Abstract
In this study, the role of RON (receptor originated from nantes) in tumor progression was further investigated in context with MET expression and activity. RON and MET expressions were not detected in an immortalized normal human pancreas cell line (HPNE), but were co-expressed in five of seven pancreatic ductal adenocarcinoma (PDAC) cell lines (PANC-1, BxPC-3, Capan-2, CFPAC-1 and AsPC-1). RON expression was knocked down by an shRNA approach in two PDAC cell lines (BxPC-3 and CFPAC-1) that co-express MET. Knockdown of RON significantly inhibited cell growth, clonogenicity and macrophage stimulating protein (MSP), RON ligand induced invasion by in vitro assays and significantly inhibited tumor growth (P<0.001) and metastasis (P<0.009) in an orthotopic pancreatic cancer mouse model at week 7. However, by week 9, the mice implanted with RON knockdown cells had developed similar size primary tumors and metastases compared with that seen in the control group at week 7. Western blotting and immunohistochemistry analyses showed that MET remains highly expressed in cells and tumor tissues where RON was knocked down. Moreover, knockdown of RON did not prevent hepatocyte growth factor (HGF) stimulated invasion in in vitro Matrigel assays. Treating cells with MSP induced the transphosphorylation of MET, suggesting that signaling may be modulated by relative levels of RON and MET receptors and their corresponding ligands. To this point, HGF treatment of RON knockdown cells caused an increase in intensity and duration of MET signaling, suggesting that MET signaling may compensate for loss of RON signaling. Treatment of cells with an MET inhibitor, PHA-665752, had minimal effects on inhibiting cell growth but significantly inhibited cell invasion induce by ligands for either MET or RON. These results suggest that HGF/MET signaling may have a more important role in tumor cell invasion and metastasis rather than in tumor cell proliferation. This study indicates that specific inhibition of RON delays but does not prevent progression of PDAC. Moreover, specific signaling may be modulated by the interaction of RON and MET receptors. This dynamic interaction of RON and MET in pancreatic cancer cells suggests that dual targeting of both RON and MET will be preferable to inhibition of either target alone.
Keywords: RON, MET, pancreatic cancer, cancer therapy
Introduction
RON (receptor originated from nantes) is a member of the MET-proto-oncogene family of receptor tyrosine kinases. RON belongs to the semaphorin superfamily composed of three protein families, the semaphorins, plexins and the MET family. All three families share an N-terminal sema domain that contains the ligand-binding domain and most possess an adjacent cysteine-rich domain.1, 2 The mature forms of RON and MET are ∼180 kD heterodimeric proteins comprised of an extracellular 40 kD α-chain and a 145 kD transmembrane β-chain with intrinsic tyrosine kinase activity. RON shares only 25% homology with MET in its extracellular domain but shares 63% homology within the tyrosine kinase domain. Macrophage stimulating protein (MSP) and hepatocyte growth factor (HGF) are ligands that activate RON and MET, respectively.1, 3
Ligand-induced activation of RON and MET transduces cell signaling through multiple targets including MAPK, PI3K/AKT, c-Src, FAK and β-catenin/TCF. RON and MET have essential functional roles in embryonic development and organogenesis4 and numerous reports3, 5, 6 show that RON and MET are overexpressed and/or aberrantly activated in various cancer types suggesting their potential importance as therapeutic targets. Overexpression of RON or MET in cancers is associated with poor prognosis.7, 8, 9 RON and MET signaling mediates tumor progression, at least in part, by increasing proliferation, by inhibiting apoptosis and by promoting metastasis pathways.7, 10, 11 Agents targeting RON and MET for cancer therapy are in various phases of clinical trials and/or pre-clinical testing and these include small molecular weight kinase inhibitors and neutralizing antibodies.12, 13, 14, 15
Aberrant expression and activities of RON and MET in cancer are attributed to various mechanisms including increased expression of their ligands or receptors and activating mutations as reviewed by Lu et al.16 and Gherardi et al.4 Overexpression of MET but seldom RON is linked to gene amplification.7, 17 TGFβ negatively regulates RON transcription in a Smad-dependent manner; therefore, cancer cells that lose or have suppressed Smad signaling may show increased RON transcription.18 HIF-1α was recently identified as a key positive regulator of RON promoter activity,19 which is also consistent with the finding that hypoxia drives MET expression by upregulating HIF-1α.20 Pro-tumorigenic activities of RON have also been attributed to different isoforms identified in cancer cells. At least six isoform variants of RON are known and these likely originate by alternative pre-mRNA processing, by alternative transcription or by truncation.16 Thus, cancer cells may possess a variety of mechanisms that account for increased expression and/or activity of RON and MET.
The homology between RON and MET suggests that they may interact. RON and MET are reported to be co-expressed in several tumor types21, 22 and cross-talk between these two receptor pathways is known to occur.23 A recent study in various types of cancer cells indicates that RON can be transphosphorylated by MET and that oncogenic addiction to MET requires co-expression of RON.24 Thus, it is likely that signaling may be modulated in tumors co-expressing these two receptors and therapeutic strategies designed to specifically target one or the other could be problematic due to compensatory mechanisms. Studies from our laboratory and others18, 25, 26 have reported that RON is overexpressed in pancreatic cancers and pancreatic cancer cell lines. A recent study showed that RON is increasingly expressed during progression of pancreatic cancer27 and that RON expression is sustained in pancreatic cancer stem cells,28 suggesting its potential value as a therapeutic target for this disease. Moreover, inhibiting RON expression suppressed growth of pancreatic cancer xenografts and increased sensitivity to gemcitabine. Interestingly, regrowth of one tumor showed increased expression of other phosphotyrosine kinase receptors.26 These findings raise the possibilities that RON signaling may be modulated by co-expression of MET and that MET signaling could mitigate anti-tumor effects of inhibiting RON and vice versa.
In this study, we investigated the effects of inhibiting RON expression on cell signaling pathways and tumorigenicity in pancreatic cancer cells that co-express RON and MET. In these cells, RON and MET formed both homo- and heterodimers and the RON ligand MSP was able to activate MET. This study indicates that specific inhibition of RON delays but does not prevent tumor progression. Moreover, MET signaling is maintained and enhanced in pancreatic cancer cells where RON is knocked down. Thus, there is a dynamic interaction of RON and MET in pancreatic cancer cells. The consequence of this interaction on modulating signaling is not fully understood. However, using combination therapy or a dual kinase inhibitor of RON and MET will be more effective than specific inhibition of either target alone.
Results
Knockdown of RON inhibits cell growth and clonogenicity
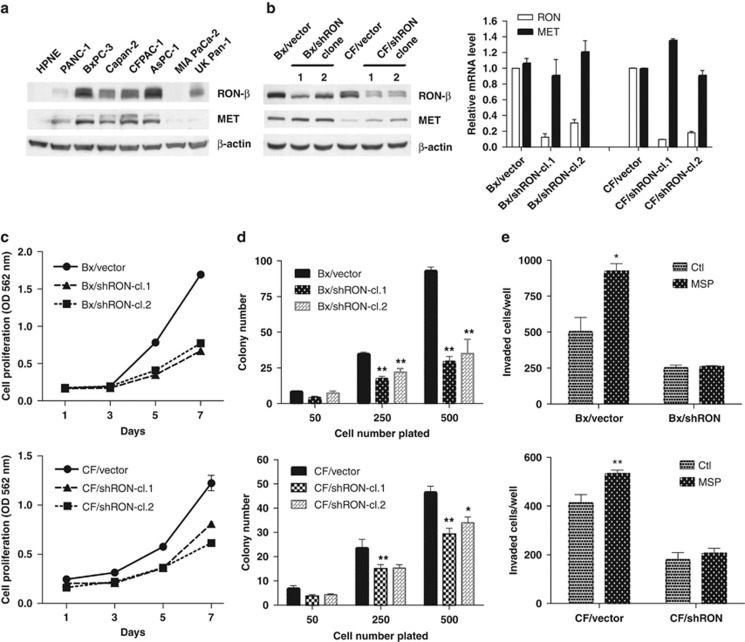
RON is overexpressed in pancreatic cancer and this overexpression is correlated with tumor progression, suggesting that RON may be a valuable target for therapy.27 Our laboratory previously found that knockdown of RON inhibited invasion of BxPC-3 cells.18 In the current study, RON and MET expression was compared in seven pancreatic ductal adenocarcinoma (PDAC) cell lines and one hTERT-immortalized human pancreatic ductal cell line (HPNE). Five of seven PDAC cell lines expressed relatively high levels of RON (BxPC-3, Capan-2, CFPAC-1, AsPC-1 and UK Pan-1), one cell line (PANC-1) expressed a low level of RON; whereas RON was not detected in MIA PaCa-2 cells and in the immortalized HPNE cells (Figure 1a). MET was readily detected in five of the seven PDAC cell lines with trace levels observed in two PDAC cell lines (MIA PaCa-2 and UK Pan-1) and as with RON, MET was not detected in HPNE cells (Figure 1a). To further investigate the potential role of RON in the development and progression of pancreatic cancer, an shRNA approach was used to knockdown RON in BxPC-3 and CFPAC-1 cells (designated as Bx/shRON and CF/shRON, respectively). Western blotting and quantitative RT–PCR confirmed that RON was knocked down in these cells (Figure 1b). These two PDAC cell lines co-express RON and MET (Figure 1b). Interestingly, Bx/shRON and CF/shRON showed a slight increase in MET expression compared with the control cells (Figure 1b). In vitro studies showed that knockdown of RON inhibited cell growth at day 7 by an average of 59% for BxPC-3 and 42% for CFPAC-1 cells (Figure 1c) and reduced colony formation by 65% for BxPC-3 cells and by 33% for CFPAC-1 cells (see cells plated at a density of 500 cells/6 cm2 plate) compared with the controls (Figure 1d). MSP induced invasion in control BxPC-3 and CFPAC-1 cells, but did not induced in cells where RON was knocked down as determined by in vitro Matrigel assays (Figure 1e).
Figure 1.
Knockdown of RON inhibits cell growth and clonogenicity. (a) Expression level of RON-β and MET protein from normal pancreatic ductal cell line (HPNE) and PDAC cell lines were analyzed by western blotting. (b) RON-β and MET expression levels were analyzed by western blotting (left) and quantitative RT–PCR (right) in RON knockdown clones (shRON cl.1 and shRON cl.2) and vector control cells. (c) 1000 cells per well were seeded in 96-well plates and cell proliferation rates were determined by MTT assay at indicated time points. Bars represent s.d. of six replicates. (d) Clonogenicity assays were performed as described previously.50 Briefly, cells were seeded at 50, 250 and 500 cells per 6 cm2 dish and cultured for 2 weeks. The cells were stained with crystal violet and colonies were counted. The data were presented as mean±s.d. of experiments performed in triplicates. *P<0.05 and **P<0.01 compared with their vector controls, respectively. (e) 3 × 104/well cells were seeded in 24-well Matrigel invasion chambers and treated with 200 ng/ml of MSP for 24 h. Invaded cells were counted under microscope and the data represent mean±s.d. from triplicate experiments. *P<0.05 and **P<0.01 compared with their vector controls, respectively.
Knockdown of RON suppresses tumor growth and delays metastasis
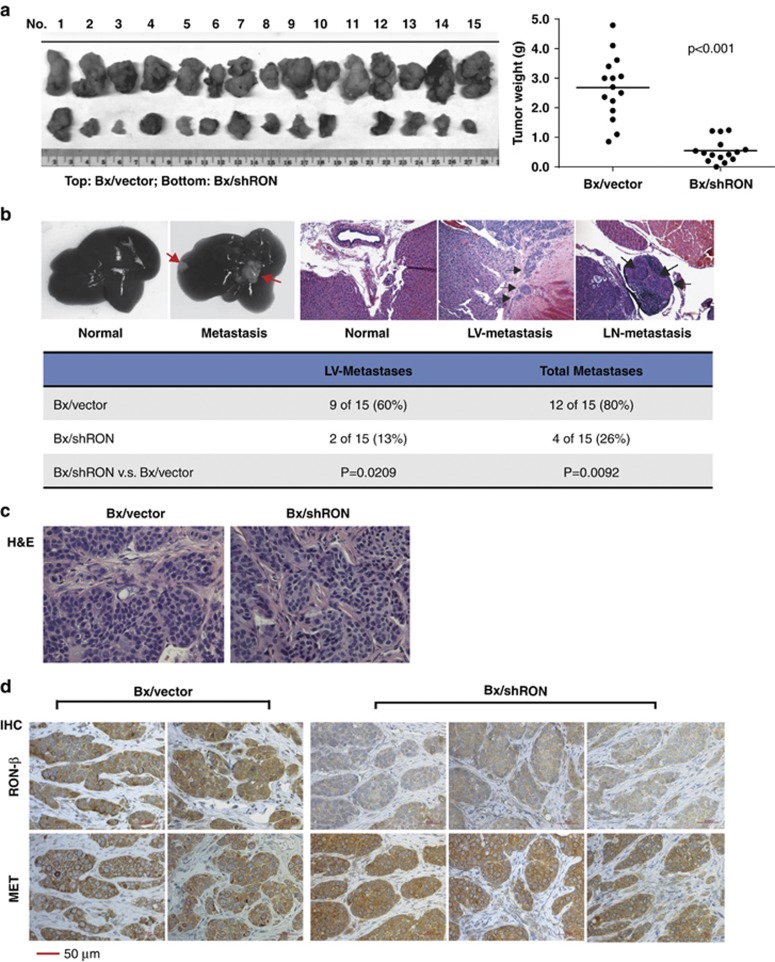
To further study the effects of RON on PDAC tumor progression in vivo, Bx/vector and Bx/shRON cells were orthotopically implanted into the pancreas of athymic nude mice. Seven weeks after implantation, primary tumor and metastases were evaluated grossly and histologically. As shown in Figure 2a, downregulation of RON significantly suppressed tumor growth (P<0.001 compared with the vector control group, n=15). The RON knockdown group revealed a striking reduction in hepatic hilar, peritoneum and mesenteric lymph-node metastases compared with the control group at week 7 after tumor cell implantation. Photographic images of resected livers and histologic tissue sections are shown in Figure 2b. Metastases observed in RON knockdown and control groups are summarized in the table at the bottom of this figure. Histological examination of the primary tumors showed no obvious differences between the control and RON knockdown groups (Figure 2c). Immunohistochemistry studies with RON and MET antibodies of primary tumors confirmed the reduced RON expression in tumors from Bx/shRON cells; whereas MET expression remained high in these tumors (Figure 2d).
Figure 2.
Knockdown of Ron suppresses tumor growth and delays metastasis. 1 × 106 Bx/vector and Bx/shRON cl.1 cells were implanted into the tail of pancreas of nude mice. Primary tumors and metastases were examined grossly and pathologically at 6–7 weeks after implantation. (a) Images of primary tumors (left) from Bx/vector and Bx/shRON cells and the distribution of tumor weights (right) from mice implanted with Bx/vector and Bx/shRON cells. The mean (n=15) of each group is represented by a horizontal line. (b) Representative photos show a normal liver from a mouse implanted with Bx/shRON cells and metastases to the liver from a mouse implanted with BxPC-3 vector control cells. The photos of H&E stained tissue sections show a tumor-free area of the liver, tumor infiltration into liver vessels (LVs) and metastases to a surrounding lymph node (LN) from Bx/vector tumors. The table summarized the total metastases of each group. (c) Representative photos of H&E stained Bx/vector and Bx/shRON tumors. (d) Immunohistochemistry staining of RON-β and MET of the orthotopic tumor section from Bx/vector and Bx/shRON cells. MET expression remains high in tumors generated from Bx/shRON cells.
Knockdown of RON delays but does not prevent tumor progression and metastasis
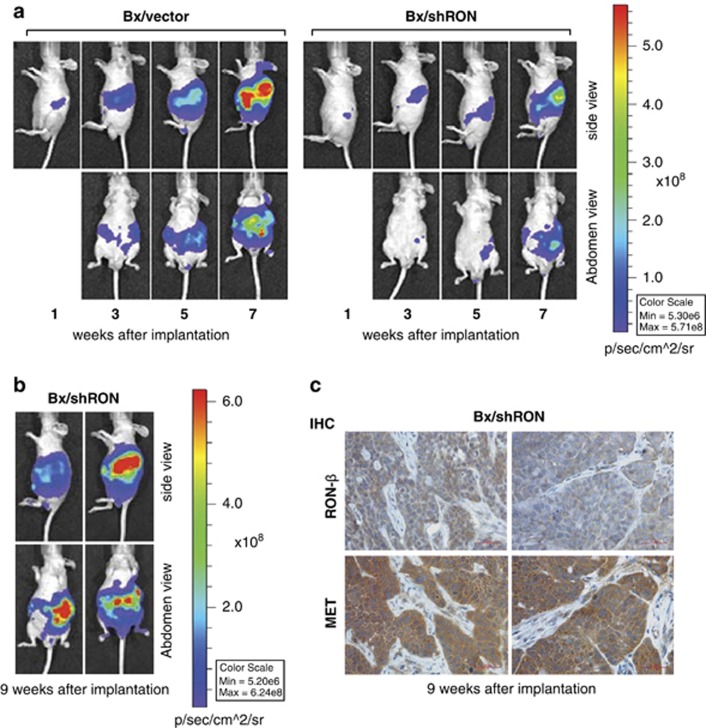
To determine the long-term effect of knockdown of RON on tumor progression, we repeated the in vivo study using luciferase expressing Bx/vector cells and Bx/shRON cells. This allowed for in vivo monitoring of tumor growth and metastasis. Representative images of each group are shown at weeks 1, 3, 5 and 7 (Figure 3a). Mice from the control group developed fast growing primary tumors and metastases. By week 7, control group mice began to develop ascites and show signs of stress, at this point this group of mice was euthanized. The mice with tumors from the RON knockdown group were maintained for 2 additional weeks and monitored for tumor progression by luciferase imaging. At week 9, the RON knockdown group developed similar metastasis as observed from the control group at week 7, as shown by representative images of two animals (Figure 3b). This result indicates that inhibition of RON delays but does not prevent tumor progression and metastasis. Decreased RON expression in RON knockdown tumors was confirmed by immunohistochemistry using an antibody to RON; however, MET was highly expressed in the same tumors from the RON knockdown group (Figure 3c).
Figure 3.
Knockdown of RON delays but does not prevent tumor progression and metastasis. (a) Representative luciferase images of mice at 1, 3, 5 and 7 weeks after orthotopically implanted with Bx/vector-Luc or Bx/shRON-Luc cells. (b) Representative luciferase images of mice at week 9 after implanted with Bx/shRON-Luc cells. (c) Immunohistochemistry staining of RON-β and MET of the primary tumor sections from Bx/shRON cells at week 9 after implantation. RON remains low, but MET is highly expressed in these tumors.
Knockdown of RON enhances HGF/MET signaling
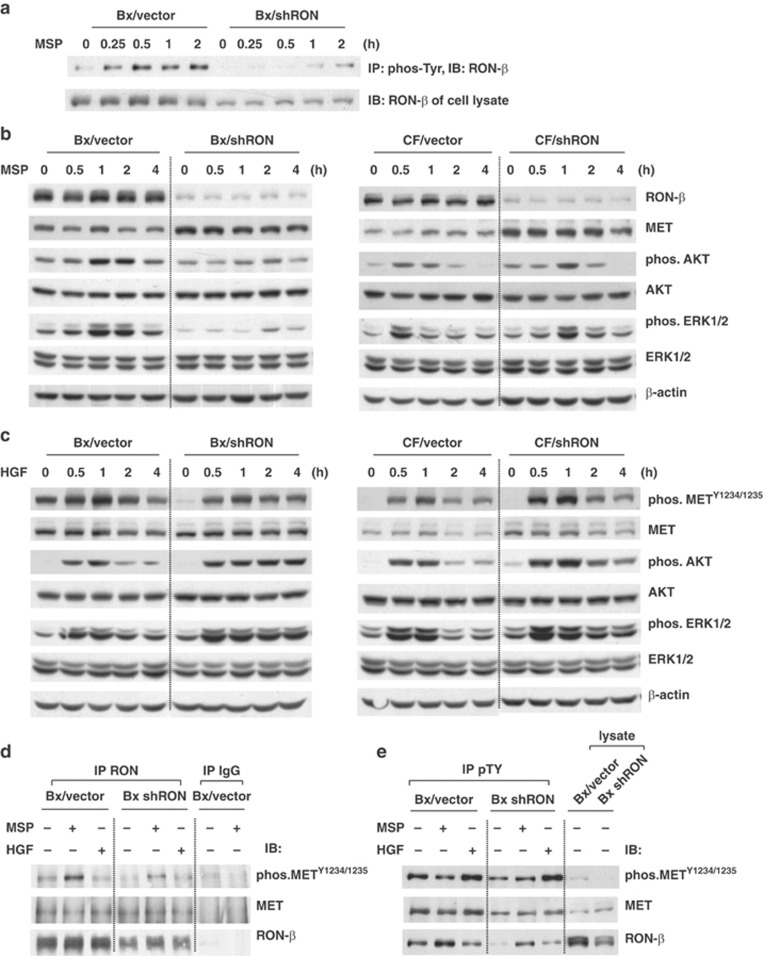
Our in vivo studies indicate that MET remains highly expressed in RON knockdown tumors, suggesting that MET signaling may contribute to tumorigenic properties of RON knockdown cells. MSP/RON and HGF/MET signaling was further compared in control and RON knockdown BxPC-3 and CFPAC-1 cells. Cells were serum deprived overnight and then stimulated with MSP for different time periods and immunoprecipitation was performed with an anti-phosphotyrosine antibody. MSP-induced phosphorylation of RON was significantly suppressed in BxPC-3 cells where RON was knocked down (Figure 4a). The phosphorylation of AKT and ERKs, two downstream targets of RON, was also inhibited in Bx/shRON cells. Unlike BxPC-3 cells, MSP caused only delayed phosphorylation of AKT and ERKs in RON knockdown CFPAC-1 cells compared with their vector control cells (Figure 4b). The delayed activation of downstream signaling in CF/shRON cells could be due to cell type differences. In contrast, cells stimulated with MET ligand, HGF, induced a stronger or prolonged phosphorylation of METY1234/1235 and its downstream targets, ERKs and AKT in both BxPC-3 and CFPAC-1 cells where RON was knocked down (Figure 4c). Immunoprecipitation studies revealed that RON and MET were co-associated and that MSP induced a transphosphorylation of METY1234/1235 in BxPC-3 cells where RON is highly expressed (Figure 4d). As anticipated MSP induced a lower level of phosphorylation of MET in RON knockdown cells (Figure 4d), suggesting that RON could form a heterodimer with MET and transphosphorylate MET upon MSP stimulation. Unlike the effect seen with MSP treatment, HGF stimulation only phosphorylated METY1234/1235 but did not transphosphorylate RON or induce MET and RON dimerization (Figure 4d and e). This suggests that MET may preferentially homodimerize when receptors are expressed in similar levels.
Figure 4.
Knockdown of RON enhances HGF/MET signaling. Subconfluent cells were serum starved for 16 h and stimulated with 200 ng/ml of MSP or 50 ng/ml of HGF for the indicated time periods (for 30 min in d and e). (a, b) Cells were stimulated with MSP. (a) 500 μg of total cell lysates was subjected to immunoprecipitation using anti-phospho-tyrosine (4G10) monoclonal antibody and MSP-induced phosphorylation of RON-β was detected with anti-RON-β antibody. (b) Cell lysates (40 μg) were analyzed by western blotting and phosphorylation of AKT and ERK1/2 was detected with indicated antibodies. β-Actin was used for the protein loading control. (c) Cells stimulated with HGF. Cell lysates (40 μg) were analyzed by western blotting for phosphorylated forms of MET, AKT and ERK1/2 using the indicated antibodies. β-Actin was used for the protein loading control. (d) 500 μg of cell lysates was subjected to immunoprecipitation with anti-RON-β antibody. MSP-induced phosphorylation of MET was detected by phospho-MET antibody. Co-association of MET with RON-β was detected with MET antibody. (e) 500 μg of cell lysates was subjected to immunoprecipitation with anti-phospho-tyrosine (4G10) antibody. The phosphorylation of RON-β or MET was detected with indicated antibodies.
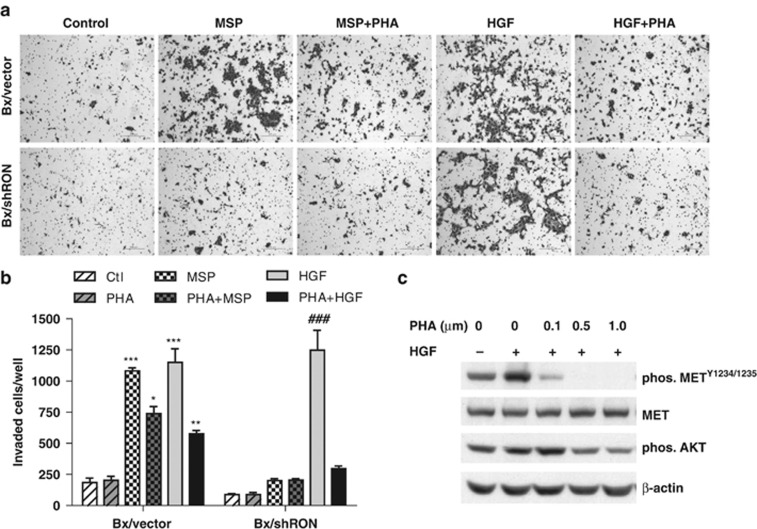
MET inhibition blocks HGF-induced invasion
To determine whether MET signaling may contribute to tumor progression in RON knockdown cells, we utilized small molecule MET inhibitor, PHA-665752, to block HGF/MET signaling pathway. Cell growth, clonogenicity and invasiveness were compared in cells where RON is expressed or was knocked down. Treatment of cells with an MET inhibitor, PHA-665752, at lower doses (⩽0.5 μM) did not significantly affect cell growth and colony formation and at higher concentration (⩾1.0 μM) caused cell death in both RON expressing and RON knockdown cells (data not shown). Next, in vitro Matrigel invasion assay was performed in Bx/vector and Bx/shRON cells. As anticipated, knockdown of RON inhibited MSP-induced invasion, but did not prevent HGF stimulated invasion (Figure 5a). Interestingly, treatment with PHA-665752 had little effect on cell growth and colony formation; however, treatment with 0.5 μM of PHA-665752 significantly blocked HGF-induced cell invasion of both RON expressing and RON knockdown cells and a more potent inhibition was seen for RON knockdown cells (Figure 5a and b). PHA-665752 also partially inhibited MSP-induced invasion in RON expressing cells. In agree with that reported by Christensen et al.,5 this compound also inhibited RON kinase activity at higher concentration (cellular IC50 as 0.9 μM). Efficacy of PHA-665752 inhibiting phosphorylation of METY1234/1235 and downstream target AKT is shown in Figure 5c.
Figure 5.
MET inhibition blocks HGF-induced cell invasion. Bx/vector and Bx/shRon cells were seeded in 24-well Matrigel invasion chambers and treated with 200 ng/ml of MSP or 50 ng/ml of HGF for 24 h in the presence or absence of 0.5 μM MET inhibitor PHA-665752. (a) Representative images of Matrigel invasion assays (6 × 104 cells/well plated). (b) The quantitative graph of invasion assays (3 × 104 cells/well plated). The data represent mean±s.d. from repeated duplicate experiments. *P<0.05; **P<0.01; ***P<0.001 compared with Bx/vector control; ###P<0.001 compared with Bx/shRon control. (c) Subconfluent BxPC-3 cells were serum starved for 16 h and pretreated cells with different doses of PHA-665752 for 4 h, then stimulated with 50 ng/ml of HGF for 30 min. Cell lysates were analyzed by western blotting. Inhibition of phosphorylation of MET and AKT by PHA-665752 was detected with phos-METY1234/1235 or phos-AKT antibodies, respectively.
Discussion
Long-term survival for patients with PDAC has improved little over the past decade. This has led to clinical trials with agents that target pathways that are aberrantly activated in PDAC in combination with conventional chemotherapy.29, 30 Traceva, an epidermal growth factor receptor (EGFR) kinase inhibitor, was approved for use in pancreatic cancer on the bases of a modest increase in 6-month survival.31 This modest response with Traceva suggests that molecules other than EGFR, including other receptor kinases, may be important drivers of cancer cell survival in PDAC. One such potential target is RON receptor tyrosine kinase that is overexpressed in many PDAC cell lines and tumor specimens.25 RON is a member of the MET family and we report here that RON and MET are often co-expressed in PDAC. RON and MET may have different as well as overlapping functions. Both RON and MET are involved in cancer cell migration, invasion, metastasis, as well as in apoptotic resistance.32, 33, 34 Studies indicate that RON and MET form both homo- and heterodimers although, whether there are differences in downstream targets activated by homo- versus heterodimers for RON and MET have not been thoroughly investigated.23 Moreover, it is not known whether targeting RON or MET alone may induce compensation through the other receptor kinases. In the current study, in vitro cell models and an orthotopic mouse model were used (1) to assess the effects of inhibiting RON on MET expression and signaling and (2) to determine whether inhibiting RON was sufficient to prevent invasive properties of PDAC.
In agreement with previous studies that RON was overexpressed in the majority of PDAC,25, 27 we found that five of seven PDAC cell lines examined showed high expression of RON and co-expressed MET. The influence of RON on tumor progression in context with MET signaling is not known. RON was knocked down in two of the cell lines that showed co-expression of RON and MET (BxPC-3 and CFPAC-1). Interestingly, there was a subtle increase in MET expression in RON knockdown cells compared with the control cells, suggesting that MET may compensate for loss of RON.
Several in vitro and pre-clinical studies have investigated the potential of targeting of RON for therapy of pancreatic cancer. O'Toole et al.35 showed that treating mice with neutralizing antibodies to RON inhibited pancreatic xenograft tumor growth by 50% and when combined with the EGFR inhibitor, Erbitux, further tumor regression was observed. Logan-Collins recently reported that silencing RON in pancreatic murine xenograft model suppressed tumor growth and increased sensitivity to gemcitabine. However, some tumors escaped from increased sensitivity to gemcitabine induced by targeting RON,26 although the mechanism by which this occurs is not fully understood. In vitro and in vivo animal studies suggest that targeting RON may prevent invasion and inhibit metastasis.26, 27, 35 In agreement with these studies, the data presented here show that knockdown of RON inhibited cell growth and colony formation in vitro and inhibited tumor growth and metastasis at week 7 in the BxPC-3 orthotopic pancreatic tumor model used here, suggesting that RON is a viable target independent of whether MET is co-expressed. The studies were repeated for confirmation and also for determining long-term effects of inhibiting RON on tumor progression. Our study demonstrates that knockdown of RON slows tumor growth but it does not prevent tumor progression and metastasis over time. Although delayed by up to 2 weeks, RON knockdown mice developed similar invasiveness and metastasis to the control group. RON expression remained suppressed in tumors from RON knockdown cells; however, MET expression was high or upregulated in RON knockdown tumors suggesting that MET may have compensatory effects. The mechanisms of upregulation of MET in RON knockdown tumors are currently not clear. Ide et al.36 showed that hypoxia drives MET expression in tumor-stromal cells and activation of paracrine HGF/MET signaling in pancreatic cancer and Qian et al.37 reported that cultured pancreatic cancer cells increased MET expression following radiation treatment. These studies suggest that targeting a specific growth factor receptor may induce physiologic stress that in turn causes tumor cells to activate alternative growth/survival pathways.
Receptor cross-talk or synergies in downstream signaling have been reported.38, 39 ERKs and AKT are known to be common and key downstream effector molecules of RON and MET that promote cell growth and survival.23, 40 It is of interest that knockdown of RON in cells inhibited or delayed MSP, a ligand specific for RON, induced activation of ERKs and AKT. However, knockdown of RON enhanced and prolonged HGF-induced activation of ERKs and AKT. MSP and HGF are known to affect cell growth and migration/invasion, and it is believed that cancer cells expressing RON or MET are prone to tissue invasion and metastasis.32, 33 The ability of HGF/MET to induce metastasis in different organs has been shown experimentally with xenografts of tumor cells that are transfected with HGF or MET, as well as in transgenic mice that overexpress HGF or MET.41, 42, 43 A role for MET in metastatic progression has also been established in patients with head and neck cancer.44 We observed that MET was highly expressed or upregulated in RON knockdown tumors and that MSP could cause the transphosphorylation of MET in vitro. In tumor microenvironments, both autocrine and paracrine MSP and HGF could activate MET signaling, which favors tumor cell growth and migration/invasion. In this study, we show that MET inhibitor PHA-665752 had little effects on cell growth and colony formation using in vitro cultures; however, PHA-665752 caused a significant inhibition of cell invasion induce by HGF and to a lesser extent inhibition induced by MSP as determined by Matrigel invasion assays. These results suggest that HGF/MET signaling may have a more important role in tumor cell invasion and metastasis rather than in tumor cell proliferation.
Activation of tyrosine kinase receptors is based on the ligand-induced dimerization of receptors that leads to transphosphorylation and activation of the intracellular kinase domain with subsequent signal transduction and pathway activation. The formation of homodimers represents the basic mechanism that triggers signaling cascade. The ability to form heterodimers was found between members of the same receptor family, in particular, heterodimerization between EGFR and other erbB family members.45, 46 In some cases, the formation of heterodimer results in more efficient signaling, such as the expanded signaling capacity of the ErbB2/ErbB3 heterodimer.47 By using immunoprecipitation, we demonstrated the formation of heterocomplexes between RON and MET, which suggests cross-talk between these two receptor kinases. The formation of heterocomplexes and transphosphorylation between MET and RON may lead to reciprocal regulation of the kinase activity. A recent study by Benvenuti et al.24 showed that RON is specifically transphosphorylated by activated MET in MET addicted cancer cells. We show here that MSP stimulation induces phosphorylation of RON and transphosphorylation of MET, suggesting that reciprocal regulation of these two receptor kinases may be the cell context dependent. In this study, the limited efficacy for suppressing tumor progression by knockdown RON could arise from unblocked MET signaling. In cells where RON was knocked down, a stronger and prolonged activation of ERKs and AKT was observed in PDAC cells stimulated with HGF. In addition to the interaction of MET with RON, MET is reported to interact with other phosphotyrosine kinases, such as EGFR and insulin-like growth factor receptor.38, 48 The activation of multiple pathways may also contribute to tumor progression in RON knockdown tumors. Further studies are needed to define the role of the interaction of RON with other phosphotyrosine kinases. The co-expression of RON and MET and their abilities to function as homodimer or heterodimer suggest that it is not sufficient to specifically target only one of these receptors. Rather, this suggests that MET should also be targeted in PDAC that co-express both receptors.
This study revealed for the first time that blocking RON delays but does not prevent tumor progression and metastatic disease. Upregulation of MET in RON knockdown tumors and increased HGF/MET signaling in RON knockdown cells could contribute to metastatic progression of these tumors. The complexity of the signaling network in tumors implies that interfering with a single component of the network will not be sufficient for a targeted therapy. Combined or multi-targeted therapies will likely be preferable strategies for the successful treatment of pancreatic cancer.
Materials and methods
Cell culture and treatment
Human PDAC cell lines BxPC-3, Canpan-2, MIA PaCa-2, CFPAC-1, PANC-1 and AsPC-1 were from American Type Culture Collection (ATCC, Rockville, MD, USA); UK Pan-1 was established in our laboratory.49 Cells were maintained in medium as recommended and supplemented with 10% fetal bovine serum. Human recombinant MSP and HGF were purchased from R&D Systems (Minneapolis, MN, USA). pSuper-shRNA-RON plasmid was kindly provided by Jenny Wang (University of Nebraska Medical Center, Omaha, NE) and PMMP-luciferase plasmids were from Ricardo Aguiar (University of Texas Health Science Center, San Antonio, TX). The plasmids were transfected into human embryonic kidney 293T amphotropic packaging cells (ATCC) using FuGENE 6 (Roche Applied Science, Indianapolis, IN, USA) according to the manufacturer's protocol. Cells were then infected with retroviral medium from the packaging cells 48 h after transfection. The stable clones were selected with 1 μg/ml puromycin for shRNA RON expression or 300 μg/ml G418 for luciferase expression. Expression levels of RON were determined by western blotting analysis and quantitative RT–PCR. The expression of luciferase was determined using the luciferase assay kit (Promega, Madison, WI, USA).
MTT assay
Cell proliferation was determined by MTT assays. Briefly, 1000 cells/well were seeded in 96-well plates. At the indicated time points, cells were incubated 0.5 mg/ml MTT (3-(4,5 dimethylthiazol-2-yl)-2,5-di-phenyltetrazolium bromide, Sigma, St Louis, MO, USA) media for 2 h and the dye was dissolved in DMSO. The absorbance was taken at 562 nm using a VersaMax Microplate Reader (Molecular Devices, Sunnyvale, CA, USA)
Clonogenicity assay
Single-cell suspension was plated at a density of 50, 250 and 500 cells per 6 cm2 dish and cultured for 2 weeks. The cells were then fixed and stained with 0.1% crystal violet/40% methanol and colonies⩾1 mm were counted.
Matrigel invasion assay
Cell invasion was analyzed by in vitro Matrigel invasion assays according to the manufacturers' protocols. Briefly, 3 × 104 cells/well (6 × 104 cells/well for the images) were plated in 24-well Matrigel invasion chambers (BD Biosciences, Bedford, MA, USA) in 0.5 ml of serum-free medium. The outer chambers contained 0.7 ml of 10% fetal bovine serum medium. Cells were treated with 200 ng/ml of MSP or 50 ng/ml of HGF in the presence or absence of 0.5 μM MET inhibitor, PHA-665752 (TOCRIS, Bioscience, Ellisville, MO, USA). After 24 h of incubation, cells on the top surface of the membrane were gently removed with cotton swabs. The cells invaded to the undersurface of the membrane were fixed in 70% ethanol and stained with 0.1% crystal violet. Invaded cells were then counted under light microscope.
Western blotting and immunoprecipitation
Whole cell lysates were obtained in Laemmli's sample buffer and western blotting analysis was performed using standard methods. For immunoprecipitation, cells were lysed in lysis buffer (50 mM HEPES, pH 7.4, 150 mM NaCl, 10% glycerol and 1% Triton X-100) with phosphatase and proteinase inhibitors (1 mM EDTA, 1 mM sodium orthovanadate, 10 mM sodium pyrophosphate, 100 mM NaF, 10 mg/ml leupeptin, 10 units/ml aprotinin, 1 mM phenylmethylsulfonyl fluoride) and 500 μg of cell lysate was subjected to immunoprecipitation with indicated antibodies. Antibodies against RON-β and MET were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-phospho-MET, anti-phospho-Erk1/2 and anti-phospho-AKT were purchased from Cell Signaling (Beverly, MA, USA). Anti-phospho-Tyr (4G10) was from Upstate Biotechnology (Lake Placid, NY, USA).
Quantitative RT–PCR
Total RNA was isolated from cells using the TRIzol reagent (Invitrogen, Carlsbad, CA, USA). In all, 100 ng of total RNA was subjected to reverse transcription and real-time PCR was performed with SYBR Green PCR mix (Bio-Rad, Life Sciences, Hercules, CA, USA) according to the manufacturer's instruction. The primer sequences for RON were described elsewhere.18 β-Actin mRNA was amplified simultaneously for an endogenous control.
Orthotopic pancreatic cancer mouse model
Four- to five-week-old athymic nude mice were from Harlan Corp (Kansas City, KS, USA). Mice were housed and maintained in accordance with the standards of The University of Texas Health Science Center Animal Care and Use Committee. Cells were grown to 80% confluence, trypsinized and resuspended in phosphate-buffered saline. In all, 1 × 106 cells/50 μl were injected into the tail of the pancreas of anesthetized mice. For the luciferase expression tumors, the tumor growth and metastasis were monitored every 2 weeks by bioluminescent imaging using the IVIS Imaging system (Caliper LifeSciences, Hopkinton, MA, USA) at Optical Imaging Facility Core of University of Texas Health Science Center (San Antonio, TX). Six to seven weeks after implantation, the mice showing stress signs were euthanized. During necropsy, mice were evaluated grossly and histologically for the evidence of tumor invading and metastasizing into the peritoneum and liver.
Histopathology and immunohistochemistry
Paraffin-embedded primary tumor and liver sections were stained with H&E and immunohistochemistry of RON-β and MET was performed with antibodies described above in the Histology and Pathology Laboratory (University of Texas Health Science Center, San Antonio, TX). The primary tumors and liver metastases were examined under the light microscopy.
Statistical analysis
Statistical analysis was performed using the GraphPad InStat software (GraphPad Software, Inc., La Jolla, CA, USA). The significance of differences among groups was determined by one-way ANOVA, t test or Fisher's exact test accordingly. Statistically significance was considered as P-value<0.05.
Acknowledgments
We thank Jenny Wang (University of Nebraska Medical Center, Omaha, NE) for the pSuper-shRNA-RON and Ricardo Aguiar (University of Texas Health Science Center, San Antonio, TX) for the PMMP-luciferase plasmids. We thank the University of Texas Health Science Center Animal Care and Use Committee and the Optical Imaging Facility Core of University of Texas Health Science Center (San Antonio, TX) for housing the mice and facility the animal images; and we thank the Histology and Pathology Laboratory (University of Texas Health Science Center, San Antonio, TX) for the histopathology and immunohistochemistry analysis. Grants to JWF, VA merit award, and NIH-RO1CA069122, Cancer Center Grant to CTRC, NIH-P30CA054174, supported this work.
Glossary
- EGFR
epidermal growth factor receptor
- HGF
hepatocyte growth factor
- MSP
Macrophage stimulating protein
- PDAC
pancreatic ductal adenocarcinoma
- RON
Receptor originated from nantes
The authors declare no conflict of interest.
References
- Gaudino G, Follenzi A, Naldini L, Collesi C, Santoro M, Gallo KA, et al. RON is a heterodimeric tyrosine kinase receptor activated by the HGF homologue MSP. EMBO J. 1994;13:3524–3532. doi: 10.1002/j.1460-2075.1994.tb06659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gherardi E, Love CA, Esnouf RM, Jones EY. The sema domain. Curr Opin Struct Biol. 2004;14:669–678. doi: 10.1016/j.sbi.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Wang MH, Padhye SS, Guin S, Ma Q, Zhou YQ. Potential therapeutics specific to c-MET/RON receptor tyrosine kinases for molecular targeting in cancer therapy. Acta Pharmacol Sin. 2010;31:1181–1188. doi: 10.1038/aps.2010.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gherardi E, Birchmeier W, Birchmeier C, Vande Woude G, Targeting MET. in cancer: rationale and progress. Nat Rev Cancer. 2012;12:89–103. doi: 10.1038/nrc3205. [DOI] [PubMed] [Google Scholar]
- Christensen JG, Schreck R, Burrows J, Kuruganti P, Chan E, Le P, et al. A selective small molecule inhibitor of c-Met kinase inhibits c-Met-dependent phenotypes in vitro and exhibits cytoreductive antitumor activity in vivo. Cancer Res. 2003;63:7345–7355. [PubMed] [Google Scholar]
- Zhang Y, Kaplan-Lefko PJ, Rex K, Yang Y, Moriguchi J, Osgood T, et al. Identification of a novel recepteur d'origine nantais/c-met small-molecule kinase inhibitor with antitumor activity in vivo. Cancer Res. 2008;68:6680–6687. doi: 10.1158/0008-5472.CAN-07-6782. [DOI] [PubMed] [Google Scholar]
- Zeng ZS, Weiser MR, Kuntz E, Chen CT, Khan SA, Forslund A, et al. c-Met gene amplification is associated with advanced stage colorectal cancer and liver metastases. Cancer Lett. 2008;265:258–269. doi: 10.1016/j.canlet.2008.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano F, Galluccio N, Lorenzini P, Ruzzo A, Canestrari E, D'Emidio S, et al. Genetic activation of the MET pathway and prognosis of patients with high-risk, radically resected gastric cancer. J Clin Oncol. 2011;29:4789–4795. doi: 10.1200/JCO.2011.36.7706. [DOI] [PubMed] [Google Scholar]
- De Oliveira AT, Matos D, Logullo AF, DA Silva SR, Neto RA, Filho AL, et al. MET Is highly expressed in advanced stages of colorectal cancer and indicates worse prognosis and mortality. Anticancer Res. 2009;29:4807–4811. [PubMed] [Google Scholar]
- Previdi S, Abbadessa G, Dalo F, France DS, Broggini M. Breast cancer-derived bone metastasis can be effectively reduced through specific c-MET inhibitor tivantinib (ARQ 197) and shRNA c-MET knockdown. Mol Cancer Ther. 2012;11:214–223. doi: 10.1158/1535-7163.MCT-11-0277. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Bilchik A, Saha S, Turner R, Wiese D, Tanaka M, et al. c-MET expression level in primary colon cancer: a predictor of tumor invasion and lymph node metastases. Clin Cancer Res. 2003;9:1480–1488. [PubMed] [Google Scholar]
- Dussault I, Bellon SF. From concept to reality: the long road to c-Met and RON receptor tyrosine kinase inhibitors for the treatment of cancer. Anticancer Agents Med Chem. 2009;9:221–229. doi: 10.2174/187152009787313792. [DOI] [PubMed] [Google Scholar]
- Eder JP, Vande Woude GF, Boerner SA, LoRusso PM. Novel therapeutic inhibitors of the c-Met signaling pathway in cancer. Clin Cancer Res. 2009;15:2207–2214. doi: 10.1158/1078-0432.CCR-08-1306. [DOI] [PubMed] [Google Scholar]
- Liu X, Newton RC, Scherle PA. Developing c-MET pathway inhibitors for cancer therapy: progress and challenges. Trends Mol Med. 2010;16:37–45. doi: 10.1016/j.molmed.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Yap TA, Sandhu SK, Alam SM, de Bono JS. HGF/c-MET Targeted Therapeutics: Novel Strategies for Cancer Medicine. Curr Drug Targets. 2011;12:2045–2058. doi: 10.2174/138945011798829348. [DOI] [PubMed] [Google Scholar]
- Lu Y, Yao HP, Wang MH. Multiple variants of the RON receptor tyrosine kinase: biochemical properties, tumorigenic activities, and potential drug targets. Cancer Lett. 2007;257:157–164. doi: 10.1016/j.canlet.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- Zhao S, Ammanamanchi S, Brattain M, Cao L, Thangasamy A, Wang J, et al. Smad4-dependent TGF-beta signaling suppresses RON receptor tyrosine kinase-dependent motility and invasion of pancreatic cancer cells. J Biol Chem. 2008;283:11293–11301. doi: 10.1074/jbc.M800154200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thangasamy A, Rogge J, Ammanamanchi S. Regulation of RON tyrosine kinase-mediated invasion of breast cancer cells. J Biol Chem. 2008;283:5335–5343. doi: 10.1074/jbc.M706957200. [DOI] [PubMed] [Google Scholar]
- Hara S, Nakashiro K, Klosek SK, Ishikawa T, Shintani S, Hamakawa H. Hypoxia enhances c-Met/HGF receptor expression and signaling by activating HIF-1alpha in human salivary gland cancer cells. Oral Oncol. 2006;42:593–598. doi: 10.1016/j.oraloncology.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Cheng HL, Liu HS, Lin YJ, Chen HH, Hsu PY, Chang TY, et al. Co-expression of RON and MET is a prognostic indicator for patients with transitional-cell carcinoma of the bladder. Br J Cancer. 2005;92:1906–1914. doi: 10.1038/sj.bjc.6602593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggiora P, Lorenzato A, Fracchioli S, Costa B, Castagnaro M, Arisio R, et al. The RON and MET oncogenes are co-expressed in human ovarian carcinomas and cooperate in activating invasiveness. Exp Cell Res. 2003;288:382–389. doi: 10.1016/s0014-4827(03)00250-7. [DOI] [PubMed] [Google Scholar]
- Follenzi A, Bakovic S, Gual P, Stella MC, Longati P, Comoglio PM. Cross-talk between the proto-oncogenes Met and Ron. Oncogene. 2000;19:3041–3049. doi: 10.1038/sj.onc.1203620. [DOI] [PubMed] [Google Scholar]
- Benvenuti S, Lazzari L, Arnesano A, Li Chiavi G, Gentile A, Comoglio PM. Ron kinase transphosphorylation sustains MET oncogene addiction. Cancer Res. 2011;71:1945–1955. doi: 10.1158/0008-5472.CAN-10-2100. [DOI] [PubMed] [Google Scholar]
- Camp ER, Yang A, Gray MJ, Fan F, Hamilton SR, Evans DB, et al. Tyrosine kinase receptor RON in human pancreatic cancer: expression, function, and validation as a target. Cancer. 2007;109:1030–1039. doi: 10.1002/cncr.22490. [DOI] [PubMed] [Google Scholar]
- Logan-Collins J, Thomas RM, Yu P, Jaquish D, Mose E, French R, et al. Silencing of RON receptor signaling promotes apoptosis and gemcitabine sensitivity in pancreatic cancers. Cancer Res. 2010;70:1130–1140. doi: 10.1158/0008-5472.CAN-09-0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas RM, Toney K, Fenoglio-Preiser C, Revelo-Penafiel MP, Hingorani SR, Tuveson DA, et al. The RON receptor tyrosine kinase mediates oncogenic phenotypes in pancreatic cancer cells and is increasingly expressed during pancreatic cancer progression. Cancer Res. 2007;67:6075–6082. doi: 10.1158/0008-5472.CAN-06-4128. [DOI] [PubMed] [Google Scholar]
- Padhye SS, Guin S, Yao HP, Zhou YQ, Zhang R, Wang MH. Sustained expression of the RON receptor tyrosine kinase by pancreatic cancer stem cells as a potential targeting moiety for antibody-directed chemotherapeutics. Mol Pharm. 2011;8:2310–2319. doi: 10.1021/mp200193u. [DOI] [PubMed] [Google Scholar]
- Vaccaro V, Melisi D, Bria E, Cuppone F, Ciuffreda L, Pino MS, et al. Emerging pathways and future targets for the molecular therapy of pancreatic cancer. Expert Opin Ther Targets. 2011;15:1183–1196. doi: 10.1517/14728222.2011.607438. [DOI] [PubMed] [Google Scholar]
- Mimeault M, Brand RE, Sasson AA, Batra SK. Recent advances on the molecular mechanisms involved in pancreatic cancer progression and therapies. Pancreas. 2005;31:301–316. doi: 10.1097/01.mpa.0000175893.04660.1b. [DOI] [PubMed] [Google Scholar]
- Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- Boccaccio C, Comoglio PM. Invasive growth: a MET-driven genetic programme for cancer and stem cells. Nat Rev Cancer. 2006;6:637–645. doi: 10.1038/nrc1912. [DOI] [PubMed] [Google Scholar]
- Camp ER, Liu W, Fan F, Yang A, Somcio R, Ellis LM. RON, a tyrosine kinase receptor involved in tumor progression and metastasis. Ann Surg Oncol. 2005;12:273–281. doi: 10.1245/ASO.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Trusolino L, Comoglio PM. Scatter-factor and semaphorin receptors: cell signalling for invasive growth. Nat Rev Cancer. 2002;2:289–300. doi: 10.1038/nrc779. [DOI] [PubMed] [Google Scholar]
- O'Toole JM, Rabenau KE, Burns K, Lu D, Mangalampalli V, Balderes P, et al. Therapeutic implications of a human neutralizing antibody to the macrophage-stimulating protein receptor tyrosine kinase (RON), a c-MET family member. Cancer Res. 2006;66:9162–9170. doi: 10.1158/0008-5472.CAN-06-0283. [DOI] [PubMed] [Google Scholar]
- Ide T, Kitajima Y, Miyoshi A, Ohtsuka T, Mitsuno M, Ohtaka K, et al. The hypoxic environment in tumor-stromal cells accelerates pancreatic cancer progression via the activation of paracrine hepatocyte growth factor/c-Met signaling. Ann Surg Oncol. 2007;14:2600–2607. doi: 10.1245/s10434-007-9435-3. [DOI] [PubMed] [Google Scholar]
- Qian LW, Mizumoto K, Inadome N, Nagai E, Sato N, Matsumoto K, et al. Radiation stimulates HGF receptor/c-Met expression that leads to amplifying cellular response to HGF stimulation via upregulated receptor tyrosine phosphorylation and MAP kinase activity in pancreatic cancer cells. Int J Cancer. 2003;104:542–549. doi: 10.1002/ijc.10997. [DOI] [PubMed] [Google Scholar]
- Peace BE, Hill KJ, Degen SJ, Waltz SE. Cross-talk between the receptor tyrosine kinases Ron and epidermal growth factor receptor. Exp Cell Res. 2003;289:317–325. doi: 10.1016/s0014-4827(03)00280-5. [DOI] [PubMed] [Google Scholar]
- Zwick E, Hackel PO, Prenzel N, Ullrich A. The EGF receptor as central transducer of heterologous signalling systems. Trends Pharmacol Sci. 1999;20:408–412. doi: 10.1016/s0165-6147(99)01373-5. [DOI] [PubMed] [Google Scholar]
- Comoglio PM, Boccaccio C. The HGF receptor family: unconventional signal transducers for invasive cell growth. Genes Cells. 1996;1:347–354. doi: 10.1046/j.1365-2443.1996.37037.x. [DOI] [PubMed] [Google Scholar]
- Takayama H, LaRochelle WJ, Sharp R, Otsuka T, Kriebel P, Anver M, et al. Diverse tumorigenesis associated with aberrant development in mice overexpressing hepatocyte growth factor/scatter factor. Proc Natl Acad Sci USA. 1997;94:701–706. doi: 10.1073/pnas.94.2.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang TJ, Reid AE, Xavier R, Cardiff RD, Wang TC. Transgenic expression of tpr-met oncogene leads to development of mammary hyperplasia and tumors. J Clin Invest. 1996;97:2872–2877. doi: 10.1172/JCI118744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego MI, Bierie B, Hennighausen L. Targeted expression of HGF/SF in mouse mammary epithelium leads to metastatic adenosquamous carcinomas through the activation of multiple signal transduction pathways. Oncogene. 2003;22:8498–8508. doi: 10.1038/sj.onc.1207063. [DOI] [PubMed] [Google Scholar]
- Di Renzo MF, Olivero M, Martone T, Maffe A, Maggiora P, Stefani AD, et al. Somatic mutations of the MET oncogene are selected during metastatic spread of human HNSC carcinomas. Oncogene. 2000;19:1547–1555. doi: 10.1038/sj.onc.1203455. [DOI] [PubMed] [Google Scholar]
- Tzahar E, Waterman H, Chen X, Levkowitz G, Karunagaran D, Lavi S, et al. A hierarchical network of interreceptor interactions determines signal transduction by Neu differentiation factor/neuregulin and epidermal growth factor. Mol Cell Biol. 1996;16:5276–5287. doi: 10.1128/mcb.16.10.5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X, LeVea CM, Freeman JK, Dougall WC, Greene MI. Heterodimerization of epidermal growth factor receptor and wild-type or kinase-deficient Neu: a mechanism of interreceptor kinase activation and transphosphorylation. Proc Natl Acad Sci USA. 1994;91:1500–1504. doi: 10.1073/pnas.91.4.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallasch C, Weiss FU, Niederfellner G, Jallal B, Issing W, Ullrich A. Heregulin-dependent regulation of HER2/neu oncogenic signaling by heterodimerization with HER3. EMBO J. 1995;14:4267–4275. doi: 10.1002/j.1460-2075.1995.tb00101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaquish DV, Yu PT, Shields DJ, French RP, Maruyama KP, Niessen S, et al. IGF1-R signals through the RON receptor to mediate pancreatic cancer cell migration. Carcinogenesis. 2011;32:1151–1156. doi: 10.1093/carcin/bgr086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fralix KD, Ahmed MM, Mattingly C, Swiderski C, McGrath PC, Venkatasubbarao K, et al. Characterization of a newly established human pancreatic carcinoma cell line, UK Pan-1. Cancer. 2000;88:2010–2021. doi: 10.1002/(sici)1097-0142(20000501)88:9<2010::aid-cncr5>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Zhao S, Venkatasubbarao K, Lazor JW, Sperry J, Jin C, Cao L, et al. Inhibition of STAT3 Tyr705 phosphorylation by Smad4 suppresses transforming growth factor beta-mediated invasion and metastasis in pancreatic cancer cells. Cancer Res. 2008;68:4221–4228. doi: 10.1158/0008-5472.CAN-07-5123. [DOI] [PubMed] [Google Scholar]