Abstract
Fibrosis is characterized by accumulation of activated fibroblasts and pathological deposition of fibrillar collagens. Activated fibroblasts overexpress matrix proteins and release factors that promote further recruitment of activated fibroblasts, leading to progressive fibrosis. The contribution of epithelial cells to this process remains unknown. Epithelium-directed injury may lead to activation of epithelial cells with phenotypes and functions similar to activated fibroblasts. Prior reports that used a reporter gene fate-mapping strategy are limited in their ability to investigate the functional significance of epithelial cell-derived mesenchymal proteins during fibrogenesis. We found that lung epithelial cell-derived collagen I activates fibroblast collagen receptor discoidin domain receptor-2, contributes significantly to fibrogenesis, and promotes resolution of lung inflammation. Alveolar epithelial cells undergoing transforming growth factor-β–mediated mesenchymal transition express several other secreted profibrotic factors and are capable of activating lung fibroblasts. These studies provide direct evidence that activated epithelial cells produce mesenchymal proteins that initiate a cycle of fibrogenic effector cell activation, leading to progressive fibrosis. Therapy targeted at epithelial cell production of type I collagen offers a novel pathway for abrogating this progressive cycle and for limiting tissue fibrosis but may lead to sustained lung injury/inflammation.
Progressive fibrosis can occur as a serious complication of lung injury, as a sequel of many inflammatory chronic diseases, or as a primary disease, such as idiopathic pulmonary fibrosis (IPF).1 Progressive fibrosis often has a devastating clinical course without good therapeutic options. Tissue fibrosis is characterized by accumulation of activated fibroblasts and extensive matrix remodeling. Primary functions of activated fibroblasts include deposition of fibrotic matrix proteins and secretion of profibrotic factors.1–4 The cellular source of activated fibroblasts remains unknown and controversial.5 The traditional model has been that resident fibroblasts respond to injury by proliferating and acquiring a profibrotic, activated phenotype accounting for all of the deposition of fibrotic matrix proteins. A newer hypothesis is that structural cells with normal physiological functions can respond to injury by down-regulating some of their physiological proteins and activities in favor of a profibrotic phenotype that overlaps with activated fibroblast activities. Proposed cells that can respond in this way include epithelial cells, endothelial cells, and pericytes. A third possible source of fibrillar collagens are from circulating bone marrow-derived fibrocytes that can be rapidly recruited to sites of injury. These different possibilities are not mutually exclusive and may potentially have nonredundant functions during fibrogenesis.5–9 In addition to the secretion of fibrotic matrix proteins, activated fibroblasts have an important function in recruiting more activated fibroblasts through secretion of specific profibrotic factors in response to transforming growth factor (TGF)-β–mediated activation.2,5 Thus, a proposed model is that injury leads to activation of structural lung cells toward a profibrotic phenotype similar to activated fibroblasts, leading to early fibrotic matrix deposition and activation of other profibrotic effector cells. Understanding these events may lead to new targets for inhibiting progressive fibrogenesis.
Activation of lung epithelial cells during fibrogenesis is an attractive hypothesis, given the likely propensity for environmental lung epithelial injury and the extensive animal model and human data suggesting that epithelial cell dysregulation is an important contributor of lung fibrogenesis.10–13 We and others have identified expression of mesenchymal genes, such as type I collagen, within epithelial cells of human fibrotic lung tissue, suggesting partial mesenchymal transition.14–16 A number of different lung epithelial cells, including alveolar and airway epithelial cells, can undergo epithelial-mesenchymal transitions (EMTs) in vitro in response to cytokines known to be highly expressed in the fibrotic lung.17–20 Fate-mapping studies that use an epithelial cell-specific Cre transgene and a lox-stop-lox reporter transgene to permanently and specifically label epithelial cells were proposed as a definitive method for proving or disproving EMTs during animal models of fibrogenesis.21 However, these studies have severe limitations leading to continued controversy.22 For example, in the lung at least three separate groups have identified EMTs during fibrogenesis by this technique, although a recent report failed to find evidence of EMTs.23–26 A similar controversy exists in fibrogenesis of the kidney and liver.6,21,22,27–29 One of the points of controversy has been the potential for artifact from overlapping cells, leading to the appearance of a cell costaining the reporter protein and a mesenchymal protein. The costaining approach lends itself to cytoskeletal or cell surface protein such as vimentin, α-smooth muscle actin (SMA), fibroblast-specific protein, or N-cadherin, but these proteins are of uncertain functional significance to fibrogenesis. Most importantly, gene expression studies in human samples and the murine fate-mapping reporter gene costaining approaches are both ultimately descriptive without addressing these more important questions: to what extent and in what way do different cell types contribute to fibrosis? Several recent reports have found that epithelial cell-specific deletion of EMT transcription factors and cell surface receptors can dramatically attenuate fibrosis well beyond the fractional contribution of epithelial-derived cells to the pool of activated fibroblasts, suggesting that activated epithelial cells may have a nonredundant function during fibrogenesis.30–33
The present study offers a new and more functional approach to understanding the contribution of epithelial cells. We have developed a novel mouse model with conditional deletion of type I collagen that enables lung epithelial cell-specific deletion of this gene to determine the ultimate consequences on fibrogenesis. In addition to type I collagen, we found that activated lung epithelial cells express a number of profibrotic proteins and can activate lung fibroblasts. These studies confirm an early and important epithelial cell expression of type I collagen and shift the focus away from arbitrary definitions of EMTs to better understanding the function of epithelial cells during fibrogenesis.
Materials and Methods
Mice
The floxed collagen, type I, alpha 1 (Col1a1) targeting vector was generated with standard cloning and recombineering techniques to incorporate loxP sites within introns 2 and 5 and a FRT [flippase (Flp) recombination target]-flanked neomycin resistance gene within intron 5 (Supplemental Figure S1). Embryonic stem (ES) cell electroporation, ES cell culture, and blastocyst injection to generate chimera were performed by inGenious Targeting Laboratory (Ronkonkoma, NY). ES clones were screened by PCR and Southern blot analysis. Southern blot analysis was performed as previously described.34 ES cell DNA was digested with SphI and 5′ probe that was generated with the following primers: forward, 5′-AATAGTGTTATGCTCTGGTTTC-3′ and reverse, 5′-CTGCAGTGGCTAGAAAAGTCA-3′. The 5′ probe recognizes a 23-kb DNA fragment for wild-type (WT) ES cells and a 15-kb fragment for correctly targeted floxed Col1a1 ES cells. ES cell DNA also was digested with HindIII and 3′ probe generated with the following primers: forward, 5′-CTGGTCGCCCCGGTGA-3′ and reverse, 5′-GACTTGGGTGTGACTATCACATAAAAAGACC-3′. The 3′ probe recognizes a 10-kb DNA fragment for WT ES cells and a 5-kb DNA fragment for correctly targeted floxed Col1a1 ES cells. For PCR analysis of floxed Col1a1, forward primer (F1), 5′-ATCCATCATGGCTGATGCAATGCG-3′, located with neomycin resistance and reverse primer (R1), 5′-TGACTTACGGGTTCTCCTTTGGCA-3′, located 7 kb from the start site confirm integration of targeting vector to the Col1a1 gene. Forward primer (F2), 5′-GGTAGCTCTGGCATGCATAAC-3′, overlaps with 5′ loxP site and reverse (R2), 5′-AGCTAGCTTGGCTGGACGTAAACT-3′, within neomycin resistance confirm integration of the 5′ loxP site and neomycin gene on the same allele. Forward primer (F3), 5′-TGGTACAGCACTTTACAGCGCACA-3′, located upstream of 5′ loxP insertion site and reverse prime (R3), 5′-TTACTCGGCCTGGGTCACTTCTTT-3′, are located downstream of 5′ loxP insertion site; WT allele yields a 138-bp PCR product, floxed Col1a1 with insertion of 40-bp loxP, and SphI site yields a 178-bp band. Removal of neomycin gene confirmed by PCR using forward primer (F4), 5′-ACTGTCCTCCAATAAACTGCAGTTTTCTTT-3′, located upstream of neomycin insertion site and reverse primer (R4), 5′-TAGCAGTAATGGGACAAACGGATGTAG-3′, located downstream of neomycin insertion site. Mice with constitutive expression of flpE (The Jackson Laboratory, Bar Harbor, ME) were used to delete the FRT-flanked neomycin resistance gene. Floxed Col1a1 mice were backcrossed with C57bl/6 mice for at least eight generations.
To delete floxed Col1a1 in lung epithelial cells mice were crossed with mice expressing the surfactant proteins-C promoter-reverse tetracycline transactivator (SPC-rtTA) and tetO-CMV (cytomegalovirus) promoter-Cre recombinase (tetO-Cre) transgenes as previously described.24 Triple transgenic SPC-rtTA/tetO-Cre/Col1a1f/f (homozygous floxed Col1a1) mice are abbreviated SCcol. Smad3-null mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Mice were genotyped by PCR. Six- to 8-week-old mice were given 50-μL intratracheal injections of saline or saline with dissolved bleomycin (1.3 U/kg) via surgical tracheotomy. At various time points after injection, mice were euthanized, and bronchoalveolar lavage (BAL) and lung samples were collected for analysis. For BAL, the lungs were lavaged with 1 mL of PBS per mouse. Samples were centrifuged, and the supernatants were used for total protein count and immunoblot analysis. The cell pellets were resuspended for total cell counting with a hemocytometer under light microscopy. Lung physiological measurements were obtained from 6- to 8-week-old anesthetized SCcol mice and littermate controls as previously described.32,35
All mice were bred and maintained in a specific pathogen-free environment, and all animal experiments were approved by the University Animal Care and Use Committee at the University of Michigan.
Reagents
Plasma fibronectin (FN), bleomycin, and phospho-Smad2 antibody were purchased from Millipore (Billerica, MA); collagen I, Matrigel (MG), biotin-conjugated rat anti-mouse CD16/32 and CD45 antibodies, and 70-μm and 40-μm nylon filters from BD Biosciences (San Jose, CA); purified human keratinocyte growth factor from PeproTech (Rocky Hill, NJ); purified human TGF-β1 from R&D Systems (Minneapolis, MN); small airway basal media and small airway growth media (SAGM) from Lonza Inc. (Allendale, NJ); horseradish peroxidase-conjugated secondary antibodies from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA); immunofluorescent-conjugated secondary antibodies, streptavidin-coated magnetic beads, and magnetic particle separator from Invitrogen (Carlsbad, CA); collagen I and discoidin domain receptor-2 (DDR2) antibodies from Abcam (Cambridge, MA); horseradish peroxidase-conjugated phospho-tyrosine and glyceraldehyde phosphate dehydrogenase (GAPDH) antibodies from Cell Signaling Technology Inc. (Danvers, MA); adenoviruses expressing Cre (AdCre) or green fluorescent protein (AdGFP) were obtained from the University of Iowa Gene Transfer Vector Core Facility; and dispase from Roche (Indianapolis, IN). All other reagents were purchased from Sigma-Aldrich (St. Louis, MO).
Mouse Fibroblast and Type II Cell Isolation and Culture
Cells were cultured in a 37°C, 5% CO2 incubator. Murine primary lung fibroblasts were harvested from 6- to 10-week-old mice as described previously.36 Cells were used at passages two to four. In some experiments cells were treated with three daily doses of AdGFP or ADCre (50 pfu/cell) and then cultured for an additional 7 days before analyzing the cells. Adult murine fibroblast cell line, MLg (CCL-206), was purchased from the American Type Culture Collection (Manassas, VA) and also maintained in Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal bovine serum, penicillin, and streptomycin. Murine type II alveolar epithelial cells (AECs) were isolated as previously described with minor modifications.24,37 Briefly, after mice were sacrificed, lungs were exposed, perfused, and lavaged. Lungs were filled with dispase, followed by low-melt agarose. Lungs were digested at room temperature for 45 minutes then dissected. Dissected lungs were then treated with DNase for 10 to 15 minutes, then serially filtered through 70-μm, 40-μm, and 20-μm filters. The crude suspensions were then labeled for CD16/32 and CD45 with the use of biotin-conjugated antibodies, streptavidin-conjugated magnetic beads, and a magnetic separator. AECs were further negatively selected by incubating for 1 to 2 hours on a tissue-culture–treated plastic plate.
H&E Staining and Masson’s Trichrome Assay
Lungs from sacrificed mice were inflated to a pressure of 25 cm H2O and fixed with formaldehyde. Lungs were embedded in paraffin, sectioned, and stained with H&E and Masson’s trichrome by the McClinchey Histology Laboratory (Stockbridge, MI).
Hydroxyproline Assay
Hydroxyproline was measured by methods previously described.36 Briefly, homogenates from the entire lungs were baked in 12N HCl overnight at 120°C. Aliquots of the samples were added to citrate buffer and chloramine T solution and incubated at room temperature for 20 minutes. Erlich’s solution was added, and the samples were incubated at 65°C for 15 minutes. Absorbance at 540 nm was measured. Hydroxyproline content was quantified by comparison against a hydroxyproline standard curve.
Immunofluorescence Staining
Lungs were perfused with PBS, inflated with intratracheal OCT, removed, and immediately frozen in a dry-ice alcohol bath. Lungs were stored at −80°C. Seven-micron lung sections were stained as previously described.24 Stained sections were visualized on an Olympus BX-51 fluorescence microscope (Olympus, Tokyo, Japan), and images were captured with an Olympus DP-70 camera and analyzed with DP controller software version 3.1.1.267.
Collagen I Deletion in Fibroblasts
Col1a1f/f lung fibroblasts were treated with three daily doses of AdGFP or AdCre (50 pfu/cell). After 1 week equal numbers of cells were plated on tissue culture plastic or collagen I-coated plates. Cells were maintained in DMEM and cultured on collagen I-coated plates, which were treated with an additional 10 μg/mL collagen I. After 24 hours, RNA was collected for gene expression analysis. In some experiments, AdCre-treated Col1a1f/f fibroblasts were seeded on six-well tissue culture plates and then stimulated with 1 mL of DMEM supplemented with 0.5 mL of BAL fluid from control and SCcol mice collected 1 week after saline or bleomycin injury or treated with 10 μg/mL purified collagen I. After 15 minutes cells were lyzed and analyzed for DDR2 phosphorylation.
CM Stimulation
AECs were cultured on either MG- or FN-coated six-well plates. Some wells were treated with 10 μmol/L TGF-β receptor inhibitor SB431642, 5 pfu per cell lentivirus-expressing siRNA, or 50 pfu/cell AdGFP or AdCre. Conditioned media (CM) was generated by changing the AEC media to serum-free SAGM plus 0.1% bovine serum albumin 24 to 48 hours before collection (free of inhibitors or lentivirus). Harvested CM was filtered, then either stored at 80°C or immediately added to cultured lung fibroblasts. After 48 hours, fibroblasts were lyzed for various assays.
Gene Expression Analysis
RNA was isolated from cells and tissue with TRIzol Reagent (Invitrogen) according to the manufacturer. Reverse transcription was performed with the SuperScript III First-strand synthesis kit (Invitrogen), and RT-PCR was performed with POWER SYBR Green PCR MasterMix Kit (Applied Biosystems, Foster City, CA) and Applied Biosystems 7000 sequence detection system. The relative expression levels of gene were calculated against β-actin or GAPDH. Eighty-four genes related to mouse fibrosis plus five housekeeping genes were evaluated by RT-PCR (RT2 Profiler PCR Arrays PAMM-120A; Qiagen, Valencia, CA) according to the manufacturer. The gene expression level was normalized to the average of five housekeeping genes and was calculated as 2−ΔΔCt. PCR primers for floxed Col1a1 DNA recombination were as follows: forward, 5′-GGTAGCTCTGGCATGCATAAC-3′, and reverse, 5′-AGCTAGCTTGGCTGGACGTAAACT-3′. PCR of cDNA isolated from whole lungs was used to detect the truncated Col1a1 with forward primer 5′-ATGGCCAAGAAGACAAACTTT-3′ (overlaps adjacent exon 1 and 6) and reverse primer 5′-GGCCTTGGAAACCTTGTGGAC-3′. RT-PCR was performed with the Power SYBR Green PCR MasterMix Kit (Applied Biosystems). Relative expression levels of genes in fold change were calculated against β-actin or GAPDH. The following primers were used: collagen 1a1, forward, 5′-TGACTGGAAGAGCGGAGAGTACT-3′, and reverse, 5′-GGTCTGACCTGTCTCCATGTTG-3′; connective tissue growth factor (CTGF), forward, 5′-CACTCCGGGAAATGCTGCAAGGAG-3′ and reverse, 5′-GTTGGGTCTGGGCCAAATGT-3′; collagen, type III, alpha 1 (Col3a1), forward, 5′-CAAGGTCTTCCTGGTCAGCCT-3′ and reverse, 5′-TGCCACCAGGAGATCCATCTC-3′; α-SMA, forward, 5′-AGAGACTCTCTTCCAGCCATC-3′ and reverse, 5′-GACGTTGTTAGCATAGAGATC-3′; β-actin, forward, 5′-CCGTGAAAAGATGACCCAGATC-3′ and reverse, 5′-CACAGCCTGGATGGCTACGT-3′; GAPDH, forward, 5′-AACTTTGGCATTGTGGAAGG-3′ and reverse, 5′-ACACATTGGGGGTAGGAACA-3′. Other primers used have been previously reported.38–41
Immunoblot Analysis
Immunoblot analysis and immunoprecipitation of tissue and cells were performed as previously described.24 Scanned immunoblots are representative of at least three separate experiments, and densitometry was quantified by ImageJ software version 1.47 (NIH, Bethesda, MD).
Statistical Analysis
Data were expressed as means ± SEM. For evaluation of group differences, the two-tailed Student’s t-test was used assuming equal variance. A P value of <0.05 was accepted as significant.
Results
Generation and Validation of Mice with Lung Epithelial Cell-Specific Deletion of Col1a1
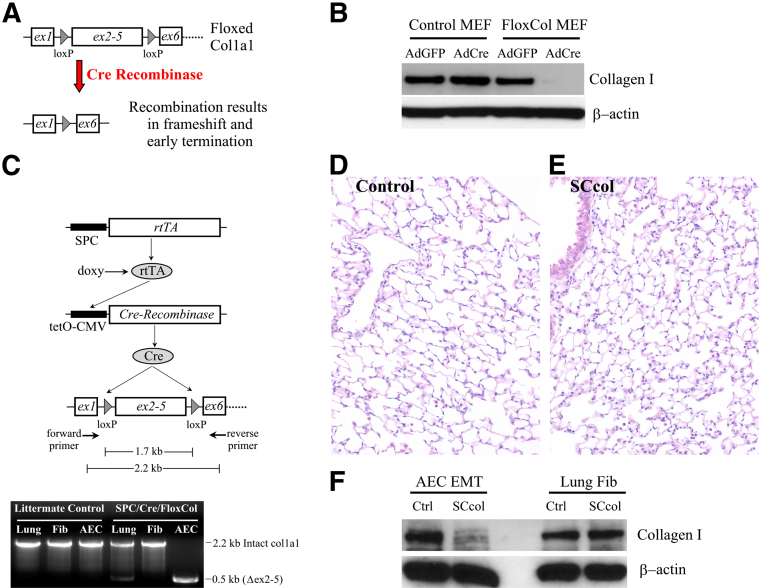
Floxed Col1a1 mice were generated by integrating loxP sites within introns 1 and 5 of the Col1a1 gene which enabled Cre-mediated deletion of exons 2 to 5, resulting in frameshift and early translational termination of the truncated mRNA (Figure 1A and Supplemental Figure S1). Conditional deletion of collagen I expression was confirmed with the use of murine embryonic fibroblast (MEF) cells from Col1a1f/f embryos. Col1a1f/f MEF cells treated with an AdCre had near complete loss of collagen I compared with cells treated with a control AdGFP or control MEFs (Col1a1w/w) treated with either AdCre or AdGFP (Figure 1B and Supplemental Figure S2A). Lung epithelial cell-specific recombination was achieved by crossing with mice carrying the human SPC-rtTA and tetO-CMV-Cre transgenes (Figure 1C).24 Recombination within lung epithelial cells was verified with PCR primers encompassing the floxed region. DNA from lungs of SCcol mice showed a 2.2-kb PCR product that corresponded to non-recombined flox Col1a1 in nonepithelial cells and a 0.5-kb PCR product consistent with removal of the 1.7-kb floxed region within epithelial cells. PCR of AECs from SCcol mice yielded only the 0.5-kb recombined band, whereas PCR from lung fibroblasts yielded only the 2.2-kb non-recombined band. DNA from littermate control mice, lacking one of the transgenes, only produced the 2.2-kb PCR product. We and others have previously reported that primary AECs cultured on provisional matrix proteins such as FN undergo EMTs through production and activation of endogenous TGF-β1.24,42 AECs from SCcol mice expressed significantly reduced levels of collagen I after FN-mediated EMTs compared with littermate control AECs (Figure 1F and Supplemental Figure S2B). Lung fibroblasts from SCcol mice showed similar levels of collagen I as control lung fibroblasts. Thus, SCcol mice have robust lung epithelial cell-specific loss of Col1a1. As expected SCcol mice have normal histology (Figure 1, D and E) and physiology (Table 1) because lung epithelial cells express little to no collagen I under physiological conditions.14,24
Figure 1.
Generation and validation of mice with lung epithelial-specific deletion of Col1a1. A: Schematic of floxed Col1a1 (Col1a1f/f) mice generated by inserting loxP sites flanking exons 2 to 5 in the Col1a1 gene. Cre-mediated recombination of the floxed Col1a1 leads to removal of exons 2 to 5, shift in the translational reading frame in subsequent exons, and permanent inactivation of collagen I expression. B: MEFs isolated from Col1a1f/f mice have deletion of collagen I expression after AdCre, whereas cells treated with AdGFP or WT Col1a1 MEFs treated with AdGFP or AdCre have normal expression of collagen I by immunoblot analysis. C: Lung epithelial cell-specific and permanent deletion of Col1a1 is achieved with the use of transgenic mice that carry the SPC-rtTA and tetO-Cre. Triple transgenic SPC-rtTA/tetO-Cre/Col1a1f/f mice are abbreviated SCcol. The SPC promoter yields rtTA expression specifically within lung epithelial cells, in the presence of doxycycline, rtTA activates the tetO-CMV promoter, leading to expression of cre recombinase and permanent deletion of DNA flanked by loxP sites. Deletion within lung epithelial cells is confirmed by PCR with the use of primers that encompass the floxed region. Intact floxed Col1a1 yields a 2.2-kb PCR product, and removal of the floxed Col1a1 yields a 0.5-kb PCR product. D and E: Uninjured lungs from SCcol (E) mice have normal histology compared with littermate genotype control mice (D), by H&E staining. F: AECs from SCcol mice have diminished EMT-induced expression of collagen I by immunoblot analysis compared with AECs from littermate control mice lacking one of the three transgenes. Primary lung fibroblasts from SCcol mice have similar collagen I expression compared with control lung fibroblasts, verifying robust lung epithelial-specific deletion of Col1a1. Original magnification: ×200 (D and E). AdCre, adenoviral-mediated expression of Cre; AdGFP, adenovirus expressing green fluorescent protein; AEC, Alveolar epithelial cell; Col1a1, collagen, type I, alpha 1; Ctrl, control; EMT, epithelial-mesenchymal transition; Fib, fibroblast; MEF, murine embryonic fibroblast; SPC-rtTA, surfactant proteins-C promoter-reverse tetracycline transactivator; tetO-Cre, tetO-cytomegalovirus promoter-Cre recombinase; WT, wild-type.
Table 1.
Baseline Physiology of Littermate Control and SCcol mice
| Control | SCcol | P value | |
|---|---|---|---|
| IC (mL) | 0.473 ± 0.027 | 0.467 ± 0.017 | 0.83 |
| VC (mL) | 0.562 ± 0.034 | 0.559 ± 0.019 | 0.93 |
| ERV (mL) | 0.089 ± 0.009 | 0.092 ± 0.003 | 0.73 |
| FRC (mL) | 0.586 ± 0.017 | 0.575 ± 0.012 | 0.64 |
| TLC (mL) | 1.059 ± 0.038 | 1.042 ± 0.029 | 0.71 |
| RV (mL) | 0.497 ± 0.016 | 0.483 ± 0.013 | 0.53 |
| Cch (mL/cm H2O) | 0.034 ± 0.002 | 0.034 ± 0.002 | 0.82 |
Values indicate means ± SEM. n = 9 to 10 per group.
Cch, chord compliance; ERV, expiratory reserve capacity; FRC, functional reserve capacity; IC, inspiratory capacity; RV, residual volume; SCcol, triple transgenic surfactant proteins-C promoter-reverse tetracycline transactivator/tetO-cytomegalovirus promoter-Cre recombinase/homozygous floxed collagen, type I, alpha 1; TLC, total lung capacity; VC, vital capacity.
Mice with Lung Epithelial Cell Deletion of Col1a1 Have Abnormal Response to Injury
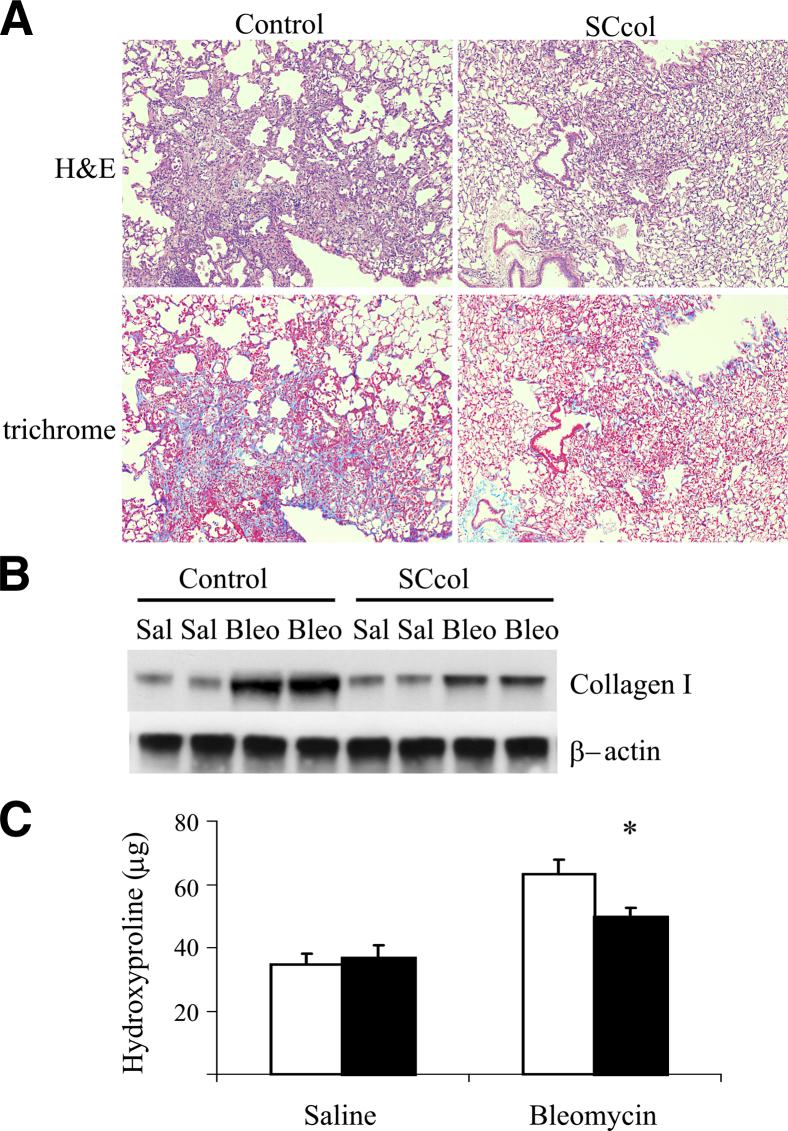
Groups of SCcol mice and littermate control mice lacking at least one of the transgenes were given intratracheal saline or bleomycin. Genotype control mice developed robust fibrosis after bleomycin, visualized by trichrome and H&E staining. In contrast, SCcol lungs appeared injured but had less fibrosis (Figure 2A and Supplemental Figure S3, A and B), resulting in similar physiological impairment (Supplemental Table S1). Immunoblot analysis of lung lysates indicated that control mice have increased levels of collagen I in response to bleomycin, whereas collagen I up-regulation was attenuated in SCcol mice (Figure 2B and Supplemental Figure S4). Hydroxyproline analysis also showed a robust increase in collagen deposition in bleomycin-injured control mice and an attenuated response in SCcol mice (Figure 2C).
Figure 2.
Lung epithelial cell-derived type I collagen contributes to fibrogenesis. A: Lung sections from littermate control and SCcol mice 3 weeks after bleomycin injection, stained with H&E and trichrome. Original magnification, ×100. Genotype control mice have robust fibrosis compared with SCcol mice. B: Whole lung lysate from mice 3 weeks after treatment with saline or bleomycin was analyzed for collagen I by immunoblot analysis. Control mice have robust induction of collagen I after bleomycin compared with SCcol mice. C: Hydroxyproline assay from entire lungs 3 weeks after saline or bleomycin in SCcol (black bars) or littermate control (white bars) mice. SCcol mice have less fibrosis after bleomycin (n = 6 to 15 per group). ∗P < 0.05 versus control bleomycin. Bleo, bleomycin; Sal, saline; SCcol, triple transgenic surfactant proteins-C promoter-reverse tetracycline transactivator/tetO-cytomegalovirus promoter-Cre recombinase/homozygous floxed collagen, type I, alpha 1.
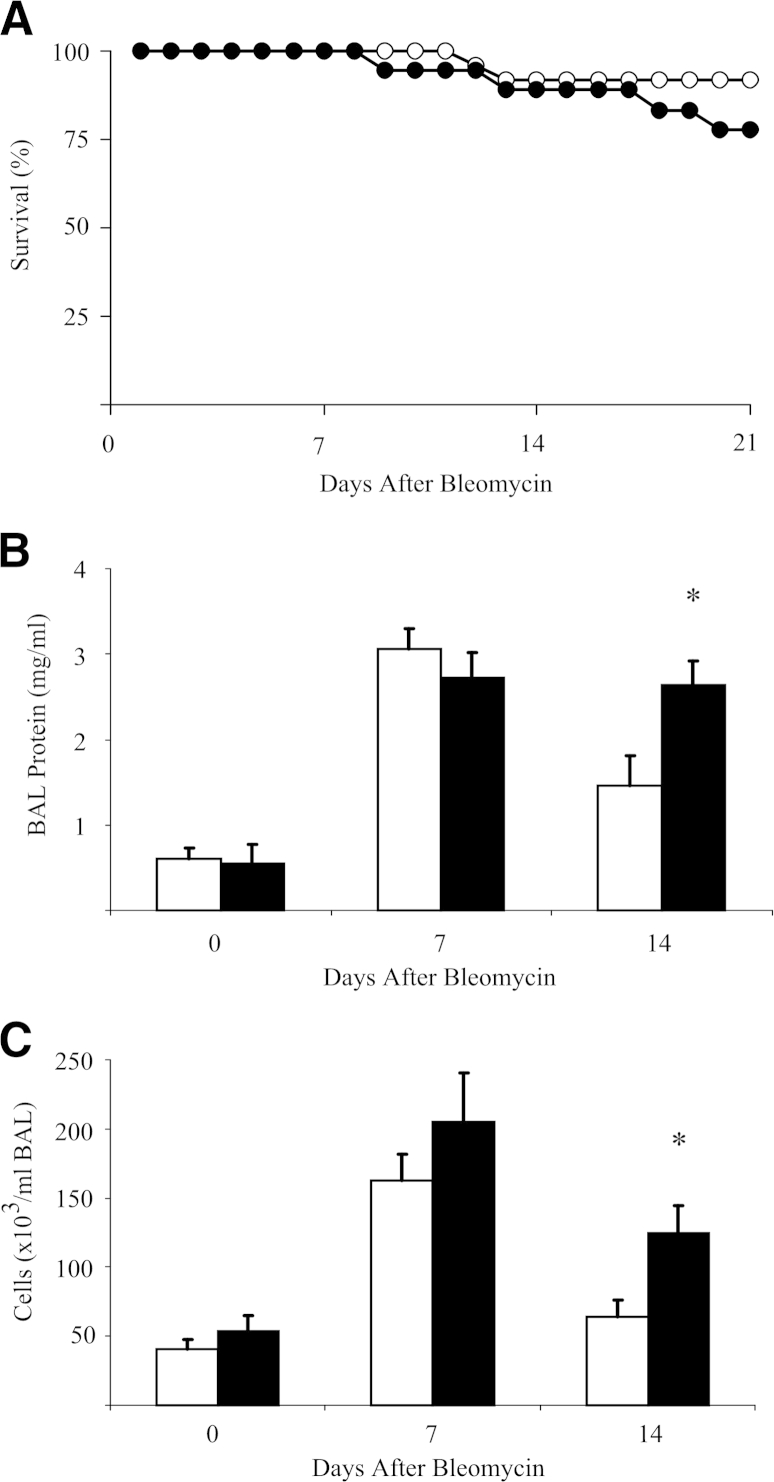
Surprisingly, a trend was observed for more death in the SCcol mice after bleomycin treatment than in the genotype control mice. No deaths were found in either saline treatment group (Figure 3A). To determine whether loss of collagen deposition in SCcol mice led to increased or sustained lung injury/inflammation we measured total protein and cell count in the BAL fluid. Genotype control mice and SCcol mice have a similar approximate fivefold increase in BAL protein and an approximate threefold increase in BAL cell count 1 week after bleomycin. However, 2 weeks after bleomycin, control mice have a reduction in BAL protein and cell count, whereas persistent elevation was observed in SCcol mice (Figure 3, B and C).
Figure 3.
Lung epithelial cell-derived collagen I inhibits sustained lung inflammation. A: Survival of littermate control (white circles; n = 25) and SCcol (black circles; n = 18) mice after bleomycin. SCcol mice have a trend toward less survival. B and C: BAL from control (white bars) and SCcol (black bars) mice at 0, 7, and 14 days after bleomycin injury was analyzed for protein (B), and cell count (C) shows that SCcol and control mice have similar increases in proteins and cell count 1 week after bleomycin, whereas SCcol mice have greater persistence of protein, n = 5 to 7 per group. ∗P < 0.05 versus control bleomycin. BAL, bronchoalveolar lavage; SCcol, triple transgenic surfactant proteins-C promoter-reverse tetracycline transactivator/tetO-cytomegalovirus promoter-Cre recombinase/homozygous floxed collagen, type I, alpha 1.
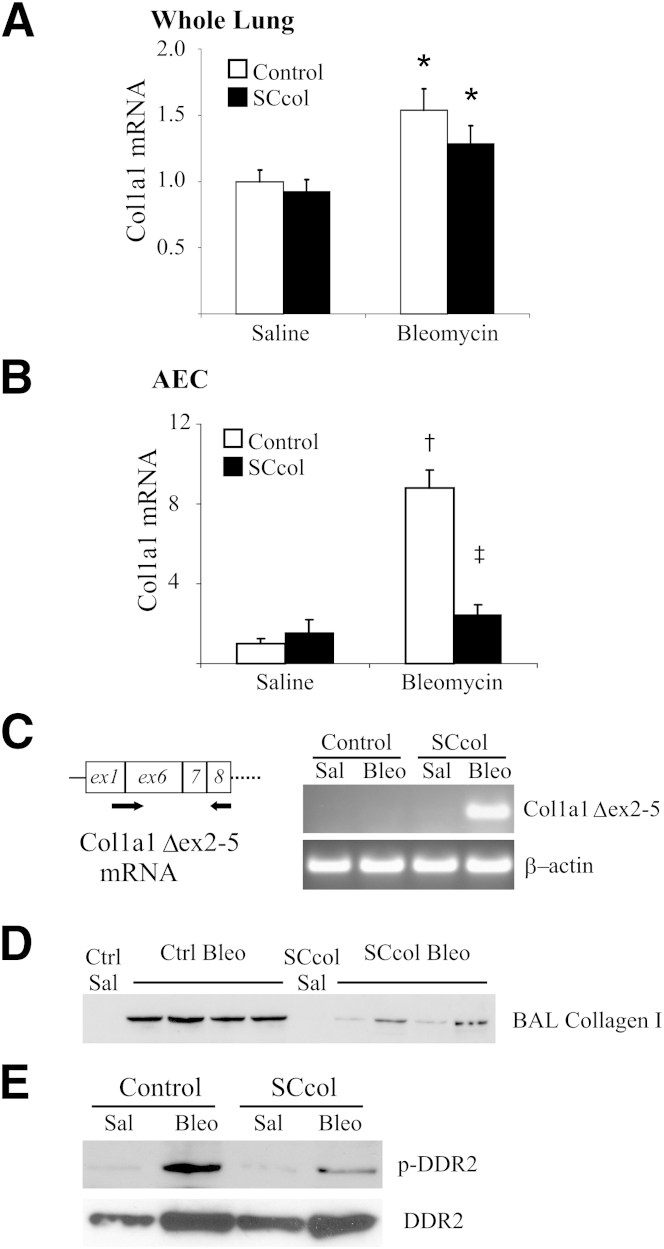
Because the trend toward increased death in SCcol mice may affect the differences observed in the hydroxyproline and collagen I immunoblot analysis, we wanted to assess for differences in collagen I production at a time point before bleomycin-induced death. One week after bleomycin, mRNA from whole lungs of genotype control mice and SCcol mice had a similar increase in Col1a1 mRNA compared with saline-treated mice (Figure 4A). However, mRNA from AECs isolated 1 week after bleomycin indicated an increased expression of Col1a1 within control AECs and a significant attenuation within isolated SCcol AECs (Figure 4B). Deletion of the floxed exons 2 to 5 resulted in frameshift and early termination of translation; however, transcription and generation of truncated Col1a1 mRNA lacking exons 2 to 5 (Col1a1 Δex2-5) may still occur within lung epithelial-derived cells. PCR primers were generated to detect the presence of a truncated Col1a1 Δex2-5 mRNA in which exon 1 is directly adjacent to exon 6. Indeed, mRNA from lungs of bleomycin-treated SCcol mice showed the presence of Col1a1 Δex2-5 mRNA, indicating transcription of Col1a1 specifically within lung epithelial-derived cells (Figure 4C). Collectively, these studies indicated bleomycin-induced expression of Col1a1 within lung epithelial cells. Furthermore, genotype control mice had small foci of collagen I deposition 1 week after bleomycin, and this was largely absent in SCcol mice (Supplemental Figure S5, A and B). Several studies have shown soluble procollagens in the BAL fluid of patients with fibrotic lung disease.43–45 BAL samples from SCcol and littermate control mice were collected 1 week after bleomycin or saline treatment and were analyzed by immunoblot for collagen I (Figure 4D and Supplemental Figure S6A). A dramatic increase was observed in collagen I in the BAL fluid of bleomycin-injured control mice and attenuation in the SCcol mice treated with bleomycin, indicating that accumulation of collagen I in the BAL after bleomycin is dependent on the presence of an intact Col1a1 gene within lung epithelial cells. Essentially, no detectable collagen I was observed in the saline-treated mice. We next wanted to determine whether deficient collagen accumulation could lead to attenuated collagen signaling. DDR2 has been identified as a novel receptor tyrosine kinase that uses type I collagen as its major ligand.46 Whole lung lysates were analyzed by immunoprecipitation against DDR2, followed by immunoblot analysis for phospho-tyrosine. As expected, genotype control mice had increased phosphorylation of DDR2 in response to bleomycin, whereas SCcol mice had attenuated DDR2 phosphorylation, indicating deficient collagen signaling in these mice (Figure 4E and Supplemental Figure S6, B and C). Collectively, these results support an important contribution of type I collagen by lung epithelial cells after lung injury.
Figure 4.
Lung epithelial cell-deletion of collagen I attenuates collagen I accumulation acutely after bleomycin treatment. A: Whole lung mRNA was analyzed for Col1a1 mRNA 1 week after bleomycin or saline, n = 4 to 9. ∗P < 0.05 versus control saline. Difference between control (white bars) and SCcol (black bars) bleo is not statistically significant. P = 0.3. B: mRNA from AECs isolated 1 week after bleomycin or saline, n = 4. †P < 0.01 versus control saline; ‡P < 0.01 versus control bleomycin. C: Whole lung mRNA was analyzed with primers specific for a truncated Col1a1 mRNA missing exons 2 to 5 (Col1a1 Δex2-5), forward primer bridged the adjacent exons 1 and 6, and reverse primer was within exon 8. Col1a1 Δex2-5 was detected in SCcol mice 1 week after bleomycin, indicating native promoter activation and transcription of the recombined Col1a1 gene within lung epithelial cells after bleomycin. D: Immunoblot of 50 μL of a 1-mL BAL from littermate control and SCcol mice 1 week after saline or bleomycin. Bleomycin induces collagen I accumulation in BAL of control mice. BAL of SCcol mice have less collagen I accumulation after bleomycin. E: One week after bleomycin, SCcol mice have attenuated activation of the collagen receptor DDR2 observed by immunopreciptiation of whole lung lysate for DDR2 followed by immunoblot analysis for phosphotyrosine. Immunoblot of 1% of the whole lung lysate indicates increase in DDR2 expression after bleomycin in SCcol and control mice. AEC, alveolar epithelial cell; BAL, bronchoalveolar lavage; Bleo, bleomycin; Ctrl, control; DDR2, discoidin domain receptor 2; Sal, saline; SCcol, triple transgenic surfactant proteins-C promoter-reverse tetracycline transactivator/tetO-cytomegalovirus promoter-Cre recombinase/homozygous floxed collagen, type I, alpha 1.
AEC Activation Promotes Fibroblast Activation
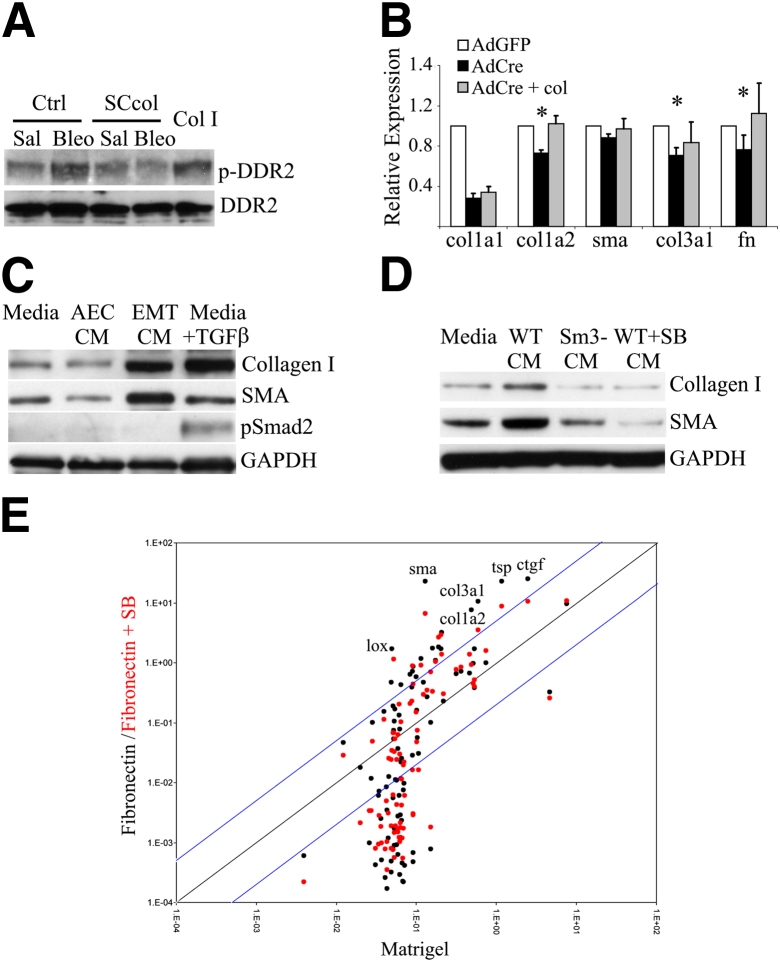
An initial collagen deposition by epithelial cells may promote activation and collagen production by lung fibroblasts. Prior studies of collagen signaling within cultured fibroblasts are limited because of significant expression of collagen I in the confined space of the culture dish. To address this problem we isolated lung fibroblasts from Col1a1f/f mice. AdCre-treated Col1a1f/f lung fibroblasts were stimulated with BAL from control and SCcol mice collected 1 week after saline or bleomycin injury. Fifteen minutes after BAL stimulation cells were lysed and analyzed for phospho-DDR2. BAL from control mice injured with bleomycin induced DDR2 phosphorylation, indicating the presence of bioactive collagen. DDR2 phosphorylation was not induced by BAL from SCcol mice (Figure 5A and Supplemental Figure S7A). To determine the influence of type I collagen signaling on fibroblast extracellular matrix (ECM) production, Col1a1f/f lung fibroblasts were treated with AdCre to delete Col1a1 or AdGFP as a control. Lung fibroblasts with Cre-mediated deletion of Col1a1 had decreased expression of Col1a1, as expected, and some suppression of Col1a2, Col3a1, and FN expression. Culturing Cre-treated Col1a1f/f lung fibroblasts in the presence of collagen I reversed the effects of AdCre treatment, suggesting that the presence of collagen I modestly enhances transcription of these genes within lung fibroblasts (Figure 5B). Treating WT or AdGFP-treated Col1a1f/f cells with exogenous type I collagen had little effect on the expression of these genes. Given the unexpected sustained inflammation in SCcol mice after bleomycin, AdGFP- and AdCre-treated Col1a1f/f lung fibroblasts were also analyzed for expression of several inflammatory cytokines. Surprisingly, deletion of Col1a1 led to increased expression of IL-1α, IL-1β, and tumor necrosis factor α (Supplemental Figure S8A).
Figure 5.
Alveolar epithelial cell-derived factors promote fibroblast activation. A: DDR2 phosphorylation of collagen I-deleted fibroblasts by BAL from control and SCcol mice collected 1 week after saline or bleomycin injury. Cells treated with 10 μg/mL exogenous type I collagen and cells treated with BAL from bleomycin-injured control mice have induction of DDR2 phosphorylation. B: Reverse transcription-PCR of primary lung fibroblasts from Col1a1f/f mice treated with AdGFP, AdCre, or AdCre plus exogenously added type I collagen (collagen I-coated plus 10 μg/mL added to media). Loss of collagen I expression led to reduction in expression of several fibroblast activation markers; the expression of these genes was restored by exogenous collagen. C: Immunoblot of lung fibroblasts stimulated with plain media, CM from AECs cultured on Matrigel (AEC CM), CM from AECs cultured on FN (EMT CM), and media supplemented with 4 ng/mL TGF-β. TGF-β and EMT CM activate fibroblast expression of α-SMA and collagen I. TGF-β promotes smad2 phosphorylation but EMT CM does not. D: Immunoblot of lung fibroblasts stimulated with plain media, CM from WT AECs cultured on FN (WT CM), smad3-null AECs (Sm3-CM) cultured on FN, and WT AECs cultured on FN with addition of TGFβ receptor inhibitor SB431542 (SB), 10 μmol/L (WT + SB CM). E: Mouse fibrosis reverse transcription-PCR array of AECs cultured on Matrigel, FN, or FN with addition of SB. Expression of genes on FN versus Matrigel (black) and FN with SB versus Matrigel (red) are shown. Blue lines indicate fivefold differential expression. Several genes of interest are indicated. AdCre, adenovirus expressing Cre; AdGFP adenovirus expressing green fluorescent protein; AEC, alveolar epithelial cell; BAL, bronchoalveolar lavage; Bleo, bleomycin; CM, conditioned media; Col I, type I collagen; Col1a1f/f, homozygous floxed Col1a1; Col1a2, collagen, type I, alpha 2; Col3a1, collagen, type III, alpha 1; ctgf, connective tissue growth factor; Ctrl, control; DDR2, discoidin domain receptor-2; EMT, epithelial-mesenchymal transition; FN, fibronectin; GAPDH, glyceraldehyde phosphate dehydrogenase; lox, lysyl oxidase; Sal, saline; SB, transforming growth factor β receptor inhibitor SB431542; SCcol, triple transgenic surfactant proteins-C promoter-reverse tetracycline transactivator/tetO-cytomegalovirus promoter-Cre recombinase/homozygous floxed collagen, type I, alpha 1; SMA, smooth muscle actin; sma, smooth muscle actin; TGF-β, transforming growth factor β; tsp, thrombospondin; WT, wild-type.
Activated AECs may secrete other factors that more profoundly promote fibroblast activation. We and others have previously reported that primary AECs cultured on laminin or MG maintain an AEC phenotype, whereas cells cultured on provisional matrix proteins such as fibrin and FN undergo TGF-β–mediated EMTs.24,42 Murine lung fibroblasts (MLg) were treated with CM from primary AECs cultured on MG (AEC CM) or FN (EMT CM). EMT CM promoted fibroblast expression of collagen I, SMA, and FN compared with fibroblasts treated with AEC CM (Supplemental Figure S8B). Enhanced expression of α-SMA and collagen I by fibroblasts stimulated with EMT CM was confirmed by immunoblot analysis (Figure 5C and Supplemental Figure S7B). Lung fibroblasts treated with EMT CM had levels of collagen I and SMA similar to TGF-β1–stimulated lung fibroblasts, whereas fibroblasts treated with AEC CM or plain media had lower levels of collagen I and SMA. EMT CM did not promote Smad2 phosphorylation consistent with our prior observations that TGF-β production and activation in this in vitro system is required for AEC EMTs, but the active TGF-β remains attached to the ECM rather than being secreted into the CM. We next wanted to confirm that production of the EMT-derived fibroblast activating factor(s) required TGF-β–mediated AEC EMTs. TGF-β signaling was inhibited in primary AECs undergoing EMTs with the use of 10 μmol/L TGF-β receptor (TGF-βR) kinase inhibitor (SB431642) and by using Smad3-deficient AECs. CM from AECs treated with SB431542 and from Smad3-null AECs had a diminished capacity to activate lung fibroblasts (Figure 5D and Supplemental Figure S7C). Collectively, these results suggest that TGF-β signaling is required for AECs to produce/secrete the fibroblast stimulating factor(s) but that the secreted factor is not TGF-β itself.
To identify candidate TGF-β–induced, AEC-derived fibroblast-activating factors we used a mouse fibroblast pathway-specific reverse transcription-PCR array. mRNA from AECs cultured on MG, FN, or FN plus TGF-βR inhibition were compared. As expected, AECs cultured on FN had marked up-regulation of collagen I, collagen III, and SMA, and expression of these genes was suppressed by TGF-βR inhibition. The highest expressed genes by AECs on FN included several secreted profibrotic proteins: CTGF, thrombospondin, collagen III, and collagen I (Figure 5E and Supplemental Table S2). Expression of these genes was suppressed by inhibition of TGF-β signaling. Several of these factors have been used as markers of fibrotic fibroblasts and have been shown to induce fibroblast expression of type I collagen, suggesting that fibroblast-activating factors secreted by activated lung epithelial cells may not be limited to type I collagen.
Discussion
These findings support a model in which lung epithelial cells can acquire functions and protein expression that overlap with activated fibroblasts in response to injury. In this model, injury leads to activation of the epithelial cells which initiates secretion of matrix proteins early in response to injury, and these proteins promote activation of fibroblasts, leading to progressive fibrosis. We found that activated lung epithelial cells produce collagen I and that deletion of type I collagen within lung epithelial cells limits fibrosis. Several prior reports have suggested the ability of lung epithelial cells to express type I collagen in vitro when stimulated with TGF-β.24,47 Recent reports indicate that deletion of the TGF-β receptor in either lung epithelial cells or resident fibroblasts leads to a similar attenuation of fibrosis in mouse models.33,48 These findings are in agreement with at least one point from a recent report from Rock et al,26 in which type II AECs were found to have an approximately twofold induction in type I collagen 10 days after bleomycin. Rock et al26 did not find other evidence for EMT, in contrast to several other reports that favored EMT during lung fibrosis. The differences among these reports are likely because of differences in the fate-mapping strategy. Studies favoring EMT labeled lung epithelial cells broadly, whereas Rock et al26 initiated fate-mapping within a subtotal population of type II AECs. Indeed in vitro evidence is strong that a number of different lung epithelial cell types can express type I collagen when activated.17,20,24,47,49 Further, the fraction of labeled type II AECs was dramatically reduced after bleomycin injury in Rock et al,26 suggesting that the dTomato reporter used in that study might be toxic to injured cells as previously suggested50 or that a plastic stem cell-like population capable of regenerating the epithelium and up-regulating mesenchymal proteins during injury was not labeled. A consensus definition of EMT has been elusive, and demonstrating an important contribution of proteins typically associated with activated fibroblasts may not be sufficient to prove EMTs. However, this approach may lead to a better understanding of the functions of different cell types during fibrogenesis, which may ultimately move the field beyond this controversy.
One critical limitation of a fate-mapping/costaining approach is the descriptive nature of the findings as opposed to a functional analysis. We found that epithelial cell-derived type I collagen affects both the overall amount of fibrosis and may have an effect on survival through regulating the lung injury response. Some of this effect may be through collagen-mediated cell signaling. We found that type I collagen is produced quickly in response to injury and leads to activation of the collagen signaling acutely after bleomycin injury. Indeed, there is mounting evidence that collagen signaling has important functions in inflammation and fibrogenesis.51,52 We used phosphorylation of the fibroblast collagen receptor, DDR2, as an indicator of epithelial cell-derived collagen signaling on neighboring fibroblasts. In vitro, we found collagen-dependent activation of fibroblasts (Figure 5). Deleting endogenous collagen expression resulted in attenuated expression of several ECM genes, and addition of exogenous collagen restored expression of these genes (Figure 5B). These studies suggest the presence of a collagen signaling pathway, leading to augmented fibroblast production of ECM. It is unclear whether the cellular source of collagen I is important in determining this response in vivo, but differences in the timing and localization of collagen production after injury are potentially important. We are currently investigating the role of specific collagen receptors in regulating this response. Several studies support an influence of DDR2 activation on fibroblast function and that collagen I can signal through other cell surface receptors.46 If type I collagen indeed promotes further production of type I collagen, as our data suggest, then epithelial cells may represent an important early source of collagen I necessary for progressive fibrosis. Thus, the contribution of type I collagen from different cell types is unlikely to be a simple sum calculation.
Deletion of type I collagen within lung epithelial cells surprisingly promotes sustained lung inflammation after bleomycin injury. These findings suggest that removing a significant source of type I collagen impairs the lungs ability to replace the damaged normal matrix with a fibrotic matrix, leading to prolonged inflammation. The bleomycin model is often criticized as a poor model of IPF in part because of the robust inflammation that occurs after bleomycin contrasted with the paucity of inflammation in IPF.53,54 However, fibrogenesis often occurs in the context of acute and chronic inflammation, and factors produced during the injury/inflammation phase are thought to facilitate the fibrogenic phase by promoting fibroblast activation.1,55 Our data suggest that factors produced during the fibrogenic phase, particularly collagen I, may regulate termination of the injury/inflammatory phase. As mentioned, some evidence supports an anti-inflammatory role for type I collagen. Indeed we found that deleting type I collagen within primary lung fibroblasts augments expression of several inflammatory cytokines, suggesting autocrine and paracrine inhibition of inflammatory cytokine expression through type I collagen signaling. Our results may thus translate better to inflammatory fibrotic disease rather than IPF and suggest that targeting collagen production after robust lung injury must be taken with caution.
This study used type I collagen as a prototypical secreted mesenchymal protein that is critical for fibrogenesis, but activated lung epithelial cells likely produce and secrete other mesenchymal proteins that may promote fibroblast activation. We found that AECs have high expression of a number of mesenchymal genes, including CTGF, fibrillar collagens, and thrombospondin. CTGF expression is increased within fibroblasts in patients with IPF.56 CTGF expression is induced by TGF-β in a number of fibroblast cell lines and studies that used a CTGF-promoter GFP transgenic reporter mouse reported CTGF promoter activity exclusively within activated fibroblasts57 in a model of skin fibrosis. CTGF has thus been used as an effective marker of an activated, fibrotic fibroblast.58 CTGF expression has been found within lung epithelial cells and endothelial cells during fibrogenesis and has been used as a marker of EMTs, but the functional importance of epithelial cell-derived CTGF has not been reported.59,60 Inhibition of CTGF has been shown to attenuate fibrosis in animal models.61,62 Thrombospondin expression has also been used as a marker of fibrogenic cells, and thrombospondin is thought to enable activation of latent TGF-β, potentially promoting fibroblast activation. A recent report however found that thrombospondin-null mice are not protected from bleomycin-induced fibrosis.63 In addition to type I collagen, type III collagen is often used as a marker of activated fibroblasts, and mice with deletion of type III collagen have impaired type I collagen fibrillogenesis.64
Future studies that use this more functional approach may help resolve controversies from descriptive fate-mapping studies. The contribution of collagen I from other cell types can be pursued in future studies, and epithelial cell-specific deletion of other genes typically associated with activated fibroblasts (collagen III, fibroblast-specific protein, SMA, CTGF, etc) could be applied to better understand the contribution of epithelial cell activation to fibrogenesis. We used PCR primers to specifically detect the presence of a truncated Col1a1 mRNA (with exon 1 directly adjacent to exon 6), indicating lung epithelial cell-specific origin of this important fibrotic protein. We cannot exclude the possibility that translation of this modified mRNA results in a truncated polypeptide affecting epithelial cell phenotype, but this limitation does not affect the overall conclusion that activated epithelial cells are actively expressing Col1a1. This PCR approach does not enable histological localization of the epithelial-derived cells with activation of the Col1a1. This approach importantly uses the intact native promoter rather than reporter transgenes with a fragment of the promoter. This is important because different cell types often activate transcription of the same genes by using different promoter regions, some of which are distant from the transcriptional start site.65,66 Expression of mesenchymal proteins from nontraditional cell types may use previously uncharacterized promoter regions. Future studies will investigate differences between epithelial cells and fibroblasts in promoter activation sites. A more complete understanding of cellular contributions to progressive fibrogenesis may lead to new targets for potential therapeutic intervention.
Acknowledgments
We thank Kelly McDonough and Zhen Geng for technical assistance.
Footnotes
Supported by NIH grants R01 HL108904 (K.K.K.), K08 HL085290 (K.K.K.), and RO1 HL44712 (H.A.C.).
Supplemental Data
Generation of floxed Col1a1 mice. A: Flox Col1a1 targeting vector has 12-kb homology (8.5-kb 5′ arm and 3.5-kb 3′ arm). LoxP site and new SphI (S) site were added to intron 1. LoxP-FRT-neomycin-FRT and new HindIII (H) site were added to intron 6. Endogenous SphI sites are located at −14 kb and 9 kb, and endogenous HindIII sites are −2 kb and 8 kb. B: Southern blot analysis of ES cell clones indicates correct integration of targeting vector. C: Targeting confirmation by PCR. Forward primer (F1) located close to neomycin resistance and reverse primer (R1) located 7 kb from start site confirms integration of targeting vector to the Col1a1 gene. Forward primer (F2) overlaps with 5′ loxP site and reverse (R2) within neomycin resistance confirms integration of the 5′ loxP site and neomycin gene on the same allele. Forward primer (F3) is located upstream of 5′ loxP insertion site and reverse prime (R3) downstream of 5′ loxP insertion site; WT allele yields a 138-bp PCR product, floxed Col1a1 with insertion of 40-bp loxP, and SphI site yields a 178-bp band. D: Removal of FRT-neomycin was achieved by crossing mice with constitutive FlpE mice. Removal of neomycin gene was confirmed by PCR with the use of forward primer (F4) located upstream of neomycin insertion site and reverse primer (R4) located downstream of neomycin insertion site. WT DNA yields a 1-kb PCR product, flox Col1a1 with neomycin gene yields a 3-kb PCR product, and flox Col1a1 allele with removal of neomycin resistance yields a 1.1-kb PCR product. Col1a1, collagen, type I, alpha 1; ES, embryonic stem; FRT, flippase (Flp) recombination target; neo, neomycin; WT, wild-type.
Densitometry of collagen I immunoblot validating floxed Col1a1. A: Densitometry of collagen I immunoblot analysis of MEFs treated with AdGFP or (AdCre. Floxed Col1a1 (FloxCol) cells treated with Cre have near complete loss of collagen I expression, n = 4. ∗P < 0.01 versus control MEFs. B: Densitometry of collagen I immunoblot analysis from primary AECs and primary lung fibroblasts from uninjured control and SCcol mice. AECs from SCcol mice have diminished EMT-induced expression of collagen I by immunoblot analysis compared with AECs from littermate control mice lacking one of the three transgenes. Primary lung fibroblasts from SCcol mice have similar collagen I expression compared with control lung fibroblasts, verifying robust and lung epithelial specific deletion of Col1a1, n = 4. ∗P < 0.05 versus control AECs. AdCre, adenovirus expressing Cre; AdGFP adenovirus expressing green fluorescent protein; AEC, alveolar epithelial cell; Col1a1, collagen, type I, alpha 1; EMT, epithelial-mesenchymal transition; Fib, fibroblast; MEF, murine embryonic fibroblast; SCol, triple transgenic surfactant proteins-C promoter-reverse tetracycline transactivator/tetO-cytomegalovirus promoter-Cre recombinase/homozygous floxed collagen, type I, alpha 1.
Lung sections from littermate control (A) and SCcol (B) mice 3 weeks after bleomycin injection stained with H&E. Original magnification, ×400. SCol, triple transgenic surfactant proteins-C promoter-reverse tetracycline transactivator/tetO-cytomegalovirus promoter-Cre recombinase/homozygous floxed collagen, type I, alpha 1.
Densitometry of whole lung collagen. Densitometry of collagen I immunoblot analysis of whole lung lysate from control and SCcol mice 3 weeks after bleomycin or saline injury. Control mice have robust induction of collagen I after bleomycin compared with SCcol mice as quantified by densitometry, n = 4 per group. ∗P < 0.01 versus control mice treated with bleomycin. Bleo, bleomycin; SCol, triple transgenic surfactant proteins-C promoter-reverse tetracycline transactivator/tetO-cytomegalovirus promoter-Cre recombinase/homozygous floxed collagen, type I, alpha 1.
Collagen I immunostaining 1 week after bleomycin treatment. Lungs from mice 1 week after bleomycin were immunostained for collagen I (red). Genotype control mouse lungs show foci of collagen I deposition (A) that is less apparent in SCcol mice (B). Original magnification: ×400 (A and B). SCol, triple transgenic surfactant proteins-C promoter-reverse tetracycline transactivator/tetO-cytomegalovirus promoter-Cre recombinase/homozygous floxed collagen, type I, alpha 1.
Densitometry of immunoblot analysis of collagen I BAL and whole lung DDR2. A: Densitometry of collagen I immunoblot of BAL from control and SCcol mice 1 week after bleomycin. Bleomycin induces collagen I expression in BAL of control mice 1 week after bleomycin. BAL of SCcol mice have less collagen I accumulation after bleomycin, n = 4 per group. ∗P < 0.01 versus control mice treated with bleomycin. B and C: Densitometry of phospho-DDR2 (B) and total DDR2 (C) immunoblot of whole lung lysate from control and SCcol mice 1 week after saline or bleomycin injury. Bleomycin induces greater DDR2 phosphorylation in control mice than in SCcol mice, n = 4 per group. ∗P < 0.05 versus control mice treated with bleomycin. BAL, bronchoalveolar lavage; Bleo, bleomycin; DDR2, discoidin domain receptor-2; Sal, saline; SCol, triple transgenic surfactant proteins-C promoter-reverse tetracycline transactivator/tetO-cytomegalovirus promoter-Cre recombinase/homozygous floxed collagen, type I, alpha 1.
Densitometry of immunoblot analysis of lung fibroblasts treated with BAL and CM. A: Densitometry of immunoblot analysis for phospho-DDR2 of collagen I-deleted fibroblasts treated with BAL from control and SCcol mice collected 1 week after saline or bleomycin injury or treated with 10 μg/mL exogenous Col I, n = 4. ∗P < 0.05 versus control saline. B: Densitometry of immunoblot analysis for collagen I, α-SMA, and pSmad2 of lung fibroblasts stimulated with plain media, AEC CM, EMT CM, and media supplemented with 4 ng/mL TGF-β, n = 4. ∗P < 0.05 versus media. C: Densitometry of immunoblot analysis of lung fibroblasts stimulated with plain media, CM from WT AECs cultured on fibronectin (WT CM), smad3-null AECs (Sm3− CM) cultured on fibronectin, and WT AECs cultured on fibronectin with addition of SB, 10 μmol/L (WT + SB CM), n = 4. ∗P < 0.05 versus media; ∗∗P < 0.05 versus WT CM. AEC, Alveolar epithelial cell; BAL, bronchoalveolar lavage; Bleo, bleomycin; CM, conditioned media; DDR2, discoidin domain receptor-2; Col I, type I collagen; EMT, epithelial-mesenchymal transitions; pSmad2, phospho-Smad2; SCol, triple transgenic surfactant proteins-C promoter-reverse tetracycline transactivator/tetO-cytomegalovirus promoter-Cre recombinase/homozygous floxed collagen, type I, alpha 1; SB, transforming growth factor β receptor inhibitor SB431542; SMA, smooth muscle actin; sma, smooth muscle actin; TGF-β, transforming growth factor β.
Lung fibroblast gene expression is influenced by EMTs CM and autocrine collagen signaling. A: Reverse transcription-PCR of primary lung fibroblasts from Col1a1f/f mice treated with AdGFP, AdCre. Fibroblast loss of collagen I expression leads to increased expression of several inflammatory cytokines, n = 4. ∗P < 0.05 versus AdGFP-treated cells. B: Reverse transcription-PCR of lung fibroblasts stimulated with CM from primary alveolar epithelial cultured on Matrigel (AEC CM) and FN (EMT CM). EMT CM induces expression of several markers of fibroblast activation by RT-PCR, n = 4. ∗P < 0.04 versus AEC CM. AdCre, adenovirus expressing Cre; AdGFP adenovirus expressing green fluorescent protein; AEC, Alveolar epithelial cell; CM, conditioned media;Col1a1f/f, floxed collagen, type I, alpha 1 EMT, epithelial-mesenchymal transitions; FN, fibronectin; TNFα, tumor necrosis factor α.
References
- 1.Thannickal V.J., Toews G.B., White E.S., Lynch J.P., III, Martinez F.J. Mechanisms of pulmonary fibrosis. Annu Rev Med. 2004;55:395–417. doi: 10.1146/annurev.med.55.091902.103810. [DOI] [PubMed] [Google Scholar]
- 2.Selman M., King T.E., Pardo A. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med. 2001;134:136–151. doi: 10.7326/0003-4819-134-2-200101160-00015. [DOI] [PubMed] [Google Scholar]
- 3.American Thoracic Society Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS) Am J Respir Crit Care Med. 2000;161:646–664. doi: 10.1164/ajrccm.161.2.ats3-00. [DOI] [PubMed] [Google Scholar]
- 4.American Thoracic Society; European Respiratory Society American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002;165:277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- 5.Scotton C.J., Chambers R.C. Molecular targets in pulmonary fibrosis: the myofibroblast in focus. Chest. 2007;132:1311–1321. doi: 10.1378/chest.06-2568. [DOI] [PubMed] [Google Scholar]
- 6.Humphreys B.D., Lin S.L., Kobayashi A., Hudson T.E., Nowlin B.T., Bonventre J.V., Valerius M.T., McMahon A.P., Duffield J.S. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol. 2010;176:85–97. doi: 10.2353/ajpath.2010.090517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hashimoto N., Jin H., Liu T., Chensue S.W., Phan S.H. Bone marrow-derived progenitor cells in pulmonary fibrosis. J Clin Invest. 2004;113:243–252. doi: 10.1172/JCI18847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hashimoto N., Phan S.H., Imaizumi K., Matsuo M., Nakashima H., Kawabe T., Shimokata K., Hasegawa Y. Endothelial-mesenchymal transition in bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol. 2010;43:161–172. doi: 10.1165/rcmb.2009-0031OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hinz B., Phan S.H., Thannickal V.J., Galli A., Bochaton-Piallat M.L., Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol. 2007;170:1807–1816. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Selman M., Pardo A. Role of epithelial cells in idiopathic pulmonary fibrosis: from innocent targets to serial killers. Proc Am Thorac Soc. 2006;3:364–372. doi: 10.1513/pats.200601-003TK. [DOI] [PubMed] [Google Scholar]
- 11.Nogee L.M., Dunbar A.E., III, Wert S.E., Askin F., Hamvas A., Whitsett J.A. A mutation in the surfactant protein C gene associated with familial interstitial lung disease. N Engl J Med. 2001;344:573–579. doi: 10.1056/NEJM200102223440805. [DOI] [PubMed] [Google Scholar]
- 12.Sisson T.H., Mendez M., Choi K., Subbotina N., Courey A., Cunningham A., Dave A., Engelhardt J.F., Liu X., White E.S., Thannickal V.J., Moore B.B., Christensen P.J., Simon R.H. Targeted injury of type II alveolar epithelial cells induces pulmonary fibrosis. Am J Respir Crit Care Med. 2010;181:254–263. doi: 10.1164/rccm.200810-1615OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maitra M., Wang Y., Gerard R.D., Mendelson C.R., Garcia C.K. Surfactant protein A2 mutations associated with pulmonary fibrosis lead to protein instability and endoplasmic reticulum stress. J Biol Chem. 2010;285:22103–22113. doi: 10.1074/jbc.M110.121467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iwashita T., Kadota J., Naito S., Kaida H., Ishimatsu Y., Miyazaki M., Ozono Y., Kohno S. Involvement of collagen-binding heat shock protein 47 and procollagen type I synthesis in idiopathic pulmonary fibrosis: contribution of type II pneumocytes to fibrosis. Hum Pathol. 2000;31:1498–1505. doi: 10.1053/hupa.2000.20378. [DOI] [PubMed] [Google Scholar]
- 15.Kakugawa T., Mukae H., Hayashi T., Ishii H., Nakayama S., Sakamoto N., Yoshioka S., Sugiyama K., Mine M., Mizuta Y., Kohno S. Expression of HSP47 in usual interstitial pneumonia and nonspecific interstitial pneumonia. Respir Res. 2005;6:57. doi: 10.1186/1465-9921-6-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marmai C., Sutherland R.E., Kim K.K., Dolganov G.M., Fang X., Kim S.S., Jiang S., Golden J.A., Hoopes C.W., Matthay M.A., Chapman H.A., Wolters P.J. Alveolar epithelial cells express mesenchymal proteins in patients with idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2011;301:L71–L78. doi: 10.1152/ajplung.00212.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hackett T.L. Epithelial-mesenchymal transition in the pathophysiology of airway remodelling in asthma. Curr Opin Allergy Clin Immunol. 2012;12:53–59. doi: 10.1097/ACI.0b013e32834ec6eb. [DOI] [PubMed] [Google Scholar]
- 18.Kasai H., Allen J.T., Mason R.M., Kamimura T., Zhang Z. TGF-beta1 induces human alveolar epithelial to mesenchymal cell transition (EMT) Respir Res. 2005;6:56. doi: 10.1186/1465-9921-6-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Willis B.C., Borok Z. TGF-beta-induced EMT: mechanisms and implications for fibrotic lung disease. Am J Physiol Lung Cell Mol Physiol. 2007;293:L525–L534. doi: 10.1152/ajplung.00163.2007. [DOI] [PubMed] [Google Scholar]
- 20.Willis B.C., Liebler J.M., Luby-Phelps K., Nicholson A.G., Crandall E.D., du Bois R.M., Borok Z. Induction of epithelial-mesenchymal transition in alveolar epithelial cells by transforming growth factor-beta1: potential role in idiopathic pulmonary fibrosis. Am J Pathol. 2005;166:1321–1332. doi: 10.1016/s0002-9440(10)62351-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalluri R., Neilson E.G. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112:1776–1784. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zeisberg M., Duffield J.S. Resolved: eMT produces fibroblasts in the kidney. J Am Soc Nephrol. 2010;21:1247–1253. doi: 10.1681/ASN.2010060616. [DOI] [PubMed] [Google Scholar]
- 23.DeMaio L., Buckley S.T., Krishnaveni M.S., Flodby P., Dubourd M., Banfalvi A., Xing Y., Ehrhardt C., Minoo P., Zhou B., Crandall E.D., Borok Z. Ligand-independent transforming growth factor-beta type I receptor signalling mediates type I collagen-induced epithelial-mesenchymal transition. J Pathol. 2012;226:633–644. doi: 10.1002/path.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim K.K., Kugler M.C., Wolters P.J., Robillard L., Galvez M.G., Brumwell A.N., Sheppard D., Chapman H.A. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci U S A. 2006;103:13180–13185. doi: 10.1073/pnas.0605669103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanjore H., Xu X.C., Polosukhin V.V., Degryse A.L., Li B., Han W., Sherrill T.P., Plieth D., Neilson E.G., Blackwell T.S., Lawson W.E. Contribution of epithelial-derived fibroblasts to bleomycin-induced lung fibrosis. Am J Respir Crit Care Med. 2009;180:657–665. doi: 10.1164/rccm.200903-0322OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rock J.R., Barkauskas C.E., Cronce M.J., Xue Y., Harris J.R., Liang J., Noble P.W., Hogan B.L. Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proc Natl Acad Sci U S A. 2011;108:E1475–E1483. doi: 10.1073/pnas.1117988108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iwano M., Plieth D., Danoff T.M., Xue C., Okada H., Neilson E.G. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002;110:341–350. doi: 10.1172/JCI15518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zeisberg M., Yang C., Martino M., Duncan M.B., Rieder F., Tanjore H., Kalluri R. Fibroblasts derive from hepatocytes in liver fibrosis via epithelial to mesenchymal transition. J Biol Chem. 2007;282:23337–23347. doi: 10.1074/jbc.M700194200. [DOI] [PubMed] [Google Scholar]
- 29.Taura K., Miura K., Iwaisako K., Osterreicher C.H., Kodama Y., Penz-Osterreicher M., Brenner D.A. Hepatocytes do not undergo epithelial-mesenchymal transition in liver fibrosis in mice. Hepatology. 2010;51:1027–1036. doi: 10.1002/hep.23368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Balli D., Ustiyan V., Zhang Y., Wang I.C., Masino A.J., Ren X., Whitsett J.A., Kalinichenko V.V., Kalin T.V. Foxm1 transcription factor is required for lung fibrosis and epithelial-to-mesenchymal transition. EMBO J. 2013;32:231–244. doi: 10.1038/emboj.2012.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rowe R.G., Lin Y., Shimizu-Hirota R., Hanada S., Neilson E.G., Greenson J.K., Weiss S.J. Hepatocyte-derived Snail1 propagates liver fibrosis progression. Mol Cell Biol. 2011;31:2392–2403. doi: 10.1128/MCB.01218-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim K.K., Wei Y., Szekeres C., Kugler M.C., Wolters P.J., Hill M.L., Frank J.A., Brumwell A.N., Wheeler S.E., Kreidberg J.A., Chapman H.A. Epithelial cell alpha3beta1 integrin links beta-catenin and Smad signaling to promote myofibroblast formation and pulmonary fibrosis. J Clin Invest. 2009;119:213–224. doi: 10.1172/JCI36940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li M., Krishnaveni M.S., Li C., Zhou B., Xing Y., Banfalvi A., Li A., Lombardi V., Akbari O., Borok Z., Minoo P. Epithelium-specific deletion of TGF-beta receptor type II protects mice from bleomycin-induced pulmonary fibrosis. J Clin Invest. 2011;121:277–287. doi: 10.1172/JCI42090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chapman H.A., Li X., Alexander J.P., Brumwell A., Lorizio W., Tan K., Sonnenberg A., Wei Y., Vu T.H. Integrin alpha6beta4 identifies an adult distal lung epithelial population with regenerative potential in mice. J Clin Invest. 2011;121:2855–2862. doi: 10.1172/JCI57673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Christensen P.J., Preston A.M., Ling T., Du M., Fields W.B., Curtis J.L., Beck J.M. Pneumocystis murina infection and cigarette smoke exposure interact to cause increased organism burden, development of airspace enlargement, and pulmonary inflammation in mice. Infect Immun. 2008;76:3481–3490. doi: 10.1128/IAI.00165-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bauman K.A., Wettlaufer S.H., Okunishi K., Vannella K.M., Stoolman J.S., Huang S.K., Courey A.J., White E.S., Hogaboam C.M., Simon R.H., Toews G.B., Sisson T.H., Moore B.B., Peters-Golden M. The antifibrotic effects of plasminogen activation occur via prostaglandin E2 synthesis in humans and mice. J Clin Invest. 2010;120:1950–1960. doi: 10.1172/JCI38369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Corti M., Brody A.R., Harrison J.H. Isolation and primary culture of murine alveolar type II cells. Am J Respir Cell Mol Biol. 1996;14:309–315. doi: 10.1165/ajrcmb.14.4.8600933. [DOI] [PubMed] [Google Scholar]
- 38.Zhang F., Tom C.C., Kugler M.C., Ching T.T., Kreidberg J.A., Wei Y., Chapman H.A. Distinct ligand binding sites in integrin alpha3beta1 regulate matrix adhesion and cell-cell contact. J Cell Biol. 2003;163:177–188. doi: 10.1083/jcb.200304065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim Y., Kugler M.C., Wei Y., Kim K.K., Li X., Brumwell A.N., Chapman H.A. Integrin alpha3beta1-dependent beta-catenin phosphorylation links epithelial Smad signaling to cell contacts. J Cell Biol. 2009;184:309–322. doi: 10.1083/jcb.200806067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Naik P.N., Horowitz J.C., Moore T.A., Wilke C.A., Toews G.B., Moore B.B. Pulmonary fibrosis induced by gamma-herpesvirus in aged mice is associated with increased fibroblast responsiveness to transforming growth factor-beta. J Gerontol A Biol Sci Med Sci. 2012;67:714–725. doi: 10.1093/gerona/glr211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He Z., Feng L., Zhang X., Geng Y., Parodi D.A., Suarez-Quian C., Dym M. Expression of Col1a1, Col1a2 and procollagen I in germ cells of immature and adult mouse testis. Reproduction. 2005;130:333–341. doi: 10.1530/rep.1.00694. [DOI] [PubMed] [Google Scholar]
- 42.Brown A.C., Fiore V.F., Sulchek T.A., Barker T.H. Physical and chemical microenvironmental cues orthogonally control the degree and duration of fibrosis-associated epithelial-to-mesenchymal transitions. J Pathol. 2013;229:25–35. doi: 10.1002/path.4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lammi L., Kinnula V., Lahde S., Risteli J., Paakko P., Lakari E., Ryhanen L. Propeptide levels of type III and type I procollagen in the serum and bronchoalveolar lavage fluid of patients with pulmonary sarcoidosis. Eur Respir J. 1997;10:2725–2730. doi: 10.1183/09031936.97.10122725. [DOI] [PubMed] [Google Scholar]
- 44.Lammi L., Ryhanen L., Lakari E., Risteli J., Paakko P., Kahlos K., Lahde S., Kinnula V. Type III and type I procollagen markers in fibrosing alveolitis. Am J Respir Crit Care Med. 1999;159:818–823. doi: 10.1164/ajrccm.159.3.9805060. [DOI] [PubMed] [Google Scholar]
- 45.Meduri G.U., Tolley E.A., Chinn A., Stentz F., Postlethwaite A. Procollagen types I and III aminoterminal propeptide levels during acute respiratory distress syndrome and in response to methylprednisolone treatment. Am J Respir Crit Care Med. 1998;158:1432–1441. doi: 10.1164/ajrccm.158.5.9801107. [DOI] [PubMed] [Google Scholar]
- 46.Leitinger B., Hohenester E. Mammalian collagen receptors. Matrix Biol. 2007;26:146–155. doi: 10.1016/j.matbio.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 47.Furuyama A., Iwata M., Hayashi T., Mochitate K. Transforming growth factor-beta1 regulates basement membrane formation by alveolar epithelial cells in vitro. Eur J Cell Biol. 1999;78:867–875. doi: 10.1016/s0171-9335(99)80088-0. [DOI] [PubMed] [Google Scholar]
- 48.Hoyles R.K., Derrett-Smith E.C., Khan K., Shiwen X., Howat S.L., Wells A.U., Abraham D.J., Denton C.P. An essential role for resident fibroblasts in experimental lung fibrosis is defined by lineage-specific deletion of high-affinity type II transforming growth factor beta receptor. Am J Respir Crit Care Med. 2011;183:249–261. doi: 10.1164/rccm.201002-0279OC. [DOI] [PubMed] [Google Scholar]
- 49.Harvey W., Amlot P.L. Collagen production by human mesothelial cells in vitro. J Pathol. 1983;139:337–347. doi: 10.1002/path.1711390309. [DOI] [PubMed] [Google Scholar]
- 50.Strack R.L., Strongin D.E., Bhattacharyya D., Tao W., Berman A., Broxmeyer H.E., Keenan R.J., Glick B.S. A noncytotoxic DsRed variant for whole-cell labeling. Nat Methods. 2008;5:955–957. doi: 10.1038/nmeth.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Furuzawa-Carballeda J., Macip-Rodriguez P., Galindo-Feria A.S., Cruz-Robles D., Soto-Abraham V., Escobar-Hernandez S., Aguilar D., Alpizar-Rodriguez D., Ferez-Blando K., Llorente L. Polymerized-type I collagen induces upregulation of Foxp3-expressing CD4 regulatory T cells and downregulation of IL-17-producing CD4(+) T cells (Th17) cells in collagen-induced arthritis. Clin Dev Immunol. 2012;2012:618608. doi: 10.1155/2012/618608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Furuzawa-Carballeda J., Ortiz-Avalos M., Lima G., Jurado-Santa Cruz F., Llorente L. Subcutaneous administration of polymerized type I collagen downregulates interleukin (IL)-17A. IL-22 and transforming growth factor-beta1 expression, and increases Foxp3-expressing cells in localized scleroderma. Clin Exp Dermatol. 2012;37:599–609. doi: 10.1111/j.1365-2230.2012.04385.x. [DOI] [PubMed] [Google Scholar]
- 53.Moeller A., Ask K., Warburton D., Gauldie J., Kolb M. The bleomycin animal model: a useful tool to investigate treatment options for idiopathic pulmonary fibrosis? Int J Biochem Cell Biol. 2008;40:362–382. doi: 10.1016/j.biocel.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peng R., Sridhar S., Tyagi G., Phillips J.E., Garrido R., Harris P., Burns L., Renteria L., Woods J., Chen L., Allard J., Ravindran P., Bitter H., Liang Z., Hogaboam C.M., Kitson C., Budd D.C., Fine J.S., Bauer C.M., Stevenson C.S. Bleomycin induces molecular changes directly relevant to idiopathic pulmonary fibrosis: a model for “active” disease. PLoS One. 2013;8:e59348. doi: 10.1371/journal.pone.0059348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wynn T.A. Integrating mechanisms of pulmonary fibrosis. J Exp Med. 2011;208:1339–1350. doi: 10.1084/jem.20110551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kelly M.M., Leigh R., Gilpin S.E., Cheng E., Martin G.E., Radford K., Cox G., Gauldie J. Cell-specific gene expression in patients with usual interstitial pneumonia. Am J Respir Crit Care Med. 2006;174:557–565. doi: 10.1164/rccm.200510-1648OC. [DOI] [PubMed] [Google Scholar]
- 57.Kapoor M., Liu S., Huh K., Parapuram S., Kennedy L., Leask A. Connective tissue growth factor promoter activity in normal and wounded skin. Fibrogenesis Tissue Repair. 2008;1:3. doi: 10.1186/1755-1536-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leask A., Abraham D.J. The role of connective tissue growth factor, a multifunctional matricellular protein, in fibroblast biology. Biochem Cell Biol. 2003;81:355–363. doi: 10.1139/o03-069. [DOI] [PubMed] [Google Scholar]
- 59.Pan L.H., Yamauchi K., Uzuki M., Nakanishi T., Takigawa M., Inoue H., Sawai T. Type II alveolar epithelial cells and interstitial fibroblasts express connective tissue growth factor in IPF. Eur Respir J. 2001;17:1220–1227. doi: 10.1183/09031936.01.00074101. [DOI] [PubMed] [Google Scholar]
- 60.Yin Q., Nan H.Y., Zhang W.H., Yan L.F., Cui G.B., Huang X.F., Wei J.G. Pulmonary microvascular endothelial cells from bleomycin-induced rats promote the transformation and collagen synthesis of fibroblasts. J Cell Physiol. 2011;226:2091–2102. doi: 10.1002/jcp.22545. [DOI] [PubMed] [Google Scholar]
- 61.Lipson K.E., Wong C., Teng Y., Spong S. CTGF is a central mediator of tissue remodeling and fibrosis and its inhibition can reverse the process of fibrosis. Fibrogenesis Tissue Repair. 2012;5(suppl 1):S24. doi: 10.1186/1755-1536-5-S1-S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sung D.K., Kong W.H., Park K., Kim J.H., Kim M.Y., Kim H., Hahn S.K. Noncovalenly PEGylated CTGF siRNA/PDMAEMA complex for pulmonary treatment of bleomycin-induced lung fibrosis. Biomaterials. 2013;34:1261–1269. doi: 10.1016/j.biomaterials.2012.09.061. [DOI] [PubMed] [Google Scholar]
- 63.Ezzie M.E., Piper M.G., Montague C., Newland C.A., Opalek J.M., Baran C., Ali N., Brigstock D., Lawler J., Marsh C.B. Thrombospondin-1-deficient mice are not protected from bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol. 2011;44:556–561. doi: 10.1165/rcmb.2009-0019OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu X., Wu H., Byrne M., Krane S., Jaenisch R. Type III collagen is crucial for collagen I fibrillogenesis and for normal cardiovascular development. Proc Natl Acad Sci U S A. 1997;94:1852–1856. doi: 10.1073/pnas.94.5.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bou-Gharios G., Garrett L.A., Rossert J., Niederreither K., Eberspaecher H., Smith C., Black C., Crombrugghe B. A potent far-upstream enhancer in the mouse pro alpha 2(I) collagen gene regulates expression of reporter genes in transgenic mice. J Cell Biol. 1996;134:1333–1344. doi: 10.1083/jcb.134.5.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rossert J.A., Chen S.S., Eberspaecher H., Smith C.N., de Crombrugghe B. Identification of a minimal sequence of the mouse pro-alpha 1(I) collagen promoter that confers high-level osteoblast expression in transgenic mice and that binds a protein selectively present in osteoblasts. Proc Natl Acad Sci U S A. 1996;93:1027–1031. doi: 10.1073/pnas.93.3.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Generation of floxed Col1a1 mice. A: Flox Col1a1 targeting vector has 12-kb homology (8.5-kb 5′ arm and 3.5-kb 3′ arm). LoxP site and new SphI (S) site were added to intron 1. LoxP-FRT-neomycin-FRT and new HindIII (H) site were added to intron 6. Endogenous SphI sites are located at −14 kb and 9 kb, and endogenous HindIII sites are −2 kb and 8 kb. B: Southern blot analysis of ES cell clones indicates correct integration of targeting vector. C: Targeting confirmation by PCR. Forward primer (F1) located close to neomycin resistance and reverse primer (R1) located 7 kb from start site confirms integration of targeting vector to the Col1a1 gene. Forward primer (F2) overlaps with 5′ loxP site and reverse (R2) within neomycin resistance confirms integration of the 5′ loxP site and neomycin gene on the same allele. Forward primer (F3) is located upstream of 5′ loxP insertion site and reverse prime (R3) downstream of 5′ loxP insertion site; WT allele yields a 138-bp PCR product, floxed Col1a1 with insertion of 40-bp loxP, and SphI site yields a 178-bp band. D: Removal of FRT-neomycin was achieved by crossing mice with constitutive FlpE mice. Removal of neomycin gene was confirmed by PCR with the use of forward primer (F4) located upstream of neomycin insertion site and reverse primer (R4) located downstream of neomycin insertion site. WT DNA yields a 1-kb PCR product, flox Col1a1 with neomycin gene yields a 3-kb PCR product, and flox Col1a1 allele with removal of neomycin resistance yields a 1.1-kb PCR product. Col1a1, collagen, type I, alpha 1; ES, embryonic stem; FRT, flippase (Flp) recombination target; neo, neomycin; WT, wild-type.
Densitometry of collagen I immunoblot validating floxed Col1a1. A: Densitometry of collagen I immunoblot analysis of MEFs treated with AdGFP or (AdCre. Floxed Col1a1 (FloxCol) cells treated with Cre have near complete loss of collagen I expression, n = 4. ∗P < 0.01 versus control MEFs. B: Densitometry of collagen I immunoblot analysis from primary AECs and primary lung fibroblasts from uninjured control and SCcol mice. AECs from SCcol mice have diminished EMT-induced expression of collagen I by immunoblot analysis compared with AECs from littermate control mice lacking one of the three transgenes. Primary lung fibroblasts from SCcol mice have similar collagen I expression compared with control lung fibroblasts, verifying robust and lung epithelial specific deletion of Col1a1, n = 4. ∗P < 0.05 versus control AECs. AdCre, adenovirus expressing Cre; AdGFP adenovirus expressing green fluorescent protein; AEC, alveolar epithelial cell; Col1a1, collagen, type I, alpha 1; EMT, epithelial-mesenchymal transition; Fib, fibroblast; MEF, murine embryonic fibroblast; SCol, triple transgenic surfactant proteins-C promoter-reverse tetracycline transactivator/tetO-cytomegalovirus promoter-Cre recombinase/homozygous floxed collagen, type I, alpha 1.
Lung sections from littermate control (A) and SCcol (B) mice 3 weeks after bleomycin injection stained with H&E. Original magnification, ×400. SCol, triple transgenic surfactant proteins-C promoter-reverse tetracycline transactivator/tetO-cytomegalovirus promoter-Cre recombinase/homozygous floxed collagen, type I, alpha 1.
Densitometry of whole lung collagen. Densitometry of collagen I immunoblot analysis of whole lung lysate from control and SCcol mice 3 weeks after bleomycin or saline injury. Control mice have robust induction of collagen I after bleomycin compared with SCcol mice as quantified by densitometry, n = 4 per group. ∗P < 0.01 versus control mice treated with bleomycin. Bleo, bleomycin; SCol, triple transgenic surfactant proteins-C promoter-reverse tetracycline transactivator/tetO-cytomegalovirus promoter-Cre recombinase/homozygous floxed collagen, type I, alpha 1.
Collagen I immunostaining 1 week after bleomycin treatment. Lungs from mice 1 week after bleomycin were immunostained for collagen I (red). Genotype control mouse lungs show foci of collagen I deposition (A) that is less apparent in SCcol mice (B). Original magnification: ×400 (A and B). SCol, triple transgenic surfactant proteins-C promoter-reverse tetracycline transactivator/tetO-cytomegalovirus promoter-Cre recombinase/homozygous floxed collagen, type I, alpha 1.
Densitometry of immunoblot analysis of collagen I BAL and whole lung DDR2. A: Densitometry of collagen I immunoblot of BAL from control and SCcol mice 1 week after bleomycin. Bleomycin induces collagen I expression in BAL of control mice 1 week after bleomycin. BAL of SCcol mice have less collagen I accumulation after bleomycin, n = 4 per group. ∗P < 0.01 versus control mice treated with bleomycin. B and C: Densitometry of phospho-DDR2 (B) and total DDR2 (C) immunoblot of whole lung lysate from control and SCcol mice 1 week after saline or bleomycin injury. Bleomycin induces greater DDR2 phosphorylation in control mice than in SCcol mice, n = 4 per group. ∗P < 0.05 versus control mice treated with bleomycin. BAL, bronchoalveolar lavage; Bleo, bleomycin; DDR2, discoidin domain receptor-2; Sal, saline; SCol, triple transgenic surfactant proteins-C promoter-reverse tetracycline transactivator/tetO-cytomegalovirus promoter-Cre recombinase/homozygous floxed collagen, type I, alpha 1.
Densitometry of immunoblot analysis of lung fibroblasts treated with BAL and CM. A: Densitometry of immunoblot analysis for phospho-DDR2 of collagen I-deleted fibroblasts treated with BAL from control and SCcol mice collected 1 week after saline or bleomycin injury or treated with 10 μg/mL exogenous Col I, n = 4. ∗P < 0.05 versus control saline. B: Densitometry of immunoblot analysis for collagen I, α-SMA, and pSmad2 of lung fibroblasts stimulated with plain media, AEC CM, EMT CM, and media supplemented with 4 ng/mL TGF-β, n = 4. ∗P < 0.05 versus media. C: Densitometry of immunoblot analysis of lung fibroblasts stimulated with plain media, CM from WT AECs cultured on fibronectin (WT CM), smad3-null AECs (Sm3− CM) cultured on fibronectin, and WT AECs cultured on fibronectin with addition of SB, 10 μmol/L (WT + SB CM), n = 4. ∗P < 0.05 versus media; ∗∗P < 0.05 versus WT CM. AEC, Alveolar epithelial cell; BAL, bronchoalveolar lavage; Bleo, bleomycin; CM, conditioned media; DDR2, discoidin domain receptor-2; Col I, type I collagen; EMT, epithelial-mesenchymal transitions; pSmad2, phospho-Smad2; SCol, triple transgenic surfactant proteins-C promoter-reverse tetracycline transactivator/tetO-cytomegalovirus promoter-Cre recombinase/homozygous floxed collagen, type I, alpha 1; SB, transforming growth factor β receptor inhibitor SB431542; SMA, smooth muscle actin; sma, smooth muscle actin; TGF-β, transforming growth factor β.
Lung fibroblast gene expression is influenced by EMTs CM and autocrine collagen signaling. A: Reverse transcription-PCR of primary lung fibroblasts from Col1a1f/f mice treated with AdGFP, AdCre. Fibroblast loss of collagen I expression leads to increased expression of several inflammatory cytokines, n = 4. ∗P < 0.05 versus AdGFP-treated cells. B: Reverse transcription-PCR of lung fibroblasts stimulated with CM from primary alveolar epithelial cultured on Matrigel (AEC CM) and FN (EMT CM). EMT CM induces expression of several markers of fibroblast activation by RT-PCR, n = 4. ∗P < 0.04 versus AEC CM. AdCre, adenovirus expressing Cre; AdGFP adenovirus expressing green fluorescent protein; AEC, Alveolar epithelial cell; CM, conditioned media;Col1a1f/f, floxed collagen, type I, alpha 1 EMT, epithelial-mesenchymal transitions; FN, fibronectin; TNFα, tumor necrosis factor α.