Abstract
Airway epithelial cells cultured at an air–liquid interface bear many hallmarks of in vivo cells and are used extensively to study the biology of the lung epithelium. Because miRNAs regulate many cellular functions, we postulated that miRNA profiling would provide an unbiased assessment of the effects of in vitro culturing. RNA was extracted from primary airway epithelial cells either immediately after cell procurement (in vivo condition) or after air–liquid interface culture was established (in vitro condition). We assessed 742 miRNAs and determined differential expression between in vivo and in vitro conditions. Air–liquid interface culturing of airway epithelial cells caused widespread changes in miRNA expression. A similarly extensive alteration in gene expression was observed in an independent set of publicly available microarray data. We integrated miRNA and gene expression results to identify culture-induced differences in transcriptional programs (including several involved in epithelial injury and repair). Air–liquid interface cultures are useful models for studying airway biology, but the present findings indicate that, despite phenotypic similarities with primary cells, these culture systems profoundly perturb miRNA and gene expression. Studies of lung epithelium based on in vitro culture should therefore be designed and interpreted with an appreciation of the limitations of air–liquid interface culture systems.
The airways are not only conduits for gas exchange but also play an important role in lung immunity.1–4 A contiguous pseudostratified epithelium lines the airways, providing the ability to respond to pathogenic insults and quickly restore breaches to barrier integrity. In addition to such homeostatic functions, the epithelial surface also plays a key role in malignant, inflammatory, and fibrotic diseases of the airways.1,3–5
A variety of airway epithelial cell lines have been developed for basic studies aimed at understanding mechanisms that underlie normal and diseased states.5,6 However, because the airway epithelium comprises a variety of highly specialized cells, each having unique qualities and characteristics, monolayers of undifferentiated cell lines cannot fully replicate the complex in vivo interactions that lead to pathological conditions. To facilitate studies of airway pathologies, an organotypic culture system of primary airway epithelial cells was developed that is now considered the gold standard in vitro model for studying airway epithelial biology.6,7 Airway epithelial cells cultured at an air–liquid interface (ALI), where cells are fed basally and exposed to air apically, differentiate into a mucociliary monolayer similar to the in vivo epithelium.7 ALI cultures are replete with various cell types (eg, ciliated, basal, goblet, club) and morphologically replicate the in vivo airway epithelium. Moreover, multiple studies have demonstrated that ALI cultures replicate many of the in vivo functions.8–12 Although these cultures undeniably replicate much of the in vivo epithelial phenotype, fundamental differences may still exist.13,14
miRNAs are small noncoding RNAs approximately 23 nucleotides in length that complement target mRNAs, causing translational repression or mRNA degradation.15 Because a given miRNA can have hundreds of targets, these regulatory factors exert widespread control over gene products and are estimated to influence one third of the genome.16,17 To date, approximately 1600 human miRNA precursors and more than 2000 mature miRNAs have been identified (miRBase version 19, http://www.mirbase.org, last accessed May 15, 2013).18 Because miRNAs are broad regulators of cellular processes, changes in their expression can lead to profound effects that fundamentally alter cellular behavior. It is not surprising, therefore, that miRNAs have been linked to lung development and pulmonary diseases.19
A critical first step in understanding the role of miRNAs in the biology of airway epithelium during health and disease is assessing the ability of ALI culture systems to accurately replicate the miRNA profile of primary airway epithelial cells. In this study, we compared global miRNA expression between in vitro and in vivo airway epithelium and found that many miRNAs were differentially expressed between these two conditions. Functional analysis of the predicted targets of differentially expressed miRNAs identified pathways associated with epithelial injury and wound healing. Our findings indicate that miRNAs undergo significant differential regulation in ALI culture, and thus that these cultures must be used with caution as a surrogate for the in vivo epithelium.
Materials and Methods
Study Population
Lung transplant patients at the University of Washington Medical Center undergoing bronchoscopic evaluation were enrolled into this study. The study protocol was approved by the Institutional Review Board at the University of Washington, and all patients provided informed consent before enrollment. The airway epithelium from 11 patients was used for immediate RNA isolation (in vivo group). Samples from eight patients were first cultured before RNA collection (in vitro group), and samples from four patients were divided for both in vivo and in vitro conditions. Patient age in the in vivo and in vitro groups was 46.8 ± 17.0 and 40.9 ± 15.2, respectively (means ± SD; P = 0.44). None of the enrolled subjects had evidence of infection, acute allograft rejection, or chronic allograft rejection at the time of tissue collection.
Airway Epithelial Cell Collection and Culture
Airway epithelial cells were collected from small airway brushings with a sheathed cytologic brush (ConMed, Utica, NY). Four to six passes of the brush were used, yielding typically between 1 × 106 and 2 × 106 cells. After the procedure, the cells were immediately transferred on ice to the laboratory, where they were pelleted and resuspended in 500 μL of cold Dulbecco’s modified Eagle’s medium. These cells were then further processed for ALI cell culture or for RNA collection from in vivo samples. Some collections were divided for both cell culture and RNA collection from the primary cells.
Airway epithelial cell cultures were established according to published protocols.7 Primary cells collected from the airway brushings were plated on type I collagen–coated dishes at an initial plating density of 2.5 × 104 cells/cm2. Cells were fed with bronchial epithelial growth medium until they were 80% confluent (typically 7 to 8 days in culture). These cells were then passaged onto type I collagen–coated Transwell inserts (Corning Life Sciences, Tewksbury, MA) at a seeding density of 105 cells/cm2 and were fed with bronchial epithelial cell growth medium in both the apical and basal chambers. Once a fully confluent monolayer was established (typically after 1 to 2 days), cell cultures were fed basally with ALI medium to allow differentiation into a mucociliary monolayer (Supplemental Figure S1).
RNA Collection
To obtain a pure population of airway epithelial cells for RNA collection, primary cells collected from bronchial brushings were first immunodepleted of inflammatory cells with a biotinylated anti-human CD45 antibody (AbD Serotec; Raleigh, NC), followed by Pierce streptavidin magnetic bead separation (Thermo Fisher Scientific, Rockford; IL). This procedure efficiently eliminates all inflammatory cells, which constituted approximately 3% of the total population of harvested cells (data not shown). TRIzol (Life Technologies, Carlsbad, CA) surfactant was then added, and RNA was collected using phase-lock tubes according to the manufacturer’s protocols (5 PRIME, Gaithersburg, MD).
RNA was isolated from cultures after they were fully matured at an ALI for 4 weeks. In brief, cells on the polyester membrane were cut out with a sterile scalpel and placed into TRIzol. Membranes were vortexed in TRIzol for 30 seconds and then processing for RNA collection with phase-lock tubes.
miRNA Profiling and Data Analysis
RNA collected from both primary cells (in vivo samples) and passage 1 ALI cultures (in vitro samples) was used for cDNA generation with a miRCURY LNA cDNA synthesis kit (Exiqon, Woburn, MA). miRNA profiling was performed using the miRCURY LNA Universal RT human miRNA PCR panel version 2.0 (Exiqon) to evaluate 742 unique human miRNAs.
Each miRNA PCR panel was normalized by subtracting a given miRNA quantification cycle (Cq) from the mean Cq of all miRNA probes. This global normalization strategy is superior to normalizing against a limited set of stable RNA controls.20 Correspondence analysis was performed based on the variability in miRNA expression across in vivo (n = 11) and in vitro (n = 8) samples. Differentially expressed miRNAs between in vivo and in vitro samples were identified using the significance analysis of microarrays (SAM) technique.21 Statistical significance was determined based on false discovery rate analysis (q-value).
Highly significant differentially expressed miRNAs (q < 0.001) were interrogated via TargetScan release 6.2 (http://www.targetscan.org, last accessed May 15, 2013) to identify putative gene targets.22 miRNAs not found with TargetScan were eliminated from further analyses. Only conserved targets within the human genome were selected.
Analysis of Gene Expression Data
We downloaded and analyzed human microarray data from the study of Pezzulo et al14 comparing the global transcriptional response of freshly isolated airway epithelial cells (ie, in vivo conditions) (n = 16) to ALI cultures (n = 16) (http://www.ncbi.nlm.nih.gov/geo; accession number GSE20502). Log-transformed probe intensities from these Affymetrix microarrays were normalized using the robust multiarray (RMA) procedure.23 Differential gene expression was determined using SAM based on the same significance q-value cutoff applied to the miRNA data set (q < 0.001).
Functional Analysis
We used the WebGestalt online analytical toolkit (http://bioinfo.vanderbilt.edu/webgestalt, last accessed May 15, 2013)24 to assess enrichment of functional categories for three sets of generated data: i) predicted gene targets for differentially expressed miRNAs (in vivo versus in vitro); ii) differentially expressed genes from the publicly accessed microarray data (in vivo versus in vitro); and iii) a common set of genes that were differentially expressed in both the microarray experiments and putative targets for differentially regulated miRNAs. Functional enrichment was assessed relative to the human genome using the hypergeometric distribution and the Benjamini–Hochberg method to adjust for multiple hypothesis testing (adjusted P value of <0.001).25
Results
miRNA and Transcriptional Profiles of Airway Epithelial Cells Are Altered in Culture
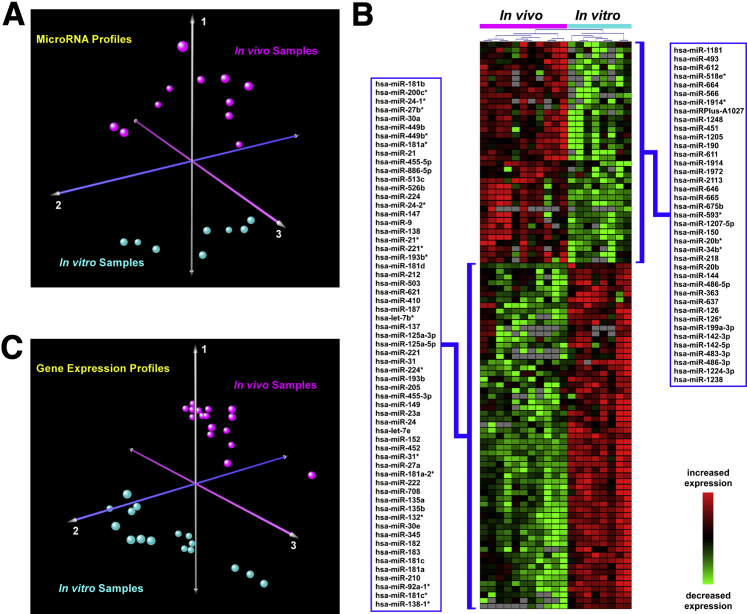
Variation in global miRNA expression between in vivo and in vitro samples was assessed using correspondence analysis and demonstrated clear segregation between the two groups (Figure 1A). This finding implies that culturing airway epithelial cells profoundly perturbs their miRNA profile. Indeed, the expression levels of 247 miRNAs were significantly different between the in vitro and in vivo conditions at a q-value of <0.05; this constitutes one third of the total miRNAs evaluated (Supplemental Tables S1 and S2). When we applied a more stringent statistical cutoff (q < 0.001), 100 miRNAs were differentially expressed between ALI cultures and in vivo samples, with a median log2 difference of 3.06 (ie, an eightfold change) in expression (Figure 1B and Supplemental Table S1). These findings indicated that the global changes observed in miRNA expression were largely attributable to condition (ie, in vitro versus in vivo) and corroborated the notion that miRNA expression is significantly altered in ALI cell cultures, compared with the in vivo epithelium.
Figure 1.
A: Correspondence analysis of miRNAs in ALI cultures and in vivo airway epithelium. In this unsupervised analysis based on all detectable human miRNAs, global variability in miRNA expression distinctly segregated in vivo samples (n = 11) from in vitro cultures (n = 8). B: Differentially regulated miRNAs between ALI cultures and in vivo airway epithelium. Heat-map depiction of 100 highly differentially expressed miRNAs between the in vitro and in vivo groups (q < 0.001). Gray color indicates miRNA levels undetectable by PCR. C: Correspondence analysis of microarray-based gene expression in ALI cultures and in vivo airway epithelium. In this analysis based on the expression profile of more than 5000 genes, global variability in gene expression clearly discriminated between in vivo samples and in vitro cultures. n = 16 samples per group.
miRNAs function by either directly repressing translation or augmenting mRNA destruction.15 However, mRNA destabilization is detectable for targets that have translation repressed by more than one third.26–29 Changes in miRNA expression will therefore induce widespread effects in gene expression, and alterations in the transcriptome of the airway epithelium due to condition (ALI versus in vivo) should reflect changes in the miRNA profile. To further assess whether culture status influences gene expression, we mined publicly available microarray data comparing the transcriptome of human ALI cultures with freshly isolated airway epithelial cells.14 Correspondence analysis of the gene expression data (Figure 1C) strongly resembled the miRNA correspondence analysis of in vitro and in vivo airway epithelium (Figure 1A), confirming large-scale transcriptional differences between the conditions.
Culture-Induced Alterations in miRNA and Gene Expression of Airway Epithelial Cells Map to Common Pathways Linked to Injury and Repair
Because miRNAs have multiple targets and influence the expression of many genes, we explored the predicted transcriptional consequences of altered miRNA expression in ALI cultures of airway epithelium. Multiple algorithms have been developed to determine putative miRNA targets.30 We used TargetScan, which is based on an experimentally validated algorithm with high predictive specificity.31 We limited our analysis to miRNAs with highly significant changes in expression between conditions (q < 0.001) (Figure 1B and Supplemental Table S2) and identified 4543 unique predicted gene targets for the differentially regulated miRNAs. These putative miRNA targets were highly enriched in pathways associated with cancer, cell signaling, epithelial injury, and repair, among others (Table 1 and Supplemental Table S3).
Table 1.
Enriched Functional Pathways Mapping to Putative Targets of Differentially Regulated miRNAs between ALI Cultures and the in Vivo Airway Epithelium
| Functional pathways∗ | Adjusted P† |
|---|---|
| Pathways in cancer | 1.06 × 10−41 |
| MAPK signaling pathway | 2.74 × 10−38 |
| Axon guidance | 1.86 × 10−35 |
| Focal adhesion | 3.97 × 10−32 |
| Endocytosis | 3.37 × 10−27 |
| Neurotrophin signaling pathway | 1.60 × 10−25 |
| Wnt signaling pathway | 6.52 × 10−25 |
| Insulin signaling pathway | 4.53 × 10−24 |
| Regulation of actin cytoskeleton | 5.71 × 10−24 |
| ErbB signaling pathway | 1.01 × 10−20 |
| Ubiquitin-mediated proteolysis | 7.80 × 10−20 |
| Long-term potentiation | 1.33 × 10−19 |
| Chronic myeloid leukemia | 9.17 × 10−18 |
| TGF-β signaling pathway | 6.38 × 10−17 |
| Melanogenesis | 9.46 × 10−17 |
Only the top 15 pathways are listed here; the full list appears in Supplemental Table S3.
Putative targets of differentially expressed miRNAs (q < 0.001) were used to determine enriched pathways based on the KEGG (Kyoto Encyclopedia of Genes and Genomes) database (http://www.genome.jp/kegg, release 66.1, last accessed May 15, 2013).
Benjamini–Hochberg adjustment.
We then analyzed publicly available microarray data measuring global transcriptional differences between ALI cultures and in vivo airway epithelium and identified 5270 differentially regulated genes using a strict threshold of q < 0.001 (Supplemental Figure S2).14 We applied the same pathway enrichment analysis pipeline used for the miRNA data to these differentially expressed genes (Table 2 and Supplemental Table S4). Interestingly, there were many similarities between the list of functional pathways over-represented in the microarray data and processes enriched among the putative miRNA targets. In fact, 7 of the top 15 enriched pathways were identical in both analyses (pathways in cancer, focal adhesion, endocytosis, MAPK signaling, actin cytoskeleton, ubiquitin-mediated proteolysis, and adherens junction). At the functional level, therefore, transcriptional alterations assessed from the microarray data overlapped with changes inferred from in silico–generated targets of differentially expressed miRNAs.
Table 2.
Enriched Functional Pathways Mapping to Differentially Expressed Genes between ALI Cultures and the in Vivo Airway Epithelium
| Functional Pathways∗ | Adjusted P† |
|---|---|
| Metabolic pathways | 8.41 × 10−76 |
| Alzheimer disease | 8.58 × 10−34 |
| Huntington disease | 1.90 × 10−33 |
| Parkinson disease | 1.69 × 10−29 |
| Oxidative phosphorylation | 2.64 × 10−26 |
| Pathways in cancer | 4.90 × 10−21 |
| Focal adhesion | 3.30 × 10−19 |
| Protein processing in endoplasmic reticulum | 8.20 × 10−19 |
| Endocytosis | 2.93 × 10−17 |
| Tight junction | 7.04 × 10−17 |
| MAPK signaling pathway | 6.18 × 10−16 |
| Leukocyte transendothelial migration | 1.64 × 10−15 |
| Regulation of actin cytoskeleton | 3.07 × 10−15 |
| Ubiquitin-mediated proteolysis | 2.05 × 10−14 |
| Adherens junction | 2.05 × 10−14 |
Only the top 15 pathways are listed here; the full list appears in Supplemental Table S4.
Differentially expressed genes (q < 0.001) were used to determine enriched pathways based on the KEGG database.
Benjamini–Hochberg adjustment.
To more precisely highlight the functional role of miRNAs in regulating biological processes that distinguish airway epithelium ALI cultures from in vivo cells, we leveraged the microarray information to filter out putative miRNA targets that were not differentially expressed. By comparing predicted targets of differentially regulated miRNAs with genes that had significant expression changes in the microarray data under in vivo versus in vitro conditions, we generated a list of 1013 common genes identified from both analyses. Functional enrichment analysis of this gene list identified miRNA-regulated pathways that drive key biological differences between cultured human airway epithelium and primary cells (Table 3 and Supplemental Table S5). Importantly, many of these processes are strongly associated with epithelial injury and repair (eg, MAPK/insulin/Wnt/ErbB signaling, focal adhesion, adherens/tight junction, and regulation of cytoskeleton).
Table 3.
Pathways Enriched among Putative Target Genes of Differentially Regulated miRNAs That Were Also Differentially Expressed between ALI Cultures and the in Vivo Airway Epithelium
| Functional Pathways∗ | Adjusted P† |
|---|---|
| MAPK signaling pathway | 8.72 × 10−20 |
| Pathways in cancer | 8.72 × 10−20 |
| Insulin signaling pathway | 8.20 × 10−15 |
| Focal adhesion | 2.08 × 10−14 |
| Adherens junction | 1.05 × 10−13 |
| Ubiquitin-mediated proteolysis | 2.32 × 10−13 |
| Regulation of actin cytoskeleton | 3.30 × 10−12 |
| Neurotrophin signaling pathway | 3.91 × 10−12 |
| Axon guidance | 4.85 × 10−12 |
| T-cell receptor signaling pathway | 1.05 × 10−11 |
| Tight junction | 5.54 × 10−11 |
| Endocytosis | 1.33 × 10−10 |
| Wnt signaling pathway | 5.73 × 10−10 |
| Chronic myeloid leukemia | 1.18 × 10−9 |
| ErbB signaling pathway | 1.55 × 10−9 |
Only the top 15 pathways are listed here; the full list appears in Supplemental Table S5.
Enriched pathways were determined based on the KEGG database.
Benjamini–Hochberg adjustment.
Discussion
ALI cultures are currently considered the gold standard of in vitro systems for studying airway epithelial biology.32 However, the present results show that there are limitations to the ALI culture system. In particular, we found that miRNA expression profiles are widely perturbed between in vitro conditions and in vivo condition. To our knowledge, evaluation of miRNA fidelity between ALI culture and in vivo airway epithelium has not been previously reported. However, two analogous studies comparing the transcriptome of in vitro and in vivo airway epithelium were recently published.13,14 We reanalyzed the data from Pezzulo et al14 and found more than 5000 genes to be differentially regulated between in vitro and in vivo conditions, which is similar to the numbers reported by Dvorak et al.13
In the present study, we first showed that similar functional pathways were affected by differentially regulated miRNAs (as assessed by their putative targets) or genes (as measured by microarrays) in ALI cultures and in in vivo airway epithelium. Next, we intersected the microarray and miRNA data to identify a subset of differentially expressed genes that were also targets of the differentially regulated miRNAs. These genes were highly enriched in pathways linked to epithelial wounding and repair, suggesting that ALI cultures remain in an injured state even after differentiation into a mucociliary monolayer.8,33 Despite the attempts to mimic in situ conditions, such as controlling growth factors and feeding cells basally while exposing cells to air apically, ALI cultures cannot fully replicate the in vivo environment (eg, extracellular matrix and substratum stiffness are lacking).34–36
miRNAs are critical regulators of gene expression, and alterations in their expression can drive various diseases of the lung.17,19,37 The present findings suggest that ALI cultures may not adequately represent the diseased in vivo airway epithelial cells and that these cultures may have limited utility as a surrogate for the in vivo airway epithelium in discovering mechanism of disease. Of course, there are advantages to culturing cells, including overcoming the limited airway samples procured via bronchoscopy and allowing for manipulation of the epithelium under controlled conditions. Even though our data suggest that these cultures do not reliably reproduce in vivo phenotypes in vitro, they are still useful in studying basic airway epithelial biology; ALI cultures can replicate many in vivo cellular functions and can respond appropriately to various stimuli.8–12
The present study has a number of limitations. TargetScan is highly specific, but it has low sensitivity in identifying miRNA targets.31 Thus, it is likely that, although the majority of putative targets identified by TargetScan are true targets, the overall number is under-represented. We opted for this conservative approach, to reduce false positivity in target identification. To overcome the limitation that gene targets of differentially expressed miRNAs were predicted in silico, we focused on enrichment of canonical pathways instead of specific genes. Furthermore, we integrated gene expression information from an independent set of experiments with our miRNA data, to systematically narrow down candidate gene lists and to identify key miRNA-regulated processes that are differentially activated in ALI cultures relative to in vivo airway epithelium. The relatively small sample size is another limitation of the present study; however, even in this limited cohort, we discovered highly significant differences in miRNA profiles depending on culture condition. Because cells were harvested from lung transplant patients, we cannot rule out the effects of transplantation itself or the associated drug treatments on differential miRNA expression between the in vivo epithelium and ALI cultures. Nevertheless, when we compared our results with the microarray data from in vivo condition and in vivo airway epithelium obtained from healthy individuals, a significant overlap was identified between differentially expressed genes and functional pathways. This observation implies that the primary cause of transcriptional differences between our samples was not transplantation status but rather resulted from culture condition.
In summary, the present findings demonstrate that miRNAs, as well as a large proportion of the transcriptome, have altered expression when airway epithelial cells are grown in ALI cultures. The primary implication of our findings is that these culture systems do not fully capture the in vivo state of diseased airways and must therefore be used with caution. Nonetheless, ALI cultures remain necessary and appropriate models for understanding basic airway biology and to complement findings derived from in vivo tissue.
Acknowledgments
We thank Ellen McCown and Sharon Kelso for their assistance with enrolling patients into this study.
Footnotes
Supported by NIH-NHLBI grants HL084396 (P.C.), HL103868 (P.C.), and HL029594 (S.A.G.), the Institute of Translational Health Sciences (P.C.), and the Cystic Fibrosis Foundation (P.C.). Additional support was also provided by the Cystic Fibrosis Research Development Program funded through the NIH-NIDDK (P30-DK089507).
Supplemental Data
PAS staining of an ALI culture. Human airway epithelial cells cultured at an ALI were fixed with 4% paraformaldehyde, embedded in paraffin, and processed for PAS staining. Brightfield images were captured using an Olympus BX-51 microscope with a Plan Apo ×60/1.4 oil objective. Scale bar = 100 μm.
Heat map of mRNA data for the approximately 5000 genes that are differentially expressed (q < 0.001) between in vivo and in vitro airway epithelial cells (based on microarray experiments by Pezzulo et al14). Columns represent human samples and rows represent normalized gene expression values. The expression patterns were generated using two-way hierarchical clustering (samples, genes) based on the Euclidean distance metric. n = 16 samples per group.
References
- 1.Lambrecht B.N., Hammad H. The airway epithelium in asthma. Nat Med. 2012;18:684–692. doi: 10.1038/nm.2737. [DOI] [PubMed] [Google Scholar]
- 2.Parker D., Prince A. Innate immunity in the respiratory epithelium. Am J Respir Cell Mol Biol. 2011;45:189–201. doi: 10.1165/rcmb.2011-0011RT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Proud D., Leigh R. Epithelial cells and airway diseases. Immunol Rev. 2011;242:186–204. doi: 10.1111/j.1600-065X.2011.01033.x. [DOI] [PubMed] [Google Scholar]
- 4.Wynn T.A. Integrating mechanisms of pulmonary fibrosis. J Exp Med. 2011;208:1339–1350. doi: 10.1084/jem.20110551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sporty J.L., Horálková L., Ehrhardt C. In vitro cell culture models for the assessment of pulmonary drug disposition. Expert Opin Drug Metab Toxicol. 2008;4:333–345. doi: 10.1517/17425255.4.4.333. [DOI] [PubMed] [Google Scholar]
- 6.Wu R., Sato G.H., Whitcutt M.J. Developing differentiated epithelial cell cultures: airway epithelial cells. Fundam Appl Toxicol. 1986;6:580–590. doi: 10.1016/0272-0590(86)90170-3. [DOI] [PubMed] [Google Scholar]
- 7.Randell S.H., Fulcher M.L., O’Neal W., Olsen J.C. Primary epithelial cell models for cystic fibrosis research. Methods Mol Biol. 2011;742:285–310. doi: 10.1007/978-1-61779-120-8_18. [DOI] [PubMed] [Google Scholar]
- 8.Ross A.J., Dailey L.A., Brighton L.E., Devlin R.B. Transcriptional profiling of mucociliary differentiation in human airway epithelial cells. Am J Respir Cell Mol Biol. 2007;37:169–185. doi: 10.1165/rcmb.2006-0466OC. [DOI] [PubMed] [Google Scholar]
- 9.Mathis C., Poussin C., Weisensee D., Gebel S., Hengstermann A., Sewer A., Belcastro V., Xiang Y., Ansari S., Wagner S., Hoeng J., Peitsch M. Human bronchial epithelial cells exposed in vitro to cigarette smoke at the air-liquid interface resemble bronchial epithelium from human smokers. Am J Physiol Lung Cell Mol Physiol. 2013;304:L489–L503. doi: 10.1152/ajplung.00181.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martinez-Anton A., Sokolowska M., Kern S., Davis A.S., Alsaaty S., Taubenberger J.K., Sun J., Cai R., Danner R.L., Eberlein M., Logun C., Shelhamer J.H. Changes in microRNA and mRNA expression with differentiation of human bronchial epithelial cells. Am J Respir Cell Mol Biol. 2013;49:384–395. doi: 10.1165/rcmb.2012-0368OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kesimer M., Kirkham S., Pickles R.J., Henderson A.G., Alexis N.E., DeMaria G., Knight D., Thornton D.J., Sheehan J.K. Tracheobronchial air-liquid interface cell culture: a model for innate mucosal defense of the upper airways? Am J Physiol Lung Cell Mol Physiol. 2009;296:L92–L100. doi: 10.1152/ajplung.90388.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shah A.S., Ben-Shahar Y., Moninger T.O., Kline J.N., Welsh M.J. Motile cilia of human airway epithelia are chemosensory. Science. 2009;325:1131–1134. doi: 10.1126/science.1173869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dvorak A., Tilley A.E., Shaykhiev R., Wang R., Crystal R.G. Do airway epithelium air-liquid cultures represent the in vivo airway epithelium transcriptome? Am J Respir Cell Mol Biol. 2011;44:465–473. doi: 10.1165/rcmb.2009-0453OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pezzulo A.A., Starner T.D., Scheetz T.E., Traver G.L., Tilley A.E., Harvey B.G., Crystal R.G., McCray P.B., Jr., Zabner J. The air-liquid interface and use of primary cell cultures are important to recapitulate the transcriptional profile of in vivo airway epithelia. Am J Physiol Lung Cell Mol Physiol. 2011;300:L25–L31. doi: 10.1152/ajplung.00256.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bartel D.P. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Selbach M., Schwanhäusser B., Thierfelder N., Fang Z., Khanin R., Rajewsky N. Widespread changes in protein synthesis induced by microRNAs. Nature. 2008;455:58–63. doi: 10.1038/nature07228. [DOI] [PubMed] [Google Scholar]
- 17.Lewis B.P., Burge C.B., Bartel D.P. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 18.Kozomara A., Griffiths-Jones S. miRBase: integrating microRNA annotation and deep-sequencing data. Nucleic Acids Res. 2011;39:D152–D157. doi: 10.1093/nar/gkq1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nana-Sinkam S.P., Hunter M.G., Nuovo G.J., Schmittgen T.D., Gelinas R., Galas D., Marsh C.B. Integrating the microRNome into the study of lung disease. Am J Respir Crit Care Med. 2009;179:4–10. doi: 10.1164/rccm.200807-1042PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mestdagh P., Van Vlierberghe P., De Weer A., Muth D., Westermann F., Speleman F., Vandesompele J. A novel and universal method for microRNA RT-qPCR data normalization. Genome Biol. 2009;10:R64. doi: 10.1186/gb-2009-10-6-r64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tusher V.G., Tibshirani R., Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci USA. 2001;98:5116–5121. doi: 10.1073/pnas.091062498. [Erratum appeared in Proc Natl Acad Sci USA 2001, 98:10515] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewis B.P., Shih I.-H., Jones-Rhoades M.W., Bartel D.P., Burge C.B. Prediction of mammalian microRNA targets. Cell. 2003;115:787–798. doi: 10.1016/s0092-8674(03)01018-3. [DOI] [PubMed] [Google Scholar]
- 23.Irizarry R.A., Bolstad B.M., Collin F., Cope L.M., Hobbs B., Speed T.P. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 2003;31:e15. doi: 10.1093/nar/gng015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang J., Duncan D., Shi Z., Zhang B. WEB-based GEne SeT AnaLysis Toolkit (WebGestalt): update 2013. Nucleic Acids Res. 2013;41:W77–W83. doi: 10.1093/nar/gkt439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang B., Kirov S., Snoddy J. WebGestalt: an integrated system for exploring gene sets in various biological contexts. Nucleic Acids Res. 2005;33:W741–W748. doi: 10.1093/nar/gki475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo H., Ingolia N.T., Weissman J.S., Bartel D.P. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature. 2010;466:835–840. doi: 10.1038/nature09267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baek D., Villén J., Shin C., Camargo F.D., Gygi S.P., Bartel D.P. The impact of microRNAs on protein output. Nature. 2008;455:64–71. doi: 10.1038/nature07242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lim L.P., Lau N.C., Garrett-Engele P., Grimson A., Schelter J.M., Castle J., Bartel D.P., Linsley P.S., Johnson J.M. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433:769–773. doi: 10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- 29.Bagga S., Bracht J., Hunter S., Massirer K., Holtz J., Eachus R., Pasquinelli A.E. Regulation by let-7 and lin-4 miRNAs results in target mRNA degradation. Cell. 2005;122:553–563. doi: 10.1016/j.cell.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 30.Witkos T.M., Koscianska E., Krzyzosiak W.J. Practical aspects of microRNA target prediction. Curr Mol Med. 2011;11:93–109. doi: 10.2174/156652411794859250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alexiou P., Maragkakis M., Papadopoulos G.L., Reczko M., Hatzigeorgiou A.G. Lost in translation: an assessment and perspective for computational microRNA target identification. Bioinformatics. 2009;25:3049–3055. doi: 10.1093/bioinformatics/btp565. [DOI] [PubMed] [Google Scholar]
- 32.Crystal R.G., Randell S.H., Engelhardt J.F., Voynow J., Sunday M.E. Airway epithelial cells: current concepts and challenges. Proc Am Thorac Soc. 2008;5:772–777. doi: 10.1513/pats.200805-041HR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baer P.C., Bereiter-Hahn J. Epithelial cells in culture: injured or differentiated cells? Cell Biol Int. 2012;36:771–777. doi: 10.1042/CBI20120060. [DOI] [PubMed] [Google Scholar]
- 34.Engler A.J., Sen S., Sweeney H.L., Discher D.E. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 35.Gruenert D.C., Finkbeiner W.E., Widdicombe J.H. Culture and transformation of human airway epithelial cells. Am J Physiol. 1995;268:L347–L360. doi: 10.1152/ajplung.1995.268.3.L347. [DOI] [PubMed] [Google Scholar]
- 36.Sherr C.J., DePinho R.A. Cellular senescence: minireview mitotic clock or culture shock. Cell. 2000;102:407–410. doi: 10.1016/s0092-8674(00)00046-5. [DOI] [PubMed] [Google Scholar]
- 37.Pandit K.V., Milosevic J., Kaminski N. MicroRNAs in idiopathic pulmonary fibrosis. Transl Res. 2011;157:191–199. doi: 10.1016/j.trsl.2011.01.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PAS staining of an ALI culture. Human airway epithelial cells cultured at an ALI were fixed with 4% paraformaldehyde, embedded in paraffin, and processed for PAS staining. Brightfield images were captured using an Olympus BX-51 microscope with a Plan Apo ×60/1.4 oil objective. Scale bar = 100 μm.
Heat map of mRNA data for the approximately 5000 genes that are differentially expressed (q < 0.001) between in vivo and in vitro airway epithelial cells (based on microarray experiments by Pezzulo et al14). Columns represent human samples and rows represent normalized gene expression values. The expression patterns were generated using two-way hierarchical clustering (samples, genes) based on the Euclidean distance metric. n = 16 samples per group.