Abstract
Eph receptors and their ephrin ligands are key conserved regulators of axon guidance and can function in a variety of signaling modes. Here we analyze the genetic and cellular requirements for Eph signaling in a Caenorhabditis elegans axon guidance choice point, the ventral guidance of axons in the amphid commissure. The C. elegans Eph receptor EFN-1 has both kinase-dependent and kinase-independent roles in amphid ventral guidance. Of the four C. elegans ephrins, we find that only EFN-1 has a major role in amphid axon ventral guidance, and signals in both a receptor kinase-dependent and kinase-independent manner. Analysis of EFN-1 and EFN-1 expression and tissue-specific requirements is consistent with a model in which VAB-1 acts in amphid neurons, interacting with EFN-1 expressed on surrounding cells. Unexpectedly, left-hand neurons are more strongly affected than right-hand neurons by loss of Eph signaling, indicating a previously undetected left–right asymmetry in the requirement for Eph signaling. By screening candidate genes involved in Eph signaling, we find that the Eph kinase-independent pathway involves the ABL-1 nonreceptor tyrosine kinase and possibly the phosphatidylinositol 3-kinase pathway. Overexpression of ABL-1 is sufficient to rescue EFN-1 ventral guidance defects cell autonomously. Our results reveal new aspects of Eph signaling in a single axon guidance decision in vivo.
Keywords: Ephrin, amphid, left–right asymmetry, Abl tyrosine kinase, phosphatidylinositol 3-kinase
EPHRINS and their cell surface receptors, the Eph receptor tyrosine kinases (EphR), play critical roles in many axon guidance processes, including midline guidance and growth cone collapse (Drescher et al. 1995; Cowan et al. 2000). In contrast to long-range guidance cues, Eph signaling involves short-range interactions between a transmembrane receptor and transmembrane (ephrin-B) or GPI-linked (ephrin-A) ligands. Eph signaling is complex and multifunctional, capable of mediating both repulsion and attraction depending on ephrin concentration even in the same neurons (Hansen et al. 2004). Many of the signaling pathways downstream of Eph receptors and ephrins regulate cell movement or cell adhesion (Kullander and Klein 2002; Pasquale 2005).
Because Eph receptors and ephrins are cell surface molecules, they can operate in a variety of signaling modes (Kullander and Klein 2002; Egea and Klein 2007; Pasquale 2008). Eph receptors can generate kinase-dependent “forward” signals, in which ligand binding triggers receptor dimerization, activating the intrinsic kinase activity of the receptor, and initiating responses in the receptor-expressing cell. Kinase-dependent forward Eph signaling contributes to many processes including retinotopic mapping (Hindges et al. 2002), axonal midline avoidance after crossing (Yokoyama et al. 2001), neural crest cell migration (Smith et al. 1997), and migration of neural progenitors (Catchpole and Henkemeyer 2011). This regulation of diverse developmental processes occurs in part via kinase-dependent interactions with downstream effectors including Src-family kinases (Zisch et al. 1998; Knoll and Drescher 2004), Rho GTPases (Wahl et al. 2000; Noren and Pasquale 2004), and RhoGEFs (Shamah et al. 2001; Sahin et al. 2005).
In addition to kinase-dependent signaling, some Eph receptors initiate kinase-independent forward signals. In HEK293 cells, EphA8 signals promote integrin activity via the phosphatidylinositol 3-kinase (PI3K) pathway; the juxtamembrane domain of EphA8 directly interacts with the PI3K catalytic subunit p110γ, independent of EphA8 kinase activity (Gu and Park 2001, 2003). EphA8 can also interact with the Anks (ankyrin and sterile alpha motif) proteins AIDA and Odin in a kinase-independent manner (Shin et al. 2007). However, the significance of kinase-independent forward signaling in vivo has not been extensively analyzed. Reverse signaling via ephrin ligands can also contribute to kinase-independent functions. Although both ephrin-B and ephrin-A ligands are capable of reverse signaling (Bruckner et al. 1999; Davy et al. 1999), ephrin-A ligands do not contain a transmembrane domain and therefore require a coreceptor, such as p75 (Lim et al. 2008), TrkB (Marler et al. 2008), or Ret (Bonanomi et al. 2012).
In contrast to the many Eph receptors and ligands in vertebrates, C. elegans encodes a single Eph receptor, VAB-1 (George et al. 1998) and four ephrins, EFN-1–4 (Chin-Sang et al. 1999; Wang et al. 1999). The C. elegans ligands resemble vertebrate ephrin-As in topology, in that they are attached to the cell membrane by a glycosylphosphatidylinositol (GPI) linker. Ephrins EFN-1–3 have partly redundant roles in VAB-1 signaling, depending on the developmental context (Chin-Sang et al. 1999; Wang et al. 1999), whereas the divergent ephrin EFN-4 functions independently of VAB-1 (Chin-Sang et al. 2002; Ikegami et al. 2004).
C. elegans Eph signaling acts in diverse cell types and processes. VAB-1 and its ephrin ligands control neuroblast migrations during embryonic morphogenesis (George et al. 1998; Chin-Sang et al. 1999; Wang et al. 1999). The VAB-1 function in embryonic neuroblast migration requires both kinase-dependent and kinase-independent signaling, and involves partly redundant signaling by all three ephrin ligands. VAB-1 signaling also regulates oocyte maturation and gonadal sheath cell contractions in response to a distinct type of ligand, the major sperm proteins (Miller et al. 2003; Cheng et al. 2008). Although the VAB-1 kinase domain is required for inhibition of oocyte maturation in the absence of sperm, it is dispensable for regulation of the basal gonadal sheath cell contraction rate (Miller et al. 2003). C. elegans Eph signaling has been implicated in outgrowth or guidance of several axon types, including PLM outgrowth (Mohamed and Chin-Sang 2006) and pathfinding of PVQ and HSN axons (Boulin et al. 2006). In most of these situations, the defects of VAB-1/Eph receptor null mutants are more severe than those of kinase-dead alleles (George et al. 1998; Boulin et al. 2006; Mohamed and Chin-Sang 2006), implying that some VAB-1 signaling is kinase independent. However, the in vivo mechanism of VAB-1 kinase-independent signaling has remained elusive.
To understand the basis of VAB-1 kinase-independent signaling at the level of individual cells, we have focused on a simple axon guidance decision, the ventral guidance of amphid sensory axons. Ventral guidance of amphid sensory axons has been shown to require VAB-1 and at least two other partly redundant guidance pathways: netrin (UNC-6/UNC-40) signaling, and the SAX-3/Robo receptor (Zallen et al. 1999). Loss of function in any one of these pathways leads to incompletely penetrant guidance defects in which the amphid commissure extends laterally instead of ventrally. In these mutants the aberrant lateral axons extend anteriorly and enter the nerve ring, indicating that it is specifically the initial ventral guidance of amphid axons that is disrupted. Moreover, double mutants between the vab-1, unc-40, and sax-3 pathways display strong synergistic enhancement of guidance defects, consistent with partial genetic redundancy. However as VAB-1 is also required for many aspects of embryonic morphogenesis, it has been unresolved whether VAB-1 acts directly in amphid axon guidance.
We show here that VAB-1 can function in amphid neurons to mediate their ventral axonal guidance, interacting with EFN-1 in nonamphid neurons. The requirement for Eph signaling displays an unexpected left–right asymmetry. VAB-1’s role in amphid axon guidance involves at least two pathways, both of which are partly kinase independent. PI3K signaling promotes amphid axon guidance, acting at least partly in parallel to VAB-1; an additional role downstream of VAB-1 cannot be excluded. Additionally, ABL-1, a nonreceptor tyrosine kinase, signals in the amphid neurons as part of a VAB-1 kinase-independent pathway. These results elucidate mechanisms of VAB-1 kinase-independent forward signaling in amphid axon guidance.
Materials and Methods
Strains and culture conditions
Worms were cultured on Escherichia coli OP50 seeded NGM agar plates. Animals were grown and analyzed at room temperature (21–23°) with the exception of pdk-1(sa709) strains, which were analyzed at 22.5°, and age-1(hx546) and aap-1(m889), which were analyzed at 25°. The following mutants were used: LGI: unc-40(e1430); aap-1(m889); shc-1(ok198); src-1(cj293), src-2(ok819), vpr-1(tm1411), daf-16(mu86), and goa-1(sa734); LGII: vab-1(ju8, e2027, ju307, ok1699, dx14, dx31, ju220, ju275, e858, e699, ju306, tn2, e118, zd118, e2, ju63, ju426, e116, ju22, e1063, qa2211), ephx-1(ok494), tag-341(ok1498), age-1(hx546), shc-2(tm328), and cog-1(sy275); LGIII: ina-1(gm144), and mig-10(ct41); LGIV: efn-1(e96, ju90), efn-2(ev658), arf-6(tm1447), daf-18(ok480), rga-5(ok2241), jac-1(ok3000), and ngn-1(ok2200); LGV: akt-1(ok525); lsy-6(ot71); fmi-1(tm306); and LGX: efn-3(ev696), abl-1(ok171), sax-3(ky123), git-1(tm1962), gap-2(tm748), nck-1(ok694), wrk-1(ok695), trk-1(tm3985, tm4054), akt-2(ok393, tm812), unc-6(ev400), sgk-1(ok538, ft15), and pdk-1(mg142, sa709). New vab-1 alleles are listed in Table 1. Published transgenes are as follows: Pstr-1-GFP (kyIs104) (Troemel et al. 1997); Pstr-3-GFP (kyIs128) (Zallen et al. 1999), Pgcy-5-GFP (ntIs1) (Altun-Gultekin et al. 2001), Pgcy-7-GFP (otIs3) (Chang et al. 2003), Pgcy-8-GFP (oyIs17) (Satterlee et al. 2001), and Pvab-1-Venus (evIs190) (Ikegami et al. 2012).
Table 1. Phenotypic strength of selected vab-1 mutations and molecular lesions.
| Allele | Mutagen | Embryonic lethality (%) | Larval lethality (%) | Adult, Vab (%) | Adult, non-Vab (%) | WT sequence | Mutant sequence | Effect |
|---|---|---|---|---|---|---|---|---|
| Strong | ||||||||
| e2027(null) | SPO | 58.2 | 31.3 | 8.9 | 2.5 | — | 74-bp deletion, removing first 7 bp of exon 5 | — |
| e721 | EMS | 58.2 | 29.3 | 11.4 | 1.0 | ACG | ATG | ATG codon in 5′-UTR |
| ok1699 | UV | 53.4 | 20.7 | 23.9 | 2.0 | — | 1016-bp deletion of exon 5 | — |
| ju220 | UV/TMP | 45.4 | 15.0 | 34.6 | 5.1 | — | Exon 1 rearrangement | — |
| ju307 | EMS | 41.9 | 27.2 | 29.1 | 1.8 | GAA | AAA | E62K |
| Intermediate | ||||||||
| ju275 | EMS | 18.0 | 7.3 | 65.0 | 9.8 | GCG | GTG | A245V |
| Weak | ||||||||
| zd118 | EMS | 13.8 | 7.5 | 24.6 | 54.0 | TGG | TGA | W934opal |
| ju426 | EMS | 13.6 | 2.1 | 55.3 | 28.9 | TGG | TGA | W934opal |
| e118(kd) | EMS | 10.1 | 8.6 | 45.6 | 35.8 | — | 326-bp deletion in exon 10 | — |
| ju306 | ENU | 1.7 | 1.4 | 32.4 | 64.4 | GTC | GAC | V220D |
| qa2211 | EMS | 1.5 | 1.1 | 18.7 | 78.7 | GGA | GAA | G256E |
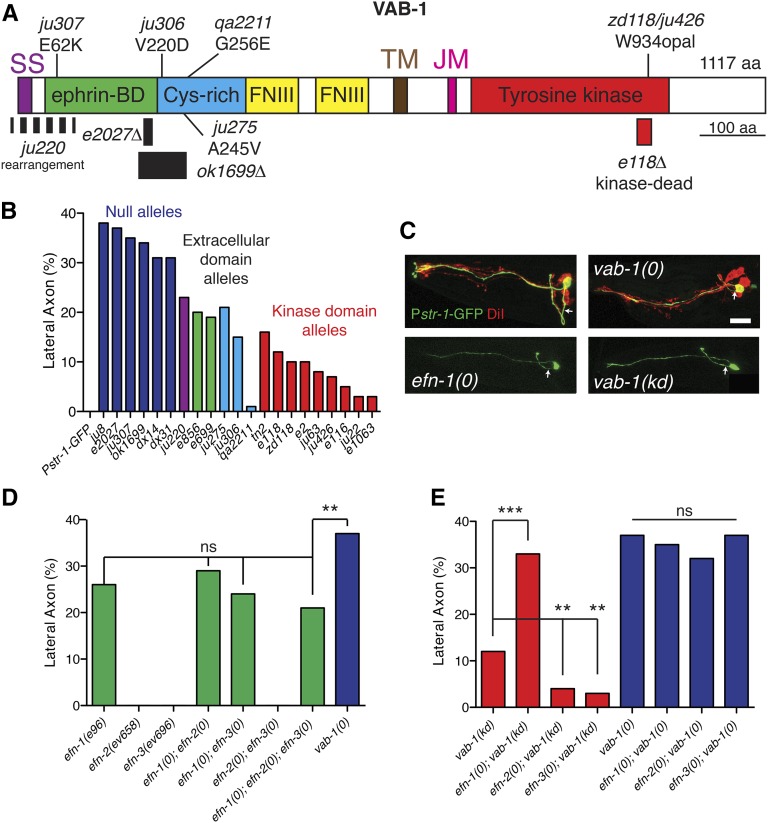
Three new alleles were characterized as genetically null based on phenotypic strength and molecular lesions: ju220 is a rearrangement of unknown structure that affects exon 1, encoding the start codon and signal sequence; ok1699 is a 1016-bp deletion of exon 5, which encodes part of the ephrin binding domain and the cysteine-rich domain in the extracellular domain; and ju307 results in the same missense alteration (E62K) as the previously described ju8 (George et al. 1998). The previously unsequenced null allele e721 creates a premature ATG in the vab-1 5′-untranslated region; translation from this out-of-frame upstream ORF likely interferes with translation from the native vab-1 ATG. Three new alleles result in missense alterations in the extracellular cysteine-rich domain, providing mutational evidence for the importance of this domain.
Scoring of amphid axon guidance and dendrite extension
To visualize amphid neuron morphology, we used dye filling (Hedgecock et al. 1985). To visualize individual neurons, we used transgenic markers labeling single amphid neuron types. We immobilized L4 stage hermaphrodites using 1% phenoxy-1-propanol in M9 and scored neuronal morphology using compound microscopy. For most genotypes, we scored >50, typically 100–200 neurons; genotypes for which <50 neurons were scored are indicated in the bar charts. In the wild type, essentially 100% of amphid axons extend ventrally in the commissure and then turn anteriorly into the nerve ring. We classified amphid axon guidance as normal, lateral, or other. The “other” category was rare (<5%) and only used if no axon was visible. As far as possible, we scored the initial guidance of the axon even if it changed direction subsequently; such changes in direction were rare. For screening, mutations were crossed into the vab-1(e118) kinase-dead background. To assess significance of differences in proportions we used the Fisher exact test or the chi-squared test.
Transgenic rescue of vab-1 and efn-1 phenotypes
To assess rescue of the vab-1 phenotypes, transgenic lines containing vab-1 genomic cosmid DNA (M03A1; pRF4 coinjection marker) (George et al. 1998), fosmid DNA (WRM0617bA10 in juEx2870) or the rescuing VAB-1::GFP minigene (pCZ55) (George et al. 1998) were generated. These lines were crossed into the vab-1(e2027); kyIs104 background and scored for rescue of guidance defects. Similarly, a transgene containing wild-type efn-1 genomic DNA (pCZ126), as well as a rescuing GFP::EFN-1 fusion (pCZ131 in juIs52) (Chin-Sang et al. 1999) were crossed into the efn-1(e96); kyIs104 mutant background.
Tissue-specific rescue experiments
We amplified full-length coding sequences and 3′-UTRs from cDNA clones yk497d6 (VAB-1A), yk338g11 (EFN-1), yk708d1 (EFN-2), yk1482h02 (ABL-1A), and cloned them into pCR8 to create Gateway entry clones pCZGY1146, pCZGY1145, pCZGY1148, pCZGY1835, and pCZGY1834. All entry clones were sequenced. We used the following tissue-specific promoters: unc-33 and rgef-1 (Altun-Gultekin et al. 2001), unc-119 (Maduro and Pilgrim 1995), myo-2 (Frøkjaer-Jensen et al. 2006), ttx-3 (Hobert et al. 1997), dyf-7 (Heiman and Shaham 2009), lin-26 (Labouesse et al. 1994), str-1 (Troemel et al. 1997), and hlh-17 (Yoshimura et al. 2008). After recombination with entry vectors containing the desired cDNA, final constructs were injected into wild-type (N2) worms at 1 ng/μl together with 15 ng/μl sur-5-mCherry as a coinjection marker; see Supporting Information, Table S1 for a list of clones and transgenes. Each transgene was crossed into the relevant mutant background; at least two transgenes per construct were scored, and representative lines are reported.
Expression analysis
To determine cellular expression patterns of VAB-1 and EFN-1, we examined animals expressing the rescuing transgenes juIs24 (VAB-1::GFP), juIs52 (EFN-1::GFP), and the vab-1 transcriptional reporters juEx101 and evIs190. To examine fixed animals, we performed fixation and staining as described (Finney and Ruvkun 1990). Fixed animals were incubated with rabbit anti-GFP polyclonal (A11122, Invitrogen, 1:1000 dilution) and mouse anti-AJM-1 monoclonal (MH27, 1:500) overnight at 4° and staining visualized with appropriate 2° antibodies.
4D lineage analysis of EFN-2 expression
To examine efn-2 embryonic expression, we generated the transgene Pefn-2-HIS-24::mCherry::let-858 3′-UTR (juEx2737), which contains 2.7 kb of efn-2 upstream sequence. We followed its expression in a HIS-72::GFP (zuIs178) background and identified Pefn-2-mCherry-expressing cells in two embryos using 4D confocal microscopy and the lineaging software NucleiTracker4D (Giurumescu et al. 2012).
Analysis of new vab-1 alleles
In addition to previously described vab-1 alleles (George et al. 1998), we characterized eight additional alleles (Table 1; Figure 1A). Mutations ju307, ju275, zd118, qa2211, and ju426 were isolated based on epidermal morphology phenotypes after EMS mutagenesis. ju306 was isolated after ENU mutagenesis and ju220 was recovered after UV/Trimethylpsoralen (TMP) mutagenesis. ok1699 was isolated following UV mutagenesis by the C. elegans Gene Knockout Consortium. DNA lesions were determined by exon sequencing and penetrance determined as described (George et al. 1998).
Figure 1.
Ventral guidance of amphid commissure axons is dependent on EFN-1-VAB-1 signaling. (A) Domains of VAB-1 and locations of molecular lesions. Previously isolated deletion alleles e2027(null) and e118(kd) are included for reference. ok1699 is a deletion and ju220 is a rearrangement of unknown structure. SS, signal sequence; TM, transmembrane domain; JM, juxtamembrane domain. (B) Quantitation of AWB guidance defects in Eph receptor null mutants (ju307, ju8, e2027, ok1699, dx14, and dx31), extracellular domain alleles (ju220, ju275, e856, e699, and ju306), and kinase dead alleles (tn2, e118, zd118, e2, ju63, e116, ju22, and e1063). Color coding corresponds to region of protein affected by mutation. (C) Amphid axon guidance in vab-1 and efn-1 mutants; AWB (Pstr-1-GFP, green) and DiI staining (red). Confocal projections, anterior is left and dorsal is up. Arrows indicate axon extending from cell body. Bar, 10 μm. (D) Quantitation of AWB guidance defects in efn mutants and double mutants. Asterisks indicate compound mutants significantly different from the efn-1 single mutant. (E) Quantitation of guidance defects in double mutants between each ligand and the vab-1(e118) kinase dead receptor strain or double mutants between each ligand and the receptor null vab-1(e2027). N > 50 neurons per genotype in this and all subsequent bar charts, except where N is indicated in the bar. Statistics, Fisher exact test: *P < 0.05; **P < 0.01; ***P < 0.001.
Results
Amphid ventral guidance involves both kinase-dependent and kinase-independent functions of VAB-1/Eph receptor
Amphids are sensory organs containing 12 bilaterally symmetric pairs of neurons whose cell bodies are located in the lateral ganglia of the head. Amphid neuron cell bodies are born in the anterior head in midembryogenesis, move anteriorly to anchor their dendrite tip, then migrate posteriorly, laying down their sensory processes by “retrograde extension” (Sulston et al. 1983; Heiman and Shaham 2009). Once the amphid cell body reaches its final place in the lateral ganglia, the amphid axons grow out ventrally then turn and extend anteriorly and dorsally into the nerve ring. Amphid axons are fasciculated in their ventral trajectories, forming two bundles known as the amphid commissures. Previous work revealed that amphid commissure guidance was strongly dependent on VAB-1/EphR signaling: vab-1(dx31) null mutants display 30–40% guidance defects in which amphid axons leave the cell body laterally and enter the nerve ring without following the normal ventral trajectory (Zallen et al. 1999). Defects were significantly less penetrant in the kinase-dead allele vab-1(e2), suggesting amphid axon guidance is a suitable model for VAB-1/EphR kinase-independent signaling.
To confirm the requirement for VAB-1, we examined amphid axon guidance in the entire vab-1 allelic series using the AWB marker Pstr-1-GFP (kyIs104) (Figure 1B). Axon guidance defect penetrance correlated strongly with penetrance of lethality or body morphology defects (Table 1; George et al. 1998). Putative vab-1 null alleles (e2027, dx31, ju8, ok1699, dx14, and ju307) cause between 31 and 38% axon guidance defects, whereas likely kinase-dead vab-1 alleles (e118, e2, and zd118) display 10% defects. The penetrance of defects in vab-1(e2027) L1 stage animals was comparable to that observed in L4 animals (not shown), suggesting guidance errors arise during initial amphid axon outgrowth in embryogenesis and do not result from a failure to maintain axon position. These results confirm that both kinase-dependent and kinase-independent VAB-1 functions are involved in amphid axon guidance. Hereafter we refer to the reference null vab-1(e2027) as vab-1(0), and to the reference kinase dead allele vab-1(e118) as vab-1(kd). In agreement with previous observations (Zallen et al. 1999), vab-1 mutations affect all amphid axons equally; guidance of the amphid axon bundle is typically either completely ventral or completely lateral. Below, we focus on a representative neuron, AWB.
Amphid axon guidance is dependent on EFN-1, which signals through both VAB-1 kinase-dependent and -independent pathways
Previous studies had not resolved which ephrin ligands were involved in amphid axon guidance. We found that of the four C. elegans ephrins, only efn-1(0) mutants displayed amphid axon ventral guidance defects like those of vab-1, at lower penetrance (25%; Figure 1, C and D). These observations suggested that EFN-1 might be partly redundant with EFN-2 and EFN-3 in regulating ventral guidance. efn-2 or efn-3 null mutants or efn-2efn-3 double mutants displayed completely normal AWB guidance (Figure 1D). efn-1efn-2 and efn-1efn-3 double mutants resembled efn-1 single mutants, as did the efn-1efn-2efn-3 triple mutant (Figure 1D). Thus, EFN-1 is the major ephrin ligand involved in amphid axon guidance. As shown below, EFN-2 may play a minor role in guidance.
To address how EFN-1 may regulate VAB-1 signaling, we examined amphid guidance in efn-1vab-1 double mutants. The phenotype of vab-1 null mutants was not enhanced by efn-1(0), consistent with EFN-1 and VAB-1 acting in a linear pathway. We next addressed whether EFN-1 acted in the VAB-1 kinase-dependent or kinase-independent pathway by examining double mutants between each ephrin ligand and the kinase dead receptor. We interpret enhancement of the kinase-dead phenotype as evidence for signaling in a kinase-independent pathway. efn-1(0) vab-1(kd) double mutants showed enhancement relative to vab-1(kd) but not to efn-1(0) alone, consistent with EFN-1 signaling at least in part through a kinase-independent pathway (Figure 1E). Conversely, efn-2vab-1(kd) and efn-3vab-1(kd) mutants displayed significant suppression of axon guidance defects relative to vab-1(kd), suggesting EFN-2 and EFN-3 have a cryptic function that antagonizes the kinase-independent pathway. efn-2 or efn-3 neither enhanced nor suppressed vab-1(0) guidance defects, implying that the antagonistic effects of EFN-2/3 require the Eph receptor (Figure 1E).
To test whether loss of EFN-2 improved VAB-1 signaling by enhancing EFN-1 activity, we examined whether efn-1 partial loss-of-function mutants could be suppressed by efn-2(lf) and found no significant suppression in these double mutants (not shown). Loss of EFN-2 may be unable to compensate for the reduced EFN-1 function in these mutants. To address the specificity of efn-2 suppression, we analyzed double mutants between efn-2 and the netrin receptor unc-40 or sax-3/Robo. efn-2 weakly suppressed guidance defects in both cases (not shown), although this was not statistically significant. These data imply that loss of efn-2 function might increase EFN-1/VAB-1 signaling via VAB-1, resulting in slight improvement of guidance in the absence of UNC-40 or SAX-3.
VAB-1 is expressed and required in amphid neurons for axon guidance
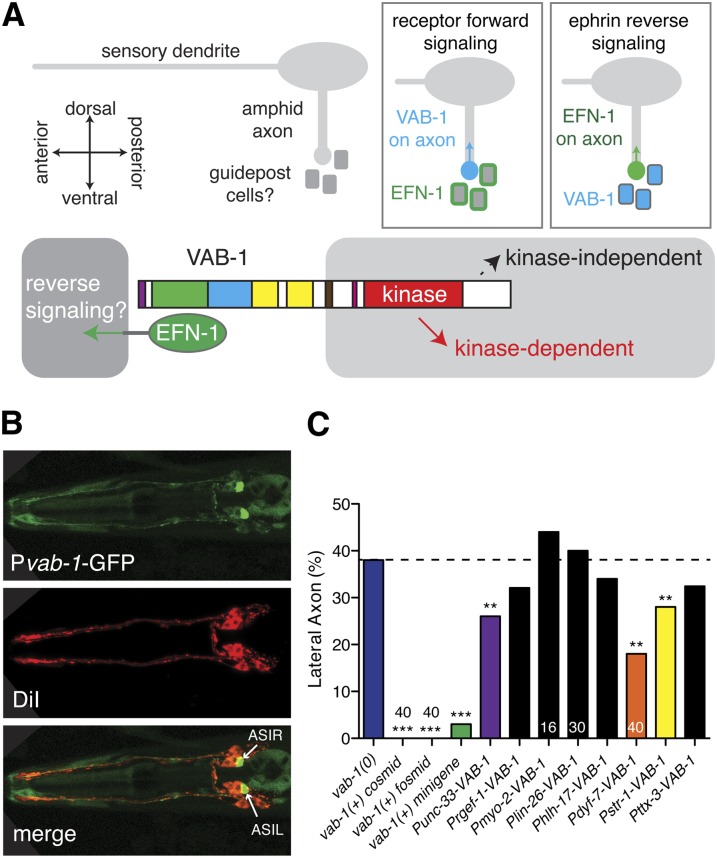
We considered two general models for how VAB-1 and EFN-1 might promote amphid guidance (Figure 2A). First, VAB-1 in amphid neurons might interact with EFN-1 on surrounding guidepost cells, mediating a receptor-dependent forward signal into axons. EFN-1 might present an attractive cue on ventral guidepost cells or EFN-1 might repel amphid axons from the more lateral pathway; the stereotyped lateral guidance of aberrant axon trajectories in vab-1, unc-40, or sax-3 suggests that the lateral path represents a “default” pathway for axons in the absence of ventral cues. In the second model EFN-1 in amphid neurons might interact with VAB-1 in surrounding cells, mediating an ephrin reverse signal into axons. More complex models in which VAB-1 and EFN-1 are coexpressed in amphids and surrounding cells are also possible.
Figure 2.
Expression and tissue-specific rescue of VAB-1. (A) Models for the cellular focus of VAB-1 and ephrins in amphid axon ventral guidance. (B) Pvab-1-GFP (evIs190) expression in amphid neurons, including ASI, and in nerve ring in larvae. Anterior is to the left, dorsal views. ASI is identified as the most posterior of the dorsal trio of large amphid neurons, double labeled with DiI (red). (C) Tissue and cell-specific rescue of vab-1 guidance defects. All transgenic rescue assays were conducted in vab-1(e2027); kyIs104 mutant background. Asterisks indicate significant differences from vab-1 single mutant by Fisher exact test: *P < 0.05; **P < 0.01; ***P < 0.001.
VAB-1 is widely expressed in anterior neurons during embryonic and larval development (George et al. 1998; Brisbin et al. 2009). During larval stages Pvab-1-GFP expression becomes more restricted, and was observed strongly in at least one amphid neuron pair, ASIR/L (Figure 2B). We therefore focused on testing the cellular requirement of VAB-1 using a variety of tissue- or cell-specific promoters (Table S1). We verified that the vab-1(0) axon guidance phenotypes were fully rescuable by genomic vab-1 DNA or by the VAB-1::GFP transgene (George et al. 1998). Expression of VAB-1 under the control of early panneuronal promoters such as unc-33 partly rescued vab-1(0) phenotypes from 38 to 27%, suggesting expression of VAB-1 in neurons is sufficient to promote normal amphid guidance; expression under the control of the later-onset rgef-1 promoter did not significantly rescue, suggesting VAB-1 expression later in neuronal differentiation is not sufficient. When we expressed VAB-1 using an amphid (Pdyf-7) or AWB-specific promoter (Pstr-1), vab-1(0) mutant phenotypes were significantly rescued (18–28%; Figure 2C). In contrast, expression of VAB-1 from nonneuronal promoters, including lin-26 (glial and epidermal cells), hlh-17 (cephalic sheath cells), or myo-2 (pharyngeal muscle), did not rescue amphid axon defects. Taken together, our expression and tissue-specific rescue experiments are most consistent with VAB-1 acting directly in amphid neurons to promote axon guidance.
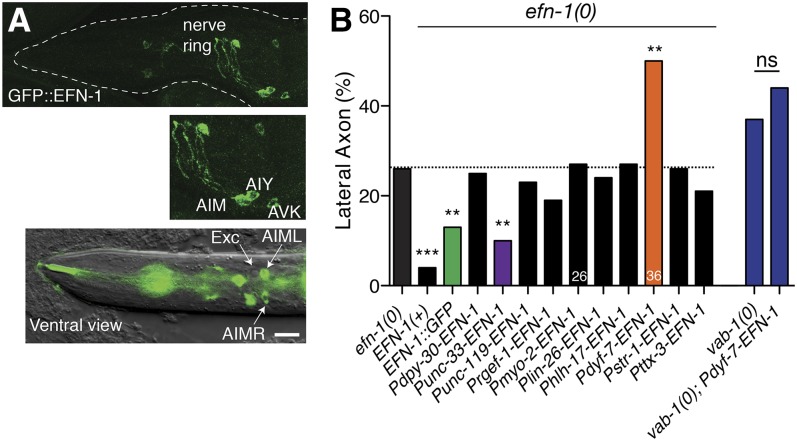
EFN-1 is expressed and required in neurons and may interfere with VAB-1 when misexpressed in amphid neurons
EFN-1::GFP is expressed widely in anterior neurons in midembryogenesis (Chin-Sang et al. 1999), but like VAB-1 becomes more restricted during larval stages. In larvae, EFN-1::GFP was not observed in amphid neurons but was seen in a set of ventral neurons including AIM, AIY, and AVK (Figure 3A). Tissue-specific expression of EFN-1 under the control of the panneuronal unc-33 promoter significantly suppressed efn-1(0) guidance defects, suggesting EFN-1 is at least partly required in neurons. However, amphid-specific expression of EFN-1 strongly enhanced guidance defects in an efn-1(0) mutant (Figure 3B). We reasoned that if EFN-1 is not normally expressed in VAB-1-expressing amphid neurons, its ectopic expression in amphid neurons could inhibit VAB-1 signaling by a cis-interaction similar to that reported between ephrin-A5 and EphA3 in retinal axons (Carvalho et al. 2006). Consistent with this hypothesis, Pdyf-7-EFN-1 did not significantly enhance guidance defects of a vab-1(0) mutant. Thus, ventral guidance defects due to misexpression of EFN-1 may reflect inhibition of VAB-1/EphR in amphid neurons. As the Pdyf-7-EFN-1 guidance defects in an efn-1(0) background are slightly more severe than those of vab-1(0), EFN-1 overexpression may also interfere with the function of SAX-3 or UNC-40, which are known to be expressed in amphid neurons (Chan et al. 1996; Zallen et al. 1998). Expression of EFN-1 under the control of nonneuronal or AWB-specific promoters (lin-26, hlh-17, myo-2, or str-1) neither rescued nor enhanced efn-1 phenotypes. Overall these data are consistent with EFN-1 functioning in nonamphid neurons, presumably guidepost cells for amphid axons.
Figure 3.
EFN-1 expression and tissue-specific rescue. (A) EFN-1::GFP (juIs52) expression in larvae and adults. EFN-1::GFP is expressed in a small number of anterior neurons, identified based on position and morphology as AIM, AIY, and AVK. (B) Tissue and cell-specific rescue of efn-1 axon guidance defects, scored in the efn-1(e96); kyIs104 background or in vab-1(e2027) as indicated. Asterisks indicate significant difference from efn-1 single mutant defects, by Fisher exact test. N > 50 except where indicated. *P < 0.05; **P < 0.01; ***P < 0.001. Bar, 10 μm.
Eph signaling in amphid axon guidance displays left–right asymmetry
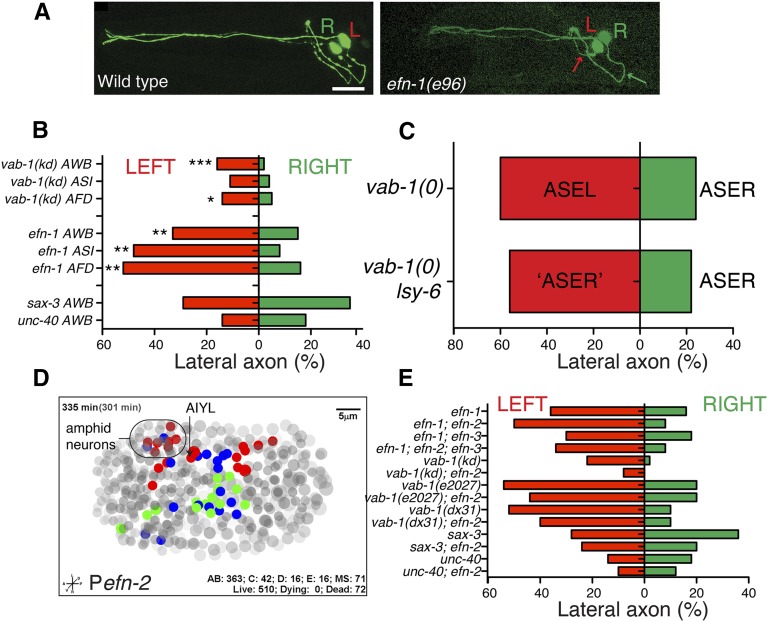
In the course of analyzing amphid ventral guidance phenotypes, we noticed that almost all vab-1 and ephrin mutant strains displayed a strong left–right bias, in that guidance defects were two to four times more frequent in the left-hand neuron of a bilateral pair (Figure 4, A and B). Such asymmetric guidance defects were seen in multiple singly labeled neuron types and in amphid neurons labeled by dye filling. In contrast, the penetrance of guidance defects in unc-40 (Netrin receptor) or sax-3 (Robo) mutants did not display left–right bias (Figure 4B). Thus, despite the overt symmetry of axon guidance in the wild type, left-hand neurons are more dependent on Eph signaling than are right-hand neurons.
Figure 4.
Ephrin signaling has asymmetric requirements in amphid commissure ventral guidance. (A) Amphid outgrowth defects display left-hand bias in Eph signaling mutants; representative example from efn-1(e96). Bar, 10 μm. (B) Quantitation of axon guidance defects in left vs. right hand AWB, ASI, or AFD axons in efn-1 or vab-1(e118) mutants. unc-40 and sax-3 do not show detectable left–right bias in penetrance. Green bars are right-side axons and red bars, left. (C) Transformation of ASEL into an ASER-like fate by lsy-6 does not alter the left-hand bias in guidance defects in vab-1(e2027) background. (D) Asymmetry in Pefn-2-mCherry (juEx2737) expression as determined by 4D lineage analysis at 335 min postfertilization; anterior is to the left and left is up. Blue nuclei are bilateral pairs that show symmetrical expression of efn-2, green nuclei are members of bilateral pairs for which only the right-hand side expresses efn-2, red nuclei are members of pairs that express only on the left-hand side. Gray nuclei are nonexpressing (grayscale depth code). See Table S2 for list of expressing nuclei. (E) Loss of efn-2 function increases asymmetry of efn-1 mutants, does not affect vab-1(e2027) or vab-1(dx31) null mutants, and decreases penetrance and asymmetry in vab-1(kd).
Amphid neurons are known to display left–right asymmetries in gene expression and function, determined cell autonomously by gene regulatory cascades (Hobert et al. 2002; Johnston and Hobert 2003). To distinguish whether an amphid neuron’s identity or its environment determines the asymmetric response to loss of ephrin signaling, we used lsy-6 mutants, in which a left-hand neuron (ASEL) is genetically transformed into its right-hand counterpart ASER (Johnston and Hobert 2003). If left-hand bias in guidance defects reflects an intrinsic aspect of the ASEL fate, ASER should not show higher guidance defects when on the left side. However if the left-side environment determines reliance on Eph signaling then the transformed ASER (left) should show enhanced defects compared to the nontransformed ASER (right). In vab-1(0) lsy-6 double mutants we observed a strong left-hand bias in defects (P < 0.01), equivalent to that in vab-1(0) alone (Figure 4C), suggesting it is not the lateralized identity of the cell, but a difference in the environment that leads to the asymmetry of Eph guidance defects.
Our analysis of VAB-1 and EFN-1 expression patterns did not reveal overt left–right asymmetries in amphid neurons or surrounding cells. We therefore examined the embryonic expression of EFN-2 using 4D lineage analysis of an efn-2 transcriptional reporter. We found that efn-2 expression shows widespread left–right asymmetry in embryos, both in amphid neurons and in other cells (Figure 4D; Table S2). This asymmetry involves expression in either the right or left member of a bilaterally symmetrical neuron pair. The asymmetric expression of efn-2 prompted us to reexamine whether efn-2 mutants might have subtle asymmetric effects on amphid guidance that were not evident from averaging defects on both sides. Loss of function in efn-1 causes a strong left-hand bias in defects that is exacerbated in efn-1efn-2: defects on the left side increase from 36 to 50%, and defects on the right side decrease from 16 to 8% (Figure 4E), resulting in slightly increased overall defects (Figure 1D). In contrast, loss of efn-2 improves left-hand guidance in vab-1(kd) mutants, suggesting the effect of EFN-2 on left-side guidance can be positive or negative depending on the presence of EFN-1. Loss of efn-2 function did not modify the left–right bias of defect in vab-1(0) consistent with the idea that the inhibitory effect of EFN-2 requires VAB-1. Asymmetric expression of EFN-2 or other guidance cues could contribute to the unequal roles of Eph signaling in left and right amphid axon guidance.
Screening candidates for components of Eph signaling in axon guidance
To identify additional components of Eph signaling involved in axon guidance, we screened candidate genes chosen based on their involvement in Eph signaling in other tissues or organisms (Table 2). We scored guidance in all single mutants and selected mutants in the vab-1(kd) and vab-1(0) backgrounds, reasoning that mutations affecting a VAB-1 kinase-independent pathway should enhance vab-1(kd) but not vab-1(0). VAB-1 kinase-independent signaling could potentially act downstream of VAB-1 in a “forward” pathway, or in a “reverse” pathway into the EFN-1-expressing cell. Loss of function in candidate coreceptors for ephrin reverse signaling such as the TrkB homolog trk-1 (Manning 2005) did not affect guidance. Other potential VAB-1 ligands such as the atypical ephrin EFN-4, VPR-1, or the Wrapper/Klingon-like receptor WRK-1 did not affect amphid axon guidance as single mutants and were not tested further (Table 2). Some sterile or maternal-effect mutants, such as src-1 were only tested as single mutants and did not affect guidance. Loss of function in mig-10/Lamellipodin or in ngn-1/Neurogenin caused highly penetrant axon guidance defects as single mutants and may affect parallel pathways or multiple signaling pathways. Most single mutants did not display axon guidance defects and did not modify the vab-1(kd) phenotype, including several genes implicated in Eph signaling in other contexts, such as nck-1/Nck or ephx-1/Ephexin. Below we focus on two pathways that displayed specific roles in amphid guidance: the PI3-kinase/Insulin signaling pathway and the nonreceptor tyrosine kinase abl-1/Abl.
Table 2. Candidate Eph signaling genes in C. elegans.
| Single mutant lateral axon phenotype (%) | Double vab-1(kd) mutant lateral axon phenotype (%) | Compound vs. vab-1(kd) (P-value) | Mammalian homolog | |
|---|---|---|---|---|
| vab-1(e118) | — | 12 | — | |
| Class I: suppressors of vab-1(kd) | ||||
| akt-1(ok525) | 0 | 3 | ***0.0009, N = 200 | Akt/PKB |
| sgk-1(ok538) | 0 | 3 | **0.002, N = 160 | SGK/serum-and glucocorticoid-inducible kinase |
| daf-16(mu86) | 0 | 3 | **0.003, N = 200 | FOXO |
| efn-3(ev696) | 0 | 3 | **0.008, N = 134 | Ephrin-A ligands |
| efn-2(ev658) | 0 | 4 | **0.006, N = 268 | Ephrin-A ligands |
| egl-19(ad695gf) | 0 | 4 | *0.024, N = 164 | VGCC α subunit |
| daf-18(ok480) | 0 | 4 | *0.036, N = 100 | PTEN |
| Class II: specific enhancers of vab-1(kd) | ||||
| efn-1(e96) | 26 | 33 | ***0.0001, N = 122 | Ephrin-A ligands |
| abl-1(ok171) 25° | 0 | 32 | **0.002, N = 100 | Abl/Abelson kinase |
| abl-1(ok171) | 1 | 22 | 0.091, N = 100 | Abl/Abelson kinase |
| aap-1(m889) 25° | 0 | 28 | *0.041, N = 50 | PI3K p50/p55 |
| age-1(hx546) 25° | 0 | 22 | 0.06, N = 130 | PI3K p110 |
| Class III: nonspecific enhancers of vab-1(kd) | ||||
| ngn-1(ok2200) | 32 | 50 | ***0.0001, N = 100 | Neurogenin |
| mig-10(ct41) | 2 | 22 | *0.05, N = 200 | Lamellipodin |
| Class IV: no significant change | ||||
| ina-1(gm144) | 3 | 11 | 0.825, N = 100 | alpha integrin subunit |
| git-1(tm1962) | 0 | 8 | 0.355, N = 100 | GIT1 |
| arf-6(tm1447) | 0 | 11 | 0.825, N = 100 | ARF6 |
| nck-1(ok694) | 0 | 14 | 0.834, N = 100 | NCK adapter protein |
| shc-1(ok198) | 0 | 8 | 0.347, N = 100 | Shc proteins |
| shc-2(tm328) | 1 | — | — | Shc proteins |
| ephx-1(ok494) | 0 | 15 | 0.683, N = 100 | Ephexin |
| rga-5(ok2241) | 0 | 12 | 1.0, N = 100 | Rho GTPase Activating protein |
| gap-2(tm748) | 0 | — | — | nGAP/synGAP |
| vpr-1(tm1411) | 0 | — | — | VAPB |
| wrk-1(ok695) | 0 | 9 | 0.490, N = 100 | Wrapper/Rega-1/Klingon |
| trk-1(tm3895)†† | 0 | 14 | 0.822, N = 72 | TRK neurotrophin receptor |
| trk-1(tm4054)†† | 0 | 6 | 0.139, N = 100 | TRK neurotrophin receptor |
| src-1(cj293) | 0 | — | — | Src family kinase member |
| src-2(ok819) | 0 | 12 | 1.0, N = 50 | Src family kinase member |
| egl-19(n2368cs) | 4 | 6 | 0.138, N = 100 | VGCC α subunit |
| jac-1(ok3000) | 0 | 5 | 0.125, N = 100 | p120 catenin |
| tag-341(ok1498) | 1 | 5 | 0.056, N = 200 | F-BAR and RhoGAP domains |
| akt-2(ok393) | 0 | 14 | 0.836, N = 100 | Akt/PKB |
| akt-2(tm812) | 0 | 18 | 0.326, N = 100 | Akt/PKB |
| lsy-6(ot71) | 0 | 19 | 0.248, N = 100 | N/A |
| cog-1(sy275)† | 5 | — | — | Nkx6 homeodomain protein |
| wsp-1(gm324) | 0 | 10 | 0.652, N = 100 | N-WASP |
| goa-1(sa734) | 1 | Sterile | — | Heterotrimeric G protein alpha subunit Go (Go/Gi class) |
| fmi-1(tm306) | 7 | — | — | Celsr/Flamingo |
| efn-4(bx80) | 3 | — | — | Ephrin-A ligands |
N, number of neurons scored. †cog-1 was tested with the Pgcy-7-GFP marker to label ASEL. ††trk-1(tm3985) is a 176-bp deletion, which removes most of exons 8 and 9 of the primary sequence and is predicted to truncate the protein between the transmembrane and kinase domain. tm4054 is an 823-bp deletion, which removes exons 6–9 and is predicted to be a null. Statistics, Fisher exact test: *, P < 0.05, **, P < 0.01, ***, P < 0.001.
PI3-kinase signaling promotes amphid axon guidance
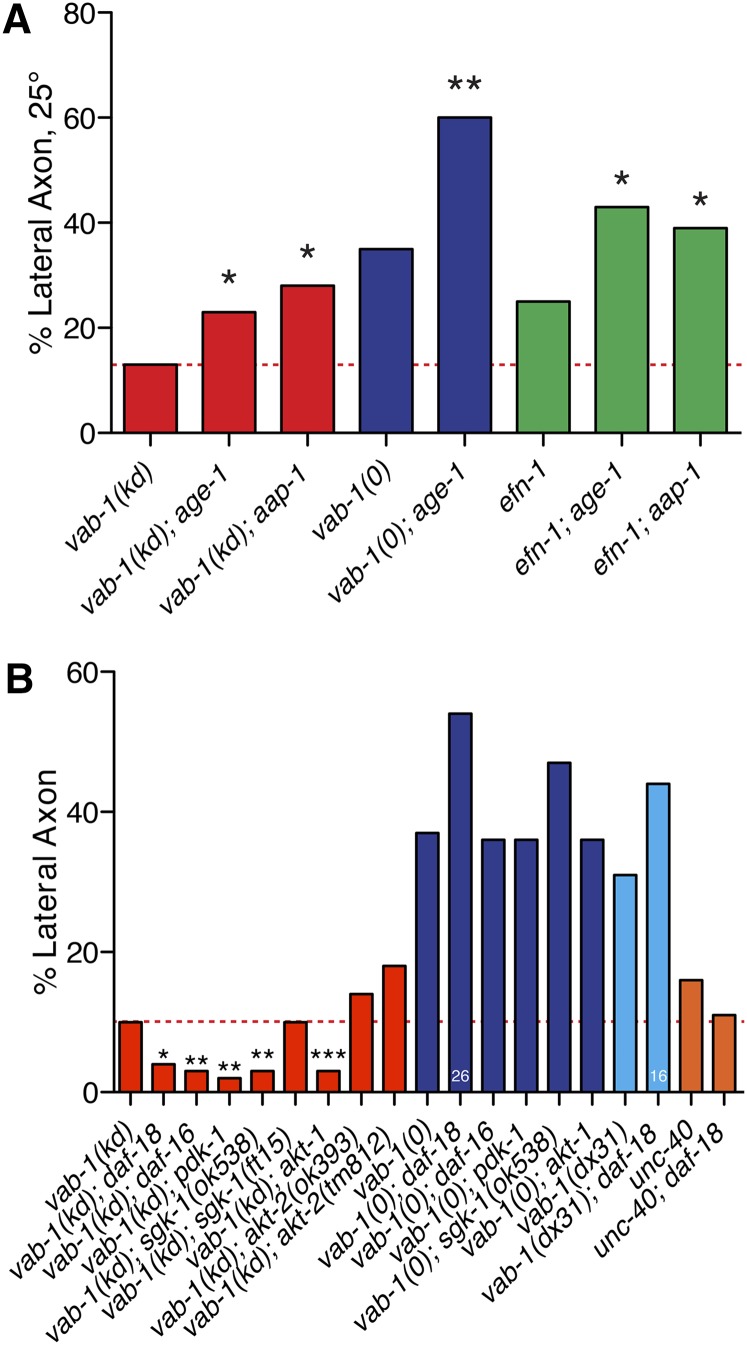
The phosphatidylinositol 3-kinase pathway plays widespread roles in Eph signaling; in C. elegans, VAB-1 promotes PI3K signaling in neurons by negatively regulating the phosphatase DAF-18/PTEN (Brisbin et al. 2009). We therefore tested age-1 and aap-1, which encode the C. elegans orthologs of the PI3K p110 catalytic and p50/p55 adaptor/regulatory subunits. age-1 or aap-1 single mutants displayed normal amphid guidance and both significantly enhanced the vab-1(kd) phenotype (Figure 5A). Loss of aap-1 or age-1 also enhanced efn-1(0) guidance defects, and age-1 enhanced vab-1 null mutant defects. PI3K signaling has recently been shown to cell-autonomously promote axon outgrowth of the AIY neuron (Christensen et al. 2011); we found that AWB also displayed outgrowth defects in PI3K mutants, but that these were independent of axon guidance or VAB-1 (not shown). These results suggest PI3-kinase signaling promotes amphid guidance and that loss of VAB-1 function sensitizes amphid axons to loss of PI3K activity. As vab-1(0) is enhanced by PI3K mutations, PI3K acts in parallel to VAB-1. An additional role for PI3K downstream of VAB-1 cannot be excluded.
Figure 5.
Phosphatidylinositol 3-kinase signaling promotes amphid axon guidance. (A) Enhancement of vab-1(kd) amphid axon guidance defects by reduction of function in PI3-kinase activity. age-1 and aap-1 were tested at 25°. Significance is relative to the single vab-1(kd) mutant (red dashed line). (B) Loss of function in daf-18/PTEN, daf-16/FOXO, and in downstream PI3K signaling kinases suppresses vab-1(kd) guidance defects. daf-18 slightly enhanced the ventral guidance defects of vab-1 null mutants but this is not statistically significant; daf-18 does not significantly affect unc-40 penetrance. Statistics, Fisher exact test: *P < 0.05; **P < 0.01; ***P < 0.001.
daf-18 encodes the C. elegans PTEN phosphatase that acts antagonistically to PI3K (Ogg and Ruvkun 1998). VAB-1 has been shown to interact with and negatively regulate PTEN expression and function in amphid neurons in a kinase-dependent manner (Brisbin et al. 2009). We found that daf-18vab-1(kd) mutants showed significant suppression of amphid guidance defects, consistent with PI3K signaling promoting guidance (Figure 5B). Loss of function in daf-18 did not suppress amphid axon guidance defects of unc-40 null mutants, suggesting loss of vab-1 kinase activity might sensitize axons to levels of PI3K signaling. In the course of these experiments, we observed that daf-18 displayed strong synergistic lethality with vab-1 or sax-3 null mutants (not shown). Analysis of a limited number of surviving vab-1(0) daf-18 double mutants revealed slight enhancement of guidance defects that was not statistically significant. Thus, DAF-18 may play both positive and negative roles in guidance depending on the signaling context.
In C. elegans PI(3,4,5)P3 (PIP3) is thought to act via a variety of kinases, including AKT-1/AKT-2 (Paradis and Ruvkun 1998) and PDK-1 (Paradis et al. 1999). The serum-and glucocorticoid-inducible kinase SGK-1 strictly depends on PDK-1 for its activation, but transduces PIP3 signals by forming a complex with AKT-1/2 to control life span through regulation of DAF-16 (Hertweck et al. 2004). We therefore tested pdk-1/PDK, sgk-1/SGK, and the Akt/PKB homologs akt-1 and akt-2. Unexpectedly, loss of function in each of these kinases except akt-2 suppressed vab-1(kd) defects (Figure 5B). Loss of function in a target of the PI3-kinase/insulin pathway, DAF-16/FOXO (Lin et al. 1997; Ogg et al. 1997), also suppressed vab-1(kd) guidance defects.
Overall, these results further demonstrate that PI3K signaling influences amphid axon guidance but suggest that the roles of PI3K-dependent kinases may differ from their canonical roles as defined by their functions in aging or dauer formation (Paradis and Ruvkun 1998; Paradis et al. 1999; Hertweck et al. 2004). Reduced PI3K activity enhanced guidance defects, yet loss of function in the kinases that transduce PIP3 activity suppressed guidance defects. These results suggest a more complex and possibly noncanonical relationship between VAB-1 and the PI3K pathway in axon guidance.
The ABL-1 tyrosine kinase acts in Eph signaling in amphid axon guidance:
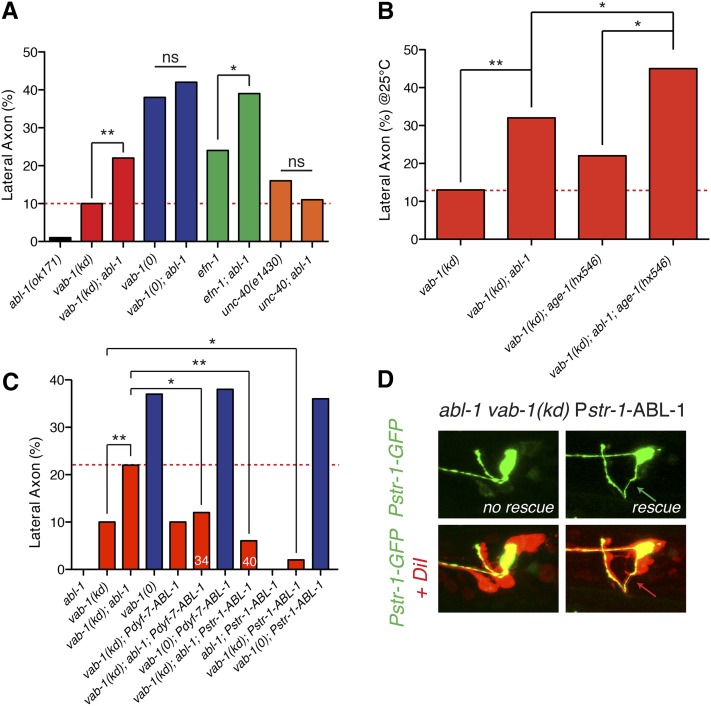
abl-1 mutants display a low penetrance (1%) lateral amphid axon phenotype and significantly enhanced vab-1(kd), but not vab-1(0) guidance defects (Figure 6A), suggesting abl-1 acts in Eph signaling but independent of the Eph kinase. abl-1unc-40 double mutants did not display genetic interactions, consistent with ABL-1 acting specifically in Eph signaling (Figure 6A). efn-1abl-1 double mutants displayed significant enhancement, suggesting that although ABL-1 acts within the Eph pathway it is not solely downstream of EFN-1 (Figure 6A). age-1abl-1 double mutants displayed normal guidance. However, the abl-1age-1vab-1(kd) triple mutant displayed 45% guidance defects, equivalent to or slightly stronger than vab-1(0) (Figure 6B). This synergistic effect in the vab-1(kd) background suggests abl-1 and age-1 act in distinct pathways in amphid guidance, which are redundant with each other and with VAB-1 kinase-dependent signals.
Figure 6.
ABL-1 signals in VAB-1-dependent amphid axon guidance. (A) Quantification of amphid axon guidance defects in abl-1 mutants. (B) Synergism of guidance defects in the vab-1(kd) abl-1 age-1 triple mutant suggests ABL-1 and PI3K act in parallel and are redundant with VAB-1 kinase signaling. (C) dyf-7 driven (panamphid) expression of ABL-1 (juEx4345) rescues abl-1 enhancement of vab-1(kd) guidance defects but not vab-1(0) defects. str-1-driven AWB-specific expression of ABL-1 (juEx5059) suppresses the vab-1(kd) abl-1(ok171) double mutant and the vab-1(kd) single mutant. (D) Rescue of guidance of multiple amphid axons by expression of ABL-1 in AWB. Images of vab-1(kd); abl-1; Pstr-1-ABL-1 kyIs104 transgenic animals with other amphid axons visualized by DiI staining. Guidance of AWB correlates with guidance of other amphid axons in nonrescued and rescued animals (N > 50 axons). Statistics, Fisher exact test: *P < 0.05; **P < 0.01; ***P < 0.001.
To determine where ABL-1 functions in amphid axon guidance, we conducted tissue- and cell-specific rescue experiments. We expressed the ABL-1 cDNA under the control of amphid-specific (dyf-7) or AWB-specific (str-1) promoters and tested for rescue of the vab-1(kd) abl-1 enhanced phenotype. Amphid-specific expression of ABL-1 rescued the vab-1(kd) abl-1 double mutant to the level of the vab-1(kd) phenotype alone, indicating ABL-1 acts in amphid neurons. Strikingly, expression of ABL-1 under the AWB-specific str-1 promoter was able to suppress vab-1(kd) abl-1 guidance defects to levels even lower than those of vab-1(kd) (Figure 6C). This suppression of vab-1 by AWB-specific ABL-1 overexpression is also consistent with ABL-1 acting in AWB downstream of VAB-1. Moreover, when we examined other amphid neurons in both the Pstr-1-ABL-1 and Pstr-1-VAB-1 rescued lines, we found that rescue of AWB guidance was almost completely correlated with rescue of other axons in the commissure (Figure 6D; vab-1 data not shown). Thus, AWB-specific expression of either ABL-1 or VAB-1 not only rescues AWB guidance but also rescues guidance of other amphid axons.
Discussion
We were interested in the roles of Eph signaling in amphid axon guidance as a simple model for kinase-independent functions of Eph receptors. Our genetic and cellular analysis suggests the role of VAB-1 signaling in amphid axons is in several respects unlike its function in other cellular contexts in C. elegans. Thus, despite expressing a single Eph receptor and a small number of ephrin ligands, C. elegans Eph signaling is highly context specific (Miller and Chin-Sang 2012).
EFN-1 is the major ephrin promoting amphid axon ventral guidance
In contrast to the previously characterized functional redundancy between EFN-1, EFN-2, and EFN-3 in embryonic morphogenesis and ventral neuroblast migration (Wang et al. 1999), only EFN-1 is required to properly guide the amphid axons and is sufficient to signal in the absence of EFN-2 and EFN-3. Unexpectedly, the efn-1,-2,-3 triple mutant fails to phenocopy the vab-1 receptor null in amphid guidance, suggesting that EFN-1,-2, and -3 do not collectively account for all VAB-1 activity. At least three explanations can be considered for this disparity between the vab-1 null phenotype and the efn-1,-2,-3 triple mutant. First, EFN ligands might antagonize one another. Removal of efn-2 appears to slightly enhance efn-1, but this effect is negated by loss of efn-3. It is possible that EFN-3 acts in an inhibitory way to disrupt the functionality of EFN-1 or EFN-2 during ephrin signaling. We also find that loss of EFN-2 or EFN-3 mildly suppressed vab-1(kd), suggesting EFN-2 and EFN-3 inhibit kinase-independent signaling. Loss of EFN-2 or EFN-3 does not affect the vab-1 null phenotype, suggesting such suppression effect requires the VAB-1 receptor.
A second possibility is that amphid axon guidance involves an additional VAB-1 ligand. However, neither the fourth ephrin EFN-4 nor the nonephrin ligand VPR-1 appear to affect amphid axon guidance. Lastly, VAB-1 could have ligand-independent activity. In cancer cells EphB4 can regulate integrin-mediated adhesion independently of ephrin stimulation (Noren et al. 2009). In axon guidance, where localized ligands give directional information, ligand-independent activity of VAB-1 might be permissive for other instructive signals such as netrins to promote ventral guidance.
Cellular sufficiency for Eph signaling in amphid axon guidance
Our tissue-specific rescue experiments suggest VAB-1 functions at least in part in the nervous system, and most likely in the amphid neurons themselves. This conclusion is consistent with our expression data and previous evidence for VAB-1 functioning in amphid neurons (George et al. 1998; Brisbin et al. 2009). Conversely, EFN-1 does not appear to be expressed or required in amphid neurons; moreover, expression of EFN-1 in amphid neurons enhances the efn-1(0) mutant phenotype. We hypothesize that this enhancement reflects a cis-inhibitory interaction of EFN-1 and VAB-1, because amphid-specific expression of EFN-1 does not enhance vab-1(0). Although we have not pinpointed the cells in which EFN-1 is expressed at the time of amphid guidance, it is noteworthy that EFN-1 is expressed in a set of ventral neurons, some of which are postsynaptic to amphid sensory neurons and whose cell bodies are close to the amphid commissure.
A caveat to our analysis is that none of our VAB-1 or EFN-1 tissue-specific transgenes fully rescued the corresponding mutant defects. As some panneural (unc-33) amphid (dyf-7) or AWB-specific (str-1) driven constructs had rescuing activity, these promoters appear to be expressed early enough to function during amphid axon guidance. We therefore suspect that the inability of transgenes to fully rescue vab-1 or efn-1 is because VAB-1 and EFN-1 are required either in multiple tissues or in a complex subset of neurons, and that panneural or panamphid expression does not sufficiently recapitulate these patterns.
VAB-1 and phosphatidylinositol 3-kinase signaling in axon guidance
PI3K signaling is a key regulator of axon guidance in many organisms. In C. elegans ventral guidance of the AVM axon depends on AGE-1/PI3K to properly respond to UNC-6/netrin and SLT-1/slit cues (Chang et al. 2006). Eph receptors can physically interact with the p85 subunit of phosphatidylinositol 3-kinase (PI3K) (Pandey et al. 1994), as well as transmit kinase-independent forward signals through a PI3K pathway (Gu and Park 2001). In C. elegans, VAB-1 directly interacts with and inhibits PTEN in axon termination (Brisbin et al. 2009). Here we have shown genetic evidence that PI3-kinase signaling contributes to amphid axon guidance and may function in a VAB-1 kinase-independent manner. vab-1(kd) phenotypes are enhanced by loss of function in PI3K and suppressed by loss of function in DAF-18/PTEN. As age-1 mutations enhance the vab-1 null mutant PI3K signals do not solely act in the VAB-1 pathway and may also contribute to UNC-40- or SAX-3-dependent guidance or neuronal polarization, as in the AVM and HSN neurons (Adler et al. 2006; Chang et al. 2006). Nevertheless given the evidence for regulation of DAF-18 by VAB-1 in amphid neurons (Brisbin et al. 2009) and the specific suppression of vab-1(kd) by daf-18, we consider the most likely model that VAB-1 promotes PI3K signaling in amphid axon guidance, either by inhibiting DAF-18 or promoting PI3K activity, or both. Our observations that loss of daf-18 function both suppresses vab-1(kd) and slightly enhances vab-1(0) guidance defects may reflect the need for a specific intermediate level of PI3K signaling: partial loss of PI3K signaling, as in vab-1(kd) mutants, may be compensated for by reduced DAF-18 activity, whereas in the context of a more complete loss of PI3K signaling as in vab-1(0), the elevated or delocalized PIP2 resulting from lack of DAF-18 function may also affect parallel pathways.
Loss of function in the PI3K target DAF-16/FOXO also suppresses vab-1 guidance defects. Unexpectedly, loss of function in PIP3-regulated kinases such as AKT-1, SGK-1, or PDK-1 also suppressed vab-1(kd) defects. The downstream mechanisms of PI3K signal transduction in axon guidance may therefore differ from those involved in dauer formation or aging.
The role of ABL-1 in VAB-1 signaling
We have shown that the Src family tyrosine kinase ABL-1 promotes amphid axon guidance, likely downstream of VAB-1 signaling. So far, abl-1 has only been indirectly implicated in C. elegans axon guidance (Fox et al. 2005); our results provide functional evidence for this role. Interestingly, in mammalian neurons Abl has been shown to mediate EphA-dependent axon repulsion (Harbott and Nobes 2005). The simplest interpretation of our findings in C. elegans is that ABL-1 promotes attractive responses to guidance cues. ABL-1 appears to be required in a kinase-independent and EFN-1-independent branch of VAB-1 signaling. ABL-1 might be activated by a nonephrin ligand, or by a ligand-independent activity of VAB-1 as discussed above. In mammalian cells Abl can interact with Eph receptors in multiple ways. In breast cancers, EphB4 can directly interact with Abl, dependent on ligand and kinase activity (Noren et al. 2006). Eph receptors can also interact with Abl independent of Eph kinase activity through an interaction mediated by the C-terminal tail of Abl (Yu et al. 2001). Although the ABL-1 SH2 domain did not interact with the kinase domain of VAB-1 in two-hybrid assays (Mohamed et al. 2012), ABL-1 might interact with VAB-1 indirectly, or via one of the other modes described by Yu et al. (2001). Further work will be required to determine the mechanism by which ABL-1 might mediate VAB-1 signals.
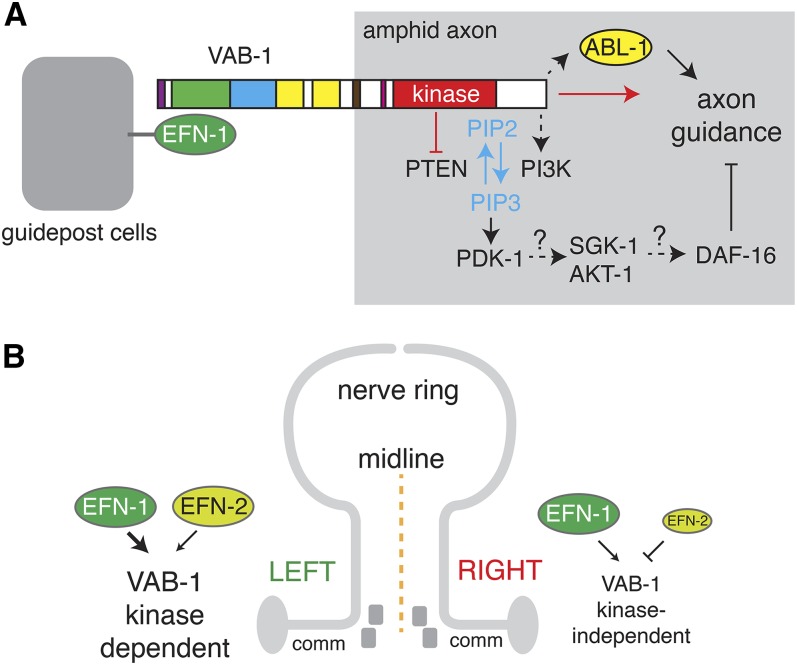
Our finding that PI3K and ABL-1 act as parallel outputs in VAB-1-mediated axon guidance (Figure 7A) is interesting in light of the finding that EphB2 signaling in intestinal stem cells also involves parallel PI3K and Abl signals (Genander et al. 2009). EphB2 regulates cell positioning in a kinase-independent pathway via PI3K and regulates cell proliferation via a kinase-dependent Abl pathway. Although our results are more compatible with ABL-1 functioning in a kinase-independent forward signaling pathway, these comparisons suggest Eph signaling operates via a small number of signaling cascades whose outputs are ultimately cell-type specific.
Figure 7.
Models of Eph signaling in amphid axon guidance. (A) Possible relationships between EFN-1, VAB-1, and the PI3K and ABL-1 pathways in axon guidance. (B) Model for the left–right bias in Eph signaling. Amphid guidance on the left side of the animal is mediated by a VAB-1 kinase-dependent signaling pathway. In the presence of EFN-1, EFN-2 inhibits ventral guidance. In the absence of EFN-1, EFN-2 can promote signaling. The right side relies mostly on a kinase-independent signaling mechanism that is inhibited by EFN-2.
Eph signaling has unexpected left–right asymmetry in axon guidance
It is now well established that amphid neurons display extensive left–right asymmetry in function and in structure. Such asymmetry can be stochastic, as in AWC receptor expression (Troemel et al. 1997), or biased, as in ASE receptor expression, sensory function, and axon diameter (Bargmann and Horvitz 1991; Pierce-Shimomura et al. 2001; Chang et al. 2003; Goldsmith et al. 2010). Left–right asymmetry in axon outgrowth has been reported in AIY amphid interneurons (Bertrand et al. 2011). Our findings are the first evidence that amphid neurons also display biased asymmetry in their axon guidance.
vab-1 and efn mutants display a consistent and significant left-hand bias in ventral guidance defects. We observed this bias in all amphid neuron classes examined. The inability of the ASEL–ASER left-to-right fate transformation in lsy-6 mutants to alter this bias is suggestive that the bias reflects an asymmetric signaling environment, although it remains possible that lsy-6 mutants fail to transform the relevant intrinsic aspects of the ASEL fate. Consistent with this, we find that at least one ephrin, EFN-2, displays highly asymmetric expression in embryonic neurons. Although these studies do not directly address the signaling environment at the time of amphid axon guidance, they are consistent with the idea that axon guidance cues can be highly left–right asymmetric. As vab-1 null mutants also display asymmetric defects, it appears that other nonephrin cues must also be asymmetric and that this asymmetry is revealed in the absence of VAB-1. However no obvious bias has been reported in the expression or function of the two other main pathways in amphid axon guidance, UNC-6 netrin/UNC-40 and SAX-3/Robo.
Our results also imply a differential reliance on kinase-dependent or kinase-independent signaling on the left and right sides (Figure 7B). As >90% of aberrantly guided axons in vab-1(kd) mutants are on the left, the kinase-dependent pathway seems to be most important in left-side guidance. Loss of efn-2 improves left-hand guidance in vab-1(kd) mutants, indicating EFN-2 is inhibitory in this context. This inhibitory effect might reflect the expression of EFN-2 in multiple left-hand amphid neurons, where it could inhibit VAB-1 signaling in cis in a manner similar to EFN-1. However in animals lacking EFN-1, loss of EFN-2 exacerbates left-hand guidance defects and improves right-hand defects. In the absence of EFN-1, EFN-2 may play a positive role in left-hand guidance, as it is also asymmetrically expressed in AIYL. EFN-2 does not appear to be expressed in right-hand amphid neurons but could influence guidance from nearby asymmetric sites of expression such as the IL neurons. An interesting possibility is that loss of function in EFN-2 suppresses ventral guidance defects because EFN-2 is involved in promoting guidance to the lateral path. In this model, EFN-2 and EFN-1 act as competing cues for the lateral and ventral paths.
Axons in the amphid commissure may be guided by pioneer neurons
A prime question in analyzing guidance of an axon bundle such as the amphid commissure is the extent to which axons are guided independently or by fasciculation with pioneer axons (Lee et al. 1997). The all-or-nothing nature of amphid commissure guidance defects has been previously noted (Bülow et al. 2002) and suggests amphid axons might follow single pioneer axons. Our analysis extends this picture in that expression of VAB-1 or ABL-1 in a single neuron, AWB, can not only cell autonomously rescue guidance of that neuron, but also may nonautonomously rescue the guidance of other amphid neurons. At present we cannot exclude the possibility that the str-1 promoter is more widely expressed in amphid neurons early in development; our attempts to use other amphid neuron-specific promoters to rescue amphid guidance were unsuccessful for technical reasons. It is possible that AWB is normally a pioneer axon whose guidance determines the direction of the commissure. Alternatively, VAB-1 or ABL-1 overexpression may sensitize or otherwise give a growth advantage to AWB such that it is able to assume a pioneer function that it does not normally have. An important future goal will be to examine the dynamics of amphid axon outgrowth in these genetic backgrounds.
Supplementary Material
Acknowledgments
We thank Max Heiman, Joe Culotti, and Ian Chin-Sang for reagents; Scott Clark, Crystal Lee, and Martin Hudson for new vab-1 alleles; Asim Alam for characterizing vab-1 alleles; and Nese Cinar, Jennifer Gotenstein, and Andy Nguyen for help with strain constructions and scoring amphid guidance. Emily Troemel, Elena Pasquale, Zhiping Wang, and Naina Kurup made helpful comments on the manuscript. We thank Sukryool Alan Kang for help with NucleiTracker4D software. Deletion mutations were generated by the C. elegans Gene Knockout Consortium and the Japan National Bioresource Project. Some mutations were provided by the Caenorhabditis Genetics Center, which is funded by the National Institutes of Health (NIH) Office of Research Infrastructure Programs (P40 OD010440). E.N.G. was supported by the University of California, San Diego (UCSD)/Salk Training Grant in Developmental Biology of Neural Diseases (T32 HD007495). C.A.G. was supported by the UCSD Neural Circuits Postdoctoral Training Program (T32 NS007220) and by a Ruth S. Kirschstein National Research Service Award (F32 GM090652). This work was supported by an award from the US Public Health Service (NIH R01 GM054657) to A.D.C.
Footnotes
Communicating editor: M. Sundaram
Literature Cited
- Adler C. E., Fetter R. D., Bargmann C. I., 2006. UNC-6/Netrin induces neuronal asymmetry and defines the site of axon initiation. Nat. Neurosci. 9: 511–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altun-Gultekin Z., Andachi Y., Tsalik E. L., Pilgrim D., Kohara Y., et al. , 2001. A regulatory cascade of three homeobox genes, ceh-10, ttx-3 and ceh-23, controls cell fate specification of a defined interneuron class in C. elegans. Development 128: 1951–1969 [DOI] [PubMed] [Google Scholar]
- Bargmann C. I., Horvitz H. R., 1991. Chemosensory neurons with overlapping functions direct chemotaxis to multiple chemicals in C. elegans. Neuron 7: 729–742 [DOI] [PubMed] [Google Scholar]
- Bertrand V., Bisso P., Poole R. J., Hobert O., 2011. Notch-dependent induction of left/right asymmetry in C. elegans interneurons and motoneurons. Curr. Biol. 21: 1225–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonanomi D., Chivatakarn O., Bai G., Abdesselem H., Lettieri K., et al. , 2012. Ret is a multifunctional coreceptor that integrates diffusible- and contact-axon guidance signals. Cell 148: 568–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulin T., Pocock R., Hobert O., 2006. A novel Eph receptor-interacting IgSF protein provides C. elegans motoneurons with midline guidepost function. Curr. Biol. 16: 1871–1883 [DOI] [PubMed] [Google Scholar]
- Brisbin S., Liu J., Boudreau J., Peng J., Evangelista M., et al. , 2009. A role for C. elegans Eph RTK signaling in PTEN regulation. Dev. Cell 17: 459–469 [DOI] [PubMed] [Google Scholar]
- Bruckner K., Pablo Labrador J., Scheiffele P., Herb A., Seeburg P. H., et al. , 1999. EphrinB ligands recruit GRIP family PDZ adaptor proteins into raft membrane microdomains. Neuron 22: 511–524 [DOI] [PubMed] [Google Scholar]
- Bülow H. E., Berry K. L., Topper L. H., Peles E., Hobert O., 2002. Heparan sulfate proteoglycan-dependent induction of axon branching and axon misrouting by the Kallmann syndrome gene kal-1. Proc. Natl. Acad. Sci. USA 99: 6346–6351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho R. F., Beutler M., Marler K. J., Knoll B., Becker-Barroso E., et al. , 2006. Silencing of EphA3 through a cis interaction with ephrinA5. Nat. Neurosci. 9: 322–330 [DOI] [PubMed] [Google Scholar]
- Catchpole T., Henkemeyer M., 2011. EphB2 tyrosine kinase-dependent forward signaling in migration of neuronal progenitors that populate and form a distinct region of the dentate niche. J. Neurosci. 31: 11472–11483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan S. S., Zheng H., Su M. W., Wilk R., Killeen M. T., et al. , 1996. UNC-40, a C. elegans homolog of DCC (Deleted in Colorectal Cancer), is required in motile cells responding to UNC-6 netrin cues. Cell 87: 187–195 [DOI] [PubMed] [Google Scholar]
- Chang C., Adler C. E., Krause M., Clark S. G., Gertler F. B., et al. , 2006. MIG-10/lamellipodin and AGE-1/PI3K promote axon guidance and outgrowth in response to slit and netrin. Curr. Biol. 16: 854–862 [DOI] [PubMed] [Google Scholar]
- Chang S., Johnston R. J., Jr, Hobert O., 2003. A transcriptional regulatory cascade that controls left/right asymmetry in chemosensory neurons of C. elegans. Genes Dev. 17: 2123–2137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H., Govindan J. A., Greenstein D., 2008. Regulated trafficking of the MSP/Eph receptor during oocyte meiotic maturation in C. elegans. Curr. Biol. 18: 705–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin-Sang I. D., George S. E., Ding M., Moseley S. L., Lynch A. S., et al. , 1999. The ephrin VAB-2/EFN-1 functions in neuronal signaling to regulate epidermal morphogenesis in C. elegans. Cell 99: 781–790 [DOI] [PubMed] [Google Scholar]
- Chin-Sang I. D., Moseley S. L., Ding M., Harrington R. J., George S. E., et al. , 2002. The divergent C. elegans ephrin EFN-4 functions inembryonic morphogenesis in a pathway independent of the VAB-1 Eph receptor. Development 129: 5499–5510 [DOI] [PubMed] [Google Scholar]
- Christensen R., de la Torre-Ubieta L., Bonni A., Colon-Ramos D. A., 2011. A conserved PTEN/FOXO pathway regulates neuronal morphology during C. elegans development. Development 138: 5257–5267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan C. A., Yokoyama N., Bianchi L. M., Henkemeyer M., Fritzsch B., 2000. EphB2 guides axons at the midline and is necessary for normal vestibular function. Neuron 26: 417–430 [DOI] [PubMed] [Google Scholar]
- Davy A., Gale N. W., Murray E. W., Klinghoffer R. A., Soriano P., et al. , 1999. Compartmentalized signaling by GPI-anchored ephrin-A5 requires the Fyn tyrosine kinase to regulate cellular adhesion. Genes Dev. 13: 3125–3135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drescher U., Kremoser C., Handwerker C., Loschinger J., Noda M., et al. , 1995. In vitro guidance of retinal ganglion cell axons by RAGS, a 25 kDa tectal protein related to ligands for Eph receptor tyrosine kinases. Cell 82: 359–370 [DOI] [PubMed] [Google Scholar]
- Egea J., Klein R., 2007. Bidirectional Eph-ephrin signaling during axon guidance. Trends Cell Biol. 17: 230–238 [DOI] [PubMed] [Google Scholar]
- Finney M., Ruvkun G., 1990. The unc-86 gene product couples cell lineage and cell identity in C. elegans. Cell 63: 895–905 [DOI] [PubMed] [Google Scholar]
- Fox R. M., Von Stetina S. E., Barlow S. J., Shaffer C., Olszewski K. L., et al. , 2005. A gene expression fingerprint of C. elegans embryonic motor neurons. BMC Genomics 6: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frøkjaer-Jensen C., Kindt K. S., Kerr R. A., Suzuki H., Melnik-Martinez K., et al. , 2006. Effects of voltage-gated calcium channel subunit genes on calcium influx in cultured C. elegans mechanosensory neurons. J. Neurobiol. 66: 1125–1139 [DOI] [PubMed] [Google Scholar]
- Genander M., Halford M. M., Xu N. J., Eriksson M., Yu Z., et al. , 2009. Dissociation of EphB2 signaling pathways mediating progenitor cell proliferation and tumor suppression. Cell 139: 679–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George S. E., Simokat K., Hardin J., Chisholm A. D., 1998. The VAB-1 Eph receptor tyrosine kinase functions in neural and epithelial morphogenesis in C. elegans. Cell 92: 633–643 [DOI] [PubMed] [Google Scholar]
- Giurumescu C. A., Kang S., Planchon T. A., Betzig E., Bloomekatz J., et al. , 2012. Quantitative semi-automated analysis of morphogenesis with single-cell resolution in complex embryos. Development 139: 4271–4279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith A. D., Sarin S., Lockery S., Hobert O., 2010. Developmental control of lateralized neuron size in the nematode Caenorhabditis elegans. Neural Dev. 5: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu C., Park S., 2001. The EphA8 receptor regulates integrin activity through p110ϒ phosphatidylinositol-3 kinase in a tyrosine kinase activity-independent manner. Mol. Cell. Biol. 21: 4579–4597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu C., Park S., 2003. The p110 gamma PI-3 kinase is required for EphA8-stimulated cell migration. FEBS Lett. 540: 65–70 [DOI] [PubMed] [Google Scholar]
- Hansen M. J., Dallal G. E., Flanagan J. G., 2004. Retinal axon response to ephrin-as shows a graded, concentration-dependent transition from growth promotion to inhibition. Neuron 42: 717–730 [DOI] [PubMed] [Google Scholar]
- Harbott L. K., Nobes C. D., 2005. A key role for Abl family kinases in EphA receptor-mediated growth cone collapse. Mol. Cell. Neurosci. 30: 1–11 [DOI] [PubMed] [Google Scholar]
- Hedgecock E. M., Culotti J. G., Thomson J. N., Perkins L. A., 1985. Axonal guidance mutants of Caenorhabditis elegans identified by filling sensory neurons with fluorescein dyes. Dev. Biol. 111: 158–170 [DOI] [PubMed] [Google Scholar]
- Heiman M. G., Shaham S., 2009. DEX-1 and DYF-7 establish sensory dendrite length by anchoring dendritic tips during cell migration. Cell 137: 344–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertweck M., Gobel C., Baumeister R., 2004. C. elegans SGK-1 is the critical component in the Akt/PKB kinase complex to control stress response and life span. Dev. Cell 6: 577–588 [DOI] [PubMed] [Google Scholar]
- Hindges R., McLaughlin T., Genoud N., Henkemeyer M., O’Leary D. D., 2002. EphB forward signaling controls directional branch extension and arborization required for dorsal-ventral retinotopic mapping. Neuron 35: 475–487 [DOI] [PubMed] [Google Scholar]
- Hobert O., Mori I., Yamashita Y., Honda H., Ohshima Y., et al. , 1997. Regulation of interneuron function in the C. elegans thermoregulatory pathway by the ttx-3 LIM homeobox gene. Neuron 19: 345–357 [DOI] [PubMed] [Google Scholar]
- Hobert O., Johnston R. J., Jr, Chang S., 2002. Left-right asymmetry in the nervous system: the Caenorhabditis elegans model. Nat. Rev. Neurosci. 3: 629–640 [DOI] [PubMed] [Google Scholar]
- Ikegami R., Zheng H., Ong S. H., Culotti J., 2004. Integration of semaphorin-2A/MAB-20, ephrin-4, and UNC-129 TGF-beta signaling pathways regulates sorting of distinct sensory rays in C. elegans. Dev. Cell 6: 383–395 [DOI] [PubMed] [Google Scholar]
- Ikegami R., Simokat K., Zheng H., Brown L., Garriga G., et al. , 2012. Semaphorin and Eph receptor signaling guide a series of cell movements for ventral enclosure in C. elegans. Curr. Biol. 22: 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. J., Hobert O., 2003. A microRNA controlling left/right neuronal asymmetry in Caenorhabditis elegans. Nature 426: 845–849 [DOI] [PubMed] [Google Scholar]
- Knoll B., Drescher U., 2004. Src family kinases are involved in EphA receptor-mediated retinal axon guidance. J. Neurosci. 24: 6248–6257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullander K., Klein R., 2002. Mechanisms and functions of Eph and ephrin signalling. Nat. Rev. Mol. Cell Biol. 3: 475–486 [DOI] [PubMed] [Google Scholar]
- Labouesse M., Sookhareea S., Horvitz H. R., 1994. The Caenorhabditis elegans gene lin-26 is required to specify the fates of hypodermal cells and encodes a presumptive zinc-finger transcription factor. Development 120: 2359–2368 [DOI] [PubMed] [Google Scholar]
- Lee R. Y., Lobel L., Hengartner M., Horvitz H. R., Avery L., 1997. Mutations in the alpha1 subunit of an L-type voltage-activated Ca2+ channel cause myotonia in Caenorhabditis elegans. EMBO J. 16: 6066–6076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim Y. S., McLaughlin T., Sung T. C., Santiago A., Lee K. F., et al. , 2008. p75(NTR) mediates ephrin-A reverse signaling required for axon repulsion and mapping. Neuron 59: 746–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin K., Dorman J. B., Rodan A., Kenyon C., 1997. daf-16: an HNF-3/forkhead family member that can function to double the life-span of Caenorhabditis elegans. Science 278: 1319–1322 [DOI] [PubMed] [Google Scholar]
- Maduro M., Pilgrim D., 1995. Identification and cloning of unc-119, a gene expressed in the Caenorhabditis elegans nervous system. Genetics 141: 977–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning G., 2005. Genomic overview of protein kinases (December 13, 2005), WormBook, ed. The C. elegansResearch Community, WormBook, /10.1895/wormbook.1.60.1,http://www.wormbook.org [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marler K. J., Becker-Barroso E., Martinez A., Llovera M., Wentzel C., et al. , 2008. A TrkB/EphrinA interaction controls retinal axon branching and synaptogenesis. J. Neurosci. 28: 12700–12712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M. A., Chin-Sang I. D., 2012. Eph receptor signaling in C. elegans (November 29, 2012), WormBook, ed. The C. elegansResearch Community, WormBook, /10.1895/wormbook.1.151.1,http://www.wormbook.org [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M. A., Ruest P. J., Kosinski M., Hanks S. K., Greenstein D., 2003. An Eph receptor sperm-sensing control mechanism for oocyte meiotic maturation in Caenorhabditis elegans. Genes Dev. 17: 187–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed A. M., Chin-Sang I. D., 2006. Characterization of loss-of-function and gain-of-function Eph receptor tyrosine kinase signaling in C. elegans axon targeting and cell migration. Dev. Biol. 290: 164–176 [DOI] [PubMed] [Google Scholar]
- Mohamed A. M., Boudreau J. R., Yu F. P., Liu J., Chin-Sang I. D., 2012. The Caenorhabditis elegans Eph receptor activates NCK and N-WASP, and inhibits Ena/VASP to regulate growth cone dynamics during axon guidance. PLoS Genet. 8: e1002513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noren N. K., Pasquale E. B., 2004. Eph receptor-ephrin bidirectional signals that target Ras and Rho proteins. Cell. Signal. 16: 655–666 [DOI] [PubMed] [Google Scholar]
- Noren N. K., Foos G., Hauser C. A., Pasquale E. B., 2006. The EphB4 receptor suppresses breast cancer cell tumorigenicity through an Abl-Crk pathway. Nat. Cell Biol. 8: 815–825 [DOI] [PubMed] [Google Scholar]
- Noren N. K., Yang N. Y., Silldorff M., Mutyala R., Pasquale E. B., 2009. Ephrin-independent regulation of cell substrate adhesion by the EphB4 receptor. Biochem. J. 422: 433–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogg S., Ruvkun G., 1998. The C. elegans PTEN homolog, DAF-18, acts in the insulin receptor-like metabolic signaling pathway. Mol. Cell 2: 887–893 [DOI] [PubMed] [Google Scholar]
- Ogg S., Paradis S., Gottlieb S., Patterson G. I., Lee L., et al. , 1997. The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature 389: 994–999 [DOI] [PubMed] [Google Scholar]
- Pandey A., Lazar D. F., Saltiel A. R., Dixit V. M., 1994. Activation of the Eck receptor protein tyrosine kinase stimulates phosphatidylinositol 3-kinase activity. J. Biol. Chem. 269: 30154–30157 [PubMed] [Google Scholar]
- Paradis S., Ruvkun G., 1998. Caenorhabditis elegans Akt/PKB transduces insulin receptor-like signals from AGE-1 PI3 kinase to the DAF-16 transcription factor. Genes Dev. 12: 2488–2498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradis S., Ailion M., Toker A., Thomas J. H., Ruvkun G., 1999. A PDK1 homolog is necessary and sufficient to transduce AGE-1 PI3 kinase signals that regulate diapause in Caenorhabditis elegans. Genes Dev. 13: 1438–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquale E. B., 2005. Eph receptor signalling casts a wide net on cell behaviour. Nat. Rev. Mol. Cell Biol. 6: 462–475 [DOI] [PubMed] [Google Scholar]
- Pasquale E. B., 2008. Eph-ephrin bidirectional signaling in physiology and disease. Cell 133: 38–52 [DOI] [PubMed] [Google Scholar]
- Pierce-Shimomura J. T., Faumont S., Gaston M. R., Pearson B. J., Lockery S. R., 2001. The homeobox gene lim-6 is required for distinct chemosensory representations in C. elegans. Nature 410: 694–698 [DOI] [PubMed] [Google Scholar]
- Sahin M., Greer P. L., Lin M. Z., Poucher H., Eberhart J., et al. , 2005. Eph-dependent tyrosine phosphorylation of ephexin1 modulates growth cone collapse. Neuron 46: 191–204 [DOI] [PubMed] [Google Scholar]
- Satterlee J. S., Sasakura H., Kuhara A., Berkeley M., Mori I., et al. , 2001. Specification of thermosensory neuron fate in C. elegans requires ttx-1, a homolog of otd/Otx. Neuron 31: 943–956 [DOI] [PubMed] [Google Scholar]
- Shamah S. M., Lin M. Z., Goldberg J. L., Estrach S., Sahin M., et al. , 2001. EphA receptors regulate growth cone dynamics through the novel guanine nucleotide exchange factor ephexin. Cell 105: 233–244 [DOI] [PubMed] [Google Scholar]
- Shin J., Gu C., Park E., Park S., 2007. Identification of phosphotyrosine binding domain-containing proteins as novel downstream targets of the EphA8 signaling function. Mol. Cell. Biol. 27: 8113–8126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A., Robinson V., Patel K., Wilkinson D. G., 1997. The EphA4 and EphB1 receptor tyrosine kinases and ephrin-B2 ligand regulate targeted migration of branchial neural crest cells. Curr. Biol. 7: 561–570 [DOI] [PubMed] [Google Scholar]
- Sulston J. E., Schierenberg E., White J. G., Thomson J. N., 1983. The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev. Biol. 100: 64–119 [DOI] [PubMed] [Google Scholar]
- Troemel E. R., Kimmel B. E., Bargmann C. I., 1997. Reprogramming chemotaxis responses: sensory neurons define olfactory preferences in C. elegans. Cell 91: 161–169 [DOI] [PubMed] [Google Scholar]
- Wahl S., Barth H., Ciossek T., Aktories K., Mueller B. K., 2000. Ephrin-A5 induces collapse of growth cones by activating Rho and Rho kinase. J. Cell Biol. 149: 263–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Roy P. J., Holland S. J., Zhang L. W., Culotti J. G., et al. , 1999. Multiple ephrins control cell organization in C. elegans using kinase-dependent and -independent functions of the VAB-1 Eph receptor. Mol. Cell 4: 903–913 [DOI] [PubMed] [Google Scholar]
- Yokoyama N., Romero M. I., Cowan C. A., Galvan P., Helmbacher F., et al. , 2001. Forward signaling mediated by ephrin-B3 prevents contralateral corticospinal axons from recrossing the spinal cord midline. Neuron 29: 85–97 [DOI] [PubMed] [Google Scholar]
- Yoshimura S., Murray J. I., Lu Y., Waterston R. H., Shaham S., 2008. mls-2 and vab-3 control glia development, hlh-17/Olig expression and glia-dependent neurite extension in C. elegans. Development 135: 2263–2275 [DOI] [PubMed] [Google Scholar]
- Yu H. H., Zisch A. H., Dodelet V. C., Pasquale E. B., 2001. Multiple signaling interactions of Abl and Arg kinases with the EphB2 receptor. Oncogene 20: 3995–4006 [DOI] [PubMed] [Google Scholar]
- Zallen J. A., Yi B. A., Bargmann C. I., 1998. The conserved immunoglobulin superfamily member SAX-3/Robo directs multiple aspects of axon guidance in C. elegans. Cell 92: 217–227 [DOI] [PubMed] [Google Scholar]
- Zallen J. A., Kirch S. A., Bargmann C. I., 1999. Genes required for axon pathfinding and extension in the C. elegans nerve ring. Development 126: 3679–3692 [DOI] [PubMed] [Google Scholar]
- Zisch A. H., Kalo M. S., Chong L. D., Pasquale E. B., 1998. Complex formation between EphB2 and Src requires phosphorylation of tyrosine 611 in the EphB2 juxtamembrane region. Oncogene 16: 2657–2670 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.