Abstract
We evaluate the long-term results of a prospective clinical study enrolling more than 100 adult patients with Burkitt lymphoma/leukemia. Depending on extent of disease, treatment consisted of six to eight rituximab infusions and four to six courses of intensive chemotherapy (attenuated in patients aged >55 years) with high-dose methotrexate, fractionated ifosfamide/cyclophosphamide, other drugs in rotation, and intrathecal chemoprophylaxis. One-hundred five patients were treated (median age 47 years, range 17–78 years); 48% had Burkitt leukemia, 25% were older than 60 years, 37% had an Eastern Cooperative Oncology Group performance score >1, and 14% were positive for human immunodeficiency virus. The complete response rate and 3-year overall and disease-free survival rates were 79%, 67% and 75%, respectively, ranging from 100% to 45% for survival (P=0.000) and from 100% to 60% for disease-free survival (P=0.01) in patients with low, intermediate and high adapted International Prognostic Index scores. In multivariate analysis, only age (≤ versus >60 years) and performance status (0–1 versus >1) retained prognostic significance, identifying three risk groups with overall and disease-free survival probabilities of 88% and 87.5%, 57% and 70.5%, 20% and 28.5% (P=0.0000 and P=0.0001), respectively. The relapse rate was only 7% in patients treated with an intercycle interval ≤25 days. This regimen achieved 100% curability in patients with low adapted International Prognostic Index scores (21% of total), and very close to 90% in patients aged ≤60 years with performance score 0–1 (48% of total). Rapid diagnosis of Burkitt lymphoma/leukemia with prompt referral of patients to prevent clinical deterioration, and careful supervision of treatment without chemotherapy delay can achieve outstanding therapeutic results. ClinicalTrials.gov ID, NCT01290120
Introduction
Burkitt lymphoma and leukemia (BL) are highly aggressive lymphoid malignancies treated with chemotherapy regimens that differ from standard schedules1 for non-Hodgkin lymphoma and acute lymphoblastic leukemia and typically include methotrexate, cyclophosphamide/ifosfamide, cytarabine, and other drugs used in high cumulative doses and according to the principles of drug rotation, hyper-fractionation, and tight sequencing.2–7
Although survival rates in excess of 60% and up to 90% have been reported, a comparative analysis of trials is difficult because of small numbers of patients, age group disparity, and occasional exclusion of Burkitt leukemia patients or patients at high risk of death. Unlike more favorable series including patients with a median age of 35 years or younger, the Cancer and Leukemia Group B reported an overall survival rate of 50% with the B-NHL 86 regimen in 54 adults aged 18–71 years (median age 44 years) and noted significant toxicity.5 Similar results were obtained at the MD Anderson Cancer Center in 26 patients treated with hyper-CVAD who had a median age of 58 years (range, 17–79 years).4 Therefore, high patient age and age-related co-morbidity and organ dysfunction can severely impair patients’ ability to withstand intensive chemotherapy and favor therapy-related toxicity. Such patients are expected to rank high on scales of clinical performance and are best recognized in unselected cohorts from a multi-institutional setting, including human immunodeficiency virus (HIV)-positive patients who are well-documented epidemiological targets of the disease.
The German Multicenter Study Group for Adult ALL (GMALL) piloted a new short intensive rituximab-chemotherapy program (B-NHL 2002) that improved outcomes compared to those achieved with the B-NHL 86 regimen.8 In this and other recent trials rituximab was added to chemotherapy because of the strong CD20 antigen expression by tumor cells.9–12
The Northern Italy Leukemia Group (NILG) adopted the B-NHL 2002 regimen to treat a cohort of 105 consecutive, unselected adult patients with BL. The objective of the present study was to assess long-term outcome and toxicity according to pre-treatment risk factors in a high-risk population predominantly identified by higher age and/or performance status (PS) according to the Eastern Cooperative Oncology Group (ECOG) score.13
Methods
Patients and diagnosis
Adult patients with BL were treated with the GMALL BALL/NHL 2002 protocol8 with no restriction based on age or HIV status, as long as highly active anti-retroviral therapy was given concomitantly. The diagnosis of Burkitt lymphoma was based on histological examination according to the World Health Organization (WHO) criteria14 and included the Burkitt-like subtype, confirmed whenever possible by detection of the c-myc rearrangement using standard cytogenetics or fluorescence in situ hybridization. All cases fulfilled essential diagnostic criteria for BL, similarly to a prior analysis in 34 BL patients treated with ALL-type regimens.15 Burkitt leukemia was diagnosed when the bone marrow analysis showed more than 25% infiltration by Burkitt-type blasts.16 Clinical staging was performed according to the Ann Arbor system as in the original protocol.8 Other details are reported in Online Supplementary Material S1A.
Treatment protocol
The protocol was approved by the relevant Institutional Review Boards and registered at ClinicalTrials.gov with identifier NCT01290120 (Figure 1, Online Supplementary Material S1B). Patients aged 55 years and younger received the full dose program with courses A–C. Patients with stage III–IV disease or mediastinal and extra-nodal involvement received a total of six courses (A1-B1-C1-A2-B2-C2), whereas patients with stage I–II disease were scheduled for four cycles (A1-B1-C1-A2). Patients aged over 55 years received courses A and B only, with reduced drug doses as detailed in Online Supplementary Material S1B (A1-B1-A2-B2-A3-B3 or A1-B1-A2-B2). Fit patients older than 55 years of age could be treated with the full intensity protocol if judged appropriate by the responsible physician.
Figure 1.
Outline of the GMALL B-ALL/NHL2002 protocol (excluding prephase and rituximab administrations, the planned median intercycle time between chemotherapy day 1 of first and last A-B-C blocks was 23 and 22 days for the four and six cycle programs, respectively; other treatment details can be found in Online Supplementary Material S1B).
Response evaluation and definitions
Evaluation of the response to treatment included bone marrow analysis after the first course in leukemia patients and computed tomography (CT) scans after the second course in lymphoma patients. The final response was assessed 4 weeks after the end of treatment by repeating the CT scans, while positron emission tomography (PET) and magnetic resonance imaging were used in doubtful cases as clinically indicated. Treatment response was evaluated according to standard definitions of complete remission (CR), no response, early death, overall and disease-free survival (OS, DFS), cumulative incidence of relapse and treatment-related mortality (Online Supplementary Material S1C). Toxicity was evaluated according to the Common Toxicity Criteria scale, and the individual PS was assessed according to the ECOG definition.13 The adapted International Prognostic Index (aIPI),17 previously validated in BL by two independent studies18,19 ranged from low (0–1 risk factors) to intermediate (2–3 risk factors) and high (4–5 risk factors). Risk factors were age >60 years, elevated lactate dehydrogenase (LDH) level, ECOG PS >1, clinical stage III–IV, and involvement of >1 extranodal site.
Study endpoints and statistics
The primary study endpoint was treatment efficacy in a prospective cohort of at least 100 unselected patients. The main efficacy indicator was the 3-year OS rate, and CR, DFS, and cumulative incidence of relapse were additional outcome parameters. Prior data from similar programs without rituximab yielded an OS rate of 50% at 3–5 years in a comparable age group.2,5 Other study endpoints concerned prognosis and toxicity in relation to the characteristics of the patients and the disease. The statistical analysis is illustrated in Online Supplementary Material S1D.
Results
Patients
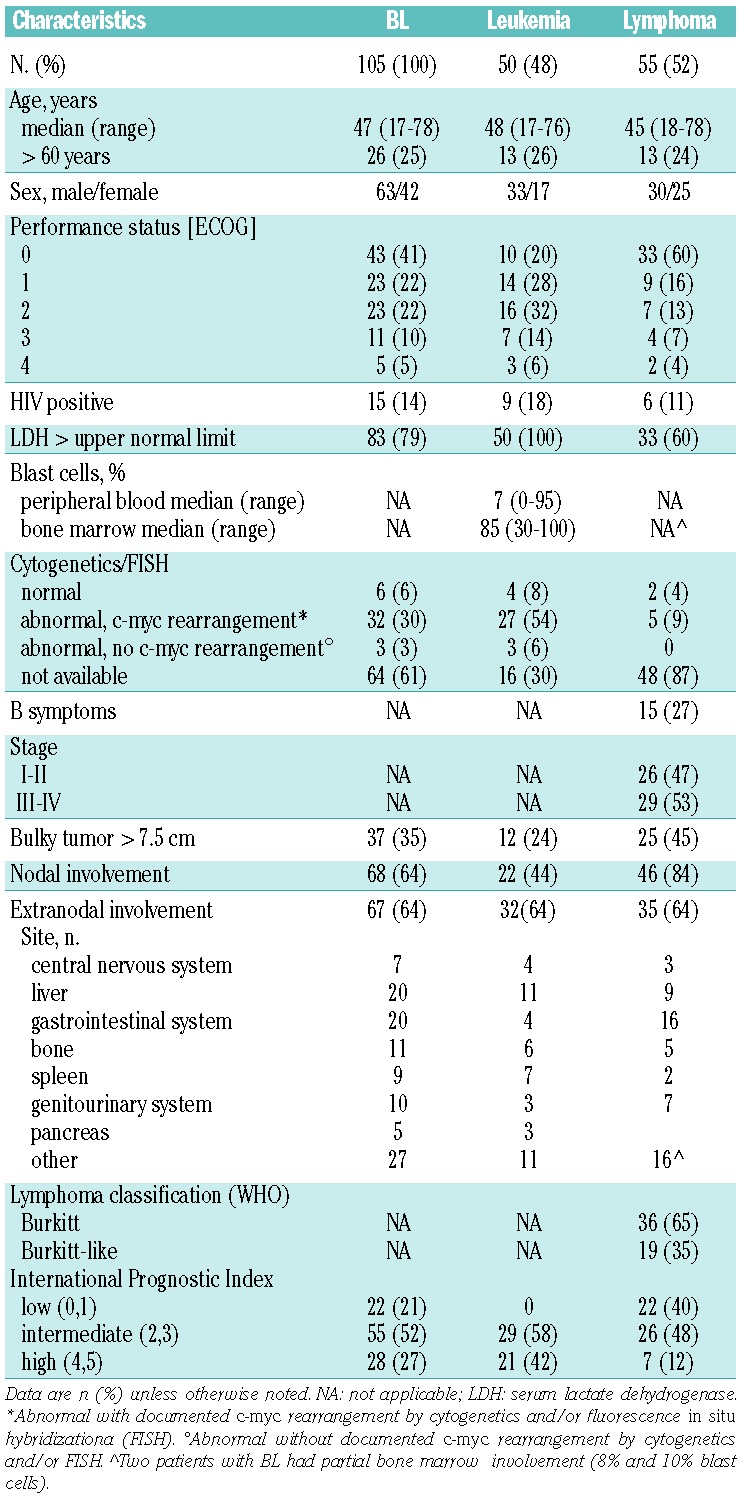
Between December 2002 and June 2010, 105 patients with BL were enrolled in the study (Table 1). The median age of the patients was 47 years, and 25% of patients were >55 years old. Among 55 patients with lymphoma, 53% had stage III–IV disease, 45% had bulky lesions, and 64% had extranodal involvement. In the total cohort, 14% of the patients were HIV-positive, 37% had an ECOG PS >1, 6.6% had central nervous system (CNS) involvement, 79% had an elevated LDH, and only 21% presented with a low aIPI.
Table 1.
Diagnostic characteristics of patients with Burkitt lymphoma and leukemia (BL).
Induction of complete remission
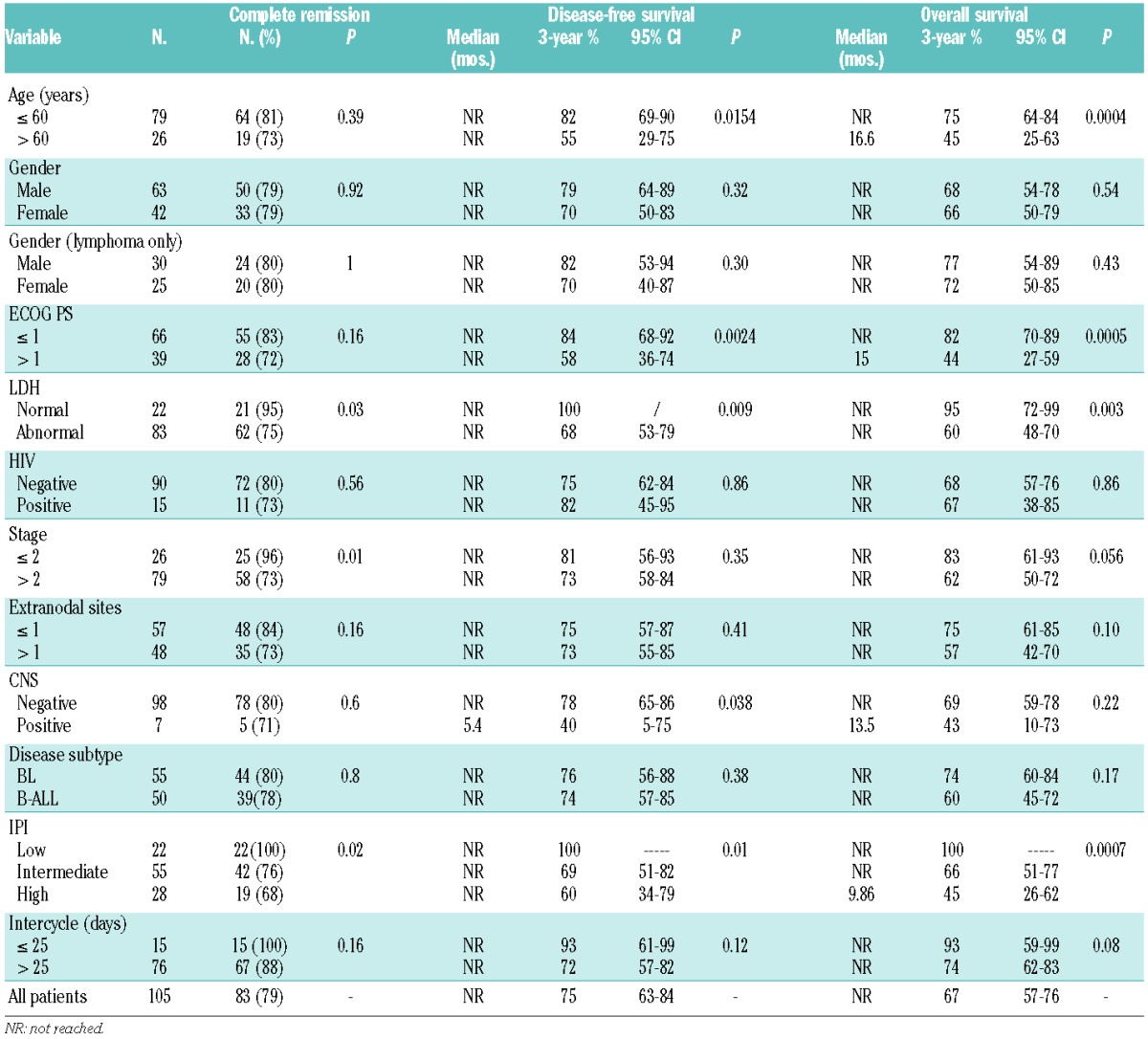
In accordance with the study design and individualized therapeutic options, 76 patients received the full intensity regimen (including five aged >55 years) and 29 received the attenuated regimen for patients older than 55 years (including one aged ≤55 years). Eighty-three patients (79%) achieved CR, 8 (8%) had no response and 14 (13%) died early. Early death occurred mostly in patients older than 45 years of age (n=12), correlated with a diagnosis of stage III–IV lymphoma or leukemia (n=13), a PS ≥1 (n=10), and was mainly caused by infections (Online Supplementary Material S2A). The probability of CR was lower in patients with elevated serum LDH (75% versus 95%, P=0.03; Table 2) and stage III–IV lymphoma (65% versus 96% in stage I–II, P=0.005).
Table 2.
Therapeutic outcome and univariate analysis of prognostic variables
Post-remission therapy
A total of 67 of the 83 patients (81%) who achieved CR received the complete planned therapy, including 15 patients scheduled to receive only four chemotherapy courses and 12 patients selected for additional radiotherapy (CNS n=2, mediastinum n=1, extranodal n=8). Reasons for study withdrawal were CR death (n=5), toxicity (n=6), early relapse (n=3), and other (n=2). The mean intercycle time (MIT) between chemotherapy courses, calculated from day 1 of the first course to day 1 of the last received course, was 29 days (range, 22–52 days), with no difference between age groups (≤55 years: median 29 days, range 22–51 days; >55 years: median 30 days, range 23–48; P=0.15).
Treatment dose reduction
Other than considering an increased MIT as a contributing factor for relapse, we analyzed the dose reductions reported in the 19 patients in whom therapy failed (Online Supplementary Material S2B). The majority of these patients were scheduled to receive six cycles (n=18, 95%) of standard (n=13, 68%) or reduced (n=6, 32%) intensity therapy. All eight unresponsive patients (one HIV+) received less therapy than planned (median 3 cycles, range 3–6) because of disease progression, with very few drug modifications in A1-B1 or subsequent cycles (5% or less). Among 11 relapsed patients (2 HIV+), only three had treatment curtailed to less than six planned cycles: a 66 year-old PS 3 leukemia patient (4 cycles, MIT 28 days and CR duration 3 months); a 78 year-old PS 4 lymphoma patient (3 cycles, MIT 48 days, CR duration 5.4 months); and a 45 year-old HIV+ PS 2 leukemia patient exhibiting very poor treatment tolerance (3 cycles, MIT 50.5 days, CR duration 3.6 months), this one also being the only patient aged <55 years to receive the attenuated treatment regimen. With regard to dose modifications, methotrexate and rituximab were omitted in three patients/six total induction courses (16%) but never in post-induction therapy, while partial reductions were reported in 10.5% and 11% of the same courses, respectively. Altogether, single drug reductions and omissions concerned 12 (13%) and 10 (11%) of 94 total courses administered to these 19 patients, respectively.
Long-term results
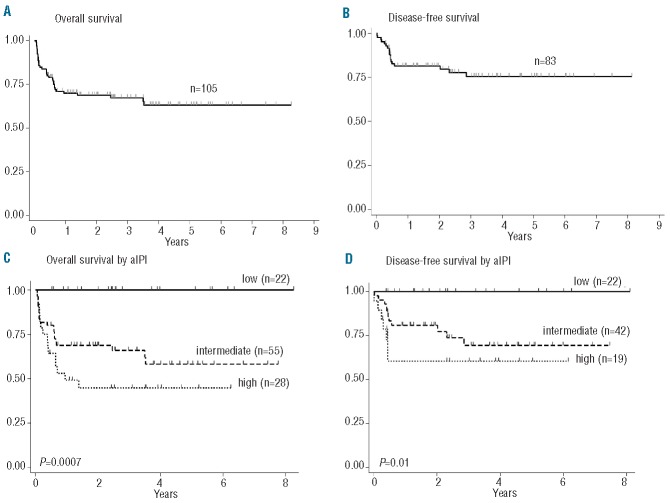
After a median follow-up of 23.8 months (range, 0.7–99 months), 65 patients (61%) were alive in first CR, 14 died of complications during induction, five during consolidation, and two late deaths were caused by secondary acute myeloid leukemia and a cardiovascular accident. Nineteen patients had refractory (n=8) or recurrent disease (bone marrow n=3, CNS n=1, bone marrow and CNS n=1, nodal n=6). Details on treatment failures are reported in the Online Supplementary Material S2. One patient with no response with PET-positive residual abdominal adenopathy at the end of therapy experienced prolonged survival following high-dose salvage including autologous blood stem transplantation. Of 11 relapses, 10 occurred among 67 patients treated with MIT >25 days (15%), compared to one of 15 patients with MIT ≤25 days (7%, P=0.34). Projected 3-year OS and DFS rates were 67% and 75%, respectively, with marked differences according to aIPI classification. Notably, OS and DFS rates were 100% in 22 patients with low aIPI (Table 2, Figure 2A–D). Only two deaths, both unrelated to BL, were reported after the third year of follow-up.
Figure 2.
Three-year probability of overall survival (OS) and disease-free survival (DFS). (A) OS: all patients, 0.67 (95% CI, 0.57 to 0.76). (B) DFS: all patients, 0.75 (95% CI, 0.63 to 0.84). (C) OS according to aIPI: low, 1.0; intermediate, 0.66 (95% CI, 0.51 to 0.77); high, 0.45 (95% CI, 0.26 to 0.62). (D) DFS according to aIPI: low, 1.0; intermediate, 0.69 (95% CI, 0.51 to 0.82); high, 0.60 (95% CI, 0.34 to 0.79).
Prognostic analysis
In univariate analysis, beside aIPI, selected risk factors such as elevated LDH, PS >1, age >60 years and CNS involvement (DFS only) were adverse prognostic indicators, whereas MIT and stage were near to significance (OS) and gender (in both unselected patients and Burkitt lymphoma patients only), HIV status and diagnosis of Burkitt-like lymphoma did not have any impact (Table 2). The effect of HIV positivity on outcome was analyzed separately looking at different treatment steps. Failure rates were similar among HIV+ (n=15) and HIV-negative (n=90) groups: early death rate 20% versus 12% (P=0.41), no response rate 7% versus 8% (P=0.88), relapse rate 13% versus 10% (P=0.69), CR death rate 0% versus 8% (P=0.26), and sum of all failures 40% versus 38% (P=0.87).
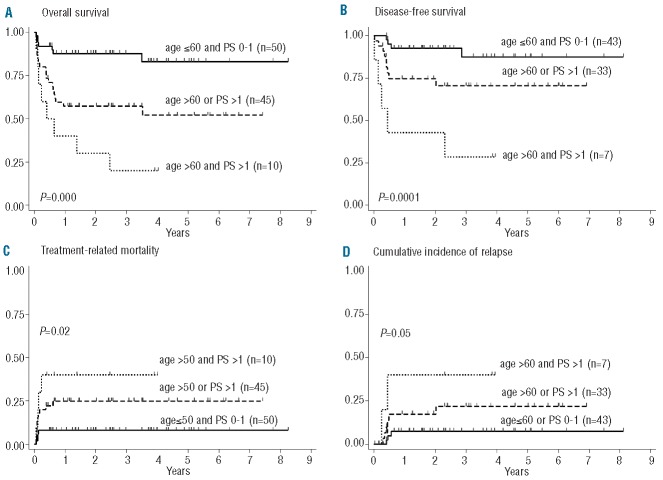
In multivariate analysis, carried out also to identify the most influential factors within the aIPI risk model, disease stage was of borderline significance in predicting the probability of CR, whereas age and PS were confirmed as being highly predictive for both OS and DFS (Table 3). Partitioning groups with different age- and PS-related hazards and clinical outcomes confirmed that patients aged ≤60 years with PS 0–1 fared exceptionally well (n=50, 3-year OS 88% and DFS 87.5%), those aged >60 years with PS >1 or aged <60 years with PS 0–1 constituted an intermediate-good risk category (n=45, OS 57% and DFS 70.5%), and those aged >60 years with PS >1 had a markedly worse outcome (n=10, OS 20% and DFS 28.5%) (Figure 3A–B, all P values highly significant). The incidence of treatment-related mortality and relapse varied significantly among the three groups, each contributing almost equally to the final outcome (Figure 3B–C).
Table 3.
Multivariable prognostic analysis of complete remission, disease-free survival, and overall survival.
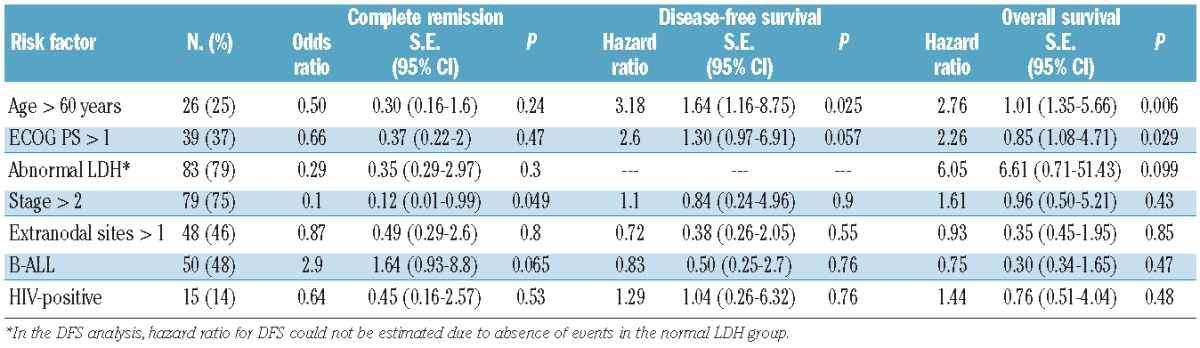
Figure 3.
Three-year probability of overall survival (OS), disease-free survival (DFS), treatment-related mortality (TRM), and cumulative incidence of relapse (CIR) according to age and PS. (A) OS: age ≤60 and PS 0–1, 0.88 (95% CI, 0.75 to 0.94); age >60 or PS >1, 0.57 (95% CI, 0.42 to 0.70); age >60 and PS >1, 0.20 (95% CI, 0.03 to 0.47). (B) DFS: age ≤ 60 and PS 0–1, 0.875 (95% CI, 0.68 to 0.95); age > 60 or PS > 1, 0.705 (95% CI, 0.51 to 0.84); age > 60 and PS > 1, 0.285 (95% CI, 0.04 to 0.61). (C) TRM: age ≤ 60 and PS 0–1, 0.08 (95% CI, 0.03 to 0.20); age >60 or PS > 1, 0.25 (95% CI, 0.15 to 0.40); age > 60 and PS > 1, 0.40 (95% CI, 0.17 to 0.75). (D) CIR: age ≤ 60 and PS 0–1, 0.07 (95% CI, 0.02 to 0.21); age > 60 or PS > 1, 0.22 (95% CI, 0.10 to 0.43); age > 60 and PS > 1, 0.40 (95% CI, 0.12 to 0.87).
Treatment toxicity
Induction deaths occurred mostly in older patients (age range 40–73 years, median 57) and were mainly caused by infections (78.5%). Five CR patients aged 45–70 years died of complications during therapy (Online Supplementary Material S2). Following pre-phase and course A1, neutropenia (<0.5×109/L) lasted a median of 7 (range, 0–25 days) and 8 days (range, 0–23 days) in patients younger and older than 55 years who were scheduled for the full or reduced intensity regimen, respectively, whereas thrombocytopenia (<20×109/L) lasted a median of 3 days (range, 0–45 days) and 5 days (range, 0–32 days), respectively. A total of 51 infectious episodes were recorded among 105 induction courses (48.5%), with a higher incidence in older patients (43% versus 62%, P=0.08, Online Supplementary Material S3A). No other differences were found in terms of gastrointestinal, renal, neurological, cardiovascular, respiratory, and hepatic toxicity, whereas older patients were more likely to develop metabolic complications (grade >2: 38% versus 10%, P=0.001). During consolidation (374 assessable courses), renal and cardiovascular toxicity was more frequent in the older age group. With regards to HIV+ patients, a comparative analysis with the HIV-negative group showed similar rates of hematotoxicity, a trend to increased gastrointestinal/hepatic toxicity (P=0.04) and a numerically modest, albeit statistically significant increase in febrile and infectious episodes other than bacterial sepsis and pneumonia (Online Supplementary Material S3B).
Discussion
In this prospective NILG study, the German short intensive rituximab-chemotherapy program B-NHL 2002 was adopted to improve the long-term outcome of adult patients with BL. The GMALL previously reported the results obtained in 185 patients,8 recently updating their experience in 363 total cases.19 In patients aged 15–55 years, the 3-year OS was 91% for Burkitt lymphoma and 79% for Burkitt leukemia, representing a significant improvement from prior results obtained with chemotherapy without rituximab.2 We treated 105 patients with a median age of 47 years and 21% incidence of low aIPI (compared with 54% in a similar CALGB study),18 achieving OS and DFS rates of 67% (75% for those aged ≤60 years) and 75% (82% for those aged ≤60 years), respectively. Considering the high median age and adverse aIPI distribution of our study population, the results are only marginally inferior to those from the German and Spanish groups8,20 and comparable to the those of the CALGB study in patients with a better aIPI profile.18 All studies found a prognostic gain of approximately 30% over rituximab-free protocols, as confirmed by the first randomized trial on 257 BL patients.21
Differing from the German data, the induction phase resulted in a slightly lower CR rate in both Burkitt lymphoma (79% compared to 90%) and leukemia (78% compared to 83%), especially in older patients who were treated with the reduced-intensity regimen and/or suffered from an increased complication rate. However, like the German series, no highly significant risk factor for CR was identified in univariable or multivariable prognostic analyses. Early treatment failures were mostly related to serious septic complications and more common in older patients, despite prophylactic measures including granulocyte colony-stimulating factor and irrespective of HIV serology. However the relatively small number of HIV+ patients could mask some critical aspects of this study. The Spanish Group, adopting the same GMALL program, reported a higher incidence of mucositis and infections in 38 HIV+ patients, associated with a slightly inferior outcome compared with HIV-negative patients (DFS 63% versus 77%, non-significant P value).20 In our study the infectious risk correlated with absolute neutropenia (<0.5×109/L) lasting more than 10 days, as observed in 28% and 41% of younger and older patients, respectively, aggravated by the immune suppression caused by rituximab, cyclophosphamide/ifosfamide, dexamethasone, and methotrexate. The present series likely consisted of a higher proportion of elderly and/or frail patients, who are typically at increased risk of complications and death. The prognostic analysis indicated that, among the aIPI risk factors, increasing age and worse PS at presentation correlated with the risk of treatment-related mortality. The second problem encountered during induction was chemoresistance, which was unrelated to age and was observed in eight patients (less than 8%), only two of whom were over 55 years old and treated with the attenuated protocol. The risk of refractory BL using this program is, therefore, low and cannot be anticipated from standard diagnostic features.
The probability of recurrence among CR patients was also low (16%) and depended on age and PS risk profile, with all relapses occurring within 6 months of CR, soon after completion of treatment. Thus, given the rapid growth kinetics of BL/B-ALL, cure was more the product of the entire chemotherapy sequence rather than single treatment elements, which was also indicated by the impact of the MIT on the risk of relapse. In contrast to a planned MIT of 22–23 days (Figure 1), the actual MIT was 29 days due to toxic complications, poor treatment compliance, or delayed hospital admission, as can be observed when treating intensively very high-risk, unselected patients in a mixed university and community hospital setting. Notably, ten of the 11 cases of relapse (91%) occurred in patients with a MIT longer than 25 days, whereas only one case of recurrence was observed among the 15 patients treated at shorter intervals (7%). This observation underlines the therapeutic potency of the protocol when it is applied correctly and strengthens the need for carefully supervised treatment in order to avoid unnecessary treatment delays, which may be highly detrimental to the outcome.
To evaluate inappropriate drug dose reductions we performed an ad hoc analysis in 19 unresponsive or relapsing patients. The omission of full treatment cycles was almost exclusively caused by documented resistance, early relapse and/or very poor individual compliance. The omission of critical therapeutic elements such as rituximab and methotrexate was relatively uncommon (11%) when compared with the total number of chemotherapy courses administered to these patients. Partial drug reductions, also prevailing in poor performance patients, were mainly limited to one-two drugs for one-two cycles in a few patients. Thus, in terms of protocol application, more problems were encountered with the ability to recycle within stated time intervals in all patients than with using planned drug dosages, at least in the group of refractory/relapsed patients. This would reflect underlying logistics problems at the treating hospitals, which become progressively more relevant in older and/or poorly compliant patients and need to be addressed to optimize the cure rates.
In general, the cumulative risk of treatment-related mortality and no response/relapse in patients older than 60 years of age and/or with suboptimal PS (>1) explained the worse survival compared to that of other patients. Both of these variables predicted OS and DFS in univariate and multivariate prognostic analyses, and were the only significant ones extracted from the aIPI risk model. The role of PS is seldom emphasized in BL, probably because the small numbers of patients in many series do not allow verification of its real prognostic weight, or because the information is not adequately collected. The substantial adequacy of the clinical PS evaluation in the present study was indirectly supported by the enrollment of patients with PS >2 (15%), who are sometimes excluded from clinical trials, and by the homogeneous distribution of patients across different PS categories. A poor performance status was previously recognized as an adverse risk factor in the MD Anderson Hospital study,4 and more recently in a large series of adult patients with acute lymphocytic leukemia22 in which a PS >0 predicted a significantly worse outcome in patients younger than 55 years of age.
These observations would support an adaption of the treatment policy in some frail patients with an increased risk of early death from treatment toxicity. All those with poor PS (>1) could be treated with a totally or in part de-intensified first induction cycle regardless of age. Subsequently, even elderly patients with leukemia or stage III–IV lymphoma, once in CR and reverted to a good PS, could benefit from an additional cycle C including high-dose cytarabine, to lower the risk of recurrence, which was higher in the high-risk cohort treated without C cycles. Furthermore, all frail/elderly patients may profit from an early PET-based response evaluation to have treatment safely curtailed to fewer cycles (3–4), as indicated by another recent study of very short intensive chemotherapy followed by routine PET assessment.23
Although older BL patients with a PS >1 constituted a very poor risk group, all of the remaining formed a numerically robust cohort (90.5% of total) with a good to very good outcome provided CR was achieved initially (DFS 70.5%–87.7%). The excellent therapeutic potential of this treatment, yielding a 100% cure rate, was further confirmed in patients with a good-risk presentation (low aIPI), even with the de-intensified regimen used in some patients in the age range of 55–60 years.
These globally positive therapeutic results would suggest the efficacy of this treatment in BL not otherwise specified, which may include cases of borderline diffuse large B-cell lymphoma and double- and triple-hit lymphoma,24,25 although this cannot be formally established from the present study without a detailed analysis of c-myc, bcl-2 and bcl-6 expression. However, in the German trial,8 the same regimen was highly effective in all BL variants as well as mediastinal large B-cell lymphoma. This could be a meaningful consideration when facing an immediate clinico-therapeutic decision, and when the distinction among BL variants is difficult or impossible.
In summary, prompt diagnosis and referral of BL patients is essential for exploiting the prognostic advantage conferred by a better PS in any age group, in conjunction with maximizing anti-infectious measures and the rapid re-cycling of chemo-immunotherapy blocks.
Acknowledgments
The seminal contribution of Prof. Dieter Hoelzer (GMALL chair, Frankfurt, Germany) to the design of the treatment protocol is gratefully recognized by us all.
Footnotes
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Blum KA, Lozanski G, Byrd JC. Adult Burkitt leukemia and lymphoma. Blood. 2004;104(10):3009–20 [DOI] [PubMed] [Google Scholar]
- 2.Hoelzer D, Ludwig WD, Thiel E, Gassmann W, Loffler H, Fonatsch C, et al. Improved outcome in adult B-cell acute lymphoblastic leukemia. Blood. 1996;87(2): 495–508 [PubMed] [Google Scholar]
- 3.Magrath I, Adde M, Shad A, Venzon D, Seibel N, Gootenberg J, et al. Adults and children with small non-cleaved-cell lymphoma have a similar excellent outcome when treated with the same chemotherapy regimen. J Clin Oncol. 1996;14(3):925–34 [DOI] [PubMed] [Google Scholar]
- 4.Thomas DA, Cortes J, O'Brien S, Pierce S, Faderl S, Albitar M, et al. Hyper-CVAD program in Burkitt's-type adult acute lymphoblastic leukemia. J Clin Oncol. 1999; 17(8):2461–70 [DOI] [PubMed] [Google Scholar]
- 5.Lee EJ, Petroni GR, Schiffer CA, Freter CE, Johnson JL, Barcos M, et al. Brief-duration high-intensity chemotherapy for patients with small noncleaved-cell lymphoma or FAB L3 acute lymphocytic leukemia: results of Cancer and Leukemia Group B study 9251. J Clin Oncol. 2001;19(20):4014–22 [DOI] [PubMed] [Google Scholar]
- 6.Di Nicola M, Carlo-Stella C, Mariotti J, Devizzi L, Massimino M, Cabras A, et al. High response rate and manageable toxicity with an intensive, short-term chemotherapy programme for Burkitt's lymphoma in adults. Br J Haematol. 2004; 126(6):815–20 [DOI] [PubMed] [Google Scholar]
- 7.Divine M, Casassus P, Koscielny S, Bosq J, Sebban C, Le Maignan C, et al. Burkitt lymphoma in adults: a prospective study of 72 patients treated with an adapted pediatric LMB protocol. Ann Oncol. 2005;16(12): 1928–35 [DOI] [PubMed] [Google Scholar]
- 8.Hoelzer D, Hiddemann W, Baumann A, Dohner H, Duhrsen U, Fietkau R, et al. High survival rate in adult Burkitt's lymphoma/leukemia and diffuse large B-cell lymphoma with mediastinal involvement [abstract]. Blood. 2007;110 (11):518a [Google Scholar]
- 9.Thomas DA, Faderl S, O'Brien S, Bueso-Ramos C, Cortes J, Garcia-Manero G, et al. Chemoimmunotherapy with hyper-CVAD plus rituximab for the treatment of adult Burkitt and Burkitt-type lymphoma or acute lymphoblastic leukemia. Cancer. 2006;106(7):1569–80 [DOI] [PubMed] [Google Scholar]
- 10.Mohamedbhai SG, Sibson K, Marafioti T, Kayani I, Lowry L, Goldstone AH, et al. Rituximab in combination with CODOX-M/IVAC: a retrospective analysis of 23 cases of non-HIV related B-cell non-Hodgkin lymphoma with proliferation index >95%. Br J Haematol. 2010;152(2): 175–81 [DOI] [PubMed] [Google Scholar]
- 11.Maruyama D, Watanabe T, Maeshima AM, Nomoto J, Taniguchi H, Azuma T, et al. Modified cyclophosphamide, vincristine, doxorubicin, and methotrexate (CODOX-M)/ifosfamide, etoposide, and cytarabine (IVAC) therapy with or without rituximab in Japanese adult patients with Burkitt lymphoma (BL) and B cell lymphoma, unclassifiable, with features intermediate between diffuse large B cell lymphoma and BL. Int J Hematol. 2010;92(5):732–43 [DOI] [PubMed] [Google Scholar]
- 12.Barnes JA, Lacasce AS, Feng Y, Toomey CE, Neuberg D, Michaelson JS, et al. Evaluation of the addition of rituximab to CODOX-M/IVAC for Burkitt's lymphoma: a retrospective analysis. Ann Oncol. 2011;22(8): 1859–64 [DOI] [PubMed] [Google Scholar]
- 13.Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649–55 [PubMed] [Google Scholar]
- 14.Diebold J, Jaffe ES, Raphael M, Warnke RA. Burkitt lymphoma, in: Pathology and Genetics of Tumours of the Haematopoietic and Lymphoid Tissues (ed): Jaffe ES, Harris NL, Stein H, Vardiman JW. IARC Press, Lyon: 2001, pp 181–184 [Google Scholar]
- 15.Lerede T, Bassan R, Rossi A, Di Bona E, Rossi G, Pogliani EM, et al. Therapeutic impact of adult-type acute lymphoblastic leukemia regimens in B-cell/L3 acute leukemia and advanced-stage Burkitt's lymphoma. Haematologica. 1996;81(5): 442–9 [PubMed] [Google Scholar]
- 16.Bene MC, Castoldi G, Knapp W, Ludwig WD, Matutes E, Orfao A, et al. Proposals for the immunological classification of acute leukemias. European Group for the Immunological Characterization of Leukemias (EGIL) Leukemia. 1995;9(10): 1783–6 [PubMed] [Google Scholar]
- 17.Shipp MA, Harrington DP, Anderson JR, Bonadonna G, Brittinger G, Cabanillas F, et al. A predictive model for aggressive non-Hodgkin's lymphoma. The International Non-Hodgkin's Lymphoma Prognostic Factors Project. N Engl J Med. 1993;329 (14):987–94 [DOI] [PubMed] [Google Scholar]
- 18.Rizzieri DA, Johnson JL, Byrd JC, Lozanski G, Powell BL, Shea TC, et al. Efficacy and toxicity of rituximab and brief duration, high intensity chemotherapy with filgrastim support for Burkitt or Burkitt - like leukemia/lymphoma: Cancer and Leukemia Group B (CALGB) Study 10002. [abstract]. Blood. 2010;116(21):858a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoelzer D, Walewski J, Dohner H, Schmid M, Hiddemann W, Baumann A, et al. Substantially improved outcome of adult Burkitt non-Hodgkin lymphoma and leukemia patients with rituximab and a short-intensive chemotherapy; report of a large prospective multicenter trial. [abstract]. Blood. 2012;120(21):667a [Google Scholar]
- 20.Ribera JM, Garcia O, Grande C, Esteve J, Oriol A, Bergua J, et al. Dose-intensive chemotherapy including rituximab in Burkitt's leukemia or lymphoma regardless of human immunodeficiency virus infection status: final results of a phase 2 study (Burkimab). Cancer. 2013;119(9):1660–8 [DOI] [PubMed] [Google Scholar]
- 21.Ribrag V, Koscielny S, Bouabdallah K, Salles G, Casasnovas O, Recher C, et al. Addition of rituximab improves outcome of HIV negative patients with Burkitt lymphoma treated with the LMBA protocol: results of the randomized intergroup (GRAALL-LYSA) LMBA02 Protocol. (IGR sponsored LMBA02, NCT00180882). [abstract]. Blood. 2012;120(21):685a [Google Scholar]
- 22.Goekbuget N, Arnold R, Baumann A, Beck J, Diedrich H, Freund M, et al. General condition and early complications have in addition to disease-specific factors a significant impact on outcome - prognostic factors revisited in 1657 adult ALL patients (15–55 years). [abstract]. Blood. 2008;112 (11):1927a [Google Scholar]
- 23.Kasamon YL, Brodsky RA, Borowitz MJ, Ambinder RF, Crilley PA, Cho SY, et al. Brief intensive therapy for older adults with newly diagnosed Burkitt or atypical Burkitt lymphoma/leukemia. Leuk Lymphoma. 2013;54(3):483–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jack A. The pathology of B-cell lymphomas with features intermediate between diffuse large B-cell lymphoma and Burkitt's lymphoma. Hematology Education. 2012:187–94 [Google Scholar]
- 25.Sweetenham JW. How to treat patients with bordeline DLBLC and Burkitt's lymphoma. Hematology Education. 2012:205–12 [Google Scholar]