Abstract
The polyphenol compound resveratrol is reported to have multiple functions, including neuroprotection, and no major adverse effects have been reported. Although the neuroprotective effects have been associated with sirtuin 1 activation by resveratrol, the mechanisms by which resveratrol exerts such functions are a matter of controversy. We examined whether resveratrol can be neuroprotective in two models of multiple sclerosis: experimental autoimmune encephalomyelitis (EAE) and Theiler’s murine encephalomyelitis virus-induced demyelinating disease (TMEV-IDD). EAE was induced in C57BL/6 mice, which were fed a control diet or a diet containing resveratrol during either the induction or effector phase or through the whole course of EAE. SJL/J mice were infected with TMEV and fed a control diet or a diet containing resveratrol during the chronic phase of TMEV-IDD. In EAE, all groups of mice treated with resveratrol had more severe clinical signs than the control group. In particular, resveratrol treatment during the induction phase resulted in the most severe EAE, both clinically and histologically. Similarly, in the viral model, the mice treated with resveratrol developed significantly more severe TMEV-IDD than the control group. Thus, surprisingly, the resveratrol treatment significantly exacerbated demyelination and inflammation without neuroprotection in the central nervous system in both models. Our findings indicate that caution should be exercised in potential therapeutic applications of resveratrol in human inflammatory demyelinating diseases, including multiple sclerosis.
Resveratrol is a polyphenol compound found in a variety of foods and beverages, including red grapes, peanuts, and red wine.1 Resveratrol has been reported to have multiple functions with anti-inflammatory, antioxidant, anti-aging, and antiviral properties that could be beneficial in diabetes and cardiovascular diseases.2,3 Some of the effects of resveratrol have been proposed to be exerted via activation of sirtuin 1 (SIRT1), which prevents axonal degeneration.4 Increased SIRT1 activity by resveratrol was associated with the suppression of apoptotic pathways and promoting neuronal survival, although the activation by resveratrol has lately been disputed.5 Three research groups demonstrated that, in the SIRT1 assay, resveratrol activates SIRT1 when Fluor de Lys peptide substrate with fluorophore is used but has no effect on SIRT1 activation when the Fluor de Lys peptide substrate without fluorophore is used.6–8 In all three reports, the authors concluded that resveratrol-mediated SIRT1 activation is an experimental artifact.
Multiple sclerosis (MS) is an inflammatory demyelinating disease with axonal degeneration in the central nervous system (CNS).9,10 We examined the neuroprotective effects of resveratrol in an autoimmune and a viral model of MS, namely, experimental autoimmune encephalomyelitis (EAE) and Theiler’s murine encephalomyelitis virus-induced demyelinating disease (TMEV-IDD).11,12 To our surprise, resveratrol was not neuroprotective, but instead exacerbated both EAE and TMEV-IDD. The lack of neuroprotective effects of resveratrol in our study is consistent with the above reports of failure to confirm previously reported interactions between resveratrol and SIRT1.6–8 Our findings indicate that caution should be exercised in potential therapeutic application of resveratrol in human diseases, including MS.
Materials and Methods
Resveratrol Treatment
Although an intake of resveratrol may be less constant via chow than via oral gavage, resveratrol is more stable in chow than in water,13 and we therefore administered the resveratrol in chow. A diet containing resveratrol (Sigma-Aldrich, St. Louis, MO) was prepared according to Banerjee et al.14 First, 50 g of a powder diet was weighed, then 55 mL of double-distilled H2O were added to the powder diet and mixed well. Next, 15 mL of 100% ethanol (control diet) or 100% ethanol containing 0.02 g of resveratrol (for 0.04% in the diet) was added to the chow and mixed well. For ethanol evaporation, the chow was placed in a vacuum oven at 50°C overnight. Because resveratrol is sensitive to light,15–17 the chow was kept away from light whenever possible and stored in the dark at 4°C.18 The mice were fed a control diet or a diet containing resveratrol at various time points as described below. A diet containing 0.04% resveratrol provided approximately 20 mg/kg per day,2 which is the most standard and effective dose reported by others.19–21 Although some research groups have used a higher dose (100 mg/kg per day) in animals, this higher dose corresponds to approximately 5 g/kg per day for humans, at which level kidney failure was reported in a clinical trial for multiple myeloma.22 One concern is that food intake of mice with full-blown EAE might be lower, which would falsify the resveratrol dosage; however, in the present study, the EAE mice fed a diet containing resveratrol did not lose weight during the full-blown EAE. We therefore concluded that there was no significant change in food intake at the peak of EAE.
Animal Experiments
EAE was induced in 6-week-old C57BL/6 mice (Harlan Laboratories, Indianapolis, IN), using myelin oligodendrocyte glycoprotein (MOG)35-55 peptide (United Peptide, Rockville, MD), as described previously.23 The mice were fed a control diet or a diet containing resveratrol during the induction (days −1 to 8; Early) or effector (days 14 to 23; Late) phase, or through the whole course (days −1 to 63; Whole) of EAE. Clinical scoring of EAE was as follows: 0, no sign; 1, paralyzed tail; 2, mild hind-limb paresis; 3, moderate hind-limb paralysis; 4, complete hind-limb paraplegia; and 5, quadriplegia or moribund state.23
Five-week-old SJL/J mice (Jackson Laboratory, Bar Harbor, ME) were infected intracerebrally with the Daniels (DA) strain of TMEV, as described previously.24 The mice were fed a control diet (DA alone) or a diet containing resveratrol during the chronic phase (days 35 to 48; Chronic) of TMEV infection. Clinical scoring of TMEV-IDD was based on impairment of the righting reflex. The proximal end of the mouse’s tail was grasped and twisted to the right and then to the left, with scoring as follows: 0, the mouse resists being turned over; 1, the mouse is flipped onto its back but immediately rights itself on one side; 1.5, the mouse is flipped onto its back but immediately rights itself on both sides; 2, the mouse rights itself in 1 to 5 seconds; 3, righting takes more than 5 seconds; and 4, the mouse cannot right itself.24 All experimental procedures involving the use of animals were reviewed and approved by the Institutional Animal Care and Use Committee of Louisiana State University Health Sciences Center and performed according to the criteria outlined by the NIH.
Neuropathology
Mice were perfused with PBS followed by a 4% paraformaldehyde solution (Sigma-Aldrich) in PBS. The CNS tissues were harvested and fixed with 4% paraformaldehyde. The spinal cords were divided into 10 to 12 transversal segments and brains into five coronal slabs before being embedded in paraffin. Sections (4 μm thick) were stained with Luxol Fast Blue (Solvent Blue 38; Sigma-Aldrich) for visualization of myelin. For scoring of spinal cord sections, each spinal cord section was divided into four quadrants (the ventral funiculus, the dorsal funiculus, and each lateral funiculus), as described previously.24,25 Any quadrant containing demyelination, meningitis, or perivascular cuffing was given a score of 1 in that pathological class. The total number of positive quadrants for each pathological class was determined and then divided by the total number of quadrants present on the slide and multiplied by 100 to give the percent involvement for each pathological class. An overall pathology score was also determined, by giving a positive score if any pathology was present in the quadrant, and was calculated as percent involvement.
Brain pathology was scored for meningitis (0, no meningitis; 1, mild cellular infiltration; 2, moderate cellular infiltration; and 3, severe cellular infiltration), perivascular cuffing (0, no cuffing; 1, 1 to 10 lesions; 2, 11 to 20 lesions; 3, 21 to 30 lesions; 4, 31 to 40 lesions; and 5, >40 lesions), and demyelination (0, no demyelination; 1, mild demyelination; 2, moderate demyelination; and 3, severe demyelination). The three scores were combined, for a maximum brain pathology score of 11 per mouse. Damaged axons were visualized by immunohistochemistry with SMI 311 (Sternberger Monoclonal; Covance, Princeton, NJ), a cocktail of monoclonal antibodies (SMI 32, 33, 37, 38, and 39) against nonphosphorylated neurofilament, using the avidin–biotin–peroxidase complex technique (Vector Laboratories, Burlingame, CA) with 3,3′-diaminobenzidine tetrahydrochloride as chromogen (Sigma-Aldrich) after antigen retrieval in double-distilled H2O using a Digital Decloaking Chamber I (Biocare Medical, Concord, CA) for 15 minutes at 120°C.26 The numbers of damaged axons were counted under a light microscope using 10 to 12 transverse spinal cord segments per mouse, as described previously.25
Statistical Analysis
Analysis of variance (ANOVA), Fisher’s exact test, and t-test were performed using OriginPro 8.1 (OriginLab Corporation, Northampton, MA) and StatView version 5.0 (SAS Institute, Cary, NC). Data are expressed as means ± SEM.
Results
Resveratrol Treatment Exacerbates MOG35-55–Induced EAE
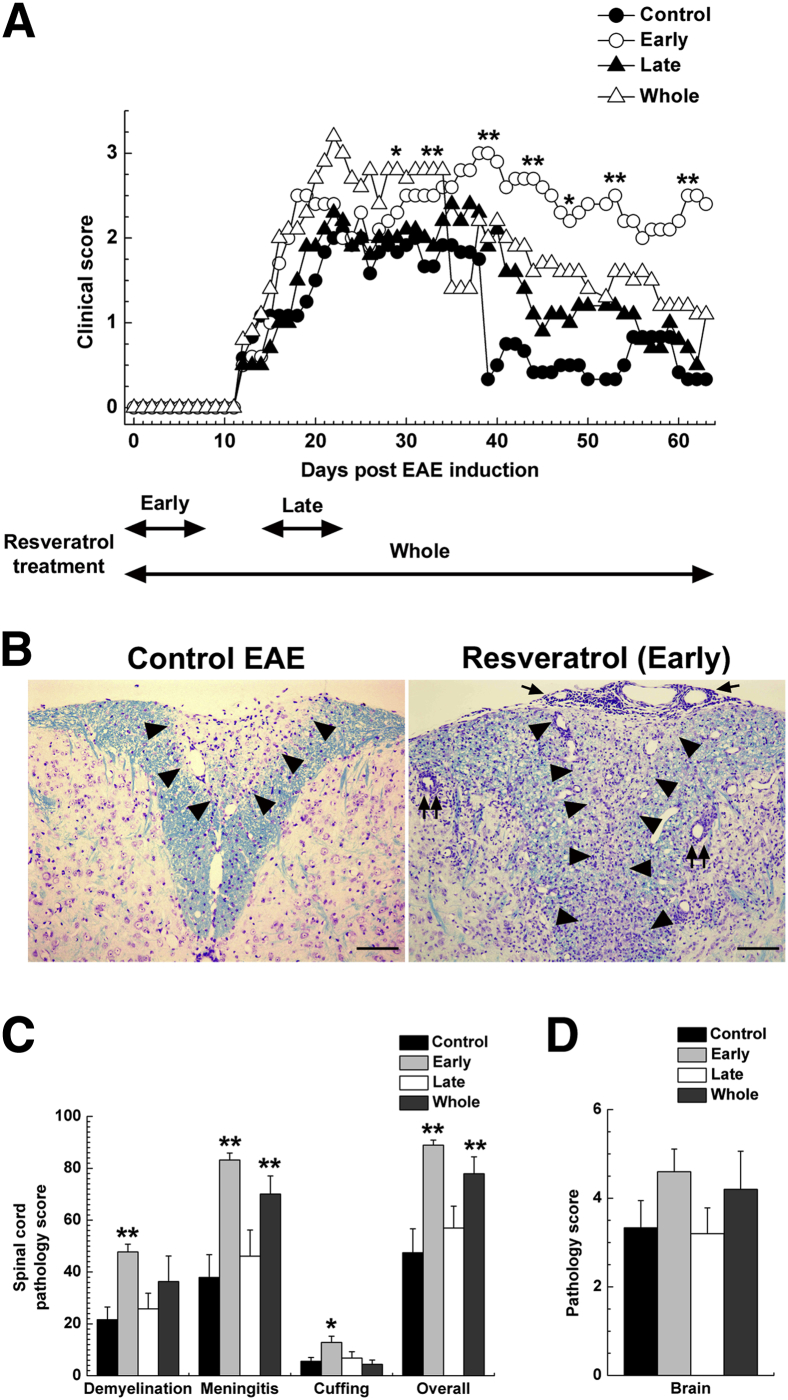
We first determined whether resveratrol treatment could alter EAE induced with MOG35-55. EAE mice were fed a control diet or a diet containing resveratrol on days −1 to 8 (Early), days 14 to 23 (Late), or days −1 to 63 (Whole). At approximately 12 days after MOG sensitization, all groups of mice started to develop clinical signs, including tail and hind-limb paralysis; there was no significant difference in the onset of EAE between groups (P > 0.05, ANOVA) (Figure 1A). In all four groups, mice exhibited disease progression and reached disease peak 3 weeks after MOG sensitization. At 5 weeks after MOG sensitization, most control EAE mice recovered completely or remained mildly paralyzed during the 2 months observation period. In contrast, all three resveratrol treatment groups recovered poorly and had significantly higher clinical scores than the control group at approximately 40 days after MOG sensitization; mean clinical scores on day 39 were 0.3 ± 0.3 (Control), 3.0 ± 0 (Early; P < 0.01 versus Control), 1.9 ± 0.4 (Late; P < 0.01 versus Control), and 2.0 ± 0.5 (Whole; P < 0.01 versus Control). Mice in the Early group experienced almost no remission and had lasting severe EAE; mean clinical scores on day 61 were 0.3 ± 0.3 (Control), 2.5 ± 0.5 (Early; P < 0.01), 0.7 ± 0.4 (Late), and 1.2 ± 0.7 (Whole). During the chronic phase of EAE, the clinical scores tended to be higher in the Late and Whole groups, compared with the Control group, although the difference did not reach statistical significance (Figure 1A). Of note, a few mice in the Early group did not develop EAE [EAE incidence: 10/13 (77%) mice], whereas all of the mice without resveratrol treatment during the induction phase developed EAE [EAE incidence of 11/11 (100%) mice]. However, no statistical difference was found between the two groups (P = 0.228, Fisher’s exact test). Thus, resveratrol might be useful only as a prophylactic, but not for therapeutic use in autoimmune-mediated demyelinating diseases.
Figure 1.
Adverse effects of resveratrol in the EAE autoimmune model of MS. C57BL/6 mice were sensitized with MOG35-55 on day 0 and were fed either a control diet or a diet containing resveratrol on days −1 to 8 (Early) or days 14 to 23 (Late) or days −1 to 63 (Whole). A: All three resveratrol-treated groups develop more severe EAE, compared with the Control group, although all groups had a similar disease onset. B: Control EAE mice have inactive demyelinating lesions in the dorsal funiculus of the spinal cord. Mice treated with resveratrol during the induction phase of EAE have more severe demyelination (arrowheads), meningitis (arrows), and active perivascular inflammation (paired arrows), compared with control mice. Luxol fast blue staining. C: Mice fed a diet containing resveratrol during the induction phase of EAE have significantly higher spinal cord pathology scores in demyelination, meningitis, perivascular cuffing (indicative of inflammation), and overall pathology, compared with mice fed a control diet. Mice treated with resveratrol during the whole course of the experiment have significantly higher scores in meningitis and overall pathology, compared with control mice. D: Brain pathology score does not differ significantly among the groups. Data are expressed as means (A) or as means ± SEM (C and D). n = 5 or 6 symptomatic mice per group. ∗P < 0.05, ∗∗P < 0.01, ANOVA. Original magnification, ×41. Scale bar = 100 μm.
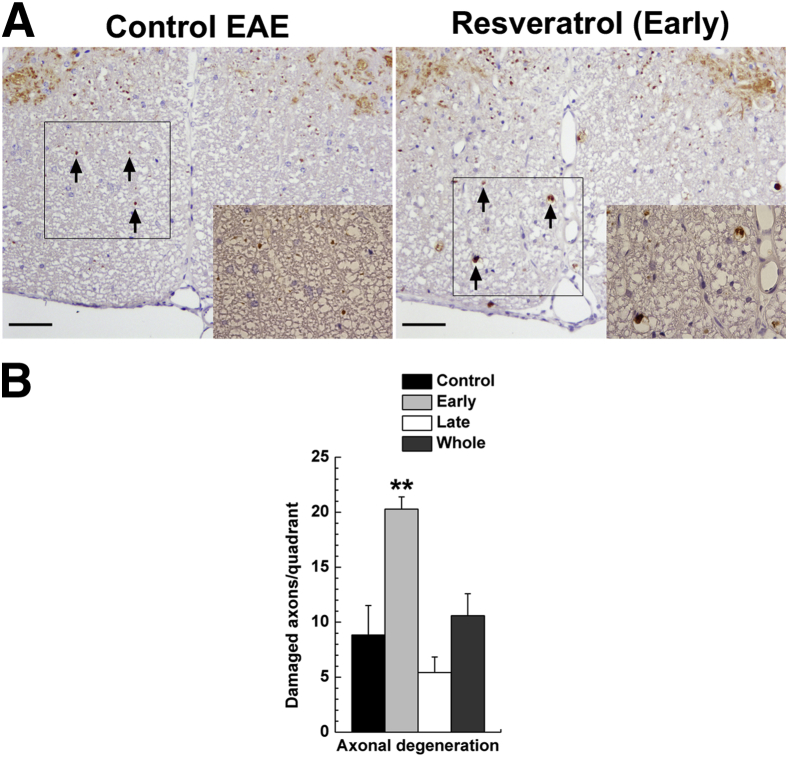
At 2 months after EAE induction, we sacrificed symptomatic mice and compared the neuropathology of symptomatic mice between the groups. In the spinal cord of the Control group, inactive demyelinating lesions in the white matter with meningitis and axonal degeneration were detected by Luxol fast blue staining for myelin (Figure 1B) and by immunohistochemistry against nonphosphorylated neurofilament as a marker of axonal degeneration (Figure 2A). Consistent with the clinical signs, the Early group had severe active inflammatory demyelinating lesions with axonal degeneration (Figures 1B and 2A). The pathology scores and numbers of damaged axons in the spinal cord were significantly higher in the Early group than in the Control group (Figures 1C and 2B). The Whole group had more severe demyelination and meningitis than the Control group, but the levels of axonal degeneration were similar. No significant difference was seen in neuropathology between the Control and the Late groups. Although the brain pathology scores were higher in the Early and Whole groups than in the Control and Late groups, the scores were mild in all groups, and there was no significant difference between groups (Figure 1D). We also compared the levels of MOG35-55–specific lymphoproliferative responses and proinflammatory cytokine production between the groups and found no significant differences (Supplemental Figure S1). Thus, even though resveratrol has been reported to have anti-inflammatory properties,3 in the present study resveratrol did not suppress autoimmune responses.
Figure 2.
Axonal degeneration, 2 months after EAE induction. EAE mice were given a control diet or a diet containing resveratrol on days −1 to 8 (Early), days 14 to 23 (Late), or days −1 to 63 (Whole). A: Immunohistochemistry for nonphosphorylated neurofilament reveals higher levels of axonal damage (arrows) in the Early group than in the Control group. Boxed regions are shown at higher magnification in the corresponding inset. B: Higher numbers of nonphosphorylated neurofilament-positive damaged axons are detected in the spinal cord of mice fed a diet containing resveratrol during the induction phase of EAE, compared with control mice. Data are expressed as means ± SEM. n = 5 or 6 symptomatic mice per group. ∗∗P < 0.01, ANOVA. Original magnification: ×83; ×115 (insets). Scale bars: 50 μm (A).
Resveratrol Treatment Exacerbates TMEV-IDD
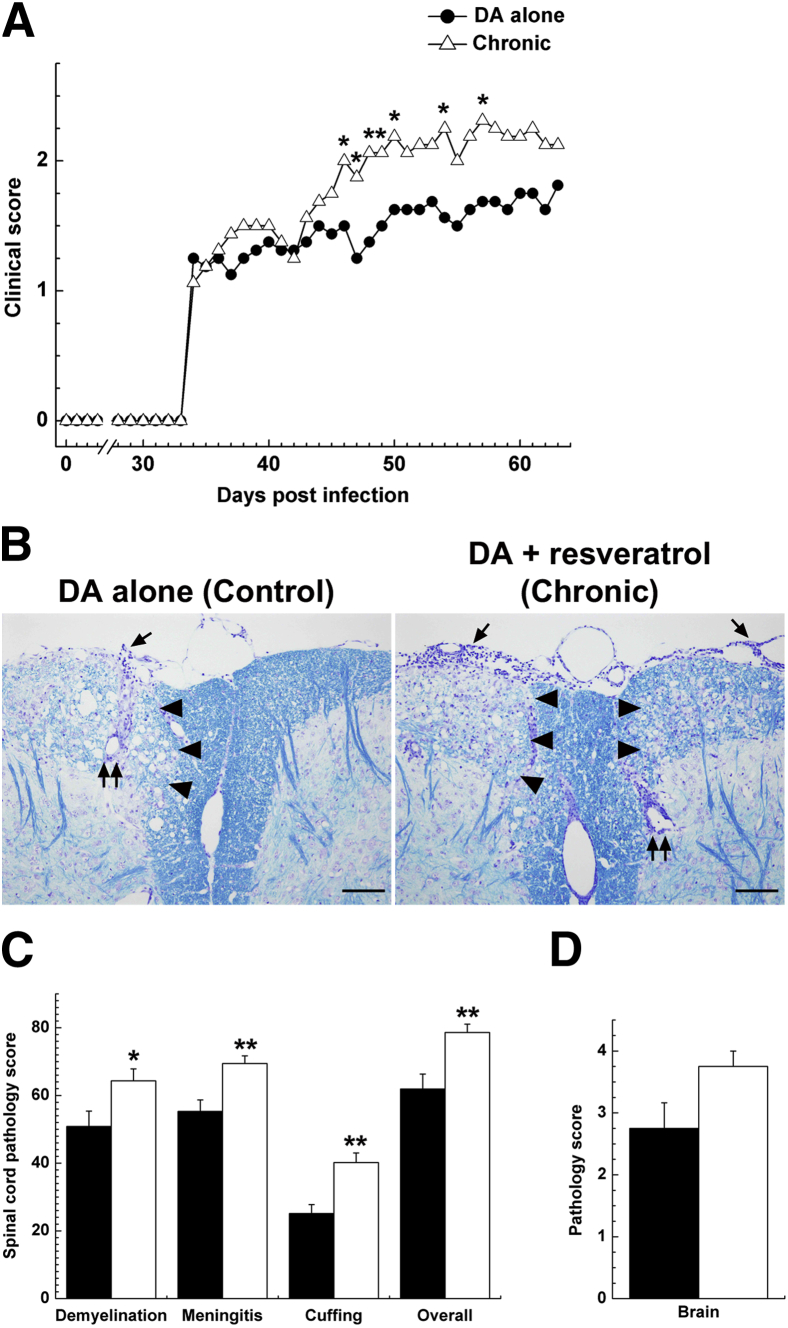
We next examined whether resveratrol treatment has suppressive effects on a viral model of MS, TMEV-IDD.27 We treated TMEV-infected mice during the chronic phase, in which mice develop inflammatory demyelinating disease at approximately 1 month after infection. Mice were infected with the DA strain of TMEV on day 0 and were fed a control diet (DA alone) or a diet containing resveratrol from day 35 to day 48 (Chronic). Beginning approximately 1 month after infection, both groups displayed impairment of righting reflexes. The Chronic group had significantly higher righting reflex scores than the DA alone group; mean clinical scores on day 48 were 1.4 ± 0.1 and 2.1 ± 0.2 for the DA alone and Chronic group, respectively (P < 0.05, t-test) (Figure 3A).
Figure 3.
Adverse effects of resveratrol in the TMEV viral model of MS. SJL/J mice were infected intracerebrally with 2 × 105 PFU of the DA strain of TMEV on day 0 and were fed a control diet (DA alone, black bars) or a diet containing resveratrol from day 35 to day 48 (Chronic, white bars). A: The TMEV-infected mice treated with a resveratrol diet develop more severe clinical signs than mice on the control diet. B: Mice treated with a resveratrol diet have more severe demyelination (arrowheads), meningitis (arrows), and active perivascular inflammation (paired arrows) than mice on the control diet. Luxol fast blue staining. C: The TMEV-infected mice fed resveratrol have significantly higher pathology scores in demyelination, meningitis, and perivascular cuffing (inflammation) than mice fed a control diet. D: There is a small but nonsignificant difference in brain pathology scores between the two groups. Data are expressed as means (A) or as means ± SEM (C and D). n = 8 mice per group. ∗P < 0.05, ∗∗P < 0.01, ANOVA. Original magnification, ×42. Scale bars: 100 μm (B).
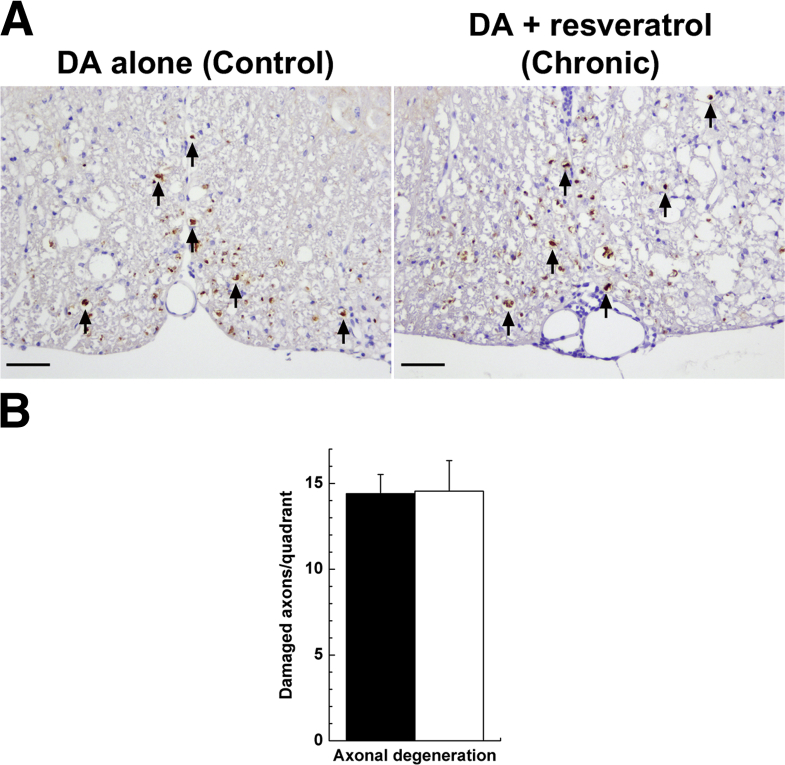
Histologically, mice treated with resveratrol developed significantly more severe demyelination, meningitis, and perivascular cuffing in the spinal cord, compared with control mice (Figure 3, B and C). Regardless of treatment, both groups of mice developed severe axonal degeneration (Figure 4A), and quantification of damaged axons revealed no significant difference between the two groups (P > 0.05, t-test) (Figure 4B). In the brain, the Chronic group had higher pathology scores than the DA alone group, although the difference did not reach statistical significance (Figure 3D). To determine whether resveratrol treatment modulated the immune responses in TMEV-IDD, we measured the levels of TMEV-specific lymphoproliferation and proinflammatory cytokine production in each group. Although no significant difference was seen in a lymphoproliferation assay between the two groups, resveratrol treatment during the chronic phase of TMEV infection resulted in approximately twofold increased IL-17 production and approximately twofold decreased interferon (IFN)-γ production (Supplemental Figure S2).
Figure 4.
Axonal degeneration, 2 months after TMEV infection. SJL/J mice were infected with the DA strain of TMEV and fed a control diet (DA alone, black bar) or a diet containing resveratrol from day 35 to day 48 (Chronic, white bar). A: Levels of damaged axons (arrows) in the spinal cord are similar in the two groups. Immunohistochemistry for nonphosphorylated neurofilament. B: Nonphosphorylated neurofilament-positive damaged axons in the spinal cord are similar in the two groups. Data are expressed as means ± SEM. n = 8 mice per group. Original magnification, ×83. Scale bars: 50 μm (A).
We also examined whether resveratrol has prophylactic effects on TMEV-IDD. Mice were infected with the DA strain of TMEV on day 0 and were fed a control diet (DA alone) or a diet containing resveratrol during the acute (days 0 to 14; Acute) or subclinical (days 21 to 35; Subclinical) phase of TMEV infection. Both clinically and histologically, the Subclinical group tended to have more severe disease signs than the DA alone group, although the difference did not reach statistical significance (Supplemental Figures S3 and S4). Using hyperimmune serum against TMEV, we observed similar levels and distributions of viral antigen-positive cells in the spinal cord in the three groups (Supplemental Figure S4). We also examined the effect of resveratrol treatment on immune responses in TMEV-IDD and found that the levels of TMEV-specific lymphoproliferative and antibody responses were similar in the three groups (Supplemental Figure S5). Production of both IL-17 and IFN-γ was lower in the Acute and Subclinical groups than in the DA-alone group, although the difference did not reach statistical significance (Supplemental Figure S5).
Resveratrol Treatment Is Not Neuroprotective in Infection with a Neurovirulent Strain of TMEV
In the above experiments, we did not observe a neuroprotective effect of the resveratrol treatment. One explanation could be enhancement of inflammation in the CNS, which might mask the neuroprotective effects of resveratrol. To address this possibility, we used the GDVII strain of TMEV, which is highly neurovirulent.24 Unlike the DA virus, GDVII virus induces neurodegeneration without induction of TMEV-specific immune responses.26 Thus, GDVII virus infection can be used to determine whether resveratrol can suppress neurodegeneration caused by direct viral infection (not by immunopathology). Mice were infected with 1 to 1000 plaque-forming units (PFU) of GDVII virus and were fed a control diet (GDVII alone) or a diet containing resveratrol (GDVII-resveratrol). Regardless of resveratrol treatment, all mice infected with 100 or 1000 PFU of GDVII virus started to exhibit encephalitic signs, such as weight loss, ruffled fur, and a hunched posture, at approximately day 6 and all died within 10 days of infection (Supplemental Table S1). A small number of mice infected with a low dose (1 or 10 PFU) of GDVII virus survived the infection. Both mortality and survival period were similar in the two groups. The lethal dose (LD)50 was similar in mice fed a control diet and mice treated with resveratrol (GDVII alone, 2.6 PFU; GDVII-resveratrol, 3.8 PFU).
As an indicator of neurodegeneration, we compared the levels of neuronal apoptosis and axonal degeneration between the two groups. Using the TUNEL method, we found that all symptomatic mice had large numbers of apoptotic neurons (particularly in the cerebral cortex, thalamus, and hippocampus), regardless of treatment (Supplemental Figure S6A). Quantification of apoptotic neurons in the brain revealed no statistical difference in the number of TUNEL-positive cells between the two groups (P > 0.05, t-test) (Supplemental Table S2). Regardless of treatment, all symptomatic mice had large numbers of damaged axons in the white matter of the spinal cord (Supplemental Figure S6A). Quantification of damaged axons revealed no significant difference in the levels of axonal degeneration between the two groups (P > 0.05, t-test) (Supplemental Figure S6B). We also scored the severity of brain pathology based on the levels of meningitis and perivascular inflammation. The brain pathology scores were similar in the two groups (Supplemental Figure S6C).
Discussion
We demonstrated that the resveratrol treatment exacerbated demyelinating disease with no neuroprotection in both EAE and TMEV-IDD, although the precise mechanism is unclear. Experimentally, animal studies for cardiovascular disease suggest that resveratrol exerts a vasodilation effect by improving function of endothelial cells, which form the inner wall of blood vessels.28 The expansion of blood vessels enhances blood flow volume and reduces blood flow velocity. Theoretically, these processes facilitate tethering, rolling, and firm adhesion of circulating inflammatory cells to endothelia, leading to the transmigration of inflammatory cells across the blood–brain barrier and into the CNS.29 In the present study, resveratrol treatment resulted in higher pathology scores for meningitis and perivascular cuffing in both EAE and TMEV-IDD. This suggests that the vasodilation effects by resveratrol enhanced infiltration of inflammatory cells into the CNS. Inflammatory cell infiltration into the CNS has been suggested to play a key role in the pathogenesis of MS,30 because large numbers of inflammatory cells infiltrate across the blood–brain barrier in active lesions from patients with MS.31 Furthermore, in MS, injection of natalizumab (Tysabri; Biogen Idec, Weston, MA), a monoclonal antibody against α4-integrin [very late antigen 4 (VLA-4)], can inhibit migration of inflammatory cells into the CNS, reducing the severity of disease.29 Similar to the case of MS, in EAE the blockade of migration of inflammatory cells into the CNS has been shown to improve clinical signs.32,33 Thus, the exacerbation of both EAE and TMEV-IDD by resveratrol treatment observed in the present study could be due to enhanced infiltration across the blood–brain barrier of inflammatory cells, accompanied by vasodilation.
We also found no significant differences in immune responses between control and resveratrol-treated EAE mice. Although resveratrol has been tested in several different conditions in EAE,34–36 resveratrol studies in EAE have not shown consistent outcomes. Singh et al34 and Imler et al35 demonstrated that EAE clinical scores were lower in mice treated with resveratrol, compared with control mice, although resveratrol treatment altered proinflammatory cytokine production either positively or negatively, depending on the disease course or the dose of resveratrol. It is unknown whether decreased clinical signs correlate with neuroprotection by resveratrol, because axonal damage was not examined in these studies. On the other hand, Fonseca-Kelly et al36 demonstrated that a high dose (250 mg/kg per day) of resveratrol delayed the onset of EAE, but a low dose (100 mg/kg per day) had no effect36; however, both resveratrol treatments neither ameliorated CNS pathology nor suppressed immune responses, although resveratrol treatments protected from the loss of retinal ganglion cells, which are the neurons forming the optic nerve. The different effects of resveratrol on EAE may be due to differences in antigens and animals. For EAE induction, Imler et al35 sensitized SJL/J mice with myelin proteolipid protein (PLP)139-151, whereas other research groups,33,35 including our own group, sensitized C57BL/6 mice with MOG35-55. In MOG-induced EAE, different groups used different concentrations of MOG and pertussis toxin. Thus, further research is needed to determine the optimal concentration of resveratrol and what factors may result in deleterious effects.
Resveratrol has been shown to have antiviral effects on some viruses relevant to MS, including herpes simplex virus (HSV) and Epstein–Barr virus (EBV),37,38 although the exact mechanisms remain unclear. In HSV infection, resveratrol inhibited viral replication by regulation of the nuclear factor-κB (NF-κB) pathway in vitro.39 Resveratrol treatment modulated EBV early antigen induction in vitro.40 In the present study, however, with the DA strain of TMEV infection, resveratrol-treated mice had levels of viral antigen-positive cells in the spinal cord similar to those of control mice. Furthermore, resveratrol treatment did not substantially alter LD50 titers in mice infected with the GDVII strain of TMEV. Thus, resveratrol did not have antiviral effects in TMEV infection.
In conclusion, we demonstrated that resveratrol treatment profoundly exacerbated demyelinating disease with enhanced CNS inflammation in EAE and TMEV-IDD, two animal models of MS. Although resveratrol has not been reported to have major adverse effects, GlaxoSmithKline reported that the safety of a proprietary formulation of resveratrol known as SRT501 was questioned in a clinical trial for patients with multiple myeloma.22 In this trial, several patients treated with SRT501 experienced kidney failure, although it is unclear whether the kidney failure was due to SRT501 treatment or was a natural consequence of the disease. Nonetheless, resveratrol may have detrimental effects in some disease conditions and its use should be discouraged for MS patients, pending further research.
Acknowledgments
We thank Drs. Monica A. Rojas, Viromi Fernando, and Masashi Tsunoda for many helpful discussions and Blair L. Wood, Sadie Faith Elliott, and Lesya Ekshyyan for excellent technical assistance.
Footnotes
Supported by the fellowships from the Malcolm Feist Cardiovascular Research Endowment, Louisiana State University Health Sciences Center (F.S.), by the NIH (National Institute of Neurological Disorders and Stroke grant R21-NS059724, National Center for Research Resources grant 5P20-RR018724, and National Institute of General Medical Sciences Centers of Biomedical Research Excellence grant 8P20-GM103433 to I.T.), and by National Multiple Sclerosis Society Pilot Research Award PP 1499 (I.T.).
Supplemental Data
Immune responses in C57BL/6 mice fed a control diet or a diet containing resveratrol on days −1 to 8 (Early), days 14 to 23 (Late), or days −1 to 63 (Whole), 2 months after EAE induction. A: Mononuclear cells (MNCs) were isolated from draining lymph nodes in each group and incubated with or without MOG35-55 peptide for 5 days. Lymphoproliferation was assessed by a [3H]thymidine incorporation assay. All groups of mice had substantial MOG-specific lymphoproliferation. There was no significant difference in lymphoproliferative responses against MOG35-55 among the groups, although all three resveratrol-treated groups tended to have lower levels of lymphoproliferation than the control group. All cultures were performed in triplicate. Data are expressed as stimulation index (calculated as experimental cpm/control cpm). B and C: MNCs isolated from draining lymph nodes were stimulated with concanavalin A for 48 hours. The levels of IL-17 (B) and IFN-γ (C) production in the culture supernatant were measured by enzyme-linked immunosorbent assay (ELISA). There was no significant difference in cytokine production among the four groups. Data are expressed as means ± SEM of two or three pools of lymph nodes from two or three mice. n = 5 or 6 mice per group.
Immune responses in SJL/J mice fed a control diet (DA alone) or a diet containing resveratrol from day 35 to day 48 (Chronic), 2 months after TMEV infection. A: MNCs isolated from spleens were stimulated with TMEV antigen or live virus. Lymphoproliferation was assessed by a [3H]thymidine incorporation assay. Both groups of mice had similar levels of substantial TMEV-specific lymphoproliferation. All cultures were performed in triplicate. Data are expressed as stimulation index. B and C: MNCs isolated from spleens of each group were stimulated with concanavalin A for 48 hours. The levels of IL-17 (B) and IFN-γ (C) production in the culture supernatant were measured by ELISA. Mice treated with resveratrol during the chronic phase of TMEV infection had higher levels of IL-17 and lower levels of IFN-γ production than those fed a control diet, but the difference did not reach statistical significance. Data are expressed as means ± SEM of four pools of spleens from two mice. n = 8 mice per group.
Clinical scores and weight changes of TMEV-infected mice with or without resveratrol treatment. SJL/J mice were infected with the DA strain of TMEV on day 0 and fed a control diet (DA alone) or a diet containing resveratrol on days 0 to 14 (Acute) or days 21 to 35 (Subclinical). A: Mice fed a diet containing resveratrol during the acute phase or subclinical phase of TMEV infection had higher clinical scores of impairment of righting reflex than TMEV-infected mice fed a control diet, but the difference did not reach statistical significance. B: Mice treated with resveratrol during the acute phase of TMEV infection had significant weight gain, compared with control mice. ∗∗P < 0.01. n = 9 mice per group.
Neuropathology and numbers of TMEV antigen-positive cells, 1 month after infection. SJL/J mice were infected with the DA strain of TMEV and fed a control diet (DA alone) or a diet containing resveratrol on days 0 to 14 (Acute) or days 21 to 35 (Subclinical). Viral antigen-positive cells were visualized by immunohistochemistry with hyperimmune serum against TMEV, using the avidin–biotin–peroxidase complex technique with 3,3′-diaminobenzidine tetrahydrochloride (DAB) as chromogen. The numbers of viral antigen-positive cells were counted under a light microscope, using 10 to 12 transverse spinal cord segments per mouse. A (top): There was no difference in severity and distributions of neuropathology among the groups; demyelination (arrowheads), meningitis (arrows), and perivascular inflammation (paired arrows). Luxol fast blue staining. A (bottom): Similar levels of TMEV antigen-positive cells (arrows) were detected in the spinal cord in the three groups. B: Immunohistochemistry for TMEV antigen. There was no significant difference in the levels of demyelination, meningitis, perivascular cuffing (inflammation), and overall pathology scores among the groups. C: In the spinal cord, the Acute and Subclinical groups had similar numbers of TMEV antigen-positive cells as in the DA-alone group. D: In the brain, the Subclinical group had higher pathology scores than the DA-alone and Acute groups, but the difference did not reach statistical significance. Data are expressed as means ± SEM. n = 9 mice per group. Original magnification, ×53 (top row); ×107 (bottom row). Scale bars: 100 μm (top row); 50 μm (bottom row).
Immune responses in SJL/J mice fed a control diet (DA alone) or a diet containing resveratrol on days 0 to 14 (Acute) or days 21 to 35 (Subclinical), 1 month after TMEV infection. A: MNCs isolated from spleens were stimulated with TMEV antigen or live virus. Lymphoproliferation was assessed by a [3H]thymidine incorporation assay. All groups of mice had substantial TMEV-specific lymphoproliferation. There was no significant difference in lymphoproliferative responses against TMEV among the three groups. B: At the time of terminal perfusion, blood was collected from the heart of TMEV-infected mice. The levels of total IgG antibody against TMEV in serum were assessed by ELISA. There was no significant difference in the levels of serum TMEV-specific IgG antibody production. C and D: MNCs isolated from spleens were stimulated with concanavalin A for 48 hours. The levels of IL-17 (C) and IFN-γ (D) production in the culture supernatant were measured by ELISA. Mice treated with resveratrol during the acute phase or subclinical phase of TMEV infection had lower levels of IL-17 and IFN-γ production, compared with mice on a control diet. Data are expressed as means ± SEM of four pools of spleens from two or three mice. n = 9 mice per group. OD, optical density.
Neuropathology of GDVII virus infection. SJL/J mice were infected with the GDVII strain of TMEV and fed a control diet (GDVII alone) or a diet containing resveratrol (GDVII-resveratrol). Apoptosis was detected using the TUNEL method. TUNEL-positive cells were visualized by the avidin–biotin–peroxidase technique with DAB as chromogen. The numbers of TUNEL-positive cells in the brain were counted under a light microscope, using five coronal brain slabs per mouse. A (top): High levels of apoptosis of neurons were observed in both groups (pyramidal cell layers of the hippocampus). The TUNEL method revealed no difference in the levels of apoptotic cells (arrowheads) in the brain between the two groups. Boxed regions are shown at higher magnification in the corresponding inset. A (bottom): Immunohistochemistry for nonphosphorylated neurofilament revealed substantial numbers of degenerated axons (ventrolateral funiculus of the spinal cord). Similar levels of damaged axons (arrows) in the spinal cord were detected in the two groups. B: Numbers of damaged axons per quadrant of the spinal cord were similar in the two groups. C: There was no significant difference in the brain pathology score in the two groups. Data are expressed as means ± SEM. n = 4 to 6 mice per group. Original magnification, ×26 (top row); ×113 (insets); ×128 (bottom row). Scale bars: 250 μm (top row); 50 μm (bottom row).
References
- 1.Jang M., Cai L., Udeani G.O., Slowing K.V., Thomas C.F., Beecher C.W.W., Fong H.H.S., Farnsworth N.R., Kinghorn A.D., Mehta R.G., Moon R.C., Pezzuto J.M. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 2.Baur J.A., Pearson K.J., Price N.L., Jamieson H.A., Lerin C., Kalra A., Prabhu V.V., Allard J.S., Lopez-Lluch G., Lewis K., Pistell P.J., Poosala S., Becker K.G., Boss O., Gwinn D., Wang M., Ramaswamy S., Fishbein K.W., Spencer R.G., Lakatta E.G., Le Couteur D., Shaw R.J., Navas P., Puigserver P., Ingram D.K., de Cabo R., Sinclair D.A. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palsamy P., Subramanian S. Ameliorative potential of resveratrol on proinflammatory cytokines, hyperglycemia mediated oxidative stress, and pancreatic beta-cell dysfunction in streptozotocin-nicotinamide-induced diabetic rats. J Cell Physiol. 2010;224:423–432. doi: 10.1002/jcp.22138. [DOI] [PubMed] [Google Scholar]
- 4.Borra M.T., Smith B.C., Denu J.M. Mechanism of human SIRT1 activation by resveratrol. J Biol Chem. 2005;280:17187–17195. doi: 10.1074/jbc.M501250200. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt C. GSK/Sirtris compounds dogged by assay artifacts. Nature Biotechnol. 2010;28:185–186. doi: 10.1038/nbt0310-185. [DOI] [PubMed] [Google Scholar]
- 6.Kaeberlein M., McDonagh T., Heltweg B., Hixon J., Westman E.A., Caldwell S.D., Napper A., Curtis R., DiStefano P.S., Fields S., Bedalov A., Kennedy B.K. Substrate-specific activation of sirtuins by resveratrol. J Biol Chem. 2005;280:17038–17045. doi: 10.1074/jbc.M500655200. [DOI] [PubMed] [Google Scholar]
- 7.Beher D., Wu J., Cumine S., Kim K.W., Lu S.-C., Atangan L., Wang M. Resveratrol is not a direct activator of SIRT1 enzyme activity. Chem Biol Drug Des. 2009;74:619–624. doi: 10.1111/j.1747-0285.2009.00901.x. [DOI] [PubMed] [Google Scholar]
- 8.Pacholec M., Bleasdale J.E., Chrunyk B., Cunningham D., Flynn D., Garofalo R.S., Griffith D., Griffor M., Loulakis P., Pabst B., Qiu X., Stockman B., Thanabal V., Varghese A., Ward J., Withka J., Ahn K. SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1. J Biol Chem. 2010;285:8340–8351. doi: 10.1074/jbc.M109.088682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pirko I., Lucchinetti C.F., Sriram S., Bakshi R. Gray matter involvement in multiple sclerosis. Neurology. 2007;68:634–642. doi: 10.1212/01.wnl.0000250267.85698.7a. [Erratum appeared in Neurology 2008, 71:2021] [DOI] [PubMed] [Google Scholar]
- 10.Martinez N.E., Sato F., Omura S., Minagar A., Alexander J.S., Tsunoda I. Immunopathological patterns from EAE and Theiler’s virus infection: is multiple sclerosis a homogenous 1-stage or heterogenous 2-stage disease? Pathophysiology. 2013;20:71–84. doi: 10.1016/j.pathophys.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mecha M., Carrillo-Salinas F.J., Mestre L., Feliú A., Guaza C. Viral models of multiple sclerosis: Neurodegeneration and demyelination in mice infected with Theiler’s virus. Prog Neurobiol. 2013;101–102:46–64. doi: 10.1016/j.pneurobio.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sato F., Omura S., Martinez N.E., Tsunoda I. Animal models of multiple sclerosis. Neuroinflammation. In: Minagar A., editor. Elsevier; London: 2011. pp. 55–79. [Google Scholar]
- 13.Niles R.M., Cook C.P., Meadows G.G., Fu Y.-M., McLaughlin J.L., Rankin G.O. Resveratrol is rapidly metabolized in athymic (nu/nu) mice and does not inhibit human melanoma xenograft tumor growth. J Nutr. 2006;136:2542–2546. doi: 10.1093/jn/136.10.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banerjee S., Bueso-Ramos C., Aggarwal B.B. Suppression of 7,12-dimethylbenz(a)anthracene-induced mammary carcinogenesis in rats by resveratrol: role of nuclear factor-κB, cyclooxygenase 2, and matrix metalloprotease 9. Cancer Res. 2002;62:4945–4954. [PubMed] [Google Scholar]
- 15.Vivekananthan D.P., Penn M.S., Sapp S.K., Hsu A., Topol E.J. Use of antioxidant vitamins for the prevention of cardiovascular disease: meta-analysis of randomised trials [Erratum appeared in Lancet 2004, 363:662] Lancet. 2003;361:2017–2023. doi: 10.1016/S0140-6736(03)13637-9. [DOI] [PubMed] [Google Scholar]
- 16.Campos-Toimil M., Elíes J., Álvarez E., Verde I., Orallo F. Effects of trans- and cis-resveratrol on Ca2+ handling in A7r5 vascular myocytes. Eur J Pharmacol. 2007;577:91–99. doi: 10.1016/j.ejphar.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 17.Rius C., Abu-Taha M., Hermenegildo C., Piqueras L., Cerda-Nicolas J.M., Issekutz A.C., Estañ L., Cortijo J., Morcillo E.J., Orallo F., Sanz M.-J. Trans- but not cis-resveratrol impairs angiotensin-II-mediated vascular inflammation through inhibition of NF-κB activation and peroxisome proliferator-activated receptor-gamma upregulation. J Immunol. 2010;185:3718–3727. doi: 10.4049/jimmunol.1001043. [DOI] [PubMed] [Google Scholar]
- 18.Figueiras T.S., Neves-Petersen M.T., Petersen S.B. Activation energy of light induced isomerization of resveratrol. J Fluoresc. 2011;21:1897–1906. doi: 10.1007/s10895-011-0886-3. [DOI] [PubMed] [Google Scholar]
- 19.Gupta Y.K., Chaudhary G., Sinha K., Srivastava A.K. Protective effect of resveratrol against intracortical FeCl3-induced model of posttraumatic seizures in rats. Methods Find Exp Clin Pharmacol. 2001;23:241–244. doi: 10.1358/mf.2001.23.5.662120. [DOI] [PubMed] [Google Scholar]
- 20.Asensi M., Medina I., Ortega A., Carretero J., Baño M.C., Obrador E., Estrela J.M. Inhibition of cancer growth by resveratrol is related to its low bioavailability. Free Radic Biol Med. 2002;33:387–398. doi: 10.1016/s0891-5849(02)00911-5. [DOI] [PubMed] [Google Scholar]
- 21.Eybl V., Kotyzova D., Koutensky J. Comparative study of natural antioxidants—curcumin, resveratrol and melatonin—in cadmium-induced oxidative damage in mice. Toxicology. 2006;225:150–156. doi: 10.1016/j.tox.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 22.GlaxoSmithKline halts all further development of resveratrol drug SRT501. The Myeloma Beacon 2010 Nov 30 [Internet]. Available at http://www.myelomabeacon.com/news/2010/11/30/glaxosmithkline-halts-all-further-development-of-resveratrol-drug-srt501, last revised November 30, 2010
- 23.Tsunoda I., Tanaka T., Terry E.J., Fujinami R.S. Contrasting roles for axonal degeneration in an autoimmune versus viral model of multiple sclerosis: when can axonal injury be beneficial? Am J Pathol. 2007;170:214–226. doi: 10.2353/ajpath.2007.060683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsunoda I., Tanaka T., Fujinami R.S. Regulatory role of CD1d in neurotropic virus infection. J Virol. 2008;82:10279–10289. doi: 10.1128/JVI.00734-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsunoda I., Wada Y., Libbey J.E., Cannon T.S., Whitby F.G., Fujinami R.S. Prolonged gray matter disease without demyelination caused by Theiler’s murine encephalomyelitis virus with a mutation in VP2 puff B. J Virol. 2001;75:7494–7505. doi: 10.1128/JVI.75.16.7494-7505.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsunoda I., Kuang L.-Q., Libbey J.E., Fujinami R.S. Axonal injury heralds virus-induced demyelination. Am J Pathol. 2003;162:1259–1269. doi: 10.1016/S0002-9440(10)63922-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sato F., Tanaka H., Hasanovic F., Tsunoda I. Theiler’s virus infection: pathophysiology of demyelination and neurodegeneration. Pathophysiology. 2011;18:31–41. doi: 10.1016/j.pathophys.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nicholson S.K., Tucker G.A., Brameld J.M. Physiological concentrations of dietary polyphenols regulate vascular endothelial cell expression of genes important in cardiovascular health. Br J Nutr. 2010;103:1398–1403. doi: 10.1017/S0007114509993485. [DOI] [PubMed] [Google Scholar]
- 29.Rice G.P.A., Hartung H.-P., Calabresi P.A. Anti-alpha4 integrin therapy for multiple sclerosis: mechanisms and rationale. Neurology. 2005;64:1336–1342. doi: 10.1212/01.WNL.0000158329.30470.D0. [DOI] [PubMed] [Google Scholar]
- 30.Steinman L. Multiple sclerosis: a coordinated immunological attack against myelin in the central nervous system. Cell. 1996;85:299–302. doi: 10.1016/s0092-8674(00)81107-1. [DOI] [PubMed] [Google Scholar]
- 31.Lassmann H., Brück W., Lucchinetti C. Heterogeneity of multiple sclerosis pathogenesis: implications for diagnosis and therapy. Trends Mol Med. 2001;7:115–121. doi: 10.1016/s1471-4914(00)01909-2. [DOI] [PubMed] [Google Scholar]
- 32.Yednock T.A., Cannon C., Fritz L.C., Sanchez-Madrid F., Steinman L., Karin N. Prevention of experimental autoimmune encephalomyelitis by antibodies against alpha 4 beta 1 integrin. Nature. 1992;356:63–66. doi: 10.1038/356063a0. [DOI] [PubMed] [Google Scholar]
- 33.Tsunoda I., Terry E.J., Marble B.J., Lazarides E., Woods C., Fujinami R.S. Modulation of experimental autoimmune encephalomyelitis by VLA-2 blockade. Brain Pathol. 2007;17:45–55. doi: 10.1111/j.1750-3639.2006.00042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh N.P., Hegde V.L., Hofseth L.J., Nagarkatti M., Nagarkatti P. Resveratrol (trans-3,5,4′-trihydroxystilbene) ameliorates experimental allergic encephalomyelitis, primarily via induction of apoptosis in T cells involving activation of aryl hydrocarbon receptor and estrogen receptor. Mol Pharmacol. 2007;72:1508–1521. doi: 10.1124/mol.107.038984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Imler T.J., Jr., Petro T.M. Decreased severity of experimental autoimmune encephalomyelitis during resveratrol administration is associated with increased IL-17+IL-10+ T cells, CD4(−) IFN-gamma+ cells, and decreased macrophage IL-6 expression. Int Immunopharmacol. 2009;9:134–143. doi: 10.1016/j.intimp.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 36.Fonseca-Kelly Z., Nassrallah M., Uribe J., Khan R.S., Dine K., Dutt M., Shindler K.S. Resveratrol neuroprotection in a chronic mouse model of multiple sclerosis. Front Neurol. 2012;3:84. doi: 10.3389/fneur.2012.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bergström T., Andersen O., Vahlne A. Isolation of herpes simplex virus type 1 during first attack of multiple sclerosis. Ann Neurol. 1989;26:283–285. doi: 10.1002/ana.410260218. [DOI] [PubMed] [Google Scholar]
- 38.Bray P.F., Culp K.W., McFarlin D.E., Panitch H.S., Torkelson R.D., Schlight J.P. Demyelinating disease after neurologically complicated primary Epstein-Barr virus infection. Neurology. 1992;42:278–282. doi: 10.1212/wnl.42.2.278. [DOI] [PubMed] [Google Scholar]
- 39.Faith S.A., Sweet T.J., Bailey E., Booth T., Docherty J.J. Resveratrol suppresses nuclear factor-κB in herpes simplex virus infected cells. Antiviral Res. 2006;72:242–251. doi: 10.1016/j.antiviral.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 40.Kapadia G.J., Azuine M.A., Tokuda H., Takasaki M., Mukainaka T., Konoshima T., Nishino H. Chemopreventive effect of resveratrol, sesamol, sesame oil and sunflower oil in the Epstein-Barr virus early antigen activation assay and the mouse skin two-stage carcinogenesis. Pharmacol Res. 2002;45:499–505. doi: 10.1006/phrs.2002.0992. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Immune responses in C57BL/6 mice fed a control diet or a diet containing resveratrol on days −1 to 8 (Early), days 14 to 23 (Late), or days −1 to 63 (Whole), 2 months after EAE induction. A: Mononuclear cells (MNCs) were isolated from draining lymph nodes in each group and incubated with or without MOG35-55 peptide for 5 days. Lymphoproliferation was assessed by a [3H]thymidine incorporation assay. All groups of mice had substantial MOG-specific lymphoproliferation. There was no significant difference in lymphoproliferative responses against MOG35-55 among the groups, although all three resveratrol-treated groups tended to have lower levels of lymphoproliferation than the control group. All cultures were performed in triplicate. Data are expressed as stimulation index (calculated as experimental cpm/control cpm). B and C: MNCs isolated from draining lymph nodes were stimulated with concanavalin A for 48 hours. The levels of IL-17 (B) and IFN-γ (C) production in the culture supernatant were measured by enzyme-linked immunosorbent assay (ELISA). There was no significant difference in cytokine production among the four groups. Data are expressed as means ± SEM of two or three pools of lymph nodes from two or three mice. n = 5 or 6 mice per group.
Immune responses in SJL/J mice fed a control diet (DA alone) or a diet containing resveratrol from day 35 to day 48 (Chronic), 2 months after TMEV infection. A: MNCs isolated from spleens were stimulated with TMEV antigen or live virus. Lymphoproliferation was assessed by a [3H]thymidine incorporation assay. Both groups of mice had similar levels of substantial TMEV-specific lymphoproliferation. All cultures were performed in triplicate. Data are expressed as stimulation index. B and C: MNCs isolated from spleens of each group were stimulated with concanavalin A for 48 hours. The levels of IL-17 (B) and IFN-γ (C) production in the culture supernatant were measured by ELISA. Mice treated with resveratrol during the chronic phase of TMEV infection had higher levels of IL-17 and lower levels of IFN-γ production than those fed a control diet, but the difference did not reach statistical significance. Data are expressed as means ± SEM of four pools of spleens from two mice. n = 8 mice per group.
Clinical scores and weight changes of TMEV-infected mice with or without resveratrol treatment. SJL/J mice were infected with the DA strain of TMEV on day 0 and fed a control diet (DA alone) or a diet containing resveratrol on days 0 to 14 (Acute) or days 21 to 35 (Subclinical). A: Mice fed a diet containing resveratrol during the acute phase or subclinical phase of TMEV infection had higher clinical scores of impairment of righting reflex than TMEV-infected mice fed a control diet, but the difference did not reach statistical significance. B: Mice treated with resveratrol during the acute phase of TMEV infection had significant weight gain, compared with control mice. ∗∗P < 0.01. n = 9 mice per group.
Neuropathology and numbers of TMEV antigen-positive cells, 1 month after infection. SJL/J mice were infected with the DA strain of TMEV and fed a control diet (DA alone) or a diet containing resveratrol on days 0 to 14 (Acute) or days 21 to 35 (Subclinical). Viral antigen-positive cells were visualized by immunohistochemistry with hyperimmune serum against TMEV, using the avidin–biotin–peroxidase complex technique with 3,3′-diaminobenzidine tetrahydrochloride (DAB) as chromogen. The numbers of viral antigen-positive cells were counted under a light microscope, using 10 to 12 transverse spinal cord segments per mouse. A (top): There was no difference in severity and distributions of neuropathology among the groups; demyelination (arrowheads), meningitis (arrows), and perivascular inflammation (paired arrows). Luxol fast blue staining. A (bottom): Similar levels of TMEV antigen-positive cells (arrows) were detected in the spinal cord in the three groups. B: Immunohistochemistry for TMEV antigen. There was no significant difference in the levels of demyelination, meningitis, perivascular cuffing (inflammation), and overall pathology scores among the groups. C: In the spinal cord, the Acute and Subclinical groups had similar numbers of TMEV antigen-positive cells as in the DA-alone group. D: In the brain, the Subclinical group had higher pathology scores than the DA-alone and Acute groups, but the difference did not reach statistical significance. Data are expressed as means ± SEM. n = 9 mice per group. Original magnification, ×53 (top row); ×107 (bottom row). Scale bars: 100 μm (top row); 50 μm (bottom row).
Immune responses in SJL/J mice fed a control diet (DA alone) or a diet containing resveratrol on days 0 to 14 (Acute) or days 21 to 35 (Subclinical), 1 month after TMEV infection. A: MNCs isolated from spleens were stimulated with TMEV antigen or live virus. Lymphoproliferation was assessed by a [3H]thymidine incorporation assay. All groups of mice had substantial TMEV-specific lymphoproliferation. There was no significant difference in lymphoproliferative responses against TMEV among the three groups. B: At the time of terminal perfusion, blood was collected from the heart of TMEV-infected mice. The levels of total IgG antibody against TMEV in serum were assessed by ELISA. There was no significant difference in the levels of serum TMEV-specific IgG antibody production. C and D: MNCs isolated from spleens were stimulated with concanavalin A for 48 hours. The levels of IL-17 (C) and IFN-γ (D) production in the culture supernatant were measured by ELISA. Mice treated with resveratrol during the acute phase or subclinical phase of TMEV infection had lower levels of IL-17 and IFN-γ production, compared with mice on a control diet. Data are expressed as means ± SEM of four pools of spleens from two or three mice. n = 9 mice per group. OD, optical density.
Neuropathology of GDVII virus infection. SJL/J mice were infected with the GDVII strain of TMEV and fed a control diet (GDVII alone) or a diet containing resveratrol (GDVII-resveratrol). Apoptosis was detected using the TUNEL method. TUNEL-positive cells were visualized by the avidin–biotin–peroxidase technique with DAB as chromogen. The numbers of TUNEL-positive cells in the brain were counted under a light microscope, using five coronal brain slabs per mouse. A (top): High levels of apoptosis of neurons were observed in both groups (pyramidal cell layers of the hippocampus). The TUNEL method revealed no difference in the levels of apoptotic cells (arrowheads) in the brain between the two groups. Boxed regions are shown at higher magnification in the corresponding inset. A (bottom): Immunohistochemistry for nonphosphorylated neurofilament revealed substantial numbers of degenerated axons (ventrolateral funiculus of the spinal cord). Similar levels of damaged axons (arrows) in the spinal cord were detected in the two groups. B: Numbers of damaged axons per quadrant of the spinal cord were similar in the two groups. C: There was no significant difference in the brain pathology score in the two groups. Data are expressed as means ± SEM. n = 4 to 6 mice per group. Original magnification, ×26 (top row); ×113 (insets); ×128 (bottom row). Scale bars: 250 μm (top row); 50 μm (bottom row).