Abstract
The role of Large tumor suppressor LATS/Warts in human cancer is not clearly understood. Here we show that hLATS1/2 cancer mutations affect their expression and kinase activity. hLATS1/2 mutants exhibit a decreased activity in inhibiting YAP and tissue growth. Therefore, hLATS1/2 alleles from human cancer can be loss-of-function mutations.
Keywords: Hippo signaling, human cancer, LATS1, LATS2, protein kinase
LARGE tumor suppressor (Lats)/Warts (Wts) plays a critical role in mediating Hippo (Hpo) growth-inhibitory signaling but its role in human cancer is less clear (recently reviewed in Harvey et al. 2013; Yu and Guan 2013). In the Catalogue of Somatic Mutation in Cancer (COSMIC) database, 58 nonsynonymous hLATS1 somatic mutations and 43 for hLATS2 have been identified from >5000 unique human cancer samples (Supporting Information, Table S1). Therefore, hLATS1/2 genes are mutated in 1–2% of human cancers. The primary tissue types of these mutations include lung, ovary, breast, and a number of other organs (Table S1). In this study, several computationally predicted damaging mutations have been experimentally investigated, which include hLATS1-V719I, hLATS1-R806P, hLATS2-G40E, hLATS2-G909R, and hLATS2-C953* (Table S2). V719, R806, and G909 are conserved from nematode to vertebrate, whereas G40 is conserved only in vertebrates (Figure S1).
Through Western blot analyses, we found that V719I and R806P mutations did not significantly alter their expression levels compared to wild type. However, V719I moderately and R806P dramatically reduced hLATS1 kinase activity (Figure S2). For hLATS2 (Figure S2), G40E abolished its expression as well as activity; G909R did not alter expression level but significantly reduced its activity. A nonsense mutation C953* deletes part of its kinase domain and C-terminal region. As predicted, C953* led to the production of a truncated inactive protein. Thus, these hLATS1/2 alleles are loss-of-function mutations.
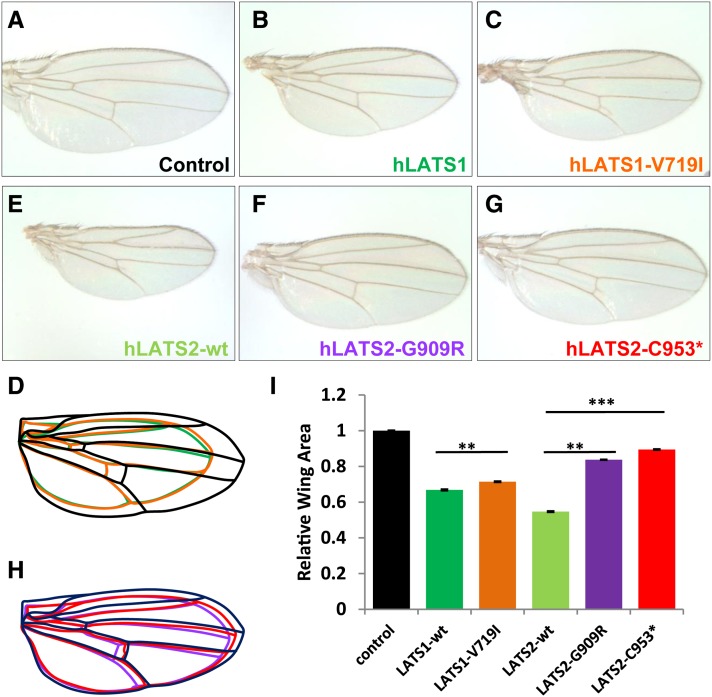
To test the activity of these hLATS1/2 alleles in tissue growth inhibition, we generated transgenic Drosophila lines as an in vivo model. hLATS1/2 and their variants were selectively expressed in larval wing epithelium under the control of wing-specific drivers such as MS1096-Gal4. As expected, overexpression of wild-type hLATS1 and hLATS2 in Drosophila wing resulted in 34 and 45% reduction in size, respectively (Figure 1, A, B, E, and I), indicating that human LATS genes are sufficient to reduce tissue growth and organ size. Interestingly, hLATS1-V719I mutation does not drastically affect hLATS1 activity, as the mutant protein can still effectively reduce the organ size (Figure 1, C and I). It appears that Drosophila cells were not sensitive to the structural change caused by V719I alteration. In the case of hLATS2, however, G909R and C953* mutations effectively reduced their growth-inhibitory activity of hLATS2, as the wings were much less reduced in hLATS2-G909R and hLATS2-C953* transgenic flies (Figure 1, E–I). Therefore, human cancer mutations such as hLATS2-G909R and hLATS2-C953* are loss-of-function mutations with reduced growth inhibitory function.
Figure 1.
In vivo analysis of hLATS1/2 and variants for their activity to inhibit tissue growth in Drosophila. (A–H) Images of female wings (anterior to the top) that express following transgenes under the control of MS1096-Gal4. (A) UAS-86Fb-lacZ as a negative control. (B) UAS-86Fb-hLATS1-wt. (C) UAS-86Fb-hLATS1-V719I. (D) Outlines of the wings shown in A–C. (E) UAS-86Fb-hLATS2-wt. (F) UAS-86Fb-hLATS2-G909R. (G) UAS-86Fb-hLATS2-C953*. (H) Outlines of the wings shown in E–G. (I) Quantification of total wing area of the genotypes (n = 40 for each genotype) displayed in A–C and E–G. Results represent mean ± SEM. **P < 10−11, ***P < 10−53. Embryos from Drosophila attP docking strains y[1] M{vas-int.Dm}ZH-2A w[*]; M{3xP3-RFP.attP}ZH-86Fb or y[1] M{vas-int.Dm}ZH-2A w[*]; M{3xP3-RFP.attP}ZH-51D (from Bloomington Drosophila Stock Center, BDSC; Bischof et al. 2007) were injected with the pUAST-attB constructs carrying wild-type hLATS1/2 and mutant variants. Fly embryo injections were partly done by Rainbow Transgenic Flies. Orange eye color was a selection marker for isolating transformants. Transgenic lines were established through standard procedures. Flies with pUAST-lacZ-attB inserted in 51D or 86Fb (gift from Johannes Bischof) were used as controls. The Drosophila strains that carry wts-RNAi on the second chromosome (no. v106174) and the third chromosome (no. 34064) were obtained from the Vienna Drosophila RNAi Center (VDRC) and BDSC, respectively. The VDRC UAS-wts-RNAi inserted in the second chromosome was combined with UAS-hLATS1/2 in the third chromosome (86Fb), while the BDSC UAS-wts-RNAi inserted in the third chromosome was combined with UAS-hLATS1/2 at the second chromosome (51D). MS1096-Gal4 was used to drive wing-specific expression. Adult wings were dissected and analyzed by ImageJ for size measurement.
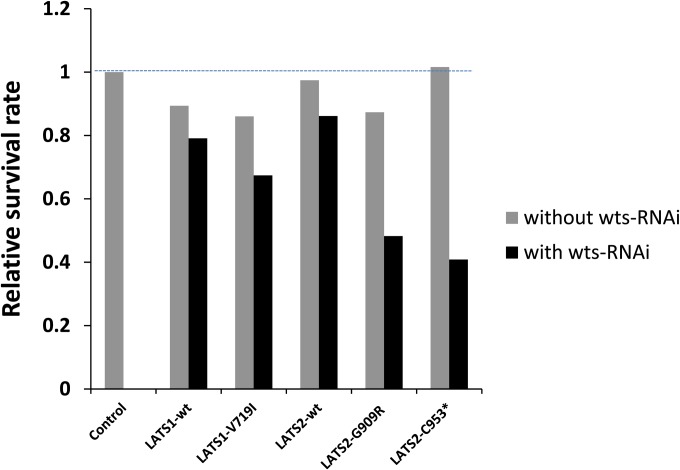
To further investigate the growth regulatory activity of hLATS1/2, we used RNAi lines for wts, the Drosophila ortholog of human LATS1/2, to monitor activities of hLATS1/2 and variants in a wts loss-of-function background. Previous studies have shown that expression of hLATS1 transgene in homozygous wts mutants rescued lethality (Tao et al. 1999). Under the control of wing-specific driver MS1096-Gal4, UAS-wts-RNAi caused pupal lethality (Figure 2), which was a critical assay to monitor in vivo activities of wild-type hLATS1/2 and variants.
Figure 2.
In vivo analysis of hLATS1/2 and mutants for their ability to rescue the lethality induced by loss of the endogenous wts gene function. Relative survival rate is measured by the number of adult females divided by the number of adult males produced by parental flies of males MS1096-Gal4 (on the X chromosome) crossed with females with genotype as indicated. The number of offspring males serves as a control to estimate an expected number of female flies that express the UAS transgenes driven by MS1096-Gal4. In a wts-RNAi (II) background, the survival rate in the absence of LATS transgenic expression was zero (the control column). Between 40 and 148 offspring males were scored from each cross.
To determine the relative survival rate of strains expressing different hLATS1/2 transgenes, we crossed XMS1096-Gal4Y males with transgenic UAS-hLATS1/2 females to generate female offspring with induced hLATS expression, and male offspring without any hLATS expression, in the presence or absence of wts-RNAi. As controls, expression of hLATS1/2 and variants in a wild-type background caused no defects in viability (Figure 2). Compared to wild-type hLATS1, hLATS1-V719I did not fully rescue the lethality caused by wts-RNAi (Figure 2). Moreover, unlike wild-type hLATS2, hLATS2 mutants G909R and C953* failed to fully rescue the lethality of wts-RNAi flies (Figure 2). Therefore, V719I, G909R, and C953* are loss-of-function mutations and unable to fully replace the endogenous wts gene for normal development.
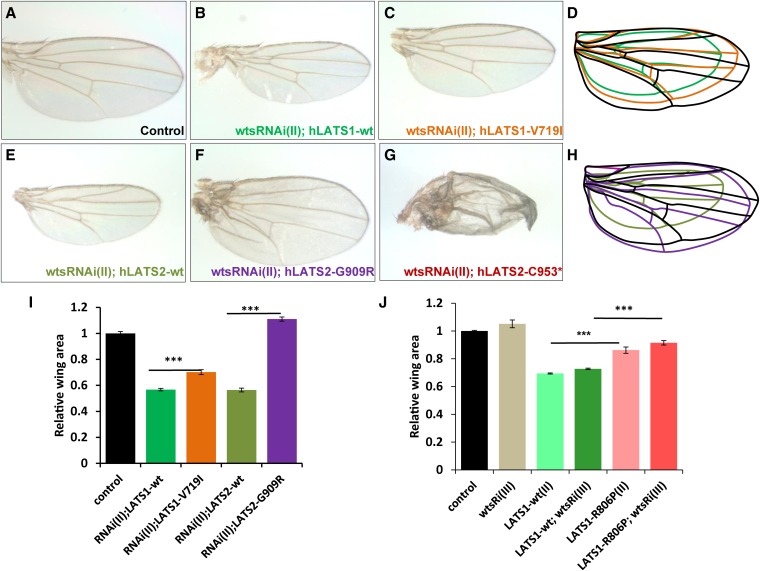
To investigate this further, the wings of female offspring were analyzed. In a wts-RNAi background, both hLATS1 and hLATS2 still exhibited potent growth-inhibiting activities (Figure 3, compare parts B and E with A). Compared with wild-type hLATS1, hLATS1-V719I was less capable in blocking tissue growth (Figure 3I, compare C with B). Moreover, hLATS1-R806P also appeared to be less active (Figure 3J). Interestingly, hLATS2 mutant G909R was inactive in growth inhibition as there was no longer reduction in wing size (Figure 3, E, F, and I). The G909R wing in a wts-RNAi background was actually 11% larger than wild-type wings, possibly due to a dominant-negative effect. LATS2-C953* failed to maintain a normal wing morphology in wts mutant tissues (Figure 3G), which makes it difficult to accurately measure its wing size. These results are consistent with what was observed in other assays described in Figure 1 and Figure 2.
Figure 3.
In vivo analysis of hLATS1/2 and variants for their activity to control organ size in the absence of the endogenous wts gene function. Representative images of female adult wings (anterior to the top). (A) MS1096-Gal4/+; UAS-lacZ/+, which only expresses a UAS-lacZ transgene in the wing. β-Galactosidase expression in the wing has no impact on wing development. (B) MS1096-Gal4/+; UAS-wtsRNAi(II)/+; UAS-hLATS1-wt. (C) MS1096-Gal4/+; UAS-wtsRNAi(II)/+; UAS-hLATS1-V719I. (E) MS1096-Gal4/+; UAS-wtsRNAi(II)/+; UAS-hLATS2-wt. (F) MS1096-Gal4/+; UAS-wtsRNAi(II)/+; UAS-hLATS2-G909R. (G) MS1096-Gal4/+; UAS-wtsRNAi(II)/+; UAS-hLATS2-C953*. Wing size comparison of samples from A–C is shown in D, and those from A, E, and F are shown in H. (I and J) Display wing size data from statistical analysis of flies with genotypes described at the bottom portion of the panels (n = 40 for each genotype). (J) UAS-wtsRNAi (III, inserted on the third chromosome) driven by MS1096-Gal4 did not cause an obvious lethality and therefore UAS-hLATS1-R806P (II, inserted on the second chromosome) was not tested in a survival rate experiment. Activities of LATS1-R806P in overexpresion and wing-size rescue experiments were analyzed and are shown in J. Results represent mean ± SEM. **P < 10−4, ***P < 10−22.
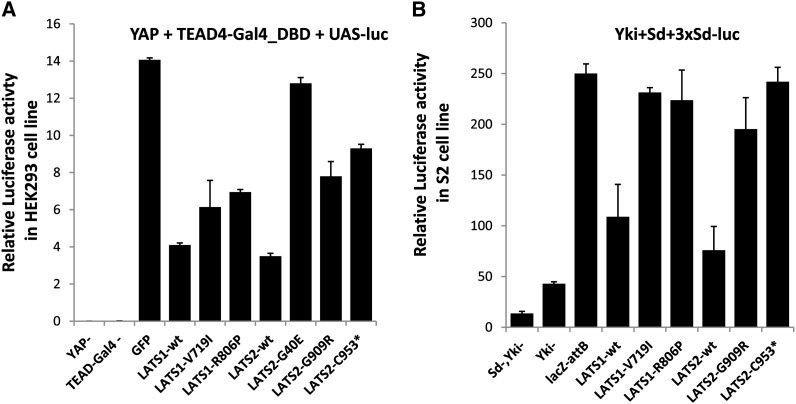
Mammalian Lats1/2 genes mediate growth control by directly phosphorylating and inactivating Yap/Taz (Dong et al. 2007; Zhao et al. 2007; Hao et al. 2008; Lei et al. 2008). To investigate whether the mutations in hLATS1/2 affect their ability to negatively regulate YAP, we have used luciferase reporter assays to directly measure YAP/Yki activities. In cultured HEK293T cells, wild-type hLATS1 and hLATS2 effectively inhibited the transcriptional activity of YAP (Figure 4A). V719I and R806P mutations in hLATS1 and G909R and C953* in hLATS2 all decreased the activity of hLATS1/2 as inhibitors of YAP (Figure 4A). As expected, YAP activity was not obviously affected in cells expressing hLATS2-G40E (Figure 4A). Similar results were observed using Drosophila S2 cells (Figure 4B). Therefore, these data further support that the hLATS1/2 mutations cause a loss or reduction of their activity in mediating Hippo signaling.
Figure 4.
Activities of hLATS1/2 and variants in regulating YAP/Yki transcriptional coactivator activity in both human and Drosophila cells. (A) Relative luciferase activity in HEK293T cells that overexpressed Renilla luciferase as an internal control, UAS-luciferase, CMV-YAP (except the first column), and fusion construct TEAD4-Gal4 that fuses TEAD4 with Gal4 DNA-binding domain (except the second column). (B) Relative luciferase activity of UAS-LATS1/2-wild type and mutants in S2 cells that overexpressed Renilla luciferase as an internal control, 3xSd (Scalloped)-luciferase, Ac-Yki (except the first and second columns), and UAS-Sd (except the first column). HEK293T cells were maintained in Dulbecco’s modified Eagle’s medium, containing 10% fetal calf serum and 0.5% penicillin–streptomycin. They were transiently transfected using PolyFect Transfection Reagent (Qiagen) and harvested 36 hr later. Plasmids include wild-type or mutant hLATS1 and hLATS2 in pcDNA3.1-Hygro-3xFLAG, pCMV-YAP, pCMV-Gal4-TEAD4, pUAS-LUC, and pCMV-Renilla (gift from Kun-Liang Guan). Cells were lysed after 36 hr and luciferase activity was detected using the Dual-Luciferase Reporter assay kit and GloMax multi detection system (Promega). Drosophila S2R+ cells were cultured in Schneider’s medium (Sigma), containing 10% fetal calf serum. S2 cells were transfected using Effectene (Qiagen) with wild-type or mutant hLATS1/2 in pUAST-attB, pAc-Yki, pUAST-Sd, p3xSd-LUC, and copia-Renilla (gift from Jin Jiang), together with pAc-Gal4. Results represent the mean of standard error of three independent experiments.
Genetic analysis of Lats/Wts family genes using Drosophila and mice models has revealed their role as a negative growth regulator and tumor suppressor (Justice et al. 1995; Xu et al. 1995; St John et al. 1999). The fact that hLATS1 can functionally replace Wts in Drosophila strongly suggests that LATS may function as a tumor suppressor in human (Tao et al. 1999). Although they are not frequently mutated genes, an increasing number of mutations in LATS1 and LATS2 have been detected in human cancers through human cancer genome projects. In this work, we have shown that LATS1 and LATS2 mutations from human cancer can lead to loss or reduction of their growth-inhibitory activity. Therefore, human LATS1 and LATS2 mutations are expected to confer tissue growth advantage to drive tumor development.
Supplementary Material
Acknowledgments
We acknowledge Johannes Bischof for fly lines; Kun-Liang Guan, Jin Jiang, and Xiaolong Yang for plasmids; Nicholas Anzalone for figure editing; and the Bloomington Drosophila Stock Center and Vienna Drosophila RNAi Center. This work was partly supported by the National Science Foundation.
Footnotes
Communicating editor: I. Hariharan
Literature Cited
- Bischof J., Maeda R. K., Hediger M., Karch F., Basler K., 2007. An optimized transgenesis system for Drosophila using germ-line-specific ϕC31 integrases. Proc. Natl. Acad. Sci. USA 104: 3312–3317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J., Feldmann G., Huang J., Wu S., Zhang N., et al. , 2007. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell 130: 1120–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Y., Chun A., Cheung K., Rashidi B., Yang X., 2008. Tumor suppressor LATS1 is a negative regulator of oncogene YAP. J. Biol. Chem. 283: 5496–5509 [DOI] [PubMed] [Google Scholar]
- Harvey K. F., Zhang X., Thomas D. M., 2013. The Hippo pathway and human cancer. Nat. Rev. Cancer 13: 246–257 [DOI] [PubMed] [Google Scholar]
- Justice R. W., Zilian O., Woods D. F., Noll M., Bryant P. J., 1995. The Drosophila tumor suppressor gene warts encodes a homolog of human myotonic dystrophy kinase and is required for the control of cell shape and proliferation. Genes Dev. 9: 534–546 [DOI] [PubMed] [Google Scholar]
- Lei Q. Y., Zhang H., Zhao B., Zha Z. Y., Bai F., et al. , 2008. TAZ promotes cell proliferation and epithelial-mesenchymal transition and is inhibited by the hippo pathway. Mol. Cell. Biol. 28: 2426–2436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St John M. A., Tao W., Fei X., Fukumoto R., Carcangiu M. L., et al. , 1999. Mice deficient of Lats1 develop soft-tissue sarcomas, ovarian tumours and pituitary dysfunction. Nat. Genet. 21: 182–186 [DOI] [PubMed] [Google Scholar]
- Tao W., Zhang S., Turenchalk G. S., Stewart R. A., St John M. A., et al. , 1999. Human homologue of the Drosophila melanogaster lats tumour suppressor modulates CDC2 activity. Nat. Genet. 21: 177–181 [DOI] [PubMed] [Google Scholar]
- Xu T., Wang W., Zhang S., Stewart R. A., Yu W., 1995. Identifying tumor suppressors in genetic mosaics: the Drosophila lats gene encodes a putative protein kinase. Development 121: 1053–1063 [DOI] [PubMed] [Google Scholar]
- Yu F. X., Guan K. L., 2013. The Hippo pathway: regulators and regulations. Genes Dev. 27: 355–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B., Wei X., W, Li, R. S. Udan, Q. Yang, et al, 2007. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 21: 2747–2761 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.