Abstract
Environments without any contact with anthropogenic antibiotics show a great abundance of antibiotic resistance genes that use to be chromosomal and are part of the core genes of the species that harbor them. Some of these genes are shared with human pathogens where they appear in mobile genetic elements. Diversity of antibiotic resistance genes in non-contaminated environments is much greater than in human and animal pathogens, and in environments contaminated with antibiotic from anthropogenic activities. This suggests the existence of some bottleneck effect for the mobilization of antibiotic resistance genes among different biomes. Bacteriophages have characteristics that make them suitable vectors between different biomes, and as well for transferring genes from biome to biome. Recent metagenomic studies and detection of bacterial genes by genomic techniques in the bacteriophage fraction of different microbiota provide indirect evidences that the mobilization of genes mediated by phages, including antibiotic resistance genes, is far more relevant than previously thought. Our hypothesis is that bacteriophages might be of critical importance for evading one of the bottlenecks, the lack of ecological connectivity that modulates the pass of antibiotic resistance genes from natural environments such as waters and soils, to animal and human microbiomes. This commentary concentrates on the potential importance of bacteriophages in transferring resistance genes from the environment to human and animal body microbiomes, but there is no doubt that transduction occurs also in body microbiomes.
Keywords: bacteriophages, transduction, lysogeny, horizontal gene transfer, antibiotic resistance
Antibiotic Resistance in Pristine Environments
Emergence and spread of resistance to antibiotics is hampering one of the major achievements of the history of medicine, which is the minimization of the effects of infectious diseases, mostly of those caused by bacteria. Traditionally, it was thought that the selective pressure caused by the overuse and misuse of antibiotics in human medicine and animal husbandry was the major, if not the unique, cause of this occurrence.1 In the last years, the number of evidences about the ubiquity and abundance of antibiotic resistance genes in diverse environments has increased. These studies have shown that there is a great abundance of antibiotic resistance genes (ARG) in many environmental ecosystems barely in contact with human produced and released antibiotics, that suggests an important role of environmental microorganisms as source and reservoirs of resistance genes.2 Thus, a controversial question about microbial resistance origin is whether it is the result of human activity or rather a result of the joint evolution of antibiotic production and antibiotic resistance pathways that evolved during millions of years in the environment, or both.3–5 Indeed, antibiotic resistance seems to be very antique.6 The diversity of resistance determinants in different bacteria is larger in natural environments not contaminated with antibiotic from anthropogenic activities than in human pathogens5 or in environments with anthropogenic influence.7 This seems to indicate the existence of bottlenecks controlling the transfer, spread and stability of antibiotic resistance genes already exiting in nature among bacteria from other biomes.
Many of the antibiotic resistance determinants present in clinical isolates are typically acquired through and located on mobile genetic elements (MGE), allowing their horizontal transfer to other strains (pathogens, commensal or even environmental) or even across bacteria from different taxa. In contrast, antibiotic resistance genes in naturally resistant environmental bacteria are typically mediated by a resistance gene belonging to the cell’s core genes. Examples of this type of natural resistance are the chromosomally encoded β-lactamases found in several species of the Enterobacteriaceae,8 many of them colonizing plants and soils, as for example in species of Rahnella and Ewingella. Such resistance genes are vertically inherited, shared by most isolates of the same species, often encoded by the chromosome and usually immobile. This maintenance and vertical transfer of antibiotic resistance genes in environments with no or very mild long lasting selective pressure appears to have minor fitness costs for the host bacteria than maintenance of and transfer by MGE.9
But the same genes have been found in pathogens and commensal bacteria of human and animal microbiomes after the introduction in clinics of a given antibiotic. There, the presence of antibiotics will push the selection of the genes either alone or located in the genetic mobile platforms found in pathogens. Usually, conjugation mediated by plasmids has been considered as the main and virtually unique mode of horizontal transfer of antibiotic resistance genes.
How transfer occurs and which are the bottlenecks modulating the transfer of these natural chromosomal and non-mobile genes from environmental bacteria to mobile gene platforms of pathogens remains intriguing.
Bacteriophages: A Potential Vector
Horizontal transfer among bacteria occurs by one of the three following mechanisms: conjugation, free DNA transformation and transduction through bacteriophages. Phage transduction can be either specialized, when the bacterial DNA adjacent to the prophage attachment site of a temperate bacteriophage is moved to a recipient host cell, or generalized transduction, that accounts for transfer of any gene of the host and can be done by temperate phages, like phage P22 of Salmonella, or virulent phages. Experimental detection of generalized transduction by virulent bacteriophages is quite challenging, and this may be the reason of the general thinking that transduction is only carried by temperate bacteriophages. The size of the DNA fragments that can be packaged into a bacteriophage particle is limited by the size of the phage capsid, but can reach upwards of 100 kilobases (kb). Transduction by bacteriophages includes any sort of bacterial DNA, including linear chromosome fragments and all sorts of mobile elements such as plasmids, islands, transposons and insertion elements.10,11 An important characteristic of transduction is that it does not require “donor” and “recipient” cells to be present at the same place or even at the same time.
Recently, the idea that bacteriophage transduction, either specialized or generalized, plays a role in this horizontal gene transfer from environmental to human and animal body associated biomes is gaining momentum. This change in the dominant belief is due to the following lines of indirect evidence; first, bacteriophages have been confirmed as the most abundant beings in many natural environments as oceans, lakes and soil and man-managed environment as for example sewage treatment plants, as well as in human body-associated biomes. In all these ecosystems, concentrations of bacteriophages use to overnumber those of bacteria by a factor most frequently ranging from 1 to 10.12 In many aquatic and terrestrial environments, as well as in body-associated microbiomes,12,13 the concentrations of bacteria and bacteriophages are high enough to guarantee a great frequency of bacteria-bacteriophages encounters that are needed for infection and subsequent transduction to occur. Second, due to the structural characteristics of phages in their extracellular phase they persist quite successfully in the environment and are quite resistant to natural and man generated stressors.14 Most frequently they persist better than their hosts. Some of the families, as for example Siphoviridae, well known for holding phages able to transduce and being among the more abundant in nature, are also among the more resistant to environmental stressors. Their persistence in the environment is also much higher than that of free DNA, which is more sensitive to nucleases, temperature and radiation.
These survival capabilities together with the fact that for transduction donor and recipient bacteria do not need to coincide in space and time make bacteriophages especially suited for gene movement between different biomes.15
Transduction has so far been considered as a rare event occurring around once every 107–109 phage infections, but recent studies show that transduction might occur at frequencies several orders of magnitudes greater than previously thought.16 With these frequencies and the concentrations of phages and hosts found in many environments, gene transfer by transduction would take place an exceptional number of times per second in any one place.
Certain bacteriophages, known as polyvalent bacteriophages, have been reported to have a wide host range that crosses the boundaries of different taxa, even at the superior levels of taxonomical hierarchy, as for example between Gammaproteobacteria and Betaproteobacteria.17 Furthermore, transduction has also been described between bacteria belonging to different taxa.18 In addition, similar prophages have been detected in bacteria of different species of Clostridium and Bacillus spp19 Considering those phages that harbor MGEs, like conjugative plasmids, the polyvalence of some transducing phages would allow the transfer of the plasmids to bacterial hosts that could not be recipients if the transfer would take place by conjugation. This would lead to simultaneous transmission of ARGs and even virulence determinants, commonly found in megaplasmids in vivo.
Metagenomic analysis of viral communities has provided a huge amount of information on the characteristics of the genetic material included in the viral particles that constitute the viral communities of the biomes of natural environments, anthropogenic environments such as wastewater treatments plants, and in the microbial communities associated with human and animal bodies. Bacteriophages are always the major fraction in these viral communities. A very important percentage, up to 50–60%, of the bacteriophage particles detected in all sort of environments contain bacterial genes. There is a clear correlation between the functional composition of viral and cellular metagenomes.20,21 The bacterial DNA seized by the viral particles in a number of biomes, in addition to the bacterial genes implicated in all core cellular functions, contains prophages, MGE,22,23 and integrases, transposases and recombinases.24 All the bacterial genes and genetic elements contained in the viral communities of most biomes studied indicate that both specialized and generalized transductions occur frequently, but also that generalized transduction may predominate. As well, sequences corresponding to antibiotic resistance genes have been detected in the viral communities of the human gut, human lungs and in an activated sludge wastewater treatment plants.23 Moreover, the presence of antibiotics, even at subinhibitory concentrations, has been shown to increase the transfer of MGEs,25 and recently it has been shown that antibiotic treatment increase the number of bacterial antibiotic resistance genes within the phage genome.26 Therapeutic agents can promote the spread of antibiotic resistance genes by causing stress and through activation of the SOS response.25,26
On the other hand, detection and quantification by qPCR of specific ARG in the bacteriophage populations of different environments indicates that the numbers of these genes are quite high; with values only one order of magnitude lower than the numbers of genes found in the corresponding bacterial populations.27,28 Additionally, DNA extracted from these bacteriophage particles successfully transforms bacteria for antibiotic resistance,27 however, to the best of our knowledge, it has not been possible to detect the transduction of antibiotic resistance determinants using phages partially purified from the different microbial communities studied. This may be due to experimental challenges in the preservation and identification of the potential transductants.29
And finally, an increasing number of phages induced from pure cultures of lysogenic bacteria, most of them isolated in clinical studies, as well as a few isolated from natural samples, have been reported to transduce ARG.12,18
All the information summarized in this section reinforces, in our opinion, two ideas. The first one is that bacteriophages play a role much more important than though up to now in horizontal gene transfer in nature, and the second is about the importance of phages in the mobilization and spread of ARG among different microbiomes.
Easing the Bottlenecks
Among the bottlenecks found in the transferability of ARG from natural ecosystems to human bacterial pathogens, the lack of ecological connectivity emerges as the first one30 This concept includes the need of spatial coincidence of microorganisms and the need of concurrence of microbes belonging to the same genetic exchange communities. Bacteriophages can facilitate evading restrictions of spatial concurrence of genes or bacteria from different biomes, as stated earlier, and phages can also enlarge the genetic exchange communities because of the existence of multivalent bacteriophages.
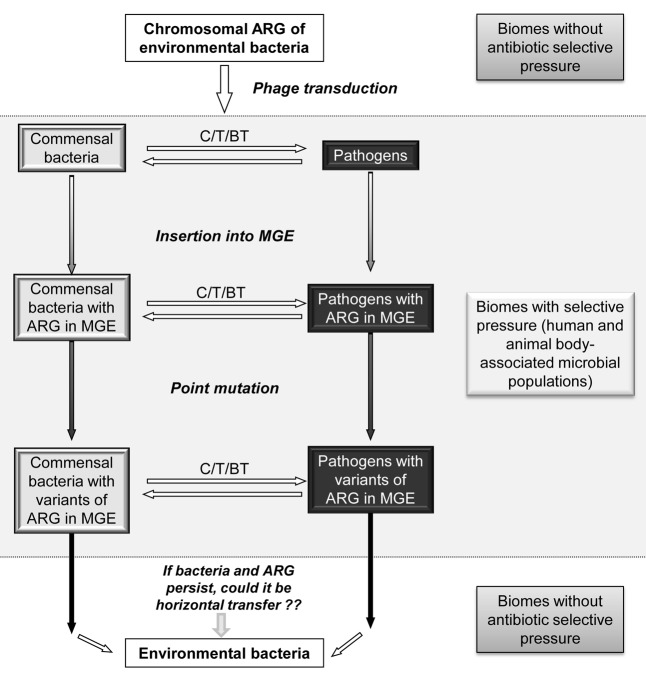
In our opinion, the bacteriophage mediated transfer might be crucial in the mobilization and transfer of chromosomally located ARG of environmental bacteria to human and animal pathogens. Most likely, the incorporation of the environmental ARG in animal and human microbiomes will be through commensal bacteria,31 because of their major abundance. From commensal ARG will move to pathogens belonging to the same genetic exchange community, for example between commensal and pathogenic Enterobacteriaceae. Once in a body-associated microbiome, where well-established genetic exchange communities exist, the maintenance and entrance of an ARG in a proficient MGE will need of recombination, point mutation and mobilization and transfer events. By sure, the pressure exerted by antibiotics will favor the process of incorporation of genes in MGE and their permanence in the body-associated microbiomes (Figure 1). Then, the spread of resistant bacteria from animals to humans and vice versa will play an important role on the spread and maintenance in anthropogenic environments of these genes from environmental bacteria.
Figure 1. Proposed model for mobilization of ARGs from environmental biomes, where there is not a selective pressure by antibiotics, to biomes related to human and animal microbiota. The model proposes phages as the first mechanism for mobilization of ARG from environmental bacteria, where ARG are located in the chromosome, to commensal bacteria or pathogens. After incorporating the ARG, it would continue its mobilization within this biome through other elements (plasmids, transposons or also phages). Mutation of ARG at this stage would explain variations of ARGs found this biome. These ARGs, if they are maintained within the bacteria, and if the bacteria themselves can persist long enough, can plausibly be mobilized back to environmental bacteria. ARG: Antibiotic resistance genes. MGE: mobile genetic element. C: conjugation. T: transformation, BT: bacteriophage transduction.
At this point, the questions of whether ARG with an initial chromosomal location in environmental bacteria can return to natural environments, mostly through sewage, and persist there in mobile genetic platforms remains to be elucidated. So far, the general believe was that fitness costs caused by the acquisition of resistance determinants will lead, in the absence of selection, to the loss of the character in the population since resistant bacteria will be outcompeted by the susceptible ones.9 However, some recent information seems to indicate that this may not always be the case.28 In such circumstance, again, the potential role of bacteriophages in the transfer of these antibiotic resistance determinants between different biomes should not be dismissed.
Glossary
Abbreviations:
- ARG
antibiotic resistance genes, MGE: mobile genetic elements
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/mge/article/25847
References
- 1.Levy SB, Marshall B. Antibacterial resistance worldwide: causes, challenges and responses. Nat Med. 2004;10(Suppl):S122–9. doi: 10.1038/nm1145. [DOI] [PubMed] [Google Scholar]
- 2.Martínez JL. Antibiotics and antibiotic resistance genes in natural environments. Science. 2008;321:365–7. doi: 10.1126/science.1159483. [DOI] [PubMed] [Google Scholar]
- 3.Stefani S. Analyzing possible intersections in the resistome among human, animal and environmental matrices. Frontiers in Microbiol. 2012;3:1–2. doi: 10.3389/fmicb.2012.00418. [editorial] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sengupta S, Chattopadhyay MK, Grossart HP. The multifaced roles of antibiotic resistance in nature. Frontiers in Microbiol. 2013;4:1–13. doi: 10.3389/fmicb.2013.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forsberg KJ, Reyes A, Wang B, Selleck EM, Sommer MO, Dantas G. The shared antibiotic resistome of soil bacteria and human pathogens. Science. 2012;337:1107–11. doi: 10.1126/science.1220761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D’Costa VM, King CE, Kalan L, Morar M, Sung WW, Schwarz C, et al. Antibiotic resistance is ancient. Nature. 2011;477:457–61. doi: 10.1038/nature10388. [DOI] [PubMed] [Google Scholar]
- 7.Tacão M, Correia A, Henriques I. Resistance to broad-spectrum antibiotics in aquatic systems: anthropogenic activities modulate the dissemination of bla(CTX-M)-like genes. Appl Environ Microbiol. 2012;78:4134–40. doi: 10.1128/AEM.00359-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poirel L, Naas T, Nordmann P. Diversity, epidemiology, and genetics of class D β-lactamases. Antimicrob Agents Chemother. 2010;54:24–38. doi: 10.1128/AAC.01512-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andersson DI, Levin BR. The biological cost of antibiotic resistance. Curr Opin Microbiol. 1999;2:489–93. doi: 10.1016/S1369-5274(99)00005-3. [DOI] [PubMed] [Google Scholar]
- 10.Schmieger H, Schicklmaier P. Transduction of multiple drug resistance of Salmonella enterica serovar typhimurium DT104. FEMS Microbiol Lett. 1999;170:251–6. doi: 10.1111/j.1574-6968.1999.tb13381.x. [DOI] [PubMed] [Google Scholar]
- 11.Mann BA, Slauch JM. Transduction of low-copy number plasmids by bacteriophage P22. Genetics. 1997;146:447–56. doi: 10.1093/genetics/146.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weinbauer MG. Ecology of prokaryotic viruses. FEMS Microbiol Rev. 2004;28:127–81. doi: 10.1016/j.femsre.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Letarov A, Kulikov E. The bacteriophages in human- and animal body-associated microbial communities. J Appl Microbiol. 2009;107:1–13. doi: 10.1111/j.1365-2672.2009.04143.x. [DOI] [PubMed] [Google Scholar]
- 14.Jofre J. Indicators of Waterborne Enteric Viruses. In Human Viruses in Water (Series Perspectives in Medical Virology). Elsevier, Amsterdam. 2007; 17:227-49 [Google Scholar]
- 15.Sano E, Carlson S, Wegley L, Rohwer F. Movement of viruses between biomes. Appl Environ Microbiol. 2004;70:5842–6. doi: 10.1128/AEM.70.10.5842-5846.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kenzaka T, Tani K, Nasu M. High-frequency phage-mediated gene transfer in freshwater environments determined at single-cell level. ISME J. 2010;4:648–59. doi: 10.1038/ismej.2009.145. [DOI] [PubMed] [Google Scholar]
- 17.Jensen EC, Schrader HS, Rieland B, Thompson TL, Lee KW, Nickerson KW, et al. Prevalence of broad-host-range lytic bacteriophages of Sphaerotilus natans, Escherichia coli, and Pseudomonas aeruginosa. Appl Environ Microbiol. 1998;64:575–80. doi: 10.1128/aem.64.2.575-580.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mazaheri Nezhad Fard R, Barton MD, Heuzenroeder MW. Bacteriophage-mediated transduction of antibiotic resistance in enterococci. Lett Appl Microbiol. 2011;52:559–64. doi: 10.1111/j.1472-765X.2011.03043.x. [DOI] [PubMed] [Google Scholar]
- 19.Shan J, Patel KV, Hickenbotham PT, Nale JY, Hargreaves KR, Clokie MR. Prophage carriage and diversity within clinically relevant strains of Clostridium difficile. Appl Environ Microbiol. 2012;78:6027–34. doi: 10.1128/AEM.01311-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dinsdale EA, Edwards RA, Hall D, Angly F, Breitbart M, Brulc JM, et al. Functional metagenomic profiling of nine biomes. Nature. 2008;452:629–32. doi: 10.1038/nature06810. [DOI] [PubMed] [Google Scholar]
- 21.Kristensen DM, Mushegian AR, Dolja VV, Koonin EV. New dimensions of the virus world discovered through metagenomics. Trends Microbiol. 2010;18:11–9. doi: 10.1016/j.tim.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parsley LC, Consuegra EJ, Kakirde KS, Land AM, Harper WF, Jr., Liles MR. Identification of diverse antimicrobial resistance determinants carried on bacterial, plasmid, or viral metagenomes from an activated sludge microbial assemblage. Appl Environ Microbiol. 2010;76:3753–7. doi: 10.1128/AEM.03080-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Breitbart M, Felts B, Kelley S, Mahaffy JM, Nulton J, Salamon P, et al. Diversity and population structure of a near-shore marine-sediment viral community. Proc Biol Sci. 2004;271:565–74. doi: 10.1098/rspb.2003.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cantalupo PG, Calgua B, Zhao G, Hundesa A, Wier AD, Katz JP, et al. Raw sewage harbors diverse viral populations. MBio. 2011;2:e00180–11. doi: 10.1128/mBio.00180-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beaber JW, Hochhut B, Waldor MK. SOS response promotes horizontal dissemination of antibiotic resistance genes. Nature. 2004;427:72–4. doi: 10.1038/nature02241. [DOI] [PubMed] [Google Scholar]
- 26.Modi SR, Lee HH, Spina CS, Collins JJ. Antibiotic treatment expands the resistance reservoir and ecological network of the phage metagenome. Nature. 2013;499:219–22. doi: 10.1038/nature12212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Colomer-Lluch M, Jofre J, Muniesa M. Antibiotic resistance genes in the bacteriophage DNA fraction of environmental samples. PLoS One. 2011;6:e17549. doi: 10.1371/journal.pone.0017549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Colomer-Lluch M, Imamovic L, Jofre J, Muniesa M. Bacteriophages carrying antibiotic resistance genes in fecal waste from cattle, pigs, and poultry. Antimicrob Agents Chemother. 2011;55:4908–11. doi: 10.1128/AAC.00535-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muniesa M, Colomer-Lluch M, Jofre J. Potential impact of environmental bacteriophages in spreading antibiotic resistance genes. Future Microbiol. 2013;8:739–51. doi: 10.2217/fmb.13.32. [DOI] [PubMed] [Google Scholar]
- 30.Martínez JL. Bottlenecks in the transferability of antibiotic resistance from natural ecosystems to human bacterial pathogens. Front Microbiol. 2011;2:265. doi: 10.3389/fmicb.2011.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van den Bogaard AE, Stobberingh EE. Epidemiology of resistance to antibiotics. Links between animals and humans. Int J Antimicrob Agents. 2000;14:327–35. doi: 10.1016/S0924-8579(00)00145-X. [DOI] [PubMed] [Google Scholar]