Summary
Investigation into the development of oxygen storage capacity in air-breathing marine predators has been performed, but little is known about the development of regulatory factors that influence oxygen utilization. Strategies for efficiently using oxygen stores should enable marine predators to optimize time spent foraging underwater.
We describe the developmental patterns of oxygen use during voluntary breath-holds in northern elephant seals (Mirounga angustirostris) at 2 and 7 weeks post-weaning. We measured 1) changes in oxygen consumption (VO2), and 2) changes in venous pH, partial pressure of oxygen (pO2), haemoglobin saturation (sO2), oxygen content (O2ct), partial pressure of carbon dioxide (pCO2), haematocrit (Hct) and total haemoglobin (tHb). To examine the effect of the dive response on the development of oxygen utilization, voluntary breath-hold experiments were conducted in and out of water.
Suppression of VO2 during voluntary breath-holds increased significantly between 2 and 7 weeks post-weaning, reaching a maximum suppression of 53% below resting metabolic rate and 56% below Kleiber’s standard metabolic rate. From 2 to 7 weeks post-weaning, breath-hold VO2 was reduced by 52%. Between the two age classes, this equates to a mean breath-hold VO2 reduction of 16% from resting VO2. Breath-hold VO2 also declined with increasing breath-hold duration, but there was no direct effect of voluntary submergence on reducing VO2.
Age did not influence rates of venous pO2 depletion during breath-holds. However, voluntary submergence did result in slower pO2 depletion rates when compared to voluntary terrestrial apnoeas. The differences in whole body VO2 during breath-holds (measured at recovery) and venous pO2 (reflective of tissue O2-use measured during breath-holds), likely reflects metabolic suppression in hypoxic, vasoconstricted tissues.
Consistent pCO2 values at the end of all voluntary breath-holds (59.0 ± 0.7 mmHg) suggests the physiological cue for stimulating respiration in northern elephant seal pups is the accumulation of CO2.
Oxygen storage capacity and metabolic suppression directly limit diving capabilities and may influence foraging success in low-weaning weight seals forced to depart to sea prior to achieving full developmental diving capacity.
Keywords: Blood gas, diving physiology, facial immersion, haemoglobin, metabolic rate pCO2, pinniped, pO2, sleep apnoea, VO2
Introduction
The reduced breath-hold capacity of juvenile marine predators limits the time available for prey localization and capture, which reduces the volume of the water column available as foraging habitat (Costa, 1991, Burns, 1999, Noren et al., 2001). In deep diving species, rapid ontogeny of oxygen storage capacity and cardiovascular regulation is needed to support independent marine foraging (Thorson & Le Boeuf, 1994, Ponganis et al., 1999). Several studies have shown the enhancement of oxygen stores through ontogenetic increases in total haemoglobin (tHb), myoglobin (Mb), haematocrit (Hct) and blood volume (BV) (Thorson et al., 1994, Burns & Castellini, 1996, Ponganis et al., 1999, Noren et al., 2002). These physiological adjustments contribute to increases in the duration of aerobic diving, i.e. the aerobic dive limit (ADL), which is defined as the dive duration at which blood lactate levels begin rising above resting levels.
Several physiological responses to diving (e.g. peripheral vasoconstriction, splenic contraction and bradycardia) can extend the ADL by optimizing the use of blood and muscle oxygen stores (Davis & Kanatous, 1999, Davis et al., 2004). This ‘dive response’ is a common physiological response to submergence that has been observed across multiple taxa (Ponganis et al., 1997, Mottishaw, Thornton & Hochachka, 1999, Jobsis, Ponganis & Kooyman, 2001, Elliott, Andrews & Jones, 2002, Davis et al., 2004, Foster & Sheel, 2005) and several features of the response are highly conserved among vertebrates (Mottishaw et al., 1999). Development of the dive response and its impact on oxygen use has been studied in a few marine mammal species, but has focused mainly on the development of sinus arrhythmia and bradycardia (Castellini et al., 1994, Noren, Cuccurullo & Williams, 2004).
Breath-holding without submergence (i.e. apnoea) produces similar cardiovascular adjustments to that observed in the dive response (Foster et al., 2005). Muscle blood flow, heart rate, cardiac output and blood oxygen depletion have been shown to be reduced during terrestrial apnoeas (Ponganis et al., 2006, Stockard et al., 2007, Ponganis et al., 2008). Although these responses to terrestrial apnoea are similar to a diving response, the impact of submergence and consistent hypoxia exposure on oxygen utilization is unclear in developing deep divers. Recent findings show that facial immersion in water enhances oxygen conservation associated with the dive response (Alboni, Alboni & Gianfranchi, 2011), though the response is not static and depends on behavioural constituents (Noren et al., 2012a). Studies on free-ranging elephant seals demonstrate that bradycardia is significantly stronger during diving when compared to terrestrial apnoea (Andrews et al., 1997), but the diving heart rate is variable in its relation to the rate of total body oxygen consumption (VO2) (Webb et al., 1998, Young et al., 2012). Together, these findings suggest greater roles for circulatory control in influencing tissue specific oxygen use in deep divers.
The northern elephant seal (Mirounga angustirostris, Gill, 1866; Fig. 1) exhibits some of the deepest and longest duration dives amongst marine air-breathing predators (Robinson et al., 2012). During foraging trips, elephant seals of all age classes (Zeno et al., 2008, Hassrick et al., 2010) routinely exhibit repetitive dives in excess of their calculated aerobic dive limit (cADL),which is a theoretical ADL that typically includes an estimated or non-diving metabolism above resting values (Costa, Gales & Goebel, 2001). A significant decrease (up to 40%) in diving VO2 from resting values has been hypothesized as the mechanism by which elephant seals often exceed their cADL (Hindell et al., 1992). Recent measures of blood oxygen tensions in free-ranging juvenile elephant seals revealed dramatic levels of hypoxia with venous blood oxygen depletion averaging 84% (Meir et al., 2009). Despite adult elephant seals routinely exceeding their cADL (Hassrick et al., 2010), the observed levels of oxygen depletion suggests that elephant seals have no need for metabolic suppression on dives of average duration (Meir et al., 2009). Attempts to assess the potential for hypometabolism in laboratory settings have been equivocal. Six elephant seals of unknown ages yielded submerged rates of oxygen consumption that averaged 26% below resting values (Webb et al., 1998). In contrast, an investigation on Steller sea lions (Eumetopias jubatus) concluded that diving metabolic rate was not significantly different from surface metabolic rate (Fahlman et al., 2008). A laboratory investigation in freely diving grey seals (Halichoerus grypus) estimated diving VO2 without exercise as 1.7 times Kleiber’s predicted standard metabolic rate (Sparling & Fedak, 2004). However, when Sparling and Fedak defined hypometabolism below resting values (Kooyman, 1989), they found a majority of dives to be hypometabolic. Thus, the extent to which hypometabolism is exhibited during dives of some air-breathing marine predators remains a controversial topic.
Fig. 1.
Weaned northern elephant seal pups (Mirounga angustirostris). Photo credit: S. Shen
The brief on-shore developmental period (2 – 3 months) in elephant seals is subsequent to a short nursing duration of around 28 days. During this period, known as the post-weaning fast (PWF), the pups rapidly develop oxygen stores despite fasting completely from food and water (Thorson et al., 1994). At the same time, the resting metabolic rate (RMR) declines (Rea & Costa, 1992). Pups with a lower weaning mass have a shortened PWF, reducing the developmental period, and potentially forcing the pups to depart to sea with a reduced oxygen storage capacity (Noren et al., 2003). While oxygen storage capacity in weaned pups is typically 25 - 45% that of adults (Thorson et al., 1994, Hassrick et al., 2010), they exhibit long duration and deep foraging dives (> 500 m) on their first trip to sea (Thorson et al., 1994).
This study investigated the developmental changes in oxygen-use during voluntary breath-holds in weaned elephant seal pups. At 2 and 7 weeks post-weaning, we examined changes in VO2, venous blood gases (pO2, pCO2), pH and oxygen carrying properties such as tHb, Hct, haemoglobin saturation (sO2) and venous oxygen content (O2ct) during voluntary breath-holds. We compared responses during terrestrial apnoeas and voluntary submergences to better understand the role that submergence plays in the development of oxygen-use in deep divers. We hypothesized that 1) VO2 would be suppressed below resting levels on a majority of all breath-holds, 2) oxygen conservation associated with the dive response would be present early in development, and 3) oxygen conservation would improve with age and breath-hold duration.
Materials and Methods
Study site and subjects
Seventeen weaned elephant seal pups (6 males and 11 females), were captured from Año Nuevo State Reserve, San Mateo County, CA, USA. At Año Nuevo State Reserve, we performed daily censuses to evaluate specific weaning dates and animals were marked with flipper-tags (Jumbo Rototag; Dalton, England) upon weaning for identification. Upon capture, unsedated animals were placed into a transfer cage and transported by truck to Sonoma State University, Rohnert Park, CA, USA. Immediately following experiments, animals were returned to Año Nuevo State Reserve. To address developmental questions, subjects were captured early (2 weeks post-weaning, n = 8) and late (7 weeks post-weaning, n = 9) within their PWF.
Metabolic chamber
Each subject was placed in a sealed respirometry chamber (Volume = 325 L; dimensions = 183 cm length × 97 cm width × 106 cm height; Den Hartog Industries, Inc., Iowa, USA) with the air volume reduced by the addition of sealed polycarbonate containers. The effective air volume after volume reduction was ~ 95 L. The chamber’s lid could be tightened on a rubber gasket during respirometry experiments to prevent air leaks and included a translucent polyethylene window for visual conformation of submergence and rest-associated apnoeas. The lid and polycarbonate containers were removed during all blood sampling procedures (see below). During all submergence experiments, the chamber was filled to 60 cm depth. At this depth of water the animals were able to fully submerge.
Metabolic rate (VO2) measurements
Oxygen consumption (VO2) was measured using a flow-through, open-circuit respirometry system (Qubit Systems, ON, Canada). The chamber was checked for leaks before every run using a nitrogen dilution technique (Fedak, Rome & Seeherman, 1981). The O2 sensor was two-point calibrated by comparing to reference gases and 100% nitrogen prior to each session. The system was corrected for sensor drift by comparing to a reference gas of known concentration every 30 min. Constant flow rates of 60 L min−1 into the chamber were controlled by a mass flow controller and were measured every second along with incurrent and excurrent O2 concentration. Air temperature and/or water temperature in the chamber was measured using a thermocouple (PTC instruments, Los Angeles, CA, USA) and averaged 20.0 ± 3.1 C. Excurrent gases were scrubbed of water using magnesium perchlorate and gases reaching the O2 sensor were scrubbed of CO2 via Ascarite II® (Thomas Scientific, Swedesboro, NJ, USA).
The animal was first placed into the dry chamber, unrestrained, and given a minimum of an hour to equilibrate to the new surroundings before any VO2 measurements commenced (average VO2 experiment duration = 132 ± 30 min). Apnoea was identified by observing when the seal’s nostrils were closed. Stopwatches were used to time breath-holds and to confirm breath-hold durations calculated from instantaneous VO2 measurements (Fig. 2). To calculate RMR for each animal, we averaged VO2 during eupnic periods between apnoeas, when the animal was stationary, calm, dry and awake for ≥ 15 minutes. Subsequent to the RMR and rest-associated apnoea VO2 measurements, the tank was filled with water. Voluntary submergence VO2 measurements were then estimated using an identical protocol to that listed above for rest-associated apnoeas. VO2 measurements collected after the animal’s nostrils breached the surface and the animal inhaled for the first time were considered recovery VO2 measurements for the duration of the recovery period (≤ 5 min), or until the next submergence began.
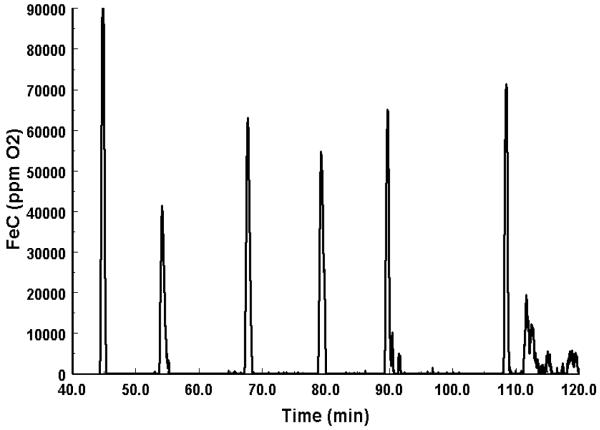
Fig. 2.
Example of a Z-transformed respirometry trace from 5 rest-associated apnoeas in one individual. Peaks in the corrected fractional extraction of oxygen (FeC) represent dramatic increases in oxygen consumption (VO2) during the recovery period. Where the corrected FeC is zero, represents periods of apnoea. These discrete periods where FeC was zero were used to define durations of breath-holds and eupnic recovery periods.
Oxygen consumption calculations
Instantaneous O2 consumption data were obtained using a Z-transformation technique (Bartholomew, Vleck & Vleck, 1981). Briefly, this method uses a pulse of pure nitrogen gas to calculate the exponential washout time of the chamber for each set of measurements. This method accounts for any lag in washout time and allows calculations of the instantaneous, corrected fractional concentration of O2 (FeC). The FeC data during submergence and apnoea were used to identify periods where gas exchange within the chamber ceased, thus enabling the duration of the breath-hold to be determined (Fig. 2). VO2 was calculated from fractional O2 and CO2 measurements, using equation 4b from (Withers, 1977). Breath-hold VO2 was calculated as the oxygen consumption over the entire recovery interval divided by the duration of the complete breath-hold cycle (i.e. breath-hold + recovery duration) (Castellini, Kooyman & Ponganis, 1992, Ponganis, Kooyman & Castellini, 1993, Webb et al., 1998). In this study, hypometabolism was defined as in (Kooyman, 1989), as any rate of metabolism (i.e. breath-hold VO2) below that of resting metabolism collected in a post-absorptive and quiet period of the 24 hour cycle. The duration for animals to recover to resting VO2 was always less than 5 minutes.
Catheterization and animal handling
Animals were chemically immobilized with an intramuscular injection (1 mg kg−1) of Telazol (tiletamine/zolazepam HCl) after completion of the respirometry measurements. Subsequent intravenous ketamine (0.5 mg kg−1) injections were used as necessary to maintain immobilization for the catheterization (all drugs from Fort Dodge Labs, Ft Dodge, IA, USA). The extradural vein was catheterized percutaneously using a long-term polyurethane catheter (MILA; 16 ga. × 25 cm). To prevent catheter displacement, we affixed the catheters to the fur of the animal using Loctite glue (Henkel Corporation, Westlake, OH, USA). An extension tube (76 cm) with a three-way stopcock system was attached to the catheter, which allowed for uncontaminated blood collection with minimal disturbance to the animal during the voluntary breath-holds and recovery periods. Animals were allowed to recover from the catheterization procedure over-night and sampling began the following day.
The mass of each seal was determined using a nylon bag suspended from a tripod and scale (MSI tension dynamometer, Seattle, WA, USA, (±1.0·kg)). Blood volume (BV) and plasma volume (PV) were determined using the methods of El-Sayed, Goodall & Hainsworth (1995), and detailed methods can be found in (Hassrick et al., 2010). Briefly, upon venous catheter placement, each animal received a 2 ml intravenous injection (0.14 - −0.31 mg kg−1) of Evan’s Blue dye at a concentration of 10 mg ml−1. Three sequential 10 ml blood samples were collected into chilled heperanized vacutainers (BD, Franklin Lakes, NJ, USA), at 10 min intervals post-injection. Samples were immediately centrifuged and plasma was stored at −80 C until analysis. To correct for hemolysis and precipitate error, PV was spectrophotometrically determined at both 624 and 740 nm. Blood volume (BV) was then calculated using PV and the maximum haematocrit collected from blood gas analysis (see below).
Blood sampling
Serial venous blood samples (~2 mL) were collected every 30 sec into chilled glass, sodium heparinized blood tubes during voluntary submergences (Sub), rest-associated apnoeas (Apnoea), and recovery periods (Recovery). Breath-holds less than 3 minutes were excluded from analysis. The recovery period consisted of samples drawn every 30 sec within the second and third minute of recovery. This time frame was chosen based on the mean surface interval of juvenile elephant seals diving at sea and the time spent in eupnea between periods of sleep apnoea in elephant seal weanlings (Blackwell & Le Boeuf, 1993, Zeno et al., 2008).
Blood draws during rest-associated apnoeas were performed first in the dry metabolic chamber. The lid from the chamber was removed and a canopy was placed above the animal’s head to minimize visual disturbance. All breath-holds and recovery durations were recorded to the nearest second using dual stopwatches. The tank was then filled with water as described above and blood samples were then collected during submergences. Submergences were voluntarily engaged by the seal and commenced when the seal’s nostrils and face were submerged. Blood samples were again collected every 30 sec into the breath-hold and at the same time during recovery as described for apnoeas in the dry chamber, i.e. 2-3 min after the animal breached the water surface and inhaled for the first time following submergence.
Blood gas analysis
Blood samples were immediately placed over ice and then gently mixed before undergoing standard blood gas analysis using a Bayer Rapidlab 845 blood gas analyzer (Siemens Medical Diagnostics, Bayer, Tarrytown, NY, USA). Blood gas parameters directly measured included the partial pressure of oxygen (pO2), partial pressure of carbon dioxide (pCO2), pH, total circulating haemoglobin (tHb), and haematocrit (Hct). Oxygen content (O2ct) and haemoglobin saturation (sO2) were calculated using the equations below.
Where O2Hb is the percent of total haemoglobin bound to oxygen, and HHb is the percent of total haemoglobin that is deoxygenated (both minus the percent of carboxyhemoglobin (COHb) and methemoglobin (MetHb)). All haemoglobin fractions were determined on the co-oximeter of the blood gas analyzer. The values of 1.34 and 0.00314 correspond to the oxygen binding factor of haemoglobin in ml O2 g−1 Hb and the solubility coefficient of oxygen, respectively.
Data Analysis
Only breath-holds with a minimum duration of 3 min were included in the analysis. We used linear mixed effect (LME) models with individual animal ID as a random subject effect to examine the fixed effects of breath-hold duration, age (Early or Late), breath-hold type (Apnoea or Submergence), and sex on blood gases or VO2 values. Akaike information criterion values (AICc) were used to choose a covariance structure for the random effect (variance components for all models) and model residuals were assessed for approximate normality. Fixed effects were evaluated using type III sum of squares. For comparisons of plasma and blood volume and RMR between ages, body mass was included as a covariate in an ANCOVA. Sex was not a significant effect in any model and was therefore removed from each model. All rates and means are reported as the mean (± SE). Statistical significance was set at α = 0.05. Data were analyzed using the statistical programs SAS 9.2 and JMP 10.0 (SAS Institute, Inc., Cary, North Carolina, USA).
To examine rates of depletion or accumulation in blood gas variables, we fit exponential functions for changes in pO2, pCO2 and pH across all breath-holds and recoveries. A coefficient of slope (β) based on the exponential function was calculated for each variable for each breath-hold and recovery and the coefficients were then compared using the same LME model structure described above. O2ct depletion rates were linear and were calculated as: (Initial value – Final value)/breath-hold duration. Calculated rates were then included in an LME with age, and breath-hold type as fixed effects and individual ID as the random effect.
“Long” (≥ 6 min) and “short” duration breath-holds (< 6 min) were separated using the median breath-hold duration of 5.9 minutes. To evaluate the impact of breath-hold duration on rates of change within blood gases, long and short duration categories were included as fixed effects in the LME model described above. The impact of the fixed effects on the β of blood gas values (pO2, pCO2, and pH) was then assessed.
To examine changes across breath-holds and in relation to recovery values, we also compared mean values of blood gases (pO2, pCO2, and pH) and haemoglobin parameters (sO2, O2ct, tHb and Hct) within the first minute of a breath-hold (Initial), the final sample (Final), and between the second and third minute of the recovery period (Recovery). This was done for every breath-hold and a LME model with Initial, Final and Recovery as fixed effects and individual ID as a random effect was used to assess variations across breath-holds. If the LME model detected a significant difference between the three measurement periods, a Tukey’s post-hoc test was used to determine which of the three periods were significantly different from one another.
Results
We obtained VO2 measurements during 154 rest-associated apnoeas and 121 submergences. From a subset of these animals (2 weeks post-weaning = 3♀ and 2♂; 7 weeks post-weaning = 6♀ and 3♂), blood gas analysis was performed on 1068 venous blood samples obtained during a total of 56 rest-associated apnoeas and 47 voluntary submergences. Mean duration of breath-holds did not differ between apnoeas and submergences (p = 0.32), which averaged 6.58 ± 0.15 minutes. Mean breath-hold duration was also not significantly different amongst age classes (p = 0.19). Mass decreased with duration of the PWF and, similar to previous studies (Thorson et al., 1994), all variables associated with blood O2 storage capacity (BV, PV, Hct, and tHb) were significantly increased late in the PWF (p < 0.05 for all; Table 1).
Table 1.
Blood oxygen storage parameters all significantly increase across the post-weaning fast (PWF), while mass decreased. Values with a letter in the superscript represent significant changes in the parameter with age. Differences were assessed using ANOVA for animals Early (2 weeks into the PWF) and Late (7 weeks into the PWF). Values are reported as mean ± SE.
| F-ratio | p-value | Early | Late | |
|---|---|---|---|---|
| Blood Volume (ml kg−1) | F1,12 = 92.3 | <0.0001 | 94.5 ± 6.7 | 170.0 ± 5.1a |
| Plasma Volume (ml kg−1) | F1,12 = 101.3 | <0.0001 | 42.6 ± 2.6 | 74.3 ± 2.0a |
| Total Haemoglobin (g dl−1) | F1,12 = 5.4 | 0.03 | 19.1 ± 0.8 | 21.5 ± 0.78a |
| Haematocrit (%) | F1,12 = 5.1 | 0.03 | 43.0 ± 1.8 | 48.6 ± 1.6a |
| Mass (kg) | F1,15 = 16.4 | 0.001 | 119.0 ± 7.0 | 81.0 ± 6.0a |
Metabolic rate (VO2)
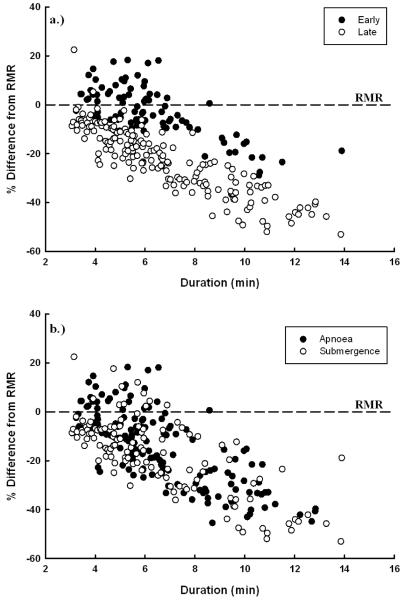
The mean RMR of animals late in the developmental fast (346.0 ± 26.0 ml O2 min−1) was significantly lower than animals early in development (544.0 ± 15.8 ml O2 min−1; F1,14 = 10.2; p =0.006). Least square means for breath-hold VO2 from the LME model containing both age and breath-hold type are shown in Table 2. Breath-hold VO2 of animals early in the PWF (522.0 ± 20.9 ml O2 min−1) was more than twice that of animals late in the PWF (273.2 ± 19.4 ml O2 min−1; Table 2). Hypometabolism was present in over 82% of all breath-holds, and the degree of hypometabolism was similar between periods of apnoea and submergence (F1,260 = 0.71, p =0.41; Fig. 3b). The degree of hypometabolism increased significantly with the duration of the breath-holds (Fig. 3). Animals late in the PWF were significantly more hypometabolic during breath-holds (20.1 ± 0.9 % below RMR) compared to animals early in the PWF (4.0 ± 1.2 % below RMR; F1,252 = 195.2, p < 0.001; Table 2). Recovery VO2 was significantly lower late in the PWF for both breath-hold types, with submergences having the lowest recovery VO2 values (F1,284 = 4.0, p = 0.046; Tukey’s: Early Apnoea = 1797 ± 74, Early Submergence= 1710 ± 85, Late Apnoea = 1195 ± 62, Late Submergence = 943 ± 62 (ml O2 min−1 for all)). Recovery VO2 values were also significantly and positively related to previous breath-hold durations (F1,285 = 22.6, p < 0.0001).
Table 2.
Least square means ± SE of oxygen consumption (VO2) and venous pO2 decay coefficients (β) during rest-associated apnoeas and voluntary submergences and at two developmental ages (Early (2 weeks into the PWF) and Late (7 weeks into the PWF)). The pO2 decay coefficient (β) is the mean exponential decay constant from changes in pO2 over the breath-holds. Rate of O2 content (O2ct) depletion is the linear rate of change in venous O2ct over the duration of breath-holds. Different superscripts denote significant differences between breath-hold type or age.
| Breath-hold VO2 (ml O2 min−1) |
Hypometabolism (% below RMR) |
pO2 decay coefficient (β) |
O2ct depletion rate (ml O2 dl−1 min−1) |
|
|---|---|---|---|---|
| Breath-hold type | ||||
| Apnoea | 399.3 ± 14.2a | 12.2 ± 0.8a | −0.095 ± .0005a | 1.38 ± 0.19a |
| Submergence | 395.9 ± 14.5a | 11.9 ± 0.9a | −0.081 ± 0.005b | 0.78 ± 0.18b |
| Age | ||||
| Early | 522.0 ± 20.9a | 4.0 ± 1.2a | −0.091 ± 0.006a | 0.92 ± 0.24a |
| Late | 273.2 ± 19.4b | 20.1 ± 0.9b | −0.084 ± 0.004a | 1.24 ± 0.17a |
Fig. 3.
Percent difference in breath-hold VO2 from resting VO2 (RMR) for all breath-holds (N = 275). The dotted line represents the mean RMR for each animal. a) Filled circles are animals early in the developmental fast and open circles represent animals late in the developmental fast. (F1,252 = 9.9, p = 0.002). b) Filled circles are rest-associated apnoeas and close circles are voluntary submergences (p > 0.05).
Blood Gases
pO2, O2ct and sO2
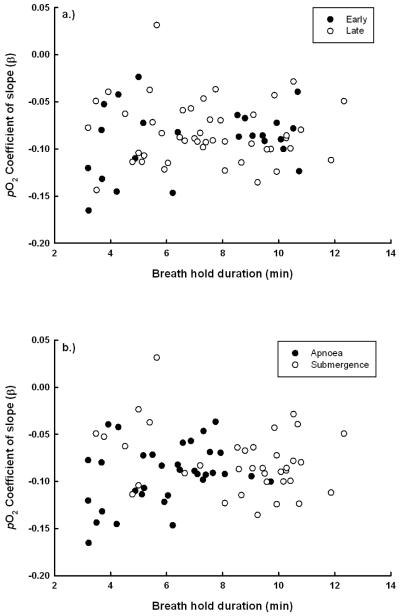
The exponential decay of venous pO2 (βpO2) was significantly faster in apnoeas than in submergences (F1, 64 = 4.5, p = 0.03; Fig. 4b). However, βpO2 did not vary with breath-hold duration (p = 0.79) or with age (p = 0.74; Fig. 4a, Table 2). Visual examination of Fig. 4b suggests this difference was largely due to increased rates of pO2 depletion on shorter duration apnoeas. Venous O2ct declined linearly across breath-holds and the rate of venous O2ct depletion was greater during apnoeas than submergences (F1,68.3 = 7.3, p < 0.01), but did not vary significantly with age (p > 0.05; Table 2).
Fig. 4.
Exponential coefficent (β) for rate of pO2 depletion across breath-holds (N = 70). a) Filled circles are animals early in the PWF (2 weeks post weaned) and open circles represent animals late in the PWF (7 weeks post weaned). (p > 0.05). b) Filled circles are rest-associated apnoeas and open circles are voluntary submergences (F1, 64 = 4.5, p = 0.03).
Initial pO2 values were positively related to subsequent breath-hold duration, with higher initial values seen on longer duration breath-holds (67.6 ± 3.6 mmHg (≥ 6 min) vs. 54.8 ± 2.8 mmHg (< 6 min); F1,41 = 8.4, p = 0.006). Average pO2 values obtained during the short recovery period were statistically similar to initial pO2 values at the beginning of breath-holds (F1,80 = 2.2, p = 0.14: Table 3), despite significant depletion of pO2 across breath-holds. Similar patterns were seen for both sO2 and O2ct (Table 3). The exponential pO2 recovery rate (β) was significantly faster during recoveries from longer duration breath-holds (F1,32 = 11.3, p = 0.002).
Table 3.
Ranges of blood gas parameters for all breath-holds. Initial (< 1 min), Final and Recovery (2 – 3 min) blood gas values compared across all breath-holds. Different letters in the superscript denote significant differences in that parameter between the three time periods. Values are reported as mean ± SE. Venous blood parameters represented in the table are partial pressure of oxygen (pO2), partial pressure of carbon dioxide (pCO2), saturation of haemoglobin (sO2), oxygen content (O2ct), total circulating haemoglobin (tHb), haematocrit (Hct), and pH. Differences were assessed using a linear mixed effect model with seal ID as a random effect. Differences in least square means were assessed using a Tukey’s test following a significant difference between periods.
| Range | Initial (< 1 min.) | Final | Recovery (2-3 min.) | F-ratio: p-value (LMM) | |
|---|---|---|---|---|---|
| pO2 (mmHg) | 16.5 - 108 | 60.2 ± 1.9 a | 35.3 ± 1.4 b | 55.1 ± 2.0 a | F2,159 = 68.6, p < 0.0001 |
| pCO2 (mmHg) | 28.2 - 77.7 | 51.3 ± 1.0 a | 59.0 ± 0.7 b | 55.7 ± 1.1 c | F2,155 = 155, p < 0.0001 |
| sO2 (%) | 24.5 - 100 | 89.7 ± 3.4 a | 64.4 ± 3.0 b | 88.0 ± 3.4 a | F2,147 = 73.3, p < 0.0001 |
| O2ct (ml O2 dl−1) | 3.4 – 31.6 | 24.0 ± 1.3 a | 17.9 ± 1.2 b | 25.5 ± 1.3 a | F2,142 = 60.5, p < 0.0001 |
| tHb (g dl−1) | 5.5 - 27 | 22.2 ± 0.4 a | 21.5 ± 0.3 a | 24.3 ± 0.5 b | F2,148 = 16.9, p < 0.0001 |
| Hct (%) | 12.4 - 58 | 50.0 ± 0.9 a | 48.2 ± 0.7 a | 53.1 ± 1.2 b | F2,132 = 11.6, p < 0.0001 |
| pH | 7.204 - 7.447 | 7.334 ± 0.005 a | 7.314 ± 0.004 b | 7.320 ± 0.006 ab | F2,142 = 8.0, p = 0.0005 |
pCO2 and pH
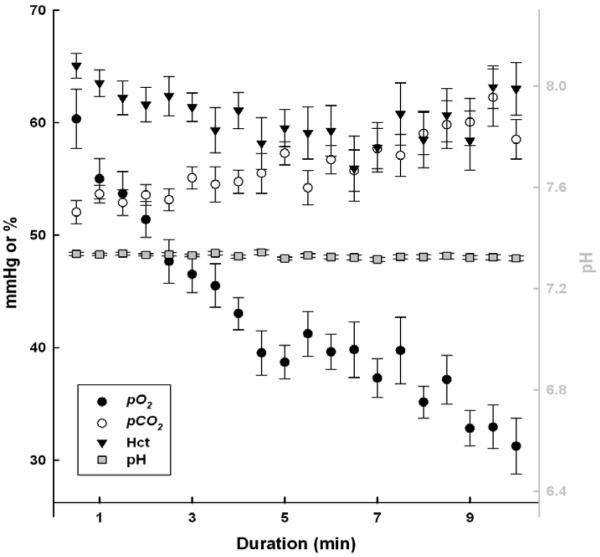
The pattern of pCO2 increasing during breath-holds was more variable among animals (i.e. linear, exponential and no change), although most frequently increases in pCO2 during longer duration breath-holds (> 6 min) appeared to be linear (Fig. 5). Despite these patterns, the mean final pCO2 values were not significantly different when comparing breath-hold types, age, and breath-hold duration (final pCO2 = 59.0 ± 0.7 mmHg; p > 0.05 for all). Contrary to the animals’ ability to rapidly recover pO2 to initial values during the short recovery period, pCO2 recovery values remained elevated during this time frame (Table 3). Although statistically significant, the decrease in mean pH from initial to final samples was minor (ΔpH = 0.021; Table 3) and pH was relatively stable across breath-holds and recoveries (Fig. 5).
Fig. 5.
Mean venous partial pressure of oxygen (pO2), partial pressure of carbon dioxide (pCO2), haematocrit (Hct) and pH at 30 second intervals combined across all breath-holds for all animals. Samples from breath-holds exceeding 10 minutes were not included in the figure. Black and white symbols (pO2, pCO2 and Hct) are associated with the left y-axis. Gray symbols with square symbols (pH) are associated with the right y-axis. Each point is represented by mean ± SE for every animal at that specific duration.
Haemoglobin
Rates of change in [tHb] across breath-holds were significantly different between age classes (F1,56 = 15.6, p = 0.0002). Early in the PWF, animals displayed exponential increases in [tHb] (βEarly = 0.041 ± 0.009) while changes were negligible for animals late into the PWF (βLate = −0.002 ± 0.007). Seals early in the PWF experienced a more rapid exponential increase in Hct (βEarly = 0.036 ± 0.011) across breath-holds than did older animals (βLate = 0.004 ± 0.008; F1,62 = 5.1, p = 0.03). No significant difference in the magnitude of change of [tHb] or Hct was seen due to breath-hold type (p > 0.05 for all). Differences in the initial and final values of tHb and Hct were significant during recovery periods (p < 0.05 for both; Table 3).
Discussion
VO2 during breath-holds
The developmental suppression in metabolism during breath-holds is critically linked to the duration of the PWF. Animals forced to depart to sea prematurely, due to a low weaning mass, not only leave with lower oxygen stores but have a reduced ability to conserve oxygen while diving (Noren et al., 2003, Noren et al., 2008). This, in turn, could impact foraging behaviour and success (Bennett et al., 2010). The degree of hypometabolism observed during breath-holds of animals late in the PWF, which is characterized relative to the RMR, is consistent with hypotheses regarding the degree of hypometabolism (~ 40% below RMR) required to consistently exceed the cADL in free-ranging elephant seals (Hindell et al., 1992). When compared to resting levels, pups late in the PWF conserved significantly more oxygen during breath-holds (564 ± 54 ml O2) than did the underdeveloped pups (150 ± 72 ml O2). Over the course of the PWF, this equates to a 73% savings in venous oxygen stores during breath-holds of older pups.
Weaned pups lowered VO2 at least 5% below RMR on a majority of breath-holds (79%); while the magnitude and frequency of hypometabolism increased on longer duration breath-holds (91% of breath-holds ≥ 6 min were hypometabolic). Despite elephant seal weanlings have O2 stores capable of supporting aerobic metabolism over the average dive durations seen in juveniles (~ 14 min; Meir et al., 2009), the O2 stores are not sufficient to support the repetitive long-duration dives matched with short surface intervals consistently seen in all age classes (Zeno et al., 2008, Robinson et al., 2012). Together with previous studies in adults, which shows a large portion (~30%) of dives exceeding the cADL (Hassrick et al., 2010), our data suggest a potential role for consistent hypometabolism in explaining extended dive durations in free-ranging, exercising animals. Additionally, previous research using the cADL to predict the duration allotted for aerobic diving assumed several fixed variables, typically including a diving metabolism above RMR (Kooyman et al., 1980, Olesiuk, 1993, Nilssen et al., 2000, Costa et al., 2001, Sparling et al., 2004). The consistent display of hypometabolism and pronounced negative relationship between VO2 and the duration of breath-holds suggests that in some species, tissue level responses to hypoxia and hypoperfusion may serve to reduce VO2 while diving, despite the metabolic costs of exercise.
This study examined the development of oxygen-use associated with the dive response in the absence of both exercise and possible pressure effects. Diving animals must exercise (swim) to some extent to either reach depths suitable for foraging or to return to the surface (Castellini et al., 1985). Exercise increases VO2 and conflicts with the O2 conservation effects associated with the dive response (Noren et al., 2012a), however elephant seals and other marine mammals have shown energy-efficient gliding behaviours which can save ~60% of the energy required to actively stroke (Williams et al., 2000). Together, an energy efficient swimming strategy, high levels of hypoxia tolerance and tissue level hypometabolism may allow elephant seals to consistently maintain aerobic metabolism over dive durations that greatly exceed previous cADL estimates.
Similar to previous studies, we found dramatic reductions in mass-controlled RMR across the PWF (Kohin, Williams & Ortiz, 1999, Noren et al., 2004). It is important to note that fasting is known to induce a reduction in the RMR that is partially dependent upon the time fasting. Thus, it is difficult to separate the relative influences of ontogeny and fasting on the RMR in the elephant seal pup. Although, the progression of the PWF is associated with increases in thyroid hormones (Ortiz et al., 2003), which typically help regulate levels of resting energy expenditure. Despite this developmental reduction in mass-controlled RMR, we note that hypometabolism during breath-holds was frequent in this study and was defined as reductions in breath-hold VO2 from the RMR (i.e. resting VO2).
The regulatory mechanism behind hypometabolism in deep divers is hypothesized to be a cellular O2 sensory system directly linked to O2 utilization and availability (Hochachka, 1994, Hochachka & Lutz, 2001). Hypoxia inducible factors (HIF) are a group of transcription factors which have widely been shown to have O2-conserving effects during periods of low cellular O2 concentrations (Iyer et al., 1998, Semenza, 1999). In a concurrent study on these same subjects, Vázquez-Medina and colleagues (2011) showed a dramatic increase (25-70%) in the muscle protein content of HIF-1α after repetitive breath-holds. Metabolic alterations due to increased HIF-1α expression in response to muscle ischemia and hypoxia may be responsible for the systemic VO2 reductions seen in the current study. Additionally, constitutive HIF-1α expression due to frequent breath-holds across the PWF may contribute to the overall reductions seen in RMR.
Blood O2 During Breath-Holds
Elephant seal pups exhibited slower depletion of venous pO2 during submergences than during terrestrial apnoeas. Slower pO2 depletion rates associated with the dive response are consistent with the idea that facial immersion enhances peripheral vasoconstriction and assists in the reduction of VO2 (Alboni et al., 2011). While there were reductions in breath-hold VO2 with age, rates of venous blood oxygen-use during breath-holds were not different between age classes. As venous pO2 represents oxygen-use by tissues, the developmental reductions in whole body breath-hold metabolism (i.e. breath-hold VO2) can then be attributed to tissue-specific VO2 reductions in the most vasoconstricted tissues during breath-holds.
The lowest blood oxygen values in this study was seen in the breath-hold of a pup early in the PWF (pO2 = 16.5 mmHg; sO2 = 24.5%; O2ct = 5.7 ml dl−1). These extremely low O2ct values exhibited early in development resemble oxygen levels witnessed at the conclusion of forced dives when the dive response is assumed to be maximal (Elsner et al., 1964, Kerem & Elsner, 1973). Further, the lowest pO2 values achieved during breath-holds did not differ with age, demonstrating that elephant seals possess an extreme tolerance to hypoxia soon after weaning. The changes in initial and final pO2 values during all breath-holds resemble those seen in cetacean species during voluntary submergences (Noren et al., 2012b), yet the rate of depletion appears much slower in elephant seals (Table 2). This variation in pO2 depletion rates between species explains the differences in pH and pCO2 patterns discussed below.
Blood CO2 and Buffering Capacity During Breath-Holds
The consistency of venous pCO2 (59.0 ± 0.7 mmHg) within the final blood sample suggests that pCO2 is the physiological cue signalling apnoeic elephant seals to breathe. This finding is consistent with the CO2-dependent stimulation of respiration in mammals (Phillipson, Duffin & Cooper, 1981, Nattie, 1999, Richerson, 2004) and is similar to results from hypercapnic seals (Kohin et al., 1999). When examining the final pCO2 values obtained in other studies on deep divers (Kooyman et al., 1980, Stockard et al., 2007), we discovered values at the end of long duration breath holds that were similar to the values found in this study. The similarities in the final mean pCO2 between ages and both types of breath-holds implies relatively little ontogenetic modification in the CO2-dependent mechanism for stimulating breathing, that it is established early in development, and that it is unaffected by the dive response.
Despite wide variations in breath-hold durations and significant depletion of O2 stores, mean pH varied little across breath-holds types for all ages. Several cetacean species lack the ability to maintain pH across long-duration breath-holds (Noren et al., 2012b), yet studies on pinnipeds display pH maintenance or even decreases in pH across breath-holds (Kooyman et al., 1980, Qvist et al., 1986, Stockard et al., 2007). This study separates from others demonstrating that the development of acid-base balance during periods of hypoxia is present at a very early age in elephant seals.
Haemoglobin and Haematocrit During Breath-Holds
Splenic contraction during a dive mobilizes oxygenated erythrocytes into circulation (Hurford et al., 1996, Thornton et al., 2001). Haematocrit is also known to increase across the duration of sleep apnoea in elephant seals (Castellini, Costa & Huntley, 1986, Stockard et al., 2007) and during diving (Kooyman et al., 1980). Despite lower blood volumes and [tHb] concentrations, the more rapid increase in [tHb] and Hct was seen during breath-holds early in development. This suggests less sympathetic control over splenic contraction and the caval sphincter, leading to a more rapid release of erythrocytes into circulation. We also witnessed significantly higher values of both Hct and [tHb] during recovery in both age classes, suggesting splenic relaxation is slow or splenic contraction is occurring at the end of breath-holds. Similar patterns of splenic relaxation following a forced dive have been observed in weanling seals through MRI visualization techniques (Thornton et al., 2001). Although this feature should facilitate the replenishment of O2 during recovery periods, the manner in which splenic relaxation occurs over the course of development, remains uncertain (i.e. splenic relaxation being quicker or more variable).
Perspectives and Significance
This study provides evidence for the ontogeny of hypometabolism during breath-holds in the elephant seal. Different responses in whole body VO2 and venous pO2 depletion rates between breath-hold types and ages suggest that hypometabolism is occurring predominantly in vasoconstricted tissues. Breath-hold VO2 decreased across the PWF suggesting an increasing ability to down-regulate tissue metabolism or an enhanced peripheral vasoconstriction response leading to greater hypoperfusion of tissues. The current study did not attempt to investigate the impact of body reserves or behaviour on oxygen use across development. For example, elephant seal pups increase the time spent in surf zone waters with the progression of the fast, but it is unknown whether small pups could compensate for a reduced developmental period (leading to decreased O2 stores) by increasing the proportion of time spent in voluntary submergence. Our findings provide strong evidence that critical changes in patterns of oxygen use occur during the PWF and that oxygen use is strongly influenced by breath-hold duration. Pups also exhibited extreme hypoxia tolerance immediately upon weaning. In addition to the well studied increases in body oxygen stores across development, these features of oxygen use may be critically important in allowing developing phocids to achieve breath-hold durations required for successful foraging.
Acknowledgements
This project was supported by National Institute of Health (NHLBI R01-HL09176) and California State University’s Council on Ocean Affairs, Science and Technology (COAST) student award. Special thanks to J. Vázquez-Medina, C. Champagne, M. Fowler, J. Jelincic, B. Kelso, S. Boaz, S. Tavoni, J. Cutler, V. Farnham, Z. Dallara and many other field volunteers for making this research possible. Thank you to the rangers and docents at Año Nuevo State Reserve. This research was conducted through the National Marine Fisheries Service permit No. 14636 and approved by the Institutional Animal Care and Use Committee (IACUC) at Sonoma State University.
References
- Alboni P, Alboni M, Gianfranchi L. Diving bradycardia: a mechanism of defense against hypoxic damage. Journal of Cardiovascular Medicine. 2011;12:422–427. doi: 10.2459/JCM.0b013e328344bcdc. [DOI] [PubMed] [Google Scholar]
- Andrews RD, Jones DR, Williams JD, Thorson PH, Oliver GW, Costa DP, Le Boeuf BJ. Heart rates of northern elephant seals diving at sea and resting on the beach. Journal of Experimental Biology. 1997;200:2083–2095. doi: 10.1242/jeb.200.15.2083. [DOI] [PubMed] [Google Scholar]
- Bartholomew GA, Vleck D, Vleck CM. Instantaneous measurements of oxygen consumption during pre-flight warm-up and post-flight cooling in sphingid and saturniid moths. Journal of Experimental Biology. 1981;90:17–32. [Google Scholar]
- Bennett KA, Mcconnell BJ, Moss SEW, Speakman JR, Pomeroy PP, Fedak MA. Effects of Age and Body Mass on Development of Diving Capabilities of Gray Seal Pups: Costs and Benefits of the Postweaning Fast. Physiological and Biochemical Zoology. 2010;83:911–923. doi: 10.1086/656925. [DOI] [PubMed] [Google Scholar]
- Blackwell SB, Le Boeuf BJ. Developmental aspects of sleep apnoea in northern elephant seals, Mirounga angustirostris. Journal of Zoology, London. 1993;231:437–447. [Google Scholar]
- Burns JM. The development of diving behavior in juvenile Weddell seals: pushing physiological limits in order to survive. Canadian Journal of Zoology. 1999;77:737–747. [Google Scholar]
- Burns JM, Castellini MA. Physiological and behavioral determinants of the aerobic dive limit in Weddell seal (Leptonychotes weddellii) pups. Journal of Comparative Physiology B: Biochemical, Systemic, and Environmental Physiology. 1996;166:473–483. [Google Scholar]
- Castellini MA, Costa DP, Huntley A. Hematocrit variation during sleep apnea in elephant seal pups. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 1986;251:R429–R431. doi: 10.1152/ajpregu.1986.251.2.R429. [DOI] [PubMed] [Google Scholar]
- Castellini MA, Kooyman GL, Ponganis PJ. Metabolic rates of freely diving weddell seals: correlations with oxygen stores, swim velocity and diving duration. Journal of Experimental Biology. 1992;165:181–194. doi: 10.1242/jeb.165.1.181. [DOI] [PubMed] [Google Scholar]
- Castellini MA, Murphy BJ, Fedak M, Ronald K, Gofton N, Hochachka PW. Potentially conflicting metabolic demands of diving and exercise in seals. Journal of Applied Physiology. 1985;58:392–399. doi: 10.1152/jappl.1985.58.2.392. [DOI] [PubMed] [Google Scholar]
- Castellini MA, Rea LD, Sanders JL, Castellini JM, Zenteno-Savin T. Developmental changes in cardiorespiratory patterns of sleep-associated apnea in northern elephant seals. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 1994;267:R1294–R1301. doi: 10.1152/ajpregu.1994.267.5.R1294. [DOI] [PubMed] [Google Scholar]
- Costa DP. Reproductive and foraging energetics of high latitude penguins, albatrosses and pinnipeds: Implications for life history patterns. American Zoologist. 1991;31:111–130. [Google Scholar]
- Costa DP, Gales NJ, Goebel ME. Aerobic dive limit: how often does it occur in nature? Comparative Biochemistry and Physiology, Part A. 2001;129:771–783. doi: 10.1016/s1095-6433(01)00346-4. [DOI] [PubMed] [Google Scholar]
- Davis RW, Kanatous SB. Convective oxygen transport and tissue oxygen consumption in Weddell seals during aerobic dives. Journal of Experimental Biology. 1999;202:1091–1113. doi: 10.1242/jeb.202.9.1091. [DOI] [PubMed] [Google Scholar]
- Davis RW, Polasek L, Watson R, Fuson A, Williams TM, Kanatous SB. The diving paradox: new insights into the role of the dive response in air-breathing vertebrates. Comparative Biochemistry and Physiology, Part A. 2004;138:263–268. doi: 10.1016/j.cbpb.2004.05.003. [DOI] [PubMed] [Google Scholar]
- El-Sayed H, Goodall SR, Hainsworth R. Re-evaluation of Evans blue dye dilution method of plasma volume measurement. Clinical and laboratory haematology. 1995;17:189–194. [PubMed] [Google Scholar]
- Elliott NM, Andrews RD, Jones DR. Pharmacological blockade of the dive response: effects on heart rate and diving behaviour in the harbour seal (Phoca vitulina) Journal of Experimental Biology. 2002;205:3757–3765. doi: 10.1242/jeb.205.23.3757. [DOI] [PubMed] [Google Scholar]
- Elsner RW, Scholander PF, Craig AB, Dimond EG, Irving L, Pilson M, Johansen K, Bradstreet E. A venous blood oxygen reservoir in the diving elephant seal. The Physiologist. 1964;7:1. [Google Scholar]
- Fahlman A, Svã¤Rd C, Rosen DAS, Jones DR, Trites AW. Metabolic costs of foraging and the management of O2 and CO2 stores in Steller sea lions. Journal of Experimental Biology. 2008;211:3573–3580. doi: 10.1242/jeb.023655. [DOI] [PubMed] [Google Scholar]
- Fedak MA, Rome L, Seeherman HJ. One-step N2-dilution technique for calibrating open-circuit VO2 measuring systems. Journal of Applied Physiology. 1981;51:772–776. doi: 10.1152/jappl.1981.51.3.772. [DOI] [PubMed] [Google Scholar]
- Foster GE, Sheel AW. The human diving response, its function, and its control. Scandinavian Journal of Medicine & Science in Sports. 2005;15:3–12. doi: 10.1111/j.1600-0838.2005.00440.x. [DOI] [PubMed] [Google Scholar]
- Hassrick JL, Crocker DE, Teutschel NM, Mcdonald BI, Robinson PW, Simmons SE, Costa DP. Condition and mass impact oxygen stores and dive duration in adult female northern elephant seals. Journal of Experimental Biology. 2010;213:585–592. doi: 10.1242/jeb.037168. [DOI] [PubMed] [Google Scholar]
- Hindell MA, Slip DJ, Burton HR, Bryden MM. Physiological implications of continuous, prolonged, and deep dives of the southern elephant seal (Mirounga leonina) Canadian Journal of Zoology. 1992;70:370–379. [Google Scholar]
- Hochachka PW. Muscles as molecular and metabolic machines. CRC Press; Boca Raton: 1994. [Google Scholar]
- Hochachka PW, Lutz PL. Mechanism, origin, and evolution of anoxia tolerance in animals. Comparative Biochemistry and Physiology B-Biochemistry & Molecular Biology. 2001;130:435–459. doi: 10.1016/s1096-4959(01)00408-0. [DOI] [PubMed] [Google Scholar]
- Hurford WE, Hochachka PW, Schneider RC, Guyton GP, Stanek KS, Zapol DG, Liggins GC, Zapol WM. Splenic contraction, catecholamine release, and blood volume redistribution during diving in the Weddell seal. Journal of Applied Physiology. 1996;80:298–306. doi: 10.1152/jappl.1996.80.1.298. [DOI] [PubMed] [Google Scholar]
- Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY, Semenza GL. Cellular and developmental control of O-2 homeostasis by hypoxia-inducible factor 1 alpha. Genes & Development. 1998;12:149–162. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobsis PD, Ponganis PJ, Kooyman GL. Effects of training on forced submersion responses in harbor seals. Journal of Experimental Biology. 2001;204:3877–3885. doi: 10.1242/jeb.204.22.3877. [DOI] [PubMed] [Google Scholar]
- Kerem D, Elsner R. Cerebral tolerance to asphyxial hypoxia in the harbor seal. Respiration Physiology. 1973;19:188–200. doi: 10.1016/0034-5687(73)90077-7. [DOI] [PubMed] [Google Scholar]
- Kohin S, Williams TM, Ortiz CL. Effects of hypoxia and hypercapnia on aerobic metabolic processes in northern elephant seals. Respiration Physiology. 1999;117:59–72. doi: 10.1016/s0034-5687(99)00050-x. [DOI] [PubMed] [Google Scholar]
- Kooyman G, Wahrenbrock E, Castellini M, Davis R, Sinnett E. Aerobic and anaerobic metabolism during voluntary diving in Weddell seals: Evidence of preferred pathways from blood chemistry and behavior. Journal of Comparative Physiology B: Biochemical, Systemic, and Environmental Physiology. 1980;138:335–346. [Google Scholar]
- Kooyman GL. Diverse divers: physiology and behaviour. Springer-Verlag; Berlin: 1989. [Google Scholar]
- Meir JU, Champagne CD, Costa DP, Williams CL, Ponganis PJ. Extreme hypoxemic tolerance and blood oxygen depletion in diving elephant seals. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2009;297:927–939. doi: 10.1152/ajpregu.00247.2009. [DOI] [PubMed] [Google Scholar]
- Mottishaw PD, Thornton SJ, Hochachka PW. The Diving Response Mechanism and its Surprising Evolutionary Path in Seals and Sea Lions. American Zoologist. 1999;39:434–450. [Google Scholar]
- Nattie E. CO2, brainstem chemoreceptors and breathing. Progress in neurobiology. 1999;59:299–331. doi: 10.1016/s0301-0082(99)00008-8. [DOI] [PubMed] [Google Scholar]
- Nilssen KT, Pedersen O-P, Folkow LP, Haug T. Food consumption estimates of Barents Sea harp seals. Vol. 2. NAMMCO Scientific Publications; 2000. pp. 9–28. [Google Scholar]
- Noren DP, Crocker DE, Williams TM, Costa DP. Energy reserve utilization in northern elephant seal (Mirounga angustirostris) pups during the postweaning fast: size does matter. Journal of Comparative Physiology B: Biochemical, Systemic, and Environmental Physiology. 2003;173:443–454. doi: 10.1007/s00360-003-0353-9. [DOI] [PubMed] [Google Scholar]
- Noren SR, Boness DJ, Iverson SJ, Mcmillan J, Bowen WD. Body condition at weaning affects the duration of the postweaning fast in gray seal pups (Halichoerus grypus) Physiological and Biochemical Zoology. 2008;81:269–277. doi: 10.1086/528777. [DOI] [PubMed] [Google Scholar]
- Noren SR, Cuccurullo V, Williams TM. The development of diving bradycardia in bottlenose dolphins (Tursiops truncatus) Journal of Comparative Physiology B: Biochemical, Systemic, and Environmental Physiology. 2004;174:139–147. doi: 10.1007/s00360-003-0398-9. [DOI] [PubMed] [Google Scholar]
- Noren SR, Kendall T, Cuccurullo V, Williams TM. The dive response redefined: underwater behavior influences cardiac variability in freely diving dolphins. The Journal of Experimental Biology. 2012a;215:2735–2741. doi: 10.1242/jeb.069583. [DOI] [PubMed] [Google Scholar]
- Noren SR, Lacave G, Wells RS, Williams TM. The development of blood oxygen stores in bottlenose dolphins (Tursiops truncatus): implications for diving capacity. Journal of Zoology, London. 2002;258:105–113. [Google Scholar]
- Noren SR, Williams TM, Pabst DA, Mclellan WA, Dearolf JL. The development of diving in marine endotherms: preparing the skeletal muscles of dolphins, penguins, and seals for activity during submergence. Journal of Comparative Physiology B: Biochemical, Systemic, and Environmental Physiology. 2001;171:127–134. doi: 10.1007/s003600000161. [DOI] [PubMed] [Google Scholar]
- Noren SR, Williams TM, Ramirez K, Boehm J, Glenn M, Cornell L. Changes in partial pressures of respiratory gases during submerged voluntary breath hold across odontocetes: is body mass important? Journal of Comparative Physiology B: Biochemical, Systemic, and Environmental Physiology. 2012b;182:299–309. doi: 10.1007/s00360-011-0612-0. [DOI] [PubMed] [Google Scholar]
- Olesiuk PF. Annual prey consumption by harbor seals (Phoca vitulina) in the Strait of Georgia, British Columbia. Fish bulletin. 1993;91:491–515. [Google Scholar]
- Ortiz RM, Houser DS, Wade CE, Leo Ortiz C. Hormonal changes associated with the transition between nursing and natural fasting in northern elephant seals (Mirounga angustirostris) General and Comparative Endocrinology. 2003;130:78–83. doi: 10.1016/s0016-6480(02)00572-5. [DOI] [PubMed] [Google Scholar]
- Phillipson EA, Duffin J, Cooper JD. Critical dependence of respiratory rhythmicity on metabolic CO2 load. Journal of Applied Physiology. 1981;50:45–54. doi: 10.1152/jappl.1981.50.1.45. [DOI] [PubMed] [Google Scholar]
- Ponganis PJ, Kooyman GL, Castellini MA. Determinants of the aerobic dive limit of weddell seals: analysis of diving metabolic rates, postdive end tidal PO2′s, and blood and muscle oxygen stores. Physiological Zoology. 1993;66:732–749. [Google Scholar]
- Ponganis PJ, Kooyman GL, Winter LM, Starke LN. Heart rate and plasma lactate responses during submerged swimming and trained diving in California sea lions, Zalophus californianus. Journal of comparative physiology. B, Biochemical, systemic, and environmental physiology. 1997;167:9–16. doi: 10.1007/s003600050042. [DOI] [PubMed] [Google Scholar]
- Ponganis PJ, Kreutzer U, Stockard TK, Lin PC, Sailasuta N, Tran TK, Hurd R, Jue T. Blood flow and metabolic regulation in seal muscle during apnea. Journal of Experimental Biology. 2008;211:3323–3332. doi: 10.1242/jeb.018887. [DOI] [PubMed] [Google Scholar]
- Ponganis PJ, Starke LN, Horning M, Kooyman GL. Development of diving capacity in emperor penguins. Journal of Experimental Biology. 1999;202:781–786. doi: 10.1242/jeb.202.7.781. [DOI] [PubMed] [Google Scholar]
- Ponganis PJ, Stockard TK, Levenson DH, Berg L, Baranov EA. Cardiac output and muscle blood flow during rest-associated apneas of elephant seals. Comparative Biochemistry and Physiology, Part A. 2006;144:105–111. doi: 10.1016/j.cbpa.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Qvist J, Hill RD, Schneider RC, Falke KJ, Liggins GC, Guppy M, Elliot RL, Hochachka PW, Zapol WM. Hemoglobin concentrations and blood gas tensions of free-diving Weddell seals. Journal of Applied Physiology. 1986;61:1560–1569. doi: 10.1152/jappl.1986.61.4.1560. [DOI] [PubMed] [Google Scholar]
- Rea LD, Costa DP. Changes in standard metabolism during long-term fasting in northern elephant seal pups (Mirounga angustirostris) Physiological Zoology. 1992;65:97–111. [Google Scholar]
- Richerson GB. Serotonergic neurons as carbon dioxide sensors that maintain pH homeostasis. Nature Reviews Neuroscience. 2004;5:449–461. doi: 10.1038/nrn1409. [DOI] [PubMed] [Google Scholar]
- Robinson PW, Costa DP, Crocker DE, Gallo-Reynoso JP, Champagne CD, Fowler MA, Goetsch C, Goetz KT, Hassrick JL, Huckstadt LA. Foraging Behavior and Success of a Mesopelagic Predator in the Northeast Pacific Ocean: Insights from a Data-Rich Species, the Northern Elephant Seal. PloS one. 2012;7:e36728. doi: 10.1371/journal.pone.0036728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annual review of cell and developmental biology. 1999;15:551–578. doi: 10.1146/annurev.cellbio.15.1.551. [DOI] [PubMed] [Google Scholar]
- Sparling CE, Fedak MA. Metabolic rates of captive grey seals during voluntary diving. Journal of Experimental Biology. 2004;207:1615–1624. doi: 10.1242/jeb.00952. [DOI] [PubMed] [Google Scholar]
- Stockard TK, Levenson DH, Berg L, Fransioli JR, Baranov EA, Ponganis PJ. Blood oxygen depletion during rest-associated apneas of northern elephant seals (Mirounga angustirostris) Journal of Experimental Biology. 2007;210:2607–2617. doi: 10.1242/jeb.008078. [DOI] [PubMed] [Google Scholar]
- Thornton SJ, Spielman DM, Pelc NJ, Block WF, Crocker DE, Costa DP, Le Boeuf BJ, Hochachka PW. Effects of forced diving on the spleen and hepatic sinus in northern elephant seal pups. Proceedings of the National Academy of Sciences. 2001;98:9413–9418. doi: 10.1073/pnas.151192098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorson PH, Le Boeuf BJ. Developmental aspects of diving in northern elephant seal pups. In: Le Boeuf BJ, Laws RM, editors. Elephant Seals: Population Ecology, Behavior, and Physiology. University of California Press; Berkeley: 1994. pp. 271–289. [Google Scholar]
- Vázquez-Medina JP, Zenteno-Saván T, Tift MS, Forman HJ, Crocker DE, Ortiz RM. Apnea stimulates the adaptive response to oxidative stress in elephant seal pups. The Journal of Experimental Biology. 2011;214:4193–4200. doi: 10.1242/jeb.063644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb PM, Costa DP, Le Boeuf BJ, Andrews RD. Heart rate and oxygen consumption of northern elephant seals during diving in the laboratory. Physiological and Biochemical Zoology. 1998;71:116–126. doi: 10.1086/515894. [DOI] [PubMed] [Google Scholar]
- Williams T, Davis RW, Fuiman LA, Francis J, Le Boeuf BJ, Horning M, Calambokidis J, Croll DA. Sink or swim: Strategies for cost-efficient diving by Marine Mammals. Science. 2000;288:133–136. doi: 10.1126/science.288.5463.133. [DOI] [PubMed] [Google Scholar]
- Withers PC. Measurement of VO2, VCO2, and evaporative water loss with a flow-through mask. Journal of Applied Physiology. 1977;42:120–123. doi: 10.1152/jappl.1977.42.1.120. [DOI] [PubMed] [Google Scholar]
- Young BL, Rosen DAS, Hindle AG, Haulena M, Trites AW. Dive behaviour impacts the ability of heart rate to predict oxygen consumption in Steller sea lions (Eumetopias jubatus) foraging at depth. The Journal of Experimental Biology. 2012;214:2267–2275. doi: 10.1242/jeb.047340. [DOI] [PubMed] [Google Scholar]
- Zeno RL, Crocker DE, Hassrick JL, Allen SG, Costa DP. Development of foraging behavior in juvenile northern elephant seals. Journal of Zoology. 2008;274:180–187. [Google Scholar]