Abstract
Objective
The cortisol/DHEA(S) ratio has demonstrated utility in studies of HPA activity and psychopathology. However, use of the cortisol/DHEA(S) ratio in adolescent populations requires additional consideration of differential changes in DHEA(S) and cortisol during the course of puberty. This study examines the relationship between pubertal status and individual cortisol and DHEAS levels as well as with the cortisol/DHEAS ratio.
Method
Morning salivary cortisol and urinary DHEAS levels were obtained for 267 young adolescents at three time points, each approximately one year apart. Growth curve modeling and repeated measures ANOVA were used to assess the effect of adrenal development on individual hormone levels and on the total ratio.
Results
Pubic hair development was a significant predictor of change over time in DHEAS but not cortisol. Development was also a significant predictor of the cortisol/DHEAS ratio when raw cortisol and DHEAS values were used.
Conclusions
Our findings indicate that, when DHEAS levels were adjusted to control for pubertal status, the ratio demonstrated stability over time. This finding is in line with the hypothesis that the ratio may tap stable individual differences in HPA functioning.
Keywords: Cortisol, DHEA, DHEAS, ratio, HPA axis, adolescent, puberty, adrenarche
Introduction
Burgeoning research on links between the Hypothalamic-Pituitary-Adrenal (HPA) system and psychopathology has focused extensively on the cortisol stress response. However, recent studies suggest that dehydroepiandrosterone (DHEA) and its sulfate (DHEAS) also play a role in the stress system. The relative level of cortisol to DHEA(S) has been examined as an indicator of HPA axis functioning that accounts for the neurotropic effects of each hormone (for review, see Maninger et al, 2009). However, comparison of findings across age groups is complicated by the differential impact of adrenal development on these hormones; specifically, DHEA(S) levels are known to increase with adrenarche whereas effects of puberty on cortisol are unclear. Yet, the few studies examining the cortisol/DHEA(S) ratio in adolescents have neglected to control for hormonal changes associated with adrenarche.
Both cortisol and DHEA(S) are synthesized from pregnenolone in response to adrenocorticotropic hormone release. It has been suggested that the ratio of cortisol and DHEA(S) taps preferential production of one hormone or the other such that markedly higher or lower ratios may reflect physiological vulnerabilities for psychopathology. In particular, significantly higher or lower cortisol/DHEA(S) ratios have been associated with depression (Angold, 2003) and aggression (Pajer et al., 2006), respectively.
Both cortisol and DHEA(S) demonstrate developmental changes throughout the lifetime (Goodyer et al., 2001). Several studies have reported developmentally-related increases in basal cortisol levels from childhood through adolescence (Gunnar and Vasquez, 2006), but others have found this effect only in females (Schiefelbein and Susman, 2006) or not at all (Knuttson et al., 1997). A recent 6-year longitudinal study by Shirtcliff and colleagues (2011) found a decrease in cortisol and identified this change as being driven by age rather than puberty. In contrast, DHEA(S) increases significantly beginning at adrenarche continuing throughout puberty (Rege and Rainey, 2012).
As indicated, the only identified studies on the cortisol/DHEA(S) ratio in adolescents have failed to control for puberty prior to calculating the ratio beyond simply classifying participants as “pre-pubertal” or “post-pubertal” (e.g., Netherton et al., 2004). In young children and in adults, variations in DHEA(S) associated with puberty are less important because the entire sample is either pre- or post-pubertal. For adolescent samples, individuals are at varying stages of adrenarche and therefore have significantly different reference ranges for DHEA(S) (Rege and Rainey, 2012). For example, Identifying an individual as having a lower cortisol/DHEA(S) ratio could indicate preferential production of DHEA(S) over cortisol or that the individual has developed earlier than his/her peers (and vice versa for high cortisol/DHEAS). This difference is notable because early pubertal maturation has also been identified as a risk factor for psychopathology in adolescents (Graber et al., 2010).
The purpose of the current study is to examine the relationship between pubertal development, adrenal hormones, and their ratio in a community sample of children seen annually during the transition from childhood into adolescence.
Methods
Two hundred sixty-seven pre- to early-adolescent boys and girls (Ngirls=138, Nboys=129; M age=9.2 years, SD=0.70 at Time 1) were seen annually for 3 years. The racial/ethnic makeup of the participants at enrollment was 39% Caucasian, 32% African-American, 12% Hispanic, and 17% multiracial or other. One hundred ninety-four participants (Ngirls=92, Nboys=102) provided data at Time 2 (27% attrition; M age=10.9 years, SD=0.77). As all participants were contacted at each assessment, 202 children (Ngirls=111, Nboys=91) participated at Time 3 (24% attrition from enrollment; M age=12.0 years, SD=0.77). Male participants were more likely than females, and white were less likely than non-white participants to have discontinued participation (T1 to T3). No significant differences were found in attrition based on T1 cortisol, DHEAS values, or pubertal status.
Procedure
The girls’ and boys’ studies were not conducted concurrently and some procedures differed between studies; however, procedures were consistent within gender over time. Procedures were approved by the IRB of Teachers College, Columbia University. Saliva samples and overnight urine samples were collected each morning for two (boys) or three (girls) days at home to assess hormone levels. Morning salivary samples for cortisol were collected immediately upon the child’s natural waking time, before eating, drinking, or brushing teeth. Because DHEAS assays for saliva collected with salivette devices were not available at the time assays were conducted, morning urine samples were used for DHEAS sampling instead. Overnight urine collection for DHEAS included first morning void and any voids between bedtime and waking. Parents labeled samples with time and date of collection.
Cortisol
Salivary cortisol was assayed in duplicate using radioimmunoassay developed by Kirschbaum and colleagues (Diagnostic Products Company) with a lower detection limit of 0.02 µg/dl per 200 µl of saliva. The inter- and intra-assay variation coefficients were less than 3% and 5%, respectively. Cortisol values for each of the two (boys) or three (girls) samples were averaged at each time point.
DHEAS
DHEAS level was determined using a commercial solid-phase, competitive chemiluminescent immunoassay (Immulite, Siemens, Los Angeles, CA) with a sensitivity of 3 g/dl. The inter- and intra-assay variation coefficients were less than 8.2% and 12.0%, respectively. Urinary DHEAS was standardized using creatinin; however, no significant differences were found in the raw and creatinin-standardized DHEAS values. Hence, raw values were used in this study. DHEAS levels for each of the two (boys) or three (girls) samples were averaged at each time point.
Pubertal Status
Mothers rated Tanner stage of pubic hair growth (TannerPH) from drawings (1–5, from no development to complete adult stage of development; Morris and Udry, 1980); child report was obtained in cases where mothers indicated they were not able to complete ratings. While Tanner staging also includes a breast (girls) or testicular (boys) development item, only reports of pubic hair were used in these analyses as this most reliably links to adrenal hormone activity (Shirtcliff et al., 2009).
Ratio Calculation
The cortisol/DHEAS ratios were calculated using untransformed raw values and using DHEAS values adjusted for TannerPH stage. For the raw ratio, the morning cortisol value was divided by the morning DHEAS value. For the residualized ratio, regressions were run using TannerPH to predict the morning DHEAS value, and unstandardized residuals were saved. Because this resulted in negative and positive DHEAS values and to put both hormones in a comparable metric, both cortisol and residualized DHEAS values were percentile rank ordered. Percentile rank cortisol was then divided by percentile rank DHEAS.
Analyses
Growth curve modeling in SPSS AMOS using maximum likelihood estimation was used to assess the relationships of TannerPH with DHEAS and with cortisol. The variance for the Time 1 TannerPH uniqueness was constrained to zero because initial analyses produced a negative variance. The path from each observed variable to its associated intercept was set at one. Each follow-up visit took place approximately one year after the previous visit, so slope loadings were set at zero, one, and two for Times 1, 2, and 3, respectively. This model was also tested with age rather than TannerPH in order to compare effects related to age versus developent.
To assess the association of adrenal development with the cortisol/DHEAS ratio, repeated measures analyses of variance (ANOVA) were run for the raw and for the residualized ratios; follow up tests used a Bonferroni adjustment.
Prior studies in adolescents have found small (Elmlinger et al., 2002) or no (Matchcock et al., 2007) sex differences in cortisol and DHEA(S) levels. The effect of sex was tested in preliminary analyses but was found to be non-significant, so it was removed from the final analyses.
Results
Mean cortisol and DHEAS values as well as raw and residualized ratio values are presented in Table 1, by gender and TannerPH stage.
Table 1.
Means and standard deviations for mean cortisol, DHEAS, raw ratio, and residualized ratio by gender and Tanner pubic hair (TannerPH) stage
| Cortisol | Boys | Girls |
|---|---|---|
| TannerPH 1 | 0.378 (0.180) | 0.395 (0.173) |
| TannerPH 2 | 0.375 (0.191) | 0.526 (0.494) |
| TannerPH 3 | 0.373 (0.210) | 0.444 (0.184) |
| TannerPH 4/5 | 0.365 (0.194) | 0.436 (0.240) |
| DHEAS | ||
| TannerPH 1 | 36.938 (37.287) | 25.187 (22.330) |
| TannerPH 2 | 47.816 (40.003) | 43.879 (35.202) |
| TannerPH 3 | 62.294 (52.229) | 61.093 (42.579) |
| TannerPH 4/5 | 72.288 (54.862) | 88.930 (53.016) |
| Raw Ratio | ||
| TannerPH 1 | 0.025 (0.030) | 0.031 (0.030) |
| TannerPH 2 | 0.016 (0.019) | 0.020 (0.017) |
| TannerPH 3 | 0.011 (0.013) | 0.012 (0.013) |
| TannerPH 4/5 | 0.007 (0.006) | 0.008 (0.008) |
| Residualized Ratio | ||
| TannerPH 1 | 1.344 (1.571) | 1.531 (1.483) |
| TannerPH 2 | 2.663 (4.830) | 2.319 (4.610) |
| TannerPH 3 | 4.064 (8.223) | 4.096 (8.936) |
| TannerPH 4/5 | 4.484 (10.519) | 2.798 (5.549) |
Note:Because few instances of TannerPH stage 5 were reported at any time point, scores of 4 and 5 were collapsed. TannerPH stage 1 n = 157 (boys), 136 (girls); TannerPH stage 2 n = 81 (boys), 74 (girls); TannerPH stage 3 n = 39 (boys), 40 (girls); TannerPH stages 4/5 n = 18 (boys), 49 (girls). Data collected as part of the Girls and Boys Health and Development Project.
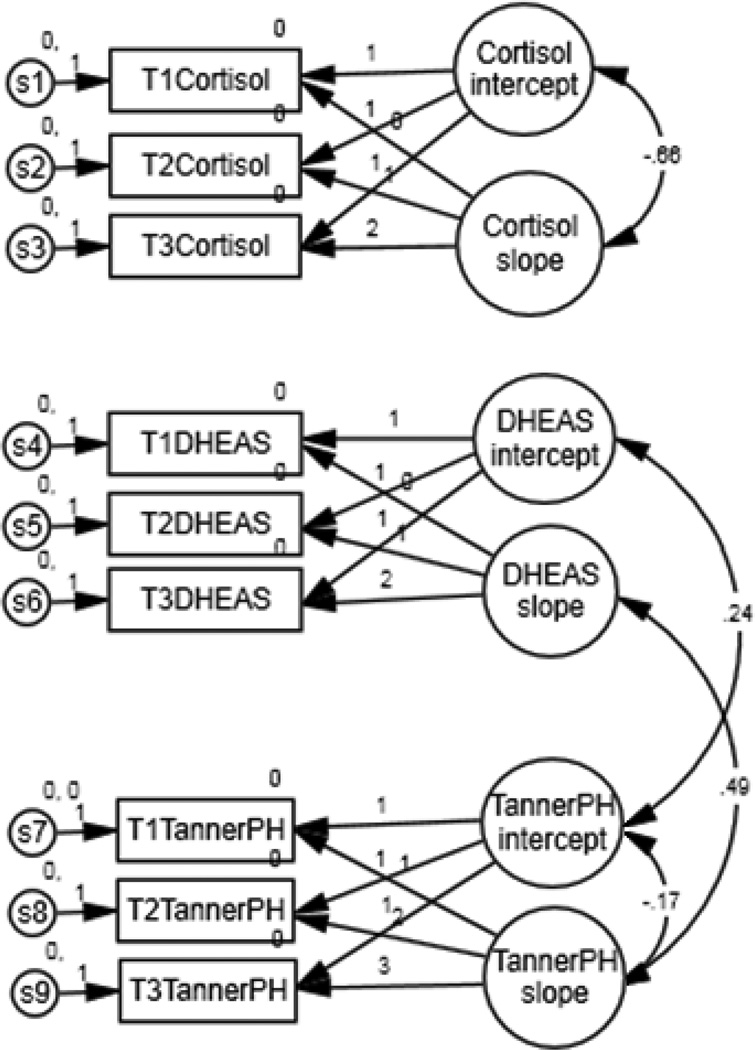
Mean and variance estimates for the higher-order slope and intercept factors are presented in Figure 1; only the paths for significant correlations between factors are shown. As expected, TannerPH score increased across the three time points, shown by a significant estimate for the TannerPH slope factor. Mean estimates indicate an average starting value of 1.46 and a yearly increase of 0.67. DHEAS scores also increased significantly, with average starting value of 28.88 ug/dl and yearly increase of 17.76 units. Cortisol value did not show a significant change over time; participants started at an average value of 0.41ug/dl and remained stable over the three time points.
Figure 1.
Path diagram illustrating relationships between cortisol, DHEAS, and adrenal development. Numbers within arrows indicate significant correlation between factors.
Variance estimates indicated significant between-subjects variability in both the initial value and the rate of change in TannerPH score, p<.001. The addition of gender into the model did not explain additional variance, indicating that the significant variance estimate is due to individual variability and not due to the inclusion of both males and females in the model. Additional analyses indicated racial differences did not predict initial TannerPH score but did predict the rate of change, with African-Americans developing significantly faster than other races, p<.01.
There is no significant between-subjects variability in DHEAS initial value, p>.05, though there was significant within-subjects variability in DHEAS, indicating change over time, p<.01. Finally, while the overall cortisol slope was not significant, there does appear to be significant between-subjects variability in cortisol slope, p<.05, and intercept, p<.01. Racial differences did not predict starting value or rate of change in cortisol or DHEAS values.
Correlations between higher-order slope and intercept factors indicated a significant negative relationship between cortisol intercept and slope, r= −.66, p<.05, and between TannerPH score intercept and slope, r=−.17, p<.001; individuals who started with the highest cortisol values or who were among the most developed at the beginning of the study increased the least. As expected, TannerPH slope and DHEAS slope were significantly positively correlated, r= .49, p<.01, as were TannerPH intercept and DHEAS intercept, r=.24, p<.001. However, since there was no significant variability in DHEAS intercept, this correlation should be interpreted with caution. The model is a good fit for the data: the chi-square test was not significant, χ2(19)=26.42, p>05; and the NFI and CFI estimates were .95 and .98, respectively, both meeting the recommended criterion of .95. As indicated, the model was also tested using age rather than TannerPH. The model was a poor fit for the data; the chi-square test was significant, χ2(19)=100.09, p>.05, and the NFI and CFI estimates were .88 and .89, respectively, both below the recommended criterion of .95.
Repeated measures ANOVA assessing change over time in the raw and residualized ratios indicated that there was significant change over time in the raw ratio, p<.001, but not in the residualized ratio, p>.05. For the raw ratio, all three occasions were significantly different from one another with significantly greater mean values at earlier occasions. This translates to smaller ratios, driven by large DHEAS values, as puberty progresses. No significant differences were found for any occasion using the residualized ratio, p>.05.
Discussion
As predicted, these analyses support prior research that DHEAS, but not cortisol, is significantly associated with puberty as assessed by TannerPH. Thus, if researchers fail to control for developmental stage when using the cortisol/DHEAS ratio, the results will not accurately represent HPA axis activity independent of puberty and may instead reflect the impact of pubertal timing (Graber et al., 2010). Although maternal or self-reported TannerPH status likely includes error variance, the residualized ratio in the present study demonstrated stability across 3 years, lending support to the hypothesis that the ratio taps individual differences in HPA activity. The study of intraindividual differences in cortisol and DHEAS is vital for the understanding of developmental processes and their effects on outcomes such as psychopathology.
While the preferential production of cortisol or DHEA(S) is largely driven by metabolic factors, psychological factors may also play a role in HPA axis activity. Some have suggested that early experience of stress and/or long-term exposure to stressors may cause a stress sensitization or inoculation; the former causing heightened cortisol production and the latter resulting in heightened DHEA(S) production (Ozbay et al., 2008). This lends explanation as to why individuals respond differently to similar social stressors, but it creates additional questions regarding what biological or psychosocial factors determine sensitization or inoculation.
Acknowledgements
The authors wish to thank the staff of the Boys and Girls Health and Development Project as well as all of the participants and families who took part in the study.
Source of Funding
This project was funded by NIMH Grants 1 K23 MH067947 (ART) and MH56557 (JBG); NIMH had no additional role in the design of the study, data collection, analysis or interpretation of data, writing of this report, or the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors have no conflicts of interest to disclose
Contributions
Mary Saczawa developed and completed the analysis plan and wrote manuscript. Drs. Graber and Brooks-Gunn designed the overall project, wrote the project protocol, and oversaw data collection. Dr. Graber also assisted in writing the manuscript. Dr. Warren contributed to the project design and conducted cortisol and DHEAS assays. All authors contributed to and approved of the final manuscript.
References
- Angold A. Adolescent depression, cortisol, and DHEA. Psychological Med. 2003;33:573–581. doi: 10.1017/s003329170300775x. [DOI] [PubMed] [Google Scholar]
- Goodyer IM, Park RJ, Netherton CM, Herbert J. Possible role of cortisol and dehydroepiandrosterone in human development and psychopathology. Brit J of Psychiatry. 2001;179:243–249. doi: 10.1192/bjp.179.3.243. [DOI] [PubMed] [Google Scholar]
- Graber JA, Nichols TR, Brooks-Gunn J. Putting pubertal timing in developmental context: Implications for prevention. Dev Psychobiology. 2010;52:254–262. doi: 10.1002/dev.20438. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Vasquez D. Stress neurobiology and developmental psychopathology. In: Cicchetti D, Cohen D, editors. Developmental psychopathology: Vol. 2. Developmental neuroscience. 2nd ed. New York: Wiley; 2006. pp. 533–577. [Google Scholar]
- Knuttson U, Dahlgreen J, Marcus C, Rosberg S, Bronnegard M, Stierna P, Albertsson-Wikland K. Circadian cortisol rhythms in healthy boys and girls: Relationship with age growth, body composition, and pubertal development. J of Clin Endocrinol & Metab. 1997;82:536–540. doi: 10.1210/jcem.82.2.3769. [DOI] [PubMed] [Google Scholar]
- Maninger N, Wolkowitz OM, Reus VI, Epel ES, Mellon SH. Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS) Frontiers in Neuroendocrinology. 2009;30:65–91. doi: 10.1016/j.yfrne.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matchcock RL, Dorn LD, Susman EJ. Diurnal and seasonal cortisol, testosterone, and DHEA rhythms in boys and girls during puberty. Chronobiology International. 2007;24:969–990. doi: 10.1080/07420520701649471. [DOI] [PubMed] [Google Scholar]
- Morris NM, Udry JR. Validation of a self-administered instrument to assess stage of adolescent development. J of Youth & Adolescence. 1980;9:271–280. doi: 10.1007/BF02088471. [DOI] [PubMed] [Google Scholar]
- Ozbay F, Fitterling H, Charney D, Southwick S. Social support and resilience to stress across the life span: A neurobiologic framework. Current Psychiatry Reports. 2008;10:304–310. doi: 10.1007/s11920-008-0049-7. [DOI] [PubMed] [Google Scholar]
- Pajer K, Tabbah R, Gardner W, Rubin RT, Czambel RK, Wang Y. Adrenal androgen and gonadal hormone levels in adolescent girls with conduct disorder. Psychoneuroendocrinology. 2006;31:1245–1256. doi: 10.1016/j.psyneuen.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Rege J, Rainey WE. The steroid metabolome of adrenarche. J of Endocrinology. 2012;214:133–143. doi: 10.1530/JOE-12-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiefelbein VL, Susman EJ. Cortisol levels and longitudinal cortisol change as predictors of anxiety in adolescents. J of Early Adolesc. 2006;26:397–413. [Google Scholar]
- Shirtcliff EA, Allison AL, Armstrong JM, Slattery MJ, Kalin NH, Essex MJ. Longitudinal stability and developmental properties of salivary cortisol levels and circadian rhythms from childhood to adolescence. Dev Psychobiol. 2011 doi: 10.1002/dev.20607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, Dahl RE, Pollak SD. Pubertal development: Correspondence between hormonal and physical development. Child Dev. 2009;80:327–337. doi: 10.1111/j.1467-8624.2009.01263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]