Abstract
Chromosomal DNA replication intermediates, revealed in ligase-deficient conditions in vivo, are of low molecular weight independently of the organism, suggesting discontinuous replication of both the leading and the lagging DNA strands. Yet, in vitro experiments with purified enzymes replicating sigma-structured substrates show continuous synthesis of the leading DNA strand in complete absence of ligase, supporting the textbook model of semi-discontinuous DNA replication. The discrepancy between the in vivo and in vitro results is rationalized by proposing that various excision repair events nick continuously-synthesized leading strands after synthesis, producing the observed low molecular weight intermediates. Here we show that, in an E. coli ligase-deficient strain with all known excision repair pathways inactivated, new DNA is still synthesized discontinuously. Furthermore, hybridization to strand-specific targets demonstrates that the low molecular weight replication intermediates come from both the lagging and the leading strands. These results support the model of discontinuous leading strand synthesis in E. coli.
Keywords: Okazaki fragments, ligA mutants, excision repair, pulse-labeling, strand-specific hybridization
Introduction
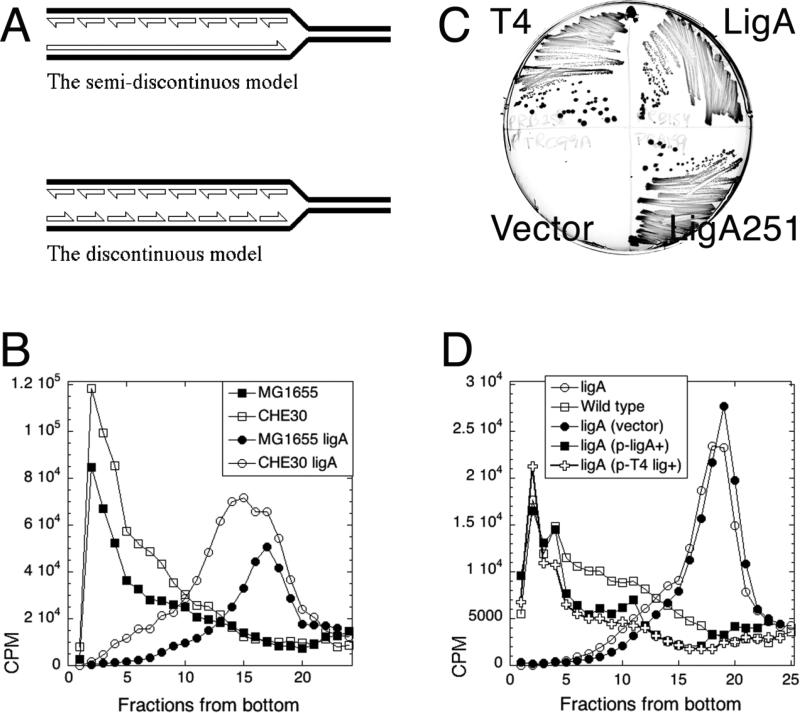
When DNA in E. coli is replicated under ligase-deficient (ligA-) conditions, pulse-labeling reveals only low molecular weight (LMW) replication intermediates, consistent with the discontinuous replication of both strands (Fig. 1A) 1; 2; 3; 4; 5; 6; 7. This thoroughly-established observation contradicts the textbook semi-discontinuous DNA replication model, according to which the lagging DNA strand is synthesized in LMW fragments, whereas the leading DNA strand is synthesized continuously to yield high molecular weight (HMW) products 8; 9 (Fig. 1A).
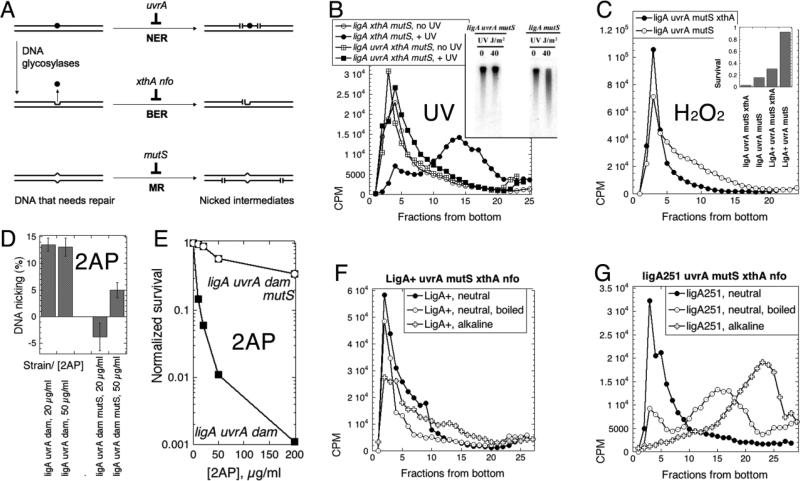
Fig. 1. The test of two models of DNA replication in the ligase mutant: background-independence and complementation of the ligase defect.
A. The two models of intermediates in DNA replication. Half-arrowheads, 3'-ends. The LMW intermediates (Okazaki fragments) are eventually linked together to form mature DNA strands. B. LMW intermediates are background-independent. Alkaline 5-20% sucrose gradients were run from right to left and collected from bottom. In this and all subsequent gradients, fractions 1-5 are considered high molecular weight (HMW), whereas fractions 15 and up are low molecular weight (LMW). Strains are: CHE30, GR523; MG1655 ligA, LA98; CHE30 ligA, GR501. C. Phenotypic complementation. MG1655 ligA251 (LA98) was transformed with plasmids expressing either T4 DNA ligase, or WT E. coli ligase, or the LigA251 mutant protein, as well as with the control vector. Strains were streaked on to agar plates and incubated overnight at 42°C. D. Complementation with functional ligase genes from plasmids. Strains are: ligA, LA98; wild type, MG1655. Plasmids are: vector, pTRC99a; p-ligA+, pRB154; p-T4 lig+, pRB258.
In vitro studies with reconstituted replication forks in the absence of DNA ligase indeed established that, while the lagging strand is synthesized discontinuously (as expected from the 5'—>3' polarity of DNA polymerases), the leading strand is synthesized continuously 10; 11; 12. These findings lead to the general acceptance that the leading strand in E. coli is primed only once, at the replication origin 8; 13, even though such single-primed leading strand synthesis could be permanently stalled by any non-coding DNA lesion. The apparent paradox was recently resolved by demonstrations in vitro that the replisome is able to reinitiate the leading strand synthesis downstream of a blocking lesion 14; 15. Yet, the overall leading strand synthesis is still considered essentially continuous.
Currently 9, the conflict between the in vitro and in vivo observations is reconciled by the idea, based on experiments with uracil-DNA incorporation, that the leading strand is synthesized continuously, only to be later fragmented by excision repair (of uracils), — thus, yielding the observed LMW replication intermediates 16; 17. Although previous attempts to test this idea did not find any contribution of uracil excision to LMW replication intermediates in E. coli 1; 7; 18, many other known DNA repair activities could potentially contribute to generation of Okazaki-like fragments on the leading strand.
E. coli has several DNA repair pathways going through the DNA strand-incision intermediate: base-excision repair, nucleotide-excision repair, alternative excision repair and mismatch repair 19; 20. Wang and Smith disabled some of these DNA repair pathways in a strong ligA7 mutant, but found no effect on the all-LMW distribution of replication intermediates 7. However, in light of the instability of daughter DNA strands synthesized in ligase-deficient conditions 1; 4; 21, it is likely that some LMW species in that study were generated by chromosomal fragmentation and subsequent linear DNA degradation, making the observation of Wang and Smith (1989) harder to interpret.
To address the DNA strand incision explanation of the discrepancy between the in vivo and in vitro results (Fig. 1A), we inactivated all DNA repair enzymes that initiate DNA excision, as an ultimate test for DNA repair contribution to LMW replication intermediates. In this work, using the ligA251(Ts) mutant that has the strongest ligase defect in E. coli 2; 22 in conditions precluding general DNA breakdown 1; 21, we test: 1) whether the discontinuous DNA replication profile is background- and ligA mutant-specific; 2) whether the LMW replication intermediates are coming from both DNA strands in specific chromosomal locations; 3) whether the discontinuities during DNA synthesis are introduced by excision repair of damaged DNA or abasic sites.
Results
The LMW species are due to the DNA ligase defect
To test against the possibility that formation of LMW replication intermediates are not due to the ligase defect but are actually endemic to the CHE30 background of the ligA251(Ts) mutant, we pulse-labeled the ligA251 mutant of MG1655 (the sequenced wild type E. coli 23) at the non-permissive temperature and found the replication intermediates to be LMW, as in the original CHE30 mutant (Fig. 1B). We also verified that the nascent LMW DNA in the ligA251 mutant is indeed due to the defect in DNA ligase, by complementation with the functional ligA+ gene on a plasmid. Not only did the complemented ligA251(Ts) mutant strain grew as well as the wild type strain at 42°C (Fig. 1C), but its replication intermediates at 42°C were of HMW, just like the replication intermediates in the wild type strain (Fig. 1D). Since a plasmid over-expressing the mutant LigA251 protein also complemented the growth defect of the ligA251 mutant at 42°C (Fig. 1C) 22, there was a possibility that E. coli DNA ligase protein plays some structural role in the replication complex, and overproduction of the mutant protein makes cells viable by restoring stability of the replication complex, instead of increasing DNA nick ligation. As a test against this possibility, we complemented the ligA251(Ts) mutant with bacteriophage T4 DNA ligase protein, which is not only smaller than E. coli ligase, but uses ATP as a high-energy cofactor in contrast to the host NADH-dependent DNA ligase 24; 25. Supplementation with T4 ligase, which fully restores the ability of the ligA251(Ts) mutant to grow at 42°C (Fig. 1C) 22, also makes all replication intermediates HMW (Fig. 1D). We conclude that: 1) the LMW replication intermediates accumulate as a result of a deficiency in the linking of juxtaposed 5’-phosphate and 3’-OH (the overall reaction catalyzed by DNA ligases) in ligA251(Ts) mutants at 42°C, rather than due to structural instability of the replisome; 2) the accumulation of LMW intermediates is linked to the inviability of the strain at 42°C (see 21 for the mechanisms of ligase-deficient death).
LMW nascent DNA in the polA rnhA double mutant
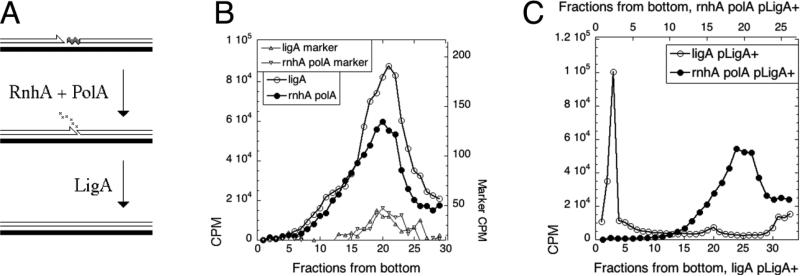
To test the possibility that the LMW species arise as a result of DNA ligase defect itself, rather than being the product of incomplete DNA syntehsis, we needed a mutant that would produce LMW nascent DNA independently of its DNA ligase activity. Since Okazaki fragments are primed with oligo-RNA primers, which are subsequently removed from the 5’ ends of Okazaki fragments by RNaseH (rnhA) and DNA polymerase I (polA) 26 (Fig. 2A), we used the rnhA polA double mutant to block Okazaki fragment maturation in a DNA ligase-positive strain. We found that the nascent DNA profile of the polA rnhA mutant at 42°C is not different from the one of ligA251(Ts) mutant (Fig. 2B). Similar results have been reported before in polA- single mutants27; 28. Moreover, while the ligase defect in maturation of Okazaki fragments is suppressed by WT ligase-producing plasmid, the rnhA polA defect is not (Fig. 2C). This indicates that formation of the LMW DNA replication intermediates is not an artifact of a specific ligase defect, but is the consequence of any defect in the maturation of Okazaki fragments.
Fig. 2. LMW replication intermediates in a non-ligase mutant.
A. A scheme of maturation of Okazaki fragments in E. coli. Filled line, template DNA strand; open line, primer (nascent) DNA strand; half-arrowhead, 3'-end; wavy gray segment, the RNA primer on the 5'-end. B. Pulse label accumulates in LMW species in the rnhA polA double mutant. The original size of the 32P-labeled linear marker was 11.2 kbp (should have peaked between fractions 13 and 15), but it became degraded upon storage. However, its characteristic profile still allows one to align the two gradients. The strains are: rnhA polA, ON2104; ligA, GR501. C. The ligase defect in Okazaki fragment maturation is complemented by the ligA+ plasmid, whereas the rnhA polA defect is not. The strains are GR501 (ligA251) and ON2104 (rnhA polA); the LigA+ plasmid is pRB154.
There was a possibility that the LMW species prematurely separate from the chromosomal DNA in maturation-deficient conditions. Such separated LMW DNA could have been preferentially released from cell debris upon cell lysis, while the HMW replication intermediates could stay trapped within the discarded cell debris pellet, never making it to the gradient. We tested this idea by lysing cells directly in the gradient — this way we would not lose any HMW material by excluding cell debris from the gradient. The result did not change: the profile of wild type cells lysed in the gradient still showed mostly HMW newly-replicated (pulse-labeled) DNA, while the profile of the ligA251 mutant lysed in the gradient still showed mostly LMW DNA and some DNA of intermediate molecular weight (IMW), but no HMW DNA (Fig. S1).
IMW species and the ΔligB mutation
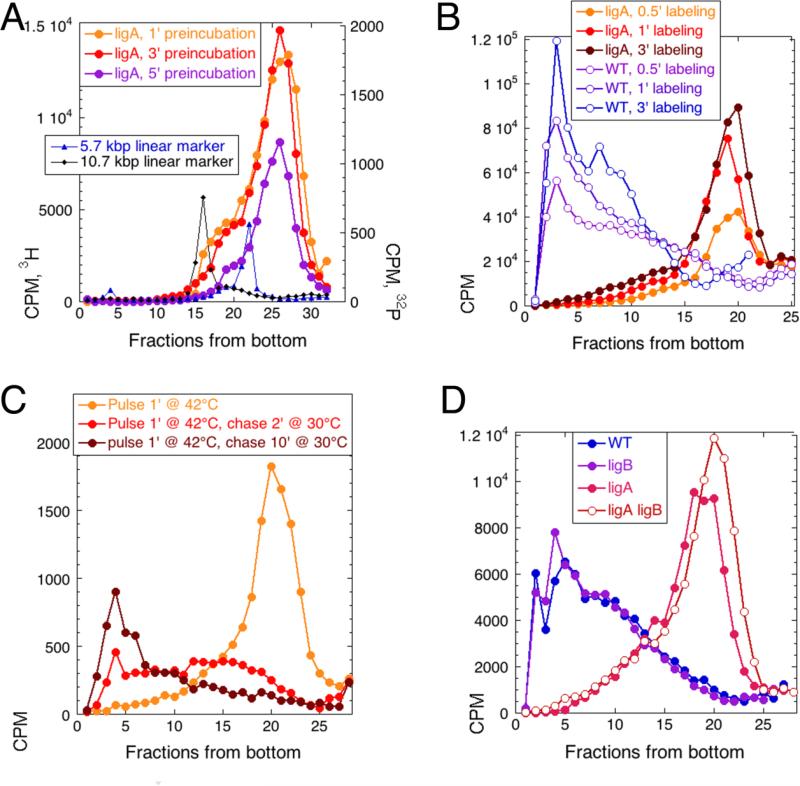
The profile of the newly-synthesized DNA in the ligA251 mutant grown at 42°C shows no HMW DNA; nevertheless, ~10% of the pulse-labeled DNA is always found in the IMW species (fractions 10-15 in our typical 25-fraction gradients (Fig. 1D and 2B)). A trivial reason for this IMW signal could be an incomplete inactivation of the LigA251 mutant protein during our short pre-incubation time at 42°C. Varying the time of pre-incubation at 42°C between 1 and 5 minutes before the standard 1 minute labeling did not change the overall profile of the replication intermediates (Fig. 3A), even though the detectable incorporation was down with 5 minute preincubation, as expected 1; 21. The only part of the gradient that was dramatically affected by longer preincubation were fractions 15-18: the disappearance of these long replication intermediates upon longer preincubation at 42°C was consistent with residual ligase activity of the LigA251 mutant protein in our standard conditions.
Fig. 3. The nature of the intermediate molecular weight (IMW) species.
In all panels, the WT strain is GR523, while the ligA251 mutant is GR501. A. Varying the time of pre-incubation. The ligA251 mutant cells were preincubated at 42°C for 1, 3 or 5 minutes and then pulsed-labeled at 42°C for 1 minute. In this experiment, the intermediate molecular weight species are defined by the two 32P-labeled MW markers, the 5.7 and the 10.7 kbp. B. Varying the time of labeling. Either WT or ligA251 mutant cells were pre-incubated at 42°C for 3 minute before being labeled at 42°C for 30 seconds, 1 minute or 3 minutes. The IMW species in this gradient are in fractions 10-15. C. The pulse-chase experiment. ligA251 mutant cells were labeled at 42°C, and the label was chased with cold thymidine (50 μg/ml) at 30°C for the indicated times. D. The effect of ΔligB mutation. The additional strains are: ligB, LA37; ligA ligB, LA38.
To address the possibility that during the first several seconds the label is preferentially incorporated into DNA species qualitatively different from those labeled later on we incubated both LigA+ and ligA251 mutant cells for 3' at 42°C and then labeled for either 30 seconds, 1 minute, or 3 minutes, still at 42°C. We saw only increasing incorporation, but no qualitative differences in the overall replication intermediate profile in either strain (Fig. 3B). A possible exception was the accumulation of counts in fractions 2-10 in the ligA mutant (Fig. 3B), again consistent with residual ligase activity in our mutant at 42°C. During the pulse-chase experiment, both the LMW and IMW products of ligase-deficient replication were gradually “chased” into HMW species if cells were switched to ligase-proficient conditions (Fig. 3C), again supporting their common nature.
Last, there was a possibility that a minor non-LigA nick-closing activity in E. coli is provided by LigB, the protein that, in contrast to LigA, lacks the DNA-binding C-terminal BRCT domain and two of the Zn-binding cysteins, essential for nick joining, but still shows some activity in vitro 29. However, we found that the ligA251 ΔligB double mutant, besides forming the usual LMW replication intermediates, still forms the IMW replication intermediates observed in the ligA single mutant (Fig. 3D). Moreover, all the pulse-labeled DNA in the ΔligB single mutant is of the size of the chromosomal DNA in the corresponding WT strain (Fig. 3D), confirming that the LigB protein is not involved in the maturation of replication intermediates. Our overall conclusion is that some IMW species must represent a true subset of longer Okazaki fragments, but most of them could be explained by the residual ligase activity in our standard conditions.
LMW replication intermediates come from both DNA strands
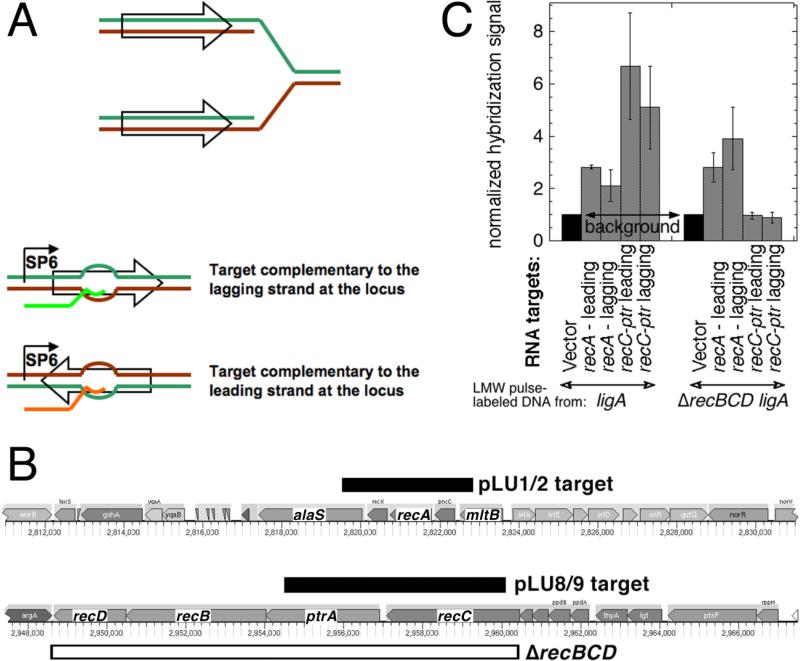
The relatively long duration of replication in ligase-deficient conditions producing only LMW intermediates (at least 6 minutes of total incubation at 42°C, Fig. 3AB) makes it unlikely that these replication products are coming only from the lagging DNA strands. To test the formal possibility that the LMW intermediates are coming only from the lagging strand, while the leading strand synthesis is inhibited in the absence of ligation, we used hybridization to strand-specific targets to ask whether, in specific chromosomal regions, replication intermediates would hybridize to a particular DNA strand or to both DNA strands. To produce strand-specific targets for hybridization, we inserted chromosomal fragments in the two possible orientations under the SP6 promoter, generating strand-specific RNA targets by in vitro transcription (Fig. 4A). As the chromosomal regions, we used either the 3.2 kb fragment centered on the recA gene, or the 5.6 kb fragment covering most of the recC and ptr genes (Fig. 4B). Since the efficiency of this in vitro transcription reaction starts decreasing beyond 2 kb, with transcripts beyond 6 kb barely synthesized 30, we did not use longer chromosomal regions.
Fig. 4. LMW replication intermediates hybridize to both DNA strands.
A. Strand-specific RNA targets were generated by in vitro SP6 polymerase transcription of chromosomal fragments (shown as empty arrows on a replication fork) cloned under the SP6 promoter in a vector (pSP72) and deposited on a hybridization membrane. The “Watson” strand at the chromosomal locus is labeled green, while the “Crick” strand is labeled brown. The generated RNA target identical to the Watson strand is shown in bright green, while the one identical to the Crick strand is shown in orange. B. The chromosomal position and the actual length of the fragments (pLU1/2 and pLU8/9 targets) is shown as black rectangles. The span of the ΔrecBCD deletion in the AK148 strain, used for the negative control, is shown as the open rectangle. The direction of replication for both regions is from right to left. C. Hybridization of pulsed-labeled LMW DNA to strand-specific RNA targets at the two chromosomal loci. Transcripts from vector-only were deposited to generate the background reading. 3H-labeled replication intermediates from ligA (GR501) or ligA ΔrecBCD (AK148) mutant cells were isolated by alkaline sucrose gradients and used as probes for the membranes with the deposited strand-specific targets. The total counts from various targets in any given experiment were normalized to the vector counts. The values are averages of 4-6 independent measurements ± SEM.
The generated strand-specific RNA targets were deposited on hybridization membranes, and the membranes were probed with isolated LMW tritium-labeled replication intermediates from the sucrose gradient fractions of ligA mutants labeled at 42°C. If the Okazaki fragments that we detect are generated in only one strand, then they should anneal to only one of the two strand-specific RNA targets from any given chromosomal region, but not to both. In contrast to this expectation, we observed no statistical difference in hybridization of Okazaki fragments between RNA targets representing the two DNA strands in the same chromosomal regions (Fig. 4C). We confirmed the specificity of hybridization by showing that Okazaki fragments from the ligA ΔrecBCD strain (the deletion covering the whole recC-ptr-recB-recD region) do not hybridize to the recC-ptr RNA targets (Fig. 4C). We conclude that not only the replication intermediates from the ligA mutants are formed as LMW species, but also that they specifically hybridize to both strands in two particular chromosomal locations, indicating discontinuous DNA replication on both strands.
LMW species are not produced by RecBCD or RecJ exonucleases
Before addressing the excision repair explanation for Okazaki fragments on the leading strand, we wanted to test one previously overlooked possibility, highlighted by our recent findings. We earlier detected by label incorporation measurements that, after 5 minutes at 42°C, the newly-synthesized DNA in ligA251 is degraded by RecBCD 1; 21, apparently because of formation of double-strand breaks in nascent DNA duplexes containing unsealed nicks 21. In one of these studies 1 we used alkaline agarose gel electrophoresis to show that the LMW replication intermediates are still present in the ligA ΔrecBCD mutants and so are unlikely to be produced by RecBCD processing of the nascent DNA duplexes under ligase-deficient conditions. Although double-strand breaks start forming after 5 minutes at 42°C in the ligA251 mutant 21, while we employed shorter 42°C incubation times in this study, there was still a possibility that the LMW character of the replication intermediates, as detected by alkaline sucrose gradients, is partially due to double-strand end processing by RecBCD. Here we show that the size of the newly-synthesized DNA detected in alkaline sucrose gradients in ligA251 recBCD and ligA251 mutants during our labeling time is no different (Fig. S2), ruling out RecBCD processing of longer linear duplex DNA molecules as a source of Okazaki fragments in ligase-deficient conditions. Separately, our previous observation that even upon neutral DNA extraction some of the LMW species became sensitive to ssDNA-specific exonuclease I 1 suggested that a significant fraction of the replication intermediates separate from the chromosome at some point, becoming targets for ss-exonucleases. We, therefore, looked at whether the size of the LMW intermediates in ligA mutants at 42°C was affected by the recJ defect in another major ssDNA-specific exonuclease, but again found no difference in the molecular weight profile (Fig. S2).
LMW species are not produced by excision repair
Our results so far indicate that the LMW replication intermediates arise from failure in maturation of the newly-replicated DNA, either at the stage of RNA primer removal, or at the stage of nick ligation. Okazaki believed that the only source of the original nicks is the discontinuous DNA synthesis itself 6; 31, but the nicks could be also formed by excision repair of DNA lesions/modifications/mismatches in the nascent DNA strand 16; 17, as was demonstrated for DNA-uracil incorporation and excision in the thyA mutants of B. subtilis (although this does not happen in the ThyA+ B. subtilis) 32. We and others have previously shown that uracil-DNA incorporation and excision in Dut+ E. coli cells do not contribute to the formation of LMW DNA replication intermediates in conditions precluding their maturation 1; 7; 18, even though this scenario does happen in the dut mutants 1; 17; 33. However, even if uracil-DNA incorporation does not contribute to the formation of Okazaki fragments, other multiple pathways of DNA repair that go through strand scission could still contribute to generation of LMW replication intermediates.
The major DNA repair pathways in E. coli that have obligate strand-scission intermediates are: 1) the nucleotide excision repair, 2) the base excision repair, 3) the methyl-directed mismatch removal 19; 20 (Fig. 5A). To test whether the LMW DNA replication intermediates in the ligA251 mutants are caused by excision repair in the nascent DNA strands, we built ligA+ and ligA251(Ts) versions of the uvrA ΔmutS ΔxthA Δnfo quadruple mutant strain, in which the nucleotide excision repair and the mismatch repair were inactivated at the level of lesion recognition (by, correspondingly, uvrA and mutS defects), while the base excision repair was inactivated at the level of incision at the abasic site intermediates (due to the double xthA nfo defect in the two abasic site endonucleases of E. coli) (Fig. 5A).
Fig. 5. Inactivation of the major DNA-scission activities does not affect the LMW replication intermediates in the ligA251 mutant.
Panels B-E are labeled with the DNA-damaging agents tested. A. A scheme of the major DNA repair pathways going through strand scission intermediates. DNA duplex is shown as a double line; DNA lesion is indicated by black circle; DNA mismatch is the bump in the duplex; abasic site is the indent in one of the DNA strands, left after the base was removed. NER, nucleotide excision repair; BER, base excision repair; MR, mismatch removal. The scenario shown (that the same DNA lesion is the substrate for both NER and BER) is not a common one and is shown for parsimony: typical DNA lesions are substrates to either NER or BER, but not both. B. Inactivation of the nucleotide excision repair prevents strand fragmentation after UV irradiation (100 J/m2), as revealed by alkaline sucrose gradient. Chromosomal DNA in this case was labeled chronically (the pre-labeling with 3H-thymidine protocol). The cells are: UvrA+, LA55; uvrA, LA44. Inset: the (A600=0.45) cultures of cells of the indicated phenotype were irradiated with the indicated dose of UV, and after 15' at 42°C the total DNA was isolated, run on an 0.7% alkaline agarose gel, that was transferred, and the membrane hybridized with the total chromosomal probe. C. Inactivation of the major abasic site endonuclease ExoIII by the xthA mutation prevents strand fragmentation after H2O2 treatment (0.5 mM for 13 minutes at 42°C) of chronically-labeled cultures. The “pre-labeling with 3H-thymidine protocol” was again used. Neutral sucrose gradient in this case, with samples boiled for 10 minutes and chilled on ice before loading. The strains are: ligA uvrA mutS, LA53; ligA uvrA mutS xthA, LA44. Inset: survival of the indicated strains (A600=0.4) after treatment with 1 mM H2O2 for 10 minutes at 30°C. D. Inactivation of mismatch removal prevents strand fragmentation after 2-aminopurine (2AP) treatment of the dam mutants. Cells of the indicated genotype were kept at 30°C throughout the assay. Once the cultures reached OD600= 0.2, they were labeled with 32P for 30 minutes, shaken in the presence of the indicated concentration of 2-AP overnight, the total DNA was prepared and ran in an alkaline agarose gel. Strand fragmentation was calculated as the percentage of DNA signal in the smear below the chromosomal DNA band relative to the total DNA signal (for example, see Fig. 5B and E). Since the background in untreated cells varied widely in this set of experiments, while the signal-to-background difference (including one negative value) was quite reproducible, we report the latter. The values are means of 4-5 independent measurements ± SEM. The strains are: ligA uvrA dam (LA73) and ligA uvrA mutsS dam (LA74). E. Sensitivity of the ligA uvrA dam strain to 2AP and its relief by the mutS defect. Cultures were shaken at 28°C throughout. When they reached OD600 = 0.4, 2AP was added to the indicated concentrations, and shaking continued for five more hours, at which time the titer of the colony-forming units was determined. The strains are: ligA uvrA dam (LA73) and ligA uvrA mutsS dam (LA74). F. Replication intermediates in the LigA+ mutant devoid of the major DNA excision activities are HMW independent of the denaturation conditions. Both neutral and alkaline sucrose gradients are shown in the same panel. The strain is LA115. G. Replication intermediates in the ligA251 mutant devoid of the major DNA excision activities are LMW independently of the denaturation conditions. Both neutral and alkaline sucrose gradients are shown in the same panel. The strain is LA114.
While the deletion mutations in the excision-minus strains were all verified by PCR and by phenotypic tests (see Methods), we also designed functional tests to verify inactivation of the DNA incision pathways. In these functional tests, we exposed mutant and control cells to specific DNA damaging treatments and then ran the isolated DNA in alkaline agarose gels or in denaturing sucrose gradients to detect single-strand scissions resulting from DNA repair activities. Specifically, while phenotypically the uvrA inactivation led to extreme sensitivity to UV irradiation, functionally the uvrA defect was verified by the inability of the mutant to fragment DNA strands after UV irradiation, which was readily detectable by alkaline sucrose gradients (Fig. 5B) and obvious in alkaline gels (Fig. 5B, inset). Phenotypically, we verified the xthA defect by increased sensitivity to H202 (Fig. 5C inset) and MMS treatments (not shown), whereas the nfo defect made cells sensitive to tert-butyl hydroperoxide (Fig. S3). Functionally, the xthA defect was assessed by the inability of the excision-minus strain to fragment DNA after H202 treatment (Fig. 5C). As a functional test for inactivation of mismatch-induced incision, the mutS defect reduced 2-aminopurine-induced strand fragmentation in the dam mutant 34 (Fig. 5D), while phenotypically it allowed the strain to survive the treatment (Fig. 5E).
With the nick-generating repair deficiencies functionally verified, we confirmed that, in the LigA+ uvrA ΔmutS ΔxthA Δnfo control strain, the newly-synthesized DNA was of high molecular weight (Fig. 5F), making sure that the four DNA repair defects do not by themselves cause breakage of nascent strands. In contrast, the profile of the newly-synthesized DNA from the ligA251 uvrA ΔmutS ΔxthA Δnfo quintuple mutant at 42°C still showed only LMW species (Fig. 5G), suggesting that none of the excision repair pathways in the cell participates in the generation of the LMW species. Qualitatively, the same results were observed whether the replication intermediates were separated from their templates by high pH (alkaline gradients) or by heat denaturation (neutral gradients) (Fig. 5FG). Compared with the alkaline denaturation conditions, our heat-denaturation conditions were specifically chosen not to yield full separation of strands, because in conditions when all strands are separated (20 minute boiling), we observed significant artefactual strand breakage (Fig. S4), — this observation should serve as a warning against unlimited boiling when intactness of denatured DNA strands is desired. We conclude that the LMW replication intermediates in the ligA minus conditions are not caused by the major DNA-incising repair pathways or by a specific denaturation protocol.
Replication intermediates are still LMW in complete absence of excision repair
In our strain lacking the major DNA repair pathways that go through strand scission (Fig. 5A), the base excision repair was blocked at the level of abasic site nicking 19. Therefore, there was still a possibility that abasic sites, if formed, would be turned into nicks by alkaline pH 35, or some modified nucleotides could be removed by glycosylases with associated DNA lyase activity that cleaves the DNA backbone 19. To exclude these possibilities, we decided to construct a mutant completely deficient in initiation of all known pathways of repair that go through strand scission. In particular, this meant inactivating all of the eight known DNA glycosylases, including those with associated DNA lyase activity 19. In such a mutant, the base excision repair would be blocked at the level of DNA damage recognition and generation of abasic sites. To this end, we have constructed a ligA251(Ts) uvrA mutS ung mutY mug nth nei tag alkA mutM nfi dodecuple mutant (12 mutations total) and then selected a 42°-growing revertant of it, verified by sequencing, to serve as a LigA+ control. In these “excisionless” mutants (Fig. 6A), not only all known DNA glycosylases, but also the lesion-recognition steps of the nucleotide excision repair (uvrA), mismatch repair (mutS) and the alternative excision repair by endonuclease V (nfi) are all inactivated.
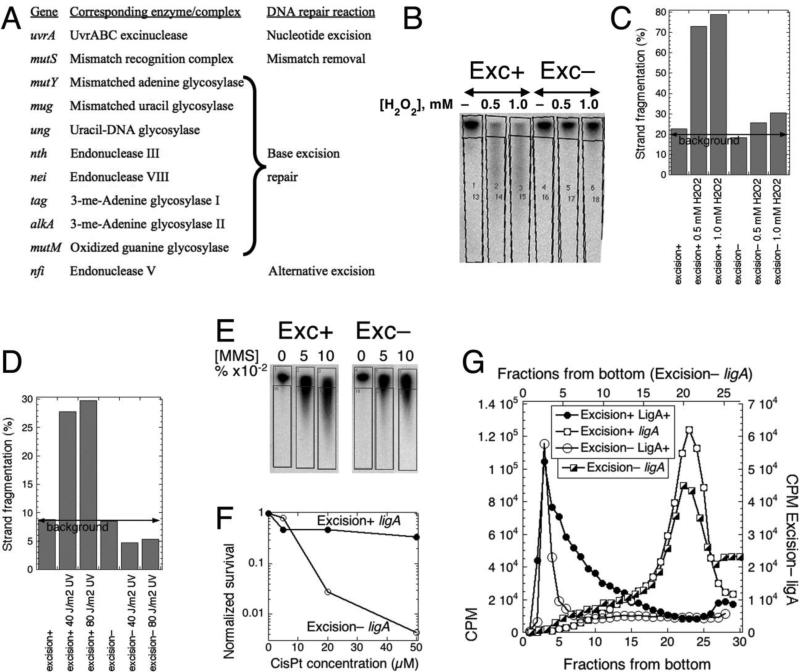
Fig. 6. Excision-less strain still synthesizes LMW replication intermediates in ligase-deficient conditions.
The strains are: excision+, GR501; excision–, LA111. A. DNA damage-recognition functions, inactivated to generate the excisionless mutant. B. Hydrogen peroxide fails to induce nicks in the excisionless mutant. 32P-labeled growing cultures (OD600 = 0.25) of GR501 (ligA251) and of its excisionless variant (LA111) were treated with the indicated concentrations of H2O2 for 15 minutes at 30°C, the total DNA was isolated by phenol extraction and ran on an alkaline agarose gel. The direct radioactivity scan shows quantification boxes along the lanes. C. Absence of strand breaks in the excisionless strain after treatment with hydrogen peroxide. Quantification of the gel in “B” according to the drawn boxes. D. Absence of strand breaks in the excisionless strain after irradiation with ultraviolet light. Method like in “A”. E. MMS-induced nicking in the excisionless strains. Treatment was like in Fig. S5A. F. Sensitivity of excisionless strain to CisPlatin. G. LMW intermediates still form in ligase-deficient conditions in excisionless strain, as revealed by alkaline sucrose gradient centrifugation. Strains: Excision+ LigA+, GR523; Excision+ ligA, GR501; Excision– LigA+, LA113; Excision– ligA, LA111.
Functionally, BER inactivation was confirmed by the inability of the excisionless mutant strain to fragment DNA strands after hydrogen peroxide treatment (Fig. 6BC). The NER defect was functionally verified by the inability of the excisionless strain to fragment DNA strands after UV irradiation (Fig. 6D). Unfortunately, the defect in the alkylated DNA BER could not be functionally verified by the same approach (Fig. 6E), as alkylation makes DNA susceptible to both thermal and alkaline hydrolysis 36; 37 (and our Fig. S5 that verifies these observations), — therefore we just confirmed the acute sensitivity of the excisionless strain to MMS treatment due to the double alkA tag defect 38 (Fig. S6). Even though the DNA backbone in the excisionless mutants is intact after DNA damaging treatments, the strain dies after the slightest DNA damage, as illustrated by strain's acute sensitivity to cisplatin (Fig. 6F).
Alkaline gradient centrifugation analysis of the pulsed-labeled DNA of the ligA251(Ts) variant of the excisionless mutant at 42°C still showed the same LMW replication intermediates at 42°C (Fig. 6G). In contrast, in the lig+ control the nascent DNA was of high molecular weight (Fig. 6G), demonstrating that the excisionless status of the strain does not cause spurious DNA breakage during DNA replication. We conclude that the LMW replication intermediates in the ligA251 mutant grown at 42°C cannot be explained by any kind of DNA repair that nicks DNA strands, — therefore they must represent true intermediates of discontinuous DNA synthesis.
Discussion
Although the semi-discontinuous model is the basis for our current thinking about the general principles of DNA replication 8; 13; 39, there is no in vivo evidence to support this model 40. In fact, all in vivo studies characterizing replication intermediates with short pulses of DNA incorporation, whether in bacteria 28; 31, in yeast 41; 42 (in both, maturation of the replication intermediates has to be inhibited) or in mammalian cells (where no interference with maturation is even required) 43; 44; 45, find them to be of low molecular weight, supporting the discontinuous model of DNA replication 6. We previously revisited the issue in E. coli 1; in the current work, we broadened the investigation and solidified our conclusions.
Following earlier experiments of others 7; 18; 46, we focused on the concern that some LMW replication intermediates in vivo are the result of excision repair processes 16; 33. This concern was once addressed by a density labeling experiment in polA mutants (polA and ligA mutants are defective not only in maturation of replication intermediates, but also in completion of DNA excision repair), where cells were grown in a “heavy” medium first, then shifted to a “light” medium for pulse labeling. LMW pulse-labeled species extracted from sucrose gradients were analyzed by CsCl density gradient and found to be “light”, indicating that they were the product of new DNA synthesis, rather than pieces of “heavy” template DNA 28.
In this paper we addressed a different concern and demonstrated that the LMW replication intermediates in ligA251 in vivo are not the result of any kind of excision repair within the nascent DNA strands. To show this we initially employed the uvrA mutS xthA nfo quadruple mutant lacking the major DNA repair pathways (NER, BER, and MR) going through DNA incision (Fig. 5A). The LMW nature of the replication intermediates in ligase-deficient conditions was unchanged in this multiple mutant (Fig. 5G). To address the potential problem of abasic site instability in alkaline conditions 35 (even though this problem seems to be much exaggerated 47), or the possibility that (the weak) DNA lyase activity of some DNA glycosylases 19 opens DNA at abasic sites, we built a dodecuple mutant with all known eight DNA glycosylase genes deleted, thus preventing the formation of abasic sites altogether (Fig. 6A). We found that, in the resulting excisionless strain, all intermediates of ligase-deficient replication were still LMW (Fig. 6G). The LMW replication intermediates are not artifacts of the high pH in the alkaline sucrose gradients either, since we also observed them after denaturing DNA strands by high temperature in neutral pH (Fig. 5G).
In an exciting recent development, eukaryotic replicative DNA polymerases were found to incorporate a detectable (still very low) amount of ribonucleotides during DNA synthesis, which are then subject to excision repair initiated by RNaseH2 enzymes 48; 49. Even though the bacterial replicative polymerases are currently not suspected to have low discrimination against ribonucleotides, since E. coli also has the RNaseH2 enzyme, coded by the rnhB gene 50; 51, the molecular weight of replication intermediates should be tested in the corresponding mutant. An apparent complication with the functional test of the rnhB mutant for the lack of DNA strand scission is the absence of conditions of high RNA nucleotide incorporation in E. coli.
Even in our strongest ligA(Ts) mutant, we regularly observe IMW replication intermediates, which could represent a native sub-population of longer Okazaki fragments, for example formed on the leading DNA strand. Alternatively, the IMW species could be the result of partial ligation of Okazaki fragments. Such partial ligation is not due to LigB activity, which we show here is not involved in the maturation of replication intermediates (Fig. 3D), but is likely the result of some residual LigA activity, as supported by the eventual appearance of higher-molecular weight species upon longer incubation at 42°C (Fig. 3B, fractions 5-10 in the ligA mutant profiles) and by their disappearance upon longer preincubation at this non-permissive temperature (Fig. 3A, fractions 15-18). The possibility of residual ligase activity in the mutant is consistent with the fact that overexpression of the mutant LigA251 protein off a plasmid allows the mutant to grow at 42°C 22.
We employed several tests to make sure that the observed discontinuous replication is not an artifact. We show that the discontinuous DNA synthesis profile is not specific to the ligase mutation by using a polA rnhA mutant, which preserves Okazaki fragments by stopping their maturation at an earlier step compared to the ligA251 mutant (Fig. 2). The completely discontinuous DNA synthesis profile is also independent of the background (Fig. 1B). To make sure that in our extraction protocol we were not selecting for LMW species while discarding HMW ones with the debris pellet, we lysed the cells directly on the gradients (Fig. S1). We also show that the LMW fragments are not products of processing by exonucleases like RecBCD or RecJ (Fig. S2).
To test the discontinuous synthesis of the leading strand in ligase-deficient conditions, we hybridized pulsed-labeled replication intermediates to ssRNA targets representing either one or the opposite DNA strand at two specific chromosomal locations. We found that pulsed-labeled replication intermediates hybridize similarly to targets representing either DNA strand and, importantly, do not bind to DNA that is deleted in the tested strain (Fig. 4). Hybridization of Okazaki fragments to specific DNA strands has been performed before, both with bacteriophage genomes 52; 53 and with bacterial genomes 46; 54, and the results were similar (Okazaki fragments hybridize to both strands), but the negative controls with deletions were not performed in those studies. The result of our hybridization experiments rule out the possibility that DNA from the leading strand was not synthesized in ligase-deficient conditions or for some reason was excluded from the analysis.
Although most of the support for the discontinuous model of DNA replication comes from in vivo experiments, recently discontinuous leading DNA strand synthesis has also been reported in vitro under special conditions. The leading strand discontinuities in these in vitro experiments were found at DNA lesions in the template DNA 14; 15, or were stimulated by collision of the replisome with a transcribing RNA polymerase, upon which the leading strand polymerase was able to use the transcript as a primer to continue synthesis 55.
As the replisome shows a remarkable plasticity 56, there is no reason to think that the leading strand cannot naturally be synthesized in fragments and that the leading strand fragments must result from alternative priming mechanisms. Analysis of the E. coli genome sequence shows the presence of DnaG priming sites in both the leading and lagging strand templates, with a higher frequency in the lagging strand template 23, supporting the possibility that the IMW signal in the gradients is coming from longer replication intermediates in the leading strand. The fact that in the polA rnhA double mutant, where the RNA primers are preserved 26, synthesis is completely discontinuous, also suggests that primers for Okazaki fragments must be distributed along both DNA strands. In conclusion, our results build on similar results of others before us and further advance the notion that short replication intermediates (Okazaki fragments) are formed on both the lagging and the leading strands in E. coli's replication forks, which is also consistent with the discontinuous replication in eukaryotes.
Materials and Methods
Construction
Strains
Gene deletions, insertions, and marker removal were made by the Datsenko and Wanner protocol 57 and were always confirmed by PCR after construction and when moving from strain to strain. Our PCR primers are always placed outside of the modified region, so the deletion/replacement alleles are confirmed by the expected changes in the size of the corresponding PCR products. Mutations were moved between strains by P1 transduction 58 and, in addition to PCR, confirmed phenotypically, as described below. Most experiments were done in the ligA251 mutant strain GR501 and its LigA+ progenitor, GR523 2. ON2104 (endA polA4113 rnh-91) is described 26.
A kanamycin-resistance marker was introduced near the ligA251 gene in GR501 yielding LA20. The mutation was then moved to MG1655, making the strain LA98. High copy number plasmids (kindly supplied by Richard Bowater 22) expressing either the E. coli WT ligase, or the LigA251 mutant protein, or the phage T4 ligase, or the corresponding empty vector, were introduced into LA98. The ligB gene was replaced with the cat gene in GR523 2 to make LA37. This ΔligB::cat allele was moved to GR501 making the double mutant strain LA38.
Strains LA53 and LA67 are, correspondingly, GR501 and GR523 carrying the uvrA277::Tn10 and ΔmutS::cat mutations. Strains LA43 and LA44 are, correspondingly, GR523 and GR501 carrying uvrA277::Tn10, ΔmutS and ΔxthA::cat mutations. LA55 is GR501 ΔxthA ΔmutS::cat. LA73 is ligA251 uvrA277::Tn10 Δdam::kan, while LA74 is ligA251 uvrA277::Tn10 Δdam::kan ΔmutS::cat. LA114 is ligA251 uvrA277::Tn10 ΔmutS ΔxthA Δnfo::cat. LA115 is the lig+ control for LA114.
LA111 is ligA251 uvrA277::Tn10 ΔmutS Δung ΔmutY Δnfi Δmug ΔnthA Δnei ΔalkA Δtag::kan ΔmutM::cat. The lig+ control (LA113) for this multiple mutant was obtained by streaking LA111 at 42°C, the non-permissive temperature for ligA251 mutants, and selecting for growing suppressors, whose frequency is increased due to the mutS deletion in the strain. Sequencing of the ligA gene showed the wild type sequence in LA113. All the deletions originally present in LA111 were verified in LA113 by PCR.
Plasmids
A 3.2 kbp DNA fragment containing the recA gene was introduced into the BamHI site of the in vitro transcription vector pSP72; one orientation was called pLU1, while the opposite orientation gave pLU2. Similarly, pLU6 and pLU7 were constructed by inserting an 8.3 kbp fragment containing the genes pulJ through recC into the PstI site of the vector. pLU8 and pLU9 carry a smaller 5.6 kbp MfeI fragment containing parts of the recC-ptr genes cloned into the EcoRI site of pSP72 — this fragment is entirely within the chromosomal region deleted in the ΔrecBCD::kan strains AK147 and AK148 1. The ribo-targets synthesized from SP6 promoter on plasmids pLU1 and pLU9 are complementary to the leading strand in the corresponding regions, while ribo-targets synthesized from SP6 promoter on plasmids pLU2 and pLU8 are complementary to the lagging strand in the same regions.
Phenotypic verification
The xthA mutation was verified by H2O2 sensitivity 59. Cells were shaken at 28°C in LB to OD600 0.4. H2O2 (Aldrich) was added to the cultures to make 1 mM final concentration, and incubation at 28°C with shaking continued for 10 more minutes. Aliquots were serially diluted in 1% NaCl and spotted on LB agar plates. Plates were incubated at 28°C, and the colonies were counted the next day.
The uvrA mutation was verified by its extreme sensitivity to UV irradiation 60. Cultures were grown in LB at 28°C to OD600 0.4, streaked with a capillary tube across an LB agar plate and, after streak drying, irradiated with doses of UV in the range between 2 and 10 J/m2 using a modified UV cross-linker (see below). Plates were incubated at 28°C overnight in the dark. The uvrA mutants showed few survivors at the lowest UV dose.
The nfo mutants were verified by increased sensitivity to 0.116 mM tert-butyl hydroperoxide using gradient plates 61. For this, cultures were shaken in LB at 28°C to OD600 0.5 and 20 μl of the culture was mixed with 2 ml of 0.6% molten agar and streaked with a capillary tube across a square agar plate containing a gradient concentration of t-butyl-hydroperoxide (from 0 to 0.116 mM).
The mutS mutants were recognized phenotypically because cultures accumulate 100 times more resistant colonies on 25 μg/ml rifampicin plates 34. In addition, the mutS defect also significantly increases the number of ligA+ revertant colonies of our ligA251 mutant at the non-permissive temperature of 42°C. The growth inhibition of ΔmutS dam cells was followed by measuring the OD600 after addition of various concentrations of 2-aminopurine (Sigma).
For the verification of the ΔalkA Δtag double mutants, rapidly growing cultures were treated with methyl methanesulfonate (MMS, Aldrich) at the final concentration of 0.1% for 30 minutes at 28°C, serially diluted in 1% NaCl and plated at 28°C. The double mutants showed extreme sensitivity to this alkylating agent 38. For treatments with cisplatin (Sigma) the cultures were grown at 28°C to OD600 0.4-0.5, the DNA-damaging agent was added for 100 minutes at 37°C at the indicated concentrations. Samples were serially diluted in 1% NaCl, spotted on LB agar plates and incubated at 28°C.
Labeling experiments
Pre-labeling with 3H-thymidine
Cells were grown in M9 minimal medium supplemented with 0.2% casamino Acids (M9CAA) 58 at 28°C to an OD600 0.2, at which point 3H-thymidine was added to 5-10 μCi/ml. After 15 more minutes of incubation at 28°C, non-labeled thymidine was added to a final concentration of 50 μg/ml, and the cultures were further grown until they reached OD600 0.4. To stop the cellular metabolism instantly, the cultures were mixed with an equal volume of 75% ethanol, 2% phenol in 21 mM sodium acetate (pH 5.3), 2 mM EDTA (the phenol-ethanol-acetate mix) 1; 62. The cells were later collected by centrifugation, and DNA was extracted for sucrose gradients.
Pre-labeling with 32P-orthophosphate
Cells were grown in MOPS CAA medium 63; 64 to OD600 0.2, at which point 32P-orthophosphoric acid was added to the cultures to the final concentration of 5-10 μCi/ml, and incubation was continued for 30 minutes at 28°C. The cells were collected by centrifugation and resuspended in the same medium without label. DNA was purified for gel electrophoresis with the Wizard genomic DNA purification kit to remove LPS and polyphosphates 63.
Pulse-labeling
10 ml cultures were shaken at 28°C in M9CAA medium, in 100 ml Erlenmeyer flasks, to OD600 0.4 followed by shift to 42°C (the non-permissive temperature) for 3 minutes, unless indicated otherwise. 5-10 μCi/ml 3H-thymidine was then added for 1 minute, unless indicated otherwise. To stop the cellular metabolism instantly, an equal volume of the phenol-ethanol-acetate mix (see above) was added to the cultures. The cells were later collected by centrifugation, and DNA was extracted for sucrose gradient centrifugation 1.
DNA purification
For gel electrophoresis, DNA was purified by SDS lysis with subsequent phenol-chloroform extraction and ethanol precipitation as described 65; 66.
For neutral sucrose gradients, a gentle phenol-chloroform extraction was performed. For this, 10 ml of MMCAA cultures (OD600 0.4) were collected by centrifugation for 6 minutes at 6,000 rpm and resuspended in 200 μl of 30% sucrose in TE. 1.4 ml of 2% SDS was added, and after careful mixing the suspension was distributed by 400 μl among four microcentrifuge tubes. Cells were lysed at 65-70°C for 5-10 minutes, and put on ice for 10 minutes. 400 μl of phenol was added, and the samples were tumbled gently for 25 minutes at room temperature. The phenol phase was removed after centrifugation, and the procedure was repeated with 400 μl of phenol-chloroform (1:1) and then with 400 μl chloroform. Samples were loaded on a gradient without prior ethanol precipitation.
For alkaline sucrose gradients, DNA was isolated by suspending cells from a 10 ml culture in 0.5 ml of 1% sodium N-lauroyl sarcosinate containing 40 mM EDTA, followed by lysis for 30 minutes at 37°C with 0.5 ml of 0.4 M NaOH 1; 62. After cooling on ice and a 5-minute centrifugation at 16,000 g, the supernatant was transferred to a fresh tube.
Separation and detection
Alkaline gel electrophoresis
1% agarose was prepared in water, cooled to 60°C and, before pouring, supplemented with alkaline electrophoresis buffer by adding NaOH to 50 mM, and EDTA (pH 8.0) to 1 mM 1; 63. DNA was ethanol precipitated, dissolved in 10 μl of a 50 mM NaOH, 1 mM EDTA solution, and mixed with 10 μl of 1x alkaline loading dye 67. Gels were run for 600-650 minutes in a 1.5 V/cm electric field.
Blot-hybridization
After completion of the run, agarose gels were soaked in 0.2 M HCl for 40 minutes, then in 0.5 M NaOH for 50 minutes, and, finally, in 1 M Tris-HCl pH 8.0 for 15 minutes. After this DNA-shearing treatment, DNA from the gels was vacuum-transferred and UV-cross-linked to a positively charged nylon membrane (Hybond-N+, Amersham Biosciences). The membranes were pre-hybridized in 5% SDS, 0.5 M sodium phosphate pH 7.4, 1 mM EDTA at 65° for one hour. Hybridization was performed overnight in the same buffer containing a 32P-labeled chromosomal DNA probe.
Sucrose gradients
For sucrose gradients, 300-500 μl of DNA were placed on top of 11 ml 5-20% linear sucrose gradient in 0.1 M NaOH, 0.9 M NaCl, and 1 mM EDTA 1; 62. For neutral gradients, the NaOH was omitted from the sucrose solutions. A 1 ml shelf of 70% sucrose was placed at the bottom of the gradient tubes to prevent pelleting of the fast-sedimenting material. Centrifugation was at 22,500 rpm and 4°C for 20 hours in a SW28 rotor, Beckman L5-75B ultracentrifuge. 500 μl fractions were collected from the bottom of the tube, and the radioactivity in 100-200 μl of the fractions was determined by liquid scintillation-counting. For alkaline gradients, the pH in the fractions was brought to neutral with 7 μl of glacial acetic acid before counting the radioactivity.
Reverse hybridization
The RiboScribe/Ampliscribe probe synthesis kits of Epicentre were used to produce (strand-specific) RNA transcripts as targets for hybridization. The templates for the transcription reaction were linearized plasmids containing chromosomal regions inserted into the pSP72 vector 68. Linearization was at the insert's 3' end, to prevent transcription of the vector sequences. Chromosomal fragments were inserted in the two opposite orientations under the SP6 RNA polymerase promoter in the vector. The reactions were performed as recommended by the manufacturer. RNA transcripts were quantified by spectrophotometer, while their quality verified by gel electrophoresis. The same amount of transcripts (either 5 μg or 10 μg) was spotted on a hybridization membrane, which was then UV cross-linked and incubated with pre-hybridization solution. 400-600 μl of 3H-thymidine labeled LMW replication intermediates isolated from the corresponding gradient fractions were used as probes for the membranes with RNA strand-specific targets. After washing, the area in the membrane where the RNA was deposited was cut out, and the radioactivity was determined in a scintillation counter.
Functional tests
The inactivation of a repair pathway after gene deletion was verified by the following functional tests:
Nucleotide excision repair (NER)
To verify the inactivation of the NER pathway in uvrA cells, 2 ml cultures were grown to OD600 0.4 at 28°C in LB for gel electrophoresis, or 10 ml cultures were pre-labeled with 3H-thymidine in MMCAA for sucrose gradient sedimentation. Cells were spun down and resuspended in 1 ml of 0.1 M MgCl2 0.1 % triton X-100 solution. The samples were spread on a ribbed Petri dish cover and UV-irradiated with the indicated doses using a UV-crosslinker from which all the lamps but one were removed, and the remaining lamp was mostly covered, to reduce the rate of irradiation, as described 69. Cells were collected, pelleted by centrifugation and resuspended in fresh medium followed by incubation in the dark (to prevent photoreactivation) for 15 minutes at 42°C. The DNA was analyzed by alkaline gel electrophoresis or alkaline gradient sedimentation.
Base excision repair (BER)
To test the inactivation of the BER pathway, cultures were grown with shaking at 28°C to OD600 0.2-0.3 in LB for gel electrophoresis or with 3H-thymidine in MMCAA for sucrose gradient sedimentation. Fresh H2O2was added to the indicated final concentrations, and shaking continued for 15 minutes at 42°C or 28°C. For neutral sucrose gradients, DNA was isolated by a gentle phenol-chloroform extraction (see above), and denatured by boiling (94°C for 6 minutes). For alkaline gel electrophoresis DNA was extracted by a regular phenol chloroform extraction (see above).
Mismatch repair (MR)
To test for the functional inactivation of mutS, cultures were grown with shaking in MOPS CAA medium to OD600 0.3-0.4 and pre-labeled with 32P-orthophosphoric acid as described above. The cells were washed once with the same volume of unlabeled medium and then resuspended in the same volume of unlabeled medium. 2-Aminopurine was added to the indicated concentrations. After overnight incubation, the DNA was purified from 2 ml cultures using the Wizard Genomic DNA purification kit (Promega) to remove lipopolysacharides and polyphosphates and run in an alkaline gel.
Supplementary Material
Highlights.
— The textbook model of semi-discontinuous DNA replication contradicts in vivo results
— Replication may seem discontinuous due to excision repair in nascent DNA strands
— We found that a completely excisionless E. coli still replicates DNA discontinuously
— Strand-specific hybridization shows that Okazaki fragments come from both strands
— We conclude that DNA replication in E. coli is fully discontinuous in vivo
Acknowledgments
We are grateful to Richard Bowater for the ligase-expressing plasmids and to Tohru Ogawa for the rnhA mutants. We wish to thank all members of this laboratory for valuable discussion of the project. This work was supported by grant # RSG-05-135-01-GMC from the American Cancer Society, by grant # GM 073115 from the National Institutes of Health and by grant F31 GM075425 from the National Institutes of Health to LA.
Abbreviations
- LMW
low molecular weight
- IMW
intermediate molecular weight
- HMW
high molecular weight
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Amado L, Kuzminov A. The replication intermediates in Escherichia coli are not the product of DNA processing or uracil excision. J. Biol. Chem. 2006;281:22635–22646. doi: 10.1074/jbc.M602320200. [DOI] [PubMed] [Google Scholar]
- 2.Dermody JJ, Robinson GT, Sternglanz R. Conditional-lethal deoxyribonucleic acid ligase mutant of Escherichia coli. J. Bacteriol. 1979;139:701–704. doi: 10.1128/jb.139.2.701-704.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gottesman MM, Hicks ML, Gellert M. Genetics and function of DNA ligase in Escherichia coli. J. Mol. Biol. 1973;77:531–547. doi: 10.1016/0022-2836(73)90221-0. [DOI] [PubMed] [Google Scholar]
- 4.Horiuchi T, Sato T, Nagata T. DNA degradation in an amber mutant of Escherichia coli K12 affecting DNA ligase and viability. J. Mol. Biol. 1975;95:271–287. doi: 10.1016/0022-2836(75)90395-2. [DOI] [PubMed] [Google Scholar]
- 5.Konrad EB, Modrich P, Lehman IR. Genetic and enzymatic characatrization of a conditional lethal mutant of Escherichia coli K12 with a temperature-sensitive DNA ligase. J. Mol. Biol. 1973;77:519–529. doi: 10.1016/0022-2836(73)90220-9. [DOI] [PubMed] [Google Scholar]
- 6.Okazaki R, Okazaki T, Sakabe K, Sugimoto K, Kainuma R, Sugino A, Iwatsuki N. In vivo mechanism of DNA chain growth. Cold Spring Harbor Symp. Quant. Biol. 1968;33:129–143. [Google Scholar]
- 7.Wang T-CV, Smith KC. Discontinuous DNA replication in a lig-7 strain of Escherichia coli is not the result of mismatch repair, nucleotide-excision repair, or the base-excision repair of DNA uracil. Biochem. Biophys. Res. Commun. 1989;165:685–688. doi: 10.1016/s0006-291x(89)80020-8. [DOI] [PubMed] [Google Scholar]
- 8.Kornberg A. DNA Replication. W.H Freeman and Company; San Francisco: 1980. [Google Scholar]
- 9.Weaver RF. Molecular Biology. 4th edit McGraw-Hill; New York: 2008. [Google Scholar]
- 10.Cha TA, Alberts BM. The bacteriophage T4 DNA replication fork. Only DNA helicase is required for leading strand DNA synthesis by the DNA polymerase holoenzyme. J. Biol. Chem. 1989;264:12220–12225. [PubMed] [Google Scholar]
- 11.Nakai H, Richardson CC. Leading and lagging strand synthesis at the replication fork of bacteriophage T7. Distinct properties of T7 gene 4 protein as a helicase and primase. J. Biol. Chem. 1988;263:9818–9830. [PubMed] [Google Scholar]
- 12.Wu CA, Zechner EL, Marians KJ. Coordinated leading- and lagging-strand synthesis at the Escherichia coli DNA replication fork, I. Multiple effectors act to modulate Okazaki fragment size. J. Biol. Chem. 1992;267:4030–4044. [PubMed] [Google Scholar]
- 13.Baker TA, Wickner SH. Genetics and enzymology of DNA replication in Escherichia coli. Annu. Rev. Genet. 1992;26:447–477. doi: 10.1146/annurev.ge.26.120192.002311. [DOI] [PubMed] [Google Scholar]
- 14.Heller RC, Marians KJ. Replication fork reactivation downstream of a blocked nascent leading strand. Nature. 2006;439:557–562. doi: 10.1038/nature04329. [DOI] [PubMed] [Google Scholar]
- 15.Yeeles JT, Marians KJ. The Escherichia coli replisome is inherently DNA damage tolerant. Science. 2011;334:235–238. doi: 10.1126/science.1209111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olivera BM. DNA intermediates at the Escherichia coli replication fork: effect of dUTP. Proc. Natl. Acad. Sci. USA. 1978;75:238–242. doi: 10.1073/pnas.75.1.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tye BK, Nyman PO, Lehman IR, Hochhauser S, Weiss B. Transient accumulation of Okazaki fragments as a result of uracil incorporation into nascent DNA. Proc. Natl. Acad. Sci. USA. 1977;74:154–157. doi: 10.1073/pnas.74.1.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tye B-K, Chien J, Lehman IR, Duncan BK, Warner HR. Uracil incorporation: a source of pulse-labeled DNA fragments in the replication of the Escherichia coli chromosome. Proc. Natl. Acad. Sci. USA. 1978;75:233–237. doi: 10.1073/pnas.75.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friedberg EC, Walker GC, Siede W, Wood RD, Schultz RA, Ellenberger T. DNA Repair and Mutagenesis. ASM Press; Washington, D.C.: 2006. [Google Scholar]
- 20.Marinus MG. Chapter 7.2.5. DNA Mismatch Repair. In: Böck A, Curtiss R III, Kaper JB, Karp PD, Neidhardt FC, Slauch JM, Squires CL, editors. EcoSal—Escherichia coli and Salmonella: Cellular and Molecular Biology. ASM Press; Washington, D.C.: 2012. [Google Scholar]
- 21.Kouzminova EA, Kuzminov A. Chromosome demise in the wake of ligase-deficient replication. Mol. Micorbiol. 2012;84:1079–1096. doi: 10.1111/j.1365-2958.2012.08076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lavesa-Curto M, Sayer H, Bullard D, MacDonald A, Wilkinson A, Smith A, Bowater L, Hemmings A, Bowater RP. Characterization of a temperature-sensitive DNA ligase from Escherichia coli. Microbiology. 2004;150:4171–4180. doi: 10.1099/mic.0.27287-0. [DOI] [PubMed] [Google Scholar]
- 23.Blattner FR, Plunkett G, III, Bloch CA, Perna NT, Burland V, Riley M, Collado-Vides J, Glasner JD, Rode CK, Mayhew GF, Gregor J, Davis NW, Kirkpatrick HA, Goeden MA, Rose DJ, Mau B, Shao Y. The complete genome sequence of Escherichia coli K-12. Science. 1997;277:1453–1462. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 24.Lehman IR. DNA ligase: structure, mechanism, and function. Science. 1974;186:790–797. doi: 10.1126/science.186.4166.790. [DOI] [PubMed] [Google Scholar]
- 25.Wilkinson A, Day J, Bowater R. Bacterial DNA ligases. Mol. Microbiol. 2001;40:1241–1248. doi: 10.1046/j.1365-2958.2001.02479.x. [DOI] [PubMed] [Google Scholar]
- 26.Kitani T, Yoda K, Ogawa T, Okazaki T. Evidence that discontinuous DNA replication in Escherichia coli is primed by approximately 10 to 12 residues of RNA starting with a purine. J. Mol. Biol. 1985;184:45–52. doi: 10.1016/0022-2836(85)90042-7. [DOI] [PubMed] [Google Scholar]
- 27.Louarn J-M, Bird RE. Size distribution and molecular polarity of newly replicated DNA in Escherichia coli. Proc. Natl. Acad. Sci. USA. 1974;71:329–333. doi: 10.1073/pnas.71.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okazaki R, Arisawa M, Sugino A. Slow joining of newly replicated DNA chains in DNA polymerase I-deficient Escherichia coli mutants. Proc. Natl. Acad. Sci. USA. 1971;68:2954–2957. doi: 10.1073/pnas.68.12.2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sriskanda V, Shuman S. A second NAD+-dependent DNA ligase (LigB) in Escherichia coli. Nucleic Acids Res. 2001;29:4930–4934. doi: 10.1093/nar/29.24.4930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Melton DA, Krieg PA, Rebagliati MR, Maniatis T, Zinn K, Green MR. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acid Res. 1984;12:7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sugimoto K, Okazaki T, Okazaki R. Mechanism of DNA chain growth, II. Accumulation of newly synthesized short chains in E. coli infected with ligase-defective T4 phages. Proc. Natl. Acad. Sci. USA. 1968;60:1356–1362. doi: 10.1073/pnas.60.4.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tamanoi F, Okazaki T. Uracil incorporation into nascent DNA of thymine-requiring mutant of Bacillus subtilis 168. Proc. Natl. Acad. Sci. USA. 1978;75:2195–2199. doi: 10.1073/pnas.75.5.2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tye B-K, Lehman IR. Excision repair of uracil incorporated in DNA as a result of a defect in dUTPase. J. Mol. Biol. 1977;117:293–306. doi: 10.1016/0022-2836(77)90128-0. [DOI] [PubMed] [Google Scholar]
- 34.Glickman BW, Radman M. Escherichia coli mutator mutants deficient in methylation-instructed DNA mismatch correction. Proc. Natl. Acad. Sci. USA. 1980;77:1063–1067. doi: 10.1073/pnas.77.2.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tamm C, Shapiro HS, Lipschtz R, Chargaff E. Distribution density of nucleotides within a desoxyribonucleic acid chain. J. Biol. Chem. 1953;203:673–688. [PubMed] [Google Scholar]
- 36.Lundin C, North M, Erixon K, Walters K, Jenssen D, Goldman AS, Helleday T. Methyl methanesulfonate (MMS) produces heat-labile DNA damage but no detectable in vivo DNA double-strand breaks. Nucleic Acids Res. 2005;33:3799–3811. doi: 10.1093/nar/gki681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strauss B, Coyle M, Robbins M. Alkylation damage and its repair. Cold Spring Harb. Symp. Quant. Biol. 1968;33:277–287. doi: 10.1101/sqb.1968.033.01.032. [DOI] [PubMed] [Google Scholar]
- 38.Clarke ND, Kvaal M, Seeberg E. Cloning of Escherichia coli genes encoding 3-methyladenine DNA glycosylases I and II. Mol. Gen. Genet. 1984;197:368–372. doi: 10.1007/BF00329931. [DOI] [PubMed] [Google Scholar]
- 39.Alberts B, Sternglanz R. Recent excitement in the DNA replication problem. Nature. 1977;269:655–661. doi: 10.1038/269655a0. [DOI] [PubMed] [Google Scholar]
- 40.Wang TC. Discontinuous or semi-discontinuous DNA replication in Escherichia coli? BioEssays. 2005;27:633–636. doi: 10.1002/bies.20233. [DOI] [PubMed] [Google Scholar]
- 41.Johnston LH, Nasmyth KA. Saccharomyces cerevisiae cell cycle mutant cdc9 is defective in DNA ligase. Nature. 1978;274:891–893. doi: 10.1038/274891a0. [DOI] [PubMed] [Google Scholar]
- 42.Nasmyth KA. Temperature-sensitive lethal mutants in the structural gene for DNA ligase in the yeast Schizosaccharomyces pombe. Cell. 1977;12:1109–1120. doi: 10.1016/0092-8674(77)90173-8. [DOI] [PubMed] [Google Scholar]
- 43.Gautschi JR, Clarkson JM. Discontinuous DNA replication in mouse P-815 cells. Eur. J. Biochem. 1975;50:403–412. doi: 10.1111/j.1432-1033.1975.tb09816.x. [DOI] [PubMed] [Google Scholar]
- 44.Goldstein NO, Rutman RJ. In vivo discontinuous DNA synthesis in Ehrlich ascites tumours. Nature. 1973;244:267–269. doi: 10.1038/newbio244267a0. [DOI] [PubMed] [Google Scholar]
- 45.Nuzzo F, Brega A, Falaschi A. DNA replication in mammalian cells. I. The size of newly synthesized helices. Proc. Natl. Acad. Sci. U S A. 1970;65:1017–1024. doi: 10.1073/pnas.65.4.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang T-CV, Chen S-H. Okazaki DNA fragments contain equal amounts of lagging-strand and leading-strand sequences. Biochem. Biophys. Res. Commun. 1994;198:844–849. doi: 10.1006/bbrc.1994.1120. [DOI] [PubMed] [Google Scholar]
- 47.Lindahl T, Andersson A. Rate of chain breakage at apurinic sites in double-stranded deoxyribonucleic acid. Biochemistry. 1972;11:3618–3623. doi: 10.1021/bi00769a019. [DOI] [PubMed] [Google Scholar]
- 48.Nick McElhinny SA, Kumar D, Clark AB, Watt DL, Watts BE, Lundström EB, Johansson E, Chabes A, Kunkel TA. Genome instability due to ribonucleotide incorporation into DNA. Nat. Chem. Biol. 2010;6:774–781. doi: 10.1038/nchembio.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nick McElhinny SA, Watts BE, Kumar D, Watt DL, Lundström EB, Burgers PM, Johansson E, Chabes A, Kunkel TA. Abundant ribonucleotide incorporation into DNA by yeast replicative polymerases. Proc. Nat. Acad. Sci. U.S.A. 2010;107:4949–4954. doi: 10.1073/pnas.0914857107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Itaya M. Isolation and characterization of a second RNase H (RNase HII) of Escherichia coli K-12 encoded by the rnhB gene. Proc. Nat. Acad. Sci. U.S.A. 1990;87:8587–8591. doi: 10.1073/pnas.87.21.8587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rydberg B, Game J. Excision of misincorporated ribonucleotides in DNA by RNase H (type 2) and FEN-1 in cell-free extracts. Proc. Nat. Acad. Sci. U.S.A. 2002;99:16654–16659. doi: 10.1073/pnas.262591699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kurosawa Y, Okazaki R. Mechanism of DNA chain growth, XIII. Evidence for discontinuous replication of both strands of P2 phage DNA. J. Mol. Biol. 1975;94:229–241. doi: 10.1016/0022-2836(75)90080-7. [DOI] [PubMed] [Google Scholar]
- 53.Sugimoto K, Okazaki T, Imae Y, Okazaki R. Mechanism of DNA chain growth, III. Equal annealing of T4 nascent short DNA chains with the separated complementary strands of the phage DNA. Proc. Natl. Acad. Sci. USA. 1969;63:1343–1350. doi: 10.1073/pnas.63.4.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sternglanz R, Wang HF, Donegan JJ. Evidence that both growing DNA chains at a replication fork are synthesized discontinuously. Biochemistry. 1976;15:1838–1843. doi: 10.1021/bi00654a008. [DOI] [PubMed] [Google Scholar]
- 55.Pomerantz RT, O'Donnell M. The replisome uses mRNA as a primer after colliding with RNA polymerase. Nature. 2008;456:762–766. doi: 10.1038/nature07527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yao NY, O'Donnell M. Replisome dynamics and use of DNA trombone loops to bypass replication blocks. Mol. Biosyst. 2008;4:1075–1084. doi: 10.1039/b811097b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miller JH. Experiments in Molecular Genetics. Cold Spring Harbor Laboratory; Cold Spring Harbor, NY: 1972. [Google Scholar]
- 59.Demple B, Halbrook J, Linn S. Escherichia coli xth mutants are hypersensitive to hydrogen peroxide. J. Bacteriol. 1983;153:1079–1082. doi: 10.1128/jb.153.2.1079-1082.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Howard-Flanders P, Boyce RP, Simson E, Theriot L. A genetic locus in E. coli K12 that controls the reactivation of UV-photoproducts associated with thymine in DNA. Proc. Nat. Acad. Sci. U.S.A. 1962;48:2109–2115. doi: 10.1073/pnas.48.12.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cunningham RP, Saporito SM, Spitzer SG, Weiss B. Endonuclease IV (nfo) mutant of Escherichia coli. J. Bacteriol. 1986;168:1120–1127. doi: 10.1128/jb.168.3.1120-1127.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Okazaki R. Short-chain intermediates. In: Wickner RB, editor. DNA Replication. Vol. 7. Marcelle-Dekker; New York: 1974. pp. 1–32. [Google Scholar]
- 63.Amado L, Kuzminov A. Polyphosphate accumulation in Escherichia coli in response to defects in DNA metabolism. J. Bacteriol. 2009;191:7410–7416. doi: 10.1128/JB.01138-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Neidhardt FC, Bloch PL, Smith DF. Culture medium for enterobacteria. J. Bacteriol. 1974;119:736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kouzminova EA, Kuzminov A. Fragmentation of replicating chromosomes triggered by uracil in DNA. J. Mol. Biol. 2006;355:20–33. doi: 10.1016/j.jmb.2005.10.044. [DOI] [PubMed] [Google Scholar]
- 66.Kuong KJ, Kuzminov A. Disintegration of nascent replication bubbles during thymine starvation triggers RecA- and RecBCD-dependent replication origin destruction. J. Biol. Chem. 2012;287:23958–23970. doi: 10.1074/jbc.M112.359687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maniatis T, Fritsch EF, Sambrook J. Molecular cloning. A laboratory manual. Cold Spring Harbor Laboratory; Cold Spring Harbor: 1982. [Google Scholar]
- 68.Krieg PA, Melton DA. In vitro RNA synthesis with SP6 RNA polymerase. Methods Enzymol. 1987;155:397–415. doi: 10.1016/0076-6879(87)55027-3. [DOI] [PubMed] [Google Scholar]
- 69.Khan SR, Kuzminov A. Replication forks stalled at ultraviolet lesions are rescued via RecA and RuvABC protein-catalyzed disintegration in Escherichia coli. J. Biol. Chem. 2012;287:6250–6265. doi: 10.1074/jbc.M111.322990. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.