Abstract
Recent studies have demonstrated that remembering past experiences and imagining future scenarios recruits a core network including the hippocampus. Even so, constructing future events engages the hippocampus more than remembering past events. This fMRI study examined whether increased hippocampal activity for future events includes both specific and general events. Participants constructed specific and general past and future events during fMRI scanning. We replicated previous findings of increased activity in the right anterior hippocampus when constructing future relative to past events, and when constructing specific relative to general events. Importantly, both effects were driven by a significant interaction between temporal direction and specificity, with specific future resulting in more activity than other conditions, including general future events. No regions exhibited greater activity during the construction of past relative to future events, or general relative to specific events. These results suggest that the process of constructing a detailed representation of a novel and specific future event differentially engages the right anterior hippocampus compared with other forms of event simulation and recall. Future work is needed to disambiguate the role of encoding, novelty and detail recombination in engaging the right anterior hippocampus during simulation.
Keywords: autobiographical, episodic memory, simulation, future, fMRI, hippocampus
Episodic memory allows humans not only to re-experience the past in rich detail but also construct vivid simulations of the future. Consistent with this idea, deficits in future simulation are evident in a number of populations that typically exhibit memory impairments, including patients with amnesia, older adults, depression and schizophrenia. Moreover, neuroimaging evidence supports the idea that memory and future simulation rely on the same neural network that includes the medial prefrontal and parietal cortex, medial temporal lobes (including hippocampus) and lateral temporal and parietal cortex (see Schacter, Addis, & Buckner, 2007, 2008, for reviews of patient and neuroimaging findings).
The hippocampus has emerged as a key node in this network, particularly with respect to episodic simulation. Several studies suggest that this region is differentially involved in imagining episodic events relative to remembering previously experienced events (e.g., Addis, Pan, Vu, Laiser, & Schacter, 2009; Addis, Wong, & Schacter, 2007; Okuda, et al., 2003; Weiler, Suchan, and Daum. 2010a, 2010b). These observations are critical to the constructive episodic simulation hypothesis (Schacter & Addis, 2007a, 2007b; for related views, see Buckner, 2010; Buckner & Carroll, 2007; Hassabis & Maguire, 2007; Suddendorf & Corballis, 1997, 2007; Szpunar & McDermott, 2008), which was advanced to explain both the reliance of memory and simulation on a common network and the increased hippocampal activity evident during future simulation. This hypothesis contends that (a) episodic memory provides a source of details for future event simulations and (b) the constructive nature of episodic memory allows the flexible recombination of such details into a coherent simulation. Thus, while the construction of both past and future events requires retrieval of details from episodic memory, leading to common recruitment of the core network, the construction of future events involves an additional process of flexibly recombining the details extracted from memory into a coherent scenario. We have suggested that such flexible recombination of details requires additional relational processing (Cohen, Poldrack, & Eichenbaum, 1997) supported by the hippocampus (Schacter & Addis, 2007a, 2009).
Consistent with this idea, neuroimaging studies have reported differential activation of right anterior hippocampus during simulation relative to remembering. Addis et al. (2007) reported that this effect was evident early on in the construction phase of a trial when one is initially trying to generate an imagined scenario, but not during the later elaboration of the event. This simulation>remembering effect during construction was replicated using an experimental recombination paradigm (Addis, et al., 2009). Here, participants imagined past or future events involving a set of details that the experimenter randomly extracted and recombined from a number of the participants’ own memories. This procedure resulted in activity in the anterior hippocampus that was unique to the process of simulation relative to remembering. Moreover, Addis and Schacter (2008) found that while posterior hippocampal activity correlated with the amount of detail comprising both past and future events, anterior hippocampal activity correlated only with the amount of detail comprising future events. More recently, Weiler, Suchan, and Daum (2010a) manipulated the occurrence probability of future events and reported that right anterior hippocampal activity increased when subjects imagined future events that had a low probability of occurring during the upcoming holidays compared with those that had a higher probability of occurring. The authors suggested that the increased hippocampal involvement for low probability events reflects higher demand on binding of event features during construction as compared with high probability events.
Interestingly, while most studies examining the reverse contrast of remembering >imagining report more activity primarily in posterior visuospatial regions, such as cuneus (Addis, et al., 2009, Weiler et al., 2010b), retrosplenial cortex (Abraham, Schubotz, & von Cramon, 2008), precuneus (Hassabis, Kumaran, & Maguire, 2007; Weiler et al., 2010a) and parahippocampal gyrus (Abraham, et al., 2008; Okuda, et al., 2003), three studies have reported greater hippocampal activity for past versus future events (Abraham, et al., 2008; Botzung, Dankova, & Manning, 2008, Weiler et al., 2010b). These contrasting findings may be related to characteristics of the paradigms used. In Botzung et al.’s paradigm, future events were already constructed prior to the scanning (and thus remembered in the scanner), meaning participants not constructing a novel future event but instead retrieving an event representation that is typically less detailed relative to previously experienced events (cf. Johnson, Foley, Suengas, & Raye, 1988). In Abraham et al.’s paradigm, they asked participants to answer yes or no to whether a particular scenario is likely to occur in future (e.g., clubbing at the age of 40) or whether it did occur in the past (e.g., giving a speech at a wedding). Thus, it is possible that while the past event statements can automatically cue past experiences, one can answer the future statements without engaging in a detailed simulation of that event that requires recombination of episodic details. Interestingly, Weiler et al. (2010b) found that the right posterior hippocampal responses to past and future events showed distinct time courses. Specifically, a past>future effect was evident during the initial construction phase, while a future>past effect was evident during the subsequent elaboration phase. While this result appears to contradict that of Addis et al. (2007), where the future>past effect in the right anterior hippocampus was evident only during construction, Weiler and colleagues suggest the absence of a future>past effect may reflect similar task demands across the conditions during construction, or differential contributions of the posterior (past>future) and anterior (future>past) regions of the right hippocampus.
Overall, neuroimaging findings suggest the anterior hippocampus may support the more intensive relational processing required when one must flexibly recombine details extracted from memory to create a coherent scenario (Schacter & Addis, 2009). However, the critical features of this imagining>remembering effect are still being established. A fundamental and as yet unanswered question is whether greater hippocampal recruitment during the construction of imagined than remembered events depends on the temporal specificity of the event. Previous work in autobiographical memory has shown that retrieving both general, repeated events (e.g., routines) and specific, unique events significantly engage the hippocampus (Addis, McIntosh, Moscovitch, Crawley, & McAndrews, 2004). By contrast, constructing imaginary events for the first time may place greater demand on recombining details to form an event representation that is specific in time and place, relative to constructing a routinized future event that may rely more closely on previously experienced routines and therefore require less relational processing. As such, specific future events likely comprise more novel detail combinations than general future events and remembered past events. Thus, we predicted that if the hippocampus is particularly responsive to the relational novelty of specific future events (Kohler, Danckert, Gati, & Menon, 2005), then hippocampal activity should be greater for specific future events relative to general future events and specific and general past events.
Twenty-three healthy, right-handed adults consented to participate in this study approved by the Massachusetts General Hospital Institutional Review Board. Eight participants were excluded due to scanner malfunction, excessive movement or an insufficient number of responses. Thus data from 15 participants (8 male; age range, 18–33 years) are presented here. None of these subjects had participated in our previous fMRI studies on future simulation.
The paradigm used here is a variation of that used in our previous study (Addis et al., 2007). Participants completed 16 trials of each of 4 autobiographical event conditions (general-future, specific-future, general-past, specific-past) in the MRI. Specific events were temporally and contextually specific, occurring over minutes or hours, but not more than one day (e.g., recalling a special dinner; imagining a marriage proposal). General events were repeated (“routine”) episodes that had happened or could happen repeatedly (e.g., high school choir practice; commuting to a future job). In order to differentiate past from future routines (i.e., to avoid events which have been done before and will be done again), participants were instructed to think only of routines in which they are not currently engaging. Future events had to be novel and plausible. Events were to be experienced from a field rather than observer perspective. Each trial consisted of a 20s phase when a cueing slide specifying the condition (remember/imagine a specific/routine event), a time period (last/next year/5–20 years) and a cue word (highly imageable, concrete and high frequency nouns; Clark & Paivio, 2004) was displayed. Participants made a button press when an event was in mind. Four rating scales followed (4s each): (1) amount of detail retrieved/imagined (1=vague; 5=vivid); (2) intensity of emotion experienced (1=detachment; 5=highly emotional); (3) a binary decision for event perspective (field/observer); and (4) a binary decision of whether the participant stayed on task for the trial (on/off task). Participants also completed 16 trials of each of 2 control tasks, each for 20s (imagery, semantic); for more information on these tasks, see Addis et al. (2007). Each control trial was followed by four rating scales (4s each): (1) amount of detail generated (1=vague; 5=vivid); (2) semantic relatedness of word/objects generated to the cue word (1=unrelated; 5=highly related); (3) a binary decision for task difficulty (easy/hard); and (4) a binary decision of whether the participant stayed on task for the trial (on/off task). All trials were followed by a fixation cross for a jittered duration (M = 4s; range 2–6s).
Participants completed a post-scan interview in which they described each event generated during scanning. Any events that could not be recounted during the post-scan interview were dropped from the analysis. Event specificity (specific, general) was confirmed by the experimenter. Only those events that could be described during the post-scan interview and were specific or general were included in the data analysis. For general events, it was confirmed that the routines were not current. Participants rated events for personal significance (1=insignificant; 5=significant), valence (positive/negative/neutral), and provided the actual/ predicted temporal distance (years from the present) of events. Future events were rated for similarity to previous thoughts/imaginings and experiences (1=I’ve never imagined/experienced this; 5=I’ve imagined/experienced this exactly) and how frequently these thoughts or events are experienced (1=never; 5=all the time).
Detailed anatomical data were collected on a 3T Siemens Allegra MRI scanner using a multiplanar rapidly acquired gradient echo (MP-RAGE) sequence. Functional images (25 coronal oblique slices, 5mm thick, no gap) were acquired at an angle perpendicular to the long axis of the hippocampus in an interleaved order using a T2*-weighted echo planar imaging (EPI) sequence (TR=2000ms, TE=23ms, FOV=200mm, flip angle=90°). Note that for two participants, data from one run (16 trials) were lost due to a scanner malfunction. Cues were projected on a screen viewed on a mirror incorporated into the head-coil. E-Prime software (Psychology Software Tools, Inc., Pittsburgh) was used for the presentation and timing of stimuli and collection of response data. Responses were made on an MR-compatible five-button box.
Pre-processing and analysis of imaging data was performed using SPM2 (http://www.fil.ion.ucl.ac.uk/spm/). Standard pre-processing of functional images was performed, including rigid-body motion correction and unwarping, slice-timing correction, spatial normalization to the Montreal Neurological Institute (MNI) template (resampled at 2×2×2 mm3 voxels), spatial smoothing (8mm full-width half maximum Gaussian kernel) and removal of linear slope to correct for drift. Each trial was modeled by SPM2’s canonical hemodynamic response function. Although the present study focused on neural activity at event construction, to be consistent with our previous study using this paradigm (Addis et al. 2007) we included regressors for both construction (2s after task-onset to allow for reading of the cue) and elaboration (1s after RT indicating an event was in mind). Trials on which the RT was less than 3s (i.e., <1s after the construction regressor) or no response was made were dropped from the analysis. Thus, the fixed-effects model for each subject comprised twelve regressors, two (construction and elaboration) for each of the six conditions. On average, participants contributed 12 general-future, 13 specific-future, 12 general-past, 13 specific-future, 15 semantic and 15 imagery trials (out of a maximum of 16 trials per condition).
To examine the future>past effect, we collapsed across specificity (specific, general) and contrasted future>past and past>future. To examine specificity, we collapsed across temporal direction (past, future) and contrasted specific>general and general>specific. We also computed an interaction contrast, (specific-future and general-past) > (general-future and specific-past). Resulting contrast images were entered into random-effects one-sample t-tests, and a small volume correction was applied to correct for multiple comparisons (P-FDR≤.05). This correction used a 10mm sphere centered on the peak right hippocampal voxel in the future>past contrast from an independent dataset (Addis et al., 2007), resulting in a search volume of 266 voxels. Peak MNI co-ordinates of activations were converted to Talairach space and localized using the Talairach atlas (Talairach & Tournoux, 1988). Percent signal change was extracted using MarsBar toolbox for SPM (Brett, Anton, Valabregue, & Poline, 2002).
Analysis of RT data by repeated-measures analyses of variance (RM-ANOVA) revealed a main effect of condition, F3, 48=11.11, p<.001, reflecting longer RTs for the imagery control condition relative to the autobiographical conditions (all p values < .001). Importantly, there were no significant RT differences between the autobiographical conditions (all p values > .25). A RM-ANOVA confirmed that temporal distance of autobiographical events did not differ according to specificity (general, specific), F1,14=3.06, p=.10, or temporal direction (past, future), F1,14=1.33, p=.27, nor did these factors interact, F1,14=.30, p=.59.
Wilcoxin Sign Ranks Tests were computed to test whether phenomenological ratings differed by temporal direction or event specificity. There were no significant effects of temporal direction (p values >.28), except for detail where there was a trend toward significance (p ≤.02) which fell short of the Bonferroni-corrected threshold (p .004). This non-significant effect was in the predicted direction, where past events were more detailed than future events. A trend was also evident for specific events being higher in detail than general events (p=.02) but this also fell short of the Bonferroni-corrected threshold. Specific events were more emotional than general events (p=.001), while valence was more positive for general than specific events (p=.002). Consistent with the idea that specific-future events are more novel than general-future events, specific-future events were rated as being less similar to previous experiences (p=.004), and a non-significant trend was evident for specific events being rated as less similar to previous thoughts (p=.02). Moreover, the previous experiences drawn upon when constructing future specific events were rated as having occurred less frequently than those drawn upon for general future events (p<.001), and a similar trend was evident for previous thoughts (p=.02). These findings suggest when constructing future routines, participants were more likely to draw on memories of past routines than when constructing future specific events. Importantly, ratings of the similarity of future events to previous events were on average 3.66 on a 5-point scale (where 5 indicates high similarity to previous events), indicating that these simulations were not identical to previous experiences. Although the average rating for similarity to previous thoughts was higher at 4.13, it was not entirely surprising that participants were drawing on previous simulations they have had about the future. Chi-square tests (Preacher, 2001) revealed that the frequencies of field and observer ratings (χ2=3.31, p=.35) and of on- or off-task ratings (χ2=5.46, p=.14) did not significantly differ across the autobiographical conditions. The majority of trials were rated in accordance to task instructions (i.e., 95% of events were classified as field perspective; for 97% of trials, participants were on-task).
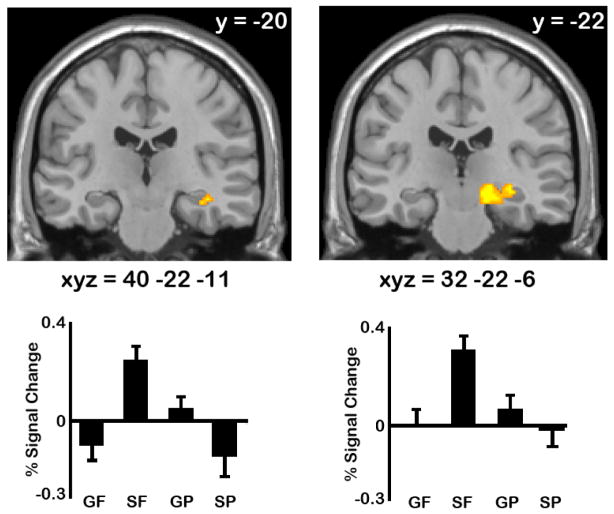
The contrast of future>past replicated previous findings of increased activity in the right anterior hippocampus (xyz = 40 −22 −11, Z=2.91, P-FDR ≤.05; see left panel, Figure 1). The patterns of activation underlying this effect of temporal direction were driven by the specific-future condition, while activity during the other autobiographical tasks was around or below baseline. The contrast of specific>general resulted in a significant effect in a proximal cluster of the right hippocampus (xyz = 32 −22 −6, Z = 2.77, P-FDR ≤.05; see right panel, Figure 1), which was also driven by the specific-future condition. Consistent with these patterns, the interaction contrast confirmed the peak voxel from each cluster showed a significant interaction effect (xyz = 40 −22 −11, Z = 3.02, P-FDR ≤.05; xyz = 32 −22 −6, Z = 3.04, P-FDR ≤.05). Note that the contrasts of past>future and general>specific did not result in any significant activations in the right anterior hippocampus. Given that both proximal clusters exhibit a significant interaction effect, it is likely both clusters are actually part of the same right hippocampal region that is maximally responsive to the specific-future condition.
Figure 1.
Right hippocampal activation identified by the future > past (left) and specific > general (right) contrasts are shown at P-uncorrected<.005 (note that both activations survive small volume correction, P-FDR≤.05). Percent signal change extracted from the peak voxel is shown for all conditions (GF=general future; SF=specific future; GP=general past; SP=specific past). All co-ordinates are in Talairach space.
Pairwise comparisons on percent signal change data were computed to confirm statistically that this interaction pattern reflected the fact that the future>past effect was evident only for specific events and did not generalize to generic events (with a Bonferroni-corrected threshold of p<.006). These comparisons confirmed that in both clusters, the future>past effect was only evident for specific events (p<.001); the future>past effect for general events was not significant in either cluster. We also examined whether these right hippocampal effects evident during construction persisted into the elaboration phase. Pairwise comparisons on percent signal change data extracted from elaboration showed that the future>past effect for specific events persisted into elaboration in both clusters (p values .≤002), but were not evident for generic events.
Although our primary focus on this report concerns the future>past effect in the right hippocampus, the whole-brain results for the contrasts of future>past and specific>general are reported in Table 2 for completeness. These maps were thresholded using a combined voxel-threshold of P-uncorrected < .001 and cluster-threshold of 68 voxels (equivalent to corrected p<.05; Slotnick, Moo, Segal, and Hart, 2003). A future>past effect was evident in bilateral medial prefrontal cortex, precuneus and inferior parietal lobule. Moreover, a specific>general effect was also evident in bilateral medial prefrontal cortex, left inferior parietal lobule, right insula and parahippocampal gyrus. Neither of the reverse contrasts (past>future; general>specific) resulted in any significant activation. We also conducted a conjunction analysis at construction. Control trials were randomly divided into two independent sets of 16, and then contrasts of past (general/ specific) >“control 1”, and future (general/specific) >“control 2” were computed. At the random-effects level, a conjunction analysis ([past>“control 1”] AND [future>“control 2”]) was performed using SPM2’s masking function (see Addis et al., 2007 for more detail). The conjoint probability estimated using Fisher’s method (Fisher, 1950) was P-uncorrected <.001. Again, a cluster threshold of 68 voxels was used, and these findings are also presented in Table 2. This analysis replicated findings of core network activation despite the fact that half of the events in this analysis were generic. Regions exhibiting this conjunction effect included bilateral medial/frontopolar cortex, medial parietal and posterior cingulate cortices, inferior parietal lobule and middle/superior temporal gyrus. No right hippocampal activity was evident in this conjunction, which is not surprising given the interaction effect. Of note, activity in the left hippocampus (xyz = −24 −22 −9) was evident subthreshold (P-uncorrected =.001) in a location proximal to that reported during construction by Addis et al. (2007; xyz = −22 −20 −12).
Table 2.
Regions activated in the conjunction and contrast analyses
| Brain Region | Co-ordinates
|
Z-score | ||
|---|---|---|---|---|
| x | y | z | ||
| Conjunction of Autobiographical Tasks > Control Tasks | ||||
|
| ||||
| B Medial Prefrontal / Anterior Cingulate Cortices (BA 9/10/32) | 6 | 53 | 10 | 5.06 |
| L Middle / Superior Temporal Gyri (BA 21/38) | −59 | −20 | −7 | 4.22 |
| R Middle / Superior Temporal Gyri (BA 21/22) | 57 | 1 | −17 | 3.15 |
| L Post-Central Gyrus (BA 2/3) | −55 | −19 | 45 | 3.49 |
| B Medial Parietal / Posterior Cingulate Cortices (BA 7/23/24/30/31) | 4 | −43 | 30 | 5.23 |
| L Inferior Parietal Lobule (BA 39) | −48 | −60 | 34 | 4.52 |
| R Inferior Parietal Lobule (BA 39) | 51 | −61 | 31 | 3.90 |
| R Middle / Inferior Occipital / Fusiform Gyri (BA 19) | 40 | −83 | 15 | 3.51 |
| R Caudate | 6 | 6 | 2 | 2.85 |
|
| ||||
| Future > Past Autobiographical Tasks | ||||
|
| ||||
| B Medial Prefrontal Cortices (BA 10) | −4 | 61 | 10 | 4.02 |
| B Superior Frontal Gyrus (BA 6) | −8 | 7 | 62 | 3.97 |
| B Anterior Cingulate Cortex (BA 23/24) | −2 | −8 | 37 | 3.51 |
| R Hippocampus* | 40 | −22 | −11 | 2.91 |
| R Pre-Central Gyrus (BA 4/6) | 38 | −23 | 53 | 3.80 |
| L Inferior Parietal Lobule / Superior Temporal Gyrus (BA 39) | −49 | −59 | 25 | 3.72 |
| R Inferior Parietal Lobule / Supramarginal Gyrus (BA 40) | 50 | −53 | 32 | 4.82 |
| B Precuneus (BA 7) | 8 | −58 | 40 | 5.30 |
| R Cuneus (BA 17/18) | 12 | −87 | 12 | 3.94 |
| L Cerebellum | −2 | −56 | −39 | 3.44 |
| R Cerebellum | 26 | −56 | −39 | 3.47 |
|
| ||||
| Specific > General Autobiographical Tasks | ||||
|
| ||||
| B Medial Frontal and Anterior Cingulate Gyri (BA 6/24) | −8 | −1 | 55 | 3.37 |
| L Anterior Cingulate Cortex (BA 32) | −4 | 43 | 5 | 3.28 |
| R Insula | 38 | 18 | 1 | 3.74 |
| R Hippocampus* | 38 | −22 | −6 | 2.00 |
| R Parahippocampal Gyrus (BA 27) | 18 | −24 | −9 | 3.81 |
| L Inferior Parietal Lobule (BA 40) | −65 | −26 | 25 | 3.51 |
| R Cerebellum | 18 | −26 | −14 | 3.72 |
For each cluster of activation, the Talairach coordinates of the maximally activated voxel is reported.
Peak voxel from hippocampal ROI analysis; BA = Brodmann area, B= Bilateral, L = left, R = right.
This study both replicated previous findings of increased activity in the right anterior hippocampus when one is simulating future events relative to remembering past events, and revealed evidence of an effect of temporal specificity, such that specific events were associated with stronger right hippocampal activity than general events. Interestingly, both the future>past and specific>general effects were driven by an interaction of temporal direction and specificity – where regions maximally responded to the construction of specific future events more than any other event type, including general future events. In contrast, the right hippocampus did not exhibit a past>future effect or a general>specific effect.
Importantly, here we establish another boundary condition to the future>past effect: this effect is not evident for all future events irrespective of temporal specificity, but is instead limited to specific future events. While the presence of a future>past effect but the absence of a past>future effect in the right anterior hippocampus replicates our previous findings (e.g., Addis et al., 2007; Addis et al., 2009), it contrasts directly with the recent findings of Abraham et al. (2008) and Weiler et al. (2010b) who report at past>future effect in the right hippocampus. While this difference could suggest there is something unique about the current paradigm that engages the hippocampus, it is notable that the future>past effect has been previously found using both the current Crovitz cueing paradigm (Addis et al., 2007) as well as an experimental recombination paradigm (Addis, Pan et al., 2009). Another difference is that the various paradigms might require different levels of simulation. While those used by Addis et al. (2007; 2009) and Weiler et al. (2010b) required participants to construct a detailed future simulation, the paradigm used by Abraham et al.(2008) required participants to make a yes/no judgment of the likelihood of future events and the occurrence of past events; such a judgment could be completed without engaging in simulation. For instance, one could answer “Is it likely that you will still go clubbing at the age of 40?” based on past and current activity (e.g., I have/still go clubbing) and preferences (e.g., I like clubbing) without constructing a specific event simulation. These differences could also reflect distinct functions of the anterior and posterior hippocampus. In the studies by Addis et al. (2007, 2009) and the current study, the future>past effect has been documented in the anterior right hippocampus, while in Weiler et al. (2010b), the past>future effect was evident in a more posterior location. Note that anterior-posterior location of the hippocampal activity in the study by Abraham et al. (2008) was not reported.
The current finding that specific-future events engage the hippocampus more than general-future and past events suggests that there is some interaction between imagination-related processes, such as novelty and encoding of newly formed representations, and the more detailed representations created when simulating specific rather than generic future events. The constructive episodic simulation hypothesis (Schacter & Addis, 2007a, 2007b, 2009) proposes that findings of greater hippocampal activity during the construction of future events reflects the increased relational processing demands of recombining details into a coherent event simulation. Specific events were rated as more detailed than general events (although this effect fell short of significance). However, the greater hippocampal response to specific future events cannot be attributed simply to detail, because past events were rated as more detailed than future events, but past events showed activity at or below baseline in the right hippocampus. Moreover, D’Argembeau, Xue, Lu, van der Linden and Bechara (2008) found no difference in hippocampal activity between near and far future events that differed in terms of subjective detail (i.e., vividness ratings). Detail could affect future-related hippocampal activity, however, in terms of varying how much information is encoded. Encoding more detail is associated with greater levels of anterior hippocampal activity (e.g., Giovanello, Schnyer, & Verfaellie, 2004; Staresina and Davachi, 2008). Disambiguating the processing of detail recombination from encoding the detailed event representation is an important step in understanding the contribution of the right anterior hippocampus to future simulation (cf. Martin, Schacter, Corballis and Addis, 2010).
Given that future events are also novel constructions, it is also conceivable that future-related hippocampal activity reflects, at least in part, a response to the inherent novelty of imagined events (e.g., Lepage, Habib, & Tulving, 1998; Ranganath & Rainer, 2003) that is not elicited when remembering already experienced events. If a simple effect of novelty detection underlies this future>past effect, it would be expected that the anterior hippocampus would be engaged more during the construction of both specific and general future events relative to past events. This pattern was not observed in the present study, where the future>past hippocampal effect was driven by specific future events – consistent with the behavioral finding that specific future events were considered more novel than general future events. Although both general and specific events were rated as containing novel information (i.e., very few events were rated as being identical to past experiences and thoughts), it is not surprising that imagined future routines drew more closely on previously experienced routines. In contrast, creating unique, specific events likely draws on information extracted and recombined from various and possibly unrelated past events, leading to more novel representations that lead to increased hippocampal engagement. This idea is consistent with recent findings from Weiler et al. (2010a) that more unique, low probability future events result in increased hippocampal activity than high probability future events, likely due to higher demand on binding of event features. Moreover, the current results are consistent with prior work showing that the hippocampus is particularly responsive to relational novelty (Kohler, et al., 2005) and the successful encoding of novel event representations (Poppenk, et al., 2010).
Aside from a difference in novelty, another explanation for the difference between specific and general future events is a more general difference between specific and generic events – that general events, whether past or future, are comprised of highly semanticized content and therefore may not even differ from the semantic control task. To rule out this explanation, we conducted supplementary analyses to determine whether past and future general events differed from each other and the semantic control task. Direct contrasts of general-future and general-past events provided evidence of neural differences between these event types (general-past>general-future; right occipital and right parahippocampal cortices, P-uncorrected< .001). Moreover, both general past and future events resulted in more activation in medial prefrontal, medial parietal and lateral parietal cortices (P-uncorrected< .001) than the semantic control task.
The present results suggest that the process of creating and encoding a detailed and novel event representation differentially engages the right anterior hippocampus compared with other forms of event simulation and recall. Thus, while it is likely that encoding, novelty and detail recombination all make contributions to episodic simulation, an important direction for future research will be to disambiguate the role of each process in engaging the anterior hippocampus during simulation.
Table 1.
Mean reaction times, temporal distance and post-scan ratings of autobiographical events.
| Mean Scores (SD) for Autobiographical Event Conditions
|
||||
|---|---|---|---|---|
| General-Future | Specific-Future | General-Past | Specific-Past | |
| Reaction time (seconds) | 7.12 (1.56) | 7.01 (1.48) | 7.31 (1.14) | 7.07 (1.70) |
| Temporal distance (years) | 7.02 (1.55) | 6.17 (1.44) | 5.67 (3.26) | 5.41 (1.46) |
| Valence** | 0.48 (0.26) | 0.27 (0.32) | 0.51 (0.23) | 0.08 (0.30) |
| Personal significance | 1.93 (0.66) | 2.19 (0.74) | 1.99 (0.56) | 1.86 (0.64) |
| Emotionality** | 2.30 (0.76) | 2.87 (0.70) | 2.39 (0.72) | 2.74 (0.66) |
| Details *† | 3.50 (0.39) | 3.77 (0.44) | 3.75 (0.52) | 3.90 (0.49) |
| Field (frequency) | 11.73 (2.28) | 12.53 (2.13) | 11.73 (3.10) | 12.40 (2.72) |
| Observer (frequency) | 0.67 (0.72) | 0.87 (1.19) | 0.67 (1.45) | 0.33 (0.62) |
| On-Task (frequency) | 11.93 (2.46) | 13.33 (1.71) | 11.93 (2.91) | 12.40 (2.75) |
| Off-Task (frequency) | 0.47 (0.64) | 0.07 (0.26) | 0.47 (0.64) | 0.33 (0.49) |
| Similarity to previous events** | 4.25 (.386) | 4.00 (.429) | n/a | n/a |
| Frequency of previous events** | 4.50 (.265) | 4.31 (.272) | n/a | n/a |
| Similarity to previous thoughts* | 3.86 (.421) | 3.47 (.546) | n/a | n/a |
| Frequency of previous thoughts* | 4.25 (.270) | 3.84 (.381) | n/a | n/a |
Note: For valence, 1=positive; 0=neutral; −1=negative.
Significant main effect of specificity, p ≤ .004 (Bonferroni-corrected threshold);
Non-significant main effect of specificity, p < .05;
Non-significant main effect of temporal direction, p < .05.
Acknowledgments
Grant sponsor: National Institute of Mental Health, Grant number: MH060941 (to DLS)
Grant sponsor: Royal Society of New Zealand Marsden Fund, Grant number: UOA0810 (to DRA)
We thank Joe Paxton and Jill Clark for assistance with data collection. This research was supported by National Institute of Mental Health (NIMH) grant MH060941 awarded to DLS and a Royal Society of New Zealand Marsden grant UOA0810 awarded to DRA.
References
- Abraham A, Schubotz RI, von Cramon DY. Thinking about the future versus the past in personal and non-personal contexts. Brain Research. 2008;1233:106–119. doi: 10.1016/j.brainres.2008.07.084. [DOI] [PubMed] [Google Scholar]
- Addis DR, McIntosh AR, Moscovitch M, Crawley AP, McAndrews MP. Characterizing spatial and temporal features of autobiographical memory retrieval networks: a partial least squares approach. Neuroimage. 2004;23:1460–1471. doi: 10.1016/j.neuroimage.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Addis DR, Pan L, Vu MA, Laiser N, Schacter DL. Constructive episodic simulation of the future and the past: Distinct subsystems of a core brain network mediate imagining and remembering. Neuropsychologia. 2009;47:2222–2238. doi: 10.1016/j.neuropsychologia.2008.10.026. [DOI] [PubMed] [Google Scholar]
- Addis DR, Schacter DL. Effects of detail and temporal distance of past and future events on the engagement of a common neural network. Hippocampus. 2008;18:227–237. doi: 10.1002/hipo.20405. [DOI] [PubMed] [Google Scholar]
- Addis DR, Wong AT, Schacter DL. Remembering the past and imagining the future: Common and distinct neural substrates during event construction and elaboration. Neuropsychologia. 2007;45:1363–1377. doi: 10.1016/j.neuropsychologia.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botzung A, Dankova E, Manning L. Experiencing past and future personal events: Functional neuroimaging evidence on the neural bases of mental time travel. Brain and Cognition. 2008;66:202–212. doi: 10.1016/j.bandc.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton JL, Valabregue R, Poline JB. Region of interest analysis using an SPM toolbox [abstract]. Presented at the 8th International Conference on Functional Mapping of the Human Brain. Neuroimage. 2002;16(2) [Google Scholar]
- Buckner RL. The role of the hippocampus in prediction and imagination. Annual Review of Psychology. 2010;61:27–48. doi: 10.1146/annurev.psych.60.110707.163508. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Carroll DC. Self-projection and the brain. Trends in Cognitive Sciences. 2007;11:49–57. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Clark JM, Paivio A. Extensions of the Paivio, Yuille, and Madigan (1968) norms. Behavior Research Methods, Instruments and Computers. 2004;36:371–383. doi: 10.3758/bf03195584. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Poldrack RA, Eichenbaum H. Memory for items and memory for relations in the procedural/declarative memory framework. Memory. 1997;5:131–178. doi: 10.1080/741941149. [DOI] [PubMed] [Google Scholar]
- D’Argembeau A, Xue G, Lu ZL, van der Linden M, Bechara A. Neural correlates of envisioning emotional events in the near and far future. Neuroimage. 2008;40:398–407. doi: 10.1016/j.neuroimage.2007.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RA. Statistical Methods for Research Workers. London: Oliver & Boyd; 1950. [Google Scholar]
- Giovanello KS, Schnyer DM, Verfaellie M. A critical role for the anterior hippocampus in relational memory: evidence from an fMRI study comparing associative and item recognition. Hippocampus. 2004;14:5–8. doi: 10.1002/hipo.10182. [DOI] [PubMed] [Google Scholar]
- Hassabis D, Kumaran D, Maguire EA. Using imagination to understand the neural basis of episodic memory. The Journal of Neuroscience. 2007;27:14365–14374. doi: 10.1523/JNEUROSCI.4549-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassabis D, Maguire EA. Deconstructing episodic memory with construction. Trends in Cognitive Sciences. 2007;11:299–306. doi: 10.1016/j.tics.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Foley MA, Suengas AG, Raye CL. Phenomenal characteristics of memories for perceived and imagined autobiographical events. Journal of Experimental Psychology: General. 1988;117:371–376. [PubMed] [Google Scholar]
- Kohler S, Danckert S, Gati JS, Menon RS. Novelty responses to relational and non-relational information in the hippocampus and the parahippocampal region: a comparison based on event-related fMRI. Hippocampus. 2005;15:763–774. doi: 10.1002/hipo.20098. [DOI] [PubMed] [Google Scholar]
- Lepage M, Habib R, Tulving E. Hippocampal PET activations of memory encoding and retrieval: the HIPER model. Hippocampus. 1998;8:313–322. doi: 10.1002/(SICI)1098-1063(1998)8:4<313::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Martin VC, Schacter DL, Corballis MC, Addis DR. Remembering the future: Hippocampal contributions to encoding future simulations [abstract]. Presented at the 16th Annual Meeting of the Organization for Human Brain Mapping.2010. [Google Scholar]
- Okuda J, Fujii T, Ohtake H, Tsukiura T, Tanji K, Suzuki K, et al. Thinking of the future and the past: The roles of the frontal pole and the medial temporal lobes. Neuroimage. 2003;19:1369–1380. doi: 10.1016/s1053-8119(03)00179-4. [DOI] [PubMed] [Google Scholar]
- Poppenk J, McIntosh AR, Craik FIM, Moscovitch M. Past experience modulates the neural mechanisms of episodic memory formation. The Journal of Neuroscience. 2010;30:4707–4716. doi: 10.1523/JNEUROSCI.5466-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ. Calculation for the chi-square test: An interactive calculation tool for chi-square tests of goodness of fit and independence [Computer software] 2001 Available from http://www.quantpsy.org.
- Ranganath C, Rainer G. Neural mechanisms for detecting and remembering novel events. Nature Reviews Neuroscience. 2003;4:193–202. doi: 10.1038/nrn1052. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR. The cognitive neuroscience of constructive memory: Remembering the past and imagining the future. Philosophical Transactions of the Royal Society of London Series B: Biological Sciences. 2007a;362:773–786. doi: 10.1098/rstb.2007.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Addis DR. The ghosts of past and future. Nature. 2007b;445:27. doi: 10.1038/445027a. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR. On the nature of medial temporal lobe contributions to the constructive simulation of future events. Philosophical Transactions of the Royal Society B-Biological Sciences. 2009;364:1245–1253. doi: 10.1098/rstb.2008.0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Buckner RL. The prospective brain: Remembering the past to imagine the future. Nature Reviews Neuroscience. 2007;8:657–661. doi: 10.1038/nrn2213. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Buckner RL. Episodic simulation of future events: concepts, data, and applications. Annals of the New York Academy of Sciences - Special Issue: The Year in Cognitive Neuroscience 2008. 2008;1124:39–60. doi: 10.1196/annals.1440.001. [DOI] [PubMed] [Google Scholar]
- Slotnick SD, Moo LR, Segal JB, Hart J. Distinct prefrontal cortex activity associated with item memory and source memory for visual shapes. Cognitive Brain Research. 2003;17:75–82. doi: 10.1016/s0926-6410(03)00082-x. [DOI] [PubMed] [Google Scholar]
- Suddendorf T, Corballis MC. Mental time travel and the evolution of the human mind. Genetic Society General Psychology Monographs. 1997;123:133–167. [PubMed] [Google Scholar]
- Suddendorf T, Corballis MC. The evolution of foresight: What is mental time travel, and is it unique to humans? Behavioral and Brain Sciences. 2007;30:299–351. doi: 10.1017/S0140525X07001975. [DOI] [PubMed] [Google Scholar]
- Szpunar KK, McDermott KB. Episodic future thought and its relation to remembering: Evidence from ratings of subjective experience. Consciousness & Cognition. 2008;17:330–334. doi: 10.1016/j.concog.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. New York, N.Y: Thieme Medical Publishers, Inc; 1988. [Google Scholar]
- Weiler JA, Suchan B, Daum I. Foreseeing the future: Occurrence probability of imagined future events modulates hippocampal activation. Hippocampus. 2010a;20:685–690. doi: 10.1002/hipo.20695. [DOI] [PubMed] [Google Scholar]
- Weiler JA, Suchan B, Daum I. When the future becomes the past: Differences in brain activation patterns for episodic memory and episodic future thinking. Behavioural Brain Research. 2010b;212:196–203. doi: 10.1016/j.bbr.2010.04.013. [DOI] [PubMed] [Google Scholar]