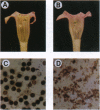
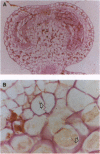
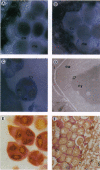
Abstract
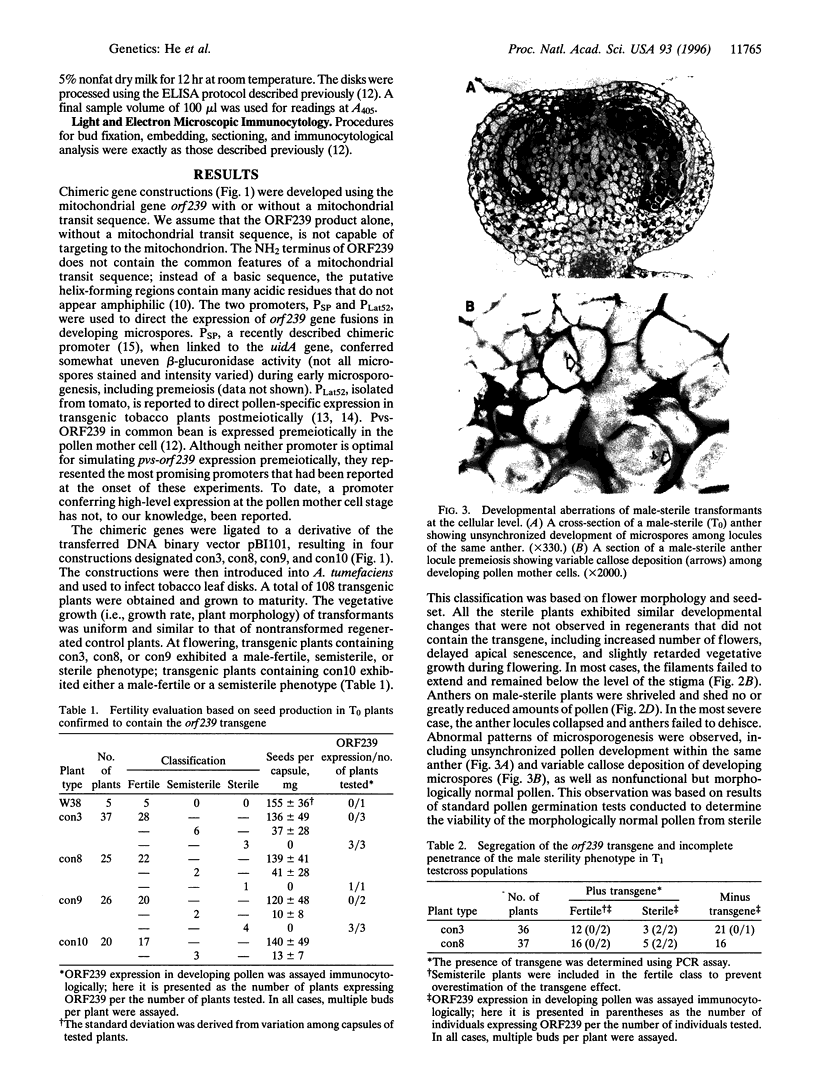
In higher plants, dominant mitochondrial mutations are associated with pollen sterility. This phenomenon is known as cytoplasmic male sterility (CMS). It is thought that the disruption in pollen development is a consequence of mitochondrial dysfunction. To provide definitive evidence that expression of an abnormal mitochondrial gene can interrupt pollen development, a CMS-associated mitochondrial DNA sequence from common bean, orf239, was introduced into the tobacco nuclear genome. Several transformants containing the orf239 gene constructs, with or without a mitochondrial targeting sequence, exhibited a semi sterile or male-sterile phenotype. Expression of the gene fusions in transformed anthers was confirmed using RNA gel blotting, ELISA, and light and electron microscopic immunocytochemistry. Immunocytological analysis showed that the ORF239 protein could associate with the cell wall of aberrant developing microspores. This pattern of extracellular localization was earlier observed in the CMS common bean line containing orf239 in the mitochondrial genome. Results presented here demonstrate that ORF239 causes pollen disruption in transgenic tobacco plants and may do so without targeting of the protein to the mitochondrion.
Full text
PDF
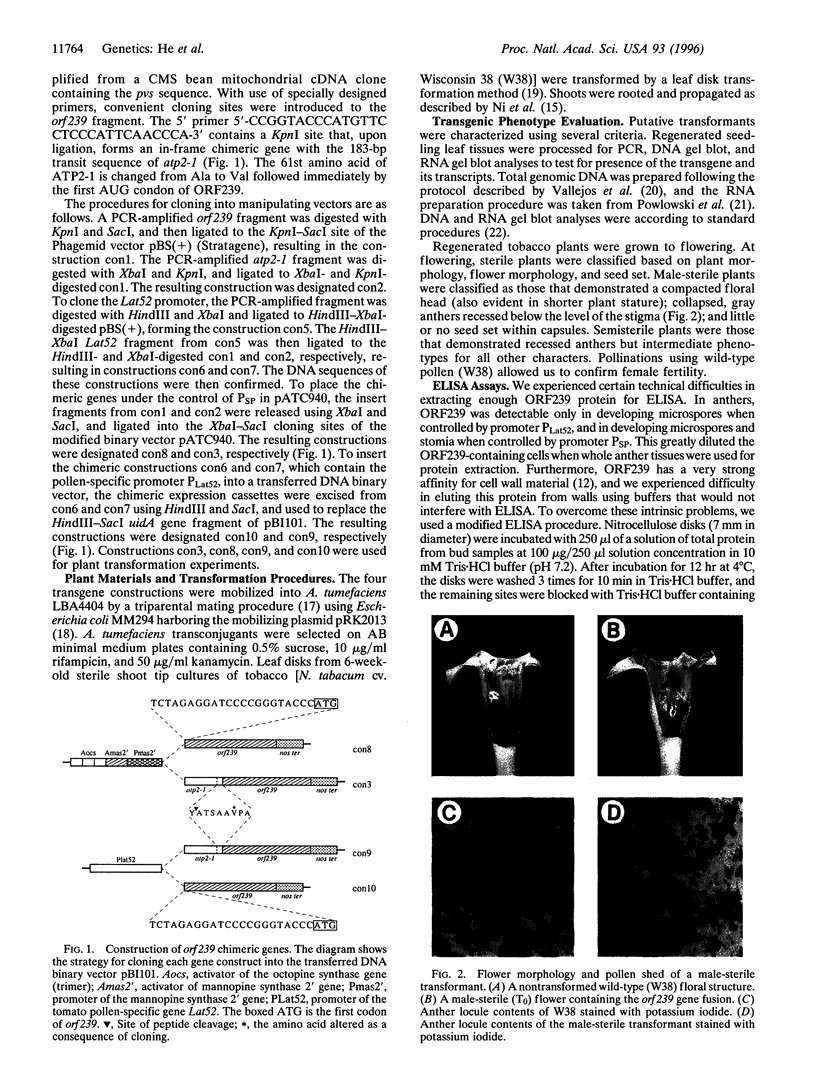


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abad A. R., Mehrtens B. J., Mackenzie S. A. Specific expression in reproductive tissues and fate of a mitochondrial sterility-associated protein in cytoplasmic male-sterile bean. Plant Cell. 1995 Mar;7(3):271–285. doi: 10.1105/tpc.7.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaumont F., Bernier B., Buxant R., Williams M. E., Levings C. S., 3rd, Boutry M. Targeting the maize T-urf13 product into tobacco mitochondria confers methomyl sensitivity to mitochondrial respiration. Proc Natl Acad Sci U S A. 1995 Feb 14;92(4):1167–1171. doi: 10.1073/pnas.92.4.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaumont F., Silva Filho M. de C., Thomas D., Leterme S., Boutry M. Truncated presequences of mitochondrial F1-ATPase beta subunit from Nicotiana plumbaginifolia transport CAT and GUS proteins into mitochondria of transgenic tobacco. Plant Mol Biol. 1994 Feb;24(4):631–641. doi: 10.1007/BF00023559. [DOI] [PubMed] [Google Scholar]
- Dewey R. E., Levings C. S., 3rd, Timothy D. H. Novel recombinations in the maize mitochondrial genome produce a unique transcriptional unit in the Texas male-sterile cytoplasm. Cell. 1986 Feb 14;44(3):439–449. doi: 10.1016/0092-8674(86)90465-4. [DOI] [PubMed] [Google Scholar]
- Dewey R. E., Timothy D. H., Levings C. S. A mitochondrial protein associated with cytoplasmic male sterility in the T cytoplasm of maize. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5374–5378. doi: 10.1073/pnas.84.15.5374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditta G., Stanfield S., Corbin D., Helinski D. R. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figurski D. H., Helinski D. R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn R., Köhler R. H., Zetsche K. A mitochondrial 16 kDa protein is associated with cytoplasmic male sterility in sunflower. Plant Mol Biol. 1991 Jul;17(1):29–36. doi: 10.1007/BF00036803. [DOI] [PubMed] [Google Scholar]
- Johns C., Lu M., Lyznik A., Mackenzie S. A mitochondrial DNA sequence is associated with abnormal pollen development in cytoplasmic male sterile bean plants. Plant Cell. 1992 Apr;4(4):435–449. doi: 10.1105/tpc.4.4.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler R. H., Horn R., Lössl A., Zetsche K. Cytoplasmic male sterility in sunflower is correlated with the co-transcription of a new open reading frame with the atpA gene. Mol Gen Genet. 1991 Jul;227(3):369–376. doi: 10.1007/BF00273925. [DOI] [PubMed] [Google Scholar]
- Leighton J., Schatz G. An ABC transporter in the mitochondrial inner membrane is required for normal growth of yeast. EMBO J. 1995 Jan 3;14(1):188–195. doi: 10.1002/j.1460-2075.1995.tb06989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie S. A., Chase C. D. Fertility Restoration Is Associated with Loss of a Portion of the Mitochondrial Genome in Cytoplasmic Male-Sterile Common Bean. Plant Cell. 1990 Sep;2(9):905–912. doi: 10.1105/tpc.2.9.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie S. A., Pring D. R., Bassett M. J., Chase C. D. Mitochondrial DNA rearrangement associated with fertility restoration and cytoplasmic reversion to fertility in cytoplasmic male sterile Phaseolus vulgaris L. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2714–2717. doi: 10.1073/pnas.85.8.2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monéger F., Smart C. J., Leaver C. J. Nuclear restoration of cytoplasmic male sterility in sunflower is associated with the tissue-specific regulation of a novel mitochondrial gene. EMBO J. 1994 Jan 1;13(1):8–17. doi: 10.1002/j.1460-2075.1994.tb06230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nivison H. T., Hanson M. R. Identification of a mitochondrial protein associated with cytoplasmic male sterility in petunia. Plant Cell. 1989 Nov;1(11):1121–1130. doi: 10.1105/tpc.1.11.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poyton R. O., Duhl D. M., Clarkson G. H. Protein export from the mitochondrial matrix. Trends Cell Biol. 1992 Dec;2(12):369–375. doi: 10.1016/0962-8924(92)90049-s. [DOI] [PubMed] [Google Scholar]
- Twell D., Wing R., Yamaguchi J., McCormick S. Isolation and expression of an anther-specific gene from tomato. Mol Gen Genet. 1989 Jun;217(2-3):240–245. doi: 10.1007/BF02464887. [DOI] [PubMed] [Google Scholar]
- Twell D., Yamaguchi J., McCormick S. Pollen-specific gene expression in transgenic plants: coordinate regulation of two different tomato gene promoters during microsporogenesis. Development. 1990 Jul;109(3):705–713. doi: 10.1242/dev.109.3.705. [DOI] [PubMed] [Google Scholar]
- Vallejos C. E., Sakiyama N. S., Chase C. D. A molecular marker-based linkage map of Phaseolus vulgaris L. Genetics. 1992 Jul;131(3):733–740. doi: 10.1093/genetics/131.3.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintz H., Chen H. C., Sutton C. A., Conley C. A., Cobb A., Ruth D., Hanson M. R. Expression of the CMS-associated urfS sequence in transgenic petunia and tobacco. Plant Mol Biol. 1995 Apr;28(1):83–92. doi: 10.1007/BF00042040. [DOI] [PubMed] [Google Scholar]
- Young E. G., Hanson M. R. A fused mitochondrial gene associated with cytoplasmic male sterility is developmentally regulated. Cell. 1987 Jul 3;50(1):41–49. doi: 10.1016/0092-8674(87)90660-x. [DOI] [PubMed] [Google Scholar]