A cute or chronic blood loss reduces the numbers of red blood cells (RBCs) in the circulation, reducing oxygen delivery and producing tissue hypoxia. One of the first cellular responses to hypoxia is increased production of reactive oxygen species (ROS) by mitochondria. With evolution, organisms have developed an efficient and rapid way to respond to erythroid challenges by using ROS as the ‘ignition key’ that activates the engine, defined stress erythropoiesis, which induces hematopoietic/stem progenitor cells to produce RBCs to compensate losses.1,2
Much has been discovered in recent years on the mechanism(s) that regulate stress erythropoiesis. ROS stimulates kidney cells to produce greater levels of erythropoietin (Epo), the hormone that specifically stimulates RBC production.2 In addition, ROS stimulates hematopoietic niches in the marrow to produce factors that induce hematopoietic stem cells to generate ‘stress-specific erythroid progenitors’ with enhanced ability to produce RBCs.3 In mice, ‘stress-specific erythroid progenitors’ egress from the marrow into the blood to home in ‘stress-specific’ niches in the spleen where they continue their maturation.4,5 The development of mechanisms that use ‘ROS’ as the sensor to increase RBC production in response to erythroid challenges is an evolutionary winner that has an important drawback. As described by Ulyanova et al. in this issue,6 maturation of erythroid cells is uniquely associated with accumulation of hemoglobin, the oxygen carrier molecule with the intrinsic property of increasing ROS production. Since above a ‘critical’ threshold, ROS activates the p53-dependent pathway of cell death, during their maturation, erythroid cells must maintain ROS constantly low by increasing production of enzymes, such as catalase, that are able to degrade ROS. The expression of these enzymes is controlled by the transcription factor forkhead-box protein O3 (FoxO3) activated by AKT7 (Figure 1). Therefore, in contrast to stem/progenitor cells, hypoxia-induced increases of ROS are deleterious to erythroblasts. Hence the need for these cells to have a safety circuitry up-regulating expression of enzymes that catabolize ROS under conditions of hypoxia. Without this circuitry, stress-specific erythroblasts would die in great numbers, eliminating the expected increases in RBC production mediated by ROS-induced proliferation of hematopoietic stem/progenitor cells.
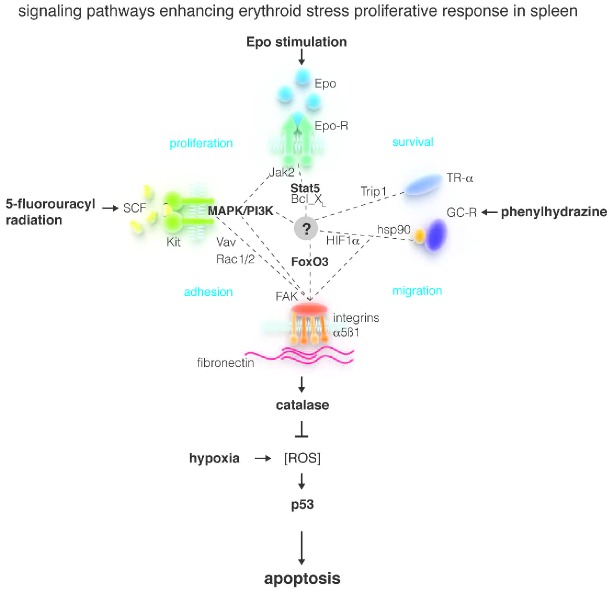
Figure 1.
A model for the control of ROS production during the maturation of erythroid cells under conditions of stress erythropoiesis. Hypoxia induced by stress challenges generates ROS that induce the formation of stress-specific erythroid progenitor cells. These progenitor cells are poised to receive signals delivered by stress-specific growth factors (SCF and Epo) and hormones (glucocorticoids and thyroid hormone) to respond to integrin outside-in signals. Stress-specific niches present in murine spleen, and equivalent niches in human marrow, induce through the integrin axes stress-specific erythroid cells to produce greater levels of catalase (and probably other enzymes) reducing the levels of ROS generated by hypoxia in these cells, allowing them to escape ROS-activated p53-dependent death, to generate great numbers of RBCs. (modified from Online Supplementary Figure 3BS by Ulyanova et al.6)
Studies have been performed to clarify how increases in ROS production as a result of hypoxia induce hematopoietic stem cells to generate stress-specific progenitor cells.1–3 ROS induce the formation of ‘stress-specific’ niches that through specific adhesion receptors attract hematopoietic stem cells exposing them to growth factors that, in synergy with Epo, induce them to generate stress-specific progenitor cells. In contrast, little is known about how the control of ROS production becomes more stringent in erythroblasts maturing under conditions of hypoxia.
Integrins are heterodimers composed by an alpha(α) and a beta(β) subunit that interact with counter receptors present on niche cells and/or with fibronectin, a component of the extracellular matrix used by cells to sense their position in the microenvironment.8 Integrins lack a kinase domain but upon activation deliver outside-in signals through association with the focal adhesion kinase (FAK).8,9 The combination of α and β subunits expressed is cell- and tissue-specific. Hematopoietic cells express mainly the β1 subunit in combination with either α4 or α5.10,11 During erythroid maturation, expression of α4 increases between the proerythroblast and the basophilic stage and then decreases between the basophilic and polychromatic stage.12–14 Expression of β1 parallels that of α4 with the exception that it is retained in terminally maturing cells. Pioneering work published, among others, by the Papayannopoulou laboratory has identified that the α4β1 heterodimer prevents mobilization of stem/progenitor cells in response to a variety of stimuli, including those that elicit the erythroid stress response.1,5 In contrast, the pattern of α5 expression and its biological functions in erythropoiesis are less clear.
In this issue, Ulyanova et al.6 present a superb review of the effect of ROS on terminal erythroid maturation and the regulation of this process by the microenvironment through the integrin axes (Figure 1). This information is used as a foundation for performing loss-of-function analysis of the role played by integrins in the regulation of the terminal phases of RBC production. The loss-of-function models utilized include mice lacking the α4 gene, mice lacking the β1 gene (and therefore presumably lacking also α5β1 heterodimers), mice lacking both the α4 and the β1 gene, and wild-type mice treated with an inhibitor of FAK, the tyrosine kinase protein that mediates their intracellular signaling. The mutants were analyzed at baseline and during the recovery from stress challenges chosen to induce progressively more profound perturbations of the erythron (Figure 1).
- Phenylhydrazine-induced hemolytic anemia: this drastically depletes the number of circulating RBCs. Mice recover from this challenge by rapidly amplifying the compartment of erythroid precursors which generates RBCs within several days. Recovery requires activation of the glucocorticoid receptor that induces proerythroblasts into a transient self-replication state.15
- Epo treatment: this induces polycythemia by increasing proliferation of cells ranging from commitment progenitor cells to proerythroblasts.16
- 5-fluorouracyl treatment and radiation: these challenges induce death of cells in proliferation such as hematopoietic progenitor cells of multiple lineages but spare quiescent hematopoietic stem cells and RBCs.17 Onset of anemia is delayed and is due to the fact that reductions in progenitor cell numbers hamper the ability of the marrow to produce new RBCs in sufficient numbers to compensate their physiological turnover due to aging. Recovery involves induction of proliferation of quiescent hematopoietic stem cells and is mediated by the interaction of cKIT, a receptor present on hematopoietic stem cells, with stem cell factor (SCF), a growth factor produced by the microenvironment.18
Both glucocorticoid receptor and cKIT activation up-regulate expression of FAK19,20 poising the cells for outside-in signaling by integrins (Figure 1).
The data presented indicate that α4β1 and α5β1 are not essential for normal erythropoiesis because the hematocrit of mice lacking either one of these integrin complexes is normal. However, careful analysis of the phenotype of α5β1 deficient mice demonstrated that they express a modest uncompensated anemia because of impaired ability to compensate for the physiological increases in ROS production occurring during the late stages of maturation. This result suggests that signaling from α5β1 integrin may be specifically responsible for regulating ROS catabolism (Figure 1).
This hypothesis was confirmed by demonstrating differences in phenotype of α4β1 and α5β1 deficient mice under conditions of stress erythropoiesis. Mice lacking the integrin α4 had delayed but quantitatively normal responses to all the challenges used. In contrast, mice lacking α5β1 were unable to respond to any of these challenges because their maturing erythroid cells expressed great levels of ROS and died of apoptosis. Further investigations indicated that erythroid cells from α5β1-deficient mice were unable to activate FAK and FOXO3 and to increase antioxidant response. These results indicate that although stress-specific erythroid cells are poised to activate the machinery that reduces ROS, an outside-in signal delivered by α5β1 integrin is necessary for activation of the machinery and ROS (and consequent p53-mediated death) reduction under hypoxic conditions.
The analyses of the phenotype of double knock-out mice provided additional information on the role exerted by the two integrins during stress erythropoiesis. Mice lacking both α4β1 and α5β1 responded to challenges perturbing only the terminal erythroid compartments (phenylhydrazine and EPO) as single α5β1 mutant mice, confirming that α5β1 is necessary and sufficient to control ROS production in maturing erythroid cells. By contrast, double knock-out mice responded to challenges perturbing the initial phases of the hematopoietic process, (5-fluorouracyl and radiation) more severely than single α5β1 mutants dying in great numbers following these challenges. This result confirms that α4β1 is necessary to induce hematopoietic stem cells to generate stress-specific erythroid progenitors.
The results described by Ulyanova et al. are important for three reasons.
They demonstrate for the first time that α4β1 and α5β1 control different levels of the response to erythroid stress. This specialization may represent an evolutionary mechanism developed by the hematopoietic system to decrease ROS in response to hypoxia only in those cells, i.e. mature erythroblasts, which, having already high ROS levels, would be induced into apoptosis by further increases.
They identify the first outside-in signaling activated by integrins represented by the FAK, AKT, FoxO3 and catalase axis. Although much is known about the activity of integrins in hematopoietic stem/progenitor cells, the outside-in signaling they activate in these cells still has to be characterized.
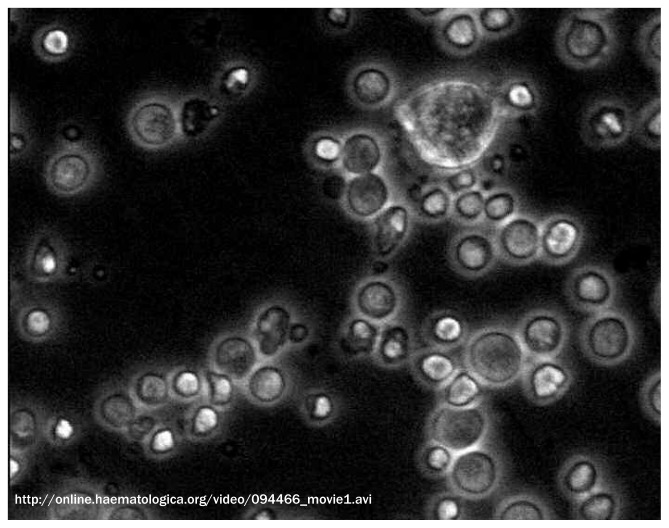
Downstream to the hematopoietic stem/progenitor cells there is a plethora of hematopoietic precursors whose appropriate maturation is essential for the generation of adequate numbers of blood cells. These precursors are embedded in a mesh of microenvironment elements. A movie documenting the complex physical contacts established by maturing erythroblasts with macrophages may be downloaded here (Movie 1). By providing evidence that the microenvironment provides not only ‘niches’ that promote specific hematopoietic stem cell functions but also ‘niches’ that control differentiation/maturation of erythroid precursors, these data provide proof-of-principle for the existance of ‘microenvironmental niches’ that may control every function of every cell type.
Movie 1.
Interaction between macrophages and human erythroblasts in vitro. Time lapse videomicroscopy of cells obtained after 14 days of culture under Human Erythroid Massive Amplification (HEMA) conditions from peripheral blood of one healthy donor. Samples were observed by an Olympus phase-contrast microscope equipped with incubator chamber and high speed, high-resolution camera (Hamamatsu), objective lens ×20. Two hours of video recording are shown. Frames were collected every 30 seconds and movie was accelerated at 10 frames per second. The movie presents erythroblasts at various stages of maturation surrounding a macrophage (top right) recognizable for being larger than the other cells, for its motility, and for its ability to protrude its cytoplasm. During the movie, the macrophage appears to explore the space in its surroundings protruding filaments of different sizes. These filaments establish contacts with numerous erythroblasts. Some of the contacts are temporary while others persist in time. The variegation of these interactions suggests that the macrophage is engaging in multiple types of biological conversations possibly in response to specific signals emitted by each erythroblast. Kindly provided by Mario Falchi PhD, National Aids Center, Istituto Superiore di Sanità, Rome, Italy.
Footnotes
Financial and other disclosures provided by the author using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are available with the full text of this paper at www.haematologica.org.
References
- 1.Suda T, Takubo K, Semenza GL. Metabolic regulation of hematopoietic stem cells in the hypoxic niche. Cell Stem Cell. 2011;9(4):298–310 [DOI] [PubMed] [Google Scholar]
- 2.Haase VH. Regulation of erythropoiesis by hypoxia-inducible factors. Blood Rev. 2013;27(1):41–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paulson RF, Shi L, Wu DC. Stress erythropoiesis: new signals and new stress progenitor cells. Curr Opin Hematol. 2011;18(3):139–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trentin JJ. Determination of bone marrow stem cell differentiation by stromal hemopoietic inductive microenvironments (HIM). Am J Pathol. 1971;65(3):621–8 [PMC free article] [PubMed] [Google Scholar]
- 5.Bonig H, Papayannopoulou T. Hematopoietic stem cell mobilization: updated conceptual renditions. Leukemia. 2013;27(1):24–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ulyanova T, Jiang Y, Padilla SM, Papayannopoulou T. Erythroid cells generated in the absence of specific β1-integrin heterodimers accumulate reactive oxygen species and are unable to mount effective antioxidant defenses. Haematologica. 2013;98(11):1769–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang P, Wang W, Wang X, Wang X, Song Y, Zhang J, et al. Focal adhesion kinase mediates atrial fibrosis via the AKT/S6K signaling pathway in chronic atrial fibrillation patients with rheumatic mitral valve disease. Int J Cardiol. 2013. April 29 pii S0167–5273(13)00749–3. [DOI] [PubMed] [Google Scholar]
- 8.Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69(1):11–25 [DOI] [PubMed] [Google Scholar]
- 9.Burridge K, Fath K, Kelly T, Nuckolls G, Turner C. Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton. Annu Rev Cell Biol. 1988;4:487–525 [DOI] [PubMed] [Google Scholar]
- 10.Papayannopoulou T, Brice M. Integrin expression profiles during erythroid differentiation. Blood. 1992;79(7):1686–94 [PubMed] [Google Scholar]
- 11.Kerst JM, Sanders JB, Slaper-Cortenbach IC, Doorakkers MC, Hooibrink B, van Oers RH, et al. Alpha 4 beta 1 and alpha 5 beta 1 are differentially expressed during myelopoiesis and mediate the adherence of human CD34+ cells to fibronectin in an activation-dependent way. Blood. 1993;81(2):344–51 [PubMed] [Google Scholar]
- 12.Ghinassi B, Ferro L, Masiello F, Tirelli V, Sanchez M, Migliaccio G, et al. Recovery and Biodistribution of Ex vivo Expanded Human Erythroblasts Injected into NOD/SCID/IL2Rγnull mice. Stem Cell Int. 2011;2011:673–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu J, Liu J, Xue F, Halverson G, Reid M, Guo A, et al. Isolation and functional characterization of human erythroblasts at distinct stages: implications for understanding of normal and disordered erythropoiesis in vivo. Blood. 2013;121(16):3246–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ulyanova T, Jiang Y, Padilla S, Nakamoto B, Papayannopoulou T. Combinatorial and distinct roles of α5 and α4 integrins in stress erythropoiesis in mice. Blood. 2011;117(3):975–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dolznig H, Grebien F, Deiner EM, Stangl K, Kolbus A, Habermann B, et al. Erythroid progenitor renewal versus differentiation: genetic evidence for cell autonomous, essential functions of EpoR, Stat5 and the GR. Oncogene. 2006;25(20):2890–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nijhof W, Goris H, Dontje B, Dresz J, Loeffler M. Optimal erythroid cell production during erythropoietin treatment of mice occurs by exploiting the splenic microenvironment. Exp Hematol. 1993; 21(4):496–501 [PubMed] [Google Scholar]
- 17.Rich IN. The effect of 5-fluorouracil on erythropoiesis. Blood. 1991;77(6):1164–70 [PubMed] [Google Scholar]
- 18.Tajima Y, Moore MA, Soares V, Ono M, Kissel H, Besmer P. Consequences of exclusive expression in vivo of kit-ligand lacking the major proteolytic cleavage site. Proc Natl Acad Sci USA. 1998;95(20): 11903–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Plotkin LI, Manolagas SC, Bellido T. Glucocorticoids induce osteocyte apoptosis by blocking focal adhesion kinase-mediated survival. Evidence for inside-out signaling leading to anoikis. J Biol Chem. 2007;282(33):24120–30 [DOI] [PubMed] [Google Scholar]
- 20.Takahira H, Gotoh A, Ritchie A, Broxmeyer HE. Steel factor enhances integrin-mediated tyrosine phosphorylation of focal adhesion kinase (pp125FAK) and paxillin. Blood. 1997;89(5):1574–84 [PubMed] [Google Scholar]