Abstract
AMP-activated protein kinases (AMPKs) are a class of serine/threonine protein kinases that are activated by an increase in intracellular AMP concentration. They are a sensitive indicator of cellular energy status and have been found to promote tumor cell survival during nutrient starvation. We recently identified a novel AMPK catalytic subunit family member, ARK5, whose activation is directly regulated by Akt, which, in turn, has been reported to be a key player in tumor malignancy. In this study, we attempted to determine whether ARK5 is involved in tumor malignancy under regulation by Akt. Matrigel invasion assays demonstrated that both overexpressed and endogenous ARK5 showed strong activity dependent on Akt. In addition, ARK5 expression induced activation of matrix metalloproteinase 2 (MMP-2) and MMP-9 following new expression of membrane type 1 MMP (MT1-MMP), and the MT1-MMP expression induced by ARK5 was initiated by rapamycin-sensitive signaling. In nude mice, ARK5 expression was associated with a significant increase in tumor growth and significant suppression of necrosis in tumor tissue. Interestingly, only the ARK5-overexpressing PANC-1 cell line (P/ARK) tumor showed invasion and metastasis in nude mice, although Akt was activated in tumors derived from both P/ARK and its parental cell line. We report that a novel AMPK catalytic subunit family member, ARK5, plays a key role in tumor malignancy downstream of Akt.
Since tumor cell survival and growth are maintained by nutrients, especially glucose, and by oxygen supplied by blood (10, 25, 51), angiogenesis is considered essential for tumor progression, and higher malignancy has been found to be associated with increased angiogenesis in many types of tumors (15, 16). Tumor angiogenesis is regulated by many factors, among which vascular endothelial growth factor has been considered the most important (53). The vascular endothelial growth factor gene is mainly regulated by hypoxia-inducible factors (7, 10, 33, 62). For these reasons, antiangiogenesis and hypoxia-targeted therapy of cancer are considered to be promising strategies (50). In addition, there are many reports suggesting a link between tumor hypoxia and tumor invasion and metastasis (13, 23, 28, 60). We have studied tumor cell physiology under hypoxic conditions and found a paradox of cell physiology: tolerance to glucose starvation is induced by hypoxia in a human hepatoma cell line and normal fibroblasts, but the tolerance seems to be independent of hypoxia-inducible factor 1 (14, 30, 31). In addition, pancreatic cancer is highly tolerant to the nutrient starvation associated with hypovascularity as determined by clinical angiography (30), and we recently demonstrated that this tolerance is dependent on Akt and AMP-activated protein kinase (AMPK) activity (14, 24, 30, 31).
The AMPK family is highly conserved in several species, including mammalian species (22, 52), and α subunits of mammalian AMPKs have been identified as catalytic subunits (22). AMPK activation has been well documented in cells exposed to metabolic stress, hypoxia, heat shock, and ischemia (22). Four proteins, AMPK-α1 and -α2 (6, 22, 40, 52), MELK (in mice [26]), and SNARK (in rats and humans [36, 57]), had previously been identified as members of the AMPK catalytic subunit family, and our group recently identified a fifth member of the AMPK catalytic subunit family, named ARK5 (56), which is directly phosphorylated and activated by Akt (56). In response to stimulation by Akt, ARK5 prevents cell death induced by glucose starvation and death receptor activation in tumor cells as a result of inhibition of caspase 8 activation via preservation of c-FLIP (55, 56). Thus, ARK5 promotes tumor cell survival under regulation by Akt.
Akt is a serine/threonine protein kinase that was first identified as the cellular homologue of viral Akt and found to mediate cell survival, proliferation, and differentiation (11). Regulation of Akt has been extensively investigated, and the phosphatidylinositol 3-kinase (PI-3K)-3-phosphoinositide-dependent protein kinase 1 (PDK-1) pathway has been clearly shown to be essential for Akt activation (11, 35). In addition, recent investigations revealed some interesting pathways for Akt activation, such as the integrin-linked kinase-PI-3K pathway (12, 21, 34, 44, 45) and the phospholipase Cγ-calcium ion-calmodulin pathway (PI-3K-independent pathway [19]). Activated Akt phosphorylates several cell death-associated factors, including Bad, caspase 9, and forkhead, and prevents cell death (35). Akt also mediates several cellular responses triggered by insulin and insulin-like growth factor (IGF), such as glycogen synthesis via glycogen synthase kinase 3 (GSK-3) phosphorylation and protein translation via mTOR phosphorylation (35). PTEN inhibits Akt activation (54), and since it is often mutated in tumors (2), the critical role of Akt in tumorigenesis has been well documented, and gene amplification, overexpression, and constitutive activation of Akt have been observed in various cancers, including colorectal and pancreatic adenocarcinoma, gastric carcinoma, and ovarian cancers (29, 42, 47). In addition, tumor malignancy, including invasion and metastasis, accelerated by Akt activation has been well documented for breast cancer, ovarian cancer, squamous cell carcinoma, and pancreatic cancer (1, 20, 41, 43, 58). Akt, therefore, plays a major role in tumor malignancy (2, 8, 34). Furthermore, tumor cell migration, one of the essential elements of tumor invasion and metastasis, is also promoted under regulation by Akt (27, 32). Akt has also recently been shown to be directly involved in the anchorage-independent survival of cancer cells (18). Although Akt-promoted tumor invasion and metastasis have been well documented, the factors downstream of Akt during tumor invasion and metastasis have not yet been identified. Since ARK5 is the substrate of Akt during nutrient starvation, we investigated in the present study whether ARK5 is involved in tumor invasion and metastasis.
MATERIALS AND METHODS
Cell culture and transfection.
Human pancreatic cancer cell line PANC-1, the stably ARK5-transfected PANC-1 cell line P/ARK, the human colorectal adenocarcinoma cell lines DLD-1 and SW480, and the stably ARK5-transfected DLD-1 cell line D/ARK were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum (Sigma Chemical Co., Ltd.). The P/ARK and D/ARK cell lines were established by transfection of the FLAG-ARK5 expression vector and G418 selection.
For transfection, cells were seeded into a six-well plate at 2.5 × 105/well, and transfection was performed with TransFast transfection reagent (5 μg of DNA/well; Promega Corporation). The time of cell exposure to the transfection reagent was 4 h. The transfection yield was measured with an expression plasmid carrying green fluorescent protein (PANC-1, 97.7%; DLD-1, 98%; SW480, 98.6%).
Antibodies, chemicals, and recombinant protein.
Antibodies to Akt (total and phosphorylated at Ser473), mTOR (total and phosphorylated at Ser2448), GSK-3β (total and phosphorylated at Ser9), and phospho-Akt substrate (for detection of ARK5; phosphorylated at Ser600) were purchased from Cell Signaling Technology, Inc. Anti-FLAG monoclonal antibody and its agarose-conjugated form and antibodies to FKHRL1 (total and phosphorylated at Thr32) were purchased from Upstate Biotechnology. LY294002 and U0126 were purchased from Cell Signaling. Human recombinant IGF-1 protein was purchased from Pepro Technology, Inc.
Plasmids.
The expression vectors of ARK5 and DN-ARK5, which was prepared by conversion of the Akt-phosphorylating serine residue (Ser600) to an alanine residue (56), were used in the experiments. The expression vectors of DN-Akt1 (K179M; ATP-unbinding mutant) and CA-Akt1 (myristoylated form) were purchased from Upstate Biotechnology.
RNAi preparation.
Interfering RNA (RNAi) for ARK5 was prepared by using the HiScribe RNAi transcription kit purchased from New England BioLabs Inc. An ARK5 cDNA fragment (positions 2022 to 2281) was prepared by PCR using full-length ARK5 cDNA as a template, and the PCR product was subcloned by ligation into pCR2.1-Topo vector (Invitrogen). The vector with the ARK5 fragment inserted was digested with BamHI and XhoI and religated into 28i-pLITMUS RNAi vector. The RNAi was prepared by in vitro transcription with T7 RNA polymerase. The efficacy of the RNAi was examined by the disappearance of ARK5 protein on a Western blot. As a control for RNAi, double-stranded RNA prepared by in vitro transcription of empty 28i-pLITMUS RNAi vector was used as recommended in by the manufacturer. In the present study, cells were seeded into a 24-well plate and 1 μg of RNAi was introduced into cells.
Matrigel invasion assay.
Matrigel invasion assays were performed with a Matrigel-coated invasion chamber (Becton Dickinson Company). Cells (5 × 104) were seeded into each chamber with culture medium, and the media in the inner and outer chamber were changed after 6 h. After 48 h, cells that had invaded were counted under a phase-contrast microscope.
Western blotting analysis for matrix metalloproteinase (MMP) expression.
The PANC-1 and P/ARK cell lines were seeded on six-well culture plates at 2.5 × 105/well. After 24 h, the medium was changed to serum-free Dulbecco's modified Eagle's medium, and serum-free culture was performed for 48 h. After culture, the media were collected and concentrated with a Centriprep instrument (Millipore Corp.), and the cells were lysed with phosphate-buffered saline-1% NP-40.
Concentrated media were used for Western blotting with antibody to MMP-2, MMP-9, or membrane type 1-MMP (MT1-MMP; Fuji Chemical Co., Ltd.) and horseradish peroxidase-conjugated anti-mouse immunoglobulin G antibody (Santa Cruz Biotechnology, Inc.). Immunostained bands were detected by an enhanced chemiluminescence system.
In vivo tumorigenicity assay.
The tumorigenicity of the PANC-1 and P/ARK cell lines was assayed by subcutaneously transplanting 5 × 106 cells into the backs of 6-week-old male BALB/c nu/nu mice. During 12 weeks, the length (L) and width (W) of each tumor were measured, and tumor volume was calculated by the formula tumor volume = (L/2) × (W/2)2 × π × (4/3). Mice were sacrificed at 12 weeks after transplantation, and tumors were fixed with 10% formaldehyde and 10% NaHPO4, embedded in paraffin, and serially sectioned at 4 μm. The sections were stained with Delafield's hematoxylin and eosin.
Immunohistochemistry with anti-mouse CD31 antibody.
Histological sections of the tumors were immunostained with anti-mouse CD31 primary antibody and horseradish peroxidase-conjugated anti-rat immunoglobulin G secondary antibody (Becton Dickinson). Frozen tissue sections were fixed in methanol containing 0.3% H2O2. After incubation with blocking buffer (2% bovine serum albumin in phosphate-buffered saline), the sections were incubated with primary antibody. Immunohistochemical staining was performed by the ABC method using a Vector Stain ABC kit (Vector Laboratories), and color development was performed with diaminobenzidine in 50 mM Tris-HCl. After the immunohistochemical procedure, sections were counterstained with hematoxylin. The intratumoral microvessel density of each tumor tissue was determined with a KS300 imaging system (Carl Zeiss Vision GmbH). All of the blood vessels on a slide were counted, and the count was normalized to the total area of the sample.
RESULTS
ARK5 stimulates invasion activity in vitro in an Akt-dependent manner.
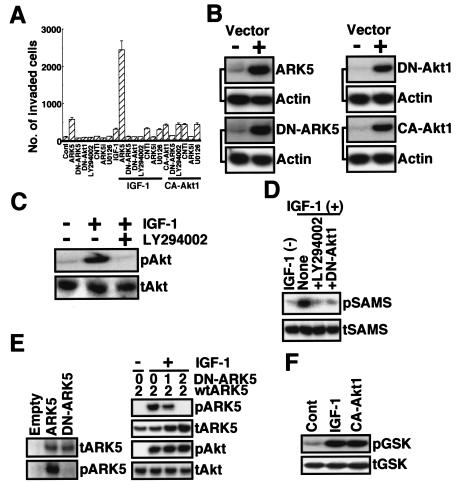
About 100 cells of the human pancreatic cancer cell line PANC-1 seeded in a Matrigel-coated invasion chamber translocated to the bottom of the lower chamber within 48 h (Fig. 1A), and the invasion activity was increased when cells were transiently transfected with FLAG-ARK5 or CA-Akt1 (myristoylated form [9]) (Fig. 1A). Exposure of cells to IGF-1 also increased their invasive activity (Fig. 1A). When cells were transfected with FLAG-ARK5, IGF-1 stimulation was greatly enhanced to about 2,500 invading cells (Fig. 1A). However, the stimulated activity was completely suppressed by expression of DN-ARK5 (lacking Akt sensitivity because of replacement of Ser600 by Ala [56]) and DN-Akt1 (lacking ATP binding ability [17]) or exposure to ARK5i or LY294002 (Fig. 1A). By contrast, the MEK1/2 inhibitor U0126 had no effect on invasion activity (Fig. 1A). As shown in Fig. 1B, transfection with FLAG-ARK5, DN-ARK5, DN-Akt1, and CA-Akt1 clearly resulted in expression of their respective protein, and LY294002 completely suppressed the phosphorylation of Akt induced by IGF-1 (Fig. 1C). Although ARK5 activity, measured as the phosphorylation of glutathione S-transferase (GST)-SAMS, was dramatically increased by IGF-1 treatment, the activity was completely suppressed by expression of DN-Akt1 or exposure to LY294002 (Fig. 1D), indicating that ARK5 is directly regulated by Akt. IGF-1-induced phosphorylation of wild-type ARK5 was efficiently suppressed by expression of DN-ARK5, indicating strongly that DN-ARK5 works in dominant-negative fashion (Fig. 1E). As shown in Fig. 1F, transient expression of CA-Akt1 dramatically induced the phosphorylation of GSK-3β, and the activity level was the same as that for the treatment with IGF-1, indicating the effectiveness of CA-Akt1.
FIG. 1.
Tumor cell invasion activity of PANC-1. (A) PANC-1 cells were transiently transfected with or without 1 μg of ARK5, DN-ARK5, DN-Akt1, CA-Akt1, control RNAi (CNTi), or ARK5i and cultured in the presence and absence of 20 μM LY294002, 20 μM U0126, or 100 ng of IGF-1/ml for 48 h. After incubation, cells that had translocated to the bottom of the lower chamber were counted. The numbers of invading cells are the means of data from three experiments, and the bars represent standard errors. (B) Expression vector (1 μg) for ARK5, DN-ARK5, DN-Akt1, or CA-Akt1 was transfected into PANC-1 cells, and 48 h after transfection, Western blotting of cell extracts was performed with antibody to ARK5 (ARK5 and DN-ARK5) or myc tag (DN-Akt1 and CA-Akt1). Western blotting of actin was performed as an internal control. (C) PANC-1 cells were exposed to 100 ng of IGF-1/ml for 2 h with or without 20 μM LY294002, and Western blotting was performed with antibody to phosphorylated (pAkt) or total (tAkt) Akt. (D) PANC-1 cells were transiently transfected with 1 μg of FLAG-ARK5 together with either 1 μg of DN-Akt1 (+DN-Akt1) or empty vector [IGF-1 (−), None, and +LY294002], and then cells were treated with [IGF-1 (+)] or without [IGF-1 (−)] 100 ng of IGF-1/ml for 2 h in the presence (+LY294002) or absence of 20 μM LY294002 at 48 h after transfection. After treatment of cells, kinase activity of ARK5 was measured as previously described (56). FLAG-ARK5 was collected with anti-FLAG antibody, and phosphorylation of GST-SAMS was measured (pSAMS, GST-SAMS phosphorylation by autoradiography; tSAMS, total GST-SAMS by Western blotting using anti-GST antibody). (E) (Left panel) Expression vector containing either wild-type FLAG-ARK5 (ARK5), DN-ARK5, or empty vector (Empty) was introduced into PANC-1 cells, and then cells were treated with 100 ng of IGF-1/ml for 2 h. After treatment, immunoprecipitates with anti-FLAG antibody were collected and Western blotting was performed using antibody to FLAG (tARK5) or Akt substrate (pARK5). (Right panel) Expression vectors containing wild-type FLAG-ARK5 (2 μg of DNA) and DN-ARK5 (0, 1, or 2 μg of DNA) were introduced into PANC-1 cells, and then IGF-1 treatment (100 ng/ml for 2 h) was performed. After treatment, cell extracts (for Akt) and immunoprecipitates by anti-FLAG antibody (for ARK5) were collected. Western blotting was performed with antibody to either FLAG (tARK5), Akt substrate (pARK5), Akt (tAkt), or phosphorylated Akt (Ser473, pAkt). (F) Cell extracts were collected from PANC-1 cells transfected with 1 μg of CA-Akt1 or treated with 100 ng of IGF-1/ml, and then Western blotting was performed with antibody to phosphorylated (pGSK) or total (tGSK) GSK-3β to assess the activity of CA-Akt1.
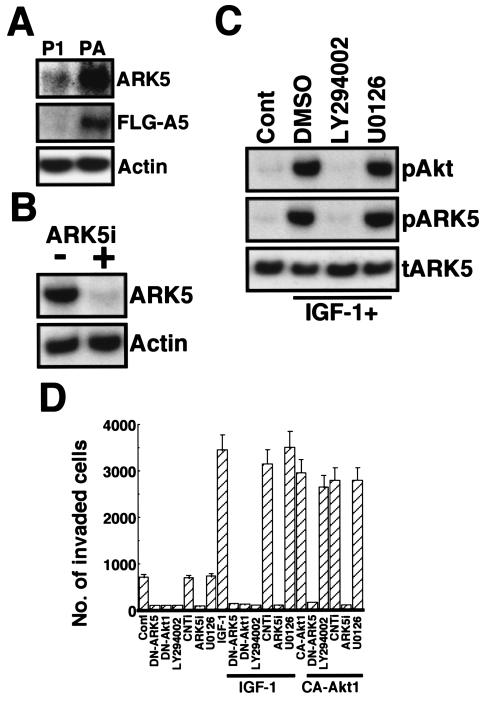
To further investigate the role of ARK5 in the invasion activity mediated by Akt, we prepared the stably FLAG-ARK5-overexpressing PANC-1 cell line P/ARK. As shown in Fig. 2A, the PANC-1 cells expressed low levels of endogenous ARK5 protein, whereas the P/ARK cells expressed much higher levels of ARK5 (about 10-fold) as a result of FLAG-ARK5 transfection (Fig. 2A). Despite the large difference in ARK5 expression level, there was little difference in proliferation rates. The doubling time of the PANC-1 cells was 22.9 h, and the doubling time of the P/ARK cells was 20 h. As shown in Fig. 2B, 1 μg of RNAi for ARK5 (ARK5i) completely suppressed ARK5 expression. Phosphorylation of Akt and ARK5 was stimulated by IGF-1 in P/ARK cells (Fig. 2C), and it was completely suppressed by LY294002, but not by the MEK1/2 inhibitor U0126 (Fig. 2C), consistent with the findings in a previous study (56). When these cells were subjected to the Matrigel invasion assay, the P/ARK cells exhibited greater activity than did the PANC-1 cells, and the activity in P/ARK cells was further increased by IGF-1 and CA-Akt1. The invasion activity was suppressed by 1 μg of DN-ARK5, DN-Akt1, and ARK5i and 20 μM LY294002, but not by 20 μM U0126 (Fig. 2D), consistent with the results of the transient-transfection experiments.
FIG. 2.
Tumor cell invasion activity of P/ARK. (A) Expression of ARK5 in PANC-1 (P1) or P/ARK (PA) cell lines was assessed by Western blotting with antibody to ARK5 (top, ARK5) or FLAG (middle, FLG-A5). (B) One microgram of ARK5i was transfected into P/ARK cells, and Western blotting was performed. (C) P/ARK cells were exposed or not exposed (dimethyl sulfoxide [DMSO]) to 20 μM LY294002 or 20 μM U0126 for 2 h in the presence of 100 ng of IGF-1/ml, cell extracts and immunoprecipitates with anti-FLAG were collected, and Western blotting was performed with antibody to phosphorylated Akt (pAkt, cell extracts); Akt substrate, which detects protein containing RXRXXpS (pARK5, immunoprecipitates); or ARK5 (tARK5). (D) P/ARK cells were transiently transfected with or without 1 μg of DN-ARK5, DN-Akt1, CA-Akt1, control RNAi (CNTi), or ARK5i and cultured for 48 h in the presence or absence of 20 μM LY294002, 20 μM U0126, or 100 ng of IGF-1/ml. After incubation, cells that had translocated to the bottom of the lower chamber were counted. The numbers of invading cells are the means of data from three experiments, and the bars represent standard errors.
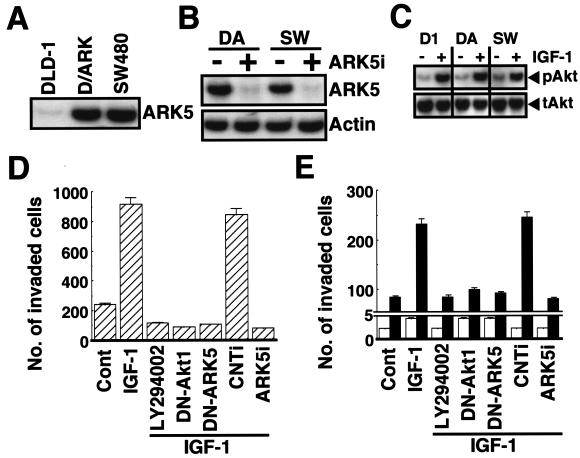
The triggering of tumor cell invasion by Akt and ARK5 in the PANC-1 cells was confirmed in human colorectal adenocarcinoma cell lines. Since the DLD-1 cell line endogenously expressed ARK5 at a very low level, the FLAG-ARK5-overexpressing cell line (D/ARK) was prepared (Fig. 3A). SW480 cells, on the other hand, very intensely expressed endogenous ARK5, and the expression level was the same as that of D/ARK cells (Fig. 3A). As in the PANC-1 cells, exposure to 1 μg of ARK5i completely suppressed ARK5 expression (Fig. 3B), and IGF-1 induced phosphorylation of Akt (Fig. 3C) to almost the same extent in all three cell lines. The Matrigel invasion assay revealed higher invasion activity by the SW480 and D/ARK cell lines, and the activity was increased by IGF-1 (Fig. 3D and E). The increase in invasion activity induced by IGF-1 in the SW480 and D/ARK cell lines was completely suppressed by LY294002, DN-Akt1, DN-ARK5, and ARK5i (Fig. 3D and E). By contrast, the invasive activity of the DLD-1 cell line was very weak, and IGF-1 did not significantly increase it (Fig. 3E). Taken together, these findings indicate that ARK5 is necessary for tumor cell invasion and is the downstream factor for Akt-induced tumor cell invasion.
FIG. 3.
Tumor invasion assay of colon cancer cell lines with a Matrigel-coated invasion chamber. (A) Expression of ARK5 in cell extracts from DLD-1, D/ARK, and SW480 cells was assessed. (B) One microgram of double-stranded RNA with (+) or without (−) ARK5i was exposed to the D/ARK (DA) or the SW480 (SW) cell lines, and Western blotting was performed. (C) Phosphorylation of Akt in cell lines exposed or not exposed to 100 ng of IGF-1/ml for 2 h was also assessed by Western blotting. (D and E) The invasion activity of SW480 (D), DLD-1 (E) (open bars), and D/ARK (E) (closed bars) cell lines exposed to 20 μM LY294002, transiently transfected with 1 μg of DN-Akt1, DN-ARK5, control RNAi (CNTi), or ARK5i, in the presence or absence (Cont) of 100 ng of IGF-1/ml for 48 h was assessed. The numbers of invading cells are the means of data from three experiments, and bars represent the standard errors.
MMP production induced by ARK5.
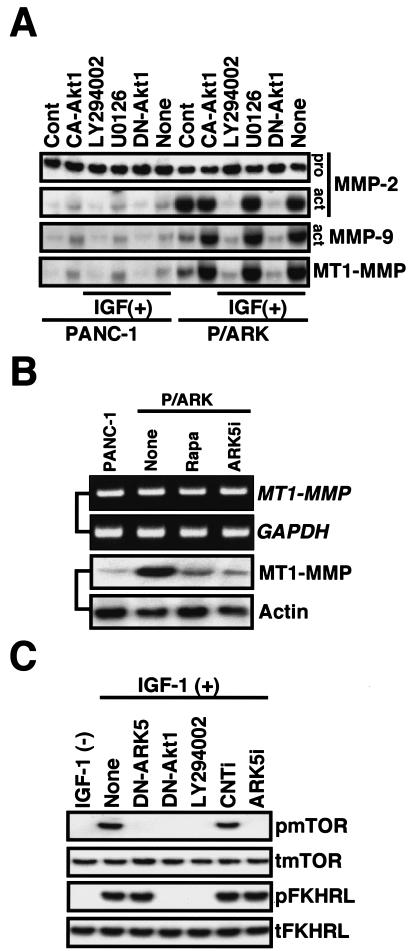
Since MMPs are known to be involved in tumor metastasis (37), we compared MMP production by the PANC-1 and P/ARK cell lines. Western blotting showed the presence of pro-MMP-2 (p72) in the culture medium of both cell lines, but the amount was not significantly changed by any treatments (Fig. 4A). The active form of MMP-2 (p43) and MMP-9 (p67) was detected in the culture medium of P/ARK cells, but the culture medium of the PANC-1 cells contained only small amounts of both active forms (Fig. 4A). The amount of active MMP-9 in the culture medium increased slightly when P/ARK cells were transiently transfected with CA-Akt1 or exposed to IGF-1 (Fig. 4A). The active form of MMP-2 was increased slightly by CA-Akt1 transfection or IGF-1 exposure of PANC-1 cells, but not P/ARK cells (Fig. 4A). Interestingly, activation of both MMP-2 and MMP-9 by IGF-1-exposed P/ARK cells was inhibited by LY294002 and DN-Akt1, but not by U0126 (Fig. 4A), indicating a critical role of the PI-3K-Akt pathway in the activation of MMPs by IGF-1. MT1-MMP, which has been identified as the membrane-bound form of MMP, induces activation of MMP-2 and MMP-9 (48). MT1-MMP was barely detectable in the PANC-1 cell line but was observed to be highly expressed in P/ARK cells (Fig. 4A). In addition, MT1-MMP in P/ARK cells was up-regulated when the cells were transiently transfected with CA-Akt1 or exposed to IGF-1 (Fig. 4A). MT1-MMP in P/ARK cells was down-regulated by LY294002 and DN-Akt1, but not by U0126 (Fig. 4A). These results strongly suggest that ARK5 stimulates MMP-2 and MMP-9 secretion and activation via MT1-MMP production.
FIG. 4.
Secretion and expression of MMPs. (A) PANC-1 and P/ARK cell lines were cultured for 48 h with serum-free medium on a six-well plate in the presence [IGF(+)] or absence of 100 ng of IGF-1/ml. After culture, the culture media were concentrated to assess MMP-2 and MMP-9 secretion, and cell extracts were collected to assess MT1-MMP expression. pro, proenzyme; act, activated form. (B) Proteins or mRNAs were extracted from P/ARK cells exposed or not exposed (None) to 100 nM rapamycin (Rapa) or 1 μg of ARK5i, and RT-PCR (upper panels) and Western blotting (lower panels) wereperformed to detect MT1-MMP mRNA or protein. RT-PCR and Western blotting were also performed on PANC-1, and Western blotting of actin and RT-PCR of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were performed as controls. (C) P/ARK cells were transfected with or without (None) 1 μg of DN-ARK5, DN-Akt1, control RNAi (CNTi), or ARK5i or exposed to 20 μM LY294002, and then the cells were treated with [IGF-1 (+)] or without [IGF-1 (−)] 100 ng of IGF-1/ml for 2 h. After treatment of cells, proteins were extracted and Western blotting using antibody to mTOR (phosphorylated, pmTOR; total, tmTOR) and FKHRL1 (phosphorylated, pFKHRL; total, tFKHRL) was performed.
We also investigated the mechanism of the effect of ARK5 on MT1-MMP expression. First, we used reverse transcription-PCR (RT-PCR) to investigate whether ARK5 stimulates MT1-MMP mRNA expression. Surprisingly, the same level of MT1-MMP mRNA expression was observed in both the PANC-1 and P/ARK cell lines (Fig. 4B), although high-level expression of MT1-MMP protein was observed in P/ARK cells. Rapamycin is known to inhibit mTOR, a key molecule in the translational control of protein synthesis. MT1-MMP mRNA expression was not suppressed by 1 μg of ARK5i or 100 nM rapamycin, but both reagents strongly inhibited MT1-MMP protein production, indicating that ARK5 may regulate MT1-MMP expression at the translational level (Fig. 4B).
In addition, we examined whether ARK5 influences mTOR phosphorylation by Western blotting. As shown in Fig. 4C, IGF-1 treatment induced the phosphorylation of mTOR in P/ARK cells; however, the phosphorylation was dramatically decreased by the transient expression of 1 μg of DN-ARK5, DN-Akt1, or ARK5i or by exposure to 20 μM LY294002. Moreover, increased phosphorylation of FKHRL1 triggered by IGF-1 was also suppressed considerably by the blockage of Akt, but not by that of ARK5 (Fig. 4C). These results suggest that mTOR phosphorylation induced by IGF-1 is mediated by ARK5 downstream of Akt.
Increased tumorigenesis and suppression of necrosis induced by ARK.
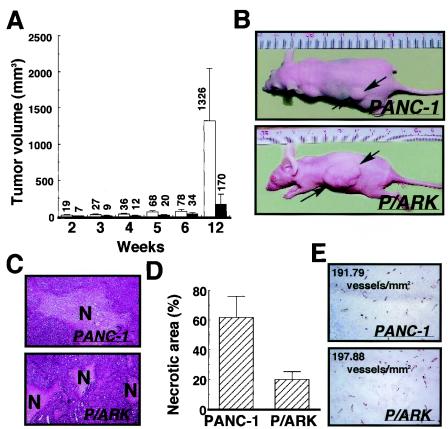
The results of in vitro studies strongly suggested that ARK5 stimulates tumorigenesis, invasion, and metastasis, and this was investigated by subcutaneous transplantation of PANC-1 and P/ARK cell lines into nude mice. As shown in Fig. 5A and B, the mean size of the P/ARK tumors was clearly larger than that of the PANC-1 tumors throughout the 12-week experiment, and at 12 weeks after transplantation the tumors formed by P/ARK cells were 10-fold larger than the PANC-1 cell tumors (Fig. 5A).
FIG. 5.
PANC-1 cells (closed bars) and P/ARK cells (open bars) were transplanted into nude mice (n = 15), and the mice were observed for 12 weeks after transplantation. (A) Tumor volume; (B) photos of tumor-bearing mice. The tumor volumes are the means of 15 data, and the bars represent the standard errors. (C) At 20 weeks after transplantation, the tumor foci formed by the PANC-1 cells and P/ARK cells were extracted and stained with hematoxylin and eosin. The necrotic area is indicated by N. (D) The necrotic area of each tumor focus was measured, the ratios of the necrotic areas are shown as the means of five data, and the bars represent the standard errors. In this study, tumor tissues were used. (E) Immunohistochemical analysis of tumors with anti-mouse CD31 antibody. The numbers on the photographs are the calculated microvessel densities.
All tumors formed by PANC-1 cells contained a large area of central necrosis (Fig. 5C). By contrast, the tumors formed by P/ARK cells showed piecemeal necrosis, but no massive central necrosis (Fig. 5C). When the total area of necrosis was measured, only about 20% of the area of the P/ARK tumors was found to be necrotic, as opposed to about 60% of the area of the PANC-1 tumors (Fig. 5D). These findings raised two possibilities: (i) P/ARK tumors might be resistant to necrosis, and (ii) angiogenesis might be stimulated in P/ARK tumors; however, immunohistochemical and microvessel density analysis with anti-mouse CD31 antibody showed no major difference in anti-CD31 staining or microvessel density between the tumors produced by the PANC-1 and the P/ARK cell lines (Fig. 5E).
Invasion and metastasis of ARK5-expressing tumors.
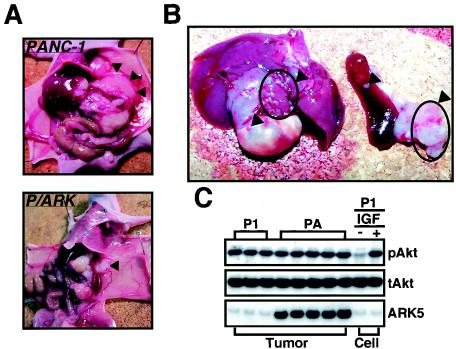
The transplanted tumors were examined macroscopically and histologically to further elucidate the effect of ARK5 overexpression in vivo. As shown in Fig. 6A, the tumors formed by P/ARK cells, but not by PANC-1 cells, had extensively invaded the muscle layer and even the abdominal cavity. Extensive metastasis to the liver, stomach, spleen, and pancreas was also observed in the nude mice transplanted with P/ARK cells (Fig. 6B). Tumor metastasis was observed in nude mice transplanted with both PANC-1 (1 of 15 mice) and P/ARK (4 of 15 mice) cells, but the P/ARK-derived tumors metastasized when the primary tumors were small (data not shown). We also examined the status of Akt and ARK5 expression in the tumors, since Akt phosphorylation is necessary for ARK5 activation (55, 56). Western blotting using proteins extracted from PANC-1 and P/ARK tumors showed that Akt was phosphorylated in tumors derived from both cell lines, but the degree of phosphorylation in the tumors from both cell lines was almost the same, and intense expression of ARK5 was detected in tumors from the P/ARK cells, but not from the PANC-1 cells (Fig. 6C), suggesting a strong correlation between the high invasion and metastasis activity of P/ARK-derived tumors and high-level expression of ARK5.
FIG. 6.
(A and B) Invasion (A) and metastasis (B) at 8 weeks in nude mice transplanted with PANC-1 cells or P/ARK cells. The arrowheads in panel A indicate the position of the transplanted tumor focus. The arrowheads in panel B indicate the metastatic foci in the liver, stomach, spleen, and pancreas of a mouse transplanted with P/ARK cells. (C) Akt phosphorylation in tumors. Proteins were extracted from tumors (Tumor) derived from PANC-1 cells (P1) and P/ARK cells (PA), and Western blotting was performed with antibodies to Akt (phosphorylated Akt, pAkt; total Akt, tAkt) and to ARK5. PANC-1 cells (Cell, P1) exposed to (+) and not exposed to (−) 100 ng of IGF-1 (IGF)/ml were also analyzed by the Western blotting procedure.
DISCUSSION
Akt is known to play key roles in tumor malignancy, including in tumor cell survival, invasion, and metastasis (11, 27, 42). Regulation of Akt has been extensively studied, and it is well established that Akt activity is under the control of PI-3K (11). More than 15 physiological substrates of Akt have been reported, including GSK-3, BAD, caspase 9, forkhead, Mdm-2, and p21WAF1 (4, 35), and the roles of Akt during cell proliferation, cell survival, and insulin response of cells have been relatively well characterized by investigation of the effects of phosphorylation of these substrates. The mechanism of Akt involvement in tumor metastasis, however, has remained unclear.
Metastasis and invasion are complex biological processes that include cell detachment, cell migration, tumor cell invasion of blood and/or lymphatic vessels, attachment to the vessel wall, and extravasation and proliferation at the target site (3). The invasion activity of tumor cells is an important characteristic of metastatic tumors. The results of the present study showed induction of a dramatic increase in invasion activity by ARK5 overexpression in human pancreatic cancer cell line PANC-1 and human colon cancer cell line DLD-1, in which endogenous ARK5 expression is very weak, and the ARK5-induced invasion activity was found to be controlled by Akt. At the present time, five factors including ARK5 have been identified as members of the AMPK catalytic subunit family, and our group has reported that AMPK-α1 and AMPK-α2 are involved in tumor growth and survival (31). However, only ARK5 of the AMPK catalytic subunit family members was regulated by Akt and showed extensive invasion activity; overexpression of α1, α2, SNARK, or MELK did not promote invasion activity (our unpublished data). In the present study, an extensive induction of invasion activity was triggered by CA-Akt1. Interestingly, the increased invasion activity induced by CA-Akt1 was not suppressed by LY294002. LY294002 suppresses Akt activation through blockage of the PI-3K-PDK-1 pathway (61), and CA-Akt1 is the membrane-targeted version (9). In the present study, IGF-1 induced both Akt and ARK5 activation, and LY294002 suppressed this activation completely. Therefore, it seems interesting that LY294002 does not suppress invasion activity stimulated by CA-Akt1 in the Matrigel assay. Recently, some reports suggested that PI-3K-PDK-1 pathway-independent phosphorylation of Akt and CA-Akt1 activation were not suppressed by LY294002 (38, 39). CA-Akt1 may be activated by a LY294002-insensitive pathway in the present setting. Since the increase in activity was completely reversed by the elimination of ARK5 by the RNAi technique and by functional suppression of ARK5 by transfection of DN-ARK5, even in the presence of constitutively active Akt, the results strongly suggest that Akt-induced invasion activity is mediated by ARK5. Human colon cancer cell line SW480, in which ARK5 is expressed at the same level as in the P/ARK and D/ARK cell lines, exhibited almost the same level of Akt/ARK5-dependent invasion activity. Moreover, the PANC-1 cell line showed weak invasion activity, and ARK5 overexpression increased it. These findings suggest that ARK5-induced invasion activity is not an artificial phenomenon.
MMP-2 and MMP-9 are well known to play key roles in tumor metastasis (37), and ARK5 overexpression has been found to be associated with increased activation of MMP-2 and MMP-9. MT1-MMP is well known to be the activator of these MMPs (48), and the results of our study demonstrated that ARK5 greatly stimulates MT1-MMP production. The ARK5-induced invasion is probably related to this increased MT1-MMP production. It has recently been reported that MT1-MMP production is regulated at the translational level by Akt during tumor cell invasion induced by IGF-1, which activates mTOR (mammalian target of rapamycin) via Akt activation (63). Although overexpressed ARK5 induced MT1-MMP production, there was no change in expression of MT1-MMP mRNA in P/ARK and PANC-1 cell lines. The results of the present study demonstrated that the increase in production of MT1-MMP was suppressed by rapamycin and ARK5i. IGF-1-induced mTOR phosphorylation was suppressed by DN-Akt1, DN-ARK5, and ARK5i in the present study. Although our present results suggested that mTOR phosphorylation induced by IGF-1 is mediated by ARK5 downstream of Akt, the phosphorylation site of mTOR (Ser2448) did not match the AMPK family member phosphorylation motif (22, 56). Therefore, it seems reasonable that ARK5 modulates mTOR phosphorylation downstream of Akt but does not phosphorylate it directly. It is also interesting that phosphorylation of FKHRL1 was not affected by antisense ARK5 or its RNAi. Because both mTOR and FKHRL1 are reported to be phosphorylated by Akt directly (5, 46), it seems likely that some factor(s) regulating mTOR phosphorylation is phosphorylated by ARK5 to permit mTOR phosphorylation. It is also possible that ARK5 might phosphorylate mTOR directly at a site other than Ser2448 to cause a conformational change allowing Ser2448 phosphorylation. These possibilities should be carefully examined in the future because mTOR regulation is more complex than previously thought and is increasingly important in cancer research (49).
When the P/ARK cell line was transplanted into nude mice, an increase in tumor volume was observed in the mice compared to the PANC-1 cell line. One remarkable feature of the P/ARK tumors was the absence of massive necrosis within the tumor. The necrotic areas in the P/ARK tumors were scattered and resembled not archipelagos but islands, a feature we refer to as piecemeal necrosis. Every area of piecemeal necrosis was significantly smaller than the area of necrosis in the PANC-1 tumors, and the total area of necrosis in the P/ARK tumors was also significantly smaller than in the PANC-1 tumors. Tumors derived from both PANC-1 and P/ARK cell lines contained high levels of phosphorylated Akt; however, P/ARK- but not PANC-1-derived tumors showed a significant decrease in the ratio of necrotic cell death and a marked induction of metastasis. The survival and growth of cells in tumors are largely dependent on the nutrient and oxygen supply (10, 25, 51). ARK5 induces tolerance to nutrient starvation in vitro (55, 56), and such tolerance has been found to be involved in tumorigenesis in nude mice (31). Tumorigenesis is also well known to be dependent on angiogenesis (15, 16); however, no significant changes in the numbers of microvessels were detected with anti-mouse CD31 antibody in the present study. Nor did angiogenesis-related gene expression profiling by DNA microarray technology show any significant changes (our unpublished data). Since our previous work showed that ARK5 overexpression conferred tolerance to the cell death caused by glucose starvation (55, 56), our present findings are highly consistent with our previous findings. These observations suggest that the accelerated tumorigenesis of P/ARK tumors is independent of angiogenesis, indicating austerity, i.e., stronger tolerance of P/ARK to oxygen and nutrient starvation (14). It is highly probable that the austerity caused by ARK5 is one of the mechanisms of accelerated tumorigenesis without massive necrosis.
The results of this study demonstrated that both tumorigenesis and invasion are stimulated by ARK5. It is noteworthy that ARK5 is a member of the AMPK catalytic subunit family, which is activated by exposure to various stresses causing elevation of the intracellular 5′-AMP concentration. Actually overexpression of ARK5 confers tolerance to glucose starvation (55, 56), which is a stress that leads to a decrease in ATP and an increase in AMP. Therefore, it is reasonable to postulate that an insufficient blood supply to tumor tissue and tumor hypoxia cause activation of ARK5 through both Akt activation and increased AMP, resulting in tolerance to nutrient starvation and up-regulation of MT1-MMP production, leading to MMP-2 and MMP-9 activation and stimulating tumor invasion and metastasis. These observations are highly consistent with the notion that tumor hypoxia is the driving force of tumor malignancy (28). Moreover, ARK5 activation is directly regulated by Akt, and Akt is overexpressed or amplified in pancreatic adenocarcinoma (29, 42, 47). The PANC-1 cell line has been widely used as a nonliver metastatic pancreatic cancer cell line (59). It has also been reported that Akt is overexpressed and activated in clinical pancreatic cancers (47). The present study showed activation of Akt in PANC-1-derived tumors, but invasion and metastasis were strongly induced only in the presence of overexpressed ARK5. This suggests that ARK5 is a key factor during the tumorigenesis of colorectal and pancreatic cancer. ARK5 and related biological processes may serve as new targets of cancer therapy.
Acknowledgments
This work was partly supported by a grant for the 2nd-Term Comprehensive 10-Year Strategy of Cancer Control from the Ministry of Health, Welfare and Labour and a grant for the Medical Frontier Program from the Ministry of Health, Welfare and Labour. A.S., G.K., and J.L. are the recipients of a Research Resident Fellowship from the Foundation for Promotion of Cancer Research.
REFERENCES
- 1.Arboleda, M. J., J. F. Lyons, F. F. Kabbinavar, M. R. Bray, B. E. Snow, R. Ayala, M. Danino, B. Y. Karlan, and D. J. Slamon. 2003. Overexpression of AKT2/protein kinase Bβ leads to up-regulation of β1 integrins, increased invasion, and metastasis of human breast and ovarian cancer cells. Cancer Res. 63:196-206. [PubMed] [Google Scholar]
- 2.Besson, A., S. M. Robbins, and V. M. Yong. 1999. PTEN/MMAC1/TEP1 in signal transduction and tumorigenesis. Eur. J. Biochem. 263:605-611. [DOI] [PubMed] [Google Scholar]
- 3.Boyd, D. 1996. Invasion and metastasis. Cancer Metastasis Rev. 15:77-89. [DOI] [PubMed] [Google Scholar]
- 4.Brazil, D. P., J. Park, and B. A. Hemmings. 2002. PKB binding proteins. Getting in on the Akt. Cell 111:293-303. [DOI] [PubMed] [Google Scholar]
- 5.Brunet, A., A. Bonni, M. J. Zigmond, M. Z. Lin, P. Juo, L. S. Hu, M. J. Anderson, K. C. Arden, J. Blenis, and M. E. Greenberg. 1999. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcriptional factor. Cell 96:857-868. [DOI] [PubMed] [Google Scholar]
- 6.Carling, D., K. Aguan, A. Woods, A. J. Verhoeven, R. K. Beri, C. H. Brennan, C. Sidebottom, M. D. Davidson, and J. Scott. 1994. Mammalian AMP-activated protein kinase is homologous to yeast and plant protein kinases involved in the regulation of carbon metabolism. J. Biol. Chem. 269:11442-11448. [PubMed] [Google Scholar]
- 7.Carmeliet, P., Y. Dor, J. M. Herbert, D. Fukumura, K. Brusselmans, M. Dewerchin, M. Neeman, F. Bono, R. Abramovitch, P. Maxwell, C. J. Koch, P. Ratcliffe, L. Moons, R. K. Jain, D. Collen, and E. Keschert. 1998. Role of HIF-1α in hypoxia-mediated apoptosis, cell proliferation and tumor angiogenesis. Nature 394:485-490. [DOI] [PubMed] [Google Scholar]
- 8.Cooper, C. R., C. H. Chay, and K. J. Pienta. 2002. The role of αvβ3 in prostate cancer progression. Neoplasia 4:191-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cross, D. A., D. R. Alessi, P. Cohen, M. Andleikovich, and B. A. Hemmings. 1995. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378:785-789. [DOI] [PubMed] [Google Scholar]
- 10.Dang, C. V., and G. L. Semenza. 1999. Oncogenic alteration of metabolism. Trends Biochem. Sci. 24:68-71. [DOI] [PubMed] [Google Scholar]
- 11.Datta, S. R., A. Brunet, and M. E. Greenberg. 1999. Cellular survival: a play in three Akts. Genes Dev. 13:2905-2927. [DOI] [PubMed] [Google Scholar]
- 12.Delcommenne, M., C. Tan, V. Gray, L. Rue, J. Woodgett, and S. Dedhar. 1998. Phosphoinositide-3-OH kinase-dependent regulation of glycogen synthase kinase 3 and protein kinase B/AKT by the integrin-linked kinase. Proc. Natl. Acad. Sci. USA 95:11211-11216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Denko, N. C., and A. J. Giaccia. 2001. Tumor hypoxia, the physiological link between Trousseau's syndrome (carcinoma-induced coagulopathy) and metastasis. Cancer Res. 61:795-798. [PubMed] [Google Scholar]
- 14.Esumi, H., K. Izuishi, K. Kato, K. Hashimoto, Y. Kurashima, A. Kishimoto, T. Ogura, and T. Ozawa. 2002. Hypoxia and nitric oxide treatment confer tolerance to glucose starvation in a 5′-AMP-activated protein kinase-dependent manner. J. Biol. Chem. 277:32791-32798. [DOI] [PubMed] [Google Scholar]
- 15.Folkman, J. 2001. Can mosaic tumor vessels facilitate molecular diagnosis of cancer? Proc. Natl. Acad. Sci. USA 98:398-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Folkman, J. 1974. Tumor angiogenesis. Adv. Cancer Res. 19:331-358. [DOI] [PubMed] [Google Scholar]
- 17.Franke, T. F., S. I. Yang, T. O. Chan, K. Datta, A. Kazlauskas, D. K. Morrison, D. R. Kaplan, and P. N. Tsichlis. 1995. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell 81:727-736. [DOI] [PubMed] [Google Scholar]
- 18.Frisch, S. M., and R. A. Screton. 2001. Anoikis mechanisms. Curr. Opin. Cell Biol. 13:555-562. [DOI] [PubMed] [Google Scholar]
- 19.Gelinas, D. S., P. N. Bernatchez, S. Rollin, N. G. Bazan, and M. G. Sirois. 2002. Immediate delayed VEGF-mediated NO synthesis in endothelial cells: role of PI3K, PKC and PLC pathways. Br. J. Pharmacol. 137:1021-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grille, S. J., A. Bellacosa, J. Upson, A. J. Klein-Szanto, F. van Roy, W. Lee-Kwon, M. Donovitz, P. N. Tsichlis, and L. Larue. 2003. The protein kinase Akt induces epithelial mesenchymal transition and promotes enhanced motility and invasiveness of squamous cell carcinoma lines. Cancer Res. 63:2172-2178. [PubMed] [Google Scholar]
- 21.Hannigan, G. E., C. Leung-Hagesteijin, L. Fitz-Gibbon, M. G. Coppolino, G. Radeva, J. Filmus, J. C. Bell, and S. Dedhar. 1996. Regulation of cell adhesion and anchorage-dependent growth by a new β1-integrin-linked protein kinase. Nature 379:91-96. [DOI] [PubMed] [Google Scholar]
- 22.Hardie, D. G., and D. Carling. 1997. The AMP-activated protein kinase—fuel gauge of the mammalian cell? Eur. J. Biochem. 246:259-273. [DOI] [PubMed] [Google Scholar]
- 23.Harris, A. L. 2002. Hypoxia—a key regulatory factor in tumor growth. Nat. Rev. Cancer 2:38-47. [DOI] [PubMed] [Google Scholar]
- 24.Hashimoto, K., K. Kato, K. Imamura, A. Kishimoto, H. Yoshikawa, Y. Taketani, and H. Esumi. 2002. 5-Amino-4-imidazolecarboxamide ribose (AICAR) confers strong tolerance to glucose starvation in a 5′-AMP-activated protein kinase-dependent fashion. Biochem. Biophys. Res. Commun. 290:263-267. [DOI] [PubMed] [Google Scholar]
- 25.Helmlinger, G., F. Yuan, M. Dellian, and R. K. Jain. 1997. Interstitial pH and pO2 gradients in solid tumors in vivo: high-resolution measurements reveal a lack of correlation. Nat. Med. 3:177-182. [DOI] [PubMed] [Google Scholar]
- 26.Heyer, B. S., J. Warsowe, D. Solter, B. B. Knowles, and S. L. Ackerman. 1997. New member of the Snf1/AMPK kinase family, Melk, is expressed in the mouse egg and preimplantation embryo. Mol. Reprod. Dev. 47:148-156. [DOI] [PubMed] [Google Scholar]
- 27.Higuchi, M., N. Masuyama, Y. Fukui, A. Suzuki, and Y. Gotoh. 2001. Akt mediates Rac/Cdc42-regulated cell motility in growth factor-stimulated cells and invasive PTEN knockout cells. Curr. Biol. 11:1958-1962. [DOI] [PubMed] [Google Scholar]
- 28.Hockel, M., and P. Vaupel. 2001. Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J. Natl. Cancer Inst. 93:266-276. [DOI] [PubMed] [Google Scholar]
- 29.Itoh, N., S. Semba, M. Ito, H. Takeda, S. Kawata, and M. Yamakawa. 2002. Phosphorylation of Akt/PKB is required for suppression of cancer cell apoptosis and tumor progression in human colorectal carcinoma. Cancer 94:3127-3134. [DOI] [PubMed] [Google Scholar]
- 30.Izuishi, K., K. Kato, T. Ogura, T. Kinoshita, and H. Esumi. 2000. Remarkable tolerance of tumor cells to nutrient deprivation: possible new biochemical target for cancer therapy. Cancer Res. 60:6201-6207. [PubMed] [Google Scholar]
- 31.Kato, K., T. Ogura, A. Kishimoto, Y. Minegishi, N. Nakajima, M. Miyazaki, and H. Esumi. 2002. Critical roles of AMP-activated protein kinase in constitutive tolerance of cancer cells to nutrient deprivation and tumor formation. Oncogene 21:6082-6090. [DOI] [PubMed] [Google Scholar]
- 32.Kim, D., S. Kim, H. Koh, S.-O. Yoon, A.-S. Chung, K. S. Cho, and J. Chung. 2001. Akt/PKB promotes cancer cell invasion via increased motility and metalloproteinase production. FASEB J. 15:1953-1962. [DOI] [PubMed] [Google Scholar]
- 33.Kimura, H., A. Weisz, Y. Kurashima, K. Hashimoto, T. Ogura, F. D'Acquisto, R. Addeo, M. Makuuchi, and H. Esumi. 2000. Hypoxia response element of the human vascular endothelial growth factor gene mediates transcriptional regulation by nitric oxide: control of hypoxia-inducible factor-1 activity by nitric oxide. Blood 95:189-197. [PubMed] [Google Scholar]
- 34.Kumar, C. C. 1998. Signaling by integrin receptors. Oncogene 17:1365-1373. [DOI] [PubMed] [Google Scholar]
- 35.Lawlor, M. A., and D. R. Alessi. 2001. PKB/Akt: a key mediator of cell proliferation, survival and insulin responses? J. Cell Sci. 114:2903-2910. [DOI] [PubMed] [Google Scholar]
- 36.Lefebvre, D. L., Y. Bai, N. Shahmolky, R. Poon, D. J. Drucker, and C. F. Rosen. 2001. Identification and characterization of a novel sucrose-non-fermenting protein kinase/AMP-activated protein kinase-related protein kinase, SNARK. Biochem. J. 355:297-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liotta, L. A. 1986. Tumor invasion and metastases—role of the extracellular matrix: Rhoads Memorial Awards lecture. Cancer Res. 46:1-7. [PubMed] [Google Scholar]
- 38.Maeda, N., Y. Inoshima, D. A. Fruman, S. M. Brachmann, and H. Fan. 2003. Transformation of mouse fibroblasts by Jaagsiekte sheep retrovirus envelope does not require phosphatidylinositol 3-kinase. J. Virol. 77:9951-9959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsumoto, J., M. Kaneda, M. Tada, J. Hamada, S. Okushiba, S. Kondo, H. Katoh, and T. Moriuchi. 2002. Differential mechanisms of constitutive Akt/PKB activation and its influence on gene expression in pancreatic cancer cells. Jpn. J. Cancer Res. 93:1317-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mitchelhill, K. I., D. Stapleton, G. Gao, C. House, B. Michell, F. Katsis, L. A. Witters, and B. E. Kemp. 1994. Mammalian AMP-activated protein kinase shares structural and functional homology with the catalytic domain of yeast Snf1 protein kinase. J. Biol. Chem. 269:2361-2364. [PubMed] [Google Scholar]
- 41.Muraoka, R. S., N. Dumont, C. A. Ritter, T. C. Dugger, D. M. Brantley, J. Chen, E. Easterly, L. R. Roebuck, S. Ryan, P. J. Gotwais, V. Koteliansky, and C. L. Arteaga. 2002. Blockade of TGF-β inhibits mammary tumor cell viability, migration, and metastases. J. Clin. Investig. 109:1551-1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nicholson, K. M., and N. G. Anderson. 2002. The protein kinase B/Akt signaling pathway in human malignancy. Cell. Signal. 14:381-395. [DOI] [PubMed] [Google Scholar]
- 43.Park, B. K., X. Zeng, and R. I. Glazer. 2001. Akt1 induces extracellular matrix invasion and matrix metalloproteinase-2 activity in mouse mammary epithelial cells. Cancer Res. 61:7647-7653. [PubMed] [Google Scholar]
- 44.Persad, S., S. Attwell, V. Gray, M. Delcommenne, A. Troussard, J. Sanghera, and S. Dedhar. 2000. Inhibition of integrin-linked kinase (ILK) suppresses activation of protein kinase B/Akt and induces cell cycle arrest and apoptosis of PTEN-mutant prostate cancer cells. Proc. Natl. Acad. Sci. USA 97:3207-3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Persad, S., and S. Dedhar. 2003. The role of integrin-linked kinase (ILK) in cancer progression. Cancer Metastasis Rev. 22:375-384. [DOI] [PubMed] [Google Scholar]
- 46.Peterson, R. T., P. A. Beal, M. J. Comb, and S. L. Schreiber. 2000. FKBP12-rapamycin-associated protein (FRAP) autophosphorylates at serine 2481 under translationally repressive conditions. J. Biol. Chem. 275:7416-7423. [DOI] [PubMed] [Google Scholar]
- 47.Ruggeri, B. A., L. Huang, M. Wood, J. Q. Cheng, and J. R. Testa. 1998. Amplification and overexpression of the AKT2 oncogene in a subset of human pancreatic ductal adenocarcinomas. Mol. Carcinog. 21:81-86. [PubMed] [Google Scholar]
- 48.Sato, H., T. Takino, Y. Okada, J. Cao, A. Shinagawa, E. Yamamoto, and M. Seiki. 1994. A matrix metalloproteinase expressed on the surface of invasive tumor cells. Nature 370:61-65. [DOI] [PubMed] [Google Scholar]
- 49.Shamji, A. F., P. Hghiem, and S. L. Schreiber. 2003. Integration of growth factor and nutrient signaling: implications for cancer biology. Mol. Cell 12:271-280. [DOI] [PubMed] [Google Scholar]
- 50.Shinkaruk, S., M. Bayler, G. Lain, and G. Deleris. 2003. Vascular endothelial cell growth factor (VEGF), an emerging target for cancer therapy. Curr. Med. Chem. Anti-Cancer Agents 3:95-117. [DOI] [PubMed] [Google Scholar]
- 51.Southerland, R. M. 1988. Cell and environment interactions in tumor microregions; the multicell spheroid model. Science 240:178-184. [DOI] [PubMed] [Google Scholar]
- 52.Stapleton, D., K. I. Mitchelhill, G. Gao, J. Widmer, B. J. Michell, T. Teh, C. M. House, C. S. Fernandez, T. Cox, L. A. Witters, and B. E. Kemp. 1996. Mammalian AMP-activated protein kinase subfamily. J. Biol. Chem. 271:611-614. [DOI] [PubMed] [Google Scholar]
- 53.Streit, M., and M. Detmar. 2003. Angiogenesis, lymphangiogenesis, and melanoma metastasis. Oncogene 22:3172-3179. [DOI] [PubMed] [Google Scholar]
- 54.Sun, H., R. Lesche, D. M. Li, J. Liliental, H. Zhang, J. Gao, N. Gavrilova, B. Mueller, X. Liu, and H. Wu. 1999. PTEN modulates cell cycle progression and cell survival by regulating phosphatidylinositol 3,4,5,-triphosphate and Akt/protein kinase B signaling pathway. Proc. Natl. Acad. Sci. USA 96:6199-6204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Suzuki, A., G. Kusakai, A. Kishimoto, J. Lu, T. Ogura, and H. Esumi. 2003. ARK5 suppresses the cell death induced by nutrient starvation and death receptors via inhibition of caspase 8 activation, but not by chemotherapeutic agents or UV irradiation. Oncogene 22:6177-6182. [DOI] [PubMed] [Google Scholar]
- 56.Suzuki, A., G. Kusakai, A. Kishimoto, J. Lu, T. Ogura, M. F. Lavin, and H. Esumi. 2003. Identification of a novel protein kinase mediating Akt survival signaling to ATM. J. Biol. Chem. 278:48-53. [DOI] [PubMed] [Google Scholar]
- 57.Suzuki, A., G. Kusakai, A. Kishimoto, Y. Minegishi, T. Ogura, and H. Esumi. 2003. Induction of cell-cell detachment during glucose starvation through F-actin conversion by SNARK, the fourth member of the AMP-activated protein kinase catalytic subunit family. Biochem. Biophys. Res. Commun. 311:156-161. [DOI] [PubMed] [Google Scholar]
- 58.Tanno, S., S. Tanno, Y. Mitsuuchi, D. A. Altomare, G. H. Xiao, and J. R. Testa. 2001. AKT-activation up-regulates insulin-like growth factor I receptor expression and promotes invasiveness of human pancreatic cancer cells. Cancer Res. 61:589-593. [PubMed] [Google Scholar]
- 59.Teraoka, H., T. Sawada, T. Nishihara, M. Yashiro, M. Ohira, T. Ishikawa, H. Nishino, and K. Hirakawa. 2001. Enhanced VEGF production and decreased immunogenicity induced by TGF-beta 1 promote liver metastasis of pancreatic cancer. Br. J. Cancer 85:612-617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vaupel, P., D. K. Kelleher, and M. Hockel. 2001. Oxygen status of malignant tumors: pathogenesis of hypoxia and significant for tumor therapy. Semin. Oncol. 28:29-35. [DOI] [PubMed] [Google Scholar]
- 61.Vlahos, C. J., W. F. Matter, K. Y. Hui, and R. F. Brown. 1994. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002). J. Biol. Chem. 269:5241-5248. [PubMed] [Google Scholar]
- 62.Zaman, K., H. Ryu, D. Hall, K. O'Donovan, K. I. Lin, M. P. Miller, J. C. Marquis, J. M. Baraban, G. L. Semenza, and R. R. Ratan. 1999. Protection from oxidative stress-induced apoptosis in cortical neuronal cultures by iron chelators is associated with enhanced DNA binding of hypoxia-inducible factor-1 and ATF/CREB and increased expression of glycolytic enzymes, p21(waf1/cip1), and erythropoietin. J. Neurosci. 19:9821-9830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang, D., and P. Brodt. 2003. Type 1 insulin-like growth factor regulates MT1-MMP synthesis and tumor invasion via PI 3-kinase/Akt signaling. Oncogene 22:974-982. [DOI] [PubMed] [Google Scholar]