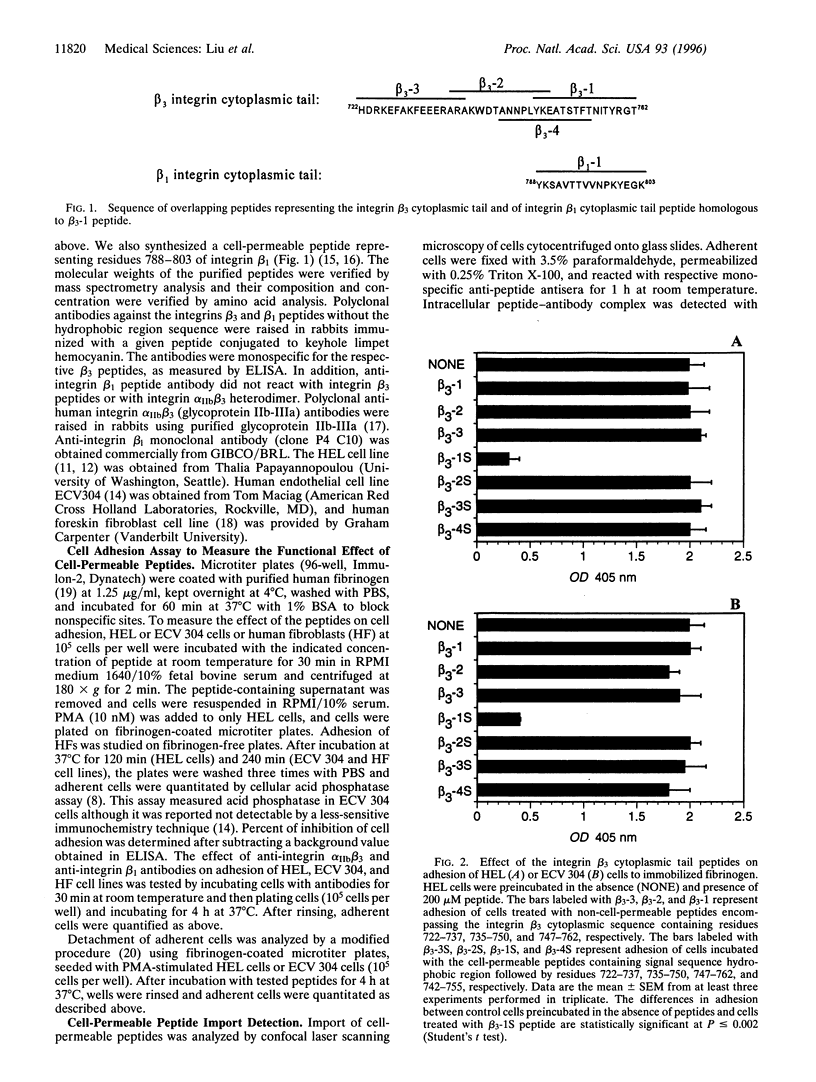
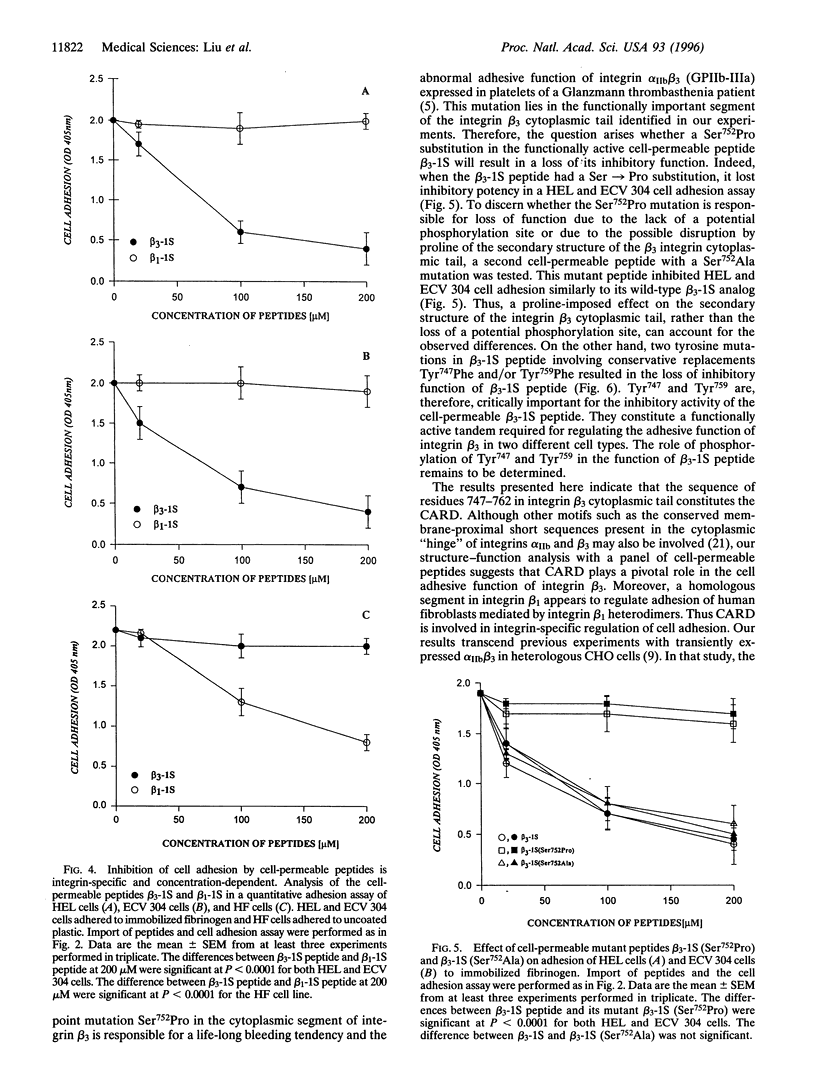
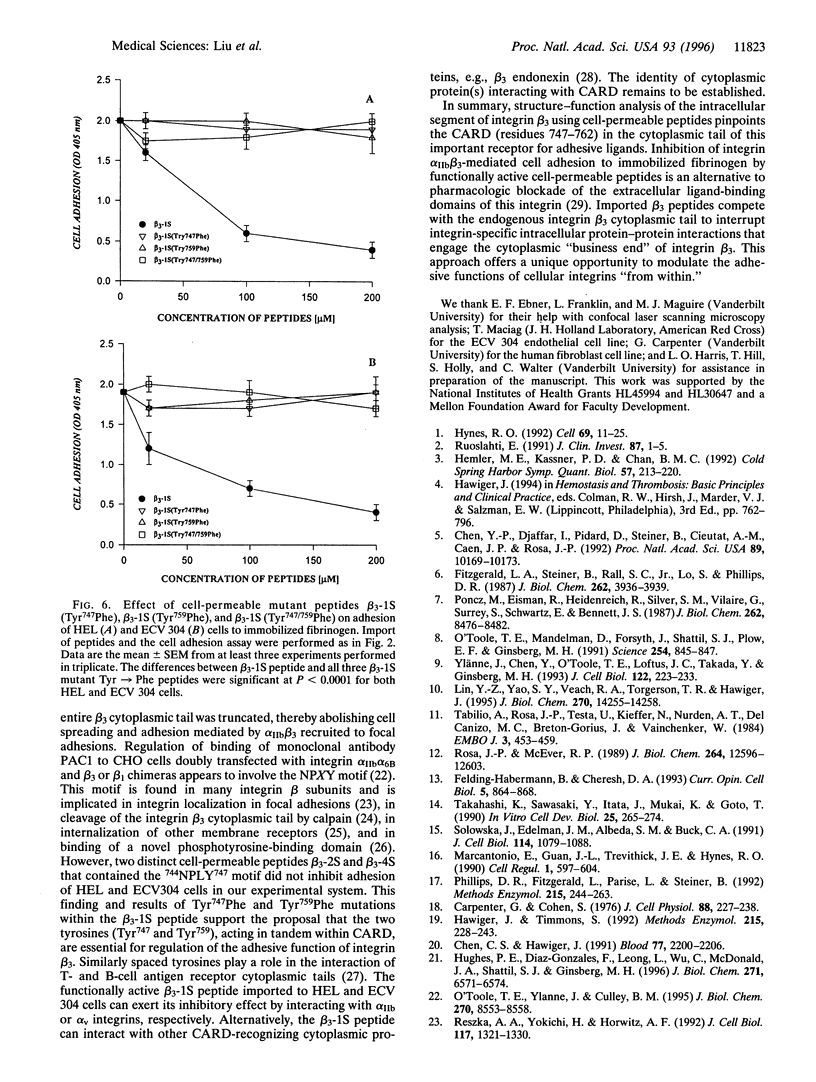
Abstract
Integrins are major two-way signaling receptors responsible for the attachment of cells to the extracellular matrix and for cell-cell interactions that underlie immune responses, tumor metastasis, and progression of atherosclerosis and thrombosis. We report the structure-function analysis of the cytoplasmic tail of integrin beta 3 (glycoprotein IIla) based on the cellular import of synthetic peptide analogs of this region. Among the four overlapping cell-permeable peptides, only the peptide carrying residues 747-762 of the carboxyl-terminal segment of integrin beta 3 inhibited adhesion of human erythroleukemia (HEL) cells and of human endothelial cells (ECV) 304 to immobilized fibrinogen mediated by integrin beta 3 heterodimers, alpha IIb beta 3, and alpha v beta 3, respectively. Inhibition of adhesion was integrin-specific because the cell-permeable beta 3 peptide (residues 747-762) did not inhibit adhesion of human fibroblasts mediated by integrin beta 1 heterodimers. Conversely, a cell-permeable peptide representing homologous portion of the integrin beta 1 cytoplasmic tail (residues 788-803) inhibited adhesion of human fibroblasts, whereas it was without effect on adhesion of HEL or ECV 304 cells. The cell-permeable integrin beta 3 peptide (residues 747-762) carrying a known loss-of-function mutation (Ser752Pro) responsible for the genetic disorder Glanzmann thrombasthenia Paris I did not inhibit cell adhesion of HEL or ECV 304 cells, whereas the beta 3 peptide carrying a Ser752Ala mutation was inhibitory. Although Ser752 is not essential, Tyr747 and Tyr759 form a functionally active tandem because conservative mutations Tyr747Phe or Tyr759Phe resulted in a nonfunctional cell permeable integrin beta 3 peptide. We propose that the carboxyl-terminal segment of the integrin beta 3 cytoplasmic tail spanning residues 747-762 constitutes a major intracellular cell adhesion regulatory domain (CARD) that modulates the interaction of integrin beta 3-expressing cells with immobilized fibrinogen. Import of cell-permeable peptides carrying this domain results in inhibition "from within" of the adhesive function of these integrins.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carpenter G., Cohen S. Human epidermal growth factor and the proliferation of human fibroblasts. J Cell Physiol. 1976 Jun;88(2):227–237. doi: 10.1002/jcp.1040880212. [DOI] [PubMed] [Google Scholar]
- Chen C. S., Hawiger J. Reactivity of synthetic peptide analogs of adhesive proteins in regard to the interaction of human endothelial cells with extracellular matrix. Blood. 1991 May 15;77(10):2200–2206. [PubMed] [Google Scholar]
- Chen W. J., Goldstein J. L., Brown M. S. NPXY, a sequence often found in cytoplasmic tails, is required for coated pit-mediated internalization of the low density lipoprotein receptor. J Biol Chem. 1990 Feb 25;265(6):3116–3123. [PubMed] [Google Scholar]
- Chen Y. P., Djaffar I., Pidard D., Steiner B., Cieutat A. M., Caen J. P., Rosa J. P. Ser-752-->Pro mutation in the cytoplasmic domain of integrin beta 3 subunit and defective activation of platelet integrin alpha IIb beta 3 (glycoprotein IIb-IIIa) in a variant of Glanzmann thrombasthenia. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10169–10173. doi: 10.1073/pnas.89.21.10169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X., Saido T. C., Tsubuki S., Indig F. E., Williams M. J., Ginsberg M. H. Calpain cleavage of the cytoplasmic domain of the integrin beta 3 subunit. J Biol Chem. 1995 Nov 3;270(44):26146–26151. doi: 10.1074/jbc.270.44.26146. [DOI] [PubMed] [Google Scholar]
- Felding-Habermann B., Cheresh D. A. Vitronectin and its receptors. Curr Opin Cell Biol. 1993 Oct;5(5):864–868. doi: 10.1016/0955-0674(93)90036-p. [DOI] [PubMed] [Google Scholar]
- Fitzgerald L. A., Steiner B., Rall S. C., Jr, Lo S. S., Phillips D. R. Protein sequence of endothelial glycoprotein IIIa derived from a cDNA clone. Identity with platelet glycoprotein IIIa and similarity to "integrin". J Biol Chem. 1987 Mar 25;262(9):3936–3939. [PubMed] [Google Scholar]
- Hawiger J. Adhesive ends of fibrinogen and its antiadhesive peptides: the end of a saga? Semin Hematol. 1995 Apr;32(2):99–109. [PubMed] [Google Scholar]
- Hawiger J., Timmons S. Binding of fibrinogen and von Willebrand factor to platelet glycoprotein IIb-IIIa complex. Methods Enzymol. 1992;215:228–243. doi: 10.1016/0076-6879(92)15067-m. [DOI] [PubMed] [Google Scholar]
- Hemler M. E., Kassner P. D., Chan B. M. Functional roles for integrin alpha subunit cytoplasmic domains. Cold Spring Harb Symp Quant Biol. 1992;57:213–220. doi: 10.1101/sqb.1992.057.01.026. [DOI] [PubMed] [Google Scholar]
- Hughes P. E., Diaz-Gonzalez F., Leong L., Wu C., McDonald J. A., Shattil S. J., Ginsberg M. H. Breaking the integrin hinge. A defined structural constraint regulates integrin signaling. J Biol Chem. 1996 Mar 22;271(12):6571–6574. doi: 10.1074/jbc.271.12.6571. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Isakov N., Wange R. L., Burgess W. H., Watts J. D., Aebersold R., Samelson L. E. ZAP-70 binding specificity to T cell receptor tyrosine-based activation motifs: the tandem SH2 domains of ZAP-70 bind distinct tyrosine-based activation motifs with varying affinity. J Exp Med. 1995 Jan 1;181(1):375–380. doi: 10.1084/jem.181.1.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y. Z., Yao S. Y., Veach R. A., Torgerson T. R., Hawiger J. Inhibition of nuclear translocation of transcription factor NF-kappa B by a synthetic peptide containing a cell membrane-permeable motif and nuclear localization sequence. J Biol Chem. 1995 Jun 16;270(24):14255–14258. doi: 10.1074/jbc.270.24.14255. [DOI] [PubMed] [Google Scholar]
- Marcantonio E. E., Guan J. L., Trevithick J. E., Hynes R. O. Mapping of the functional determinants of the integrin beta 1 cytoplasmic domain by site-directed mutagenesis. Cell Regul. 1990 Jul;1(8):597–604. doi: 10.1091/mbc.1.8.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Toole T. E., Mandelman D., Forsyth J., Shattil S. J., Plow E. F., Ginsberg M. H. Modulation of the affinity of integrin alpha IIb beta 3 (GPIIb-IIIa) by the cytoplasmic domain of alpha IIb. Science. 1991 Nov 8;254(5033):845–847. doi: 10.1126/science.1948065. [DOI] [PubMed] [Google Scholar]
- O'Toole T. E., Ylanne J., Culley B. M. Regulation of integrin affinity states through an NPXY motif in the beta subunit cytoplasmic domain. J Biol Chem. 1995 Apr 14;270(15):8553–8558. doi: 10.1074/jbc.270.15.8553. [DOI] [PubMed] [Google Scholar]
- Phillips D. R., Fitzgerald L., Parise L., Steiner B. Platelet membrane glycoprotein IIb-IIIa complex: purification, characterization, and reconstitution into phospholipid vesicles. Methods Enzymol. 1992;215:244–263. doi: 10.1016/0076-6879(92)15068-n. [DOI] [PubMed] [Google Scholar]
- Poncz M., Eisman R., Heidenreich R., Silver S. M., Vilaire G., Surrey S., Schwartz E., Bennett J. S. Structure of the platelet membrane glycoprotein IIb. Homology to the alpha subunits of the vitronectin and fibronectin membrane receptors. J Biol Chem. 1987 Jun 25;262(18):8476–8482. [PubMed] [Google Scholar]
- Reszka A. A., Hayashi Y., Horwitz A. F. Identification of amino acid sequences in the integrin beta 1 cytoplasmic domain implicated in cytoskeletal association. J Cell Biol. 1992 Jun;117(6):1321–1330. doi: 10.1083/jcb.117.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa J. P., McEver R. P. Processing and assembly of the integrin, glycoprotein IIb-IIIa, in HEL cells. J Biol Chem. 1989 Jul 25;264(21):12596–12603. [PubMed] [Google Scholar]
- Ruoslahti E. Integrins. J Clin Invest. 1991 Jan;87(1):1–5. doi: 10.1172/JCI114957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shattil S. J., O'Toole T., Eigenthaler M., Thon V., Williams M., Babior B. M., Ginsberg M. H. Beta 3-endonexin, a novel polypeptide that interacts specifically with the cytoplasmic tail of the integrin beta 3 subunit. J Cell Biol. 1995 Nov;131(3):807–816. doi: 10.1083/jcb.131.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solowska J., Edelman J. M., Albelda S. M., Buck C. A. Cytoplasmic and transmembrane domains of integrin beta 1 and beta 3 subunits are functionally interchangeable. J Cell Biol. 1991 Sep;114(5):1079–1088. doi: 10.1083/jcb.114.5.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabilio A., Rosa J. P., Testa U., Kieffer N., Nurden A. T., Del Canizo M. C., Breton-Gorius J., Vainchenker W. Expression of platelet membrane glycoproteins and alpha-granule proteins by a human erythroleukemia cell line (HEL). EMBO J. 1984 Feb;3(2):453–459. doi: 10.1002/j.1460-2075.1984.tb01827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Sawasaki Y., Hata J., Mukai K., Goto T. Spontaneous transformation and immortalization of human endothelial cells. In Vitro Cell Dev Biol. 1990 Mar;26(3 Pt 1):265–274. doi: 10.1007/BF02624456. [DOI] [PubMed] [Google Scholar]
- Ylänne J., Chen Y., O'Toole T. E., Loftus J. C., Takada Y., Ginsberg M. H. Distinct functions of integrin alpha and beta subunit cytoplasmic domains in cell spreading and formation of focal adhesions. J Cell Biol. 1993 Jul;122(1):223–233. doi: 10.1083/jcb.122.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Geer P., Pawson T. The PTB domain: a new protein module implicated in signal transduction. Trends Biochem Sci. 1995 Jul;20(7):277–280. doi: 10.1016/s0968-0004(00)89043-x. [DOI] [PubMed] [Google Scholar]



