Abstract
The proliferative capability of many invasive pathogens is limited by the bioavailability of iron. Pathogens have thus developed strategies to obtain iron from their host organisms. In turn, host defense strategies have evolved to sequester iron from invasive pathogens. This review explores the mechanisms employed by bacterial pathogens to gain access to host iron sources, the role of iron in bacterial virulence, and iron-related genes required for the establishment or maintenance of infection. Host defenses to limit iron availability for bacterial growth during the acute-phase response and the consequences of iron overload conditions on susceptibility to bacterial infection are also examined. The evidence summarized herein demonstrates the importance of iron bioavailability in influencing the risk of infection and the ability of the host to clear the pathogen.
INTRODUCTION
Iron's capacity to readily donate or accept electrons makes it essential for important cellular redox processes of nearly all organisms. However, this redox reactivity can also be deleterious if uncontrolled. Ferrous iron is potentially toxic through its ability to catalyze the production of reactive oxygen and nitrogen species, including the highly reactive hydroxyl radical (1). These reactive species can damage biological molecules, including DNA (2). Iron within heme is in the ferrous (Fe2+) state and readily participates in redox reactions. Moreover, the heme molecule is lipophilic and can disrupt membrane permeability (3) and alter cytoskeletal protein conformation in certain cell types (4). Redox reactions of bound heme (e.g., myoglobin and hemoglobin) are similar to those of free heme, although they occur more slowly (5). Autooxidation of globin-bound Fe2+-protoporphyrin (heme) produces the ferric (Fe3+) form (hemin) with concomitant production of superoxide (O2−), generating methemoglobin and metmyoglobin. Hydrogen peroxide can also oxidize these hemin-containing proteins, generating ferryl (Fe4+) iron, which decays to regenerate ferric iron (6, 7). The potential toxicity of iron is managed in both pathogen and host by highly sophisticated and tightly controlled systems dedicated to balancing cellular and whole organismal iron acquisition, storage, and utilization.
IRON HOMEOSTASIS IN HUMANS
The human body contains approximately 3 to 4 g of total iron. Iron loss arises from epithelial cell sloughing and minor bleeding and totals less than 2 mg per day on average (8). Because regulated iron excretion systems do not exist in humans, total body iron homeostasis is regulated at the level of dietary absorption (9, 10). Dietary nonheme iron is ferric and must be reduced to the ferrous state for membrane transport. This is accomplished by membrane-associated reductases at the duodenal brush border (11, 12). The ferrous iron is then transported into the enterocyte by the membrane transporter, divalent metal transporter 1 (DMT1) (13). Redox cycling is a conserved mechanism that minimizes exposure to reactive ferrous iron by oxidizing it to the relatively inert ferric form upon release from the cell. Conversely, ferric iron reductases return it to the active state prior to its transport across the membrane and incorporation into cellular machinery (14). Cellular iron can either be stored in ferritin or released into the plasma by ferroportin; iron oxidation is coupled to basolateral transport by the ferroxidase hephaestin (15). Ceruloplasmin functions as a ferroxidase in the plasma, where it is most important in situations involving high levels of iron demand, such as stress erythropoiesis (16). Plasma Fe3+ is bound to the transport protein transferrin for delivery to sites of storage (as intracellular ferritin) and utilization (primarily as heme but also in iron-sulfur proteins and other iron-containing enzymes) (9, 17). The related protein lactoferrin binds iron with higher affinity than transferrin and is able to retain it under acidic conditions (18, 19). It is found in most exocrine secretions and is a component of the secondary granules of neutrophils (20). Consequently, it is able to bind iron at mucosal surfaces and in plasma.
Iron stored within ferritin is in the ferric state and sequestered from availability to participate in redox reactions. Hemosiderin, a lysosomal degradation product of ferritin, is produced more abundantly under conditions associated with iron overload, hemorrhage, or hemolysis (21–23). Hemosiderin contains heterogenous iron mineralization products that differ from that of ferritin (24). Iron release from hemosiderin is inefficient at neutral pH but does occur under acidic conditions and has been implicated in hydroxyl radical production in vitro (25).
The majority of transferrin-bound iron uptake occurs in the bone marrow, where erythroid precursors incorporate the iron into the heme moiety during synthesis of hemoglobin (26). Hemoglobin in circulating erythrocytes accounts for the vast majority of iron-containing heme proteins in the body (27). This pool is salvaged by phagocytosis of senescent erythrocytes by reticuloendothelial (RE) macrophages. Recycled iron in the macrophage can be either stored in ferritin or released into circulation through ferroportin (28). Reoxidation is mediated by ceruloplasmin (28, 29) and followed by binding to transferrin. Ferroportin-mediated iron release from RE cells is one of the primary mechanisms for controlling plasma iron concentrations.
The hepatocyte is central to iron homeostasis, serving as both a storage site for iron and the principal site of production of the iron regulatory hormone hepcidin (28, 30, 31). Hepcidin is the master regulator of plasma iron concentration. It is induced in response to iron (32) and inflammation and suppressed in response to anemia, hypoxia, and erythropoiesis (33, 34). It regulates the concentration of iron in plasma through its ability to bind with and promote the internalization and subsequent degradation of ferroportin (35). As a consequence, the release of iron into the circulation from sites of storage (RE cells) and absorption (enterocytes) is decreased. Iron released into the circulation is bound to transferrin for transport under normal conditions. Under pathological conditions, such as hemochromatosis, iron release can exceed the binding capacity of transferrin. Iron is then bound to low-molecular-weight molecules (e.g., citrate), resulting in the generation of “non-transferrin-bound iron” (36). Thus, unbound iron is probably not present in serum. In the unlikely event that iron release surpasses the binding capacity of low-molecular-weight molecules, any free iron present will rapidly form insoluble ferric hydroxide. Estimates derived from the solubility constant of ferric hydroxide predict that free Fe3+ at a concentration above ∼10−18 M is insoluble at physiologic pH (37, 38).
BACTERIAL IRON AND HEME ACQUISITION SYSTEMS
The optimal iron concentration for growth of most bacteria is much higher than the concentration that is freely accessible in the mammalian host (39). For example, in vitro studies indicate that a siderophore mutant strain of Escherichia coli requires 0.05 μM iron for growth, and the growth rate of this strain increases as iron concentrations are increased up to 2 μM (40). This disparity provides strong selective pressure favoring the evolution of systems able to overcome the severe iron sequestration encountered in the mammalian host, and successful bacterial pathogens can exploit almost every major host iron-binding protein (Table 1). In fact, many of the components of these systems are required for pathogenesis in animal models of infection (Table 2). Notably, many pathogens possess multiple iron acquisition systems. When several different iron sources are available in any given host niche, the elimination of a single system may not be sufficient to attenuate virulence. Alternatively, a particular iron system may be required for virulence in one animal model but not another, depending on the iron sources available in each model.
Table 1.
Examples of bacterial heme and iron uptake systems
| System type | Example | Representative organism(s) (reference) |
|---|---|---|
| Ferrous iron uptake | Feo | V. cholerae (51), E. coli (52), Shigella flexneri (141), S. enterica serovar Typhimurium (55) |
| Mts | Streptococcus pyogenes (142) | |
| Ferric iron receptor | FecA | E. coli (143), S. flexneri (144) |
| Siderophore system | Ybt | Y. pestis (46) |
| Fhu | Haemophilus influenzae (145), Streptococcus agalactiae (146) | |
| Snfa | S. aureus (147) | |
| Mbt | M. tuberculosis (148) | |
| Vib | V. cholerae (149) | |
| Ent | E. coli (150), Shigella dysenteriae (151), S. enterica serovar Typhimurium (152) | |
| Transferrin receptor | Tbp | N. meningitidis (48), H. influenzae (153) |
| Tpn | S. aureus (154) | |
| Lactoferrin receptor | Lbp | Neisseria gonorrhoeae (155) |
| Unnamed | H. influenzae (156) | |
| Heme receptor | HutA | Bartonella quintana (157), V. cholerae (158) |
| HxuC | H. influenzae (159) | |
| Shr | S. pyogenes (65) | |
| ChuA | E. coli (160) | |
| ShuA | S. dysenteriae (161) | |
| Hemoglobin receptor | IsdB | S. aureus (44) |
| HmbR | N. meningitidis (162) | |
| Haptoglobin receptor | HarA | S. aureus (163) |
| HhuA | H. influenzae (164) | |
| HpuAB | N. meningitidis (165) | |
| Hemophore system | IsdX1X2 | B. anthracis (74) |
| Rv0203 | M. tuberculosis (166) | |
| HasA | Serratia marcescens (167) | |
| Hemoglobin protease | Hbp | E. coli EB1 (08-K43) (168) |
| Ferritin receptor | IlsA | B. cereus (61) |
Table 2.
Examples of iron-related bacterial genes required to establish or maintain infection
| Organism | Gene(s) | Function | Disease model | Reference |
|---|---|---|---|---|
| S. aureus | fur | Iron-responsive regulator | Murine model of pneumonia | 169 |
| isdB | Hemoglobin receptor | Murine model of abscess formation | 44 | |
| V. cholerae | irgA | Enterobactin receptor | Newborn mouse model of cholera | 170 |
| Brucella abortus | bhuA | Heme receptor | Murine macrophage and chronic spleen infection | 171 |
| Y. pestis KIM5 | yfeAB | Components of iron and manganese ABC transport system | Murine models of plague | 172 |
| irp2 | Yersiniabactin biosynthetic enzyme | Murine models of plague | 173 | |
| psn | Yersiniabactin receptor | Murine models of plague | 173 | |
| Bordetella pertussis | tonB | Energy transducer | Murine respiratory infection model | 174 |
| K. pneumoniae | tonB | Energy transducer | Murine model (intraperitoneal and intragastric inoculation) | 175 |
| S. enterica serovar Typhimurium | fur | Iron-responsive regulator | Systemic murine infection model | 176 |
| H. influenzae type b | hbpA | Heme binding lipoprotein | Weanling rat model of bacteremia | 177 |
| E. coli 018:K1:H7 | iroN | Salmochelin receptor | Rat model of neonatal meningitis | 178 |
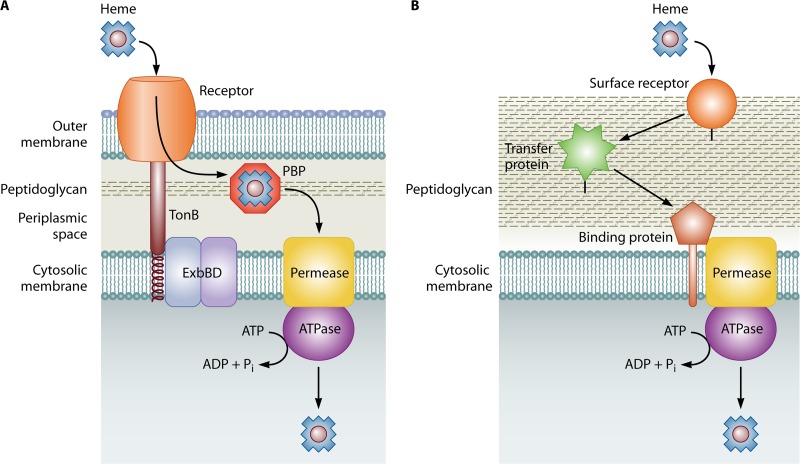
Microbial iron acquisition systems have been extensively characterized in Gram-negative bacteria. In general, an outer membrane protein receptor binds a specific iron- or heme-containing compound or protein and transports the iron or heme from it into the periplasmic space in an energy-dependent manner. The proton motive force at the cytosolic membrane supplies energy through the ExbB/ExbD complex which, in turn, induces conformational changes in TonB that allow the transduction of energy to outer membrane proteins (41). Many virulence studies have taken advantage of the fact that TonB is universally required for iron and heme uptake in Gram-negative bacteria and they have targeted it directly to circumvent issues with overlapping iron acquisition systems (discussed above). TonB-dependent receptors are structurally conserved and share an amino acid consensus sequence termed the “TonB box,” which interacts with TonB (42, 43). Once the heme or iron has been transported across the outer membrane, a substrate-specific periplasmic binding protein ferries the substrate to an ATP-binding cassette (ABC) transport system that moves it into the cytosol (39) (Fig. 1A).
Fig 1.
Schematic of bacterial heme acquisition systems. (A) In Gram-negative bacteria, heme binds an outer membrane (OM) receptor that transports it to the periplasmic space by using energy transduced from the cytosolic membrane via the TonB/ExbBD complex. A periplasmic binding protein (PBP) transfers the heme to a membrane-spanning permease, and transport across the cell membrane is mediated by an ATPase. (B) In Gram-positive bacteria, the absence of an OM eliminates the need for TonB/ExbBD and the PBP. In general, a cell wall-anchored surface receptor binds heme and relays it to an intermediate cell wall-anchored receptor (labeled a transfer protein in this diagram), which then transfers the heme to the binding protein and permease of an ABC transporter at the cell membrane. Energy for transport across the membrane is provided by an ATPase. Peptidoglycan is shown for orientation.
Considerably less is known about iron and heme uptake systems in Gram-positive bacteria. The absence of an outer membrane results in some differences in the overall design of iron and heme acquisition systems; neither outer membrane receptors nor the TonB/ExbB/ExbD system is necessary. In the most well-studied examples, substrate specificity is determined by cell wall-anchored proteins that transfer the substrate to an ABC transporter, which then delivers it to the cytoplasm (Fig. 1B) (39, 44, 45). Specific microbial nonheme iron and heme acquisition systems, as well as iron storage systems, are discussed in detail below.
Nonheme iron acquisition systems.
One bacterial strategy for the acquisition of ferric iron is the production and secretion of high-affinity siderophores that, in addition to being able to bind free iron ions, are able to extract iron from mammalian iron-binding proteins and deliver it to specific outer membrane receptors or lipoproteins. Hundreds of siderophores have been described, and they are generally classified by the nature of the ligand employed to bind iron (e.g., hydroxamates and catechols) (39). A typical example of siderophore-mediated iron acquisition is the yersiniabactin system of Yersinia pestis, which is able to extract iron from mammalian transferrin and lactoferrin proteins and deliver it to a specific outer membrane protein. Unless inoculated directly into the bloodstream, mutant strains of Y. pestis lacking yersiniabactin are avirulent in mouse models of bubonic plague, indicating that siderophore-mediated iron uptake is essential to pathogenesis in this model (46).
Citrate can be used by some pathogens as an iron-chelating molecule, and receptors specific for the uptake of ferric citrate are typically grouped with siderophore receptors (47). Whether the bacteria produce the citrate or utilize host citrate is uncertain, as it has been noted that certain pathogens may not produce sufficient citrate for iron uptake (39). In cases of iron overload, some evidence suggests that ferric citrate may be the primary form of non-transferrin-bound iron present in humans (36). Although this does not rule out the possibility that bacterial pathogens produce citrate as a siderophore, it does suggest that ferric citrate may be a biologically relevant iron source in either case.
An alternative strategy to siderophore-mediated iron uptake is the utilization of outer membrane receptors that directly recognize mammalian iron-binding proteins, such as transferrin and lactoferrin. For example, all clinical isolates of pathogenic Neisseria spp. encode a set of transferrin-binding proteins (TbpA and TbpB) dedicated to the acquisition of iron from human transferrin. TbpA is a TonB-dependent receptor that binds the C-terminal lobe of transferrin, where it extracts iron through a distortion of the iron-binding site and transports it across the outer membrane. TbpA alone is sufficient for transferrin utilization, but the coreceptor TbpB enhances the efficiency of uptake by binding transferrin and increasing its concentration for subsequent utilization by TbpA (48). A distinct set of neisserial proteins (LbpA and LbpB) is dedicated to the utilization of human lactoferrin. Molecular modeling, based on limited sequence homology, suggests that the Lbps are functionally similar to the Tbps. In contrast to TbpB, LbpB does not increase the efficiency of lactoferrin uptake. In addition to functioning as a coreceptor, it may play a protective role by binding and thereby neutralizing the antimicrobial cleavage product of lactoferrin, lactoferricin (49). These examples illustrate the fact that pathogens have several strategies to circumvent mammalian iron sequestration mechanisms.
The Feo system of Gram-negative bacteria supports direct uptake of ferrous iron. FeoB is a transport GTPase protein located in the cytosolic membrane and is usually associated with FeoA, a small cytoplasmic protein of unknown function (50). An additional open reading frame, designated feoC, may be present in the operon, and its importance is unknown (51). Feo systems appear to be preferentially expressed and used under anaerobic and microaerobic conditions when ferrous iron is expected to be the dominant species (52). Attenuated colonization of mouse gastric mucosa and intestine by feo mutants of E. coli (53), Helicobacter sp. (54), and Salmonella sp. (55) has been reported. In contrast, a feo mutant of Vibrio cholerae is fully virulent in mouse models, suggesting that redundant or alternative systems may be present (51). Uptake systems dedicated to the acquisition of ferrous iron suggest that extracellular reduction of ferric iron and subsequent uptake might be an alternative strategy to uptake of ferric iron. Accordingly, evidence has been presented for the secretion of extracellular ferric reductases by several pathogens (56–58).
Some bacterial pathogens utilize human ferritin as an iron source. Neisseria meningitidis does so indirectly by decreasing transferrin uptake, which induces a cellular iron starvation response. Ferritin is thought to be degraded by the host cell to meet its own iron needs; Neisseria is able to access the released iron as well (59). In contrast, in Burkholderia cenocepacia, an opportunistic pathogen of cystic fibrosis patients, ferritin degradation and subsequent iron release appear to be directly mediated by a secreted or surface-bound serine protease (60). Ferritin levels are known to be higher in the lungs of people with cystic fibrosis relative to healthy individuals, suggesting a direct link to the pathogenesis of this organism. Bacillus cereus is also able to use ferritin through a surface-localized NEAT (near iron transporter) domain protein (61). Although not clearly defined, extracellular pathogens, such as Streptococcus pneumoniae, may access ferritin following its release from cells damaged or lysed by virulence determinants or may utilize serum or secreted ferritin (62). Additional studies are needed to fully define the mechanisms of ferritin utilization, but these reports provide yet another example of the fact that bacterial pathogens employ multiple mechanisms to promote their survival in the iron-limiting environment of the mammalian host.
Heme acquisition systems.
In accordance with the abundance of heme in mammals, many pathogens use dedicated heme acquisition systems to obtain iron and/or heme. In addition to preserving the structural architecture characteristic of TonB-dependent receptors, Gram-negative heme receptors share amino acid homology, including conservation of FRAP/NPNL domains. Two conserved histidine residues, one of which is in the FRAP/NPNL motif, are required for heme utilization in Yersinia (63). The Gram-negative heme receptors are classified by substrate specificity into either “heme scavenger” receptors, which are able to obtain heme from a variety of heme-containing proteins, such as the HemR receptor of Yersinia enterocolitica, or more specific hemoglobin receptors, as exemplified by HmbR of N. meningitidis (63, 64). Dedicated mechanisms for the utilization of myoglobin as a heme source have not been reported to date. However, myoglobin can serve as an iron source for some organisms in vitro. Potential mechanisms include recognition and binding of myoglobin by broad-specificity hemoprotein receptors (63, 65), utilization of spontaneously released heme following oxidation of myoglobin (66), and utilization of haptoglobin-bound myoglobin (67). The degree of myoglobin availability and utilization in vivo is uncertain.
In pathogenic E. coli, heme acquisition is facilitated by the production of autotransporter proteins that have hemoglobin protease activity. In general, autotransporter proteins have diverse functions but share a unique secretion mechanism (type V secretion system) whereby a signal sequence traffics the protein to the periplasm. Once there, the C terminus of the protein generates a β-barrel in the outer membrane that allows the passenger domain to exit the cell (68). Hemoglobin proteases from E. coli belong to the SPATE (serine protease autotransporter proteins of Enterobacteriaceae) family of proteins and are only found in pathogenic strains (68, 69). E. coli hemoglobin proteases are thought to bind the released heme and deliver it to an unknown surface receptor (68).
Heme acquisition systems in Gram-positive bacteria differ somewhat from those of Gram-negative bacteria. The iron-regulated surface determinant system (Isd) of Staphylococcus aureus, encoded by 10 genes, extracts heme from hemoglobin with the cell wall-anchored proteins IsdH and IsdB and then sequentially passes the heme to various proteins in the Isd system until it is transported into the cytosol (70). Once there, it is either incorporated into bacterial heme proteins or degraded by the heme oxygenases, IsdG and IsdI (71). The cell wall-anchored components of this system share one or more NEAT domains, which facilitate heme binding and transfer (72).
Analogous to the extracellular scavenging of iron by siderophores, hemophores are high-affinity binding molecules that either extract heme from hemoproteins or bind free, extracellular heme and deliver it to appropriate surface receptors. In the Gram-positive pathogen Bacillus anthracis, two secreted proteins, IsdX1 and IsdX2, are involved in extracellular heme capture (73). IsdX1 binds heme and delivers it to either IsdX2 or the cell wall-associated IsdC protein. Akin to the S. aureus heme acquisition system, the interaction of these proteins with heme and each other is thought to be mediated by the NEAT domains of each respective protein (74). Gram-negative hemophores have also been described (42).
Once heme has been transported into the cytosol, it can be directly incorporated into bacterial proteins or degraded by bacterial heme oxygenases (HOs) to release the iron. One class of heme oxygenase, homologous to human heme oxygenase 1 (HO-1), generates iron, carbon monoxide, and biliverdin upon cleavage of heme (75). A second class is exemplified by the IsdG family. Through a mechanism that hasn't been fully defined, this family of enzymes generates iron, the novel oxo-bilirubin chromophore staphylobilin, and formaldehyde, rather than carbon monoxide, upon the cleavage of heme (76–78). ChuS of E. coli O157:H7 may represent a third, structurally distinct class of HOs (79).
In other Gram-negative pathogens, ChuS homologues protect against heme toxicity at high heme concentrations and are required for efficient heme utilization at low heme concentrations, but they appear to function as heme trafficking proteins rather than heme oxygenases (80, 81). In particular, PhuS of Pseudomonas aeruginosa has been shown to bind heme and deliver it to the HO, HemO (82). In certain Gram-positive pathogens, protection against heme toxicity is conferred by the ABC transporter proteins HrtAB. These proteins are proposed to function in heme efflux, effectively detoxifying excess heme by exporting it from the cell (83, 84). In parallel with the HOs, the heme trafficking and putative efflux proteins promote the use of heme as an iron source and protect pathogens against heme toxicity.
Bacterial iron storage systems.
Irrespective of the mechanism used to acquire iron, once it has been obtained it can be stored intracellularly. Bacterial storage systems with similarities to mammalian ferritin have been described, including ferritin and bacterioferritin, both of which consist of a hollow sphere comprised of 24 subunits (85, 86). The ferroxidase center of these proteins is highly similar to the ferroxidase center of the mammalian ferritin heavy (H) chain (87). E. coli ferritin is reportedly able to accommodate ∼2,000 iron atoms, while bacterioferritin has the capacity for ∼1,800 iron atoms (85, 86). Bacterioferritin is unique in that a heme b molecule is bound between every two subunits, and evidence suggests that it promotes electron transfer for the reduction and subsequent release of iron from the core (88). These proteins provide a system for the accumulation and storage of iron reserves that can be tapped when iron becomes scarce.
Dps (DNA-binding protein from starved cells) may represent an alternative iron storage system; homologues have been identified in many species, although not all of them are able to bind DNA. The E. coli Dps consists of 12 identical subunits that generate a hollow core for iron storage and possess a markedly different ferroxidase center than that found in ferritin and bacterioferritin. Dps reduces one hydrogen peroxide molecule for every pair of ferrous iron ions oxidized, which bypasses generation of the hydroxyl radical through a single electron transfer and neutralizes the hydrogen peroxide. These activities contribute to its role in oxidative stress resistance, regardless of its ability to physically shield DNA (89). It has, in fact, been hypothesized that the primary function of Dps is stress resistance rather than iron storage. In support of this hypothesis, the Dps core only contains ∼500 iron atoms (89). Additional studies are needed to fully explore the capacity of the Dps protein to serve as an iron source under iron-limiting conditions.
ALTERATION OF HOST IRON-RELATED PROTEINS DURING THE ACUTE-PHASE RESPONSE
The extensive iron acquisition strategies employed by bacterial pathogens may render the host's basal iron homeostasis suboptimal for a successful defense. A marked change in iron metabolism is a central component of a larger systemic host response to infection (as well as other stressors, such as tissue injury, inflammation, and cancer), collectively termed the acute-phase response (APR). The APR is initiated by the innate immune system in an attempt to neutralize the source of infection or injury while minimizing collateral tissue damage. The characteristic APR includes fever, certain hormonal changes, leukocytosis, and alterations in the hepatocellular production of multiple plasma proteins. Plasma proteins that increase in concentration during the APR are termed “positive” acute-phase proteins (APPs), while those that decrease are termed “negative” APPs (90). The APPs include protease inhibitors, secreted pathogen recognition receptors, clotting and coagulation factors, and complement proteins, as well as multiple proteins relevant to iron metabolism (91).
Alterations in hepatocellular APP synthesis are initiated by certain inflammatory cytokines produced by activated macrophages and monocytes. Interleukin-6 (IL-6) and IL-1 are the principal regulators of the APPs, but additional cytokines have been implicated, including tumor necrosis factor alpha (TNF-α) and gamma interferon (IFN-γ) (91). For some cytokines, including IL-6, homodimerization of the receptor-associated molecule gp130 is required to transduce the signal upon binding to their cognate receptors (92, 93). Intracellular signaling molecules include STAT3 (for IL-6) and NF-κB (92). Glucocorticoids have also been shown to modify expression levels of certain APPs (91).
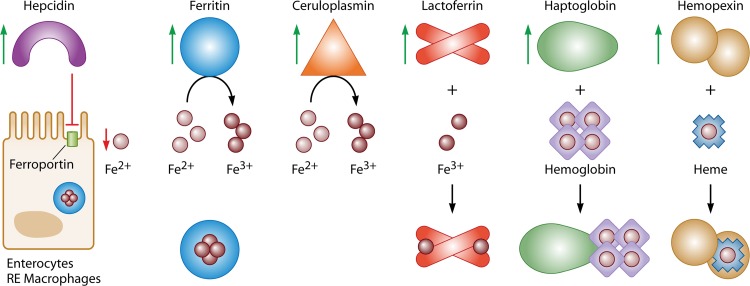
The APR markedly alters host iron metabolism in ways that serve to minimize iron bioavailability to pathogens (Fig. 2). These include (i) decreased iron release into the circulation (for which the APP mediator is hepcidin), (ii) increased intracellular iron storage (for which the APP mediator is ferritin), (iii) decreased accessibility of extracellular nonheme iron (for which the APP mediators are ceruloplasmin and lactoferrin), and (iv) decreased accessibility of extracellular heme iron (for which the APP mediators are hemopexin and haptoglobin). Each of these processes is discussed in detail below.
Fig 2.
Iron-related APPs coordinate hypoferremia in response to infection and inflammation. Infection and inflammation result in the production of proinflammatory cytokines, such as IL-6, by immune effector cells. In turn, proinflammatory cytokines bind to cognate receptors on hepatocytes, triggering a signaling cascade that results in increased synthesis (indicated by green arrows) of several iron-related APPs. Decreased release of iron into the circulation is facilitated by hepcidin upregulation, which results in a net decrease in plasma iron by binding to and promoting degradation of ferroportin on hepatocytes, RE macrophages, and duodenal enterocytes. Increased intracellular iron storage results from the induction of ferritin. Decreased bioavailability of nonheme iron is mediated by ceruloplasmin's ferroxidase activity, combined with binding of Fe3+ by lactoferrin. Decreased availability of extracellular heme iron is mediated by the induction of hemopexin, which results in the binding of free heme, while haptoglobin binds free hemoglobin and promotes its clearance. This coordinated response is thought to deprive invading microorganisms of iron while simultaneously protecting tissue from unnecessary oxidative stress resulting from the interaction of iron with immune mediators.
Decreased iron release into the circulation.
Originally described as an antimicrobial peptide, hepcidin is arguably the major APP that contributes to the hypoferremia associated with the APR (94). The hepcidin-mediated degradation of ferroportin decreases the efflux of iron by RE macrophages and hepatocytes, resulting in a net decrease in the plasma iron concentration. Dietary iron absorption is likewise decreased through the inhibition of iron efflux in duodenal enterocytes (95). IL-6 is the major inflammatory cytokine that upregulates hepcidin expression in the APR (96); however, IL-1 and IL-22 have also been implicated (97). In cultured cells, induction of hepcidin is synergistic when cells are treated with BMP6 (a signaling molecule for the BMP/SMAD pathway that is responsible, in part, for the hepatic upregulation of hepcidin in response to iron), when combined with either IL-6 or IL-22. This is of particular interest because iron-loaded ferritin has been shown to increase hepatic Bmp6 mRNA levels in mice (98). These observations suggest a potential feed-forward mechanism for maintaining high levels of hepcidin despite decreased circulating iron. Activin B, itself upregulated by lipopolysaccharide (LPS) challenge in mice, also induces hepcidin via the BMP/SMAD pathway in response to inflammation and also synergizes with the IL-6/STAT3 pathway in human hepatoma cells (99). The observation that mice with a liver-specific knockout of Smad4 do not upregulate hepcidin in response to IL-6 injection provides additional evidence suggesting that an intact BMP signaling pathway is required for the upregulation of hepcidin by inflammation (100).
It has been proposed that, in addition to altering iron efflux, the hepcidin-mediated internalization of ferroportin modulates the macrophage production of inflammatory cytokines via the activation of JAK2 and subsequent phosphorylation of STAT3. In support of this hypothesis, hepcidin treatment is protective against lethal challenge with LPS in murine models (101). However, recent evidence suggests that internalization of ferroportin by hepcidin is mediated by ubiquitination and is independent of JAK2/STAT3 activation (102). Direct antimicrobial activity, including disruption of bacterial membrane integrity and inhibition of growth, has also been reported for hepcidin present at a concentration of 200 μg/ml (103). Whether such a concentration is locally achievable in vivo is uncertain.
While decreased iron release into the circulation may be beneficial in limiting iron availability to extracellular pathogens, it is possible that the concomitant increase of intracellular iron may facilitate its availability to certain intracellular pathogens. For organisms that proliferate intracellularly, whether a low hepcidin state or a high hepcidin state protects against or facilitates iron availability may depend on the cell type in which it proliferates. It has been suggested, for example, that the low hepcidin state in HFE-associated hereditary hemochromatosis (HH) may provide a selective advantage by decreasing macrophage iron and protecting the host against Salmonella enterica serovar Typhimurium, a bacterium that proliferates in this cell type (discussed below) (104).
Increased intracellular iron storage.
Expression of genes encoding the L-type and H-type ferritin proteins is increased in the APR (105). Moreover, hepcidin-mediated cellular iron retention serves to increase translation of the ferritin proteins (through an iron response element in the ferritin mRNAs) (106). The ferritin H-chain possesses ferrroxidase activity, facilitating a rapid decrease in redox-active iron from both the extracellular space and the intracellular labile iron pool. The increased retention and storage of iron as ferritin in macrophages (107) contributes to the characteristic fall in serum iron concentrations and increase in serum ferritin concentrations observed in the APR. In general, increased intracellular iron storage is expected to increase resistance to infection. The decreased intracellular labile iron pool is expected to limit the growth of intracellular pathogens, and the decreased serum iron concentration is expected to limit the growth of extracellular pathogens. However, as noted above, certain pathogens are able to circumvent these decreases in available iron by utilizing ferritin as an iron source. Therefore, the increased ferritin in macrophages may ultimately be beneficial to a pathogen, such as B. cenocepacia, that can survive in a macrophage and utilize ferritin (60, 108).
Decreased bioavailability of extracellular nonheme iron.
Ceruloplasmin is a positive APP, with blood concentrations increasing up to 6-fold in response to inflammation (109). By virtue of its ferroxidase activity, ceruloplasmin promotes the loading of iron onto transferrin and lactoferrin. The oxidation of extracellular ferrous iron also prevents it from participating in Fenton chemistry. Ceruloplasmin scavenges superoxide, a neutrophil immune effector (110). This property decreases the availability of reactants for the Haber-Weiss cycle, which can generate Fe2+ required to interact with H2O2 during Fenton chemistry from Fe3+ and superoxide (111).
Transferrin is a negative APP (112), i.e., transferrin synthesis and circulating transferrin levels decrease during inflammatory states. The biological significance of this downregulation is not fully understood. Because circulating iron and transferrin levels both decrease during the APR, the measured transferrin saturation is generally normal, or only moderately decreased. Nonetheless, the total concentration of iron-loaded transferrin in the circulation is decreased. At the same time, iron availability to the host erythron for hemoglobin synthesis is decreased. The consequent iron-restrictive erythropoiesis contributes to the anemia of inflammation (or anemia of chronic disease) (113). Circulating inflammatory cytokines also contribute to the hypoproliferative state by disrupting erythrocyte maturation (114).
Lactoferrin is a positive APP produced in sufficient amounts to compensate for the concomitant decrease in transferrin (115). Increased hepatic synthesis of lactoferrin occurs in response to IL-6, IFN-γ, and TNF-α in mice. Serum concentrations of lactoferrin also increase due to proinflammatory cytokine-mediated neutrophil degranulation at sites of inflammation (20, 116). Apolactoferrin binds and sequesters iron, thereby limiting the amount of iron available to support pathogen growth and react with oxygen-dependent immune effectors, such as hydrogen peroxide and superoxide. Moreover, positively charged peptides derived from lactoferrin have direct antimicrobial activity through their interaction with negatively charged bacterial membrane components (117, 118).
Overall, decreased bioavailability of extracellular nonheme iron is expected to target the iron acquisition of extracellular pathogens and limit their growth. Increased ceruloplasmin decreases the concentration of any available extracellular Fe2+ and promotes the loading of Fe3+ onto transferrin and lactoferrin. The decreased production of transferrin likely limits iron availability to those pathogens capable of extracting iron from it. Increased production of lactoferrin is expected to bind and sequester iron. This may result in increased iron availability for those pathogens able to use lactoferrin as an iron source. However, the potential benefit of additional lactoferrin-bound iron may be fully offset by the antimicrobial activity of lactoferrin-derived peptides (117, 118). Facultative intracellular pathogens, such as Neisseria spp., able to utilize these molecules are likely to be affected in the same manner as extracellular pathogens, whereas these changes would presumably not have a major impact on the accessibility of iron to obligate intracellular pathogens (48, 49).
Decreased bioavailabilty of heme iron.
The APR leads to an increase in liver haptoglobin synthesis, a process mediated by IL-6 and glucocorticoids. Neutrophils also contribute to increased concentrations of haptoglobin through local degranulation at sites of inflammation and injury (119). Haptoglobin binds free hemoglobin released during hemolysis and facilitates its uptake through the CD163 hemoglobin scavenger receptor present on monocytes and macrophages. Of particular relevance during an inflammatory response, haptoglobin binding protects hemoglobin from peroxide-mediated damage that would otherwise prevent its uptake by CD163 (120). Thus, increased haptoglobin production has an antimicrobial function through its iron-sequestering activities and an antioxidant function by preventing hemoglobin-mediated generation of oxidative species.
When the binding capacity of haptoglobin has been surpassed, free hemoglobin is rapidly oxidized to methemoglobin. Hemin (Fe3+-protoporphyrin) dissociates more readily from globin than heme (Fe2+-protoporphyrin) and is released as a free molecule. Although albumin binds heme in the bloodstream, hemopexin, another positive APP, does so with much higher affinity and is considered the primary heme-scavenging molecule (121). The heme-hemopexin molecule binds the scavenger receptor LRP1 (low-density lipoprotein receptor-related protein 1); upon internalization, the two molecules dissociate, and intracellular heme can be used directly, catabolized by a heme oxygenase, or exported (122).
Extracellular and facultative intracellular pathogens are most likely to experience restricted heme access as a consequence of the upregulation of haptoglobin and hemopexin. This expectation is qualified by the fact that many pathogens have receptors that recognize the heme-hemopexin complex and the haptoglobin-hemoglobin complex. For intracellular pathogens, these changes may result in an increase in the availability of heme and/or iron, depending on the host cell type. The haptoglobin-hemoglobin complex is taken up by macrophages and monocytes; LRP1 is present on many cell types, including macrophages, hepatocytes, and neurons (122).
IRON STATUS AND SUSCEPTIBILITY TO INFECTION
Additional evidence supporting a pivotal role for iron in infection and immunity comes from studies that have examined susceptibility to infection as a function of host iron status. These studies have demonstrated the effects of iron overload, both clinically and in animal models, on infection and inflammatory responses. Mutations in several genes participating in hepcidin regulation can give rise to HH. HFE-associated HH is the most common; HFE encodes the major histocompatibility complex class I-like hemochromatosis protein (123). Mutations in the repulsive guidance molecule family member hemojuvelin result in juvenile hemochromatosis (124). Mutations in the genes encoding hepcidin or transferrin receptor 2 or gain-of-function mutations in ferroportin also cause HH. In these settings, serum and tissue iron concentrations are high, while iron concentrations in RE macrophages are comparatively low. Individuals with hemochromatosis are more susceptible to infection by certain pathogens. For example, increased risks for infections with E. coli (125), V. cholerae (126), Y. enterocolitica (127), and Listeria monocytogenes (128) are associated with iron overload. HH patients are also vulnerable to infection by Vibrio vulnificus. The specific role for iron in this susceptibility has been suggested by studies in which the addition of ferric ammonium citrate or hematin to whole blood from control subjects was sufficient to promote the same degree of V. vulnificus growth as observed in whole blood taken from hemochromatosis patients (129). Studies in macrophages from murine Hfe knockout models indicated that the Toll-like receptor 4 signaling in response to LPS challenge is impaired. Evidence suggests that this signaling impairment involves the TRAM/TRIF adaptor molecules, rather than MYD88, and is associated with decreased production of IL-6 and TNF-α. Moreover, these changes are associated with decreased intestinal inflammation in response to Salmonella-mediated enterocolitis (130). However, as discussed above, the decreased macrophage iron consequent to loss of HFE may be advantageous in the context of certain infections. For example, Hfe+/− and Hfe−/− mice injected intraperitoneally with S. enterica serovar Typhimurium (a pathogen that proliferates in macrophages) show decreased hepatic and splenic bacterial loads and increased survival compared to Hfe+/+ mice, and isolated Hfe-deficient macrophages show enhanced antimicrobial activity following infection with S. enterica serovar Typhimurium compared to wild-type macrophages (104). Likewise, decreased iron acquisition is associated with decreased growth of Mycobacterium tuberculosis in monocyte-derived macrophages from HH patients with HFE mutations compared to control macrophages (131).
Iron overload results from ineffective erythropoiesis in thalassemia intermedia (132) and from both blood transfusion and ineffective erythropoiesis in thalassemia major. Several factors contribute to increased susceptibility to infection in this population. In particular, the iron chelator deferoxamine (DFO) is a siderophore that can enhance the growth of several pathogens commonly associated with thalassemia, including Y. enterocolitica and Klebsiella pneumoniae (133, 134). Nonetheless, iron overload is an independent factor predisposing thalassemic patients to infection, as evidenced by the occurrence of these infections in the absence of chelation therapy and when alternative chelators that are unable to enhance the growth of these organisms are used (134, 135).
Dietary iron loading, as measured indirectly in rural Africans based on consumption levels of traditional beer, which is high in ferrous iron, was associated with a 3.5-fold increase in the odds of developing active tuberculosis, and among those treated for pulmonary tuberculosis, there was a 1.3-fold increase in the hazard ratio of death compared to individuals without increased dietary iron intake (136). Of note, heavy alcohol consumption is associated with the incidence of tuberculosis, as well as the outcome; dose-response relationships have been reported (137). For example, a retrospective study examining the effects of alcohol consumption, based on family member reports, on cause of mortality in Russia found a dose-response relationship between alcohol and tuberculosis. Those in the highest alcohol consumption category, defined by reported consumption of three or more 0.5-liter bottles of vodka per week, had a relative risk of tuberculosis greater than 3.0 compared to controls, who reportedly consumed less than one 0.5-liter bottle of vodka per week (138). Thus, it is possible that the increased odds of developing tuberculosis reported in rural Africans are influenced by alcohol consumption, but the authors of that study pointed out that African traditional beer is low in alcohol content and that histological changes consistent with alcoholism are notably absent in liver biopsy specimens from patients with African iron overload. In a different study, the effects of dietary iron intake as a function of traditional beer consumption and single nucleotide polymorphisms (SNPs) in the gene encoding ferroportin were examined in tuberculosis patients relative to controls. Overall, four SNPs were associated with an increased risk of tuberculosis. Gene-environment interactions were also reported for four SNPs; two of them were associated with a significant increase in the risk of tuberculosis when iron intake was high. Interestingly, the SNP encoding the Q248H ferroportin mutation was not associated with risk for tuberculosis in this study (139). In contrast, the prevalence rates of both pulmonary tuberculosis and Pneumocystis jirovecii pneumonia were increased in HIV-positive Rwandese women with the Q248H ferroportin mutation relative to those without the mutation (140). On the whole, these studies emphasize the importance of host iron status with respect to susceptibility and clearance of infection.
FUTURE DIRECTIONS
Several areas of future research are warranted. Targeting conserved bacterial iron uptake systems and mechanisms may result in novel, broad-spectrum therapeutics, a strategy that is especially relevant given the expanding problem of antimicrobial resistance. Expanded exploration of iron-related genes required for virulence may help to identify new targets for vaccine candidates. The role of iron in the outcome of infection implies that both anemia and iron supplementation should be studied carefully to ensure successful management in areas where certain infectious diseases are endemic. Continued investigation of iron-related APPs may improve the treatment of disease. Elucidation of the mechanisms regulating iron metabolism in both the host and pathogen may ultimately result in novel strategies to promote a successful host defense.
ACKNOWLEDGMENTS
We thank H. Martin Garraffo for helpful discussions of iron solubility.
N.L.P. is supported by the Intramural Research Program of the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases.
The contents of this paper are solely the responsibility of the authors and do not necessarily reflect the official views of the National Institutes of Health.
Footnotes
Published ahead of print 8 July 2013
REFERENCES
- 1.Graf E, Mahoney JR, Bryant RG, Eaton JW. 1984. Iron-catalyzed hydroxyl radical formation. Stringent requirement for free iron coordination site. J. Biol. Chem. 259:3620–3624 [PubMed] [Google Scholar]
- 2.Bergeron F, Auvre F, Radicella JP, Ravanat JL. 2010. HO* radicals induce an unexpected high proportion of tandem base lesions refractory to repair by DNA glycosylases. Proc. Natl. Acad. Sci. U. S. A. 107:5528–5533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmitt TH, Frezzatti WA, Jr, Schreier S. 1993. Hemin-induced lipid membrane disorder and increased permeability: a molecular model for the mechanism of cell lysis. Arch. Biochem. Biophys. 307:96–103 [DOI] [PubMed] [Google Scholar]
- 4.Shaklai N, Avissar N, Rabizadeh E, Shaklai M. 1986. Disintegration of red cell membrane cytoskeleton by hemin. Biochem. Int. 13:467–477 [PubMed] [Google Scholar]
- 5.Shikama K. 2006. Nature of the FeO2 bonding in myoglobin and hemoglobin: a new molecular paradigm. Prog. Biophys. Mol. Biol. 91:83–162 [DOI] [PubMed] [Google Scholar]
- 6.Giulivi C, Cadenas E. 1998. Heme protein radicals: formation, fate, and biological consequences. Free Radic. Biol. Med. 24:269–279 [DOI] [PubMed] [Google Scholar]
- 7.Reeder BJ, Wilson MT. 2005. Hemoglobin and myoglobin associated oxidative stress: from molecular mechanisms to disease States. Curr. Med. Chem. 12:2741–2751 [DOI] [PubMed] [Google Scholar]
- 8.Bothwell T, Charlton R, Cook J, Finch C. 1979. Iron metabolism in man. Blackwell, Oxford, England [Google Scholar]
- 9.Hahn PF, Bale WF, Lawrence EO, Whipple GH. 1939. Radioactive Iron and its metabolism in anemia: its absorption, transportation, and utilization. J. Exp. Med. 69:739–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hahn PF, Bale WF, Hettig RA, Kamen MD, Whipple GH. 1939. Radioactive iron and its excretion in urine, bile, and feces. J. Exp. Med. 70:443–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McKie AT, Barrow D, Latunde-Dada GO, Rolfs A, Sager G, Mudaly E, Mudaly M, Richardson C, Barlow D, Bomford A, Peters TJ, Raja KB, Shirali S, Hediger MA, Farzaneh F, Simpson RJ. 2001. An iron-regulated ferric reductase associated with the absorption of dietary iron. Science 291:1755–1759 [DOI] [PubMed] [Google Scholar]
- 12.Choi J, Masaratana P, Latunde-Dada GO, Arno M, Simpson RJ, McKie AT. 2012. Duodenal reductase activity and spleen iron stores are reduced and erythropoiesis is abnormal in Dcytb knockout mice exposed to hypoxic conditions. J. Nutr. 142:1929–1934 [DOI] [PubMed] [Google Scholar]
- 13.Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, Boron WF, Nussberger S, Gollan JL, Hediger MA. 1997. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature 388:482–488 [DOI] [PubMed] [Google Scholar]
- 14.Kosman DJ. 2010. Redox cycling in iron uptake, efflux, and trafficking. J. Biol. Chem. 285:26729–26735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vulpe CD, Kuo YM, Murphy TL, Cowley L, Askwith C, Libina N, Gitschier J, Anderson GJ. 1999. Hephaestin, a ceruloplasmin homologue implicated in intestinal iron transport, is defective in the sla mouse. Nat. Genet. 21:195–199 [DOI] [PubMed] [Google Scholar]
- 16.Cherukuri S, Tripoulas NA, Nurko S, Fox PL. 2004. Anemia and impaired stress-induced erythropoiesis in aceruloplasminemic mice. Blood Cells Mol. Dis. 33:346–355 [DOI] [PubMed] [Google Scholar]
- 17.Yang F, Lum JB, McGill JR, Moore CM, Naylor SL, van Bragt PH, Baldwin WD, Bowman BH. 1984. Human transferrin: cDNA characterization and chromosomal localization. Proc. Natl. Acad. Sci. U. S. A. 81:2752–2756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aisen P, Leibman A. 1972. Lactoferrin and transferrin: a comparative study. Biochim. Biophys. Acta 257:314–323 [DOI] [PubMed] [Google Scholar]
- 19.Mazurier J, Spik G. 1980. Comparative study of the iron-binding properties of human transferrins. I. Complete and sequential iron saturation and desaturation of the lactotransferrin. Biochim. Biophys. Acta 629:399–408 [DOI] [PubMed] [Google Scholar]
- 20.Masson PL, Heremans JF, Schonne E. 1969. Lactoferrin, an iron-binding protein in neutrophilic leukocytes. J. Exp. Med. 130:643–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyazaki E, Kato J, Kobune M, Okumura K, Sasaki K, Shintani N, Arosio P, Niitsu Y. 2002. Denatured H-ferritin subunit is a major constituent of haemosiderin in the liver of patients with iron overload. Gut 50:413–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pereira CG, Silva AL, de Castilhos P, Mastrantonio EC, Souza RA, Romao RP, Rezende RJ, Pena JD, Beletti ME, Souza MA. 2009. Different isolates from Leishmania braziliensis complex induce distinct histopathological features in a murine model of infection. Vet. Parasitol. 165:231–240 [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Juan LV, Ma X, Wang D, Ma H, Chang Y, Nie G, Jia L, Duan X, Liang XJ. 2010. Specific hemosiderin deposition in spleen induced by a low dose of cisplatin: altered iron metabolism and its implication as an acute hemosiderin formation model. Curr. Drug Metab. 11:507–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ward RJ, Legssyer R, Henry C, Crichton RR. 2000. Does the haemosiderin iron core determine its potential for chelation and the development of iron-induced tissue damage? J. Inorg. Biochem. 79:311–317 [DOI] [PubMed] [Google Scholar]
- 25.Ozaki M, Kawabata T, Awai M. 1988. Iron release from haemosiderin and production of iron-catalysed hydroxyl radicals in vitro. Biochem. J. 250:589–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Donohue DM, Gabrio BW, Finch CA. 1958. Quantitative measurement of hematopoietic cells of the marrow. J. Clin. Invest. 37:1564–1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larsen L, Milman N. 1975. Normal iron absorption determined by means of whole body counting and red cell incorporation of 59Fe. Acta Med. Scand. 198:271–274 [DOI] [PubMed] [Google Scholar]
- 28.Fleming RE, Ponka P. 2012. Iron overload in human disease. N. Engl. J. Med. 366:348–359 [DOI] [PubMed] [Google Scholar]
- 29.Osaki S, Johnson DA, Frieden E. 1971. The mobilization of iron from the perfused mammalian liver by a serum copper enzyme, ferroxidase I. J. Biol. Chem. 246:3018–3023 [PubMed] [Google Scholar]
- 30.Krause A, Neitz S, Magert HJ, Schulz A, Forssmann WG, Schulz-Knappe P, Adermann K. 2000. LEAP-1, a novel highly disulfide-bonded human peptide, exhibits antimicrobial activity. FEBS Lett. 480:147–150 [DOI] [PubMed] [Google Scholar]
- 31.Park CH, Valore EV, Waring AJ, Ganz T. 2001. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J. Biol. Chem. 276:7806–7810 [DOI] [PubMed] [Google Scholar]
- 32.Mazur A, Feillet-Coudray C, Romier B, Bayle D, Gueux E, Ruivard M, Coudray C, Rayssiguier Y. 2003. Dietary iron regulates hepatic hepcidin 1 and 2 mRNAs in mice. Metabolism 52:1229–1231 [DOI] [PubMed] [Google Scholar]
- 33.Nicolas G, Chauvet C, Viatte L, Danan JL, Bigard X, Devaux I, Beaumont C, Kahn A, Vaulont S. 2002. The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J. Clin. Invest. 110:1037–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vokurka M, Krijt J, Sulc K, Necas E. 2006. Hepcidin mRNA levels in mouse liver respond to inhibition of erythropoiesis. Physiol. Res. 55:667–674 [DOI] [PubMed] [Google Scholar]
- 35.Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J. 2004. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 306:2090–2093 [DOI] [PubMed] [Google Scholar]
- 36.Grootveld M, Bell JD, Halliwell B, Aruoma OI, Bomford A, Sadler PJ. 1989. Non-transferrin-bound iron in plasma or serum from patients with idiopathic hemochromatosis. Characterization by high performance liquid chromatography and nuclear magnetic resonance spectroscopy. J. Biol. Chem. 264:4417–4422 [PubMed] [Google Scholar]
- 37.Bullen JJ, Rogers HJ, Griffiths E. 1978. Role of iron in bacterial infection. Curr. Top. Microbiol. Immunol. 80:1–35 [DOI] [PubMed] [Google Scholar]
- 38.Chaberek S, Martell AE. 1959. Organic sequestering agents. John Wiley & Sons, Inc, New York, NY [Google Scholar]
- 39.Andrews SC, Robinson AK, Rodriguez-Quinones F. 2003. Bacterial iron homeostasis. FEMS Microbiol. Rev. 27:215–237 [DOI] [PubMed] [Google Scholar]
- 40.Hartmann A, Braun V. 1981. Iron uptake and iron limited growth of Escherichia coli K-12. Arch. Microbiol. 130:353–356 [DOI] [PubMed] [Google Scholar]
- 41.Higgs PI, Myers PS, Postle K. 1998. Interactions in the TonB-dependent energy transduction complex: ExbB and ExbD form homomultimers. J. Bacteriol. 180:6031–6038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krieg S, Huche F, Diederichs K, Izadi-Pruneyre N, Lecroisey A, Wandersman C, Delepelaire P, Welte W. 2009. Heme uptake across the outer membrane as revealed by crystal structures of the receptor-hemophore complex. Proc. Natl. Acad. Sci. U. S. A. 106:1045–1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gudmundsdottir A, Bell PE, Lundrigan MD, Bradbeer C, Kadner RJ. 1989. Point mutations in a conserved region (TonB box) of Escherichia coli outer membrane protein BtuB affect vitamin B12 transport. J. Bacteriol. 171:6526–6533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Torres VJ, Pishchany G, Humayun M, Schneewind O, Skaar EP. 2006. Staphylococcus aureus IsdB is a hemoglobin receptor required for heme iron utilization. J. Bacteriol. 188:8421–8429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dryla A, Gelbmann D, von Gabain A, Nagy E. 2003. Identification of a novel iron regulated staphylococcal surface protein with haptoglobin-haemoglobin binding activity. Mol. Microbiol. 49:37–53 [DOI] [PubMed] [Google Scholar]
- 46.Sebbane F, Jarrett C, Gardner D, Long D, Hinnebusch BJ. 2010. Role of the Yersinia pestis yersiniabactin iron acquisition system in the incidence of flea-borne plague. PLoS One 5(12):e14379. 10.1371/journal.pone.0014379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wagegg W, Braun V. 1981. Ferric citrate transport in Escherichia coli requires outer membrane receptor protein fecA. J. Bacteriol. 145:156–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Noinaj N, Easley NC, Oke M, Mizuno N, Gumbart J, Boura E, Steere AN, Zak O, Aisen P, Tajkhorshid E, Evans RW, Gorringe AR, Mason AB, Steven AC, Buchanan SK. 2012. Structural basis for iron piracy by pathogenic Neisseria. Nature 483:53–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Noinaj N, Cornelissen CN, Buchanan SK. 2013. Structural insight into the lactoferrin receptors from pathogenic Neisseria. J. Struct. Biol. [Epub ahead of print.] 10.1016/j.jsb.2013.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lau CK, Ishida H, Liu Z, Vogel HJ. 2013. Solution structure of Escherichia coli FeoA and its potential role in bacterial ferrous iron transport. J. Bacteriol. 195:46–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wyckoff EE, Mey AR, Leimbach A, Fisher CF, Payne SM. 2006. Characterization of ferric and ferrous iron transport systems in Vibrio cholerae. J. Bacteriol. 188:6515–6523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kammler M, Schon C, Hantke K. 1993. Characterization of the ferrous iron uptake system of Escherichia coli. J. Bacteriol. 175:6212–6219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stojiljkovic I, Cobeljic M, Hantke K. 1993. Escherichia coli K-12 ferrous iron uptake mutants are impaired in their ability to colonize the mouse intestine. FEMS Microbiol. Lett. 108:111–115 [DOI] [PubMed] [Google Scholar]
- 54.Velayudhan J, Hughes NJ, McColm AA, Bagshaw J, Clayton CL, Andrews SC, Kelly DJ. 2000. Iron acquisition and virulence in Helicobacter pylori: a major role for FeoB, a high-affinity ferrous iron transporter. Mol. Microbiol. 37:274–286 [DOI] [PubMed] [Google Scholar]
- 55.Tsolis RM, Baumler AJ, Heffron F, Stojiljkovic I. 1996. Contribution of TonB- and Feo-mediated iron uptake to growth of Salmonella typhimurium in the mouse. Infect. Immun. 64:4549–4556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vartivarian SE, Cowart RE. 1999. Extracellular iron reductases: identification of a new class of enzymes by siderophore-producing microorganisms. Arch. Biochem. Biophys. 364:75–82 [DOI] [PubMed] [Google Scholar]
- 57.Barchini E, Cowart RE. 1996. Extracellular iron reductase activity produced by Listeria monocytogenes. Arch. Microbiol. 166:51–57 [DOI] [PubMed] [Google Scholar]
- 58.Chatfield CH, Cianciotto NP. 2007. The secreted pyomelanin pigment of Legionella pneumophila confers ferric reductase activity. Infect. Immun. 75:4062–4070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Larson JA, Howie HL, So M. 2004. Neisseria meningitidis accelerates ferritin degradation in host epithelial cells to yield an essential iron source. Mol. Microbiol. 53:807–820 [DOI] [PubMed] [Google Scholar]
- 60.Whitby PW, Vanwagoner TM, Springer JM, Morton DJ, Seale TW, Stull TL. 2006. Burkholderia cenocepacia utilizes ferritin as an iron source. J. Med. Microbiol. 55:661–668 [DOI] [PubMed] [Google Scholar]
- 61.Daou N, Buisson C, Gohar M, Vidic J, Bierne H, Kallassy M, Lereclus D, Nielsen-LeRoux C. 2009. IlsA, a unique surface protein of Bacillus cereus required for iron acquisition from heme, hemoglobin and ferritin. PLoS Pathog. 5(11):e1000675. 10.1371/journal.ppat.1000675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gupta R, Shah P, Swiatlo E. 2009. Differential gene expression in Streptococcus pneumoniae in response to various iron sources. Microb. Pathog. 47:101–109 [DOI] [PubMed] [Google Scholar]
- 63.Bracken CS, Baer MT, Abdur-Rashid A, Helms W, Stojiljkovic I. 1999. Use of heme-protein complexes by the Yersinia enterocolitica HemR receptor: histidine residues are essential for receptor function. J. Bacteriol. 181:6063–6072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Perkins-Balding D, Baer MT, Stojiljkovic I. 2003. Identification of functionally important regions of a haemoglobin receptor from Neisseria meningitidis. Microbiology 149:3423–3435 [DOI] [PubMed] [Google Scholar]
- 65.Bates CS, Montanez GE, Woods CR, Vincent RM, Eichenbaum Z. 2003. Identification and characterization of a Streptococcus pyogenes operon involved in binding of hemoproteins and acquisition of iron. Infect. Immun. 71:1042–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mocny JC, Olson JS, Connell TD. 2007. Passively released heme from hemoglobin and myoglobin is a potential source of nutrient iron for Bordetella bronchiseptica. Infect. Immun. 75:4857–4866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Morton DJ, Van Wagoner TM, Seale TW, Whitby PW, Stull TL. 2006. Utilization of myoglobin as a heme source by Haemophilus influenzae requires binding of myoglobin to haptoglobin. FEMS Microbiol. Lett. 258:235–240 [DOI] [PubMed] [Google Scholar]
- 68.Otto BR, Sijbrandi R, Luirink J, Oudega B, Heddle JG, Mizutani K, Park SY, Tame JR. 2005. Crystal structure of hemoglobin protease, a heme binding autotransporter protein from pathogenic Escherichia coli. J. Biol. Chem. 280:17339–17345 [DOI] [PubMed] [Google Scholar]
- 69.Drago-Serrano ME, Parra SG, Manjarrez-Hernandez HA. 2006. EspC, an autotransporter protein secreted by enteropathogenic Escherichia coli (EPEC), displays protease activity on human hemoglobin. FEMS Microbiol. Lett. 265:35–40 [DOI] [PubMed] [Google Scholar]
- 70.Mazmanian SK, Skaar EP, Gaspar AH, Humayun M, Gornicki P, Jelenska J, Joachmiak A, Missiakas DM, Schneewind O. 2003. Passage of heme-iron across the envelope of Staphylococcus aureus. Science 299:906–909 [DOI] [PubMed] [Google Scholar]
- 71.Wu R, Skaar EP, Zhang R, Joachimiak G, Gornicki P, Schneewind O, Joachimiak A. 2005. Staphylococcus aureus IsdG and IsdI, heme-degrading enzymes with structural similarity to monooxygenases. J. Biol. Chem. 280:2840–2846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Andrade MA, Ciccarelli FD, Perez-Iratxeta C, Bork P. 2002. NEAT: a domain duplicated in genes near the components of a putative Fe3+ siderophore transporter from Gram-positive pathogenic bacteria. Genome Biol. 3:RESEARCH0047. 10.1186/gb-2002-3-9-research0047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Maresso AW, Garufi G, Schneewind O. 2008. Bacillus anthracis secretes proteins that mediate heme acquisition from hemoglobin. PLoS Pathog. 4(8):e1000132. 10.1371/journal.ppat.1000132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fabian M, Solomaha E, Olson JS, Maresso AW. 2009. Heme transfer to the bacterial cell envelope occurs via a secreted hemophore in the Gram-positive pathogen Bacillus anthracis. J. Biol. Chem. 284:32138–32146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wilks A, Schmitt MP. 1998. Expression and characterization of a heme oxygenase (Hmu O) from Corynebacterium diphtheriae. Iron acquisition requires oxidative cleavage of the heme macrocycle. J. Biol. Chem. 273:837–841 [DOI] [PubMed] [Google Scholar]
- 76.Skaar EP, Gaspar AH, Schneewind O. 2004. IsdG and IsdI, heme-degrading enzymes in the cytoplasm of Staphylococcus aureus. J. Biol. Chem. 279:436–443 [DOI] [PubMed] [Google Scholar]
- 77.Reniere ML, Ukpabi GN, Harry SR, Stec DF, Krull R, Wright DW, Bachmann BO, Murphy ME, Skaar EP. 2010. The IsdG-family of haem oxygenases degrades haem to a novel chromophore. Mol. Microbiol. 75:1529–1538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Matsui T, Nambu S, Ono Y, Goulding CW, Tsumoto K, Ikeda-Saito M. 2013. Heme degradation by Staphylococcus aureus IsdG and IsdI liberates formaldehyde rather than carbon monoxide. Biochemistry 52:3025–3027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Suits MD, Pal GP, Nakatsu K, Matte A, Cygler M, Jia Z. 2005. Identification of an Escherichia coli O157:H7 heme oxygenase with tandem functional repeats. Proc. Natl. Acad. Sci. U. S. A. 102:16955–16960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wyckoff EE, Lopreato GF, Tipton KA, Payne SM. 2005. Shigella dysenteriae ShuS promotes utilization of heme as an iron source and protects against heme toxicity. J. Bacteriol. 187:5658–5664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kaur AP, Lansky IB, Wilks A. 2009. The role of the cytoplasmic heme-binding protein (PhuS) of Pseudomonas aeruginosa in intracellular heme trafficking and iron homeostasis. J. Biol. Chem. 284:56–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lansky IB, Lukat-Rodgers GS, Block D, Rodgers KR, Ratliff M, Wilks A. 2006. The cytoplasmic heme-binding protein (PhuS) from the heme uptake system of Pseudomonas aeruginosa is an intracellular heme-trafficking protein to the delta-regioselective heme oxygenase. J. Biol. Chem. 281:13652–13662 [DOI] [PubMed] [Google Scholar]
- 83.Bibb LA, Schmitt MP. 2010. The ABC transporter HrtAB confers resistance to hemin toxicity and is regulated in a hemin-dependent manner by the ChrAS two-component system in Corynebacterium diphtheriae. J. Bacteriol. 192:4606–4617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Torres VJ, Stauff DL, Pishchany G, Bezbradica JS, Gordy LE, Iturregui J, Anderson KL, Dunman PM, Joyce S, Skaar EP. 2007. A Staphylococcus aureus regulatory system that responds to host heme and modulates virulence. Cell Host Microbe 1:109–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hudson AJ, Andrews SC, Hawkins C, Williams JM, Izuhara M, Meldrum FC, Mann S, Harrison PM, Guest JR. 1993. Overproduction, purification and characterization of the Escherichia coli ferritin. Eur. J. Biochem. 218:985–995 [DOI] [PubMed] [Google Scholar]
- 86.Andrews SC, Smith JM, Hawkins C, Williams JM, Harrison PM, Guest JR. 1993. Overproduction, purification and characterization of the bacterioferritin of Escherichia coli and a C-terminally extended variant. Eur. J. Biochem. 213:329–338 [DOI] [PubMed] [Google Scholar]
- 87.Andrews SC, Smith JM, Yewdall SJ, Guest JR, Harrison PM. 1991. Bacterioferritins and ferritins are distantly related in evolution. Conservation of ferroxidase-centre residues. FEBS Lett. 293:164–168 [DOI] [PubMed] [Google Scholar]
- 88.Yasmin S, Andrews SC, Moore GR, Le Brun NE. 2011. A new role for heme, facilitating release of iron from the bacterioferritin iron biomineral. J. Biol. Chem. 286:3473–3483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhao G, Ceci P, Ilari A, Giangiacomo L, Laue TM, Chiancone E, Chasteen ND. 2002. Iron and hydrogen peroxide detoxification properties of DNA-binding protein from starved cells. A ferritin-like DNA-binding protein of Escherichia coli. J. Biol. Chem. 277:27689–27696 [DOI] [PubMed] [Google Scholar]
- 90.Gabay C, Kushner I. 1999. Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 340:448–454 [DOI] [PubMed] [Google Scholar]
- 91.Bode JG, Albrecht U, Haussinger D, Heinrich PC, Schaper F. 2012. Hepatic acute phase proteins: regulation by IL-6- and IL-1-type cytokines involving STAT3 and its crosstalk with NF-κB-dependent signaling. Eur. J. Cell Biol. 91:496–505 [DOI] [PubMed] [Google Scholar]
- 92.Quinton LJ, Blahna MT, Jones MR, Allen E, Ferrari JD, Hilliard KL, Zhang X, Sabharwal V, Algul H, Akira S, Schmid RM, Pelton SI, Spira A, Mizgerd JP. 2012. Hepatocyte-specific mutation of both NF-κB RelA and STAT3 abrogates the acute phase response in mice. J. Clin. Invest. 122:1758–1763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Murakami M, Hibi M, Nakagawa N, Nakagawa T, Yasukawa K, Yamanishi K, Taga T, Kishimoto T. 1993. IL-6-induced homodimerization of gp130 and associated activation of a tyrosine kinase. Science 260:1808–1810 [DOI] [PubMed] [Google Scholar]
- 94.Nemeth E, Valore EV, Territo M, Schiller G, Lichtenstein A, Ganz T. 2003. Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood 101:2461–2463 [DOI] [PubMed] [Google Scholar]
- 95.Ganz T. 2011. Hepcidin and iron regulation, 10 years later. Blood 117:4425–4433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, Ganz T. 2004. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J. Clin. Invest. 113:1271–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Armitage AE, Eddowes LA, Gileadi U, Cole S, Spottiswoode N, Selvakumar TA, Ho LP, Townsend AR, Drakesmith H. 2011. Hepcidin regulation by innate immune and infectious stimuli. Blood 118:4129–4139 [DOI] [PubMed] [Google Scholar]
- 98.Feng Q, Migas MC, Waheed A, Britton RS, Fleming RE. 2012. Ferritin upregulates hepatic expression of bone morphogenetic protein 6 and hepcidin in mice. Am. J. Physiol. Gastrointest Liver Physiol. 302:G1397–G1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Besson-Fournier C, Latour C, Kautz L, Bertrand J, Ganz T, Roth MP, Coppin H. 2012. Induction of activin B by inflammatory stimuli up-regulates expression of the iron-regulatory peptide hepcidin through Smad1/5/8 signaling. Blood 120:431–439 [DOI] [PubMed] [Google Scholar]
- 100.Wang RH, Li C, Xu X, Zheng Y, Xiao C, Zerfas P, Cooperman S, Eckhaus M, Rouault T, Mishra L, Deng CX. 2005. A role of SMAD4 in iron metabolism through the positive regulation of hepcidin expression. Cell Metab. 2:399–409 [DOI] [PubMed] [Google Scholar]
- 101.De Domenico I, Zhang TY, Koening CL, Branch RW, London N, Lo E, Daynes RA, Kushner JP, Li D, Ward DM, Kaplan J. 2010. Hepcidin mediates transcriptional changes that modulate acute cytokine-induced inflammatory responses in mice. J. Clin. Invest. 120:2395–2405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ross SL, Tran L, Winters A, Lee KJ, Plewa C, Foltz I, King C, Miranda LP, Allen J, Beckman H, Cooke KS, Moody G, Sasu BJ, Nemeth E, Ganz T, Molineux G, Arvedson TL. 2012. Molecular mechanism of hepcidin-mediated ferroportin internalization requires ferroportin lysines, not tyrosines or JAK-STAT. Cell Metab. 15:905–917 [DOI] [PubMed] [Google Scholar]
- 103.Sow FB, Florence WC, Satoskar AR, Schlesinger LS, Zwilling BS, Lafuse WP. 2007. Expression and localization of hepcidin in macrophages: a role in host defense against tuberculosis. J. Leukoc. Biol. 82:934–945 [DOI] [PubMed] [Google Scholar]
- 104.Nairz M, Theurl I, Schroll A, Theurl M, Fritsche G, Lindner E, Seifert M, Crouch ML, Hantke K, Akira S, Fang FC, Weiss G. 2009. Absence of functional Hfe protects mice from invasive Salmonella enterica serovar Typhimurium infection via induction of lipocalin-2. Blood 114:3642–3651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Naz N, Moriconi F, Ahmad S, Amanzada A, Khan S, Mihm S, Ramadori G, Malik IA. 2013. Ferritin L is the sole serum ferritin constituent and a positive hepatic acute-phase protein. Shock 39:520–526 [DOI] [PubMed] [Google Scholar]
- 106.Sammarco MC, Ditch S, Banerjee A, Grabczyk E. 2008. Ferritin L and H subunits are differentially regulated on a post-transcriptional level. J. Biol. Chem. 283:4578–4587 [DOI] [PubMed] [Google Scholar]
- 107.Birgegard G, Caro J. 1984. Increased ferritin synthesis and iron uptake in inflammatory mouse macrophages. Scand. J. Haematol. 33:43–48 [DOI] [PubMed] [Google Scholar]
- 108.Lamothe J, Huynh KK, Grinstein S, Valvano MA. 2007. Intracellular survival of Burkholderia cenocepacia in macrophages is associated with a delay in the maturation of bacteria-containing vacuoles. Cell. Microbiol. 9:40–53 [DOI] [PubMed] [Google Scholar]
- 109.Broadley C, Hoover RL. 1989. Ceruloplasmin reduces the adhesion and scavenges superoxide during the interaction of activated polymorphonuclear leukocytes with endothelial cells. Am. J. Pathol. 135:647–655 [PMC free article] [PubMed] [Google Scholar]
- 110.Goldstein IM, Kaplan HB, Edelson HS, Weissmann G. 1979. Ceruloplasmin: a scavenger of superoxide anion radicals. J. Biol. Chem. 254:4040–4045 [PubMed] [Google Scholar]
- 111.Haber F, Weiss J. 1934. The catalytic decomposition of hydrogen peroxide by iron salts. Proc. R Soc. Lond. A 147:332–351 [Google Scholar]
- 112.Chiarla C, Giovannini I, Siegel JH. 2009. Hypotransferrinemia and changes in plasma lipid and metabolic patterns in sepsis. Amino Acids 36:327–331 [DOI] [PubMed] [Google Scholar]
- 113.Fleming RE, Bacon BR. 2005. Orchestration of iron homeostasis. N. Engl. J. Med. 352:1741–1744 [DOI] [PubMed] [Google Scholar]
- 114.Prince OD, Langdon JM, Layman AJ, Prince IC, Sabogal M, Mak HH, Berger AE, Cheadle C, Chrest FJ, Yu Q, Andrews NC, Xue QL, Civin CI, Walston JD, Roy CN. 2012. Late stage erythroid precursor production is impaired in mice with chronic inflammation. Haematologica 97:1648–1656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ahmad G, Sial GZ, Ramadori P, Dudas J, Batusic DS, Ramadori G. 2011. Changes of hepatic lactoferrin gene expression in two mouse models of the acute phase reaction. Int. J. Biochem. Cell Biol. 43:1822–1832 [DOI] [PubMed] [Google Scholar]
- 116.Topham MK, Carveth HJ, McIntyre TM, Prescott SM, Zimmerman GA. 1998. Human endothelial cells regulate polymorphonuclear leukocyte degranulation. FASEB J. 12:733–746 [DOI] [PubMed] [Google Scholar]
- 117.Flores-Villasenor H, Canizalez-Roman A, Reyes-Lopez M, Nazmi K, de la Garza M, Zazueta-Beltran J, Leon-Sicairos N, Bolscher JG. 2010. Bactericidal effect of bovine lactoferrin, LFcin, LFampin and LFchimera on antibiotic-resistant Staphylococcus aureus and Escherichia coli. Biometals 23:569–578 [DOI] [PubMed] [Google Scholar]
- 118.Appelmelk BJ, An YQ, Geerts M, Thijs BG, de Boer HA, MacLaren DM, de Graaff J, Nuijens JH. 1994. Lactoferrin is a lipid A-binding protein. Infect. Immun. 62:2628–2632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Theilgaard-Monch K, Jacobsen LC, Nielsen MJ, Rasmussen T, Udby L, Gharib M, Arkwright PD, Gombart AF, Calafat J, Moestrup SK, Porse BT, Borregaard N. 2006. Haptoglobin is synthesized during granulocyte differentiation, stored in specific granules, and released by neutrophils in response to activation. Blood 108:353–361 [DOI] [PubMed] [Google Scholar]
- 120.Buehler PW, Abraham B, Vallelian F, Linnemayr C, Pereira CP, Cipollo JF, Jia Y, Mikolajczyk M, Boretti FS, Schoedon G, Alayash AI, Schaer DJ. 2009. Haptoglobin preserves the CD163 hemoglobin scavenger pathway by shielding hemoglobin from peroxidative modification. Blood 113:2578–2586 [DOI] [PubMed] [Google Scholar]
- 121.Morgan WT, Liem HH, Sutor RP, Muller-Ebergard U. 1976. Transfer of heme from heme-albumin to hemopexin. Biochim. Biophys. Acta 444:435–445 [DOI] [PubMed] [Google Scholar]
- 122.Hvidberg V, Maniecki MB, Jacobsen C, Hojrup P, Moller HJ, Moestrup SK. 2005. Identification of the receptor scavenging hemopexin-heme complexes. Blood 106:2572–2579 [DOI] [PubMed] [Google Scholar]
- 123.Feder JN, Penny DM, Irrinki A, Lee VK, Lebron JA, Watson N, Tsuchihashi Z, Sigal E, Bjorkman PJ, Schatzman RC. 1998. The hemochromatosis gene product complexes with the transferrin receptor and lowers its affinity for ligand binding. Proc. Natl. Acad. Sci. U. S. A. 95:1472–1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Niederkofler V, Salie R, Arber S. 2005. Hemojuvelin is essential for dietary iron sensing, and its mutation leads to severe iron overload. J. Clin. Invest. 115:2180–2186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Christopher GW. 1985. Escherichia coli bacteremia, meningitis, and hemochromatosis. Arch. Intern. Med. 145:1908. [PubMed] [Google Scholar]
- 126.Fernandez JM, Serrano M, De Arriba JJ, Sanchez MV, Escribano E, Ferreras P. 2000. Bacteremic cellulitis caused by non-01, non-0139 Vibrio cholerae: report of a case in a patient with hemochromatosis. Diagn. Microbiol. Infect. Dis. 37:77–80 [DOI] [PubMed] [Google Scholar]
- 127.Hopfner M, Nitsche R, Rohr A, Harms D, Schubert S, Folsch UR. 2001. Yersinia enterocolitica infection with multiple liver abscesses uncovering a primary hemochromatosis. Scand. J. Gastroenterol. 36:220–224 [DOI] [PubMed] [Google Scholar]
- 128.Manso C, Rivas I, Peraire J, Vidal F, Richart C. 1997. Fatal Listeria meningitis, endocarditis and pericarditis in a patient with haemochromatosis. Scand. J. Infect. Dis. 29:308–309 [DOI] [PubMed] [Google Scholar]
- 129.Bullen JJ, Spalding PB, Ward CG, Gutteridge JM. 1991. Hemochromatosis, iron and septicemia caused by Vibrio vulnificus. Arch. Intern. Med. 151:1606–1609 [PubMed] [Google Scholar]
- 130.Wang L, Harrington L, Trebicka E, Shi HN, Kagan JC, Hong CC, Lin HY, Babitt JL, Cherayil BJ. 2009. Selective modulation of TLR4-activated inflammatory responses by altered iron homeostasis in mice. J. Clin. Invest. 119:3322–3328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Olakanmi O, Schlesinger LS, Britigan BE. 2007. Hereditary hemochromatosis results in decreased iron acquisition and growth by Mycobacterium tuberculosis within human macrophages. J. Leukoc. Biol. 81:195–204 [DOI] [PubMed] [Google Scholar]
- 132.Pippard MJ, Callender ST, Warner GT, Weatherall DJ. 1979. Iron absorption and loading in beta-thalassaemia intermedia. Lancet ii:819–821 [DOI] [PubMed] [Google Scholar]
- 133.Chambers CE, Sokol PA. 1994. Comparison of siderophore production and utilization in pathogenic and environmental isolates of Yersinia enterocolitica. J. Clin. Microbiol. 32:32–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chan GC, Chan S, Ho PL, Ha SY. 2009. Effects of chelators (deferoxamine, deferiprone and deferasirox) on the growth of Klebsiella pneumoniae and Aeromonas hydrophila isolated from transfusion-dependent thalassemia patients. Hemoglobin 33:352–360 [DOI] [PubMed] [Google Scholar]
- 135.Chiesa C, Pacifico L, Renzulli F, Midulla M, Garlaschi L. 1987. Yersinia hepatic abscesses and iron overload. JAMA 257:3230–3231 [DOI] [PubMed] [Google Scholar]
- 136.Gangaidzo IT, Moyo VM, Mvundura E, Aggrey G, Murphree NL, Khumalo H, Saungweme T, Kasvosve I, Gomo ZA, Rouault T, Boelaert JR, Gordeuk VR. 2001. Association of pulmonary tuberculosis with increased dietary iron. J. Infect. Dis. 184:936–939 [DOI] [PubMed] [Google Scholar]
- 137.Rehm J, Samokhvalov AV, Neuman MG, Room R, Parry C, Lonnroth K, Patra J, Poznyak V, Popova S. 2009. The association between alcohol use, alcohol use disorders and tuberculosis (TB): a systematic review. BMC Public Health 9:450. 10.1186/1471-2458-9-450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zaridze D, Brennan P, Boreham J, Boroda A, Karpov R, Lazarev A, Konobeevskaya I, Igitov V, Terechova T, Boffetta P, Peto R. 2009. Alcohol and cause-specific mortality in Russia: a retrospective case-control study of 48,557 adult deaths. Lancet 373:2201–2214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Baker MA, Wilson D, Wallengren K, Sandgren A, Iartchouk O, Broodie N, Goonesekera SD, Sabeti PC, Murray MB. 2012. Polymorphisms in the gene that encodes the iron transport protein ferroportin 1 influence susceptibility to tuberculosis. J. Infect. Dis. 205:1043–1047 [DOI] [PubMed] [Google Scholar]
- 140.Masaisa F, Breman C, Gahutu JB, Mukiibi J, Delanghe J, Philippe J. 2012. Ferroportin (SLC40A1) Q248H mutation is associated with lower circulating serum hepcidin levels in Rwandese HIV-positive women. Ann. Hematol. 91:911–916 [DOI] [PubMed] [Google Scholar]
- 141.Runyen-Janecky LJ, Reeves SA, Gonzales EG, Payne SM. 2003. Contribution of the Shigella flexneri Sit, Iuc, and Feo iron acquisition systems to iron acquisition in vitro and in cultured cells. Infect. Immun. 71:1919–1928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sun X, Ge R, Chiu JF, Sun H, He QY. 2008. Lipoprotein MtsA of MtsABC in Streptococcus pyogenes primarily binds ferrous ion with bicarbonate as a synergistic anion. FEBS Lett. 582:1351–1354 [DOI] [PubMed] [Google Scholar]
- 143.Ferguson AD, Chakraborty R, Smith BS, Esser L, van der Helm D, Deisenhofer J. 2002. Structural basis of gating by the outer membrane transporter FecA. Science 295:1715–1719 [DOI] [PubMed] [Google Scholar]
- 144.Luck SN, Turner SA, Rajakumar K, Sakellaris H, Adler B. 2001. Ferric dicitrate transport system (Fec) of Shigella flexneri 2a YSH6000 is encoded on a novel pathogenicity island carrying multiple antibiotic resistance genes. Infect. Immun. 69:6012–6021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Morton DJ, Turman EJ, Hensley PD, VanWagoner TM, Seale TW, Whitby PW, Stull TL. 2010. Identification of a siderophore utilization locus in nontypeable Haemophilus influenzae. BMC Microbiol. 10:113. 10.1186/1471-2180-10-113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Clancy A, Loar JW, Speziali CD, Oberg M, Heinrichs DE, Rubens CE. 2006. Evidence for siderophore-dependent iron acquisition in group B streptococcus. Mol. Microbiol. 59:707–721 [DOI] [PubMed] [Google Scholar]
- 147.Cotton JL, Tao J, Balibar CJ. 2009. Identification and characterization of the Staphylococcus aureus gene cluster coding for staphyloferrin A. Biochemistry 48:1025–1035 [DOI] [PubMed] [Google Scholar]
- 148.McMahon MD, Rush JS, Thomas MG. 2012. Analyses of MbtB, MbtE, and MbtF suggest revisions to the mycobactin biosynthesis pathway in Mycobacterium tuberculosis. J. Bacteriol. 194:2809–2818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wyckoff EE, Stoebner JA, Reed KE, Payne SM. 1997. Cloning of a Vibrio cholerae vibriobactin gene cluster: identification of genes required for early steps in siderophore biosynthesis. J. Bacteriol. 179:7055–7062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Liu J, Duncan K, Walsh CT. 1989. Nucleotide sequence of a cluster of Escherichia coli enterobactin biosynthesis genes: identification of entA and purification of its product 2,3-dihydro-2,3-dihydroxybenzoate dehydrogenase. J. Bacteriol. 171:791–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lawlor KM, Payne SM. 1984. Aerobactin genes in Shigella spp. J. Bacteriol. 160:266–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Pollack JR, Neilands JB. 1970. Enterobactin, an iron transport compound from Salmonella typhimurium. Biochem. Biophys. Res. Commun. 38:989–992 [DOI] [PubMed] [Google Scholar]
- 153.Loosmore SM, Yang YP, Coleman DC, Shortreed JM, England DM, Harkness RE, Chong PS, Klein MH. 1996. Cloning and expression of the Haemophilus influenzae transferrin receptor genes. Mol. Microbiol. 19:575–586 [DOI] [PubMed] [Google Scholar]
- 154.Modun B, Kendall D, Williams P. 1994. Staphylococci express a receptor for human transferrin: identification of a 42-kilodalton cell wall transferrin-binding protein. Infect. Immun. 62:3850–3858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Biswas GD, Sparling PF. 1995. Characterization of lbpA, the structural gene for a lactoferrin receptor in Neisseria gonorrhoeae. Infect. Immun. 63:2958–2967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Schryvers AB. 1988. Characterization of the human transferrin and lactoferrin receptors in Haemophilus influenzae. Mol. Microbiol. 2:467–472 [DOI] [PubMed] [Google Scholar]
- 157.Parrow NL, Abbott J, Lockwood AR, Battisti JM, Minnick MF. 2009. Function, regulation, and transcriptional organization of the hemin utilization locus of Bartonella quintana. Infect. Immun. 77:307–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Henderson DP, Payne SM. 1994. Characterization of the Vibrio cholerae outer membrane heme transport protein HutA: sequence of the gene, regulation of expression, and homology to the family of TonB-dependent proteins. J. Bacteriol. 176:3269–3277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Cope LD, Yogev R, Muller-Eberhard U, Hansen EJ. 1995. A gene cluster involved in the utilization of both free heme and heme:hemopexin by Haemophilus influenzae type b. J. Bacteriol. 177:2644–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Torres AG, Payne SM. 1997. Haem iron-transport system in enterohaemorrhagic Escherichia coli O157:H7. Mol. Microbiol. 23:825–833 [DOI] [PubMed] [Google Scholar]
- 161.Mills M, Payne SM. 1997. Identification of shuA, the gene encoding the heme receptor of Shigella dysenteriae, and analysis of invasion and intracellular multiplication of a shuA mutant. Infect. Immun. 65:5358–5363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Stojiljkovic I, Larson J, Hwa V, Anic S, So M. 1996. HmbR outer membrane receptors of pathogenic Neisseria spp.: iron-regulated, hemoglobin-binding proteins with a high level of primary structure conservation. J. Bacteriol. 178:4670–4678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Dryla A, Hoffmann B, Gelbmann D, Giefing C, Hanner M, Meinke A, Anderson AS, Koppensteiner W, Konrat R, von Gabain A, Nagy E. 2007. High-affinity binding of the staphylococcal HarA protein to haptoglobin and hemoglobin involves a domain with an antiparallel eight-stranded beta-barrel fold. J. Bacteriol. 189:254–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Maciver I, Latimer JL, Liem HH, Muller-Eberhard U, Hrkal Z, Hansen EJ. 1996. Identification of an outer membrane protein involved in utilization of hemoglobin-haptoglobin complexes by nontypeable Haemophilus influenzae. Infect. Immun. 64:3703–3712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lewis LA, Dyer DW. 1995. Identification of an iron-regulated outer membrane protein of Neisseria meningitidis involved in the utilization of hemoglobin complexed to haptoglobin. J. Bacteriol. 177:1299–1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Owens CP, Du J, Dawson JH, Goulding CW. 2012. Characterization of heme ligation properties of Rv0203, a secreted heme binding protein involved in Mycobacterium tuberculosis heme uptake. Biochemistry 51:1518–1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ghigo JM, Letoffe S, Wandersman C. 1997. A new type of hemophore-dependent heme acquisition system of Serratia marcescens reconstituted in Escherichia coli. J. Bacteriol. 179:3572–3579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Otto BR, van Dooren SJ, Nuijens JH, Luirink J, Oudega B. 1998. Characterization of a hemoglobin protease secreted by the pathogenic Escherichia coli strain EB1. J. Exp. Med. 188:1091–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Torres VJ, Attia AS, Mason WJ, Hood MI, Corbin BD, Beasley FC, Anderson KL, Stauff DL, McDonald WH, Zimmerman LJ, Friedman DB, Heinrichs DE, Dunman PM, Skaar EP. 2010. Staphylococcus aureus fur regulates the expression of virulence factors that contribute to the pathogenesis of pneumonia. Infect. Immun. 78:1618–1628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Goldberg MB, DiRita VJ, Calderwood SB. 1990. Identification of an iron-regulated virulence determinant in Vibrio cholerae, using TnphoA mutagenesis. Infect. Immun. 58:55–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Paulley JT, Anderson ES, Roop RM., II 2007. Brucella abortus requires the heme transporter BhuA for maintenance of chronic infection in BALB/c mice. Infect. Immun. 75:5248–5254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bearden SW, Perry RD. 1999. The Yfe system of Yersinia pestis transports iron and manganese and is required for full virulence of plague. Mol. Microbiol. 32:403–414 [DOI] [PubMed] [Google Scholar]
- 173.Fetherston JD, Kirillina O, Bobrov AG, Paulley JT, Perry RD. 2010. The yersiniabactin transport system is critical for the pathogenesis of bubonic and pneumonic plague. Infect. Immun. 78:2045–2052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Pradel E, Guiso N, Menozzi FD, Locht C. 2000. Bordetella pertussis TonB, a Bvg-independent virulence determinant. Infect. Immun. 68:1919–1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hsieh PF, Lin TL, Lee CZ, Tsai SF, Wang JT. 2008. Serum-induced iron-acquisition systems and TonB contribute to virulence in Klebsiella pneumoniae causing primary pyogenic liver abscess. J. Infect. Dis. 197:1717–1727 [DOI] [PubMed] [Google Scholar]
- 176.Troxell B, Sikes ML, Fink RC, Vazquez-Torres A, Jones-Carson J, Hassan HM. 2011. Fur negatively regulates hns and is required for the expression of HilA and virulence in Salmonella enterica serovar Typhimurium. J. Bacteriol. 193:497–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Morton DJ, Seale TW, Bakaletz LO, Jurcisek JA, Smith A, VanWagoner TM, Whitby PW, Stull TL. 2009. The heme-binding protein (HbpA) of Haemophilus influenzae as a virulence determinant. Int. J. Med. Microbiol. 299:479–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Negre VL, Bonacorsi S, Schubert S, Bidet P, Nassif X, Bingen E. 2004. The siderophore receptor IroN, but not the high-pathogenicity island or the hemin receptor ChuA, contributes to the bacteremic step of Escherichia coli neonatal meningitis. Infect. Immun. 72:1216–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]