Abstract
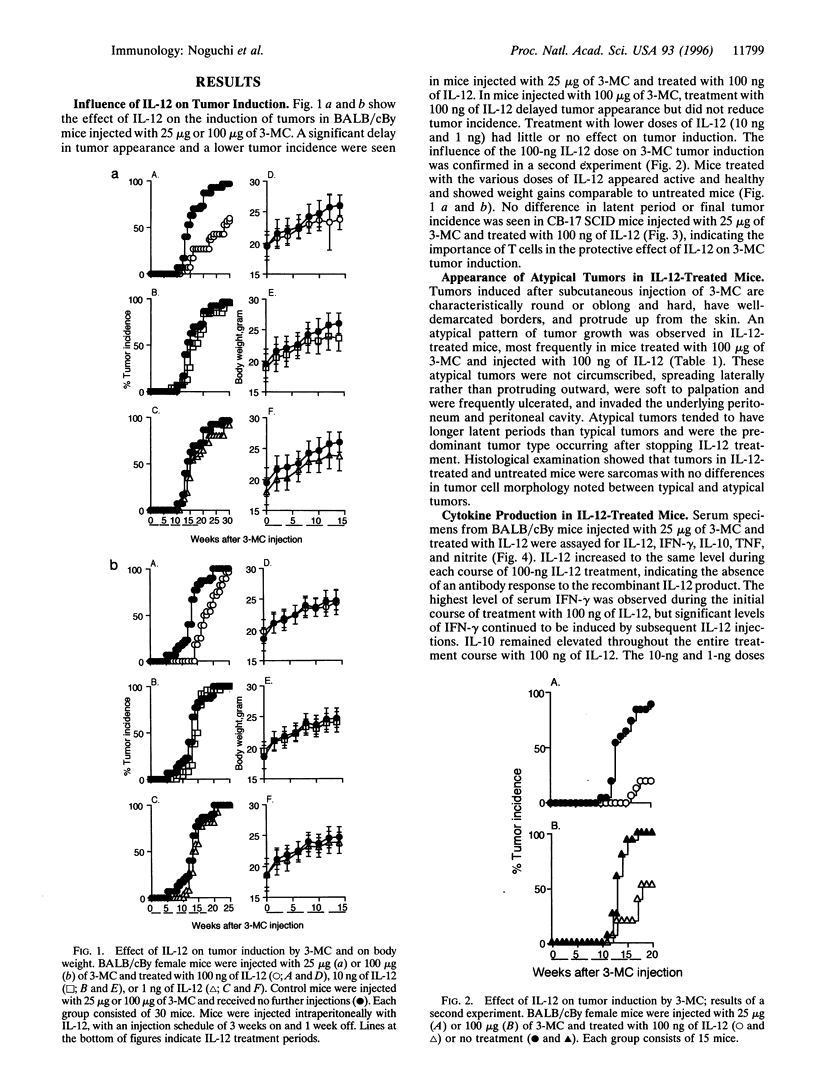
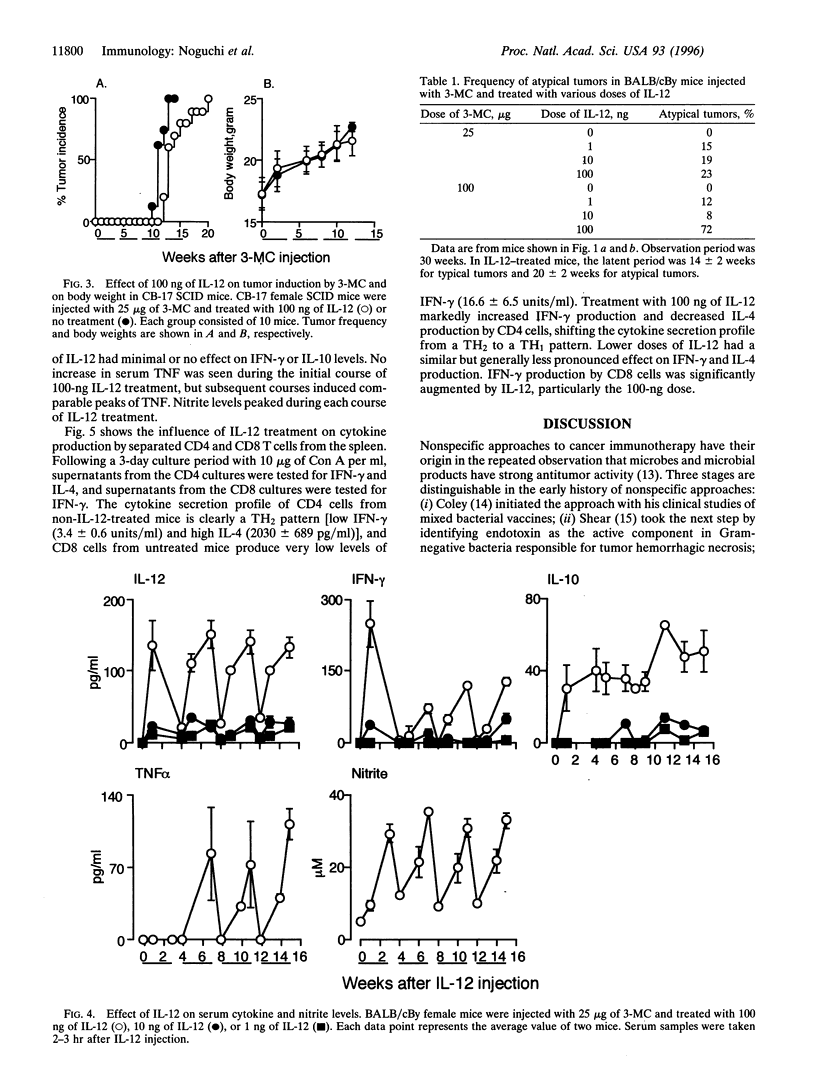
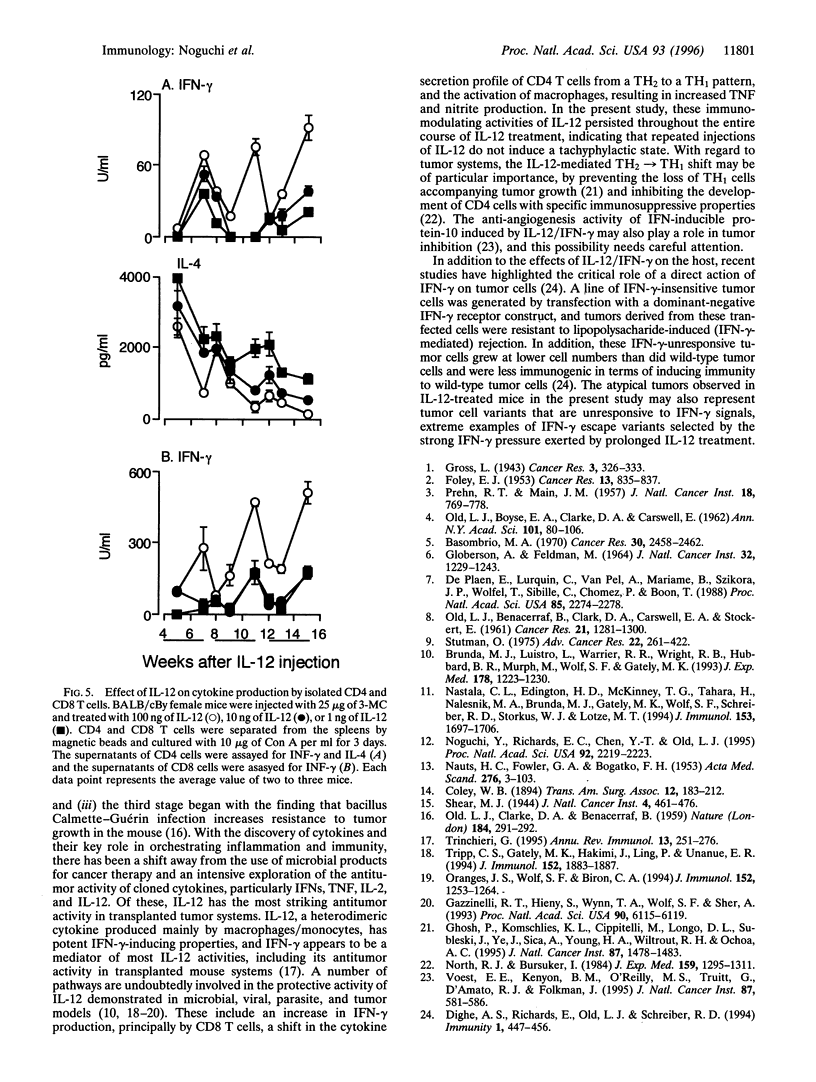
Interleukin (IL)-12 has strong antitumor activity in transplantable tumor systems in the mouse. The present study was designed to determine whether tumor induction by 3-methylcholanthrene (3-MC), a carcinogenic hydrocarbon, can be inhibited by IL-12. BALB/cBy mice were injected subcutaneously with 25 micrograms or 100 micrograms of 3-MC and treated with 100 ng, 10 ng, or 1 ng of IL-12 for 5 days a week for 18 weeks, with a schedule of 3 weeks on and 1 week off. In mice injected with 25 micrograms of 3-MC, treatment with 100 ng of IL-12 delayed tumor appearance and reduced tumor incidence. Tumor appearance was also delayed in mice injected with 100 micrograms of 3-MC and treated with 100 ng of IL-12, but the final tumor incidence was the same as in non-IL-12-treated mice. In contrast to the characteristically round, hard, well-circumscribed, and protruding tumor induced by 3-MC, a percentage of tumors induced in IL-12-treated mice had atypical characteristics: flat, soft, and invasive. Atypical tumors had a longer latent period and were more frequently seen in mice injected with 100 micrograms of 3-MC and treated with 100 ng of IL-12. Interferon gamma, IL-10, and tumor necrosis factor could be induced throughout the treatment period by IL-12, indicating that repeated injections of IL-12 do not induce a state of tachyphylaxis. High production of interferon gamma by CD8 T cells and a TH2-->TH1 or TH0 shift in the cytokine secretion profile of CD4 T cells were also seen in the IL-12-treated mice. IL-12 provides a powerful new way to explore the defensive role of the immune system in tumorigenesis.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basombrío M. A. Search for common antigenicities among twenty-five sarcomas induced by methylcholanthrene. Cancer Res. 1970 Oct;30(10):2458–2462. [PubMed] [Google Scholar]
- Brunda M. J., Luistro L., Warrier R. R., Wright R. B., Hubbard B. R., Murphy M., Wolf S. F., Gately M. K. Antitumor and antimetastatic activity of interleukin 12 against murine tumors. J Exp Med. 1993 Oct 1;178(4):1223–1230. doi: 10.1084/jem.178.4.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Plaen E., Lurquin C., Van Pel A., Mariamé B., Szikora J. P., Wölfel T., Sibille C., Chomez P., Boon T. Immunogenic (tum-) variants of mouse tumor P815: cloning of the gene of tum- antigen P91A and identification of the tum- mutation. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2274–2278. doi: 10.1073/pnas.85.7.2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dighe A. S., Richards E., Old L. J., Schreiber R. D. Enhanced in vivo growth and resistance to rejection of tumor cells expressing dominant negative IFN gamma receptors. Immunity. 1994 Sep;1(6):447–456. doi: 10.1016/1074-7613(94)90087-6. [DOI] [PubMed] [Google Scholar]
- FOLEY E. J. Antigenic properties of methylcholanthrene-induced tumors in mice of the strain of origin. Cancer Res. 1953 Dec;13(12):835–837. [PubMed] [Google Scholar]
- GLOBERSON A., FELDMAN M. ANTIGENIC SPECIFICITY OF BENZO(A)PYRENE-INDUCED SARCOMAS. J Natl Cancer Inst. 1964 Jun;32:1229–1243. doi: 10.1093/jnci/32.6.1229. [DOI] [PubMed] [Google Scholar]
- Gazzinelli R. T., Hieny S., Wynn T. A., Wolf S., Sher A. Interleukin 12 is required for the T-lymphocyte-independent induction of interferon gamma by an intracellular parasite and induces resistance in T-cell-deficient hosts. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6115–6119. doi: 10.1073/pnas.90.13.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh P., Komschlies K. L., Cippitelli M., Longo D. L., Subleski J., Ye J., Sica A., Young H. A., Wiltrout R. H., Ochoa A. C. Gradual loss of T-helper 1 populations in spleen of mice during progressive tumor growth. J Natl Cancer Inst. 1995 Oct 4;87(19):1478–1483. doi: 10.1093/jnci/87.19.1478. [DOI] [PubMed] [Google Scholar]
- NAUTS H. C., FOWLER G. A., BOGATKO F. H. A review of the influence of bacterial infection and of bacterial products (Coley's toxins) on malignant tumors in man; a critical analysis of 30 inoperable cases treated by Coley's mixed toxins, in which diagnosis was confirmed by microscopic examination selected for special study. Acta Med Scand Suppl. 1953;276:1–103. [PubMed] [Google Scholar]
- Nastala C. L., Edington H. D., McKinney T. G., Tahara H., Nalesnik M. A., Brunda M. J., Gately M. K., Wolf S. F., Schreiber R. D., Storkus W. J. Recombinant IL-12 administration induces tumor regression in association with IFN-gamma production. J Immunol. 1994 Aug 15;153(4):1697–1706. [PubMed] [Google Scholar]
- Noguchi Y., Richards E. C., Chen Y. T., Old L. J. Influence of interleukin 12 on p53 peptide vaccination against established Meth A sarcoma. Proc Natl Acad Sci U S A. 1995 Mar 14;92(6):2219–2223. doi: 10.1073/pnas.92.6.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J., Bursuker I. Generation and decay of the immune response to a progressive fibrosarcoma. I. Ly-1+2- suppressor T cells down-regulate the generation of Ly-1-2+ effector T cells. J Exp Med. 1984 May 1;159(5):1295–1311. doi: 10.1084/jem.159.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OLD L. J., BENACERRAF B., CLARKE D. A., CARSWELL E. A., STOCKERT E. The role of the reticuloendothelial system in the host reaction to neoplasia. Cancer Res. 1961 Oct;21:1281–1300. [PubMed] [Google Scholar]
- OLD L. J., CLARKE D. A., BENACERRAF B. Effect of Bacillus Calmette-Guerin infection on transplanted tumours in the mouse. Nature. 1959 Jul 25;184(Suppl 5):291–292. doi: 10.1038/184291a0. [DOI] [PubMed] [Google Scholar]
- Orange J. S., Wolf S. F., Biron C. A. Effects of IL-12 on the response and susceptibility to experimental viral infections. J Immunol. 1994 Feb 1;152(3):1253–1264. [PubMed] [Google Scholar]
- PREHN R. T., MAIN J. M. Immunity to methylcholanthrene-induced sarcomas. J Natl Cancer Inst. 1957 Jun;18(6):769–778. [PubMed] [Google Scholar]
- Stutman O. Immunodepression and malignancy. Adv Cancer Res. 1975;22:261–422. doi: 10.1016/s0065-230x(08)60179-7. [DOI] [PubMed] [Google Scholar]
- Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- Tripp C. S., Gately M. K., Hakimi J., Ling P., Unanue E. R. Neutralization of IL-12 decreases resistance to Listeria in SCID and C.B-17 mice. Reversal by IFN-gamma. J Immunol. 1994 Feb 15;152(4):1883–1887. [PubMed] [Google Scholar]
- Voest E. E., Kenyon B. M., O'Reilly M. S., Truitt G., D'Amato R. J., Folkman J. Inhibition of angiogenesis in vivo by interleukin 12. J Natl Cancer Inst. 1995 Apr 19;87(8):581–586. doi: 10.1093/jnci/87.8.581. [DOI] [PubMed] [Google Scholar]


