Abstract
Interconnection of epithelial tubules is a crucial process during organogenesis. Organisms have evolved sets of molecular and cellular strategies to generate an interconnected tubular network during animal development. Spatiotemporal control of common cellular strategies includes dissolution of the basement membrane, apoptosis, rearrangements of cell adhesion junctions, and mesenchymal-like invasive cellular behaviors prior to tubular interconnection. Different model systems exhibit varying degrees of active invasive-like behaviors that precede tubular interconnection, which may reflect changes in cell polarity or differential adhesive cell states. Studies in this newly-emerging field of tubular interconnections will provide a greater understanding of pediatric diseases and cancer metastasis, as well as generate fundamentally new insights into lumen formation pathology, or lumopathies.
Keywords: kidney, juxtaposition, luminal interconnection, lumopathies, invasive behavior
Introduction
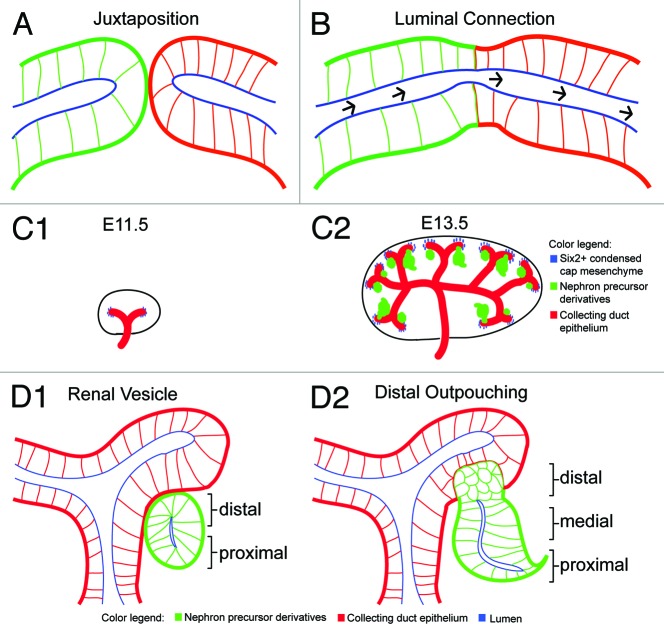
Interconnecting epithelial tubules is an essential process during organogenesis. After the juxtaposition of two tubular systems with pre-existing lumens, the formation of a continuous lumen involves precise cell and tissue rearrangments to fuse epithelial lumens (Figs. 1A and B). In my review, I will illustrate four sets of cellular strategies associated with tubular interconnections that are found with varying degrees across different animal phyla. First, modulation of the intervening basement membrane between epithelia is achieved through at least two types of mechanisms: (1) diminished synthesis of matrix proteins in the basement membrane (Xenopus primary mouth),1 and (2) sliding of the basement membrane (C. elegans uterus-vulva connection).2 Second, programmed cell death (apoptosis) is a shared cellular strategy among ureter repositioning in mouse, cloacal formation in zebrafish, and Xenopus primary mouth formation;3-5 however, in other developmental systems, such as during foregut morphogenesis in C. elegans, no visible signs of cell death is observed.6 The final two cellular strategies, namely cell rearrangements and invasive behaviors at the future site of luminal connection, are quite widespread between animal phyla; these two thematic cellular strategies have tissue-specific variations at the molecular and cellular levels. In the mammalian kidney, for example, two distinct epithelial tubules—namely the nephron and collecting duct epithelium—are derived independently: one from the progenitor pool kidney mesenchyme that gives rise to the nephron, while the other is a product of reiterative branching morphogenesis from the pre-existing epithelium called the Wolffian duct (Fig. 1C1–1D2).7,8 These two separate epithelia ultimately fuse to form the functional filtration system of the kidney. A longstanding yet important question remains: how does the distal segment of the nephron connect and form a continuous lumen with the collecting duct epithelium? Similarly, in the mammalian urogenital system, the ureteric bud epithelium must fuse with the bladder to achieve passage of fluid removal from the developing organism; failure to remove urine may result in human diseases such as hydronephrosis. A comparative view of the literature suggests that many fusion events proceed through similar stages and cellular mechanisms. Basement membrane modification between adjacent epithelia, apoptosis of juxtaposed cells, repositioning of cell-cell junctions, and active protrusive behavior of epithelial cells are all common features of epithelial fusion events in different organisms. By examining cellular mechanisms that underlie the spatiotemporal control of connecting tubular networks across different organisms, this review provides a cellular framework of tubule fusion for future molecular and cellular studies of how the kidney and other branched organs form, and may also guide development of therapeutic strategies for a novel class of human diseases resulting from defects in lumen formation; lumopathies.
Figure 1. Connecting epithelial networks during organogenesis. (A) Two juxtaposed epithelial networks (green or red) with pre-existing lumens (blue). Polarized epithelial cells have basal lamina (thick red or green lines), lateral (thin green or red lines), and apical (blue) surfaces. (B) Luminal connection established between two epithelial systems (continuous lumen labeled blue) allows unidirectional fluid flow (black arrows mark unidirectional fluid flow). (C1-C2) Highlighted stages of embryonic mouse kidney development. (C1) Embryonic day 11.5 (E11.5) depicts collecting duct epithelium (red) invading into neighboring kidney mesenchyme. Reciprocal mesenchymal-epithelial interactions result in formation of condensed cap mesenchyme (blue) surrounding tips of collecting duct. (C2) Embryonic day 13.5 (E13.5) shows induced formation of nephron precursors7,8,56 (green) that are derived from Six2+ cap mesenchymal cells during nephrogenesis. (D1-D2) Close-up views at early stages of nephron precursor morphogenesis. (D1) Cross-section of renal vesicle stage depicting intact basal lamina at future site of luminal interconnection. Arrows mark distal and proximal cell types. (D2) Distal outpouching at early S-shaped stage illustrating two epithelia are juxtaposed to each other, yet no luminal interconnection is established.
Breaking down barriers: spatiotemporal control of basement membrane dissolution
Across different vertebrate organisms, the temporal and spatial control of basement membrane dissolution is crucial for generating functional tubular networks (Fig. 2A, Table 1). During primary mouth formation in the Xenopus laevis larvae, basement membrane dissolution is an early process that occurs between the juxtaposed endoderm and ectoderm tissues located at the extreme anterior of the animal.9,4 Here, the breakdown of basal lamina is achieved by inhibiting canonical β-catenin mediated Wnt signaling through two antagonist factors: the secreted Frizzled related protein (Frzb-1) and Crescent.1 It remains to be determined how the molecular actions of Wnt antagonists leads to the diminished synthesis of laminin and collagen. In contrast, during the development of the hermaphrodite worm C. elegans, different sets of molecular pathways control the breakdown of the intervening basement membrane that resides between the juxtaposed uterus and vulva. By locally removing the basement membrane at the future site of luminal interconnection, fertilized eggs are able pass from the uterus and hatch outside of the mother. First, Sherwood and Sternberg in 2003 described how the anchor cell from the gonad breaks down the basal lamina between the uterus and vulva to form a continuous lumen during C. elegans larva development.10 Recent studies further defined a diffusive signal cue from vulva cells that is required for the anchor cell’s invasive cellular property. It has been been reported that UNC-6/Netrin is the secreted factor emanating from the vulva uterine cells that first polarizes the distribution of actin regulators (filamentous actin and phosphatidylinositol 4,5-biphosphate) through the netrin receptor unc-40.11 Recent work by Ihara et al. have shown that basement membrane sliding, achieved through a homolog of tumor suppressor Kank (VAB-19) and integrin INA-1/PAT-3, creates a gap in the intervening basement membrane.2 In addition, transcription factors fos-1, hlh-2/e/daughterless, as well as integrin signaling are crucial molecules that regulate anchor cell invasion through the basement membrane.12-14 Once the anchor cell has broken down the basal lamina, it then fuses to form the multinucleate uterine seam ‘utse’ cell that joins these two epithelial organs.15 Later, MAPK and NOTCH signals mediate lumen expansion of the vulva.16 In the mammalian kidney, two recent studies show that the basal lamina is largely discontinuous at the future site of luminal interconnection between the renal vesicle and late-S shaped nephron precursor stages.17,18 It is possible that the punctate distribution of the basal lamina may provide an inhibitory cue by an unknown signaling mechanism that may act in preventing luminal interconnection. Since the intervening basal lamina is largely denuded at the future interconnection zone, it is less likely that breakdown of basal lamina alone achieves luminal interconnection in the developing kidney. Future molecular loss and gain of function studies will be needed to test how basement membrane breakdown, Laminin or Collagen-IV deposition and synthesis are modulated at the interconnection zone during kidney development.
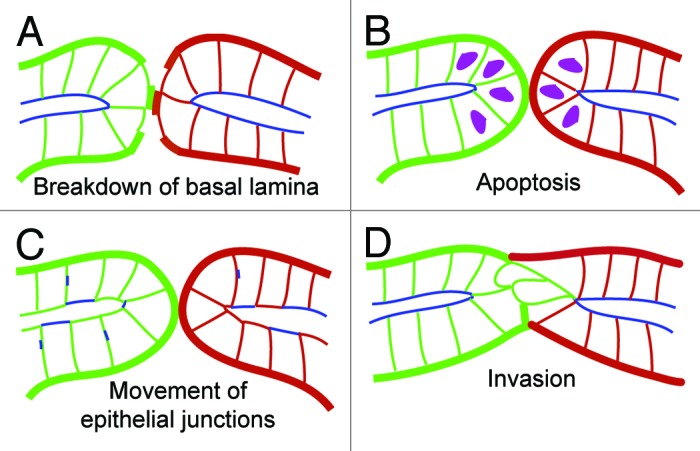
Figure 2. Spatiotemporal control of cellular strategies at the future interconnection zone. (A-D) Schematics highlighting cellular mechanisms occurring at the future site of luminal interconnections. (A) Basement membrane dissolution. Basal lamina shown in either thick green or red lines between two epithelial tubes. (B) Selected burst of apoptosis at juxtaposed cells located at the future site of luminal interconnection (hypothetical apoptotic cells depicted in magenta colored nuclei that may occur at either end of each tubular networks). (C) Cell rearrangements and reorganization of epithelial junctions. Tight junction complexes indicated in blue. (D) Invasive behaviors emerging from one tubular network at the future site of luminal interconnection (labeled in green).
Table 1. Highlighted examples of molecular and cellular processes prior to luminal connection across different organisms.
| Cellular Process |
Developmental System |
Associated molecules |
Organism | References |
|---|---|---|---|---|
| Basement membrane dissolution |
Primary mouth formation |
Wnt antagonists |
Xenopus laevis |
Dickinson and Sive1,4 |
| Uterus-vulva connection |
Netrin Fos-1 HLH-2 INA-1/PAT-3 VAB-19 |
C. elegans |
Ziel et al. 11 Sherwood et al.10 Schindler et al.14 Ihara et al.2 |
|
| Apoptosis |
Primary mouth |
Unknown |
Xenopus laevis |
Dickinson and Sive 4 |
| Cloacal formation |
Bmp |
Danio rerio |
Pyati et al.5 |
|
| Ureter repositioning |
Ret, Raldh |
Mus musculus |
Batourina et al.3 |
|
| Cell rearrangement and/or movement of adhesive junctions |
Thinning and perforation |
Unknown |
Xenopus laevis |
Dickinson and Sive4 |
| Cloacal formation |
Bmp Wtip (Ajuba) |
Danio rerio |
Pyati et al.5 Bubenshchikova et al. 25 |
|
| Branchial fusion |
Unknown |
Botryllus schlosseri |
Manni et al.24 |
|
| Vessel fusion |
Moesin1,Ve-cadherin ZO-1 |
Danio rerio |
Wang et al. 38 Herwig et al.36 Blum et al.37 |
|
| Invasive behavior |
Anchor cell invasion |
Netrin Integrin |
C. elegans |
Ziel et al.11 Hagedorn et al.12 |
| Cytoplasmic extensions (Primary ureter- bladder fusion) |
Gata3 Raldh |
Mus musculus |
Chia et al.45 |
|
| Distal cell invasion (mouse kidney) | Unknown | Mus musculus | Kao et al.18 |
To live or to die: temporal control of apoptosis prior to lumen formation
At the future tubule interconnection zone, apoptosis is another crucial process demonstrated across several vertebrate organisms (Fig. 2B, Table 1). For instance, programmed cell death of ectodermal cells is associated with the formation of the primary mouth in Xenopus laevis,4 as well as the formation of the pronephric opening in zebrafish.5 While it remains to be investigated whether Wnt antagonists or other signaling modules are required for localized apoptosis, Bone morphogenetic protein (Bmp) signaling appears to be one of the shared signaling mechanisms required for both primary mouth development in frog larvae and cloacal formation during zebrafish embryogenesis. It has been demonstrated that either expression of Bmp antagonist chordin or dominant negative Bmp receptor block primary mouth formation in the frog larvae.1 Likewise, during zebrafish cloacal development, Pyati et al.5 demonstrated that sustained Bmp signaling coupled with cell death appears are required for the pronephric duct connection to the proctodeum after somitogenesis. By using a ventral epidermal promoter msxb to label ventral epidermal cells, they demonstrated that these epidermal cells undergo cell death visualized by acridine orange staining.5 It remains to be investigated whether the selected ventral epidermal cell death undergoes caspase dependent or caspase independent cell death. While Parkin et al.19 demonstrated that Sonic hedgehog signaling zebrafish mutants exhibit no changes in TUNEL positive cells compared with wildtype mutants, Slanchev et al.20 found that nephrocystin-4 mutant zebrafish exhibit decreased numbers of acridine orange positive cell death in the ventral epidermis. In addition to cloacal development, apoptosis plays an important role in repositioning the ureter to join with the bladder in the developing mouse urogenital system.3 Here, heroic time-lapse cinematography and immunostainings of cleaved-caspase 3 labeling desmonstrated that a burst of apoptosis severs the connection between the ureter and Wolffian duct during ureter repositioning, which subsequently leads to trigone expansion and the joining between the ureter and bladder. In contrast, during mammalian kidney development, the future site of luminal interconnection between juxtaposed renal vesicle derivatives and collecting duct epithelium do not reveal signs of cleaved-caspase 3 nor TUNEL positive cells, suggesting that apoptosis is not associated with joining the late-S shaped renal vesicle derivative and collecting duct epithelium.18 Likewise, during C. elegans development, apoptosis is not observed in either foregut morphogenesis or the joining between the uterus and vulva.6,10,21,22 In sum, localized apoptosis may be utilized as one of the mechanisms leading to the assembly of a continuous lumen between tubular systems.
The intricate choreography of cell rearrangements and epithelial junction dynamics associated with luminal interconnections
Dynamic cell rearrangements and changes in apico-basolateral junctions is another important feature during the fusion between tubule systems across different organisms (Fig. 2C, Table 1). Epithelial junction plasticity may contribute to generating localized perforation in sheet of epithelia or tubular epithelia networks. In a process reminiscent to Drosophila wing disc eversion,23 Manni and colleagues identified cell rearrangements that occur at the future site of fusion between branchial fissure in the tunicate Botryllus schlosseri.24 Using electron microscopy, they demonstrated changes in the localization of tight junction complexes after breakdown of the basal lamina. It will be important in future studies to determine how apico-basolateral polarity is controlled by either molecular signals or mechanical forces prior to fusion of the branchial fissures; additionally, whether modification of apico-basolateral polarity is a common theme during the thinning and intercalation events of primary mouth formation during Xenopus development deserves future investigations. Cell rearrangements are not only involved during primary mouth formation in the frog larvae, but also crucial during two phases of zebrafish cloacal development: (1) the fusion between the pronephros and the ventral epidermis, (2) the joining of the posterior gut tube to the proctodeum.5 While future studies will need to address how cell polarity is established by examining dynamics of desmosomal complexes, adherens junctions, and tight junction assembly, several reported zebrafish mutants exhibiting cloacal development defects may provide clues to the mechanisms that drive these extensive cell rearrangements. Since genetic zebrafish mutant studies of Sonic hedgehog signaling, as well as Nephrocystin-4, Par-6, Wtip (Ajuba), and Polycystin-2 exhibit occluded distal segments of the pronephros,5,19,20,25,26 it will be important to determine how these signaling components are integrated to carry out dynamic cell rearrangements and/or establishing cell polarity during cloacal morphogenesis. Interestingly, during forgut development in C. elegans, two studies demonstrated that loss-of-function mutants zen-4 and laminin mutant lam-1 fail to establish arcade cell epithelial polarity.27,28 Finally, while there are several excellent reviews and recent mouse aorta lumen formation and in vitro studies,29-33 insights into epithelial tubular interconnections could also be drawn from recent work on blood vessel fusion events during zebrafish embryogensis. After Folkman and Haudenschild’s electron microscopy studies of human capillary lumen formation,34 Kamei et al. suggested that pinocytosis via intracellular vacuoles may be a cellular process that forms a continuous lumen during blood vessel fusion.35 However, several groundbreaking studies show dynamic cellular rearrangements, as well as extensively-modified cell-cell adhesion junctions to generate a functional intersegmental vasculature network during zebrafish development.36,37 Recent work by Herwig et al.36 demonstrated that two diverse cellular strategies are used in assembling dorsal longitudinal anastomotic vessels (DLAV) during zebrafish embryogenesis. On one hand, cord hollowing is used to form multicellular tubular connections, while membrane invagination is employed for unicellular tubular fusion.36 For investigating the cord hollowing mechanism, Wang et al. not only demonstrated that the cytoskeletal protein Moesin1 is required for intersegmental vessel (ISV) lumen formation, but also VE-cadherin positive adherens junctions (rather than ZO-1 tight junctions) is required for ISV tubulogenesis;38 here, Moesin1 may act to establish proper endothelial polarity for ISV tubule formation. These studies collectively may provide new insights into human vascular diseases, such as Bland-White-Garland disease or arteriovenous fistula. In the mammalian kidney, distal precursor cells at the distal outpouching early S-shaped body stage during nephron morphogenesis have immature epithelial cell junctions compared with their proximal cell neighbors (Fig. 1, panel D2).18 Recently, Yang et al. revealed that afadin, an adaptor protein that connects the actin cytoskeleton to nectin adhesion junctions, is required for de novo lumen formation in the developing nephron.39 Here, removal of Afadin in the developing nephron precursors leads to not only microdomains of pre-apical patches of Nectin 2/aPKC and aberrant apico-basolateral polarity, but also ectopic Laminin deposition; these results suggest that Afadin may act to establish apical surfaces and/or cell to cell adherens junctions or cues from the extracellular matrix may also signal to the cell. Future studies will need to address how distal cells’ immature epithelial junction phenotype is acquired or maintained during nephrogenesis, and whether cell rearrangements or cell intercalations (as recently observed in dorsal appendage formation during Drosophila eggshell development by Osterfield, et al.40) may occur at the future site of luminal interconnection.
Invasive-like behaviors preceding luminal interconnection: from ureters to kidneys
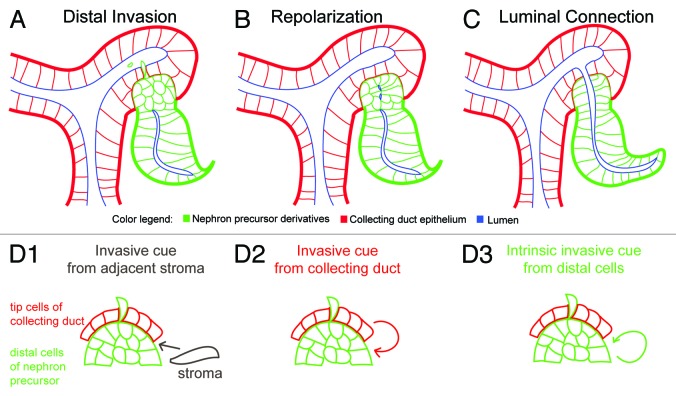
Across different organisms, there are varying degrees of invasive-like behaviors at the future site of luminal interconnection (Fig. 2D, Table 1). Anchor cell invasion (C. elegans), tracheal and blood vessel tip cell filopodial extensions (Drosophila and zebrafish), and cytoplasmic extensions in capillary endothelial cells during angiogenesis are a variety of different forms of invasive properties associated with lumen formation.10,41-44 In the assembly of the mammalian urogenital system, two recent studies report different invasive behaviors prior to types of luminal interconnections: (1) the joining of the ureter-bladder, and (2) the fusion between the nephron and collecting duct. First, Chia and colleagues have identified cell extensions projecting from the proximal end of the nephric duct prior to initial insertion to the cloacal epithelium at embryonic day 9.0 (E9.0).45 This invasive behavior was absent in Ret, Raldh2, and Gata3 mutants, and correlated with the failure of nephric duct insertion. The observation of cytoplasmic extensions is reminiscent of filopodial extensions sent by tip cells prior to the fusion of intersegmental blood vessels.36 Interestingly, removal of Fgfr2 in the surrounding stroma of the peri-Wolffian duct resulted in ureter insertion abnormalities.46 On the other hand, recent work lay the foundation for future research into tubule fusion in the developing mammalian kidney. In the early 20th century, Schreiner had used the term Verlöthungsstelle or soldering site—perhaps a process reminiscent of attaching two pieces of metal pipes—to describe the fusion between these two epithelial tubes in the developing kidney.47 However, recent findings suggest that an intricate spatiotemporal orchestration of unusual invasive-like properties of the distal nephron precursor cells closest to the neighboring collecting duct epithelium occurs prior to luminal interconnection.18 By combining cellular imaging with genetic mutant analysis, my colleagues and I identified another novel group of distal invasive epithelial cells that actively protrude and delaminate into the lumen of the adjacent collecting duct network prior to luminal interconnection (Fig. 3A).18 Future work will examine how repolarization and extracellular signaling cues control the formation of luminal interconnections in the developing kidney (Fig. 3B and C), and when this process is compromised, how this contributes to lumopathies in the kidney and other branched networks, such as the mammary duct and lung formation. In particular, it will be important to determine the following: (1) what molecular and mechanical cues control distal invasion, and (2) whether this cue originates from the neighboring stromal cells, collecting duct tip cells, or an intrinsic distal signaling cue (Fig. 3D1-3). Since previous studies reported that Rho GDIalpha knockout mice display signs of chronic renal disease with distended connecting segments between the nephron and collecting duct,48,49 it is conceivable that RhoGTPase activity may be one of the factors required for either distal outpouching, distal invasion, or repolarization at the future site of luminal interconnection. It will be important to re-examine these mouse mutants for interconnection defects. In light of recent literature, possible polarity cues include modulators of RhoGTPase activity and p120 catenins.48-50 More recently, Yang et al. demonstrated that while Afadin is required for de novo lumen formation in the developing nephron, Afadin kidney mutants display normal spatially polarized proximo-distal cell identities of the nephron precursor and luminal connection between the nephron and collecting duct is not affected compared with wildtype controls;39 these data suggests that additional molecular factors, such as p120 catenins, are also required for tubule fusion.50 Novel reagents, such as renal lumography in mice or gut injection assays in zebrafish combined with molecular studies, will provide a framework for developing useful diagnostic and therapeutic strategies for detecting and treating human lumopathies.
Figure 3. Distal invasion and framework for investigating mechanisms of luminal interconnections during kidney development. (A) Prior to luminal interconnection, nephron precursor cells of distal compartment exhibit unusual invasive-like properties that precede epithelial tubule interconnection. (B) A repolarization step between the mid to late S-shaped body stages showing re-establishment of tight junction complexes (depicted in blue). (C) Luminal connection is spatiotemporally controlled at late S-shaped body stage. (D1-D3) Possible signaling modules that control distal invasion. These cues may be secreted or membrane-tethered chemical signals and/or biomechanical cues. (D1) Stromal signaling cue that is closest to future site of luminal interconnection (gray arrow). (D2) Invasive cue may come from adjacent epithelial cells of the ureteric bud closest to distal cells of nephron precusor (red arrow). (D3) An inherent distal invasive cue (green arrow) may come as a self-signaling module independent of stromal and ureteric bud.
Emerging perspectives in interconnecting epithelial tubules
Across different organisms, connecting pre-existing lumens is performed by a combination of cellular strategies: breakdown of basement membrane, selective apoptosis, cell rearrangements, and invasive cell behavior. Over the course of evolution, how and why are certain molecular and cellular processes selected for luminal interconnections that occur in different organisms? Interestingly, each cellular process across different organisms may be performed by common or divergent molecular signals, and future work will need to determine to what extent shared mechanical forces are used across different types of luminal interconnections. Furthermore, while selective localized apoptosis is crucial for both primary mouth formation in Xenopus and cloacal development in zebrafish, there appears to be variations in the types of cellular rearrangements, adherens/tight junction reorganization, and invasive cell behaviors across different organisms. It remains imperative to elucidate the underlying molecular and mechanical cues that control the spatiotemporal orchestration of these cellular events at the future site of luminal interconnection. One of the challenges will be to determine whether invasive behavior, such as sending directional filopodial processes to neighboring cells, function as a signaling center that facilitates future luminal connection. Another alternative possibility is active cell protrusion may serve as a local mechanical perforation for future formation of a continuous lumen. By integrating recent molecular profiling data from GUDMAP51,52 and high-throughput transcript localization of proximal and distal factors during stages of nephrogenesis,17 optical clearing solutions,53-55 and inducible optogenetic and genetic mutant studies, an array of outstanding questions could be addressed. It will be important to determine whether morphogenetic signals, such as Bmps or Wnts, are involved in regulating the spatiotemporal luminal interconnection during kidney development, in particular, the repolarization step in lumen formation. Other questions include how distal invasion is acquired or maintained during nephrogenesis? It will remain important to compare the regulatory modules of normal and lumopathy states to distinguish between common and divergent molecular, mechanical, and cellular strategies. As the exciting field of tubular interconnections of the kidney is beginning to unfold, future cutting-edge molecular, cellular, and biomechanical studies and modeling approaches will provide new insights into this important luminal connection process during normal development and lumopathies.
Acknowledgments
I thank Andy McMahon and Iain Drummond for their input and suggestions; Kenneth Kronenberg for translating Kristian Schreiner’s work in 1902; Bruce Popp for translating Carlo Emery’s work in 1883; Dorothy Barr at Harvard University’s Museum of Comparative Zoology library for helping me unearth classic kidney articles written by Schreiner and Emery. I also wish to thank anonymous reviewers, as well as Katherine Rogers, Patrick Müller, Stephen Von Stetina, Julien Dubrulle, and Laila Akhmetova for their comments. This review was supported by the National Science Foundation graduate research fellowship program grant numbers DGE0644491 and DGE-0946799.
Submitted
06/23/12
Revised
05/28/13
Accepted
05/30/13
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/organogenesis/article/25225
References
- 1.Dickinson AJ, Sive HL. The Wnt antagonists Frzb-1 and Crescent locally regulate basement membrane dissolution in the developing primary mouth. Development. 2009;136:1071–81. doi: 10.1242/dev.032912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ihara S, Hagedorn EJ, Morrissey MA, Chi Q, Motegi F, Kramer JM, et al. Basement membrane sliding and targeted adhesion remodels tissue boundaries during uterine-vulval attachment in Caenorhabditis elegans. Nat Cell Biol. 2011;13:641–51. doi: 10.1038/ncb2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Batourina E, Tsai S, Lambert S, Sprenkle P, Viana R, Dutta S, et al. Apoptosis induced by vitamin A signaling is crucial for connecting the ureters to the bladder. Nat Genet. 2005;37:1082–9. doi: 10.1038/ng1645. [DOI] [PubMed] [Google Scholar]
- 4.Dickinson AJ, Sive H. Development of the primary mouth in Xenopus laevis. Dev Biol. 2006;295:700–13. doi: 10.1016/j.ydbio.2006.03.054. [DOI] [PubMed] [Google Scholar]
- 5.Pyati UJ, Cooper MS, Davidson AJ, Nechiporuk A, Kimelman D. Sustained Bmp signaling is essential for cloaca development in zebrafish. Development. 2006;133:2275–84. doi: 10.1242/dev.02388. [DOI] [PubMed] [Google Scholar]
- 6.Portereiko MF, Mango SE. Early morphogenesis of the Caenorhabditis elegans pharynx. Dev Biol. 2001;233:482–94. doi: 10.1006/dbio.2001.0235. [DOI] [PubMed] [Google Scholar]
- 7.Costantini F, Kopan R. Patterning a complex organ: branching morphogenesis and nephron segmentation in kidney development. Dev Cell. 2010;18:698–712. doi: 10.1016/j.devcel.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Little MH, McMahon AP. Mammalian kidney development: principles, progress, and projections. Cold Spring Harb Perspect Biol. 2012;4:4. doi: 10.1101/cshperspect.a008300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dickinson A, Sive H. Positioning the extreme anterior in Xenopus: cement gland, primary mouth and anterior pituitary. Semin Cell Dev Biol. 2007;18:525–33. doi: 10.1016/j.semcdb.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Sherwood DR, Sternberg PW. Anchor cell invasion into the vulval epithelium in C. elegans. Dev Cell. 2003;5:21–31. doi: 10.1016/S1534-5807(03)00168-0. [DOI] [PubMed] [Google Scholar]
- 11.Ziel JW, Hagedorn EJ, Audhya A, Sherwood DR. UNC-6 (netrin) orients the invasive membrane of the anchor cell in C. elegans. Nat Cell Biol. 2009;11:183–9. doi: 10.1038/ncb1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hagedorn EJ, Yashiro H, Ziel JW, Ihara S, Wang Z, Sherwood DR. Integrin acts upstream of netrin signaling to regulate formation of the anchor cell’s invasive membrane in C. elegans. Dev Cell. 2009;17:187–98. doi: 10.1016/j.devcel.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sherwood DR, Butler JA, Kramer JM, Sternberg PW. FOS-1 promotes basement-membrane removal during anchor-cell invasion in C. elegans. Cell. 2005;121:951–62. doi: 10.1016/j.cell.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 14.Schindler AJ, Sherwood DR. The transcription factor HLH-2/E/Daughterless regulates anchor cell invasion across basement membrane in C. elegans. Dev Biol. 2011;357:380–91. doi: 10.1016/j.ydbio.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Newman AP, White JG, Sternberg PW. The Caenorhabditis elegans lin-12 gene mediates induction of ventral uterine specialization by the anchor cell. Development. 1995;121:263–71. doi: 10.1242/dev.121.2.263. [DOI] [PubMed] [Google Scholar]
- 16.Farooqui S, Pellegrino MW, Rimann I, Morf MK, Müller L, Fröhli E, et al. Coordinated lumen contraction and expansion during vulval tube morphogenesis in Caenorhabditis elegans. Dev Cell. 2012;23:494–506. doi: 10.1016/j.devcel.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 17.Georgas K, Rumballe B, Valerius MT, Chiu HS, Thiagarajan RD, Lesieur E, et al. Analysis of early nephron patterning reveals a role for distal RV proliferation in fusion to the ureteric tip via a cap mesenchyme-derived connecting segment. Dev Biol. 2009;332:273–86. doi: 10.1016/j.ydbio.2009.05.578. [DOI] [PubMed] [Google Scholar]
- 18.Kao RM, Vasilyev A, Miyawaki A, Drummond IA, McMahon AP. Invasion of distal nephron precursors associates with tubular interconnection during nephrogenesis. J Am Soc Nephrol. 2012;23:1682–90. doi: 10.1681/ASN.2012030283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parkin CA, Allen CE, Ingham PW. Hedgehog signalling is required for cloacal development in the zebrafish embryo. Int J Dev Biol. 2009;53:45–57. doi: 10.1387/ijdb.082669cp. [DOI] [PubMed] [Google Scholar]
- 20.Slanchev K, Pütz M, Schmitt A, Kramer-Zucker A, Walz G. Nephrocystin-4 is required for pronephric duct-dependent cloaca formation in zebrafish. Hum Mol Genet. 2011;20:3119–28. doi: 10.1093/hmg/ddr214. [DOI] [PubMed] [Google Scholar]
- 21.Newman AP, Sternberg PW. Coordinated morphogenesis of epithelia during development of the Caenorhabditis elegans uterine-vulval connection. Proc Natl Acad Sci U S A. 1996;93:9329–33. doi: 10.1073/pnas.93.18.9329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Newman AP, White JG, Sternberg PW. Morphogenesis of the C. elegans hermaphrodite uterus. Development. 1996;122:3617–26. doi: 10.1242/dev.122.11.3617. [DOI] [PubMed] [Google Scholar]
- 23.Pastor-Pareja JC, Grawe F, Martín-Blanco E, García-Bellido A. Invasive cell behavior during Drosophila imaginal disc eversion is mediated by the JNK signaling cascade. Dev Cell. 2004;7:387–99. doi: 10.1016/j.devcel.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 24.Manni L, Lane NJ, Zaniolo G, Burighel P. Cell reorganisation during epithelial fusion and perforation: the case of ascidian branchial fissures. Dev Dyn. 2002;224:303–13. doi: 10.1002/dvdy.10112. [DOI] [PubMed] [Google Scholar]
- 25.Bubenshchikova E, Ichimura K, Fukuyo Y, Powell R, Hsu C, Morrical SO, et al. Wtip and Vangl2 are required for mitotic spindle orientation and cloaca morphogenesis. Biol Open. 2012;1:588–96. doi: 10.1242/bio.20121016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Obara T, Mangos S, Liu Y, Zhao J, Wiessner S, Kramer-Zucker AG, et al. Polycystin-2 immunolocalization and function in zebrafish. J Am Soc Nephrol. 2006;17:2706–18. doi: 10.1681/ASN.2006040412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Portereiko MF, Saam J, Mango SE. ZEN-4/MKLP1 is required to polarize the foregut epithelium. Curr Biol. 2004;14:932–41. doi: 10.1016/j.cub.2004.05.052. [DOI] [PubMed] [Google Scholar]
- 28.Rasmussen JP, Reddy SS, Priess JR. Laminin is required to orient epithelial polarity in the C. elegans pharynx. Development. 2012;139:2050–60. doi: 10.1242/dev.078360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dejana E, Orsenigo F, Molendini C, Baluk P, McDonald DM. Organization and signaling of endothelial cell-to-cell junctions in various regions of the blood and lymphatic vascular trees. Cell Tissue Res. 2009;335:17–25. doi: 10.1007/s00441-008-0694-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dejana E, Simionescu M, Wolburg H. Endothelial cell biology and pathology. Cell Tissue Res. 2009;335:1–3. doi: 10.1007/s00441-008-0697-2. [DOI] [PubMed] [Google Scholar]
- 31.Dejana E, Tournier-Lasserve E, Weinstein BM. The control of vascular integrity by endothelial cell junctions: molecular basis and pathological implications. Dev Cell. 2009;16:209–21. doi: 10.1016/j.devcel.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 32.Strilić B, Kucera T, Eglinger J, Hughes MR, McNagny KM, Tsukita S, et al. The molecular basis of vascular lumen formation in the developing mouse aorta. Dev Cell. 2009;17:505–15. doi: 10.1016/j.devcel.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 33.Bayless KJ, Davis GE. The Cdc42 and Rac1 GTPases are required for capillary lumen formation in three-dimensional extracellular matrices. J Cell Sci. 2002;115:1123–36. doi: 10.1242/jcs.115.6.1123. [DOI] [PubMed] [Google Scholar]
- 34.Folkman J, Haudenschild C. Angiogenesis by capillary endothelial cells in culture. Trans Ophthalmol Soc U K. 1980;100:346–53. [PubMed] [Google Scholar]
- 35.Kamei M, Saunders WB, Bayless KJ, Dye L, Davis GE, Weinstein BM. Endothelial tubes assemble from intracellular vacuoles in vivo. Nature. 2006;442:453–6. doi: 10.1038/nature04923. [DOI] [PubMed] [Google Scholar]
- 36.Herwig L, Blum Y, Krudewig A, Ellertsdottir E, Lenard A, Belting HG, et al. Distinct cellular mechanisms of blood vessel fusion in the zebrafish embryo. Curr Biol. 2011;21:1942–8. doi: 10.1016/j.cub.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 37.Blum Y, Belting HG, Ellertsdottir E, Herwig L, Lüders F, Affolter M. Complex cell rearrangements during intersegmental vessel sprouting and vessel fusion in the zebrafish embryo. Dev Biol. 2008;316:312–22. doi: 10.1016/j.ydbio.2008.01.038. [DOI] [PubMed] [Google Scholar]
- 38.Wang Y, Kaiser MS, Larson JD, Nasevicius A, Clark KJ, Wadman SA, et al. Moesin1 and Ve-cadherin are required in endothelial cells during in vivo tubulogenesis. Development. 2010;137:3119–28. doi: 10.1242/dev.048785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang Z, Zimmerman S, Brakeman PR, Beaudoin GM, 3rd, Reichardt LF, Marciano DK. De novo lumen formation and elongation in the developing nephron: a central role for afadin in apical polarity. Development. 2013;140:1774–84. doi: 10.1242/dev.087957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Osterfield M, Du X, Schüpbach T, Wieschaus E, Shvartsman SY. Three-dimensional epithelial morphogenesis in the developing Drosophila egg. Dev Cell. 2013;24:400–10. doi: 10.1016/j.devcel.2013.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Caussinus E, Colombelli J, Affolter M. Tip-cell migration controls stalk-cell intercalation during Drosophila tracheal tube elongation. Curr Biol. 2008;18:1727–34. doi: 10.1016/j.cub.2008.10.062. [DOI] [PubMed] [Google Scholar]
- 42.Folkman J, Haudenschild C. Angiogenesis in vitro. Nature. 1980;288:551–6. doi: 10.1038/288551a0. [DOI] [PubMed] [Google Scholar]
- 43.Samakovlis C, Manning G, Steneberg P, Hacohen N, Cantera R, Krasnow MA. Genetic control of epithelial tube fusion during Drosophila tracheal development. Development. 1996;122:3531–6. doi: 10.1242/dev.122.11.3531. [DOI] [PubMed] [Google Scholar]
- 44.Tanaka-Matakatsu M, Uemura T, Oda H, Takeichi M, Hayashi S. Cadherin-mediated cell adhesion and cell motility in Drosophila trachea regulated by the transcription factor Escargot. Development. 1996;122:3697–705. doi: 10.1242/dev.122.12.3697. [DOI] [PubMed] [Google Scholar]
- 45.Chia I, Grote D, Marcotte M, Batourina E, Mendelsohn C, Bouchard M. Nephric duct insertion is a crucial step in urinary tract maturation that is regulated by a Gata3-Raldh2-Ret molecular network in mice. Development. 2011;138:2089–97. doi: 10.1242/dev.056838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walker KA, Sims-Lucas S, Di Giovanni VE, Schaefer C, Sunseri WM, Novitskaya T, et al. Deletion of fibroblast growth factor receptor 2 from the peri-wolffian duct stroma leads to ureteric induction abnormalities and vesicoureteral reflux. PLoS One. 2013;8:e56062. doi: 10.1371/journal.pone.0056062. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 47.Schreiner KE. Uber die Entwicklung der Amniotenniere. 1902:128-35. [Google Scholar]
- 48.Shibata S, Nagase M, Yoshida S, Kawarazaki W, Kurihara H, Tanaka H, et al. Modification of mineralocorticoid receptor function by Rac1 GTPase: implication in proteinuric kidney disease. Nat Med. 2008;14:1370–6. doi: 10.1038/nm.1879. [DOI] [PubMed] [Google Scholar]
- 49.Togawa A, Miyoshi J, Ishizaki H, Tanaka M, Takakura A, Nishioka H, et al. Progressive impairment of kidneys and reproductive organs in mice lacking Rho GDIalpha. Oncogene. 1999;18:5373–80. doi: 10.1038/sj.onc.1202921. [DOI] [PubMed] [Google Scholar]
- 50.Marciano DK, Brakeman PR, Lee CZ, Spivak N, Eastburn DJ, Bryant DM, et al. p120 catenin is required for normal renal tubulogenesis and glomerulogenesis. Development. 2011;138:2099–109. doi: 10.1242/dev.056564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harding SD, Armit C, Armstrong J, Brennan J, Cheng Y, Haggarty B, et al. The GUDMAP database--an online resource for genitourinary research. Development. 2011;138:2845–53. doi: 10.1242/dev.063594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McMahon AP, Aronow BJ, Davidson DR, Davies JA, Gaido KW, Grimmond S, et al. GUDMAP project GUDMAP: the genitourinary developmental molecular anatomy project. J Am Soc Nephrol. 2008;19:667–71. doi: 10.1681/ASN.2007101078. [DOI] [PubMed] [Google Scholar]
- 53.Hama H, Kurokawa H, Kawano H, Ando R, Shimogori T, Noda H, et al. Scale: a chemical approach for fluorescence imaging and reconstruction of transparent mouse brain. Nat Neurosci. 2011;14:1481–8. doi: 10.1038/nn.2928. [DOI] [PubMed] [Google Scholar]
- 54.Chung K, Wallace J, Kim SY, Kalyanasundaram S, Andalman AS, Davidson TJ, et al. Structural and molecular interrogation of intact biological systems. Nature. 2013;497:332–7. doi: 10.1038/nature12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kuwajima T, Sitko AA, Bhansali P, Jurgens C, Guido W, Mason C. ClearT: a detergent- and solvent-free clearing method for neuronal and non-neuronal tissue. Development. 2013;140:1364–8. doi: 10.1242/dev.091844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Emery C. Embryological Research on Mammal Kidneys. 1883. [Google Scholar]