Abstract
In kinetoplastid protists, several metabolic pathways, including glycolysis and purine salvage, are located in glycosomes, which are microbodies that are evolutionarily related to peroxisomes. With the exception of some potential transporters for fatty acids, and one member of the mitochondrial carrier protein family, proteins that transport metabolites across the glycosomal membrane have yet to be identified. We show here that the phosphatidylcholine species composition of Trypanosoma brucei glycosomal membranes resembles that of other cellular membranes, which means that glycosomal membranes are expected to be impermeable to small hydrophilic molecules unless transport is facilitated by specialized membrane proteins. Further, we identified 464 proteins in a glycosomal membrane preparation from Leishmania tarentolae. The proteins included approximately 40 glycosomal matrix proteins, and homologues of peroxisomal membrane proteins - PEX11, GIM5A and GIM5B; PXMP4, PEX2 and PEX16 - as well as the transporters GAT1 and GAT3. There were 27 other proteins that could not be unambiguously assigned to other compartments, and that had predicted trans-membrane domains. However, no clear candidates for transport of the major substrates and intermediates of energy metabolism were found. We suggest that, instead, these metabolites are transported via pores formed by the known glycosomal membrane proteins.
1. Introduction
In kinetoplastid protists, several metabolic pathways, including glycolysis, purine salvage and ether lipid biosynthesis, are located in a microbody, the glycosome 1, 2, which is evolutionarily related to peroxisomes. All evidence so far indicates that the glycosomal membrane, like the peroxisomal membrane, is impermeable to nucleotides, notably adenosine phosphates and NAD(P)(H) 3, 4. Its permeability to smaller molecules, however, is subject to debate 5. Specific transporters would be required if the membrane were impermeable to molecules of the size of glycolytic intermediates, such as glucose, phosphate, malate, pyruvate, phosphoenolpyruvate and various triosephosphates.
In 1987, the first protein profile of glycosomal membranes from Trypanosoma brucei was published 6. It revealed two abundant proteins of 24 and 26 kDa, which were later shown to be trypanosome homologues of the peroxisome biogenesis protein PEX11 7– 9. Subsequent studies, including two of the glycosomal proteome 1, 10, revealed several more trypanosome PEX proteins that are predicted to be membrane-bound, such as PEX2 11, 12, PEX10 13, PEX12 13, PEX13 14 and PEX14 15. The only transporters known to be associated with the glycosomal membrane are the ABC transporters GAT1, GAT2 and GAT3, which might transport fatty acids 16. In addition, a member of the mitochondrial carrier protein family was found: MCP6, which is a candidate for nucleotide transport 17. MCP6 is found preferentially in the glycosomal membranes of bloodstream-form trypanosomes, whereas in procyclic forms, it is predominantly targeted to the mitochondria 17.
No analysis has yet yielded evidence for glycosomal transporters of metabolites smaller than about 400 Da. In contrast, lipid bilayers that were reconstituted with glycosomal membrane proteins revealed evidence for the presence of anion- and cation-selective pores 5. The identities of these pore-forming proteins are still unknown: they could be dedicated exclusively to metabolite transport, or they might be involved in protein import as well 5.
If proteins other than PEX components were indeed involved in metabolite transport, it ought to be possible to find them by mass spectrometry, using highly purified glycosomal membrane protein preparations. A similar proteomics approach has been previously used for mammalian peroxisomes. Analysis of carbonate-washed rat liver peroxisomes initially yielded only two peroxisomal membrane proteins, PMP70 and PMP22 18, whereas a later analysis of whole mouse kidney peroxisomes led to the identification of 12 putative glycosomal membrane proteins. These included one tetratricopeptide domain protein, four different ABC transporters, three members of the PMP22 family, PMP34, Pxmp4/PMP4, and the putative solute carrier PMP47 19.
Specific transporters for glycolytic metabolites might have been missed in previous glycosomal proteomic analyses, since glycosomal membrane proteins are likely to comprise a rather small proportion of the total protein content. We have therefore set out to identify the proteins in a highly enriched glycosomal membrane preparation from Leishmania tarentolae, using 30-times more starting material than used for our previously published T. brucei glycosomal proteome study 1. Comprehensive mass spectrometry analysis of these highly purified glycosomal membrane protein fractions did not, however, lead to the identification of any novel glycosomal transporters. We therefore postulate that the recently described porin activity 5 in the glycosomal membrane might be provided by known components of the glycosomal protein import machinery (peroxins), as has also been suggested for peroxisomes 20. In addition, we compared the phospholipid compositions of the glycosomal membranes from bloodstream- and procyclic-form T. brucei, with the lipid composition of the T. brucei cell membrane to see if this could give us more information regarding glycosomal membrane transport.
2. Materials and methods
2.1. Isolation of glycosomes from Trypanosoma brucei and Leishmania tarentolae
Leishmania tarentolae promastigotes were cultured at 28°C in 3.7 L hemin-supplemented brain-heart infusion medium to a maximum density of 2 × 10 8 cells/ml. Procyclic-form Trypanosoma brucei Lister 427 was grown at 30°C in 10% (v/v) foetal calf serum-supplemented MEM-PROS medium to a maximum density of 5 × 10 6 cells/ml 21. Bloodstream-form T. brucei 427 was grown at 37°C in 10% (v/v) foetal calf serum-supplemented HMI-9 medium 22 to a maximum density of 2 × 10 6 cells/ml 21, 23.
Procyclic-form and bloodstream-form T. brucei (10 10 cells each), and promastigote L. tarentolae (10 12 cells) were harvested by centrifugation for 10 min at 2,000x g, and were washed once in 50 ml of TEDS (25 mM Tris, 1 mM EDTA, 1 mM DTT, 250 mM sucrose, pH 7.8). After centrifugation, the cell pellet was resuspended in 2 ml homogenization medium (250 mM sucrose, 1 mM EDTA, 0.1% (v/v) ethanol, 5 mM MOPS, pH 7.2) containing protease inhibitor (complete EDTA-free, Roche Applied Science) and was grinded in a pre-chilled mortar with 1 volume of wet-weight silicon carbide (Crysalon: Norton Company: porous <400 mesh). Cells were checked for at least 90% disruption by light microscopy. The cell lysate was centrifuged sequentially for 5 minutes each at 100x g and 3,000x g to remove abrasive, intact cells, cell rests and nuclei. The supernatant was centrifuged for 15 minutes at 17,000x g to yield the glycosome-enriched pellet fraction. This fraction was resuspended in 3 ml of homogenization buffer and loaded on top of a 32 ml linear 20–40% (v/v) Optiprep (iodixanol-sucrose, Sigma Biochemicals) gradient, mounted on a 3.5 ml 50% (v/v) Optiprep cushion (Optiprep Application Sheet S9, Axis-shield). Centrifugation was performed for 1 h at 170,000x g and 4°C using a Beckman VTi-50 Rotor. 1 ml aliquots were collected from the bottom of the tube after puncture, and the protein concentration of each fraction was determined using the BioRad Bradford protein assay. Of each fraction, 100 µl was TCA-precipitated and the resulting pellets resuspended in denaturing Laemmli SDS-PAGE buffer. Proteins were separated on a 12% SDS-PAGE gel and analysed by western blotting.
2.2. Isolation and analysis of glycosomal membrane proteins from Leishmania tarentolae glycosomes
Glycosomes (corresponding to about 0.5 mg protein) were diluted 1:5 in TEDS (see 2.1), subjected to two freeze-thaw cycles, and centrifuged for 40 minutes at 140,000x g and 4°C. The resulting pellet was washed with 5 M urea for 1 h at 4°C to remove proteins that were not tightly associated with the glycosomal membranes. The glycosomal membranes were pelleted by centrifugation for 40 minutes at 140,000x g and 4°C. This 5 M urea wash-step was repeated once. The glycosomal membrane-enriched fraction was resuspended in denaturing Laemmli SDS-PAGE buffer and separated by SDS PAGE. In-gel trypsin digestion and nanoLC-MS/MS analysis of the obtained protein bands were performed as previously described 1. The obtained MS/MS spectra were analysed using MASCOT software and visualised in Scaffold. The comparison shown in Supplementary Table 1 was done using the 2012 version of the shotgun sequence of L. tarentolae ( http://tritrypdb.org/) 24; only proteins for which at least 2 different peptides could be identified with >95% confidence were included. Leishmania proteins were first scanned for the presence of a PTS1 signal based on a published analysis for L. major and T. brucei 2 and by manually examining the C-terminal sequences. Additional PTS1-containing proteins were identified using PTS1 Predictor 25, 26. Trans-membrane domains were identified using the TritrypDB annotation database. For potential glycosomal proteins with no known function and without an annotated trans-membrane domain, we also scanned for trans-membrane domains using the HMMTOP and SOSUI algorithms 27, 28.
2.3. Phospholipid analysis
Lipids were extracted in triplicate from bloodstream and procyclic T. brucei samples and from a single batch of isolated glycosomes due to the limited amount of purified material. Lipids were extracted according to the method of Bligh and Dyer (1959) 29 with the minor modification that 0.5% (v/v) 6 M HCl was added to the second chloroform wash to increase recovery of acidic phospholipids. The phospholipids and free fatty acids were separated from neutral lipids (cholesterol, cholesterol esters and triacylglycerols) by fractionation on a 1 ml silica column prepared from 0.063–0.200 mm silica 60 (Merck, Darmstadt, Germany). Lipid extracts were dissolved in chloroform and loaded on the silica column, then eluted successively with acetone (4 volumes) and methanol (4 volumes). The last fraction, which contained the purified phospholipids, was dried under nitrogen and stored at -20°C until HPLC-MS analysis.
The purified phospholipids were dissolved in methanol:acetonitrile:chloroform:water (46:20:17:17). Separation of molecular lipid species was performed on a Synergi 4 µm MAX-RP 18A column (250 × 3 mm; Phenomenex, CA, USA). Elution was performed with a linear gradient of water in methanol/acetonitrile (60/40 (v/v)) decreasing from 12.5% to 0% in 25 min, followed by further isocratic elution for another 25 minutes. The flow rate was kept constant at 0.425 ml•min -1 and 1 µM serine and 2.5 mM ammonium acetate were used in all solvents as additives.
Mass spectrometry of lipids was performed using electrospray ionization, on a 4000 QTRAP system (Applied Biosystems, Nieuwerkerk aan de IJssel, The Netherlands). Source temperature was set to 450°C and nitrogen was used as curtain gas. The declustering potential was optimized using lipid standards. The optimal collision energy was dependent on the type of experiment performed and was set to +45V (precursor scanning m/z 184), -45V (precursor scanning m/z -196), +35V (neutral loss 141), -30V (precursor scanning m/z -241), and -40V (neutral loss scanning 87 Da) respectively. For quantification of molecular species, samples were measured in multiple-reaction monitoring mode (MRM), monitoring for 95 head-group specific mass transitions with a total dwell time of 1 s, using the same settings as above. Data analysis was performed with Analyst™ v 1.4.1 software (MDS Sciex, Concord, ON).
All proteins, possible glycosomal proteins, and a short list of glycosome membrane proteins.
3. Results
3.1. Glycosome preparation from Leishmania tarentolae
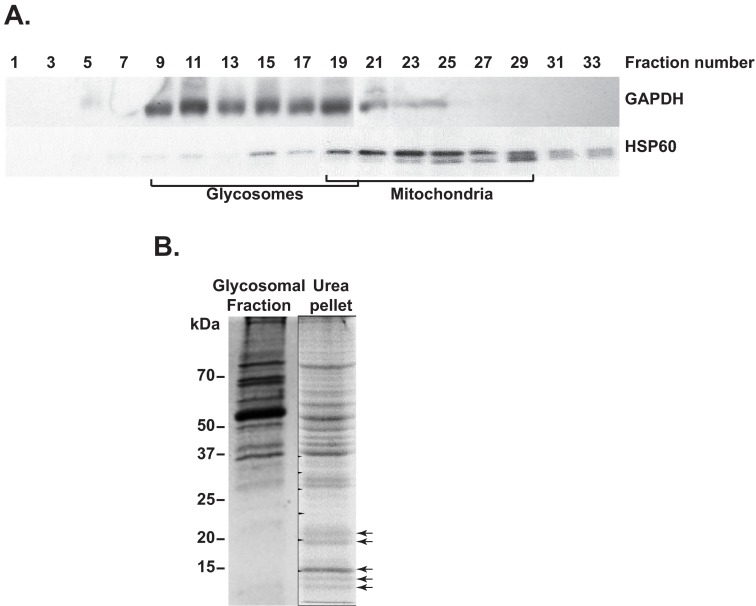
In preliminary experiments (not shown), we tested various methods for purification of membranes of iodixanol gradient-enriched T. brucei glycosomes 1. The methods tested included methanol/chloroform extraction 30; ultracentrifugation of glycosomes that had been subjected to osmotic shock with cold water 31, 32; and different high salt (0–1 M NaCl or 5 M urea) washes of glycosomal membranes 32. Although we were able to enrich glycosomal membrane proteins, as judged by the presence of PEX11 7, the matrix protein aldolase persisted. In addition, the total amount of membrane protein obtained from 3 × 10 10 T. brucei was so low that we doubted that any lower-abundance proteins would be detected by mass spectrometry. We therefore decided to isolate glycosomes from the related kinetoplastid L. tarentolae to increase the sensitivity for the detection of even low-abundant glycosomal membrane proteins. In contrast to T. brucei, L. tarentolae can be grown to far higher cell densities, enabling us to isolate glycosomes from as much as 10 12 cells. The different fractions obtained after differential fractionation and subsequent density gradient (Optiprep) centrifugation were analysed by western blotting ( Figure 1). The gradient distributions of the two marker proteins glyceraldehyde phosphate dehydrogenase (glycosomes) and HSP60 (mitochondria) are shown in Figure 1A. Comparison of previously published density gradient results from T. brucei 1 with those from L. tarentolae ( Figure 1A) showed that the mitochondria were enriched at similar gradient densities (fractions 22–25), whereas the glycosomes isolated from L. tarentolae appeared to have a higher buoyant density than those of T. brucei. In addition, both the mitochondria and glycosome-containing fractions were spread out over a wide range of fractions for the L. tarentolae gradient, which could be the result of breakage of the organelles during isolation. Judging from the western blotting results (alternate gradient fractions shown in Figure 1A), fractions 9, 11 and 13 contained about 42% of the total GAPDH measured, but only 2% of the total HSP60. We therefore decided to use fractions 9–13 for further glycosomal membrane purification.
Figure 1. Purification of glycosomal membrane-enriched fractions from L. tarentolae.
A. Western blot analysis of the different L. tarentolae fractions obtained after density gradient centrifugation. Equal volumes of only the odd-numbered fractions were loaded for analysis. Antibodies used for detection are indicated next to the western blot panels. Mitochondrial 19– 29 and glycosomal 9– 19 density gradient fractions are indicated. B. SDS-PAGE gel stained with Coomassie brilliant blue, showing protein bands from intact glycosomes (glycosomal fraction, and from the glycosomal membrane-enriched pellet (urea pellet). Arrows indicate enriched proteins in the urea-treated glycosomal membrane fraction.
To purify glycosomal membranes, we found that the protocol that gave least matrix protein contamination was one that was successfully employed to isolate the cell membrane of E. coli 33. It involved washing the glycosomal pellet with 5 M urea, and resulted in strong depletion of some prominent bands (presumably matrix proteins) and the enrichment of various proteins in the 10–25 kDa range ( Figure 1B) - similar to the expected sizes of the PEX11 protein homologues 7, 9. The entire SDS-PAGE lane containing the enriched glycosomal protein fraction ( Figure 1B) was subsequently subjected to mass spectrometry.
3.2. Identification of putative glycosomal proteins
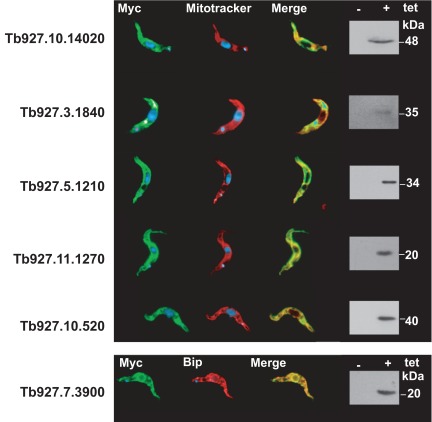
By comparison with the predicted proteome of L. tarentolae 24, 464 polypeptides were identified ( Supplementary Table S1). The first step that we undertook was to identify homologues of all identified proteins from the T. brucei genome ( http://tritrypdb.org/tritrypdb/). This was done to facilitate the retrieval of information because most experimental data is available exclusively for T. brucei. All identified proteins were screened for database annotation, including user comments, and in some cases we also updated annotations from publications. We further screened all proteins for their presence in previously published glycosomal 1, 10 and mitochondrial 34 proteomes. The results are summarised in Supplementary Table S1, Sheet 1. Proteins that were clearly located in compartments other than the glycosome were then excluded, resulting in Supplementary Table S1, Sheet 2. Some candidates predicted to contain at least one trans-membrane domain were tested for their locations, by expression of N-terminally and/or C-terminally tagged versions (none has a PTS1 signal). The proteins encoded by Tb927.3.1840 (putative 3-oxo-5-alpha-steroid 4-dehydrogenase), Tb927.5.1210 (putative short-chain dehydrogenase) and Tb927.10.14020 (unknown function) were all targeted to mitochondria, while Tb927.7.3900 (annotated as a vacuolar transporter chaperone) was in the ER (Supplementary Figure S1). All identified proteins were further searched for the presence of known peroxisomal targeting signals in the L. major or T. brucei homologues 35; in addition, the L. tarentolae protein sequences in Supplementary Table S1, Sheet 2 were manually scanned for PTS1 signals.
3.3. Glycosomal enzymes
The L. tarentolae glycosomal membrane preparations revealed the presence of 40 known or predicted glycosomal matrix proteins, and some novel proteins ( Supplementary Table S1, sheet 2). A putative glycosomal pathway scheme, incorporating all available information for L. tarentolae and T. brucei, is shown in Figure 2. Glycolytic enzymes, enzymes involved in the conversion of glycerone phosphate to glycerol, the pentose phosphate pathway, steroid and nucleotide biosynthesis as well as enzymes of the succinic fermentation branch were detected. Similar to results obtained for the T. brucei glycosome 1, fumarase (EC 4.2.1.2) is the only enzyme of the glycosomal succinic fermentation branch that was not found in the L. tarentolae glycosomal membrane preparation. It is possible that fumarase was removed in the membrane purification; alternatively, the activity may be supplied by one of the four proteins of unknown function that are conserved in kinetoplastids and have an unambiguous PTS1: LtaP34.3290/Tb927.4.1360, LtaP33.2650/Tb927.11.2620, LtaP18.0870/Tb927.10.13240, or LtaP24.1780/Tb927.8.6640 - although fumarase activity would be surprising since they lack known functional domains. Fumarase catalyses the conversion of malate to fumarate, which is an indispensable step towards the generation of succinate in the glycosomal matrix. If indeed the glycosome lacks fumarase then the glycosomal membrane must harbour a malate-fumarate shuttle. This shuttle would be responsible for the transport of glycosome-derived malate in exchange with mitochondria-derived fumarate. Inside the glycosome fumarate could then be converted to succinate by fumarate reductase (EC 1.3.1.6) to maintain the glycosomal NADH balance.
Figure 2. Putative glycosomal pathway scheme.
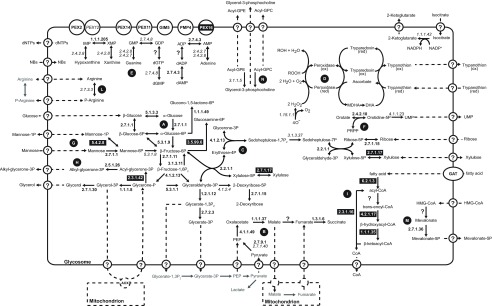
The scheme summarizes metabolic pathways identified so far in the glycosomes of Leishmania and T. brucei. EC numbers of enzymes identified only in the Leishmania glycosome are indicated in black boxes with white text, those identified only in T. brucei are indicated in italics, and those found in the glycosome of both species are indicated in bold. Predicted transport processes across the glycosomal membrane are indicated by the circled question marks and dashed arrows. Letters in black circles indicate the different metabolic pathways as follows: A: glycolysis; B: succinic fermentation; C: pentose phosphate pathway; D: superoxide and trypanothione metabolism; E: purine salvage; F: pyrimidine metabolism; G: mannose metabolism; H: glycerolipid biosynthesis; I: β-oxidation of fatty acids; L: phosphoarginine metabolism; M: mevalonate pathway; N: phospholipid degradation. Abbreviations used are: Acyl-GPC, 1-acyl-glycero-phosphocholine; Acyl-GPE, 1-acyl-glycero-phosphoethanolamine; AOX, alternative oxidase; DHA, dehydroascorbate; MDHA, monodehydroxyascorbate; NB, nucleobases; PRPP, 5-phosphoribosyl 1-pyrophosphate.
The L. tarentolae glycosomal membrane preparation contained several enzymes that were not detected during LC-MS analysis of the T. brucei glycosome. For example, glucosamine-6-phosphate isomerase was found in the glycosomes of Leishmania, but not in trypanosomes 1, 10. In addition, a PTS1-containing D-lactate dehydrogenase-like protein is found in Leishmania, for which there is no obvious substrate, as well as a PTS1-containing xylulokinase and a glucokinase-like protein. These additional enzymes involved in the metabolism of sugars might indicate a higher metabolic flexibility of L. tarentolae compared to African trypanosomes. In T. brucei, the phosphomannomutase Tb927.10.6440 was found in the glycosome, where it can act as phosphoglucomutase during glycolysis 36. T. brucei phospho-N-acetylglucosamine mutase (Tb927.8.980) was also partially glycosomal 36. Neither has an obvious PTS1 targeting signal so it is possible that they have either an internal glycosomal targeting signal or that they are co-imported via association with other glycosomal targeting signal-containing proteins 37, 38. The syntenic L. tarentolae homologues LtaP36.1960 and LtaP07.0850 were not present in our dataset, but the different non-syntenic phosphomannomutase-like protein LtaP34.3710, containing the conserved C-terminal PTS1 signal -SKL, was found. The T. brucei aldose 1-epimerase, Tb927.4.1360, is the homologue of LtaP34.3290, but Leishmanias have an additional isoform, LtaP35.110, containing a conserved PTS1 targeting signal.
The first three steps of ether-lipid biosynthesis in T. brucei, namely the conversion of glycerone 3-phosphate to 1-alkyl-glycerol-3-phosphate, are associated with the glycosome 39. T. brucei glycosome proteomic analysis confirmed the presence of the second enzyme of the biosynthetic pathway, namely alkyl-glycerone-phosphate synthetase (EC 2.5.1.26). The first and the third enzymes, glycerone-phosphate acyl-transferase (EC 2.3.1.42) and 1-acyl-glycerol-3-phosphate oxidoreductase (EC 1.1.1.101) were not identified. The T. brucei glycerone 3-phosphate acetyltransferase/acyltransferase homologue, Tb927.4.3160, has a C-terminal PTS1, SRM. Analysis of the glycosomal membrane proteome of L. tarantolae identified not only alkyl-glycerone 3-phosphate synthetase (EC 2.5.1.26) but also glycerone 3-phosphate acetyltransferase/acyltransferase (EC 2.3.1.42, LtaP34.1280, C-terminal PTS1 -SKM) supporting the idea that the first two steps of the ether-lipid biosynthesis can occur inside the glycosome.
The β-oxidation of long chain fatty acids (LCFA) is one of the hallmark catabolic pathways attributed to peroxisomes ( 40 and references therein). Our previous analysis suggested that the glycosome of T. brucei was devoid of LCFA β-oxidation enzymes 1. On the other hand, 2-enoyl coenzyme A hydratase (EC 4.2.1.17) and NADP-dependent 3-hydroxyacyl-CoA dehydrogenase (EC 1.1.1.35) were reported in glycosomal fractions from procyclic-form T. brucei 40. According to the results from this proteome analysis, the L. tarentolae glycosome might contain all enzymes involved in the β-oxidation of LCFA except acyl-CoA dehydrogenase ( Figure 2, Supplementary Table S1, sheet 2). We found one protein (LtaP24.1780, annotated as hypothetical), which is clearly a fatty acyl Co-A reductase and contains a C-terminal -SSL; another hypothetical protein (LtaP16.0130) contains -AKL and an acyl CoA dehydrogenase domain; and the enoyl-CoA hydratase/enoyl-CoA isomerase/3-hydroxyacyl-CoA dehydrogenase trifunctional enzyme homologues (EC 4.2.1.17/5.3.3.8/1.1.1.35, LtaP26.1590 and LtaP33.2830) containing putative PTS2 signals 35. Other detected enzymes of this pathway without obvious peroxisomal targeting signals were long-chain-fatty-acyl-CoA synthetase (EC 6.2.1.3) and thiolase (EC 2.3.1.16); these might have internal signals or could be contaminants from another compartment.
Mevalonate kinase is known to be in the glycosome 41, and another enzyme of the mevalonate pathway, isopentenyl-diphosphate delta-isomerase, was also present in our glycosomal preparation; but intermediate enzymes (phosphomevalonate kinase and mevalonate-5-pyrophosphate decarboxylase) were not ( Figure 2, Supplementary Table S1, sheet 2). Finally, we found a PTS1-containing phosphoribulokinase/uridine kinase family protein, LtaP14.0950, belonging to the P-loop NTPase superfamily; we speculate that this enzyme might be involved in pyrimidine salvage.
3.4. Putative glycosomal membrane proteins
We next focussed on the identification of putative membrane proteins. Known glycosomal membrane proteins and unassigned proteins that had predicted membrane-spanning domains in both L. tarentolae and T. brucei are listed in Supplementary Table S1, sheet 3. These proteins were manually analysed for conserved functional domains, and their protein sequences were aligned to the complete predicted proteomes of Homo sapiens, Saccharomyces cerevisiae, Hansenula polymorpha and Pichia pastoris on the NCBI Web site using Blastp. This enabled the exclusion of further proteins that were deemed likely to be in other (non-peroxisomal) subcellular compartments.
The list of known glycosomal membrane proteins that we identified is shown in Table 1. It included three proteins related to PEX11 (PEX11, GIM5A and GIM5B), PEX2 and PEX14. The protein encoded by Tb09.160.4700/Tb927.9.6450 has a very weak match to a conserved PEX16 domain (E-value 9e -3). We have therefore annotated this as a putative PEX16. It could therefore be involved in the incorporation of peroxisomal membrane proteins 42, although high-throughput RNAi screening revealed no growth defects for RNAi targeting this locus 43.
Table 1. Known proteins of the glycosomal membrane.
This list includes all mass-spectrometry-detected glycosomal membrane proteins.
| Lta gene ID | Peptides | Coverage | Tb gene ID | Function |
|---|---|---|---|---|
| LtaP28.2340 | 16 | 59% | Tb927.11.11520 | PEX11 |
| LtaP24.0140 | 7 | 25% | ||
| LtaP28.2330 | 3 | 14% | ||
| LtaP25.2350 | 3 | 8% | Tb927.3.2340 | PEX2 |
| LtaP15.0800 | 6 | 13% | Tb927.9.6450 | PEX16 |
| LtaP28.1050 | 3 | 15% | Tb11.0300, Tb11.0400 | |
| LtaP26.2550 | 2 | 13% | Tb927.9.1720 | PMP4 |
| LtaP35.3750 | 12 | 48% | Tb927.9.11580, Tb927.9.11600 | GIM5A,B |
| LtaP31.0560 | 5 | 5% | Tb927.11.3130, Tb927.4.4050 | GAT2 |
| LtaP27.0470 | 4 | 6% | Tb927.11.1070 | GAT3 |
Abbreviations used: Lta, Leishmania tarentolae; Tb, Trypanosoma brucei.
The trypanosome homologue of the mammalian peroxisomal membrane protein PMP24 44 (also called PXMP4 or PMP4) was also found. Like other PMP24/PMP4 proteins, a conserved TIM27 superfamily domain is present in Tb927.9.1720; the function of this domain (and of PMP4) is still unknown. Although S. cerevisiae that lack it show abnormal peroxisome size and numbers 45, no growth defects were seen in the high-throughput RNAi screens in T. brucei 43. Of the previously reported T. brucei glycosomal ABC transporters GAT1-3 16, only GAT1 and GAT3 were found in the L. tarentolae glycosomal proteome. This was not unexpected since the annotated L. tarentolae GAT2 protein sequence is severely truncated. Either L. tarentolae lacks a functional GAT2, or this is a genome assembly error; the former is quite possible since no peptides matching L. major and L. infantum GAT2 were found.
Of the remaining potential membrane proteins, four could be tentatively assigned to the mitochondrion, three to the endoplasmic reticulum, two to the flagellum, and one to the nucleus ( Supplementary Table S1, sheet 3). The remaining proteins are listed in Table 2. A protein of the major facilitator family (Tb927.3.4070-110, LtaP29.1650) was the only conserved multi-pass membrane protein that had no clear assignment to another subcellular compartment, but the 3 identified peptides covered only 3% of the protein. Best matches to this sequence are ion transporters. The Tb927.9.4310 protein sequence matches a yeast possible alpha-isopropylmalate carrier, which exports alpha-isopropylmalate from the mitochondrion to the cytoplasm for use in leucine biosynthesis. The remaining candidates have either one or two potential trans-membrane domains, usually predicted by only one algorithm.
Table 2. Possible additional glycosomal membrane proteins.
This list includes all detected proteins with predicted trans-membrane (TM) domains, for which there is no evidence for location in any particular compartment.
| Lta gene ID | Pep nr | Cov (%) | Tb gene ID | Function | TMs (a) | TMs (b) | ER sp |
|---|---|---|---|---|---|---|---|
| LtaP29.1650 | 3 | 3 | Tb927.3.4070 Tb927.3.4110 | Major facilitator family, nitrate and chloride transporter? | 12 | nd | no |
| LtaP01.0560 | 4 | 17 | Tb927.9.4310 | putative tricarboxylate carrier, matches yeast putative alpha-isopropylmalate carrier, which exports alpha-isopropylmalate from the mitochondrion to the cytoplasm for use in leucine biosynthesis | 5 | nd | no |
| LtaP05.0670 | 2 | 6 | Tb927.9.4310 | putative tricarboxylate carrier, matches yeast putative alpha-isopropylmalate carrier, which exports alpha-isopropylmalate from the mitochondrion to the cytoplasm for use in leucine biosynthesis | 5 | nd | no |
| LtaP36.4130 | 3 | 6 | Tb927.11.10260 | hypothetical protein, conserved | 0 | 2 | no |
| LtaP33.2680 | 4 | 4 | Tb927.11.2590 | hypothetical protein, conserved | 0 | 2 | no |
| LtaP29.0320 | 2 | 21 | Tb927.3.5350 | hypothetical protein, conserved | 1 | 1 | no |
| LtaP36.6980 | 4 | 4 | Tb927.10.8000 | P-loop nucleoside triphosphate hydrolase, contains EF hand domains | 0 | 1 | no |
| LtaP21.0440 | 4 | 6 | Tb927.10.2240 | NTF2-like domain | 0 | 1 | no |
| LtaP11.1210 | 2 | 5 | Tb927.11.6070 | ARM repeat superfamily | 0 | 1 | no |
| LtaP31.3020 | 2 | 3 | Tb927.4.5000, Tb927.8.7420 | TERD-like domains | 0 | 1 | yes |
| LtaP35.4380 | 2 | 4 | Tb927.9.10470 | hypothetical protein, conserved | 0 | 1 | no |
| LtaP34.2250 | 2 | 5 | Tb927.4.2250 | hypothetical protein, conserved | 0 | 1 | no |
| LtaP06.1080 | 3 | 11 | Tb927.7.5700 | hypothetical protein, conserved | 1 | nd | yes |
| LtaP26.0630 | 3 | 12 | Tb927.7.1290 | hypothetical protein, DUF2012, peptidase superfamily, starch binding domain, similarity to human C15ORF24 | 1 | nd | yes |
| LtaP36.3480 | 2 | 8 | Tb927.11.10040 | hypothetical protein, conserved | 1 | nd | no |
| LtaP17.1410 | 2 | 36 | Tb927.5.2560 | hypothetical protein, conserved | 1 | nd | no |
| LtaP23.0390 | 3 | 10 | Tb927.8.2300 | hypothetical protein, conserved | 1 | nd | no |
Abbreviations used: Lta, Leishmania tarentolae; Tb, Trypanosoma brucei; Pep nr, number of identified peptides; Cov (%), percentage of protein coverage; TMs (a), number of annotated trans-membrane domains; TMs (b), number of transmembrane-domains identified by THMM TOP; ER sp, endoplasmic reticulum signal peptide.
Overall, our proteomics analysis revealed no clear candidates for major novel glycosomal metabolite transporters.
3.5. Phosphatidylcholine composition of cellular and glycosomal membranes
The phosphatidylcholine species composition in membranes from total T. brucei cells and glycosomes were analysed to detect possible differences in membrane composition between glycosomal and the other membranes of T. brucei, and to allow comparison in membrane composition between the two cultivatable replicating life cycle stages, the bloodstream form and procylic form. The phosphatidylcholine species composition of total trypanosome membranes differed to some extent between bloodstream and procyclic stages ( Table 3), which is consistent with earlier reports on the phospholipid composition in T. brucei 46. Membranes of both stages contain predominantly diacyl-phosphatidylcholine species, comprising common fatty acids consisting of 16 to 22 carbon atoms with 0 to 6 desaturations. In addition, membranes of intact trypanosomes also contained ether phospholipids species, both 1-alkyl-2-alkyl phosphatidylcholine species and 1-alkyl-2-acyl phosphatidylcholine species. The phosphatidylcholine species composition of glycosomal membranes differed only to a minor extent from the composition observed in total membranes of both bloodstream form and procyclic form trypanosomes ( Table 3).
Table 3. Phosphatidylcholine composition of procyclic-form and bloodstream-form T. brucei total cells and glycosomes.
| PCF | BSF | |||||
|---|---|---|---|---|---|---|
| Peak | Component | Glycosomes | Total cells | Glycosomes | Total cells | |
| Mol % | Mol % | Mol % | Mol % | |||
| 1 | PtdCho | 16:0, 16:1 | 2,9 | 1,7 ± 0,0 | 0,5 | 0,2 ± 0,2 |
| 2 | AlkCho | 16:0, 18:2 | 2,3 | 2,1 ± 0,3 | 0,7 | nd |
| 3 | PtdCho | 16:0, 18:3 | 3,3 | 1,7 ± 0,3 | 0,5 | nd |
| 4 | PtdCho | 16:0, 18:2 | 7,9 | 3,9 ± 0,5 | 2,5 | 3,9 ± 0,6 |
| 5 | PtdCho | 16:0, 18:1 | 2,8 | 3,1 ± 0,9 | 3,2 | 1,2 ± 0,3 |
| 6 | AlkCho | 16:0, 20:3 | 1,0 | 0,6 ± 0,0 | 0,2 | nd |
| 7 | EnylCho | 18:0, 18:2 | 0,9 | 1,0 ± 0,2 | 1,4 | 1,0 ± 0,2 |
| 8 | AlkCho | 18:0, 18:2 | 9,3 | 12,8 ± 0,7 | 9,6 | 6,6 ± 0,3 |
| 9 | PtdCho | 18:2, 18:2, 18:1, 18:3 | 12,6 | 6,1 ± 0,5 | 5,1 | 4,4 ± 0,4 |
| 10 | PtdCho | 18:1, 18:2 | 8,1 | 6,8 ± 0,2 | 4,3 | 5,6 ± 0,7 |
| 11 | PtdCho | 18:0, 18:2, 18:1, 18:1 | 23,2 | 13,4 ± 0,7 | 26,0 | 32,7± 4,6 |
| 12 | PtdCho | 18:0, 18:1 | 2,4 | 1,2 ± 0,2 | 2,4 | 2,2 ± 0,4 |
| 13 | AlkCho | 16:0, 22:1, 18:0, 20:1 | 0,5 | 0,6 ± 0,1 | 3,5 | 0,4 ± 0,7 |
| 14 | PtdCho | 16:0, 22:6 | 1,4 | 0,9 ± 0,1 | nd | nd |
| 15 | PtdCho | 18:1, 20:5 | 2,8 | 3,9 ± 0,3 | 2,3 | 3,7 ± 0,3 |
| 16 | PtdCho | 16:0, 22:5 | 1,1 | 0,8 ± 0,0 | 1,1 | 2,1 ± 0,6 |
| 17 | PtdCho | 18:1, 20:4 | 1,3 | 2,6 ± 0,1 | nd | nd |
| 18 | PtdCho | 16:0, 22:4 | 0,9 | 0,8 ± 0,2 | 2,3 | 2,2 ± 0,6 |
| 19 | PtdCho | 18:0, 20:3 | 2,2 | 1,8 ± 0,7 | 3,0 | 4,1 ± 0,4 |
| 20 | PtdCho | 18:3, 22:5 | 4,0 | 12,2 ± 0,1 | 4,9 | 4,5 ± 0,2 |
| 21 | PtdCho | 18:2, 22:5 | 0,3 | nd | 2,4 | 1,7 ± 0,3 |
| 22 | PtdCho | 18:1, 22:6 | 3,3 | 9,5 ± 2,1 | 3,5 | 3,5 ± 0,7 |
| 23 | PtdCho | 18:3, 22:3 | 0,4 | 0,9 ± 0,3 | 3,0 | 2,6 ± 1,0 |
| 24 | PtdCho | 18:0, 22:6 | 2,4 | 3,2 ± 0,3 | 3,2 | 5,3 ± 0,8 |
| 25 | PtdCho | 18:0, 22:5 | 2,6 | 5,5 ± 0,4 | 3,7 | 3,6 ± 0,3 |
| 26 | PtdCho | 18:2, 22:3 | 0,0 | nd | 3,2 | 3,4 ± 0,3 |
| 27 | PtdCho | 18:0, 22:4 | 0,3 | 0,5 ± 0,1 | 6,7 | 3,7 ± 0,4 |
| 28 | PtdCho | 18:0, 22:3 | 0,2 | 0,2 ± 0,1 | 0,9 | 1,3 ± 0,5 |
| 29 | PtdCho | 20:4, 22:6 | 0,0 | 1,2 ± 0,0 | nd | nd |
| 30 | PtdCho | 20:4, 22:5 | 0,0 | 1,0 ± 0,1 | nd | nd |
The phosphatidylcholine species description comprises the sn-1 linkage type followed by the radyl chains on the sn-1 and sn-2 position, respectively. "Total cell" values are mean of three independent experiments. Most abundant species representing over 5 Mol % are marked in bold. Abbreviations: AlkCho, 1-alkyl, 2-acyl phosphatidylcholine; BSF, bloodstream-form T. brucei; EnylCho, 1-alkyl-1-enyl-2-acyl phosphatidylcholine; nd, not detected; PCF, procyclic-form T. brucei; PtdCho, diacyl phosphatidylcholine.
4. Discussion
The glycosome is a major contributor to kinetoplastid energy metabolism and essential for glycolysis 3, 4, 47. Flux rates through the glycolytic pathway are high in trypanosomes and - judging from the peptide counts and protein coverage in our analysis - the enzymes are also abundant in Leishmania. A model of trypanosome glycolysis that assumes free exchange of glucose between the organelle and the cytosol mirrors the in vivo kinetics 3, 47. This suggests that the protein responsible for glucose transport should be very active and probably also abundant. Our analysis of the glycosomal membrane proteome, however, failed to identify abundant membrane proteins that might fulfil such a role. Given the large number of proteins that we identified - including multiple membrane proteins from other compartments - it seems unlikely that our failure to detect transporters for such major metabolites could be due solely to lack of sensitivity. Dual subcellular locations could be a possible explanation for some proteins, as previously shown for MCP6 17. The five additional mitochondrial carrier proteins that we detected are predominantly in the mitochondrion of procyclic T. brucei 48, but the presence of a minor amount in glycosomes cannot be ruled out. If so, they could possibly function as putative glycosomal isocitrate/2-ketoglutarate and fumarate/malate shuttles.
We investigated the lipid composition of glycosomal membranes in T. brucei by analysis of the species composition of phosphatidylcholine, the most abundant phospholipid class in membranes of both procyclic and bloodstream form T. brucei 46. The phospholipid composition of peroxisomal membranes has been investigated in peroxisomes isolated from rat liver and from the yeasts Saccharomyces cerevisiae and Pichia pastoris 49– 51. In rat liver peroxisomes the phospholipid classes and their fatty acid composition were similar to those of homogenates and microsomes, although exposure to the endotoxin lipopolysaccharide (LPS) induced significant changes in phospholipid species composition: in particular, the abundance of both long chain fatty acids (>20 C atoms) and poly-unsaturated fatty acids increased in peroxisomal membranes 49. The lipid composition of peroxisomes in yeasts was shown to be rather flexible, predominantly depending on the type and amount of fatty acid supply in the medium 50, 51. The lipid composition of glycosomes in Trypanosomatidae has not been investigated before and our results showed that the phosphatidylcholine species composition in glycosomal membranes resembled that of other cellular membranes in both bloodstream-form and procyclic-form T. brucei. These results suggest that the lipid composition, and thus the biophysical properties, of the glycosomal membrane is similar to that of the other membranes in trypanosomes. Because of this similarity to other cellular membranes, glycosomal membranes are expected to be also impermeable to small hydrophilic molecules unless transport is facilitated by specialized membrane proteins.
There is accumulating evidence that small solutes enter microbodies through pores 5, 20, 52, 53. Evidence from a mammalian Pxmp2 (PMP22) knock-out mouse suggested loss of peroxisomal pores for solutes of under 300 Da 53 and this type of function was confirmed when the protein was expressed in insect cells. No Pxmp2 homologue appears to exist in kinetoplastids. Instead, it has been speculated that PEX11 family proteins might contribute to glycosomal porin activity 5, 8. Our failure to find abundant novel glycosomal transporters is consistent with the hypothesis that the PEX11 family proteins are indeed responsible for the transfer of small solutes in and out of the glycosome.
Funding Statement
Work by C. Colasante and F. Voncken was supported by the Deutsche Forschungsgemeinschaft (DFG) (Cl112/10).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
v1; ref status: indexed
Supplementary figure
Supplementary Figure S1. Immunofluorescence analysis of the putative glycosomal membrane proteins in procyclic T. brucei.
N-terminally or C-terminally myc-tagged versions of the proteins were detected (green) by using a commercial Myc antibody (Sigma-Aldrich). Mitochondria were visualized (red) using mitotracker. The endoplasmatic reticulum (ER) was detected using an antibody directed against the ER lumen protein BiP (red). Overlays (Merge) of the green staining and the red staining are shown to visualize the common compartmentalization of the proteins. On the right side, western blots are shown to illustrate the expression of the myc-tagged membrane proteins. (+) and (-) indicate tetracycline-induced and -uninduced cells respectively.
References
- 1.Colasante C, Ellis M, Ruppert T, et al. : Comparative proteomics of glycosomes from bloodstream form and procyclic culture form Trypanosoma brucei brucei. Proteomics. 2006;6(11):3275–3293 10.1002/pmic.200500668 [DOI] [PubMed] [Google Scholar]
- 2.Vertommen D, Van Roy J, Szikora JP, et al. : Differential expression of glycosomal and mitochondrial proteins in the two major life-cycle stages of Trypanosoma brucei. Mol Biochem Parasitol. 2008;158(2):189–201 10.1016/j.molbiopara.2007.12.008 [DOI] [PubMed] [Google Scholar]
- 3.Haanstra JR, van Tuijl A, van Dam J, et al. : Proliferating bloodstream-form Trypanosoma brucei use a negligible part of consumed glucose for anabolic processes. Int J Parasitol. 2012;42(7):667–673 10.1016/j.ijpara.2012.04.009 [DOI] [PubMed] [Google Scholar]
- 4.Bakker BM, Mensonides FI, Teusink B, et al. : Compartmentation protects trypanosomes from the dangerous design of glycolysis. Proc Natl Acad Sci U S A. 2000;97(5):2087–2092 10.1073/pnas.030539197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gualdron-López M, Vapola MH, Miinalainen IJ, et al. : Channel-forming activities in the glycosomal fraction from the bloodstream form of Trypanosoma brucei . PLoS One. 2012;7(4):e34530 10.1371/journal.pone.0034530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aman RA, Wang CC: Identification of two integral glycosomal membrane proteins in Trypanosoma brucei. Mol Biochem Parasitol. 1987;25(1):83–92 10.1016/0166-6851(87)90021-1 [DOI] [PubMed] [Google Scholar]
- 7.Lorenz P, Maier AG, Erdmann R, et al. : Elongation and clustering of glycosomes in Trypanosoma brucei overexpressing the glycosomal Pex11p. EMBO J. 1998;17(13):3542–3555 10.1093/emboj/17.13.3542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Voncken F, van Hellemond JJ, Pfisterer I, et al. : Depletion of GIM5 causes cellular fragility, a decreased glycosome number, and reduced levels of ether-linked phospholipids in trypanosomes. J Biol Chem. 2003;278(37):35299–35310 10.1074/jbc.M301811200 [DOI] [PubMed] [Google Scholar]
- 9.Maier A, Lorenz P, Voncken F, et al. : An essential dimeric membrane protein of trypanosome glycosomes. Mol Microbiol. 2001;39(6):1443–1451 10.1046/j.1365-2958.2001.02333.x [DOI] [PubMed] [Google Scholar]
- 10.Vertommen D, Van Roy J, Szikora JP, et al. : Differential expression of glycosomal and mitochondrial proteins in the two major life-cycle stages of Trypanosoma brucei. Mol Biochem Parasitol. 2008;158(2):189–201 10.1016/j.molbiopara.2007.12.008 [DOI] [PubMed] [Google Scholar]
- 11.Flaspohler JA, Rickoll WL, Beverley SM, et al. : Functional identification of a Leishmania gene related to peroxin 2 reveals common ancestry of glycosomes and peroxisomes. Mol Cell Biol. 1997;17(3):1093–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guerra-Giraldez C, Quijada L, Clayton CE: Compartmentation of enzymes in a microbody, the glycosome, is essential in Trypanosoma brucei. J Cell Sci. 2002;115(Pt 13):2651–8 [DOI] [PubMed] [Google Scholar]
- 13.Krazy H, Michels PA: Identification and characterization of three peroxins–PEX6, PEX10 and PEX12–involved in glycosome biogenesis in Trypanosoma brucei. Biochim Biophys Acta. 2006;1763(1):6–17 10.1016/j.bbamcr.2005.11.002 [DOI] [PubMed] [Google Scholar]
- 14.Brennand A, Rigden DJ, Michels PA: Trypanosomes contain two highly different isoforms of peroxin PEX13 involved in glycosome biogenesis. FEBS Lett. 2012;586(13):1765–71 10.1016/j.febslet.2012.05.019 [DOI] [PubMed] [Google Scholar]
- 15.Moyersoen J, Choe J, Kumar A, et al. : Characterization of Trypanosoma brucei PEX14 and its role in the import of glycosomal matrix proteins. Eur J Biochem. 2003;270(9):2059–67 10.1046/j.1432-1033.2003.03582.x [DOI] [PubMed] [Google Scholar]
- 16.Yernaux C, Fransen M, Brees C, et al. : Trypanosoma brucei glycosomal ABC transporters: identification and membrane targeting. Mol Membr Biol. 2006;23(2):157–72 10.1080/09687860500460124 [DOI] [PubMed] [Google Scholar]
- 17.Colasante C, Alibu VP, Kirchberger S, et al. : Characterization and developmentally regulated localization of the mitochondrial carrier protein homologue MCP6 from Trypanosoma brucei. Eukaryotic Cell. 2006;5(8):1194–1205 10.1128/EC.00096-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Islinger M, Luers GH, Zischka H, et al. : Insights into the membrane proteome of rat liver peroxisomes: microsomal glutathione-S-transferase is shared by both subcellular compartments. Proteomics. 2006;6(3):804–816 10.1002/pmic.200401347 [DOI] [PubMed] [Google Scholar]
- 19.Wiese S, Gronemeyer T, Ofman R, et al. : Proteomics characterization of mouse kidney peroxisomes by tandem mass spectrometry and protein correlation profiling. Mol Cell Proteomics. 2007;6(12):2045–2057 10.1074/mcp.M700169-MCP200 [DOI] [PubMed] [Google Scholar]
- 20.Visser WF, van Roermund CW, Ijlst L, et al. : Metabolite transport across the peroxisomal membrane. Biochem J. 2007;401(12):365–375 10.1042/BJ20061352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alibu VP, Storm L, Haile S, et al. : A doubly inducible system for RNA interference and rapid RNAi plasmid construction in Trypanosoma brucei. Mol Biochem Parasitol. 2005;139(1):75–82 10.1016/j.molbiopara.2004.10.002 [DOI] [PubMed] [Google Scholar]
- 22.Hirumi H, Hirumi K: Continuous cultivation of Trypanosoma brucei bloodstream forms in a medium containing a low concentration of serum protein without feeder cell layers. J Parasitol. 1989;75(6):985–989 [PubMed] [Google Scholar]
- 23.van Deursen FJ, Shahi SK, Turner CM, et al. : Characterisation of the growth and differentiation in vivo and in vitro of bloodstream-form Trypanosoma brucei strain TREU 927. Mol Biochem Parasitol. 2001;112(2):163–171 10.1016/S0166-6851(00)00359-5 [DOI] [PubMed] [Google Scholar]
- 24.Raymond F, Boisvert S, Roy G, et al. : Genome sequencing of the lizard parasite Leishmania tarentolae reveals loss of genes associated to the intracellular stage of human pathogenic species. Nucleic Acids Res. 2012;40(3):1131–1147 10.1093/nar/gkr834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neuberger G, Maurer-Stroh S, Eisenhaber B, et al. : Prediction of peroxisomal targeting signal 1 containing proteins from amino acid sequence. J Mol Biol. 2003;328(3):581–592 10.1016/S0022-2836(03)00319-X [DOI] [PubMed] [Google Scholar]
- 26.Neuberger G, Maurer-Stroh S, Eisenhaber B, et al. : Motif refinement of the peroxisomal targeting signal 1 and evaluation of taxon-specific differences. J Mol Biol. 2003;328(3):567–579 10.1016/S0022-2836(03)00318-8 [DOI] [PubMed] [Google Scholar]
- 27.Hirokawa T, Boon-Chieng S, Mitaku S: SOSUI: classification and secondary structure prediction system for membrane proteins. Bioinformatics. 1998;14(4):378–379 10.1093/bioinformatics/14.4.378 [DOI] [PubMed] [Google Scholar]
- 28.Tusnády GE, Simon I: The HMMTOP transmembrane topology prediction server. Bioinformatics. 2001;17(9):849–850 10.1093/bioinformatics/17.9.849 [DOI] [PubMed] [Google Scholar]
- 29.Bligh EG, Dyer WJ: A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37(8):911–917 10.1139/o59-099 [DOI] [PubMed] [Google Scholar]
- 30.Ferro M, Seigneurin-Berny D, Rolland N, et al. : Organic solvent extraction as a versatile procedure to identify hydrophobic chloroplast membrane proteins. Electrophoresis. 2000;21(16):3517–3526 [DOI] [PubMed] [Google Scholar]
- 31.Da Cruz S, Xenarios I, Langridge J, et al. : Proteomic analysis of the mouse liver mitochondrial inner membrane. J Biol Chem. 2003;278(42):41566–41571 10.1074/jbc.M304940200 [DOI] [PubMed] [Google Scholar]
- 32.Quinones W, Urbina JA, Dubourdieu M, et al. : The glycosome membrane of Trypanosoma cruzi epimastigotes: protein and lipid composition. Exp Parasitol. 2004;106(3–4):135–149 10.1016/j.exppara.2004.03.006 [DOI] [PubMed] [Google Scholar]
- 33.Soualhine H, Brochu V, Menard F, et al. : A proteomic analysis of penicillin resistance in Streptococcus pneumoniae reveals a novel role for PstS, a subunit of the phosphate ABC transporter. Mol Microbiol. 2005;58(5):1430–1440 10.1111/j.1365-2958.2005.04914.x [DOI] [PubMed] [Google Scholar]
- 34.Panigrahi AK, Ogata Y, Zíková A, et al. : A comprehensive analysis of Trypanosoma brucei mitochondrial proteome. Proteomics. 2008;9(2):434–450 10.1002/pmic.200800477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Opperdoes FR, Szikora JP: In silico prediction of the glycosomal enzymes of Leishmania major and trypanosomes. Mol Biochem Parasitol. 2006;147(2):193–206 10.1016/j.molbiopara.2006.02.010 [DOI] [PubMed] [Google Scholar]
- 36.Bandini G, Marino K, Guther ML, et al. : Phosphoglucomutase is absent in Trypanosoma brucei and redundantly substituted by phosphomannomutase and phospho-N-acetylglucosamine mutase. Mol Microbiol. 2012;85(3):513–534 10.1111/j.1365-2958.2012.08124.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Galland N, de Walque S, Voncken FG, et al. : An internal sequence targets Trypanosoma brucei triosephosphate isomerase to glycosomes. Mol Biochem Parasitol. 2010;171(1):45–49 10.1016/j.molbiopara.2010.01.002 [DOI] [PubMed] [Google Scholar]
- 38.Penha LL, Sant'Anna CB, Mendonca-Previato L, et al. : Sorting of phosphoglucomutase to glycosomes in Trypanosoma cruzi is mediated by an internal domain. Glycobiology. 2009;19(12):1462–1472 10.1093/glycob/cwp121 [DOI] [PubMed] [Google Scholar]
- 39.Zomer AW, Michels PA, Opperdoes FR: Molecular characterisation of Trypanosoma brucei alkyl dihydroxyacetone-phosphate synthase. Mol Biochem Parasitol. 1999;104(1):55–66 10.1016/S0166-6851(99)00141-3 [DOI] [PubMed] [Google Scholar]
- 40.Wiemer EA, IJlst L, van Roy J, et al. : Identification of 2-enoyl coenzyme A hydratase and NADP(+)-dependent 3-hydroxyacyl-CoA dehydrogenase activity in glycosomes of procyclic Trypanosoma brucei. Mol Biochem Parasitol. 1996;82(1):107–111 10.1016/0166-6851(96)02710-7 [DOI] [PubMed] [Google Scholar]
- 41.Carrero-Lerida J, Perez-Moreno G, Castillo-Acosta VM, et al. : Intracellular location of the early steps of the isoprenoid biosynthetic pathway in the trypanosomatids Leishmania major and Trypanosoma brucei. Int J Parasitol. 2009;39(3):307–314 10.1016/j.ijpara.2008.08.012 [DOI] [PubMed] [Google Scholar]
- 42.Matsuzaki T, Fujiki Y: The peroxisomal membrane protein import receptor Pex3p is directly transported to peroxisomes by a novel Pex19p- and Pex16p-dependent pathway. J Cell Biol. 2008;183(7):1275–1286 10.1083/jcb.200806062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alsford S, Turner DJ, Obado SO, et al. : High throughput phenotyping using parallel sequencing of RNA interference targets in the African trypanosome. Genome Res. 2011;21(6):915–924 10.1101/gr.115089.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reguenga C, Oliveira ME, Gouveia AE, et al. : Identification of a 24 kDa intrinsic membrane protein from mammalian peroxisomes. Biochim Biophys Acta. 1999;1445(3):337–341 10.1016/S0167-4781(99)00061-5 [DOI] [PubMed] [Google Scholar]
- 45.Marshall PA, Krimkevich YI, Lark RH, et al. : Pmp27 promotes peroxisomal proliferation. J Cell Biol. 1995;129(129):345–355 10.1083/jcb.129.2.345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patnaik PK, Field MC, Menon AK, et al. : Molecular species analysis of phospholipids from Trypanosoma brucei bloodstream and procyclic forms. Mol Biochem Parasitol. 1993;58(1):97–105 10.1016/0166-6851(93)90094-E [DOI] [PubMed] [Google Scholar]
- 47.Bakker BM, Michels PA, Opperdoes FR, et al. : Glycolysis in bloodstream-form Trypanosoma brucei can be understood in terms of the kinetics of the glycolytic enzymes. J Biol Chem. 1997;272(6):3207–3215 10.1074/jbc.272.6.3207 [DOI] [PubMed] [Google Scholar]
- 48.Colasante C, Peña Diaz P, Clayton C, et al. : Mitochondrial carrier family inventory of Trypanosoma brucei brucei: Identification, expression and subcellular localisation. Mol Biochem Parasitol. 2009;167(2):104–17 10.1016/j.molbiopara.2009.05.004 [DOI] [PubMed] [Google Scholar]
- 49.Khan M, Contreras M, Singh I: Endotoxin-induced alterations of lipid and fatty acid compositions in rat liver peroxisomes. J Endotoxin Res. 2000;6(1):41–50 10.1177/09680519000060010601 [DOI] [PubMed] [Google Scholar]
- 50.Tuller G, Nemec T, Hrastnik C, et al. : Lipid composition of subcellular membranes of an FY1679-derived haploid yeast wild-type strain grown on different carbon sources. Yeast. 1999;15(14):1555–1564 [DOI] [PubMed] [Google Scholar]
- 51.Wriessnegger T, Gübitz G, Leitner E, et al. : Lipid composition of peroxisomes from the yeast Pichia pastoris grown on different carbon sources. Biochim Biophys Acta. 2007;1771(4):455–461 10.1016/j.bbalip.2007.01.004 [DOI] [PubMed] [Google Scholar]
- 52.Antonenkov VD, Hiltunen JK: Peroxisomal membrane permeability and solute transfer. Biochim Biophys Acta. 2006;1763(12):1697–1706 10.1016/j.bbamcr.2006.08.044 [DOI] [PubMed] [Google Scholar]
- 53.Rokka A, Antonenkov VD, Soininen R, et al. : Pxmp2 is a channel-forming protein in mammalian peroxisomal membrane. PLoS One. 2009;4(4):e5090 10.1371/journal.pone.0005090 [DOI] [PMC free article] [PubMed] [Google Scholar]