agents Chemother. 51:1055–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Qian L, Ortiz de Montellano PR. 2006. Oxidative activation of thiacetazone by the Mycobacterium tuberculosis flavin monooxygenase EtaA and human FMO1 and FMO3. Chem. Res. Toxicol. 19:443–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Aires JR, Köhler T, Nikaido H, Plésiat P. 1999. Involvement of an active efflux system in the natural resistance of Pseudomonas aeruginosa to aminoglycosides. Antimicrob. Agents Chemother. 43:2624–2628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Ma D, Alberti M, Lynch C, Nikaido H, Hearst JE. 1996. The local repressor AcrR plays a modulating role in the regulation of acrAB genes of Escherichia coli by global stress signals. Mol. Microbiol. 19:101–112 [DOI] [PubMed] [Google Scholar]
- 113. Rahmati S, Yang S, Davidson AL, Zechiedrich EL. 2002. Control of the AcrAB multidrug efflux pump by quorum-sensing regulator SdiA. Mol. Microbiol. 43:677–685 [DOI] [PubMed] [Google Scholar]
- 114. White DG, Goldman JD, Demple B, Levy SB. 1997. Role of the acrAB locus in organic solvent tolerance mediated by expression of marA, soxS, or robA in Escherichia coli. J. Bacteriol. 179:6122–6126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Gibson JS, Cobbold RN, Kyaw-Tanner MT, Heisig P, Trott DJ. 2010. Fluoroquinolone resistance mechanisms in multidrug-resistant Escherichia coli isolated from extraintestinal infections in dogs. Vet. Microbiol. 146:161–166 [DOI] [PubMed] [Google Scholar]
- 116. Su CC, Rutherford DJ, Yu EW. 2007. Characterization of the multidrug efflux regulator AcrR from Escherichia coli. Biochem. Biophys. Res. Commun. 361:85–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Li XZ, Nikaido H. 2009. Efflux-mediated drug resistance in bacteria: an update. Drugs 69:1555–1623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Vogne C, Aires JR, Bailly C, Hocquet D, Plésiat P. 2004. Role of the multidrug efflux system MexXY in the emergence of moderate resistance to aminoglycosides among Pseudomonas aeruginosa isolates from patients with cystic fibrosis. Antimicrob. Agents Chemother. 48:1676–1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Poole K. 2007. Efflux pumps as antimicrobial resistance mechanisms. Ann. Med. 39:162–176 [DOI] [PubMed] [Google Scholar]
- 120. Helling B, Janes K, Kimball H, Tran T, Bundesmann M, Check P, Phelan D, Miller C. 2002. Toxic waste disposal in Escherichia coli. J. Bacteriol. 184:3699–3703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Caughlan RE, Sriram S, Daigle DM, Woods AL, Buco J, Peterson RL, Dzink-Fox J, Walker S, Dean CR. 2009. Fmt bypass in Pseudomonas aeruginosa causes induction of MexXY efflux pump expression. Antimicrob. Agents Chemother. 53:5015–5021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Morita Y, Tomida J, Kawamura Y. 2012. Primary mechanisms mediating aminoglycoside resistance in the multidrug-resistant Pseudomonas aeruginosa clinical isolate PA7. Microbiology 158:1071–1083 [DOI] [PubMed] [Google Scholar]
- 123. Hay T, Fraud S, Lau CH, Gilmour C, Poole K. 2013. Antibiotic inducibility of the mexXY multidrug efflux operon of Pseudomonas aeruginosa: involvement of the MexZ anti-repressor ArmZ. PLoS One 8:e56858. 10.1371/journal.pone.0056858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Tanaka N, Shuman S. 2011. RtcB is the RNA ligase component of an Escherichia coli RNA repair operon. J. Biol. Chem. 286:7727–7731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Baranov PV, Vestergaard B, Hamelryck T, Gesteland RF, Nyborg J, Atkins JF. 2006. Diverse bacterial genomes encode an operon of two genes, one of which is an unusual class-I release factor that potentially recognizes atypical mRNA signals other than normal stop codons. Biol. Direct 1:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Khokhlov AS, Tovarova II, Borisova LN, Pliner SA, Shevchenko LN, Kornitskaia EI, Ivkina NS, Rapoport IA. 1967. The A-factor, responsible for streptomycin biosynthesis by mutant strains of Actinomyces streptomycini. Dokl. Akad. Nauk. SSSR 177:232–235 [PubMed] [Google Scholar]
- 127. Kitani S, Hoshika M, Nihira T. 2008. Disruption of sscR encoding a gamma-butyrolactone autoregulator receptor in Streptomyces scabies NBRC 12914 affects production of secondary metabolites. Folia Microbiol. (Praha) 53:115–124 [DOI] [PubMed] [Google Scholar]
- 128. Corre C, Song L, O'Rourke S, Chater KF, Challis GL. 2008. 2-Alkyl-4-hydroxymethylfuran-3-carboxylic acids, antibiotic production inducers discovered by Streptomyces coelicolor genome mining. Proc. Natl. Acad. Sci. U. S. A. 105:17510–17515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. D'Alia D, Eggle D, Nieselt K, Hu WS, Breitling R, Takano E. 2011. Deletion of the signalling molecule synthase ScbA has pleiotropic effects on secondary metabolite biosynthesis, morphological differentiation and primary metabolism in Streptomyces coelicolor A3(2). Microb. Biotechnol. 4:239–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Takano E. 2006. Gamma-butyrolactones: Streptomyces signalling molecules regulating antibiotic production and differentiation. Curr. Opin. Microbiol. 9:287–294 [DOI] [PubMed] [Google Scholar]
- 131. Gottelt M, Kol S, Gomez-Escribano JP, Bibb M, Takano E. 2010. Deletion of a regulatory gene within the cpk gene cluster reveals novel antibacterial activity in Streptomyces coelicolor A3(2). Microbiology 156:2343–2353 [DOI] [PubMed] [Google Scholar]
- 132. Wang J, Wang W, Wang L, Zhang G, Fan K, Tan H, Yang K. 2011. A novel role of ‘pseudo'γ-butyrolactone receptors in controlling γ-butyrolactone biosynthesis in Streptomyces. Mol. Microbiol. 82:236–250 [DOI] [PubMed] [Google Scholar]
- 133. Xu G, Wang J, Wang L, Tian X, Yang H, Fan K, Yang K, Tan H. 2010. “Pseudo” gamma-butyrolactone receptors respond to antibiotic signals to coordinate antibiotic biosynthesis. J. Biol. Chem. 285:27440–27448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Onaka H, Nakagawa T, Horinouchi S. 1998. Involvement of two A-factor receptor homologues in Streptomyces coelicolor A3(2) in the regulation of secondary metabolism and morphogenesis. Mol. Microbiol. 28:743–753 [DOI] [PubMed] [Google Scholar]
- 135. Boyer M, Wisniewski-Dyé F. 2009. Cell-cell signalling in bacteria: not simply a matter of quorum. FEMS Microbiol. Ecol. 70:1–19 [DOI] [PubMed] [Google Scholar]
- 136. Michael B, Smith JN, Swift S, Heffron F, Ahmer BM. 2001. SdiA of Salmonella enterica is a LuxR homolog that detects mixed microbial communities. J. Bacteriol. 183:5733–5742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Rutherford ST, van Kessel JC, Shao Y, Bassler BL. 2011. AphA and LuxR/HapR reciprocally control quorum sensing in vibrios. Genes Dev. 25:397–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Kim Y, Kim BS, Park YJ, Choi WC, Hwang J, Kang BS, Oh TK, Choi SH, Kim MH. 2010. Crystal structure of SmcR, a quorum-sensing master regulator of Vibrio vulnificus, provides insight into its regulation of transcription. J. Biol. Chem. 285:14020–14030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. De Silva RS, Kovacikova G, Lin W, Taylor RK, Skorupski K, Kull FJ. 2007. Crystal structure of the Vibrio cholerae quorum-sensing regulatory protein HapR. J. Bacteriol. 189:5683–5691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Lenz DH, Mok KC, Lilley BN, Kulkarni RV, Wingreen NS, Bassler BL. 2004. The small RNA chaperone Hfq and multiple small RNAs control quorum sensing in Vibrio harveyi and Vibrio cholerae. Cell 118:69–82 [DOI] [PubMed] [Google Scholar]
- 141. Tsou AM, Liu Z, Cai T, Zhu J. 2011. The VarS/VarA two-component system modulates the activity of the Vibrio cholerae quorum-sensing transcriptional regulator HapR. Microbiology 157:1620–1628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Zhang L, Li W, He ZG. 2013. DarR, a TetR-like transcriptional factor, is a cyclic-di-AMP responsive repressor in Mycobacterium smegmatis. J. Biol. Chem. 288:3085–3096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Poelarends J, Kulakov A, Larkin J, JET van Hylckama Vlieg Janssen B. 2000. Roles of horizontal gene transfer and gene integration in evolution of 1,3-dichloropropene- and 1,2-dibromoethane-degradative pathways. J. Bacteriol. 182:2191–2199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Providenti MA, Shaye RE, Lynes KD, McKenna NT, JM O'brien, Rosolen S, Wyndham RC, Lambert IB. 2006. The locus coding for the 3-nitrobenzoate dioxygenase of Comamonas sp. strain JS46 is flanked by IS1071 elements and is subject to deletion and inversion events. Appl. Environ. Microbiol. 72:2651–2660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Krug A, Wendisch VF, Bott M. 2005. Identification of AcnR, a TetR-type repressor of the aconitase gene acn in Corynebacterium glutamicum. J. Biol. Chem. 280:585–595 [DOI] [PubMed] [Google Scholar]
- 146. Garcia-Nafria J, Baumgart M, Turkenburg JP, Wilkinson AJ, Bott M, Wilson KS. 2013. Crystal and solution studies reveal that the transcriptional regulator AcnR of Corynebacterium glutamicum is regulated by citrate:Mg2+ binding to a non-canonical pocket. J. Biol. Chem. 288:15800–15812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Park H, Ro YT, Kim YM. 2011. MdoR is a novel positive transcriptional regulator for the oxidation of methanol in Mycobacterium sp. strain JC1. J. Bacteriol. 193:6288–6294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Jiménez JI, Juárez JF, García JL, Díaz E. 2011. A finely tuned regulatory circuit of the nicotinic acid degradation pathway in Pseudomonas putida. Environ. Microbiol. 13:1718–1732 [DOI] [PubMed] [Google Scholar]
- 149. Li T, Zhao K, Huang Y, Li D, Jiang CY, Zhou N, Fan Z, Liu SJ. 2012. The TetR-type transcriptional repressor RolR from Corynebacterium glutamicum regulates resorcinol catabolism by binding to a unique operator, rolO. Appl. Environ. Microbiol. 78:6009–6016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Sakamoto K, Agari Y, Kuramitsu S, Shinkai A. 2011. Phenylacetyl coenzyme A is an effector molecule of the TetR family transcriptional repressor PaaR from Thermus thermophilus HB8. Bacteriol. 193:4388–4395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Amon J, Titgemeyer F, Burkovski A. 2009. A genomic view on nitrogen metabolism and nitrogen control in mycobacteria. J. Mol. Microbiol. Biotechnol. 17:20–29 [DOI] [PubMed] [Google Scholar]
- 152. Fink D, Weissschuh N, Reuther J, Wohlleben W, Engels A. 2002. Two transcriptional regulators GlnR and GlnRII are involved in regulation of nitrogen metabolism in Streptomyces coelicolor A3(2). Mol. Microbiol. 46:331–347 [DOI] [PubMed] [Google Scholar]
- 153. Shimada T, Hirao K, Kori A, Yamamoto K, Ishihama A. 2007. RutR is the uracil/thymine-sensing master regulator of a set of genes for synthesis and degradation of pyrimidines. Mol. Microbiol. 66:744–757 [DOI] [PubMed] [Google Scholar]
- 154. Hidese R, Mihara H, Kurihara T, Esaki N. 2012. Pseudomonas putida PydR, a RutR-like transcriptional regulator, represses the dihydropyrimidine dehydrogenase gene in the pyrimidine reductive catabolic pathway. J. Biochem. 152:341–346 [DOI] [PubMed] [Google Scholar]
- 155. Nguyen Ple M, Bervoets I, Maes D, Charlier D. 2010. The protein-DNA contacts in RutR · carAB operator complexes. Nucleic Acids Res. 38:6286–6300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Hillerich B, Westpheling J. 2008. A new TetR family transcriptional regulator required for morphogenesis in Streptomyces coelicolor. J. Bacteriol. 190:61–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Nickel J, Irzik K, van Ooyen J, Eggeling L. 2010. The TetR-type transcriptional regulator FasR of Corynebacterium glutamicum controls genes of lipid synthesis during growth on acetate. Mol. Microbiol. 78:253–265 [DOI] [PubMed] [Google Scholar]
- 158. Chen CH, Cheng JC, Cho YC, Hsu WH. 2005. A gene cluster for the fatty acid catabolism from Pseudonocardia autotrophica BCRC12444. Biochem. Biophys. Res. Commun. 329:863–868 [DOI] [PubMed] [Google Scholar]
- 159. Chen L, Chen J, Jiang Y, Zhang W, Jiang W, Lu Y. 2009. Transcriptomics analyses reveal global roles of the regulator AveI in Streptomyces avermitilis. FEMS Microbiol. Lett. 298:199–207 [DOI] [PubMed] [Google Scholar]
- 160. Lee HN, Huang J, Im JH, Kim SH, Noh JH, Cohen SN, Kim ES. 2010. Putative TetR family transcriptional regulator SCO1712 encodes an antibiotic downregulator in Streptomyces coelicolor. Appl. Environ. Microbiol. 76:3039–3043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Matsuoka H, Hirooka K, Fujita Y. 2007. Organization and function of the YsiA regulon of Bacillus subtilis involved in fatty acid degradation. J. Biol. Chem. 282:5180–5194 [DOI] [PubMed] [Google Scholar]
- 162. Anand S, Singh V, Singh AK, Mittal M, Datt M, Subramani B, Kumaran S. 2012. Equilibrium binding and kinetic characterization of putative tetracycline repressor family transcription regulator Fad35R from Mycobacterium tuberculosis. FEBS. J. 279:3214–3228 [DOI] [PubMed] [Google Scholar]
- 163. Kang Y, Nguyen DT, Son MS, Hoang TT. 2008. The Pseudomonas aeruginosa PsrA responds to long-chain fatty acid signals to regulate the fadBA5 beta-oxidation operon. Microbiology 154:1584–1598 [DOI] [PubMed] [Google Scholar]
- 164. Zhang YM, Marrakchi H, Rock CO. 2002. The FabR (YijC) transcription factor regulates unsaturated fatty acid biosynthesis in Escherichia coli. J. Biol. Chem. 277:15558–15565 [DOI] [PubMed] [Google Scholar]
- 165. Feng Y, Cronan JE. 2011. Complex binding of the FabR repressor of bacterial unsaturated fatty acid biosynthesis to its cognate promoters. Mol. Microbiol. 80:195–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Zhang YM, Zhu K, Frank MW, Rock CO. 2007. A Pseudomonas aeruginosa transcription factor that senses fatty acid structure. Mol. Microbiol. 66:622–632 [DOI] [PubMed] [Google Scholar]
- 167. de Eugenio LI, Galán B, Escapa IF, Maestro B, Sanz JM, García JL, Prieto MA. 2010. The PhaD regulator controls the simultaneous expression of the pha genes involved in polyhydroxyalkanoate metabolism and turnover in Pseudomonas putida KT2442. Environ. Microbiol. 12:1591–1603 [DOI] [PubMed] [Google Scholar]
- 168. Rehm BH. 2010. Bacterial polymers: biosynthesis, modifications and applications. Nat. Rev. Microbiol. 8:578–592 [DOI] [PubMed] [Google Scholar]
- 169. Kendall SL, Burgess P, Balhana R, Withers M, Ten Bokum A, Lott JS, Gao C, Uhia-Castro I, Stoker NG. 2010. Cholesterol utilization in mycobacteria is controlled by two TetR-type transcriptional regulators: kstR and kstR2. Microbiology 156:1362–1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Kendall SL, Withers M, Soffair CN, Moreland NJ, Gurcha S, Sidders B, Frita R, Ten Bokum A, Besra GS, Lott JS, Stoker NG. 2007. A highly conserved transcriptional repressor controls a large regulon involved in lipid degradation in Mycobacterium smegmatis and Mycobacterium tuberculosis. Mol. Microbiol. 65:684–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Uhía I, Galán B, Kendall SL, Stoker NG, García JL. 2012. Cholesterol metabolism in Mycobacterium smegmatis. Environ. Microbiol. Rep. 4:168–182 [DOI] [PubMed] [Google Scholar]
- 172. Van der Geize R, Yam K, Heuser T, Wilbrink MH, Hara H, Anderton MC, Sim E, Dijkhuizen L, Davies JE, Mohn WW, Eltis LD. 2007. A gene cluster encoding cholesterol catabolism in a soil actinomycete provides insight into Mycobacterium tuberculosis survival in macrophages. Proc. Natl. Acad. Sci. U. S. A. 104:1947–1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Rengarajan J, Bloom BR, Rubin EJ. 2005. Genome-wide requirements for Mycobacterium tuberculosis adaptation and survival in macrophages. Proc. Natl. Acad. Sci. U. S. A. 102:8327–8332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Förster-Fromme K, Jendrossek D. 2010. AtuR is a repressor of acyclic terpene utilization (atu) gene cluster expression and specifically binds to two 13 bp inverted repeat sequences of the atuA-atuR intergenic region. FEMS Microbiol. Lett. 308:166–174 [DOI] [PubMed] [Google Scholar]
- 175. Eaton RW. 1996. p-Cumate catabolic pathway in Pseudomonas putida Fl: cloning and characterization of DNA carrying the cmt operon. J. Bacteriol. 178:1351–1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Eaton RW. 1997. p-Cymene catabolic pathway in Pseudomonas putida F1: cloning and characterization of DNA encoding conversion of p-cymene to p-cumate. J. Bacteriol. 179:3171–3180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Grogan G, Roberts GA, Bougioukou D, Turner NJ, Flitsch SL. 2001. The desymmetrization of bicyclic beta-diketones by an enzymatic retro-Claisen reaction. A new reaction of the crotonase superfamily. J. Biol. Chem. 276:12565–12572 [DOI] [PubMed] [Google Scholar]
- 178. Lee K, Ryu EK, Choi KS, Cho MC, Jeong JJ, Choi EN, Lee SO, Yoon DY, Hwang I, Kim CK. 2006. Identification and expression of the cym, cmt, and tod catabolic genes from Pseudomonas putida KL47: expression of the regulatory todST genes as a factor for catabolic adaptation. J. Microbiol. 44:192–199 [PubMed] [Google Scholar]
- 179. Puskás LG, Inui M, Kele Z, Yukawa H. 2000. Cloning of genes participating in aerobic biodegradation of p-cumate from Rhodopseudomonas palustris. DNA Seq. 11:9–20 [DOI] [PubMed] [Google Scholar]
- 180. Satriano J. 2003. Agmatine: at the crossroads of the arginine pathways. Ann. N. Y. Acad. Sci. 1009:34–43 [DOI] [PubMed] [Google Scholar]
- 181. Nakada Y, Jiang Y, Nishijyo T, Itoh Y, Lu CD. 2001. Molecular characterization and regulation of the aguBA operon, responsible for agmatine utilization in Pseudomonas aeruginosa. PAO1. J. Bacteriol. 183:6517–6524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Williams BJ, Du RH, Calcutt MW, Abdolrasulnia R, Christman BW, Blackwell TS. 2010. Discovery of an operon that participates in agmatine metabolism and regulates biofilm formation in Pseudomonas aeruginosa. Mol. Microbiol. 76:104–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Kazakov AE, Rodionov DA, Alm E, Arkin AP, Dubchak I, Gelfand MS. 2009. Comparative genomics of regulation of fatty acid and branched-chain amino acid utilization in proteobacteria. J. Bacteriol. 191:52–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Rey DA, Pühler A, Kalinowski J. 2003. The putative transcriptional repressor McbR, member of the TetR-family, is involved in the regulation of the metabolic network directing the synthesis of sulfur containing amino acids in Corynebacterium glutamicum. J. Biotechnol. 103:51–65 [DOI] [PubMed] [Google Scholar]
- 185. Rey DA, Nentwich SS, Koch DJ, Rückert C, Pühler A, Tauch A, Kalinowski J. 2005. The McbR repressor modulated by the effector substance S-adenosylhomocysteine controls directly the transcription of a regulon involved in sulphur metabolism of Corynebacterium glutamicum ATCC 13032. Mol. Microbiol. 56:871–887 [DOI] [PubMed] [Google Scholar]
- 186. Krömer JO, Bolten CJ, Heinzle E, Schröder H, Wittmann C. 2008. Physiological response of Corynebacterium glutamicum to oxidative stress induced by deletion of the transcriptional repressor McbR. Microbiology 154:3917–3930 [DOI] [PubMed] [Google Scholar]
- 187. Howard PK, Shaw J, Otsuka AJ. 1985. Nucleotide sequence of the birA gene encoding the biotin operon repressor and biotin holoenzyme synthetase functions of Escherichia coli. Gene 35:321–331 [DOI] [PubMed] [Google Scholar]
- 188. Rodionov DA, Gelfand MS. 2006. Computational identification of BioR, a transcriptional regulator of biotin metabolism in Alphaproteobacteria, and of its binding signal. FEMS Microbiol. Lett. 255:102–107 [DOI] [PubMed] [Google Scholar]
- 189. Brune I, Götker S, Schneider J, Rodionov DA, Tauch A. 2012. Negative transcriptional control of biotin metabolism genes by the TetR-type regulator BioQ in biotin-auxotrophic Corynebacterium glutamicum ATCC 13032. J. Biotechnol. 159:225–234 [DOI] [PubMed] [Google Scholar]
- 190. Hashimoto Y, Yamashita M, Murooka Y. 1997. The Propionibacterium freudenreichii hemYHBXRL gene cluster, which encodes enzymes and a regulator involved in the biosynthetic pathway from glutamate to protoheme. Appl. Microbiol. Biotechnol. 47:385–392 [DOI] [PubMed] [Google Scholar]
- 191. Lechardeur D, Cesselin B, Liebl U, Vos MH, Fernandez A, Brun C, Gruss A, Gaudu P. 2012. Discovery of intracellular heme-binding protein HrtR, which controls heme efflux by the conserved HrtB-HrtA transporter in Lactococcus lactis. J. Biol. Chem. 287:4752–4758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Cho H, McManus HR, Dove SL, Bernhardt TG. 2011. Nucleoid occlusion factor SlmA is a DNA-activated FtsZ polymerization antagonist. Proc. Natl. Acad. Sci. U. S. A. 108:3773–3778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Tonthat NK, Arold ST, Pickering BF, Van Dyke MW, Liang S, Lu Y, Beuria TK, Margolin W, Schumacher MA. 2011. Molecular mechanism by which the nucleoid occlusion factor, SlmA, keeps cytokinesis in check. EMBO. J. 30:154–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Wu LJ, Errington J. 2012. Nucleoid occlusion and bacterial cell division. Nat. Rev. Microbiol. 10:8–12 [DOI] [PubMed] [Google Scholar]
- 195. Wagner-Herman JK, Bernard R, Dunne R, Bisson-Filho AW, Kumar K, Nyguen T, Mulcahy L, Koullias J, Gueiros-Filho FJ, Rudner DZ. 2012. RefZ facilitates the switch from medial to polar division during spore formation in Bacillus subtilis. J. Bacteriol. 194:4608–4618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Martin RG, Rosner JL. 2001. The AraC transcriptional activators. Curr. Opin. Microbiol. 4:132–137 [DOI] [PubMed] [Google Scholar]
- 197. Cherney LT, Cherney MM, Garen CR, Lu GJ, James MN. 2008. Crystal structure of the arginine repressor protein in complex with the DNA operator from Mycobacterium tuberculosis. J. Mol. Biol. 384:1330–1340 [DOI] [PubMed] [Google Scholar]
- 198. Busenlehner LS, Pennella MA, Giedroc DP. 2003. The SmtB/ArsR family of metalloregulatory transcriptional repressors: Structural insights into prokaryotic metal resistance. FEMS Microbiol. Rev. 27:131–143 [DOI] [PubMed] [Google Scholar]
- 199. Yokoyama K, Ishijima SA, Clowney L, Koike H, Aramaki H, Tanaka C, Makino K, Suzuki M. 2006. Feast/famine regulatory proteins (FFRPs): Escherichia coli Lrp, AsnC and related archaeal transcription factors. FEMS Microbiol. Rev. 30:89–108 [DOI] [PubMed] [Google Scholar]
- 200. Körner H, Sofia HJ, Zumft WG. 2003. Phylogeny of the bacterial superfamily of Crp-Fnr transcription regulators: exploiting the metabolic spectrum by controlling alternative gene programs. FEMS Microbiol. Rev. 27:559–592 [DOI] [PubMed] [Google Scholar]
- 201. Zeng G, Ye S, Larson TJ. 1996. Repressor for the sn-glycerol 3-phosphate regulon of Escherichia coli K-12: primary structure and identification of the DNA-binding domain. J. Bacteriol. 178:7080–7089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Pennella MA, Giedroc DP. 2005. Structural determinants of metal selectivity in prokaryotic metal-responsive transcriptional regulators. Biometals 18:413–428 [DOI] [PubMed] [Google Scholar]
- 203. Rigali S, Derouaux A, Giannotta F, Dusart J. 2002. Subdivision of the helix-turn-helix GntR family of bacterial regulators in the FadR, HutC, MocR, and YtrA subfamilies. J. Biol. Chem. 277:12507–12515 [DOI] [PubMed] [Google Scholar]
- 204. Molina-Henares AJ, Krell T, Eugenia Guazzaroni M, Segura A, Ramos JL. 2006. Members of the IclR family of bacterial transcriptional regulators function as activators and/or repressors. FEMS Microbiol. Rev. 30:157–186 [DOI] [PubMed] [Google Scholar]
- 205. Swint-Kruse L, Matthews KS. 2009. Allostery in the LacI/GalR family: variations on a theme. Curr. Opin. Microbiol. 12:129–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Chen J, Xie J. 2011. Role and regulation of bacterial LuxR-like regulators. J. Cell Biochem. 112:2694–2702 [DOI] [PubMed] [Google Scholar]
- 207. Maddocks SE, Oyston PC. 2008. Structure and function of the LysR-type transcriptional regulator (LTTR) family proteins. Microbiology 154:3609–3623 [DOI] [PubMed] [Google Scholar]
- 208. Wilkinson SP, Grove A. 2006. Ligand-responsive transcriptional regulation by members of the MarR family of winged helix proteins. Curr. Issues Mol. Biol. 8:51–62 [PubMed] [Google Scholar]
- 209. Hobman JL, Wilkie J, Brown NL. 2005. A design for life: prokaryotic metal-binding MerR family regulators. Biometals 18:429–436 [DOI] [PubMed] [Google Scholar]
- 210. Self WT, Grunden AM, Hasona A, Shanmugam KT. 1999. Transcriptional regulation of molybdoenzyme synthesis in Escherichia coli in response to molybdenum: ModE-molybdate, a repressor of the modABCD (molybdate transport) operon is a secondary transcriptional activator for the hyc and nar operons. Microbiology 145:41–55 [DOI] [PubMed] [Google Scholar]
- 211. Huillet E, Velge P, Vallaeys T, Pardon P. 2006. LadR, a new PadR-related transcriptional regulator from Listeria monocytogenes, negatively regulates the expression of the multidrug efflux pump MdrL. FEMS Microbiol. Lett. 254:87–94 [DOI] [PubMed] [Google Scholar]
- 212. McDonnell GE, McConnell DJ. 1994. Overproduction, isolation, and DNA-binding characteristics of Xre, the repressor protein from the Bacillus subtilis defective prophage PBSX. J. Bacteriol. 176:5831–5834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Xu Q, van Wezel GP, Chiu HJ, Jaroszewski L, Klock HE, Knuth MW, Miller MD, Lesley SA, Godzik A, Elsliger MA, Deacon AM, Wilson IA. 2012. Structure of an MmyB-like regulator from C. aurantiacus, member of a new transcription factor family linked to antibiotic metabolism in actinomycetes. PLoS One 7:e41359. 10.1371/journal.pone.0041359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Gottardi EM, Krawczyk JM, von Suchodoletz H, Schadt S, Mühlenweg A, Uguru GC, Pelzer S, Fiedler HP, Bibb MJ, Stach JE, Süssmuth RD. 2011. Abyssomicin biosynthesis: formation of an unusual polyketide, antibiotic-feeding studies and genetic analysis. Chembiochem 12:1401–1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Wang X, Tabudravu J, Rateb ME, Annand KJ, Qin Z, Jaspars M, Deng Z, Yu Y, Deng H. 2013. Identification and characterization of the actinomycin G gene cluster in Streptomyces iakyrus. Mol. Biosyst. 9:1286–1289 [DOI] [PubMed] [Google Scholar]
- 216. Johnson TJ, Jordan D, Kariyawasam S, Stell AL, Bell NP, Wannemuehler YM, Alarcón CF, Li G, Tivendale KA, Logue CM, Nolan LK. 2010. Sequence analysis and characterization of a transferable hybrid plasmid encoding multidrug resistance and enabling zoonotic potential for extraintestinal Escherichia coli. Infect. Immun. 78:1931–1942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Curson ARJ, Sullivan MJ, Todd JD, Johnston AWB. 2011. DddY, a periplasmic dimethylsulfoniopropionate lyase found in taxonomically diverse species of Proteobacteria. ISME. J. 5:1191–1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Sullivan MJ, Curson AR, Shearer N, Todd JD, Green RT, Johnston AW. 2011. Unusual regulation of a leaderless operon involved in the catabolism of dimethylsulfoniopropionate in Rhodobacter sphaeroides. PLoS One 6:e15972. 10.1371/journal.pone.0015972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Rosenfeld N, Bouchier C, Courvalin P, Périchon B. 2012. Expression of the resistance-nodulation-cell division pump AdeIJK in Acinetobacter baumannii is regulated by AdeN, a TetR-type regulator. Antimicrob. Agents Chemother. 56:2504–2510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Oja T, Palmu K, Lehmussola H, Leppäranta O, K Hännikäinen, Niemi J, P Mäntsälä, Metsä-Ketelä M. 2008. Characterization of the alnumycin gene cluster reveals unusual gene products for pyran ring formation and dioxan biosynthesis. Chem. Biol. 15:1046–1057 [DOI] [PubMed] [Google Scholar]
- 221. Bunet R, Mendes MV, Rouhier N, Pang X, Hotel L, Leblond P, Aigle B. 2008. Regulation of the synthesis of the angucyclinone antibiotic alpomycin in Streptomyces ambofaciens by the autoregulator receptor AlpZ and its specific ligand. J. Bacteriol. 190:3293–3305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Bunet R, Song L, Mendes MV, Corre C, Hotel L, Rouhier N, Framboisier X, Leblond P, Challis GL, Aigle B. 2011. Characterization and manipulation of the pathway-specific late regulator AlpW reveals Streptomyces ambofaciens as a new producer of kinamycins. J. Bacteriol. 193:1142–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Peng WT, Nester EW. 2001. Characterization of a putative RND-type efflux system in Agrobacterium tumefaciens. Gene 270:245–252 [DOI] [PubMed] [Google Scholar]
- 224. Zhang H, Wang H, Wang Y, Cui H, Xie Z, Pu Y, Pei S, Li F, Qin S. 2012. Genomic sequence-based discovery of novel angucyclinone antibiotics from marine Streptomyces sp. W007. FEMS Microbiol. Lett. 332:105–112 [DOI] [PubMed] [Google Scholar]
- 225. Onaka H, Ando N, Nihira T, Yamada Y, Beppu T, Horinouchi S. 1995. Cloning and characterization of the A-factor receptor gene from Streptomyces griseus. J. Bacteriol. 177:6083–6092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Kieboom J, de Bont J. 2001. Identification and molecular characterization of an efflux system involved in Pseudomonas putida S12 multidrug resistance. Microbiology 147:43–51 [DOI] [PubMed] [Google Scholar]
- 227. Ng D, Chin HK, Wong VV. 2009. Constitutive overexpression of asm2 and asm39 increases AP-3 production in the actinomycete Actinosynnema pretiosum. J. Ind. Microbiol. Biotechnol. 36:1345–1351 [DOI] [PubMed] [Google Scholar]
- 228. Novakova R, Bistakova J, Homerova D, Rezuchova B, Kormanec J. 2002. Cloning and characterization of a polyketide synthase gene cluster involved in biosynthesis of a proposed angucycline-like polyketide auricin in Streptomyces aureofaciens CCM 3239. Gene 297:197–208 [DOI] [PubMed] [Google Scholar]
- 229. Novakova R, Kutas P, Feckova L, Kormanec J. 2010. The role of the TetR-family transcriptional regulator Aur1R in negative regulation of the auricin gene cluster in Streptomyces aureofaciens CCM 3239. Microbiology 156:2374–2383 [DOI] [PubMed] [Google Scholar]
- 230. Miyamoto KT, Kitani S, Komatsu M, Ikeda H, Nihira T. 2011. The autoregulator receptor homologue AvaR3 plays a regulatory role in antibiotic production, mycelial aggregation and colony development of Streptomyces avermitilis. Microbiology 157:2266–2275 [DOI] [PubMed] [Google Scholar]
- 231. Chen L, Lu Y, Chen J, Zhang W, Shu D, Qin Z, Yang S, Jiang W. 2008. Characterization of a negative regulator AveI for avermectin biosynthesis in Streptomyces avermitilis NRRL8165. Appl. Microbiol. Biotechnol. 80:277–286 [DOI] [PubMed] [Google Scholar]
- 232. Zhao Q, He Q, Ding W, Tang M, Kang Q, Yu Y, Deng W, Zhang Q, Fang J, Tang G, Liu W. 2008. Characterization of the azinomycin B biosynthetic gene cluster revealing a different iterative type I polyketide synthase for naphthoate biosynthesis. Chem. Biol. 15:693–705 [DOI] [PubMed] [Google Scholar]
- 233. Okamoto S, Nakamura K, Nihira T, Yamada Y. 1995. Virginiae butanolide binding protein from Streptomyces virginiae. Evidence that VbrA is not the virginiae butanolide binding protein and reidentification of the true binding protein. J. Biol. Chem. 270:12319–12326 [DOI] [PubMed] [Google Scholar]
- 234. Matsuno K, Yamada Y, Lee CK, Nihira T. 2004. Identification by gene deletion analysis of barB as a negative regulator controlling an early process of virginiamycin biosynthesis in Streptomyces virginiae. Arch. Microbiol. 181:52–59 [DOI] [PubMed] [Google Scholar]
- 235. Kawachi R, Akashi T, Kamitani Y, Sy A, Wangchaisoonthorn U, Nihira T, Yamada Y. 2000. Identification of an AfsA homologue (BarX) from Streptomyces virginiae as a pleiotropic regulator controlling autoregulator biosynthesis, virginiamycin biosynthesis and virginiamycin M1 resistance. Mol. Microbiol. 36:302–313 [DOI] [PubMed] [Google Scholar]
- 236. Jørgensen H, Degnes KF, Sletta H, Fjaervik E, Dikiy A, Herfindal L, Bruheim P, Klinkenberg G, Bredholt H, Nygård G, Døskeland SO, Ellingsen TE, Zotchev SB. 2009. Biosynthesis of macrolactam BE-14106 involves two distinct PKS systems and amino acid processing enzymes for generation of the aminoacyl starter unit. Chem. Biol. 16:1109–1121 [DOI] [PubMed] [Google Scholar]
- 237. Martin FA, Posadas DM, Carrica MC, Cravero SL, O'Callaghan D, Zorreguieta A. 2009. Interplay between two RND systems mediating antimicrobial resistance in Brucella suis. J. Bacteriol. 191:2530–2540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Rkenes TP, Lamark T, Strøm AR. 1996. DNA-binding properties of the BetI repressor protein of Escherichia coli: the inducer choline stimulates BetI-DNA complex formation. J. Bacteriol. 178:1663–1670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Chan YY, Tan TM, Ong YM, Chua KL. 2004. BpeAB-OprB, a multidrug efflux pump in Burkholderia pseudomallei. Antimicrob. Agents Chemother. 48:1128–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Santamarta I, Pérez-Redondo R, Lorenzana LM, Martín JF, Liras P. 2005. Different proteins bind to the butyrolactone receptor protein ARE sequence located upstream of the regulatory ccaR gene of Streptomyces clavuligerus. Mol. Microbiol. 56:824–835 [DOI] [PubMed] [Google Scholar]
- 241. Schwartz KT, Carleton JD, Quillin SJ, Rollins SD, Portnoy DA, Leber JH. 2012. Hyper-induction of host beta interferon by a Listeria monocytogenes strain naturally over-expressing the multi-drug efflux pump MdrT. Infect. Immun. 80:1537–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Sun GW, Chen Y, Liu Y, Tan GY, Ong C, Tan P, Gan YH. 2010. Identification of a regulatory cascade controlling type III secretion system 3 gene expression in Burkholderia pseudomallei. Mol. Microbiol. 76:677–689 [DOI] [PubMed] [Google Scholar]
- 243. Kudo F, Numakura M, Tamegai H, Yamamoto H, Eguchi T, Kakinuma K. 2005. Extended sequence and functional analysis of the butirosin biosynthetic gene cluster in Bacillus circulans SANK 72073. J. Antibiot. (Tokyo) 58:373–379 [DOI] [PubMed] [Google Scholar]
- 244. Ahlert J, Shepard E, Lomovskaya N, Zazopoulos E, Staffa A, Bachmann BO, Huang K, Fonstein L, Czisny A, Whitwam RE, Farnet CM, Thorson JS. 2002. The calicheamicin gene cluster and its iterative type I enediyne PKS. Science 297:1173–1176 [DOI] [PubMed] [Google Scholar]
- 245. Koga H, Aramaki H, Yamaguchi E, Takeuchi K, Horiuchi T, Gunsalus IC. 1986. camR, a negative regulator locus of the cytochrome P-450cam hydroxylase operon. J. Bacteriol. 166:1089–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Xi C, Schoeters E, Vanderleyden J, Michiels J. 2000. Symbiosis-specific expression of Rhizobium etli casA encoding a secreted calmodulin-related protein. Proc. Natl. Acad. Sci. U. S. A. 97:11114–11119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Itou H, Watanabe N, Yao M, Shirakihara Y, Tanaka I. 2010. Crystal structures of the multidrug binding repressor Corynebacterium glutamicum CgmR in complex with inducers and with an operator. J. Mol. Biol. 403:174–184 [DOI] [PubMed] [Google Scholar]
- 248. Jia XY, Tian ZH, Shao L, Qu XD, Zhao QF, Tang J, Tang GL, Liu W. 2006. Genetic characterization of the chlorothricin gene cluster as a model for spirotetronate antibiotic biosynthesis. Chem. Biol. 13:575–585 [DOI] [PubMed] [Google Scholar]
- 249. Kharel MK, Subba B, Basnet DB, Woo JS, Lee HC, Liou K, Sohng JK. 2004. A gene cluster for biosynthesis of kanamycin from Streptomyces kanamyceticus: comparison with gentamicin biosynthetic gene cluster. Arch. Biochem. Biophys. 429:204–214 [DOI] [PubMed] [Google Scholar]
- 250. MacEachran DP, Stanton BA, O'Toole GA. 2008. Cif is negatively regulated by the TetR family repressor CifR. Infect. Immun. 76:3197–3206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Takano E, Kinoshita H, Mersinias V, Bucca G, Hotchkiss G, Nihira T, Smith CP, Bibb M, Wohlleben W, Chater K. 2005. A bacterial hormone (the SCB1) directly controls the expression of a pathway-specific regulatory gene in the cryptic type I polyketide biosynthetic gene cluster of Streptomyces coelicolor. Mol. Microbiol. 56:465–479 [DOI] [PubMed] [Google Scholar]
- 252. Todd JD, Curson AR, Nikolaidou-Katsaraidou N, Brearley CA, Watmough NJ, Chan Y, Page PC, Sun L, Johnston AW. 2010. Molecular dissection of bacterial acrylate catabolism—unexpected links with dimethylsulfoniopropionate catabolism and dimethyl sulfide production. Environ. Microbiol. 12:327–343 [DOI] [PubMed] [Google Scholar]
- 253. Lee LF, Huang YJ, Chen CW. 2003. Repressed multidrug resistance genes in Streptomyces lividans. Arch. Microbiol. 180:176–184 [DOI] [PubMed] [Google Scholar]
- 254. Lee LF, Chen YJ, Kirby R, Chen C, Chen CW. 2007. A multidrug efflux system is involved in colony growth in Streptomyces lividans. Microbiology 153:924–934 [DOI] [PubMed] [Google Scholar]
- 255. Watanabe K, Hotta K, Praseuth AP, Koketsu K, Migita A, Boddy CN, Wang CC, Oguri H, Oikawa H. 2006. Total biosynthesis of antitumor nonribosomal peptides in Escherichia coli. Nat. Chem. Biol. 2:423–428 [DOI] [PubMed] [Google Scholar]
- 256. Hearn EM, Dennis JJ, Gray MR, Foght JM. 2003. Identification and characterization of the emhABC efflux system for polycyclic aromatic hydrocarbons in Pseudomonas fluorescens cLP6a. J. Bacteriol. 185:6233–6240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Tian T, Wu XG, Duan HM, Zhang LQ. 2010. The resistance-nodulation-division efflux pump EmhABC influences the production of 2,4-diacetylphloroglucinol in Pseudomonas fluorescens 2P24. Microbiology 156:39–48 [DOI] [PubMed] [Google Scholar]
- 258. Piel J, Hertweck C, Shipley PR, Hunt DM, Newman MS, Moore BS. 2000. Cloning, sequencing and analysis of the enterocin biosynthesis gene cluster from the marine isolate ‘Streptomyces maritimus': evidence for the derailment of an aromatic polyketide synthase. Chem. Biol. 7:943–955 [DOI] [PubMed] [Google Scholar]
- 259. Hirakawa H, Takumi-Kobayashi A, Theisen U, Hirata T, Nishino K, Yamaguchi A. 2008. AcrS/EnvR represses expression of the acrAB multidrug efflux genes in Escherichia coli. J. Bacteriol. 190:6276–6279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Rodríguez-García A, Santamarta I, Pérez-Redondo R, Martín JF, Liras P. 2006. Characterization of a two-gene operon epeRA involved in multidrug resistance in Streptomyces clavuligerus. Res. Microbiol. 157:559–568 [DOI] [PubMed] [Google Scholar]
- 261. Rui Z, Ye M, Wang S, Fujikawa K, Akerele B, Aung M, Floss HG, Zhang W, Yu TW. 2012. Insights into a divergent phenazine biosynthetic pathway governed by a plasmid-born esmeraldin gene cluster. Chem. Biol. 19:1116–1125 [DOI] [PubMed] [Google Scholar]
- 262. Waki M, Nihira T, Yamada Y. 1997. Cloning and characterization of the gene (farA) encoding the receptor for an extracellular regulatory factor (IM-2) from Streptomyces sp. strain FRI-5. J. Bacteriol. 179:5131–5137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Wenzel M, Lang K, T Günther, Bhandari A, Weiss A, Lulchev P, Szentgyörgyi E, Kranzusch B, Göttfert M. 2012. Characterization of the flavonoid-responsive regulator FrrA and its binding sites. J. Bacteriol. 194:2363–2370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Jobling MG, Holmes RK. 1997. Characterization of hapR, a positive regulator of the Vibrio cholerae HA/protease gene hap, and its identification as a functional homologue of the Vibrio harveyi luxR gene. Mol. Microbiol. 26:1023–1034 [DOI] [PubMed] [Google Scholar]
- 265. Budarina ZI, Nikitin DV, Zenkin N, Zakharova M, Semenova E, Shlyapnikov MG, Rodikova EA, Masyukova S, Ogarkov O, Baida GE, Solonin AS, Severinov K. 2004. A new Bacillus cereus DNA-binding protein, HlyIIR, negatively regulates expression of B. cereus haemolysin II. Microbiology 150:3691–3701 [DOI] [PubMed] [Google Scholar]
- 266. Sandu C, Chiribau CB, Brandsch R. 2003. Characterization of HdnoR, the transcriptional repressor of the 6-hydroxy-d-nicotine oxidase gene of Arthrobacter nicotinovorans pAO1, and its DNA-binding activity in response to l- and d-nicotine derivatives. J. Biol. Chem. 278:51307–51315 [DOI] [PubMed] [Google Scholar]
- 267. Conlon KM, Humphreys H, O'Gara JP. 2002. icaR encodes a transcriptional repressor involved in environmental regulation of ica operon expression and biofilm formation in Staphylococcus epidermidis. J. Bacteriol. 184:4400–4408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Palumbo JD, Kado CI, Phillips DA. 1998. An isoflavonoid-inducible efflux pump in Agrobacterium tumefaciens is involved in competitive colonization of roots. J. Bacteriol. 180:3107–3113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Wang L, White RL, Vining LC. 2002. Biosynthesis of the dideoxysugar component of jadomycin B: genes in the jad cluster of Streptomyces venezuelae ISP5230 for l-digitoxose assembly and transfer to the angucycline aglycone. Microbiology 148:1091–1103 [DOI] [PubMed] [Google Scholar]
- 270. Yang K, Han L, Vining LC. 1995. Regulation of jadomycin B production in Streptomyces venezuelae ISP5230: involvement of a repressor gene, jadR2. J. Bacteriol. 177:6111–6117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Kharel MK, Nybo SE, Shepherd MD, Rohr J. 2010. Cloning and characterization of the ravidomycin and chrysomycin biosynthetic gene clusters. Chembiochem 11:523–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Zhang H, White-Phillip JA, Melançon CE, Kwon HJ, Yu WL, Liu HW. 2007. Elucidation of the kijanimicin gene cluster: insights into the biosynthesis of spirotetronate antibiotics and nitrosugars. J. Am. Chem. Soc. 129:14670–14683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Gould SJ, Hong ST, Carney JR. 1998. Cloning and heterologous expression of genes from the kinamycin biosynthetic pathway of Streptomyces murayamaensis. J. Antibiot. (Tokyo) 51:50–57 [DOI] [PubMed] [Google Scholar]
- 274. Weber T, Laiple KJ, Pross EK, Textor A, Grond S, Welzel K, Pelzer S, Vente A, Wohlleben W. 2008. Molecular analysis of the kirromycin biosynthetic gene cluster revealed beta-alanine as precursor of the pyridone moiety. Chem. Biol. 15:175–188 [DOI] [PubMed] [Google Scholar]
- 275. Choi SU, Kim MK, Ha HS, Hwang YI. 2008. In vivo functions of the gamma-butyrolactone autoregulator receptor in Streptomyces ambofaciens producing spiramycin. Biotechnol. Lett. 30:891–897 [DOI] [PubMed] [Google Scholar]
- 276. Zhang X, Alemany LB, Fiedler HP, Goodfellow M, Parry RJ. 2008. Biosynthetic investigations of lactonamycin and lactonamycin z: cloning of the biosynthetic gene clusters and discovery of an unusual starter unit. Antimicrob. Agents Chemother. 52:574–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Fidopiastis PM, Miyamoto CM, Jobling MG, Meighen EA, Ruby EG. 2002. LitR, a new transcriptional activator in Vibrio fischeri, regulates luminescence and symbiotic light organ colonization. Mol. Microbiol. 45:131–143 [DOI] [PubMed] [Google Scholar]
- 278. Si D, Urano N, Shimizu S, Kataoka M. 2012. LplR, a repressor belonging to the TetR family, regulates expression of the l-pantoyl lactone dehydrogenase gene in Rhodococcus erythropolis. Appl. Environ. Microbiol. 78:7923–7930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Miyamoto CM, Chatterjee J, Swartzman E, Szittner R, Meighen EA. 1996. The role of lux autoinducer in regulating luminescence in Vibrio harveyi; control of luxR expression. Mol. Microbiol. 19:767–775 [DOI] [PubMed] [Google Scholar]
- 280. Lin YH, Miyamoto C, Meighen EA. 2000. Cloning and functional studies of a luxO regulator LuxT from Vibrio harveyi. Biochim. Biophys. Acta 1494:226–235 [DOI] [PubMed] [Google Scholar]
- 281. de la Paz Santangelo M, Klepp L, Nuñez-García J, Blanco FC, Soria M, García-Pelayo MC, Bianco MV, Cataldi AA, Golby P, Jackson M, Gordon SV, Bigi F. 2009. Mce3R, a TetR-type transcriptional repressor, controls the expression of a regulon involved in lipid metabolism in Mycobacterium tuberculosis. Microbiology 155:2245–2255 [DOI] [PubMed] [Google Scholar]
- 282. Ichinose K. 2003. Cloning, sequencing and heterologous expression of the medermycin biosynthetic gene cluster of Streptomyces sp. AM-7161: towards comparative analysis of the benzoisochromanequinone gene clusters. Microbiology 149:1633–1645 [DOI] [PubMed] [Google Scholar]
- 283. Fukumori F, Hirayama H, Takami H, Inoue A, Horikoshi K. 1998. Isolation and transposon mutagenesis of a Pseudomonas putida KT2442 toluene-resistant variant: involvement of an efflux system in solvent resistance. Extremophiles 2:395–400 [DOI] [PubMed] [Google Scholar]
- 284. He M, Haltli B, Summers M, Feng X, Hucul J. 2006. Isolation and characterization of meridamycin biosynthetic gene cluster from Streptomyces sp. NRRL 30748. Gene 377:109–118 [DOI] [PubMed] [Google Scholar]
- 285. Chuanchuen R, Gaynor JB, Karkhoff-Schweizer R, Schweizer HP. 2005. Molecular characterization of MexL, the transcriptional repressor of the mexJK multidrug efflux operon in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 49:1844–1851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Westbrock-Wadman S, Sherman DR, Hickey MJ, Coulter SN, Zhu YQ, Warrener P, Nguyen LY, Shawar RM, Folger KR, Stover CK. 1999. Characterization of a Pseudomonas aeruginosa efflux pump contributing to aminoglycoside impermeability. Antimicrob. Agents Chemother. 43:2975–2983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. O'Rourke S, Wietzorrek A, Fowler K, Corre C, Challis GL, Chater KF. 2009. Extracellular signalling, translational control, two repressors and an activator all contribute to the regulation of methylenomycin production in Streptomyces coelicolor. Mol. Microbiol. 71:763–778 [DOI] [PubMed] [Google Scholar]
- 288. Aguilar A, Hopwood DA. 1982. Determination of methylenomycin A synthesis by the pSV1 plasmid from Streptomyces violaceusruber SANK 95570. J. Gen. Microbiol. 128:1893–1901 [DOI] [PubMed] [Google Scholar]
- 289. Oliynyk M, Stark CB, Bhatt A, Jones MA, Hughes-Thomas ZA, Wilkinson C, Oliynyk Z, Demydchuk Y, Staunton J, Leadlay PF. 2003. Analysis of the biosynthetic gene cluster for the polyether antibiotic monensin in Streptomyces cinnamonensis and evidence for the role of monB and monC genes in oxidative cyclization. Mol. Microbiol. 49:1179–1190 [DOI] [PubMed] [Google Scholar]
- 290. Yang M, Gao C, Cui T, An J, He ZG. 2012. A TetR-like regulator broadly affects the expressions of diverse genes in Mycobacterium smegmatis. Nucleic Acids. Res. 40:1009–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Hagman KE, Shafer WM. 1995. Transcriptional control of the mtr efflux system of Neisseria gonorrhoeae. J. Bacteriol. 177:4162–4165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. Cao L, Srikumar R, Poole K. 2004. MexAB-OprM hyperexpression in NalC-type multidrug-resistant Pseudomonas aeruginosa: identification and characterization of the nalC gene encoding a repressor of PA3720-PA3719. Mol. Microbiol. 53:1423–1436 [DOI] [PubMed] [Google Scholar]
- 293. Daigle DM, Cao L, Fraud S, Wilke MS, Pacey A, Klinoski R, Strynadka NC, Dean CR, Poole K. 2007. Protein modulator of multidrug efflux gene expression in Pseudomonas aeruginosa. J. Bacteriol. 189:5441–5451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Ghosh S, Cremers CM, Jakob U, Love NG. 2011. Chlorinated phenols control the expression of the multidrug resistance efflux pump MexAB-OprM in Pseudomonas aeruginosa by interacting with NalC. Mol. Microbiol. 79:1547–1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Starr LM, Fruci M, Poole K. 2012. Pentachlorophenol Induction of the Pseudomonas aeruginosa mexAB-oprM Efflux Operon: involvement of Repressors NalC and MexR and the Antirepressor ArmR. PLoS One 7:e32684. 10.1371/journal.pone.0032684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296. Morita Y, Sobel ML, Poole K. 2006. Antibiotic inducibility of the MexXY multidrug efflux system of Pseudomonas aeruginosa: involvement of the antibiotic-inducible PA5471 gene product. J. Bacteriol. 188:1847–1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Winter JM, Moffitt MC, Zazopoulos E, McAlpine JB, Dorrestein PC, Moore BS. 2007. Molecular basis for chloronium-mediated meroterpene cyclization: cloning, sequencing, and heterologous expression of the napyradiomycin biosynthetic gene cluster. J. Biol. Chem. 282:16362–16368 [DOI] [PubMed] [Google Scholar]
- 298. Liu W, Nonaka K, Nie L, Zhang J, Christenson SD, Bae J, Van Lanen SG, Zazopoulos E, Farnet CM, Yang CF, Shen B. 2005. The neocarzinostatin biosynthetic gene cluster from Streptomyces carzinostaticus ATCC 15944 involving two iterative type I polyketide synthases. Chem. Biol. 12:293–302 [DOI] [PubMed] [Google Scholar]
- 299. Umezawa Y, Shimada T, Kori A, Yamada K, Ishihama A. 2008. The uncharacterized transcription factor YdhM is the regulator of the nemA gene, encoding N-ethylmaleimide reductase. J. Bacteriol. 190:5890–5897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. Poole K, Gotoh N, Tsujimoto H, Zhao Q, Wada A, Yamasaki T, Neshat S, Yamagishi J, Li XZ, Nishino T. 1996. Overexpression of the mexC-mexD-oprJ efflux operon in nfxB-type multidrug-resistant strains of Pseudomonas aeruginosa. Mol. Microbiol. 21:713–724 [DOI] [PubMed] [Google Scholar]
- 301. Walczak RJ, Woo AJ, Strohl WR, Priestley ND. 2000. Nonactin biosynthesis: the potential nonactin biosynthesis gene cluster contains type II polyketide synthase-like genes. FEMS Microbiol. Lett. 183:171–175 [DOI] [PubMed] [Google Scholar]
- 302. Bililign T, Hyun CG, Williams JS, Czisny AM, Thorson JS. 2004. The hedamycin locus implicates a novel aromatic PKS priming mechanism. Chem. Biol. 11:959–969 [DOI] [PubMed] [Google Scholar]
- 303. Lombó F, Braña AF, Salas JA, Méndez C. 2004. Genetic organization of the biosynthetic gene cluster for the antitumor angucycline oviedomycin in Streptomyces antibioticus ATCC 11891. Chembiochem 5:1181–1187 [DOI] [PubMed] [Google Scholar]
- 304. Rost R, Haas S, Hammer E, Herrmann H, Burchhardt G. 2002. Molecular analysis of aerobic phenylacetate degradation in Azoarcus evansii. Mol. Genet. Genomics 267:656–663 [DOI] [PubMed] [Google Scholar]
- 305. Mast Y, Weber T, Gölz M, Ort-Winklbauer R, Gondran A, Wohlleben W, Schinko E. 2011. Characterization of the ‘pristinamycin supercluster' of Streptomyces pristinaespiralis. Microb. Biotechnol. 4:192–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. Boutrin MC, Wang C, Aruni W, Li X, Fletcher HM. 2012. Nitric oxide stress resistance in Porphyromonas gingivalis is mediated by a putative hydroxylamine reductase. J. Bacteriol. 194:1582–1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307. Metsä-Ketelä M, Ylihonko K, Mäntsälä P. 2004. Partial activation of a silent angucycline-type gene cluster from a rubromycin beta producing Streptomyces sp. PGA64. J. Antibiot. (Tokyo) 57:502–510 [DOI] [PubMed] [Google Scholar]
- 308. Bottiglieri M, Keel C. 2006. Characterization of PhlG, a hydrolase that specifically degrades the antifungal compound 2,4-diacetylphloroglucinol in the biocontrol agent Pseudomonas fluorescens CHA0. Appl. Environ. Microbiol. 72:418–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Gristwood T, Fineran PC, Everson L, Salmond GP. 2008. PigZ, a TetR/AcrR family repressor, modulates secondary metabolism via the expression of a putative four-component resistance-nodulation-cell-division efflux pump, ZrpADBC, in Serratia sp. ATCC 39006. Mol. Microbiol. 69:418–435 [DOI] [PubMed] [Google Scholar]
- 310. Butcher RA, Schroeder FC, Fischbach MA, Straight PD, Kolter R, Walsh CT, Clardy J. 2007. The identification of bacillaene, the product of the PksX megacomplex in Bacillus subtilis. Proc. Natl. Acad. Sci. U. S. A. 104:1506–1509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311. Dürr C, Schnell HJ, Luzhetskyy A, Murillo R, Weber M, Welzel K, Vente A, Bechthold A. 2006. Biosynthesis of the terpene phenalinolactone in Streptomyces sp. Tü6071: analysis of the gene cluster and generation of derivatives. Chem. Biol. 13:365–377 [DOI] [PubMed] [Google Scholar]
- 312. Huang X, Zhu D, Ge Y, Hu H, Zhang X, Xu Y. 2004. Identification and characterization of pltZ, a gene involved in the repression of pyoluteorin biosynthesis in Pseudomonas sp. M18. FEMS Microbiol. Lett. 232:197–202 [DOI] [PubMed] [Google Scholar]
- 313. Vargas P, Felipe A, Michán C, Gallegos MT. 2011. Induction of Pseudomonas syringae pv. tomato DC3000 MexAB-OprM multidrug efflux pump by flavonoids is mediated by the repressor PmeR. Mol. Plant Microbe Interact. 24:1207–1219 [DOI] [PubMed] [Google Scholar]
- 314. Cho YH, Kim EJ, Chung HJ, Choi JH, Chater KF, Ahn BE, Shin JH, Roe JH. 2003. The pqrAB operon is responsible for paraquat resistance in Streptomyces coelicolor. J. Bacteriol. 185:6756–6763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315. Zhang X, Parry RJ. 2007. Cloning and characterization of the pyrrolomycin biosynthetic gene clusters from Actinosporangium vitaminophilum ATCC 31673 and Streptomyces sp. strain UC 1106. Antimicrob. Agents Chemother. 51:946–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316. Huang T, Wang Y, Yin J, Du Y, Tao M, Xu J, Chen W, Lin S, Deng Z. 2011. Identification and characterization of the pyridomycin biosynthetic gene cluster of Streptomyces pyridomyceticus NRRL B-2517. J. Biol. Chem. 286:20648–20657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317. Hirooka K, Fujita Y. 2011. Identification of aromatic residues critical to the DNA binding and ligand response of the Bacillus subtilis QdoR (YxaF) repressor antagonized by flavonoids. Biosci. Biotechnol. Biochem. 75:1325–1334 [DOI] [PubMed] [Google Scholar]
- 318. Baucheron S, Coste F, Canepa S, Maurel MC, Giraud E, Culard F, Castaing B, Roussel A, Cloeckaert A. 2012. Binding of the RamR repressor to wild-type and mutated promoters of the RamA gene involved in efflux-mediated multidrug resistance in Salmonella enterica serovar Typhimurium. Antimicrob. Agents Chemother. 56:942–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319. Heinzelmann E, Berger S, Müller C, Härtner T, Poralla K, Wohlleben W, Schwartz D. 2005. An acyl-CoA dehydrogenase is involved in the formation of the delta cis3 double bond in the acyl residue of the lipopeptide antibiotic friulimicin in Actinoplanes friuliensis. Microbiology 151:1963–1974 [DOI] [PubMed] [Google Scholar]
- 320. Jakobi K, Hertweck C. 2004. A gene cluster encoding resistomycin biosynthesis in Streptomyces resistomycificus; exploring polyketide cyclization beyond linear and angucyclic patterns. J. Am. Chem. Soc. 126:2298–2299 [DOI] [PubMed] [Google Scholar]
- 321. Sun Y, Hahn F, Demydchuk Y, Chettle J, Tosin M, Osada H, Leadlay PF. 2010. In vitro reconstruction of tetronate RK-682 biosynthesis. Nat. Chem. Biol. 6:99–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322. Hernández-Mendoza A, Nava N, Santana O, Abreu-Goodger C, Tovar A, Quinto C. 2007. Diminished redundancy of outer membrane factor proteins in rhizobiales: a nodT homolog is essential for free-living Rhizobium etli. J. Mol. Microbiol. Biotechnol. 13:22–34 [DOI] [PubMed] [Google Scholar]
- 323. González-Pasayo R, Martínez-Romero E. 2000. Multiresistance genes of Rhizobium etli CFN42. Mol. Plant Microbe Interact. 13:572–577 [DOI] [PubMed] [Google Scholar]
- 324. Kawasaki T, Sakurai F, Nagatsuka SY, Hayakawa Y. 2009. Prodigiosin biosynthesis gene cluster in the roseophilin producer Streptomyces griseoviridis. J. Antibiot. (Tokyo) 62:271–276 [DOI] [PubMed] [Google Scholar]
- 325. Ou X, Zhang B, Zhang L, Zhao G, Ding X. 2009. Characterization of rrdA, a TetR family protein gene involved in the regulation of secondary metabolism in Streptomyces coelicolor. Appl. Environ. Microbiol. 75:2158–2165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326. Shimada T, Ishihama A, Busby SJ, Grainger DC. 2008. The Escherichia coli RutR transcription factor binds at targets within genes as well as intergenic regions. Nucleic Acids Res. 36:3950–3955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327. Bolla JR, Do SV, Long F, Dai L, Su CC, Lei HT, Chen X, Gerkey JE, Murphy DC, Rajashankar KR, Zhang Q, Yu EW. 2012. Structural and functional analysis of the transcriptional regulator Rv3066 of Mycobacterium tuberculosis. Nucleic Acids Res. 40:9340–9355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328. Choi SU, Lee CK, Hwang YI, Kinoshita H, Nihira T. 2004. Cloning and functional analysis by gene disruption of a gene encoding a gamma-butyrolactone autoregulator receptor from Kitasatospora setae. J. Bacteriol. 186:3423–3430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329. Pan Y, Wang L, He X, Tian Y, Liu G, Tan H. 2011. SabR enhances nikkomycin production via regulating the transcriptional level of sanG, a pathway-specific regulatory gene in Streptomyces ansochromogenes. BMC Microbiol. 11:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330. Healy FG, Eaton KP, Limsirichai P, Aldrich JF, Plowman AK, King RR. 2009. Characterization of gamma-butyrolactone autoregulatory signaling gene homologs in the angucyclinone polyketide WS5995B producer Streptomyces acidiscabies. J. Bacteriol. 191:4786–4797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331. Han S, Song P, Ren T, Huang X, Cao C, Zhang B. 2011. Identification of SACE_7040, a member of TetR family related to the morphological differentiation of Saccharopolyspora erythraea. Curr. Microbiol. 63:121–125 [DOI] [PubMed] [Google Scholar]
- 332. Duong CT, Lee HN, Choi SS, Lee SY, Kim ES. 2009. Functional expression of SAV3818, a putative TetR-family transcriptional regulatory gene from Streptomyces avermitilis, stimulates antibiotic production in Streptomyces species. J. Microbiol. Biotechnol. 19:136–139 [DOI] [PubMed] [Google Scholar]
- 333. Agari Y, Sakamoto K, Yutani K, Kuramitsu S, Shinkai A. 2013. Structure and function of a TetR family transcriptional regulator, SbtR, from Thermus thermophilus HB8. Proteins 81:1166–1178 [DOI] [PubMed] [Google Scholar]
- 334. Seipke RF, Song L, Bicz J, Laskaris P, Yaxley AM, Challis GL, Loria R. 2011. The plant pathogen Streptomyces scabies 87-22 has a functional pyochelin biosynthetic pathway that is regulated by TetR- and AfsR-family proteins. Microbiology 157:2681–2693 [DOI] [PubMed] [Google Scholar]
- 335. Basnet DB, Oh TJ, Vu TT, Sthapit B, Liou K, Lee HC, Yoo JC, Sohng JK. 2006. Angucyclines Sch 47554 and Sch 47555 from Streptomyces sp. SCC-2136: cloning, sequencing, and characterization. Mol. Cells 22:154–162 [PubMed] [Google Scholar]
- 336. Rodríguez-García A, Combes P, Pérez-Redondo R, Smith MC, Smith MC. 2005. Natural and synthetic tetracycline-inducible promoters for use in the antibiotic-producing bacteria Streptomyces. Nucleic Acids Res. 33:e87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337. Okada U, Kondo K, Hayashi T, Watanabe N, Yao M, Tamura T, Tanaka I. 2008. Structural and functional analysis of the TetR-family transcriptional regulator SCO0332 from Streptomyces coelicolor. Acta Crystallogr. D Biol. Crystallogr. 64:198–205 [DOI] [PubMed] [Google Scholar]
- 338. Kim SH, Lee HN, Kim HJ, Kim ES. 2011. Transcriptome analysis of an antibiotic downregulator mutant and synergistic actinorhodin stimulation via disruption of a precursor flux regulator in Streptomyces coelicolor. Appl. Environ. Microbiol. 77:1872–1877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339. Xu D, Seghezzi N, Esnault C, Virolle MJ. 2010. Repression of antibiotic production and sporulation in Streptomyces coelicolor by overexpression of a TetR family transcriptional regulator. Appl. Environ. Microbiol. 76:7741–7753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340. Li L, Deng W, Song J, Ding W, Zhao QF, Peng C, Song WW, Tang GL, Liu W. 2008. Characterization of the saframycin A gene cluster from Streptomyces lavendulae NRRL 11002 revealing a nonribosomal peptide synthetase system for assembling the unusual tetrapeptidyl skeleton in an iterative manner. J. Bacteriol. 190:251–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341. Olano C, Gómez C, Pérez M, Palomino M, Pineda-Lucena A, Carbajo RJ, Braña AF, Méndez C, Salas JA. 2009. Deciphering biosynthesis of the RNA polymerase inhibitor streptolydigin and generation of glycosylated derivatives. Chem. Biol. 16:1031–1044 [DOI] [PubMed] [Google Scholar]
- 342. Lee JH, Rhee JE, Park U, Ju HM, Lee BC, Kim TS, Jeong HS, Choi SH. 2007. Identification and functional analysis of Vibrio vulnificus SmcR, a novel global regulator. J. Microbiol. Biotechnol. 17:325–334 [PubMed] [Google Scholar]
- 343. McDougald D, Rice SA, Kjelleberg S. 2000. The marine pathogen Vibrio vulnificus encodes a putative homologue of the Vibrio harveyi regulatory gene, luxR: a genetic and phylogenetic comparison. Gene 248:213–221 [DOI] [PubMed] [Google Scholar]
- 344. Sánchez P, Alonso A, Martinez JL. 2002. Cloning and characterization of SmeT, a repressor of the Stenotrophomonas maltophilia multidrug efflux pump SmeDEF. Antimicrob. Agents Chemother. 46:3386–3393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345. Chattoraj P, Mohapatra SS, Rao JL, Biswas I. 2011. Regulation of transcription by SMU.1349, a TetR family regulator, in Streptococcus mutans. J. Bacteriol. 193:6605–6613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346. Wu C, Cichewicz R, Li Y, Liu J, Roe B, Ferretti J, Merritt J, Qi F. 2010. Genomic island TnSmu2 of Streptococcus mutans harbors a nonribosomal peptide synthetase-polyketide synthase gene cluster responsible for the biosynthesis of pigments involved in oxygen and H2O2 tolerance. Appl. Environ. Microbiol. 76:5815–5826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347. Lee KM, Lee CK, Choi SU, Park HR, Kitani S, Nihira T, Hwang YI. 2005. Cloning and in vivo functional analysis by disruption of a gene encoding the gamma-butyrolactone autoregulator receptor from Streptomyces natalensis. Arch. Microbiol. 184:249–257 [DOI] [PubMed] [Google Scholar]
- 348. Lee K, Shimkets LJ. 1994. Cloning and characterization of the socA locus which restores development to Myxococcus xanthus C-signaling mutants. J. Bacteriol. 176:2200–2209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349. Folcher M, Gaillard H, Nguyen LT, Nguyen KT, Lacroix P, Bamas-Jacques N, Rinkel M, Thompson CJ. 2001. Pleiotropic functions of a Streptomyces pristinaespiralis autoregulator receptor in development, antibiotic biosynthesis, and expression of a superoxide dismutase. J. Biol. Chem. 276:44297–44306 [DOI] [PubMed] [Google Scholar]
- 350. Sun X, Zahir Z, Lynch KH, Dennis JJ. 2011. An antirepressor, SrpR, is involved in transcriptional regulation of the SrpABC solvent tolerance efflux pump of Pseudomonas putida S12. J. Bacteriol. 193:2717–2725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351. Wery J, Hidayat B, Kieboom J, de Bont JA. 2001. An insertion sequence prepares Pseudomonas putida S12 for severe solvent stress. J. Biol. Chem. 276:5700–5706 [DOI] [PubMed] [Google Scholar]
- 352. Arakawa K, Mochizuki S, Yamada K, Noma T, Kinashi H. 2007. γ-Butyrolactone autoregulator-receptor system involved in lankacidin and lankamycin production and morphological differentiation in Streptomyces rochei. Microbiology 153:1817–1827 [DOI] [PubMed] [Google Scholar]
- 353. Suzuki T, Mochizuki S, Yamamoto S, Arakawa K, Kinashi H. 2010. Regulation of lankamycin biosynthesis in Streptomyces rochei by two SARP genes, srrY and srrZ. Biosci. Biotechnol. Biochem. 74:819–827 [DOI] [PubMed] [Google Scholar]
- 354. Kitani S, Miyamoto KT, Takamatsu S, Herawati E, Iguchi H, Nishitomi K, Uchida M, Nagamitsu T, Omura S, Ikeda H, Nihira T. 2011. Avenolide, a Streptomyces hormone controlling antibiotic production in Streptomyces avermitilis. Proc. Natl. Acad. Sci. U. S. A. 108:16410–16415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355. Udwary DW, Zeigler L, Asolkar RN, Singan V, Lapidus A, Fenical W, Jensen PR, Moore BS. 2007. Genome sequencing reveals complex secondary metabolome in the marine actinomycete Salinispora tropica. Proc. Natl. Acad. Sci. U. S. A. 104:10376–10381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356. Carlson JC, Fortman JL, Anzai Y, Li S, Burr DA, Sherman DH. 2010. Identification of the tirandamycin biosynthetic gene cluster from Streptomyces sp. 307-9. Chembiochem 11:564–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357. Jaques S, McCarter LL. 2006. Three new regulators of swarming in Vibrio parahaemolyticus. J. Bacteriol. 188:2625–2635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358. Engel P, Scharfenstein LL, Dyer JM, Cary JW. 2001. Disruption of a gene encoding a putative gamma-butyrolactone-binding protein in Streptomyces tendae affects nikkomycin production. Appl. Microbiol. Biotechnol. 56:414–419 [DOI] [PubMed] [Google Scholar]
- 359. Fang J, Zhang Y, Huang L, Jia X, Zhang Q, Zhang X, Tang G, Liu W. 2008. Cloning and characterization of the tetrocarcin A gene cluster from Micromonospora chalcea NRRL 11289 reveals a highly conserved strategy for tetronate biosynthesis in spirotetronate antibiotics. J. Bacteriol. 190:6014–6025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360. Guilfoile PG, Hutchinson CR. 1992. The Streptomyces glaucescens TcmR protein represses transcription of the divergently oriented tcmR and tcmA genes by binding to an intergenic operator region. J. Bacteriol. 174:3659–3666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361. Sosio M, Kloosterman H, Bianchi A, de Vreugd P, Dijkhuizen L, Donadio S. 2004. Organization of the teicoplanin gene cluster in Actinoplanes teichomyceticus. Microbiology 150:95–102 [DOI] [PubMed] [Google Scholar]
- 362. Tovar K, Ernst A, Hillen W. 1988. Identification and nucleotide sequence of the class E tet regulatory elements and operator and inducer binding of the encoded purified Tet repressor. Mol. Gen. Genet. 215:76–80 [DOI] [PubMed] [Google Scholar]
- 363. Navarro-Llorens JM, Drzyzga O, Perera J. 2008. Genetic analysis of phenylacetic acid catabolism in Arthrobacter oxydans CECT386. Arch. Microbiol. 190:89–100 [DOI] [PubMed] [Google Scholar]
- 364. Demirev AV, Khanal A, Hanh NP, Nam KT, Nam DH. 2011. Biochemical characteristization of propionyl-coenzyme a carboxylase complex of Streptomyces toxytricini. J. Microbiol. 49:407–412 [DOI] [PubMed] [Google Scholar]
- 365. Demydchuk Y, Sun Y, Hong H, Staunton J, Spencer JB, Leadlay PF. 2008. Analysis of the tetronomycin gene cluster: insights into the biosynthesis of a polyether tetronate antibiotic. Chembiochem 9:1136–1145 [DOI] [PubMed] [Google Scholar]
- 366. Nash KA, Andini N, Zhang Y, Brown-Elliott BA, Wallace RJ. 2006. Intrinsic macrolide resistance in rapidly growing mycobacteria. Antimicrob. Agents Chemother. 50:3476–3478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367. Mo X, Wang Z, Wang B, Ma J, Huang H, Tian X, Zhang S, Zhang C, Ju J. 2011. Cloning and characterization of the biosynthetic gene cluster of the bacterial RNA polymerase inhibitor tirandamycin from marine-derived Streptomyces sp. SCSIO1666. Biochem. Biophys. Res. Commun. 406:341–347 [DOI] [PubMed] [Google Scholar]
- 368. Teran W, Felipe A, Segura A, Rojas A, Ramos L, Gallegos T. 2003. Antibiotic-dependent induction of Pseudomonas putida DOT-T1E TtgABC efflux pump Is mediated by the drug binding repressor TtgR. Antimicrob. Agents Chemother. 47:3067–3072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369. Terán W, Krell T, Ramos JL, Gallegos MT. 2006. Effector-repressor interactions, binding of a single effector molecule to the operator-bound TtgR homodimer mediates derepression. J. Biol. Chem. 281:7102–7109 [DOI] [PubMed] [Google Scholar]
- 370. Rojas A, Segura A, Guazzaroni ME, Terán W, Hurtado A, Gallegos MT, Ramos JL. 2003. In vivo and in vitro evidence that TtgV is the specific regulator of the TtgGHI multidrug and solvent efflux pump of Pseudomonas putida. J. Bacteriol. 185:4755–4763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371. Preiter K, Brooks DM, Penaloza-Vazquez A, Sreedharan A, Bender CL, Kunkel BN. 2005. Novel virulence gene of Pseudomonas syringae pv. tomato strain DC3000. J. Bacteriol. 187:7805–7814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372. Bignell DR, Bate N, Cundliffe E. 2007. Regulation of tylosin production: role of a TylP-interactive ligand. Mol. Microbiol. 63:838–847 [DOI] [PubMed] [Google Scholar]
- 373. Stratigopoulos G, Gandecha AR, Cundliffe E. 2002. Regulation of tylosin production and morphological differentiation in Streptomyces fradiae by TylP, a deduced gamma-butyrolactone receptor. Mol. Microbiol. 45:735–744 [DOI] [PubMed] [Google Scholar]
- 374. Novel M, Novel G. 1976. Regulation of beta-glucuronidase synthesis in Escherichia coli K-12: constitutive mutants specifically derepressed for uidA expression. J. Bacteriol. 127:406–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375. Croxatto A, Chalker VJ, Lauritz J, Jass J, Hardman A, Williams P, Cámara M, Milton DL. 2002. VanT, a homologue of Vibrio harveyi LuxR, regulates serine, metalloprotease, pigment, and biofilm production in Vibrio anguillarum. J. Bacteriol. 184:1617–1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376. Borges-Walmsley MI, Du D, McKeegan KS, Sharples GJ, Walmsley AR. 2005. VceR regulates the vceCAB drug efflux pump operon of Vibrio cholerae by alternating between mutually exclusive conformations that bind either drugs or promoter DNA. J. Mol. Biol. 349:387–400 [DOI] [PubMed] [Google Scholar]
- 377. Garg RP, Ma Y, Hoyt JC, Parry RJ. 2002. Molecular characterization and analysis of the biosynthetic gene cluster for the azoxy antibiotic valanimycin. Mol. Microbiol. 46:505–517 [DOI] [PubMed] [Google Scholar]
- 378. Hasegawa H, Häse CC. 2009. TetR-type transcriptional regulator VtpR functions as a global regulator in Vibrio tubiashii. Appl. Environ. Microbiol. 75:7602–7609 [DOI] [PMC free article] [PubMed] [Google Scholar]