Abstract
The contribution of the bone marrow microenvironment in myelodysplastic syndrome is controversial. We therefore analyzed the functional properties of primary mesenchymal stromal cells from patients with myelodysplastic syndrome in the presence or absence of lenalidomide. Compared to healthy controls, clonality and growth were reduced across all disease stages. Furthermore, differentiation defects and particular expression of adhesion and cell surface molecules (e.g. CD166, CD29, CD146) were detected. Interestingly, the levels of stromal derived factor 1-alpha in patients’ cells culture supernatants were almost 2-fold lower (P<0.01) than those in controls and this was paralleled by a reduced induction of migration of CD34+ hematopoietic cells. Co-cultures of mesenchymal stromal cells from patients with CD34+ cells from healthy donors resulted in reduced numbers of cobblestone area-forming cells and fewer colony-forming units. Exposure of stromal cells from patients and controls to lenalidomide led to a further reduction of stromal derived factor 1-alpha secretion and cobblestone area formation, respectively. Moreover, lenalidomide pretreatment of mesenchymal stromal cells from patients with low but not high-risk myelodysplastic syndrome was able to rescue impaired erythroid and myeloid colony formation of early hematopoietic progenitors. In conclusion, our analyses support the notion that the stromal microenvironment is involved in the pathophysiology of myelodysplastic syndrome thus representing a potential target for therapeutic interventions.
Introduction
Myelodysplastic syndromes (MDS) comprise a heterogeneous group of clonal hematopoietic diseases with ineffective hematopoiesis, peripheral blood cytopenias and a substantial risk of progression to acute myeloid leukemia. The World Health Organization classifies MDS according to the percentage of blasts in the bone marrow, number and type of hematologic lineage(s) affected, presence of ringed sideroblasts, and the cytogenetic aberrations in hematopoietic cells, for example interstitial deletion of the long arm of chromosome 5 [del(5q)], which leads to a well-defined subset of MDS mostly with a rather favorable prognosis.1 In some MDS categories a specific cytogenetic aberration, mutation and/or epigenetic alteration in the hematopoietic cells explains, at least partially, the behavior of the MDS.2,3 In fact, the del(5q) subtype is associated with more responsiveness to lenalidomide (LEN, an immunomodulatory agent, IMiD®) in low/intermediate-1-risk MDS patients.4,5
In spite of intensive research on the biology of CD34+ hematopoietic stem and progenitor cells (HSPC) in MDS, cytogenetic and molecular data do not completely clarify how the progression from low risk/less advanced MDS with dysplastic progenitors and apoptosis in the bone marrow to high risk/advanced MDS with an excess of blasts occurs. While mutations might lead to the transformation of a HSPC, the latter cannot survive and expand in the absence of a supportive microenvironment.6–8
Recent striking findings indicate a decisive role of the bone marrow microenvironment in the pathophysiology and disease progression of MDS;9–12 nevertheless, the heterogeneity of the disease has generated conflicting results. Mesenchymal stromal cells (MSC) are a main component of the hematopoietic niche; MSC and their progeny – endosteal osteoprogenitors, perivascular cells, nestin+ cells, CXCL12 abundant reticular cells, among others – regulate the localization, number, and fate of HSPC.13–15 MSC also possess immunomodulatory activity and some reports point to a defect in this regard in MDS.16–17 A recent study by our group also showed that the properties of MSC from healthy donors can be modulated by LEN treatment,18 but whether this also applies to MSC derived from MDS patients is unknown.
Here we investigated the functionality of MSC and their modulation by LEN from patients with different disease subtypes including MDS associated with single del(5q), and characterized their clonogenicity and differentiation potential.
Methods
Human samples
Twenty MDS patients were studied; their characteristics are detailed in Online Supplementary Table S1. Our control cohort consisted of one male and five female healthy individuals undergoing orthopedic surgery with a median age of 60 years (range, 56–65). MSC were obtained as previously described.18 Patients were classified for the study as “lower risk” with and without del(5q) (LR, LR-5q ) (<5% bone marrow blasts and IPSS-low/int-1), and as “higher-risk” (HR) (>5% bone marrow blasts and IPSS-int-2/high). Magnetically sorted HSPC were obtained from peripheral blood of healthy individuals after mobilization. Samples were collected after approval by our Institutional Review Board, and written informed consent was obtained. A detailed description is available in the Online Supplementary Material.
Lenalidomide treatment
LEN was kindly provided by Celgene (Munich, Germany) and prepared using 10% dimethyl-sulfoxide as a vehicle. The experiments were performed with 0.1 μM and 1 μM of the drug.
Characterization of the mesenchymal stromal cells
Clonality, self-renewal, generation of single cell-derived colonies (SCD), expression of cell surface and adhesion molecules with flow cytometry, differentiation potential, proliferation, viability, apoptosis, senescence, and cell cycle were studied. Detailed information about the experiments is provided in the Online Supplementary Material.
Co-culture of myelodysplastic syndrome-mesenchymal stromal cells with hematopoietic stem and progenitor cells, clonogenicity and apoptosis assay
The capacity of MSC to support HSPC was tested using a 4-week cobblestone area-forming (CAF-C) assay and a 14-day colony-forming unit granulocyte-erythrocyte-monocyte/macrophage (CFU-GEMM) assay as described in the Online Supplementary Material. HSPC or the cell line KG1-α were labeled with carboxyfluorescein-diacetate-succinimidyl-ester (CFSE) prior to co-culture with MSC for 72 h, or left unlabeled and co-cultured for 24 h, with or without the addition of 100 nM recombinant tumor necrosis factor alpha (TNF-α, Peprotech). Apoptosis and proliferation were studied by annexin-V staining and CFSE dilution using flow cytometry.
Cytokine measurement and transwell migration assay
Supernatant from MSC was obtained after culture with or without LEN and used for enzyme-linked immunoabsorbent assay (ELISA) determinations of SDF-1α, angiopoietin-1 (ANG1), and stem cell factor (SCF) levels. Supernatant was also used for a 4-h transwell migration assay with 1×105 HSPC, as described previously.18 The CXCR4 antagonist AMD3100 (Sigma) was used at a concentration of 10 μM.
RNA extraction, complementary DNA synthesis and real time polymerase chain reaction
mRNA extracted with Trizol reagent (Invitrogen) was transcribed into complementary-DNA and used for real time polymerase chain reaction (RT-PCR) analysis for fatty acid binding protein 4 (FABP4), SDF-1α, ANG1, and SCF. Abelson murine leukemia viral oncogene homolog 1 (ABL-1) was used to normalize values of relative gene expression. Primer sequences are provided in the Online Supplementary Material.
Statistical analysis
Significance was assessed with the unpaired t-test using Graphpad Prism software version 5 (GraphPad Software, San Diego, CA, USA). The results are presented as mean ± SEM from at least three different donors per group. The level of statistical significance was set at P≤0.05.
Results
Mesenchymal stromal cells from myelodysplastic syndrome patients display abnormal characteristics
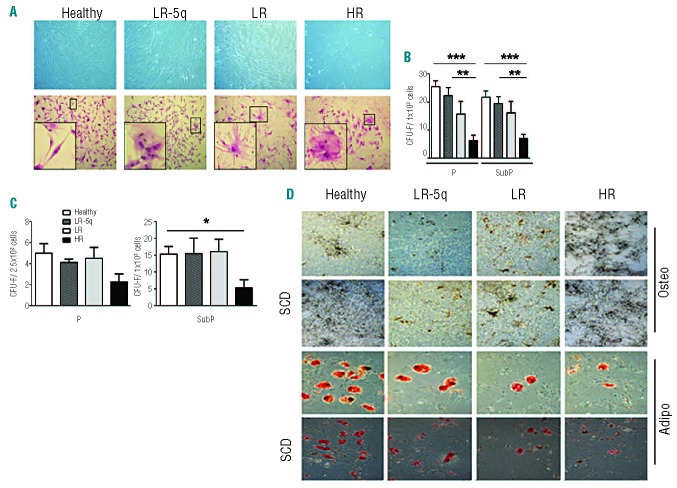
Compared to MSC from healthy age-matched controls, those from MDS patients presented altered morphology and in some MDS cases appeared disorganized (Figure 1A). They were clonal and able to generate SCD colonies; however, both LR- and HR-MSC displayed reduced numbers of colonies, while all groups presented conserved self-renewal (Figure 1B,C). The studied cells were positive for known MSC markers (CD90, CD73, and CD105) and negative for the hematopoietic markers CD34 and CD45 (Online Supplementary Figure S1). Interestingly, CD105 expression was lower in HR-MSC than in LR-5q-MSC (P=0.0162) (Online Supplementary Figure S2). MSC derived by plastic adherence and by SCD plating had altered potential for osteogenic differentiation compared to those of healthy donors (LR-5q-MSC 49%, LR-MSC 69%, and HR-MSC 96%). Cell-specific alkaline phosphatase activity measurement confirmed this observation (Figure 1D, Online Supplementary Figure S2). Adipogenic differentiation potential was also evidenced using oil red staining and was 9-fold higher in MSC from healthy subjects than in MSC from LR-5q patients, 37-fold higher than LR-MSC, and 22-fold higher than HR-MSC. This difference in adipogenic differentiation potential was reflected by the mRNA expression levels of FABP4 (Figure 1D, Online Supplementary Figure S2). LEN treatment did not affect any of the aforementioned characteristics in healthy and MDS-MSC (data not shown).
Figure 1.
MSC originated from MDS patients and healthy donors possess clonality and have different characteristics. (A) MSC are characterized by increased frequency of cells with a dysplastic appearance (lower panel, Giemsa staining 10× magnification, insets showing a cell in detail) and produce disorganized stromal layers (upper panel, 10× magnification). (B) MSC are able to generate fibroblastic colonies across two passages (P, and SubP) in all studied groups. (C) MSC can produce single cell derived (SCD) colonies; these colonies retain self-renewal and clonogenic potential (SubP-SCD). (D) MSC derived by plastic adherence and by SCD plating have distinct degrees of osteogenic and adipogenic potential, as seen by van Kossa staining (first 2 rows, 20× magnification) and by oil red staining (second 2 rows, 20× magnification). Results are expressed as mean ± SEM of independent cases, for (B) numbers were Healthy=6, LR-5q=6, LR=6 and HR=5; for (C) n=4 for all groups and n=3 for SubP. Significance was set as *P≤0.05; **P≤0.005; ***P≤0.001.
Proliferation of myelodysplastic syndrome-mesenchymal stromal cells is disturbed as a result of increased senescence
MSC from healthy donors could be expanded from 5×104 seeded cells to 1.42±0.22×105 cells after 14 days of culture; this number was similar to that reached by LR-5q-MSC (1.29±0.18×105), and higher than that of LR-MSC (8.39±0.1.54×104, P<0.01) and HR-MSC (6.13±1.92×104, P<0.001; Figure 2A, Online Supplementary Figure S3). Pulse labeling with 5-ethynyl-2′-deoxyuridine for 96 h showed reduced short-term proliferation while tracking of CFSE confirmed the impaired growth during the whole culture period (14 days) (Online Supplementary Figure S3). There were no differences between groups at any studied time point for cell viability as measured by trypan blue exclusion (data not shown) and annexin V+ cells (Figure 2B). β-Gal staining of MSC showed a significantly higher percentage of senescent MSC from MDS patients (Figure 2C). The described proliferation defects were compatible with an increased percentage of G1-arrested cells in LR and HR (Online Supplementary Figure S3). LEN treatment did not improve the proliferation impairment of MDS-MSC (measured by MTT assay) and had no influence on the percentage of apoptotic cells (Online Supplementary Figure S4).
Figure 2.
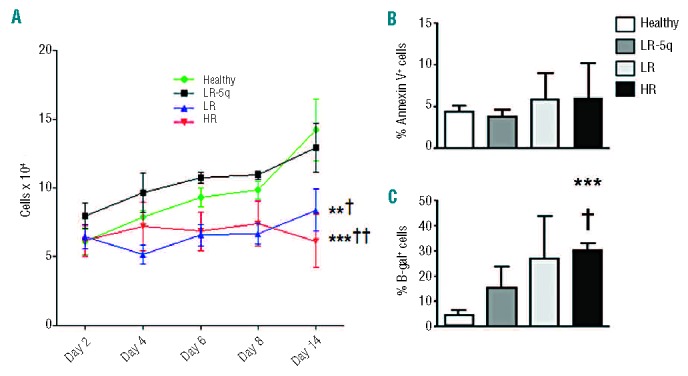
Proliferation of MDS-MSC is impaired. (A) Proliferation curves for each group were determined by seeding 5×104 cells and measuring cell number at days 2, 4, 6, 8, and 14. (B) Apoptosis of MSC after 14 days of culture was determined by annexin V staining. (C) Senescence of MSC was demonstrated by β-galactosidase (β-gal) staining after 48 h of culture of 1×103 cells per donor. Results are expressed as mean ± SEM of independent cases. For (A) numbers were Healthy=6, LR-5q=6, LR=6 and HR=5; for (B) Healthy n=6, LR-5q n=9, LR n=3 and HR n=3; (C) all groups n=4. Significance was set as */P≤0.05; **/††/‡‡P≤0.005; ***P≤0.001.
Specific adhesion molecule profiles in myelodysplastic syndrome groups
The interaction of MSC with HSPC is mediated by adhesion molecules expressed on their surface; we, there-fore, studied molecules relevant to intercellular interactions in hematopoietic homeostasis. Expression (mean fluorescence intensity, MFI) of CD146 was significantly higher in LR-5q-MSC (P=0.0449) than in MSC from healthy donors; higher levels of CD166 and CD29 were expressed in LR-MSC (P=0.0005 and P=0.0115, respectively) compared to healthy controls. The MFI of CD49e in LR-5q-MSC was higher than in HR-MSC (P=0.003); CD44 and CD54 were not expressed in healthy donors, LR-MSC or HR-MSC (MFI<5) while LR-5q-MSC were positive for these molecules (Online Supplementary Figure S5). LEN treatment did not modify the expression of adhesion molecules (data not shown).
Reduced provision of hematopoietic niche cytokines by myelodysplastic syndrome-mesenchymal stromal cells and its modulation by lenalidomide
Cytokines secreted by MSC control the fate and behavior of HSPC; alterations of their levels might result in defective support of hematopoiesis and contribute to malignancy. We investigated three important niche cytokines: SDF-1α, SCF, and ANG-1. The relative mRNA expression of SCF and SDF-1α was lower in all MDS groups (albeit only statistically significantly so for SCF: LR-5q-MSC P=0.015; LR-MSC P=0.005; HR-MSC P=0.16) while ANG1 expression was only slightly reduced in LR-MSC and HR-MSC compared to in healthy donors (Figure 3A); LEN treatment did not affect mRNA expression of the cytokines (data not shown). The concentration of SCF in MSC-supernatant was below the level of detection in all groups (data not shown). ANG1 secretion was higher in LR-5q-MSC than in other MDS groups (P=0.02 versus LR-MSC, P=0.0002 versus HR-MSC); HR-MSC also presented with a lower secretion compared to healthy MSC (P=0.0008) and LR-MSC (P<0.0001) (Figure 3B). We did not detect changes in ANG1 secretion during LEN treatment (data not shown). SDF-1α levels in supernatant from MDS-MSC were lower than in healthy donors (healthy 450.8±63.36 pg/μL; LR-5q-MSC 275±30.96 pg/μL; LR-MSC 264.4±46.15 pg/μL; and HR-MSC 297.1±58.12 pg/μL) (Figure 3C). The two concentrations of LEN used to treat MSC affected SDF-1α levels by further reducing them 1.52- and 1.86-fold in healthy donors, 1.68- and 5.83-fold in LR-5q-MSC, 1.37- and 1.81-fold in LR-MSC, and 1.16- and 1.7-fold in HR-MSC (Figure 3C-1). The effect of LEN treatment was observed up to 72 h after removal of the drug (Figure 3C-2). A reduced SDF-1α concentration in supernatant resulted in less migration of normal CD34+ cells when used as chemoattractant and MSC priming by LEN caused a further reduction of SDF-1α and migration (Figure 3C,D). The specificity of the investigated chemokine-receptor interaction was confirmed by using the CXCR-4 (SDF-1α receptor) antagonist AMD3100, resulting in equally reduced migration in experiments with a high concentration of LEN (and the lowest SDF-1α concentration) and experiments with or without LEN and antagonist (Figure 3D).
Figure 3.
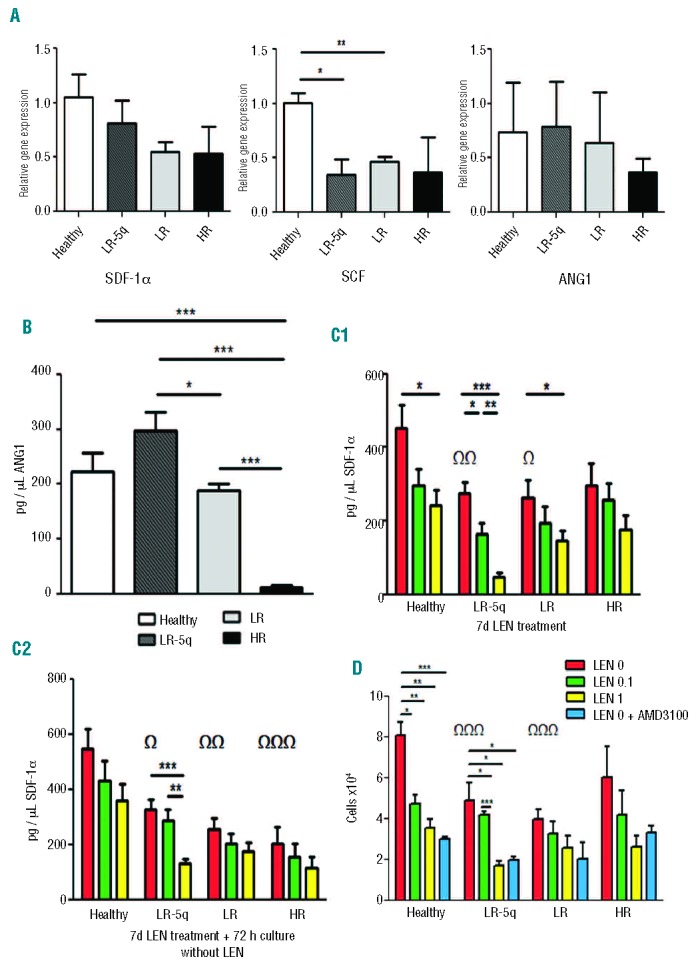
MSC provision of hematopoietic niche cytokines is altered in MDS. (A) RT-PCR with ABL-1 as reference gene and RNA extracted from MSC cultured for 14 days was used to determine the relative gene expression of SDF-1α, ANG1 and SCF. (B) ANG-1 concentration in MSC-supernatant (SN) was measured with an ELISA. (C) SDF-1α secretion by MSC with and without LEN treatment was measured with an ELISA on MSC-SN after 7 days of culture (C1) or 7 days culture + wash + 72 h of further culture without drug (C2). (D) The previously shown defect of SDF-1α secretion was functionally coupled to reduced migration of CD34+ healthy cells towards MSC-SN collected 72 h after washing and culture without drug; to show dependence of the effect on the SDF-1α/CXCR4 axis, AMD300 (CXCR4 inhibitor) was used at a concentration of 10 μM. Results are expressed as mean ± SEM of fold expression compared to controls which were set to 1 of independent cases. For (A) the numbers were Healthy=3, LR-5q=4, LR=4 and HR=4 for SDF-1 α and n=3 for all groups for SCF and ANG1; for (B) n=4 for all groups; for (C) the numbers were Healthy=6, LR-5q=6, LR=5 and HR=5 in (C1) and Healthy=4, LR-5q=4, LR=3 and HR=4 in (C2); for (D) n=3 for all groups. ΩIndicates difference between healthy and MDS groups without treatment. Significance was set as */ΩP≤0.05; **/Ω ΩP≤0.005; ***/Ω Ω ΩP≤0.001.
Reduced support of primitive healthy hematopoietic stem and progenitor cells by myelodysplastic syndrome-mesenchymal stromal cells is modulated by lenalidomide
HSPC purified from the peripheral blood of healthy donors were supported to a lesser extent by MDS-MSC, as shown by a CAF-C assay (healthy versus LR-5q-MSC P≤0.0001; versus LR-MSC P=0.0026; versus HR-MSC P≤0.0001), and co-culture of HSPC with HR-MSC led to significantly reduced numbers of CAF-C when compared to other MDS subgroups (Figure 4). Priming of MSC with LEN produced further CAF-C reductions in all groups: healthy donors, 1.45- and 2.08-fold reductions; LR-5q-MSC, 2.09- and 6.67-fold reductions; LR-MSC, 1.53- and 1.69-fold reductions, and HR-MSC, 1.79- and 1.95-fold reductions (Figure 4).
Figure 4.
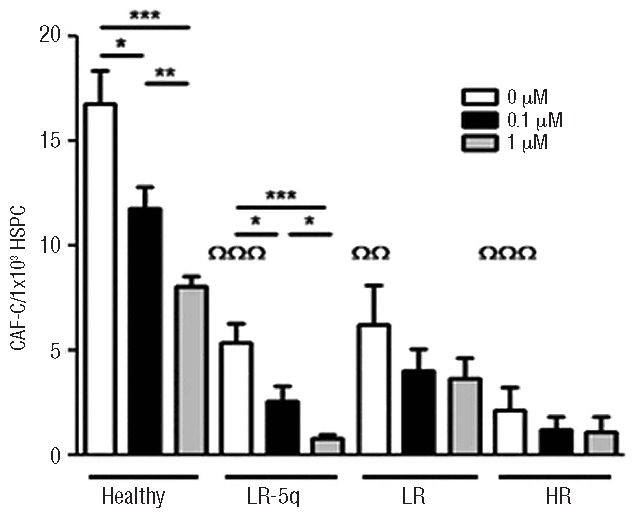
Hematopoietic support by MDS-MSC is impaired and affected by LEN treatment. CAF-C numbers were determined after seeding 1×103 CD34+ healthy cells on MSC layers with or without LEN conditioning and co-culture for 28 days. Results are expressed as mean ± SEM of independent cases. Numbers were Healthy=6, LR-5q=6, LR=5 and HR=5. ΩIndicates difference between healthy and MDS groups without treatment. Significance was set as *P≤0.05; **/ΩΩP≤0.005; ***/ΩΩΩP≤0.001.
Myelodysplastic syndrome-mesenchymal stromal cell support of expanding normal clonogenic hematopoietic progenitors is defective and influenced by lenalidomide
MSC not only support primitive HPSC but also provide signals to these cells for fate determination and expansion of differentiating progeny. We performed CFU assays with HSPC in the presence of MSC. HSPC co-cultured with healthy donors’ MSC for 14 days resulted in greater expansion of all types of CFU as compared to HSPC without co-culture (Figure 5). Compared to HSPC alone, co-culture with LR-MSC resulted in increased numbers of granulocytic colonies (CFU-G, 1.45-fold) and granulocyte-macrophage colonies (CFU-GM, 3.93-fold), with LR-5q-MSC there was a 1.36-fold increase in CFU-G and co-culture with HR-MSC generated a modest increase of macrophage colonies (CFU-M 1.16-fold) (Figure 5). LEN pre-treatment of MSC led to a further increase of CFU-GME in the healthy group, a significant rescue of all types of colonies in the LR-5q-MSC co-cultures, and expansion of BFU-E and CFU-E in LR-MSC, and an increase of CFU-GM was observed when LEN pre-treated HR-MSC were used (Online Supplementary Figure S6).
Figure 5.
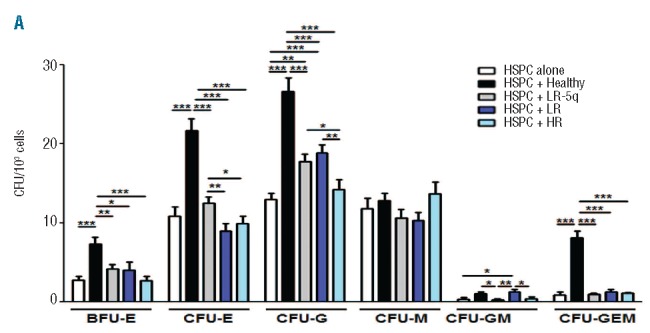
Support of expansion of healthy clonogenic progenitors by MDS-MSC is defective and modified by LEN. MSC were seeded on 35 mm plates and used for co-culture with 1×103 healthy CD34+ cells using semi-solid media. After 14 days of co-culture, hematopoietic colony output was determined under an inverted microscope; the figure shows differences of colony type and numbers of each studied group and compares them to the baseline CFU-GEMM potential of the HSPC on an assay without stroma present. Results are expressed as mean ± SEM of independent cases. Numbers were HSPC alone n=3, Healthy n=5, LR-5q n=6, LR n=5 and HR n=5. Significance was set as * P≤0.05; **P≤0.005; *** P≤0.001.
Myelodysplastic syndrome-mesenchymal stromal cell contact induces differential apoptotic and proliferative responses in healthy hematopoietic stem and progenitor cells and leukemic blasts
The bone marrow of MDS patients contains a mixture of healthy and malignant cells. Their proliferation and apoptosis might be differentially affected by stroma contact and the cytokine microenvironment with TNF-α being the most important player.19 Proliferation as measured by CFSE staining of healthy HSPC was increased when in contact with MDS-MSC, markedly so in LR-MSC and HR-MSC compared to healthy donors’ stroma, and was unaffected by the addition of TNF-α or LEN (data not shown, Figure 6A). In terms of apoptosis of HSPC, stroma contact without addition of TNF-α did not produce an effect irrespective of MSC group (data not shown). The addition of TNF-α led to increased apoptosis of leukemic cells (increased number of apoptotic cells in the fraction of cells detached from stroma) which increased by disease stage (healthy 14.68±2.59%; LR-5q-MSC 21.27±3.56%; LR-MSC 41.94± 6.8%; HR-MSC 65.36±7.1%) (Figure 6B). Pretreatment of MSC with LEN did not significantly increase the sensitivity of HSPC and KG1-α to TNF-α (Figure 6B and data not shown).
Figure 6.
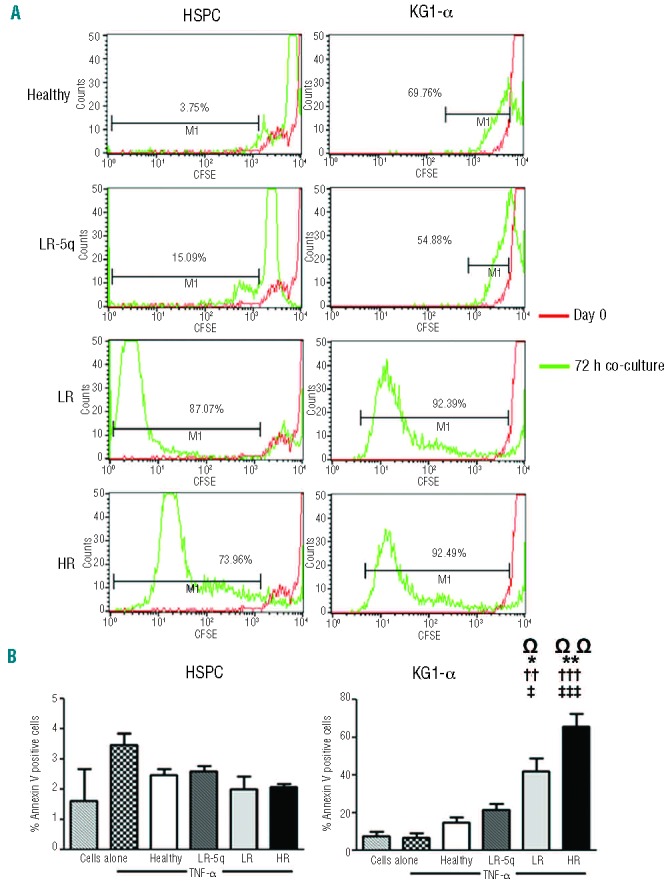
MSC from MDS patients induce different apoptotic and proliferative responses on healthy HSPC and KG1-α leukemic cells. (A) Proliferation of CD34+ healthy cells and KG1-α cells was determined using CFSE staining and co-culture with stroma with or without addition of 100 nM of TNF-α measuring the dilution of the dye after 72 h by flow cytometry. The histograms show the percentage of cells that diluted CFSE beyond the level at day 0 of representative examples in the non-adherent fraction after co-culture with TNF-α (results were similar in all fractions regardless of cytokine presence). (B) Effect on apoptosis after co-culture with stroma was only evident in KG1-α cells and in the fraction of cells that after 24 h were not attached to the MSC layers; the percentage of annex-in+ cells is shown for both HSPC and KG1-α in the non-adherent fraction (apoptosis was similarly low to that of cells without co-culture in all fractions with HSPC and in the adherent fraction of KG1-α). Ω indicates differences between cells alone with TNF-α and cells co-cultured with stroma. Results are expressed as mean ± SEM of independent cases. For (A) numbers were Healthy=6, LR-5q=6, LR=5 and HR=5; for (B)n=3 for HSPC experiments and n=4 for KG1-α for all groups. Significance was set as */‡/ΩP≤0.05; **/††/ΩΩP≤0.005; †††/‡‡‡P≤0.001.
Discussion
Primary MSC from MDS patients at different stages of disease have not been systematically characterized so far and this characterization was, therefore, the goal of our current study. The biology of MDS subtypes according to current classification systems is different and we hypothesized that MSC from patients at distinct stages of the disease might have variable functional properties. The clinical observation that some cases of LR-MDS, including patients with single del(5q), respond to LEN treatment was of special interest for our work. We, therefore, tried to investigate whether this agent modulates MSC from MDS patients differentially.
Although MSC do not seem to be part of the malignant clone,20 targeted deletion of parts of the microRNA processing machinery in cells of the osteogenic lineage has been shown to be sufficient to induce clonal hematopoietic disorders including MDS and leukemia in an animal model.10 CFU-F assays showed that MDS-MSC were capable of generating colonies even at very low density seeding, proving that single cells possess clonality. The generated colonies had self-renewal and differentiation potential. Sarugaser et al. demonstrated that seeding 0.2 cells per well isolates SCD clones with similar capacities in vitro and in vivo.21 To our knowledge, this cloning strategy has now been applied for the first time in MSC from MDS patients. Overall, clonality and proliferation of LR- and HR-MSC was reduced, partially in line with the findings of Klaus et al. as well as others. However, these authors did not study cases in relation to any specific disease group.22,23 In other studies it was shown that MDS-MSC have reduced proliferative capacity although the investigators succeeded in expanding them in vitro.22,24,25 Two studies by Aanei et al. demonstrated again that MDS-MSC present intrinsic growth deficiencies that are related to focal adhesion protein abnormalities; the MSC from patients with refractory cytopenia (mostly LR-MDS patients) had reduced proliferation capacity while cells from patients with an excess of blasts (HR-MDS patients) had increased proliferation.26,27 This differs from our observations and may be explained by differences in the patients’ characteristics, cell sorting strategy, and culture conditions.
MDS-MSC were positive (>90%) in all cases for CD73, CD90 and CD105; these markers are homogeneously expressed on MSC and useful for robust in vitro assays.28,29 However the MSC derived from patients with HR-MDS had a significantly reduced expression of CD105. Campioni et al. demonstrated that reduced CD105 and CD90 expression in MSC from patients with hematologic malignancies is related to increased angiogenesis, a feature of HR-MDS.30 Our findings are in agreement with those of Lopez-Villar but these investigators did not report on distinct MDS subgroups.24
MDS-MSC showed various degrees of osteogenic differentiation, while the adipogenic potential was significantly lower in all MDS groups than in healthy controls. Mellibovsky et al. showed that MDS patients have a typical adynamic bone with decreased mineral deposition31 and a recent study by Raaijmakers demonstrated how Dicer1 deletion in mice osteoprogenitors leads to bone dysfunction and development of MDS that progresses to AML.10 Varga et al.25 previously described similar defective adipogenic differentiation in MDS-MSC. Defects of differentiation potential of MSC might have a role in MDS pathophysiology.
The support that MDS-MSC provided HSPC was impaired. CAF-C (representing primitive HSPC) numbers were reduced in co-cultures with all MDS groups as was the short-term expansion of committed CD34+ progenitors (CFU-GEMM). Previous reports state that MDS-MSC are able to sustain the growth of healthy HSPC, but the investigators did not provide a detailed analysis of different clonogenic precursor subsets according to MDS groups.20,23,32,33 Varga et al. showed that MDS-MSC provide reduced hematopoietic support.25 Regarding the influence of MSC on CD34+ cell differentiation, we provide the first evidence of a specific deficiency of MDS-MSC correlating with disease category. It remains to be investigated which pathways are involved in the dysregulated induction of differentiation.
SDF-1α secretion by MDS-MSC was reduced and functionally coupled with lower induction of migration of normal CD34+ cells. Expression of the gene for SDF-1α has been reported to be decreased in MDS-MSC.24 Interestingly, a leukemia mouse model showed that leukemic infiltration of the bone marrow is accompanied by a reduction of SDF-1α activity.34 Another mouse model suggests that SDF-1α knock-down in stromal cells results in reduction of long-term HSPC and an increased progenitor pool;35 this might be reflected in the case of our patients’ cells by reduced CAF-C numbers. An imbalance of SDF-1α in MDS-MSC might be involved in the proliferation defects of MSC and the increased senescence of these cells: decreased SDF-1α gene expression in MSC or disturbances in its intracellular pathways leads to impaired cell survival, cytoskeletal disorganization and defective differentiation.36,37
SCF gene expression was down-regulated in MDS-MSC. It has been reported that SCF levels are elevated in bone marrow plasma of MDS patients, but with no statistical significance and without identification of the specific source of the cytokine.38,39 It has been demonstrated that loss of cells expressing SCF in the perivascular niche leads to a reduction of HSPC with long-term repopulating capability.40 ANG1 expression was also reduced in HR-MSC. This cytokine is responsible for the maintenance of HSPC quiescence,15 and its lower level in this MDS subset might also explain the reduced support of HSPC.
Adhesion molecules play a pivotal role in MSC-HSPC communication. CD146 (melanoma cell adhesion molecule, MCAM) was up-regulated in LR-5q-MSC. Interestingly, we have recently shown that CD146 expression in MSC participates in HSPC support.41 It could be speculated that a higher expression of CD146 in this group represents a compensatory mechanism in response to stress hematopoiesis. CD166 expression was up-regulated in LR-MSC and HR-MSC. This is the first report on CD166 expression in MDS-MSC. CD166 (activated leukocyte cell adhesion molecule, ALCAM) has been related to increased aggressiveness of solid tumors,42 but its significance in the context of MDS requires further studies. CD29 (integrin β-1) MFI was also elevated in LR-MSC and HR-MSC; MSC overexpression of this molecule is related to increased proliferation of HSPC and loss of primitiveness.43 Only LR-5q-MSC expressed CD44 (cell surface glycoprotein) and CD54 (intercellular adhesion molecule 1) and had the highest expression of CD49e (integrin α-5). Low expression of CD44 and CD49e is related to low proliferation on MDS-MSC,26 as is in our case with LR-MSC and HR-MSC. Moreover, CD44 expression has been shown to be negative in normal MSC.44
Increased apoptosis of hematopoietic progenitors is a prominent feature of MDS and is mainly mediated by TNF-α; in this regard It has been shown that healthy and MDS-MSC are able to induce apoptosis in KG1-α cells in the presence of TNF-α, while normal CD34+ cells are not affected by this combination.45 Nevertheless, the apoptosis and proliferation profiles of healthy HSPC and KG1-α cells in contact with MSC from different MDS subsets have never been studied. We showed that both healthy and KG1-α cells exhibit excessive proliferation with stroma contact, especially with LR-MSC and HR-MSC, regardless of the presence of the cytokine, while stroma contact and TNF-α only affect leukemic cells increasing their rate of apoptosis, markedly so in the LR-MSC and HR-MSC groups. The increased proliferation of HSPC in short-term cultures might explain our findings of impaired support of CAF-C during longer periods of culture. The suspected stromal dysfunction might in part also explain the paradoxical finding of hypercellularity in patients’ bone marrow despite peripheral pancytopenia, and could contribute to oncogenesis by inducing genetic instability in HSPC.7,19,35
LEN has been successfully used to treat MDS patients with IPSS low to intermediate risk, and to a greater extent in those presenting with del(5q). We show that this therapeutic agent does not affect the clonality, vitality, growth and differentiation potential of MDS-MSC and MSC from age-matched healthy individuals. We and others have reported the absence of toxicity of this drug on healthy MSC or other non-malignant cells.46 The influence of LEN on the expression of cell surface markers was also investigated; pharmacological concentrations of the drug did not affect the phenotype of MSC. Regarding the differentiation potential of MSC, Munemasa et al. tested LEN and other IMiD® on MSC osteogenic potential and found no increase or impairment when using the drug.47
LEN incubation with MSC further decreased SDF-1α secretion in all groups (significantly in LR-5q-MSC and LR-MSC), and this could be translated functionally into reduced CAF-C numbers. This effect was described in healthy MSC by our group.18 It might contribute to the cytopenia found in patients treated with LEN but at the same time it could be beneficial by increasing egress of malignant dormant cells from the niches and increasing their sensitivity to cytotoxicity of the drug;48 in a murine model of acute promyelocytic leukemia, blocking the SDF-1α receptor resulted in sensitization of blasts to cytarabine and prolonged survival of the mice.49 Interestingly, LEN administration before the expansion of clonogenic CD34+ cells generated an increased number of erythropoietic and granulocytic colonies in the healthy group, a global rescue of CFU output in LR-5q-MSC co-culture, and an improvement of CFU-E and BFU-E in CD34 cells cultured on LR-MSC. Anemia is reversed in a high percentage of patients treated with LEN, and transfusion independence is reached; Ximeri et al. found that after 24 weeks of LEN administration, bone marrow from MDS patients contained an increased proportion of erythroid progenitors and the clonogenic potential of myeloid colonies was increased.50 Since the intercellular crosstalk and soluble factors responsible for the support of CAF-C and CFU-GEMM are different, the influence of LEN on MSC support for each population may be diverse.
In conclusion, our work suggests that MSC from patients with MDS are functionally different from those of healthy donors, and that MDS subcategories are each characterized by specific biological properties. In addition, the expression of adhesion molecules and chemokines by MSC from MDS patients seem to be altered leading to a defect in the migratory capacity and support of HSPC. LEN was able to modulate important functions of MSC. These findings call for further investigations to clarify whether the functional defects in MSC have a definitive role in the pathogenesis of MDS and, if so, whether this might open new alternatives for therapeutic interventions.
Acknowledgments
The authors would like to thank Dr. Ute Hempel and the Institute of Physiological Chemistry, Medical Faculty Dresden (MFD), for help with ALP measurements; Mrs. Katrin Müller, Mrs. Kristin Heidel, Mrs. Ivonne Habermann, and Mrs. Anja Liebkopf from University Hospital Dresden, and Mrs. Bärbel Löbel, Institute of Immunology MFD, for excellent technical support; Mrs. Henriette Friedrich MD and Mr. Mohamed Shosha MD for assistance with healthy donors; Dr. Ulrich Germing and the LEMON5 trial for providing samples from del(5q) patients; and Mr. Abhishek Dhawan M.Sc. for language editing, scientific discussion and helpful comments.
Footnotes
The online version of this article has a Supplementary Appendix.
Funding
This work was supported by a grant from the DFG: SFB 655 (GE, MB, LCH, UP) and by the DFG Research Group-1586 SKELMET (LCH, MB and MW). The work was also supported by the BMBF-funded Consortium Therapeutic Potential of Mesenchymal Stem Cells (M Schmitz and MB).
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Vardiman J. The classification of MDS: from FAB to WHO and beyond. Leuk Res. 2012;36(12):1453–8 [DOI] [PubMed] [Google Scholar]
- 2.Nimer SD. Myelodysplastic syndromes. Blood. 2008;111(10):4841–51 [DOI] [PubMed] [Google Scholar]
- 3.Bejar R, Levine R, Ebert BL. Unraveling the molecular pathophysiology of myelodysplastic syndromes. J Clin Oncol. 2011;29(5):504–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.List A, Dewald G, Bennett J, Giagounidis A, Raza A, Feldman E, et al. Myelodysplastic Syndrome-003 Study Investigators Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. N Engl J Med. 2006;355(14):1456–65 [DOI] [PubMed] [Google Scholar]
- 5.Raza A, Reeves JA, Feldman EJ, Dewald GW, Bennet JM, Deeg HJ, et al. Phase 2 study of lenalidomide in transfusion-dependent, low-risk, and intermediate-1 risk myelodysplastic syndromes with karyotypes other than deletion 5q. Blood. 2008;111(1):86–93 [DOI] [PubMed] [Google Scholar]
- 6.Ishibashi M, Tamura H, Ogata K. Disease progression mechanism in myelodysplastic syndromes: insight into the role of the microenvironment. Leuk Res. 2011;35(11): 1449–52 [DOI] [PubMed] [Google Scholar]
- 7.Raza A, Cruz R, Latif T, Mukherjee S, Galili N. The biology of myelodysplastic syndromes: unity despite heterogeneity. Hematol Rep. 2010;2(1):e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raaijmakers MH. Niche contributions to oncogenesis: emerging concepts and implications for the hematopoietic system. Haematologica. 2011;96(7):1041–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li L, Neaves WB. Normal stem cells and cancer stem cells: the niche matters. Cancer Res. 2006;S 66(9):4553–7 [DOI] [PubMed] [Google Scholar]
- 10.Raaijmakers MH, Mukherjee S, Guo S, Zhang S, Kobayash T, Schoonmaker JA, et al. Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. Nature. 2010;464(7290):852–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walkley CR, Olsen GH, Dworkin S, Fabb SA, Swann J, McArthur GA, et al. A microenvironment-induced myeloproliferative syndrome caused by retinoic acid receptor gamma deficiency. Cell. 2007;129(6):1097–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deeg HJ. Marrow stroma in MDS: culprit or bystander? Leuk Res. 2002;26(7):687–8 [DOI] [PubMed] [Google Scholar]
- 13.Omatsu Y, Sugiyama T, Kohara H, Kondoh G, Fujii N, Kohno K, et al. The essential functions of adipo-osteogenic progenitors as the hematopoietic stem and progenitor cell niche. Immunity. 2010;33(3):387–99 [DOI] [PubMed] [Google Scholar]
- 14.Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466(7308):829–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lo Celso C, Scadden DT. The hematopoietic stem cell niche at a glance. J Cell Sci. 2011; 124:3529–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao S, Wehner R, Bornhäuser M, Wassmuth R, Bachmann M, Schmitz M. Immunomodulatory properties of mesenchymal stromal cells and their therapeutic consequences for immune-mediated disorders. Stem Cells Dev. 2010;19(5):607–14 [DOI] [PubMed] [Google Scholar]
- 17.Zhao Z, Wang Z, Li Q, Li W, You Y, Zou P. The different immunoregulatory functions of mesenchymal stem cells in patients with low-risk or high-risk myelodysplastic syndromes. PLoS One. 2012;7(9):e45675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wobus M, Benath G, Ferrer RA, Wehner R, Schmitz M, Hofbauer LC, et al. Impact of lenalidomide on the functional properties of human mesenchymal stromal cells. Exp Hematol. 2012;40(10):867–76 [DOI] [PubMed] [Google Scholar]
- 19.Kerbauy DB, Deeg HJ. Apoptosis and anti-apoptotic mechanisms in the progression of myelodysplastic syndrome. Exp Hematol. 2007;35(11):1739–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soenen-Cornu V, Tourino C, Bonnet ML, Guillier M, Flamant S, Kotb R, et al. Mesenchymal cells generated from patients with myelodysplastic syndromes are devoid of chromosomal clonal markers and support short- and long-term hematopoiesis in vitro. Oncogene. 2005;24(15):2441–8 [DOI] [PubMed] [Google Scholar]
- 21.Sarugaser R, Hanoun L, Keating A, Stanford WL, Davies JE. Human mesenchymal stem cells self-renew and differentiate according to a deterministic hierarchy. PLoS One. 2009;4 (8):e6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klaus M, Stavroulaki E, Kastrinaki MC, Fragioudaki P, Giannikou K, Psyllaki M, et al. Reserves, functional, immunoregulatory, and cytogenetic properties of bone marrow mesenchymal stem cells in patients with myelodysplastic syndromes. Stem Cells Dev. 2010;19(7):1043–54 [DOI] [PubMed] [Google Scholar]
- 23.Kastrinaki MC, Pontikoglou C, Klaus M, Stavroulaki E, Pavlaki K, Papadaki HA. Biologic characteristics of bone marrow mesenchymal stem cells in myelodysplastic syndromes. Curr Stem Cell Res Ther. 2011;6(2): 122–30 [DOI] [PubMed] [Google Scholar]
- 24.Lopez-Villar O, Garcia JL, Sanchez-Guijo FM, Robledo C, Villaron EM, Hernández-Campo P, et al. Both expanded and uncultured mesenchymal stem cells from MDS patients are genomically abnormal, showing a specific genetic profile for the 5q- syndrome. Leukemia. 2009;23(4):664–72 [DOI] [PubMed] [Google Scholar]
- 25.Varga G, Kiss J, Várkonyi J, Vas V, Farkas P, Pálóczi K, Uher F. Inappropriate Notch activity and limited mesenchymal stem cell plasticity in the bone marrow of patients with myelodysplastic syndromes. Pathol Oncol Res. 2007;13(4):311–9 [DOI] [PubMed] [Google Scholar]
- 26.Aanei CM, Flandrin P, Eloae FZ, Carasevici E, Guyotat D, Wattel E, et al. Intrinsic growth deficiencies of mesenchymal stromal cells in myelodysplastic syndromes. Stem Cells Dev. 2012;21(10):1604–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aanei CM, Eloae FZ, Flandrin-Gresta P, Tavernier E, Carasevici E, Guyotat D, et al. Focal adhesion protein abnormalities in myelodysplastic mesenchymal stromal cells. Exp Cell Res. 2011;317(18):2616–29 [DOI] [PubMed] [Google Scholar]
- 28.Jones E, McGonagle D. Human bone marrow mesenchymal stem cells in vivo. Rheumatology (Oxford). 2008;47(2):126–31 [DOI] [PubMed] [Google Scholar]
- 29.Halfon S, Abramov N, Grinblat B, Ginis I. Markers distinguishing mesenchymal stem cells from fibroblasts are downregulated with passaging. Stem Cells Dev. 2011;20 (1):53–66 [DOI] [PubMed] [Google Scholar]
- 30.Campioni D, Moretti S, Ferrari L, Punturieri M, Castoldi GL, Lanza F. Immunophenotypic heterogeneity of bone marrow-derived mesenchymal stromal cells from patients with hematologic disorders: correlation with bone marrow microenvironment. Haematologica. 2006;91(3):364–8 [PubMed] [Google Scholar]
- 31.Mellibovsky L, Diez A, Serrano S, Aubia J, Pérez-Vila E, Mariñoso ML, et al. Bone remodeling alterations in myelodysplastic syndrome. Bone. 1996;19(4):401–5 [DOI] [PubMed] [Google Scholar]
- 32.Deeg HJ, Appelbaum FR. Hematopoietic stem cell transplantation in patients with myelodysplastic syndrome. Leuk Res. 2000; 24(8):653–63 [DOI] [PubMed] [Google Scholar]
- 33.Deeg HJ, Beckham C, Loken MR, Bryant E, Lesnikova M, Shulman HM, et al. Negative regulators of hemopoiesis and stroma function in patients with myelodysplastic syndrome. Leuk Lymphoma. 2000;37(3–4):405–14 [DOI] [PubMed] [Google Scholar]
- 34.Colmone A, Amorim M, Pontier AL, Wang S, Jablonski E, Sipkins DA. Leukemic cells create bone marrow niches that disrupt the behavior of normal hematopoietic progenitor cells. Science. 2008;322(5909):1861–5 [DOI] [PubMed] [Google Scholar]
- 35.Tzeng YS, Li H, Kang YL, Chen WC, Cheng WC, Lai DM. Loss of Cxcl12/Sdf-1 in adult mice decreases the quiescent state of hematopoietic stem/progenitor cells and alters the pattern of hematopoietic regeneration after myelosuppression. Blood. 2011;117(2):429–39 [DOI] [PubMed] [Google Scholar]
- 36.Kortesidis A, Zannettino A, Isenmann S, Shi S, Lapidot T, Gronthos S. Stromal-derived factor-1 promotes the growth, survival, and development of human bone marrow stromal stem cells. Blood. 2005;105(10):3793–801 [DOI] [PubMed] [Google Scholar]
- 37.Herberg S, Fulzele S, Yang N, Shi X, Hess M, Periyasamy-Thandavan S, et al. Stromal cell-derived factor-1β potentiates bone morphogenetic protein-2-stimulated osteoinduction of genetically engineered bone marrow-derived mesenchymal stem cells in vitro. Tissue Eng Part A. 2013;19(1–2):1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Flores-Figueroa E, Montesinos JJ, Flores-Guzmán P, Gutiérrez-Espíndola G, Arana-Trejo RM, Castillo-Medina S, et al. Functional analysis of myelodysplastic syndromes-derived mesenchymal stem cells. Leuk Res. 2008;32(9):1407–16 [DOI] [PubMed] [Google Scholar]
- 39.Matsuda M, Morita Y, Hanamoto H, Tatsumi Y, Maeda Y, Kanamaru A. CD34+ progenitors from MDS patients are unresponsive to SDF-1, despite high levels of SDF-1 in bone marrow plasma. Leukemia. 2004;18(5):1038–40 [DOI] [PubMed] [Google Scholar]
- 40.Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481(7382):457–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stopp S, Bornhäuser M, Ugarte F, Wobus M, Kuhn M, Brenner S, Thieme S. Expression of the melanoma cell adhesion molecule in human mesenchymal stromal cells regulates proliferation, differentiation, and maintenance of hematopoietic stem and progenitor cells. Haematologica. 2013; 98(4):505–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tachezy M, Zander H, Marx AH, Stahl PR, Gebauer F, Izbicki JR, et al. ALCAM (CD166) expression and serum levels in pancreatic cancer. PLoS One. 2012;7(6):e39018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Walenda T, Bork S, Horn P, Wein F, Saffrich R, Diehlmann A, et al. Co-culture with mesenchymal stromal cells increases proliferation and maintenance of haematopoietic progenitor cells. J Cell Mol Med. 2010;14(1–2):337–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Quian H, Le Blanc K, Sigvardsson M. Primary mesenchymal stem and progenitor cells from bone marrow lack expression of CD44 protein. J Biol Chem. 2012; 287:25795–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mhyre AJ, Marcondes AM, Spaulding EY, Deeg HJ. Stroma-dependent apoptosis in clonal hematopoietic precursors correlates with expression of PYCARD. Blood. 2009; 113(3):649–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matsuoka A, Tochigi A, Kishimoto M, Nakahara T, Kondo T, Tsujioka T, et al. Lenalidomide induces cell death in an MDS-derived cell line with deletion of chromosome 5q by inhibition of cytokinesis. Leukemia. 2010;24(4):748–55 [DOI] [PubMed] [Google Scholar]
- 47.Munemasa S, Sakai A, Kuroda Y, Okikawa Y, Katayama Y, Asaoku H, et al. Osteoprogenitor differentiation is not affected by immunomodulatory thalidomide analogs but is promoted by low bortezomib concentration, while both agents suppress osteoclast differentiation. Int J Oncol. 2008;33(1):129–36 [PubMed] [Google Scholar]
- 48.Teicher BA, Fricker SP. CXCL12 (SDF-1)/CXCR4 pathway in cancer. Clin Cancer Res. 2010;16(11):2927–31 [DOI] [PubMed] [Google Scholar]
- 49.Nervi B, Ramirez P, Rettig MP, Uy GL, Holt MS, Ritchey JK, et al. Chemosensitization of acute myeloid leukemia (AML) following mobilization by the CXCR4 antagonist AMD3100. Blood. 2009;113(24):6206–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ximeri M, Galanopoulos A, Klaus M, Parcharidou A, Giannikou K, Psyllaki M, et al. Effect of lenalidomide therapy on hematopoiesis of patients with myelodysplastic syndrome associated with chromosome 5q deletion. Haematologica. 2010;95(3): 406–14 [DOI] [PMC free article] [PubMed] [Google Scholar]