Abstract
Background
Giardia duodenalis infection and malnutrition are still considered as public health problems in many developing countries especially among children in rural communities. This study was carried out among Aboriginal (Orang Asli) primary schoolchildren in rural peninsular Malaysia to investigate the burden and the effects of Giardia infection on growth (weight and height) of the children.
Methods/Findings
Weight and height of 374 children aged 7–12 years were assessed before and after treatment of Giardia infection. The children were screened for Giardia parasite using trichrome staining technique. Demographic and socioeconomic data were collected via face-to-face interviews using a pre-tested questionnaire. Overall, 22.2% (83/374) of the children were found to be infected with Giardia. Nutritional status of children was assessed and the results showed that the mean weight and height were 23.9 kg (95% CI = 23.3, 24.5) and 126.6 cm (95% CI = 125.6, 127.5), respectively. Overall, the prevalence of severe underweight, stunting and wasting were 28.3%, 23.8% and 21.0%, respectively. Multiple linear regression analyses showed sex, Giardia infection and household monthly income as the significant determinants of weight while sex and level of mother's education were the significant determinants of height. Weight and height were assessed at 3 and 6 months after treatment of Giardia infection. It was found that Giardia infection has a significant association with the weight of children but not with height.
Conclusions/Significance
This study reveals high prevalence of Giardia infection and malnutrition among Aboriginal children in rural Malaysia and clearly highlights an urgent need to identify integrated measures to control these health problems in the rural communities. Essentially, proper attention should be given to the control of Giardia infection in Aboriginal communities as this constitutes one of the strategies to improve the nutritional status of Aboriginal children.
Author Summary
Giardia infection, a neglected infection caused by the protozoan parasite Giardia duodenalis is prevalent worldwide especially among young children in rural areas of the tropics and subtropics. In Malaysia, Giardia infection and protein-energy malnutrition coexist in Aboriginal (Orang Asli) communities with high prevalence among school-aged children. We screened 374 schoolchildren in Lipis and Raub districts, Pahang, Malaysia for the presence of Giardia infection and investigated the effects of this infection on the growth of children. Overall, 22.2% of the children studied were infected with Giardia. Nutritional status of children was assessed and we found that the prevalence of severe underweight, stunting and wasting were 28.3%, 23.8% and 21.0%, respectively. Giardia infection was identified as a significant determinant of weight among these children. Moreover, it was found that Giardia infection has a significant association with the weight of children but not with height. The control of Giardia infection should be given prominence as it is one of the strategies to enhance the nutritional status of Aboriginal children.
Introduction
Giardia duodenalis (syn. G. intestinalis; G. lamblia) is the most frequently reported intestinal parasite worldwide, especially among children in developing countries, with a prevalence rate of 10–50% [1]. It has also been identified as a main cause of diarrhoea among young children in day care centers and among travellers from developed countries [2], [3]. Waterborne and foodborne transmission is the most frequent mode of spread besides person-to-person transmission [4], [5]. Giardia infection may cause acute or chronic diarrhea or be present as an asymptomatic infection [6]. Often, patients suffering from acute infection present with diarrhoea, abdominal pain and the clinical manifestations of malabsorption [7]. Chronic infection is usually associated with clinical manifestations of malnutrition and micronutrient deficiencies, especially vitamin A deficiency (VAD) and iron deficiency anaemia (IDA) [8], [9]. A significant association between the chronic infection and poor cognitive function has also been reported among children [6].
Protein-energy malnutrition (PEM) is probably the world's major public health problem especially in Africa and Southern Asia where about 70% of all the children are malnourished [10]. Despite the reduction in the global prevalence of stunting and underweight from 40% and 25% in 1990 to 26% and 16%, respectively in 2011, the target of a 40% reduction by 2025 in the global number of children under-five years of age who are stunted could not be reached under the current rates of decline [11]. Malnutrition and parasitic infections coexist in poor socioeconomic communities of the developing countries [12], [13]. The association of Giardia infection with malnutrition has been investigated throughout many studies in different countries and the studies have yielded a variety of results. Whilst some studies showed negative impacts of the infections on the nutritional status of children [14]–[17], other studies found no association [18]–[20].
In Malaysia, although there is increasing concern about the burden of these problems in rural areas, data on the association of Giardia infection with malnutrition are largely lacking. Previous cross-sectional studies among Aboriginal children in peripheral and rural areas of Selangor and Pahang identified Giardia infection as a significant risk factor of malnutrition and poor vitamin A status [9], [21]. However, these studies were based on a single point data analysis (cross-sectional). Within this context, the aim of the present study was to investigate the burden of G. duodenalis infection and its effect on the growth (weight and height) of Aboriginal primary school-age children in rural Malaysia.
Materials and Methods
Ethical statement
The protocol of the study was approved by the Medical Ethics Committee of the University of Malaya Medical Centre, Malaysia (Reference Number: 788.74). Before the commencement of the present study, meetings were held with the headmaster, teachers, heads of the villages, parents, and the schoolchildren. The purpose, procedures, potential risks and benefits of the study were explained to the parents and children. During the meeting, they were also informed that their participation was totally voluntarily and they could withdraw from the study at any time without citing reasons for doing so. Written and signed or thumb-printed informed consents were taken from parents or guardians on behalf of their children, and these procedures were approved by the Medical Ethics Committee of the University of Malaya Medical Centre. All the infected children were treated with a 3-day course of 400 mg albendazole tablets. Albendazole is also considered as the drug of choice for Ascaris, Trichuris and hookworm infections. By the end of 6 months assessment, drugs were distributed to all children who were found to be infected.
Study area
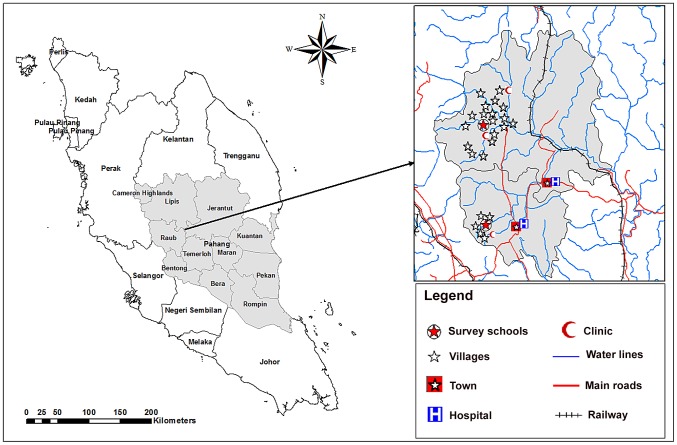
A longitudinal study with before and after treatment follow up assessments was carried out in primary schools for Aboriginal children in Raub and Lipis districts, Pahang state, Malaysia. Data collection was carried out between March and October 2010. Sekolah Kebangsaan Satak (National School of Satak) in Raub and Sekolah Kebangsaan Betau (National School of Betau) in Lipis were selected for this study (Figure 1). The study area in Raub district, located about 140 km northeast of Kuala Lumpur, has five main Aboriginal settlements namely; Satak, Rensong, Ruai Hulu, Ruai Hilir, and Kelang. The study area in Lipis district, located about 200 km northeast of Kuala Lumpur, has 18 villages. Adequate sanitation facilities is the main predictor for acquiring intestinal parasitic infections, especially Giardia and soil-transmitted helminthes, in the Aboriginal and rural communities. The food supply in these communities is constantly poor in energy and periodically low in protein [22], [23]. Most of these impoverished people are subsistence farmers, completely dependent on their environment for survival.
Figure 1. A geographic map showing Pahang state and the location of the selected schools and villages in Lipis and Raub districts.
Study population
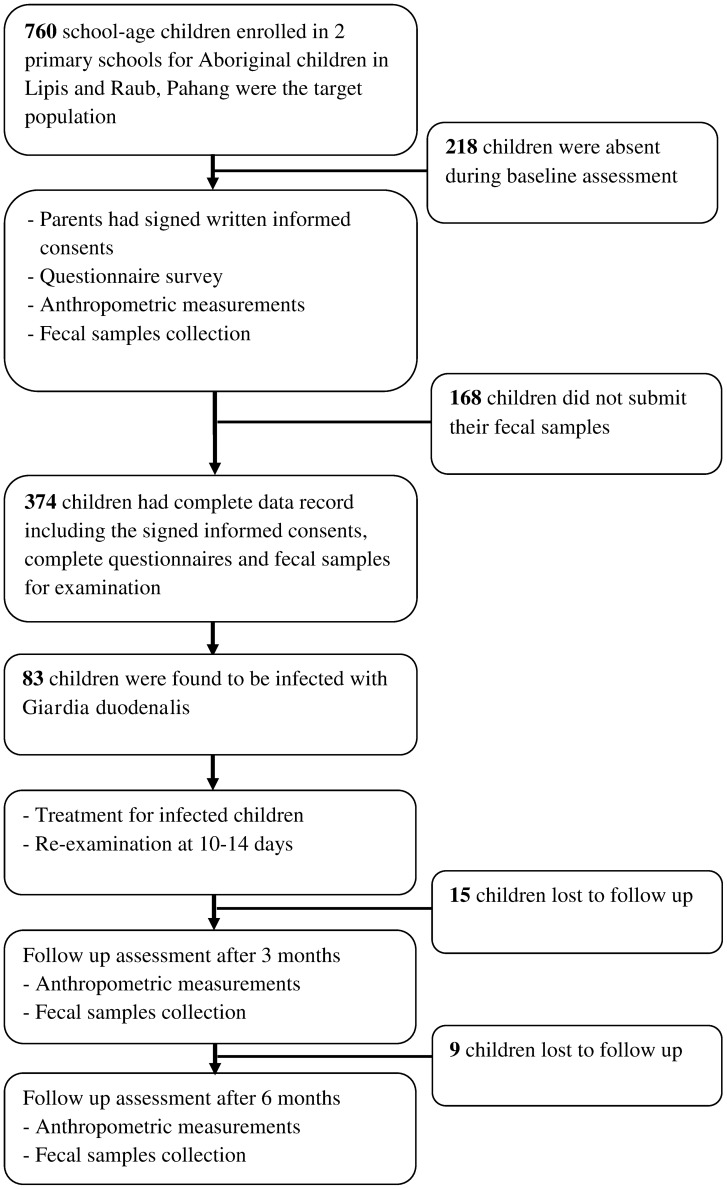
The schools had a total enrolment of 760 children in grades one to six. However, 218 students were absent during the enrolment day. All attending children were invited to participate in the study and those who agreed were enrolled. Out of the 542 students, 374 eligible children, aged 7–12 years, agreed to participate in this study and had delivered fecal samples for examination. Subsequently, 15 and 9 children were lost to follow up at 3 and 6 months assessments, respectively (Figure 2). For ethical reasons, a placebo control group was not included.
Figure 2. Flow chart of the participation and compliance in the present study.
Questionnaire
A structured questionnaire was prepared in English and then translated to Bahasa Malaysia (the national language for Malaysia). The questionnaire was designed to collect information on the demographic, socioeconomic and environmental background, personal hygiene and practices and health status of the participants. For this, the parents were interviewed in their home settings by two field assistants, one from the school and the other from the Department of Parasitology, University of Malaya. Both assistants were trained by the principal investigator on the purpose of the study and on how to administer the questionnaire.
Anthropometric measurements
All children underwent anthropometric measurements for weight and height according to this procedure: children were weighed wearing the school uniforms (empty pockets, without belts and barefooted) using a calibrated 0.1 kg intervals SECA scale (709-USA). Using the same instrument which has a scaled sliding head piece, height of children was measured to the nearest millimeter. For quality control, the scale was calibrated regularly and measurements were taken twice by different persons and the mean value was recorded. To assess the nutritional status of children, weight-for-age Z-score (WAZ), height-for-age Z-score (HAZ) and weight-for-height Z-score (WHZ) were calculated and used as indicators for underweight (an overall indicator for malnutrition), stunting or shortness (chronic malnutrition) and wasting or thinness (acute malnutrition), respectively. These Z-scores were derived from the measurements of weight and height using EpiNut Anthropometry in Epi Info program.
The Z-scores were evaluated according to the median values of the National Center for Health Statistics (NCHS), which are based on those recommended by the World Health Organization. Using these reference values, children who had Z-score below −2 standard deviations of the NCHS median values were considered to have severe malnutrition and Z-scores between −1 and −2 standard deviations were considered to have moderate malnutrition [24].
Fecal sample examination
Fresh fecal samples were collected into clean 100 mL wide-mouth screw cap containers. The participants were instructed to scoop a suitable amount of fecal sample, using a provided scoop into the container. Then, the containers were placed into zip-locked plastic bags and transported for examination at the stool processing laboratory in the Department of Parasitology, University of Malaya. Approximately 10 g of each fecal sample was kept in Poly Vinyl Alcohol (PVA). Detection of G. duodenalis was performed using trichrome staining technique [25]. G. duodenalis infection was recorded as positive when cysts and/or trophozoites were detected in the stained fecal smear. Negative fecal samples were re-examined by formalin ether sedimentation technique as described by Cheesbrough [26] before the negative results were confirmed. The unpreserved fecal samples were examined using the Kato-Katz and Harada-Mori fecal cultivation techniques for the presence of soil-transmitted helminths: Ascaris lumbricoides, Trichuris trichiura and hookworm eggs [27], [28]. To determine the worm burden, egg counts were taken and recorded as eggs per gram of feces (epg) for each positive sample and the intensity of infections was graded as heavy, moderate or light according to the criteria proposed by the WHO [29].
Fecal samples were collected and examined by the same methods at 14 days, 3 and 6 months after the administration of treatment for the infected children. For quality control, duplicate analysis was performed on 93 (25%) randomly collected samples. Moreover, the samples were coded only with numbers and the technicians at the diagnostic laboratory were blinded to the code.
Treatment
After the baseline assessment for the presence of Giardia infections, all the infected children were treated with 400 mg albendazole tablets. Penggabean et al. have evaluated the efficacy of albendazole in treating Giardia infection among 917 subjects in rural Malaysia and found that a 3-day course of 400 mg albendazole is highly effective against Giardia with a cure rate of 96.6% [30]. Similar findings were reported in a remote Aboriginal community in Australia and in Bangladesh [31], [32]. Moreover, a previous meta-analysis on the effectiveness of albendazole compared with metronidazole concluded that albendazole could be used as an alternative and/or a replacement for the metronidazole in the treatment of Giardia infection [33].
The tablets were available in small packets of two tablets; each white chewable tablet contains 200 mg albendazole as the active ingredient. The orange flavor encouraged the children to chew the tablets before swallowing. Each child was given two tablets daily for 3 days. A researcher and a medical officer supervised the treatment and asked each child to open their mouth to confirm the tablets have been swallowed. Fecal samples were collected on the 10–14 days post treatment and examined to ascertain the effectiveness of the treatment. All children who received the treatment were reported free from Giardia parasites.
Statistical analysis
Data was double-entered by two different researchers into Microsoft Office Excel 2007 spreadsheets. A third researcher cross-checked the two data sets for accuracy and created a single data set. Data analysis was performed by using Statistical Package for Social Sciences for Windows (SPSS) version 13. Demographic, socioeconomic, environmental and behavioural characteristics were treated as categorical variables and presented as frequencies and percentages. All continuous variables were evaluated for normality by Kolmogorov-Smirnov Z test and means and 95% confidence intervals (95% CI) were calculated. For inferential statistics, the dependent variables were weight and height while the independent variables were Giardia infection (main factor), demographic factors (age and gender) and socioeconomic factors (parents' educational levels, parents' employment status, household monthly income, family size). To investigate the impact of Giardia infection on growth, independent t-test was used to examine the differences in weight and height between the infected and non-infected children. The changes in these parameters between the baseline and the follow up assessment were examined by paired t-test. These comparisons were adjusted for age and sex. Chi-square test was used to examine the significance of the associations and differences in frequency distribution of variables. A repeated-measures ANOVA on the means of weight and height was used to investigate the trend of incremental change over time by Giardia infection status. Wilks' Lambda statistics and multivariate eta squared were reported to demonstrate the effect size in ANOVA tests.
Multiple linear regression analyses were used to identify the determinants of weight and height. Beta (β) coefficient and its standard error and 95% CI were reported for the significant determinants. All variables that showed significant difference with P≤0.25 in the univariate analysis were used to develop the multiple linear regression models as suggested by Bendel and Afifi [34]. All tests were considered significant at P<0.05.
Results
General characteristics of the study population
Three hundred and seventy four children aged 7–12 years with a mean age of 9.4 years (95% CI = 9.2, 9.6) participated in this study. School absenteeism and dropout rates in these communities were found to be high and this could be attributed to poverty, in general, as children of poor families being forced to work or to help their parents in their work and daily activities. Moreover, other factors such as diseases or the lack of interest in gaining an education could also be associated with school dropout and absenteeism among these children. The general characteristics of the subjects and their families are shown in Table 1. Approximately half of the fathers and mothers had no formal education and about two-thirds (63.4%) of the families had low household monthly income (<RM500; US$1 = MYR3.05). Only one-third and one-fifth of the fathers and mothers were working, respectively, as laborers in the palm oil or rubber plantations. Most of the houses were made of woods or bamboo and about half of the houses had piped water supply (gravity-fed) and electricity. The socioeconomic characteristics were found to be homogeneous in these two different Aboriginal communities.
Table 1. General baseline characteristics of Aboriginal schoolchildren who participated in this study (n = 374).
| Characteristics | Frequency (%) |
| Age groups | |
| ≥10 years | 129 (34.5) |
| <10 years | 245 (65.5) |
| Gender | |
| Females | 198 (52.9) |
| Males | 176 (47.1) |
| Socioeconomic status | |
| Fathers' education level (at least 6 years) | 191 (51.1) |
| Mothers' education level (at least 6 years) | 175 (46.8) |
| Low household income (<RM500) | 237 (63.4) |
| Working fathers | 114 (30.5) |
| Working mothers | 80 (21.4) |
| Large family size (>7 members) | 80 (21.4) |
| Piped water supply | 194 (51.9) |
| Electricity | 200 (53.5) |
| Presence of toilet in house | 176 (47.1) |
Parasitology
The prevalence and distribution of Giardia infection according to age and gender are shown in Table 2. Of the 374 participants, 83 (22.2%) were found to be positive for Giardia infection. Overall, the prevalence of Giardia infection was higher among children aged <10 years compared to those aged ≥10 years (27.4% vs 16.0%), however, the difference was not statistically significant (χ2 = 2.171; P = 0.141). Similarly, there was no significant difference in the prevalence of Giardia between males and females (24.6% vs 20.0%; χ2 = 1.134; P = 0.287). Trichuris trichiura, Ascaris lumbricoides, hookworm infections and Entamoeba histolytica/dispar were detected in 71.6%, 40.2%, 10.1% and 9.2% of the samples, respectively. Regarding co-infections, about 60% of the children had Giardia with Ascaris and/or Trichuris. Fecal samples were collected and examined at 3 and 6 months and infected child were treated. There were 1 and 2 cases of Giardia infection at 3 and 6 months among those reported negative at baseline. On the other hand, there were 3 and 5 cases of reinfection at 3 and 6 months among those reported positive at baseline.
Table 2. Prevalence of Giardia infection among Aboriginal primary schoolchildren in Satak, Raub, Pahang according to age and gender (n = 374).
| Age/Gender | Giardia infection | ||
| No. examined | No. infected | Prevalence (%) | |
| Age group (years) | |||
| ≥10 | 129 | 23 | 17.8 |
| <10 | 245 | 60 | 24.5 |
| Gender | |||
| Male | 179 | 44 | 24.6 |
| Female | 195 | 39 | 20.0 |
| Total | 374 | 83 | 22.2 |
Nutritional status
Results of the weight, height and the prevalence of malnutrition (underweight, stunting and wasting) are presented in Table 3. The mean weight of these participants was 23.9 kg (95% CI = 23.3, 24.5) and their mean height was 126.6 cm (95% CI = 125.6, 127.7). The mean height of females was significantly higher than males (127.5 cm; 95% CI = 126.7, 129.0 vs 125.6 cm; 95% CI = 124.2, 126.6; t = 1.985; P = 0.048). However, there was no significant difference in the mean weight between female and male participants (24.3 kg; 95% CI = 23.3, 25.3 vs 23.5 kg; 95% CI = 22.7, 24.2; t = 1.300; P = 0.188). The overall prevalence of severe underweight, severe stunting and severe wasting were 28.3%, 23.8% and 21.0%, respectively.
Table 3. Prevalence of malnutrition at baseline among Aboriginal primary schoolchildren in Satak, Raub, Pahang (n = 374).
| Age/Gender | Weight (kg)* | Height (cm)* | Criteria | |||||
| Underweight | Stunting | Wasting | ||||||
| Normal/Mild n (%) | Severe n (%) | Normal/Mild n (%) | Severe n (%) | Normal/Mild n (%) | Severe n (%) | |||
| Age group (years) | ||||||||
| ≥10 | 29.4(28.4, 30.4) | 134.9(133.7, 136.0) | 85(65.9) | 44(34.1) | 62(48.1) | 67(51.9) | 41(82.0) | 9(18.0) |
| <10 | 21.0(20.5, 21.5) | 122.2(121.2, 123.1) | 183(75.0) | 62(25.3) | 27(11.0) | 218(89.0) | 192(78.4) | 53(21.6) |
| Gender | ||||||||
| Male | 23.5(22.7, 24.2) | 125.6(124.2, 126.6) | 114(65.1) | 62(35.2) | 115(63.3) | 61(34.7) | 119(85.6) | 20(14.4) |
| Female | 24.3(23.3, 25.3) | 127.5(126.7, 129.0) | 154(77.8) | 44(22.2) | 170(85.9) | 14(14.1) | 114(73.1) | 42(26.9) |
| Total | 23.9(23.3, 24.5) | 126.6(125.6, 127.7) | 268(71.8) | 106(28.3) | 285(76.2) | 89(23.8) | 233(79.0) | 62(21.0) |
n represents the number of subjects.
Values are mean (95% confidence interval).
Determinants of nutritional status (weight and height)
Differences in mean weight and height of participants according to independent variables were examined by independent samples t-test and the results are shown in Table 4. Besides the biological effect of age and gender, the mean weight was found to be significantly lower among children of mothers with <6 years of education (22.9 kg; 95% CI = 22.1, 23.7; t = 3.336; P = 0.001), those from families with low household monthly income (<RM500) (22.9 kg; 95% CI = 22.2, 23.7; t = 3.914; P = 0.001), those infected with Giardia (22.1 kg; 95% CI = 21.0, 23.2; t = 2.993; P = 0.003) and those who live in large families (>7 members) (23.5 kg; 95% CI = 22.8, 24.2; t = 2.267; P = 0.024) when compared to their peers. The results of the stepwise multiple linear regression model (adjusted for age) showed that the weight of children was found to be significantly influenced by the low household monthly income (β = −1.713; 95% CI = −2.56, −0.87; P<0.001), and Giardia infection (β = −1.658; 95% CI = −2.64, −0.68; P = 0.001) (Table 5). The overall R2 value of the regression model indicated that 58.9% of the variation in the weight of these children was explained by these variables.
Table 4. Mean weight and height at baseline by demographic, socioeconomic and parasitic infections factors of Aboriginal children who participated in the study.
| Factors | Weight (kg) | Height (cm) | ||
| Mean (95% CI) | Statistics | Mean (95% CI) | Statistics | |
| Age groups | ||||
| ≥10 years | 29.4(28.4, 30.4) | t = 16.162 | 134.9(133.7, 136.0) | t = 16.460 |
| <10 years | 21.0(20.5, 21.5) | P<0.001* | 122.2(121.3, 123.1) | P<0.001* |
| Gender | ||||
| Female | 24.3(23.3, 25.3) | t = 1.300 | 127.5(126.7, 129.0) | t = 1.985 |
| Male | 23.5(22.7, 24.2) | P = 0.188 | 125.6(124.2, 126.6) | P = 0.048* |
| Household monthly income | ||||
| ≥MYR500 | 25.5(24.4, 26.6) | t = 3.914 | 128.3(126.8, 130.2) | t = 2.755 |
| <MYR500 (low) | 22.9(22.2, 23.7) | P = 0.001* | 125.6(124.5, 126.6) | P = 0.006* |
| Fathers' education | ||||
| ≥6 years formal education | 24.4(23.4, 25.3) | t = 1.492 | 127.2(125.8, 128.6) | t = 1.349 |
| No formal education | 23.4(22.6, 24.2) | P = 0.136 | 125.9(124.7, 127.1) | P = 0.178 |
| Mothers' education | ||||
| ≥6 years formal education | 25.1(24.0, 26.0) | t = 3.336 | 128.6(127.1, 130.0) | t = 3.992 |
| No formal education | 22.9(22.1, 23.7) | P = 0.001* | 124.9(123.7, 126.0) | P<0.001* |
| Fathers' employment status | ||||
| Not working | 24.1(23.4, 24.8) | t = −0.977 | 127.0(125.9, 128.1) | t = −1.407 |
| Working | 23.4(22.2, 24.7) | P = 0.329 | 125.6(123.7, 127.5) | P = 0.160 |
| Mothers' employment status | ||||
| Not working | 23.8(23.1, 24.6) | t = −0.304 | 126.5(125.4, 127.5) | t = 0.376 |
| Working | 24.2(22.8, 25.4) | P = 0.761 | 127.0 (124.9, 125.0) | P = 0.707 |
| Family size | ||||
| ≤7 members | 25.2(24.2, 26.6) | t = 2.267 | 128.7(127.2, 130.6) | t = 2.501 |
| >7 members (large) | 23.5(22.8, 24.1) | P = 0.024* | 125.9(124.8, 127.0) | P = 0.013* |
| Giardia infection | ||||
| Non infected | 24.4(23.7, 25.2) | t = 2.993 | 127.0(125.9, 128.1) | t = 1.896 |
| Infected | 22.1(21.0, 23.2) | P = 0.003* | 124.9(122.9, 126.9) | P = 0.059 |
| Ascaris infection | ||||
| Negative to light infection | 24.2(22.8, 25.9) | t = −0.854 | 126.9(125.2, 128.8) | t = −1.153 |
| Moderate-to-heavy infection | 25.5(24.2, 27.3) | P = 0.394 | 129.3(128.1, 130.7) | P = 0.250 |
| Trichuris infection | ||||
| Negative to light infection | 24.4(23.3, 26.3) | t = −0.096 | 126.9(124.5, 129.3) | t = −0.179 |
| Moderate-to-heavy infection | 24.5(23.5, 26.5) | P = 0.924 | 127.8(125.1, 129.7) | P = 0.858 |
MYR = Malaysian Ringgit; (US$1 = MYR3.05) [25th February 2013].
Significant difference (P<0.05; Independent samples t-test).
Table 5. Results of multiple linear regression of potential predictors for weight and height at baseline among Aboriginal schoolchildren who participated in the study *.
| Variables | Weight | Height | ||||||
| β coefficient | Standard error | 95% CI | P | β coefficient | Standard error | 95% CI | P | |
| Constant | 3.241 | 1.148 | - | - | 81.743 | 1.454 | - | - |
| Household monthly income (<MYR500) | −1.713 | 0.413 | −2.56, −0.87 | <0.001 | - | - | - | - |
| Giardia infection (positive) | −1.658 | 0.499 | −2.64, −0.68 | 0.001 | - | - | - | - |
| Sex (male) | - | - | - | - | −2.677 | 0.544 | −3.75, −1.61 | <0.001 |
| Mothers' educational level (<6 years) | - | - | - | - | −1.473 | 0.549 | −2.55, −0.39 | 0.008 |
Variables included in the multiple linear regression models were sex (male and female), household monthly income (<RM500 and ≥RM500), family size (≤7 members and >7 members), Giardia infection (infected and non infected) and mothers' educational level (≥6 years and <6 years).
On the other hand, the mean height was found to be significantly lower among those whose mothers had <6 years of education (124.8 cm; 95% CI = 123.7, 126.0; t = 3.992; P<0.001), those from families with a household monthly income of <RM500 (125.6 cm; 95% CI = 124.5, 126.6; t = 2.755; P = 0.006), and those who live in large families (125.9 cm; 95% CI = 124.8, 127.0; t = 2.501; P = 0.013) compared to their counterparts (Table 4). The output of the stepwise multiple linear regression model showed that the height of children was significantly predicted by the sex of children (male) (β = −2.677; 95% CI = −3.75, −1.61; P<0.001), and the low educational level of mothers (β = −1.473; 95% CI = −2.55, −0.39; P = 0.008) (Table 5). The overall R2 value of the regression model indicated that 68.3% of the variation in the height of these children was explained by these variables.
Effects of Giardia infection on nutritional status
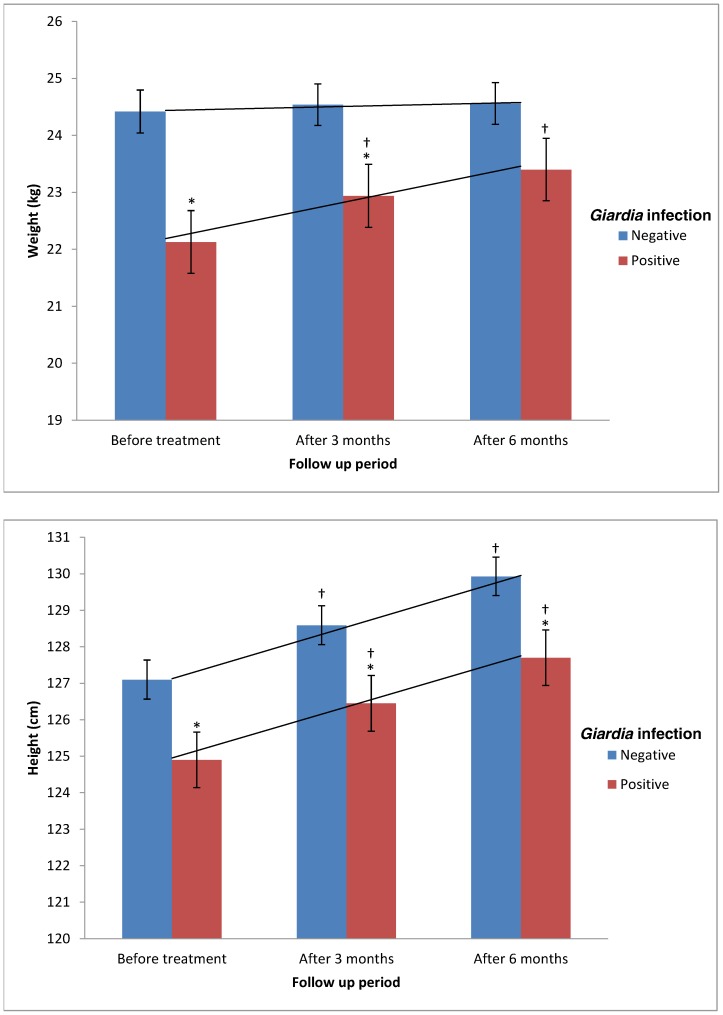
The mean values of weight and height were compared according to Giardia infection status over time and the results are presented in Figure 3. The mean weight of Giardia-infected participants was significantly lower than the mean weight of non infected participants (22.1 kg, 95% CI = 21.0, 23.2 vs 24.4 kg, 95% CI = 23.7, 25.2; t = 2.993; P = 0.003). Although the mean height of Giardia-infected participants was lower than the mean height of those non infected (124.9 cm, 95% CI = 122.9, 126.9 vs 127.1 cm, 95% CI = 125.9, 128.1), this difference was statistically not significant (t = 1.896; P = 0.059).
Figure 3. Mean weight and height of children according to Giardia infection over time.
All values are mean (SEM). * Significant difference (lower) compared to non infected group (Independent t-test, P<0.05). † Significant difference (higher) compared to previous assessment (Paired t-test, P<0.05). Trend lines represent the linear weight and height increments (repeated measures ANOVA).
Three months after treatment for Giardia infection, a significant improvement in the mean weight of treated children (from 22.1 kg to 22.9 kg; paired t = 15.769; P<0.001) was observed. This significant improvement continued at 6 months assessment with mean increment of 0.5 kg (paired t = 5.861; P<0.001). Results of repeated-measures ANOVA confirmed the significant effect for time among Giardia-infected children, Wilks' Lambda = 0.161, F = 171.045, P<0.001 and multivariate eta squared = 0.676 (0.01 = small effect size, 0.06 = moderate effect, 0.14 = large effect). On the other hand, the improvement in weight among non infected children was found to be not significant at 3 months (from 24.4 kg to 24.5 kg; paired t = 1.788; P<0.075) and 6 months assessments (paired t = 1.590; P = 0.113). With regards to height, there were significant increments in the mean height of both groups (infected and non infected participants) after 3 and 6 months of treatment (P<0.001).
On the other hand, by adjusting the mean weight and height increments for age and sex (Table 6), the weight gain was found to be significantly higher among Giardia-infected children when compared to non infected children (P<0.05), regardless of age and sex. There was no significant difference in the mean height gain between both groups (Giardia-infected and non infected children) among those aged <10 years and male children. However, in children aged ≥10 years, height gain was significantly higher among Giardia-infected children when compared to those not infected. Similar results were reported among female children.
Table 6. Adjusted mean weight and height increments of children according to Giardia infection.
| Group | Giardia infection | Weight | Height | ||||
| 0–3 months | 3–6 months | 0–6 months | 0–3 months | 3–6 months | 0–6 months | ||
| Age group (years) | |||||||
| ≥10 | Positive | 0.9(0.6, 1.1)* | 0.5(0.3, 0.7)* | 1.3(1.0, 1.6)* | 1.7(1.5, 1.9) | 1.3(1.2,1.5)* | 3.0(2.9, 3.3)* |
| Negative | 0.2(0.1, 0.3) | 0.1(0.05, 0.2) | 0.3(0.1, 0.5) | 1.5(1.3, 1.7) | 1.1(0.9, 1.1) | 2.6(2.4, 2.8) | |
| <10 | Positive | 0.8(0.6, 0.9)* | 0.5(0.3, 0.7)* | 1.3(1.0, 1.5)* | 1.5(1.3, 1.7) | 1.4(1.3, 1.7) | 2.9(2.5, 3.3) |
| Negative | 0.2(0.1, 0.3) | 0.1(0.07, 0.2) | 0.3(0.1, 0.4) | 1.5(1.3, 1.7) | 1.4(1.2, 1.6) | 2.9(2.6, 3.3) | |
| Sex | |||||||
| Male | Positive | 0.9(0.7, 1.1)* | 0.4(0.2, 0.6)* | 1.3(1.0, 1.5)* | 1.5(1.3, 1.7) | 1.4(1.2, 1.7) | 2.9(2.6, 3.2) |
| Negative | 0.1(0.05, 0.2) | 0.1(0.05, 0.2) | 0.2(0.1, 0.4) | 1.5(1.3, 1.7) | 1.5(1.3, 1.8) | 3.0(2.7, 3.4) | |
| Female | Positive | 0.8(0.6, 0.9)* | 0.5(0.3, 0.8)* | 1.3(1.1, 1.6)* | 1.6(1.3, 1.8) | 1.3(1.2, 1.5)* | 2.9(2.7, 3.3)* |
| Negative | 0.1(0.05, 0.2) | 0.1(0.05, 0.2) | 0.2(0.1, 0.3) | 1.4(1.2, 1.6) | 1.1(0.9, 1.2) | 2.5(2.3, 2.7) | |
| Overall | Positive | 0.8(0.7, 0.9)* | 0.5(0.3, 0.6)* | 1.3(1.1, 1.4)* | 1.6(1.4, 1.7) | 1.3(1.1, 1.5) | 2.9(2.7, 3.3) |
| Negative | 0.1(0.05, 0.2) | 0.1(0.05, 0.2) | 0.2(0.1, 0.3) | 1.5(1.3, 1.6) | 1.3(1.1, 1.5) | 2.8(2.5, 3.0) | |
All values are mean (95% confidence interval).
Significant difference compared to Giardia-negative children (P<0.05; Independent samples t-test).
Tables 7 and 8 show the mean changes of weight and height-for-age Z-scores adjusted for age and sex. Overall, significant improvements in the WAZ were reported in children treated for Giardia infection compared to those non infected, with significantly higher changes among those aged less than 10 years compared to their counterparts (P<0.05). Similar significant improvements in the WHZ were also reported.
Table 7. Age-adjusted mean changes in weight and height-for-age Z-scores among children according to Giardia infection.
| Variable | Age group (years) | Overall | ||||
| ≥10 years | <10 years | |||||
| Giardia +ve | Giardia −ve | Giardia +ve | Giardia −ve | Giardia +ve | Giardia −ve | |
| Δ WAZ (0–3 mo) | 0.20(0.07,0.35) | 0.14(0.06,0.22) | 0.26(0.14,0.38)* | 0.09(0.03,0.13) | 0.23(0.14,0.33)* | 0.11(0.06,0.15) |
| Δ HAZ (0–3 mo) | 0.11(0.02,0.24) | 0.08(0.01,0.18) | 0.13(0.02,0.32) | 0.05(0.01,0.13) | 0.12(0.04,0.27) | 0.06(0.01,0.16) |
| Δ WHZ (0–3 mo) | 0.34(0.15,0.48)* | 0.22(0.13,0.36) | 0.26(0.12,0.33) | 0.14(0.01,0.24) | 0.30(0.22,0.50)* | 0.17(0.13,0.25) |
| Δ WAZ (3–6 mo) | 0.21(0.14,0.30) | 0.18(0.11,0.24) | 0.25(0.20,0.33)* | 0.15(0.11,0.19) | 0.23(0.19,0.30)* | 0.16(0.12,0.19) |
| Δ HAZ (3–6 mo) | 0.09(−0.01,0.25) | 0.16(0.05,0.26) | 0.15(0.05,0.26) | 0.15(0.10,0.20) | 0.12(0.04,0.21) | 0.15(0.12,0.21) |
| Δ WHZ (3–6 mo) | 0.21(0.08,0.44) | 0.17(−0.06,0.26) | 0.21(0.14,0.32)* | 0.10(0.02,0.13) | 0.21(0.15,0.32) | 0.13(0.07,0.19) |
| Δ WAZ (0–6 mo) | 0.40(0.31,0.57) | 0.33(0.24,0.43) | 0.49(0.34,0.59)* | 0.24(0.19,0.32) | 0.46(0.35,0.54)* | 0.27(0.22,0.33) |
| Δ HAZ (0–6 mo) | 0.20(0.10,0.38) | 0.23(0.11,0.29) | 0.30(0.16,0.54) | 0.19(0.10,0.26) | 0.26(0.12,0.44) | 0.21(0.13,0.26) |
| Δ WHZ (0–6 mo) | 0.50(0.41,0.71)* | 0.28(0.16,0.39) | 0.45(0.32,0.63)* | 0.22(0.14,0.29) | 0.48(0.36,0.62)* | 0.27(0.16,0.35) |
All values are mean (95% confidence interval); Δ: change; mo: months; WAZ: weight-for-age Z-scores; HAZ: height-for-age Z-scores; WHZ: weight-for- height Z-scores.
Significant difference compared to Giardia-negative children (P<0.05; Independent samples t-test).
Table 8. Sex-adjusted mean changes in weight and height-for-age Z-scores among children according to Giardia infection.
| Variable | Sex | |||
| Male | Female | |||
| Giardia +ve | Giardia −ve | Giardia +ve | Giardia −ve | |
| Δ WAZ (0–3 mo) | 0.23(0.15,0.35)* | 0.09(0.03,0.14) | 0.24(0.12,0.40) | 0.12(0.05,0.19) |
| Δ HAZ (0–3 mo) | 0.05(0.01,0.24) | 0.06(0.01,0.12) | 0.09(0.01,0.27) | 0.05(0.01,0.12) |
| Δ WHZ (0–3 mo) | 0.38(0.13,0.63) | 0.20(0.11,0.29) | 0.29(0.10,0.47) | 0.18(0.09,0.27) |
| Δ WAZ (3–6 mo) | 0.20(0.12,0.28) | 0.16(0.11,0.22) | 0.29(0.20,0.37)* | 0.15(0.10,0.19) |
| Δ HAZ (3–6 mo) | 0.18(0.01,0.22) | 0.17(0.10,0.23) | 0.16(0.02,0.30) | 0.15(0.09,0.22) |
| Δ WHZ (3–6 mo) | 0.22(0.09,0.39) | 0.17(0.10,0.24) | 0.25(0.14,0.35) | 0.13(0.06,0.19) |
| Δ WAZ (0–6 mo) | 0.45(0.34,0.57)* | 0.26(0.18,0.33) | 0.51 (0.38,0.59)* | 0.27(0.22,0.36) |
| Δ HAZ (0–6 mo) | 0.24(0.05,0.43) | 0.22(0.10,0.29) | 0.31(0.05,0.61) | 0.19(0.10,0.27) |
| Δ WHZ (0–6 mo) | 0.48(0.16,0.66)* | 0.16(0.08,0.26) | 0.36(0.19,0.55) | 0.22(0.10, 0.33) |
All values are mean (95% confidence interval); Δ: change; mo: months; WAZ: weight-for-age Z-scores; HAZ: height-for-age Z-scores; WHZ: weight-for- height Z-scores.
Significant difference compared to Giardia-negative children (P<0.05; Independent samples t-test).
Discussion
Parasitic diseases and malnutrition have a strikingly similar geographical distribution with the same people experiencing both insults together for much of their lives [35]. Our findings showed that the prevalence of Giardia infection among Aboriginal primary schoolchildren living in rural Malaysia was high with 22.0% of the participants infected. This prevalence is in agreement with other reports in Malaysia [5], [21], [36], [37]. The present study found that the overall prevalence of severe underweight, stunting and wasting was 26.9%, 24.8% and 21.0%, respectively. These high prevalence were in agreement with previous studies among Aboriginal children in different states [22], [38], [39].
The findings of the present study showed that age, low household monthly income and Giardia infection were the significant determinants influencing the weight of Aboriginal children. Similarly, the height of children was found to be significantly influenced by the age of children, sex (male) and the low educational level of mothers. Aside from age, which would be expected to affect stature, mothers' educational attainment and sex influenced height; paradoxically, females were taller than males in both age groups. From the results of linear regression models, it can be predicted that holding other variables unchanged, the weight of a child infected with Giardia is lower on average by 1.7 kg than non infected child. Previous studies have identified Giardia as a significant predictor of weight and height [17], [21], [40]. Moreover, Giardia infection has been identified as a significant risk factor of vitamin A deficiency among Aboriginal children in rural Malaysia [9].
It is well known that poverty is the root cause of malnutrition and many other health problems including parasitic infections in developing communities. Poverty limits the purchasing power of families and therefore, either the quantity or quality of food or may be both are compromised in these families. Education is also an important factor that contributes to the selection of the good quality and nutritious food. The low household monthly income and the low educational level of mothers have been identified as significant risk factors of malnutrition by previous studies from Malaysia [22], [23], [41]. Moreover, previous studies from Lao PDR, China and Bangladesh have reported similar findings [42], [43], [44]. Similarly, we found that children belonging to big families are more prone to be malnourished. This is common in rural and poor socioeconomic communities due to the inadequate purchasing power and also the distribution of food among family members.
Our findings showed that the mean weight and height of children were significantly lower among Giardia-infected children. This is consistent with previous findings of few reports in Malaysia and abroad. A previous study among Aboriginal children aged below 15 years reported that Giardia infection was a significantly associated with severe wasting (weight-for-height Z scores), but not with severe stunting (height-for-age) or severe underweight (weight-for-age) [21].
In comparison with studies from different countries, previous reports from Zimbabwe, Iran and Colombia found a strong association between Giardia infection and under-nutrition, wasting and stunting among children [45]–[47]. Previous studies among Brazilian children showed that Giardia-infected children had a double risk for stunted growth as compared to other children [48]. In a large, population-based survey of schoolchildren in Tehran, Nematian et al. [49] showed that among nine parasite species detected among the participants only Giardia and Enterobius infections were found to be significantly associated with the weight and height. In contrast, many studies have examined the association between Giardia infection and malnutrition and found no significant association [18]–[20]. This could be attributed to the low prevalence of G. duodenalis reported by these studies as compared to the present study.
Parasitic infections are thought to contribute to child malnutrition through subtle reduction in digestion and absorption, chronic inflammation and loss of nutrients [50]. Giardia is known to cause acute diarrhoea, fat, vitamins and D-xylose malabsorption, and lactose intolerance especially among children [8]. Moreover, it is well documented that Giardia trophozoites cause derangement of the normal villous architecture with shortening of villi and inflammatory foci in the crypts and lamina propria, resulting in malabsorption [50], [51]. All these mechanisms contribute to the development of malnutrition among infected individuals.
The findings of the present study showed a significantly higher post treatment weight gain among children who were reported to be infected with Giardia at baseline assessment as compared to those who were not infected. With regards to height, our findings showed significant post treatment height gain among all children throughout the follow up assessments, regardless of the Giardia infection status. However, the height gain among Giardia-infected females and those aged ≥10 years was significantly higher when compared to their non infected counterparts. This extra height gain among female and elder children could be due to physiological variation. Therefore, these findings confirmed the significant association of Giardia with weight of children but not with the height. Post treatment changes in the weight and height-for-age Z scores were found to be significantly higher among Giardia-infected children compared to those non infected, particularly among children aged below 10 years. This could be explained by their significantly higher prevalence of Giardia infection at baseline compared to their counterparts. Although more than 70% of the children studied were found to be infected with at least one species of soil-transmitted helminths (Trichuris, Ascaris and hookworm), a previous study showed no significant improvement in the weight and height of infected children after three months of complete deworming [52].
Evaluating the effects of Giardia on the nutritional status of children after treatment has been carried out by few studies and yielded different findings. In agreement with the findings of the present study, previous clinical trials reported that Giardia-infected children who have been successfully treated with metronidazole showed significantly higher improvement in weight and height as compared with those untreated [53], [54]. By contrast, Rousham and Mascie-Taylor [55] showed that after successful elimination of infection, Giardia was still found to be associated with poorer weight gain in children as compared to non treated children. In a randomized double placebo-controlled trial, Goto et al. [16] examined the relationship between Giardia and growth indicators (weight and height) of young children for 36 weeks and found no significant differences in the anthropometric variables between the intervention groups, although there were associations between improvement in small intestinal mucosal function and better weight-for-age and weight-for-height Z-scores. This study considered two approaches to define Giardia infection; the Giardia antibody titre contrasted and stool examination. However, the prevalence of Giardia was between 1% and 3% throughout the study period and this very low prevalence may mask any possible difference in growth improvement between the groups [16].
Although there is a broad agreement on the negative impacts of Giardia infection, a recent study showed that Giardia infection is associated with protection against diarrhea and fever without localizing signs [56]. A recent meta-analysis supported this finding and suggested that the presence of Giardia infection reduce the likelihood of having acute diarrhea but not the persistent diarrhea among children from developing countries [57]. Although the mechanism involved in this protection is still unclear, it was suggested that the secretion of innate antimicrobial products having anti-Giardia activity (eg, defensin) by the intestinal epithelium and the secretion of mucins and glycoproteins of the intestinal mucus layer can reduce attachment of many other enteropathogens to the mucosal surface [58]. Moreover, a significant reduction in the severity of rotavirus gastroenteritis was reported among infants in the presence of Giardia coinfection [59].
The present findings should be treated with some caution as a double blind randomization and comparison of treatment group with a placebo group were not possible. Several threats to internal validity of the before and after study design might be raised such as the instrumentation/reporting threat, regression-to-the-mean threat, maturation threat and dropout threats. To minimize the potential threats to the present study, non infected children served as control group and intention-to-treat approach for data analysis was used. Moreover, this study had to rely on a single fecal sample at each assessment stage (baseline, 3 months and 6 months) because of limitation of resources, the cultural belief of the Aboriginal people and to avoid causing interruption in the schoolchildren's schooling. Thus, the prevalence rate is expected to be higher if three fecal samples were collected. However, we have applied standard procedures during fecal collection and examination to overcome this limitation. The detection methods employed- trichrome staining and formalin ether sedimentation- are sufficiently sensitive to detect low numbers of Giardia parasite in fecal samples [26]. Aboriginal communities in rural Malaysia share similar socioeconomic, environmental and health profiles. Thus, we may speculate that our findings can be generalized to other rural Aboriginal children in other states. However, these results may not be generalizable to the entire Malaysian rural population as other ethnic groups (Malay, Chinese and Indians) have a better socioeconomic and environmental situation. Further studies are required to confirm these speculations.
Conclusion
The present study reveals a high prevalence of Giardia infection and malnutrition among Aboriginal schoolchildren in rural Malaysia. In addition to the age, household monthly income and Giardia infection appears to be a strong predictor of weight but not height of the children, and a significant association of Giardia with the weight of children was reported. Thus, effective control measures to reduce the prevalence of Giardia infection should be considered in public health strategies to improve the nutritional status and quality of life of children in Aboriginal and other rural communities.
Supporting Information
STROBE checklist.
(DOC)
Acknowledgments
We gratefully acknowledge the Department of Orang Asli Development (JAKOA), Ministry of Rural and Regional Development, Kuala Lumpur, Malaysia for their cooperation and support during this study. We acknowledge the support and cooperation of the Headmaster and staff of the National School of Betau and National School of Satak in making this survey successful. We also wish to express our appreciation to the parents and their children for their voluntary participation in this study.
Funding Statement
The work presented in this paper was funded by the University of Malaya High Impact Research Grant UM-MOHE UM.C/625/1/HIR/MOHE/MED/18 from the Ministry of Higher Education, Malaysia and the University of Malaya Research Grant (RG439/12HTM). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Savioli L, Smith H, Thompson A (2006) Giardia and Cryptosporidium join the ‘Neglected Diseases Initiative’. Trends Parasitol 22: 203–208. [DOI] [PubMed] [Google Scholar]
- 2. Faustini A, Marinacci C, Fabrizi E, Marangi M, Recchia O, et al. (2006) The impact of Catholic Jubilee in 2000 on infectious diseases. A case–control study of giardiasis, Rome, Italy 2000–2001. Epidemiol Infect 134: 649–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Julio C, Vilares A, Oleastro M, Ferreira I, Gomes S, et al. (2012) Prevalence and risk factors for Giardia duodenalis infection among children: a case study in Portugal. Parasit Vectors 5: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baldursson S, Karanis P (2011) Waterborne transmission of protozoan parasites: review of worldwide outbreaks - an update 2004–2010. Water Res 45: 6603–6614. [DOI] [PubMed] [Google Scholar]
- 5. Anuar TS, Al-Mekhlafi HM, Ghani MK, Osman E, Yasin AM, et al. (2012) Giardiasis among different tribes of Orang Asli in Malaysia: Highlighting the presence of other family members infected with Giardia intestinalis as a main risk factor. Int J Parasitol 42: 871–880. [DOI] [PubMed] [Google Scholar]
- 6. Berkman DS, Lescano AG, Gilman RH, Lopez SL, Black MM (2002) Effects of stunting, diarrhoeal disease and parasitic infection during infancy on cognition in late childhood: a follow-up study. Lancet 359: 564–571. [DOI] [PubMed] [Google Scholar]
- 7.Farthing MJ, Cevallos AM, Kelly P (1996) Intestinal protozoa. 20th ed. In: Manson's Tropical Diseases. Cook, G. C. London: W.B. Saunders.
- 8. Gendrel D, Treluyer JM, Richard-Lenoble D (2003) Parasitic diarrhea in normal and malnourished children. Fundam Clin Pharmacol 17: 189–198. [DOI] [PubMed] [Google Scholar]
- 9. Al-Mekhlafi HM, Surin J, Sallam AA, Abdullah AW, Mahdy MA (2010) Giardiasis and poor vitamin A status among Aboriginal school children in rural Malaysia. Am J Trop Med Hyg 83: 523–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Khor GL (2005) Micronutrient status and intervention programs in Malaysia. Food Nutr Bull 26: S281–S285. [DOI] [PubMed] [Google Scholar]
- 11.UNICEF/WHO/World Bank (2012) UNICEF-WHO-World Bank joint child malnutrition estimates. New York: United Nations Children's Fund, Geneva: World Health Organization, Washington DC: The World Bank.
- 12. Stephension LS, Latham MC, Ottesen EA (2002) Malnutrition and parasitic infections. Parasitol 121: 532–538. [DOI] [PubMed] [Google Scholar]
- 13. Koukounari A, Estambale B, Njagi J, Cundill B, Ajanga A, et al. (2008) Relationships between anaemia and parasitic infections in Kenyan schoolchildren: a Bayesian hierarchical modelling approach. Int J Parasitol 38: 1663–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Black RE, Brown KH, Becker S (1984) Effects of diarrhea associated with specific enteropathogens on the growth of children in rural Bangladesh. Pediatrics 73: 799–805. [PubMed] [Google Scholar]
- 15. Prado M, Cairncross S, Strina A, Barreto ML, Oliveira-Assis AM, Rego S (2005) Asymptomatic giardiasis and growth in young children; a longitudinal study in Salvador, Brazil. Parasitol 131: 51–56. [DOI] [PubMed] [Google Scholar]
- 16. Goto R, Mascie-Taylor CG, Lunn PG (2009) Impact of anti-Giardia and anthelminthic treatment on infant growth and intestinal permeability in rural Bangladesh: a randomised double-blind controlled study. Trans R Soc Trop Med Hyg 103: 520–529. [DOI] [PubMed] [Google Scholar]
- 17. Abou-Shady O, El Raziky MS, Zaki MM, Mohamed RK (2011) Impact of Giardia lamblia on growth serum levels of zinc, copper, and iron in Egyptian children. Biol Trace Elem Res 140: 1–6. [DOI] [PubMed] [Google Scholar]
- 18. Lunn PG, Erinoso HO, Northrop-Clewes CA, Boyce SA (1999) Giardia intestinalis is unlikely to be a major cause of the poor growth of rural Gambian infants. J Nutr 129: 872–877. [DOI] [PubMed] [Google Scholar]
- 19. Sackey ME, Weigel MN, Armijos RX (2003) Predictors and nutritional consequences of intestinal parasitic infections in rural Ecuadorian children. J Trop Pediatr 49: 17–23. [DOI] [PubMed] [Google Scholar]
- 20. Hollm-Delgado MG, Gilman RH, Bern C, Cabrera L, Sterling CR, et al. (2008) Lack of an adverse effect of Giardia intestinalis infection on the health of Peruvian children. Am J Epidemiol 168: 647–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Al-Mekhlafi HM, Azlin M, Nor Aini U, Shaik A, Sa'iah A, et al. (2005) Giardiasis is a predictor of childhood malnutrition in Orang Asli children in Malaysia. Trans R Soc Trop Med Hyg 99: 686–691. [DOI] [PubMed] [Google Scholar]
- 22. Norhayati M, Noorhayati MI, Mohammod CG, Oothuman P, Azizi O, et al. (1997) Malnutrition and its risk factors among children 1–7 years old in rural Malaysian communities. Asia Pac J Clin Nutr 6: 260–264. [PubMed] [Google Scholar]
- 23. Al-Mekhlafi HM, Azlin M, Nor Aini, U, Shaik A, Sa'iah A, et al. (2005) Malnutrition and soil-transmitted helminthiasis among Orang Asli children in Selangor, Malaysia. Asia Pac J Clin Nutr 14: 188–194. [PubMed] [Google Scholar]
- 24.WHO (1995) Physical status: the use and interpretation of anthropometry. Technical report series: 854. Geneva: World Health Organization. [PubMed]
- 25.Fleck SL, Moody AH (1993) Diagnostic techniques in medical parasitology. 11th ed. Cambridge: ELBS with Tropical Health technology/Butterworth-Heinemann.
- 26.Cheesbrough M (1992) Medical laboratory manual for tropical countries. 2nd edition. Cambridge: ELBS with Tropical Health technology/Butterworth-Heinemann.
- 27. Martin LK, Beaver PC (1968) Evaluation of Kato thick smears technique for quantitative diagnosis of helminth infections. Am J Trop Med Hyg 17: 382–391. [DOI] [PubMed] [Google Scholar]
- 28. Jozefzoon LM, Oostburg BF (1994) Detection of hookworm and hookworm-like larvae in human fecocultures in Suriname. Am J Trop Med Hyg 51: 501–505. [PubMed] [Google Scholar]
- 29.Montresor A, Crompton DWT, Gyorkos TW, Savioli L (2002) Helminth control in school-age children. A guide for managers of control programmes. Geneva: World Health Organization.
- 30. Penggabean M, Oothuman P, Fatmah M (1998) Efficacy of albendazole in the treatment of Trichuris trichuria and Giardia intestinalis infection in rural Malay communities. Med J Malaysia 53: 408–412. [PubMed] [Google Scholar]
- 31. Reynoldson JA, Behnke JM, Gracey M, Horton RJ, Spargo R, et al. (1998) Efficacy of albendazole against Giardia and hookworm in a remote Aboriginal community in the north of Western Australia. Acta Trop 71 1: 27–44. [DOI] [PubMed] [Google Scholar]
- 32. Hall A, Nahar Q (1993) Albendazole as a treatment for infections with Giardia duodenalis in children in Bangladesh. Trans R Soc Trop Med Hyg 87 1: 84–86. [DOI] [PubMed] [Google Scholar]
- 33. Solaymani-Mohammadi S, Genkinger JM, Loffredo CA, Singer SM (2010) A meta-analysis of the effectiveness of albendazole compared with metronidazole as treatments for infections with Giardia duodenalis . PLoS Negl Trop Dis 4 5: e682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bendel RB, Afifi AA (1977) Comparison of stopping rules in forward regression. J Am Stat Assoc 72: 46–53. [Google Scholar]
- 35. Crompton DW (1986) Nutritional aspect of infection. Trans R Soc Trop Med Hyg 80: 697–705. [DOI] [PubMed] [Google Scholar]
- 36. Azian NMY, San YM, Gan CC, Yusri MY, Nurulsyamzawaty Y, et al. (2007) Prevalence of intestinal protozoa in an aborigine community in Pahang, Malaysia. Trop Biomed 24: 55–62. [PubMed] [Google Scholar]
- 37. Mohammed Mahdy AK, Lim YAL, Johari Surin, Wan KL, Al-Mekhlafi MS (2008) Risk factors for endemic giardiasis-highlighting the association with contaminated water and food. Trans Royal Soc Trop Med Hygiene 102: 465–470. [DOI] [PubMed] [Google Scholar]
- 38. Zulkifli A, Anuar AK, Atiya AS, Yano A (2000) The prevalence of malnutrition and geo-helminth infections among primary schoolchildren in Rural Kelantan. Southeast Asian J Trop Med Public Health 31: 339–345. [PubMed] [Google Scholar]
- 39. Osman A, Zaleha MI (1995) Nutritional status of women and children in Malaysian rural populations. Asia Pac J Clin Nutr 4: 319–324. [PubMed] [Google Scholar]
- 40. Ignatius R, Gahutu JB, Klotz C, Steininger C, Shyirambere C, et al. (2012) High prevalence of Giardia duodenalis Assemblage B infection and association with underweight in Rwandan children. PLoS Negl Trop Dis 6 6: e1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zamaliah M, Mohd N, Khor G, Siong T (1998) Socio-economic determinants of nutritional status of children in rural Peninsular Malaysia. Asia Pac J Clin Nutr 7: 307–310. [PubMed] [Google Scholar]
- 42. Phimmasone K, Douangpoutha I, Fauveau V, Pholsena P (1997) Nutritional status of children in Lao PDR. J Trop Pediatr 43: 5–11. [DOI] [PubMed] [Google Scholar]
- 43. Li Y, Guo G, Shi A, Li Y, Anme T, et al. (1999) Prevalence and correlates of malnutrition among children in rural minority areas of China. Pediatr Intern 41: 549–556. [DOI] [PubMed] [Google Scholar]
- 44. Abbas BMA, Susan ZMK, D'Souza S (1986) Socioeconomic differentials in child nutrition and morbidity in rural areas of Bangladesh. J Trop Pediatr 32: 17–23. [DOI] [PubMed] [Google Scholar]
- 45. Loewenson R, Mason PR, Patterson BA (1986) Giardiasis and the nutritional status of Zimbabwean schoolchildren. Ann Trop Paediatr 6: 73–78. [DOI] [PubMed] [Google Scholar]
- 46. Sadjjadi SM, Tanideh N (2005) Nutritional status of preschool children infected with Giardia intestinalis . Iranian J Publ Health 34: 51–57. [Google Scholar]
- 47. Botero-Garces JH, Garcia-Montoya GM, Grisales-Patino D, Aguirre-Acevedo DC, Alvarez-Uribe MC (2009) Giardia intestinalis and nutritional status in children participating in the complementary nutrition program, Antioquia, Colombia, May to October 2006. Rev Inst Med Trop Sao Paulo 51: 155–162. [DOI] [PubMed] [Google Scholar]
- 48. Muniz-Junqueira MI, Queiroz EO (2008) Relationship between protein-energy malnutrition, vitamin A and parasitoses in children living in Brasilia, Brazil. Rev Soc Bras Med Trop 35: 133–141. [DOI] [PubMed] [Google Scholar]
- 49. Nematian J, Gholamrezanezhad A, Nematian E (2008) Giardiasis and other intestinal parasitic infections in relation to anthropometric indicators of malnutrition: a large, population-based survey of schoolchildren in Tehran. Ann Trop Med Parasitol 102: 209–214. [DOI] [PubMed] [Google Scholar]
- 50. Northrop-Clewes CA, Rousham EK, Muscie-Taylor CG, Lunn PG (2001) Anthelminthic treatment of rural Bangladeshi children: effect on host physiology, growth, and biochemical status. Am J Clin Nutr 73: 53–60. [DOI] [PubMed] [Google Scholar]
- 51.Neava FA, Brown HW (1995) Basic clinical parasitology. 6th ed. London: Prentice-Hall Incooporation.
- 52. Ahmed A, Al-Mekhlafi HM, Al-Adhroey AH, Ithoi I, Abdulsalam AM, et al. (2012) The nutritional impacts of soil-transmitted helminths infections among Orang Asli schoolchildren in rural Malaysia. Parasit Vectors 5: 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gupta S, Srivastava G (1978) Drug therapy for Giardia infestation. Indian Pediatr 15: 687–689. [PubMed] [Google Scholar]
- 54. Gupta MC, Urrutia JJ (1982) Effect of periodic antiascaris and antigiardia treatment on nutritional status of preschool children. Am J Clin Nutr 36: 79–86. [DOI] [PubMed] [Google Scholar]
- 55. Rousham EK, Mascie-Taylor CGN (1994) An 18-month study of the effect of periodic anthelminthic treatment on the growth and nutritional status of pre-school children in Bangladesh. Ann Hum Biol 21: 315–324. [DOI] [PubMed] [Google Scholar]
- 56. Veenemans J, Mank T, Ottenhof M, Baidjoe A, Mbugi EV, et al. (2011) Protection against diarrhea associated with Giardia intestinalis is lost with multi-nutrient supplementation: A study in Tanzanian children. PLoS Negl Trop Dis 5 6: e1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Muhsen K, Levine MM (2012) A systematic review and meta-analysis of the association between Giardia lamblia and endemic pediatric diarrhea in developing countries. Clin Infect Dis Suppl 4: S271–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Muller N, von Allmen N (2005) Recent insights into the mucosal reactions associated with Giardia lamblia infections. Int J Parasitol 35: 1339–1347. [DOI] [PubMed] [Google Scholar]
- 59. Bilenko N, Levy A, Dagan R, Deckelbaum RJ, El-On Y, et al. (2004) Does co-infection with Giardia lamblia modulate the clinical characteristics of enteric infections in young children? Eur J Epidemiol 19: 877–883. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
STROBE checklist.
(DOC)