Abstract
Objective
Saphenous vein grafts suffer from neointima formation following bypass surgery. Matrix metalloproteinases (MMPs) play important roles in this process. We examined MMP-3 for its therapeutic potential to prevent smooth muscle cell migration and neointima formation in venous bypass grafts using adenovirus-mediated gene transfer.
Methods
Human aortic smooth muscle cells (hAoSMC) were transduced with adenoviral vectors encoding β-galactosidase (Ad.βgal) or human MMP-3 (Ad.hMMP-3), and characterized for migration in the amniotic membrane stroma as an in vitro model of the vascular wall. Cholesterol-fed New Zealand White rabbits underwent jugular vein bypass grafting into carotid arteries. Before insertion, grafts were incubated ex vivo with either Ad.βgal or Ad.hMMP-3. Transgene expression was characterized by immunohistochemistry and in situ zymography. Grafts (n=6) were explanted after 28 days and intimal hyperplasia was quantified.
Results
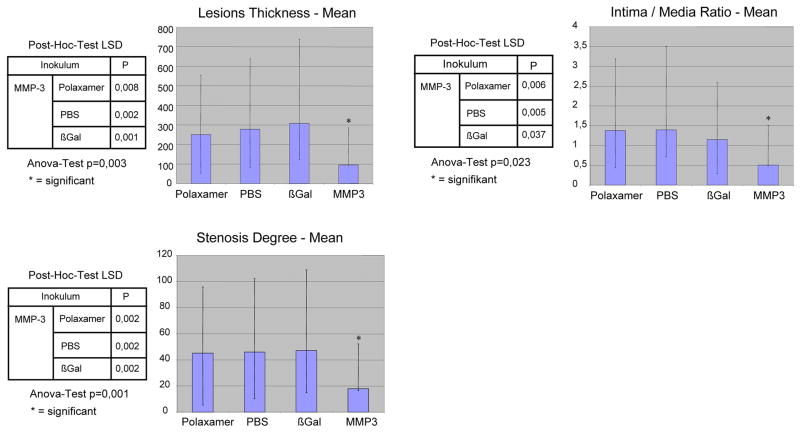
Migration of hAoSMC was significantly reduced when transduced with Ad.hMMP-3 compared to controls (p<0.001). Immunocytochemistry of Ad.hMMP-3 transduced venous grafts localized this protein to the intima. In situ-zymography showed increased MMP activity in the intima of Ad.hMMP-3 transfected grafts. Stenosis degree (p=0,001), intima/media-ratio (p=0,023) and lesion thickness (p=0,003) were significantly reduced in grafts transduced with Ad.MMP-3 in comparison to controls. There was no difference inside control groups.
Conclusions
MMP-3 overexpression inhibits formation of intimal hyperplasia in arterialized vein grafts. Adenovirus mediated gene transfer of MMP-3 may be of clinical use to prevent vein graft stenosis following bypass surgery.
Keywords: matrix-metalloproteinases, gene therapy, bypass, vascular diseases, surgery
INTRODUCTION
Autologous vein grafts are the conduits of choice for lower extremity and coronary artery bypass graft surgery. However, only 50 %–80 % of vein grafts remain patent at 5 to 15 years [1–5]. Intimal hyperplasia is the major cause of vein graft stenosis. Smooth muscle cell (SMC) proliferation and migration into to the intima and their proliferation are key mechanisms in this process [6,7]. Cell migration requires extracellular matrix (ECM) degradation by a variety of extracellular proteinase, among which the matrix metalloproteinases (MMPs) play an important role. MMPs can collectively degrade virtually all ECM components and have been implicated in a variety of tissue remodeling processes, including atherosclerosis and intimal thickening [8]. MMP-3 (stromelysin-1) has the broadest substrate specificity of all MMPs; it degrades collagen types III, IV and V, laminin, fibronectin, elastin and proteoglycans. MMP-3 is secreted by a variety of cell types including SMC [9,10]. High levels of MMP-3 have been described in atherosclerotic plaques [11] and in the SMC of atherosclerotic coronary arteries [10]. MMP-3 is therefore considered as a protease supporting SMC-migration and development of intimal hyperplasia in vascular remodeling.
Paradoxically, a common mutation of the MMP-3-promoter resulting in reduced MMP-3 expression is associated with development of atherosclerosis [12] and restenosis [13]. In MMP-3 knock-out mice, atherosclerotic plaques are more instable than in MMP-3 expressing mice [14]. Conversely, high levels of MMP-3 are associated with increased risk of myocardial infarction [15]. Therefore, the role of MMP-3 in vascular remodelling remains unclear.
Based on the previous findings we hypothesized that high levels of MMP-3 inhibit vein graft stenosis. Here we report that adenovirus-mediated overexpression of MMP-3 blocks SMC cell migration in vitro and reduces the formation of intimal hyperplasia in arterialized vein grafts.
MATERIALS AND METHODS
Cells and Culture Medium
Human aortic smooth muscle cells (hAoSMC) were purchased from Clonetics (San Diego, CA) and grown in Smooth Muscle Basal Medium supplemented with 10 ng/ml of human Epidermal Growth Factor (hEGF), 2 ng/ml of human Fibroblast Growth Factor (hFGF), 0.39 μg/ml of Dexamethasone, 50 μg/ml of Gentamycin, 50 ng/ml of Amphotericin-B, and 5% fetal bovine serum. The cells were used between passage 2 and 8 in culture. Human 293 embryonic kidney cells stably transfected with the E1A and E1B genes (kindly provided by Dr. R. Schneider, NYU Medical Center) were cultured in DMEM supplemented with 10 % FBS.
Generation of recombinant adenoviruses and transduction conditions
Adenoviral vectors encoding either β-galactosidase or human MMP-3 (hMMP-3) were generated as described [16,17]. Briefly, full length DNA for human MMP-3 (or β-galactosidase) was subcloned into a pCMVAd. The resulting construct, pCMVAd.MMP-3, contained the MMP-3 cDNA flanked on its 5′ end by the cytomegalovirus (CMV) promoter and on its 3′ end by a polyadenylation sequence and several hundred nucleotides of adenoviruy type 5 (Ad dl 309) genome lacking the E1A region. Linearized plasmids and the Ad dl 309 viral genome were cotransfected into 293 cells or, for animal experiments, in N52.E6 cells.
hAoSMC were transduced with 2 × 108 pfu/ml (100 pfu/cell). Transduction efficiency ranged 24%–40% as assessed by β-Galactosidase staining of cells transduced with Ad.βgal.
Smooth Muscle Cell Invasion of the Amniotic Membrane
Human amniotic membranes were prepared as described [16].
Northern Blotting
Northern blotting was performed as described [18] using a DIG-labeled cDNA probe to MMP-3 kindly provided by Dr. Markku Kurkinen (Wayne State University), or an 18S DIG-labeled rRNA probe (Boehringer)as a control.
Western Blotting and Casein Zymography
Western blotting and casein zymography were performed as described [19]
Ex Vivo Transduction of Vein Explants with Adenoviral Vectors
For animal experiments, N52.E6 cell line (human amniocytes, a generous gift from G. Schiedner and S. Kochanek, University of Cologne, Germany) was used for homologous recombination of adenoviral vectors to avoid the generation of replication-competent adenoviral vectors. Before grafting into the carotid arteries, excised jugular veins were incubated for 30 min at 37°C under sterile conditions with adenoviral vectors encoding hMMP-3 or β-galactosidase (2×109 pfu/ml of Poloxamer 407). Poloxamer 407, reported to increase virus-mediated transduction efficiency 100-fold [20], was used to reduce adenovirus concentration and minimize viral toxicity. After incubation, transduced vein were washed twice with PBS to remove residual adenoviral vectors.
Vein Graft Model of Intimal Hyperplasia
Female New Zealand White Rabbits [Charles River GmbH, Germany] of approx. 4 kg were fed a 1 % cholesterol diet from day 7 before operation until euthanasia. The rabbits were pre-anesthetized with ketamine (25 mg/kg) and midazolam (0.1 mg/kg) i.m. An iv-line was placed, 0.04 mg/kg buprenorphine injected and propofol-1% was triggered i.v. for intubation. After intubation, anesthesia was maintained by spontaneous breathing of 40% oxygen and 2%-vol isoflurane. Deepness of narcosis and life parameters were monitored continuously. Standard vein graft dissection techniques were used to collect the left jugular vein, which was transferred to a laboratory designed for work with genetically hazardous material (level SII). Under sterile conditions, the explanted veins were flushed with PBS, Poloxamer 407 or transduced with adenoviral vectors encoding for β-galactosidase or hMMP-3. After injection of 100 i.E/kg heparin, the veins were grafted end-to-side into the carotid artery with prolene 10-0 (Ethicon). The animals were extubated and monitored in a recovery room for 6 h, before being transferred back to the animal housing facility. Perioperatively the animals received a single dose of Penicillin/Streptomycin (0.5 ml Tardomycel s.c., Bayer AG, Leverkusen), and three daily s.c injections of carprofene (25 mg). For harvesting the grafts, the animals were anesthetized with ketamine (25 mg/kg) and midazolam (0.1 mg/kg) i.m., and sacrificed with an i.v. overdose of pentobarbital (300 mg/kg) before making the skin incision. Harvested grafts were washed with PBS and either embedded in OCT and snap-frozen in liquid nitrogen, or fixed for later paraffin embedding. Triplicate animals were used for analyzing hMMP-3 overexpression at postoperative day 7, and 6 animals/group for characterizing intimal hyperplasia. Two animals in our series had occluded grafts due to technical errors and were excluded from analysis.
The animals were treated in accordance with the German animal welfare law and European Union guidelines. Institutional approval for animal experiments was obtained (No. 509.6-42502-99/201). The investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996).
Immunohistochemistry
Ten-micrometer frozen sections were incubated with mouse anti-MMP3 monoclonal antibody [No. 1339, Chemicon] diluted 1:100 overnight at 4° C, followed by 30 min incubation with FITC-labeled goat-anti-mouse antibody [No. AP 124 F Chemicon], and fixation with mowiol (Hoechst, Germany). These MMP-3 antibodies do not cross react with rabbit-MMP-3.
In situ-Zymography
MMP-3 activity was analyzed by in situ casein zymography. Vessels were snap frozen in OCT medium after harvesting and cut into 8-μm sections. The sections were put onto microscope slides, and incubated at 37°C for 48 h in Tris 50 mM, CaCl2 10 mM, Brij 35 0.05% containing agarose 1% and resorufin-labeled casein (10mg/ml; Boehringer Mannheim). Casein degradation was observed with a fluorescence microscope.
Measurement of Neointima Formation
Neointima formation was quantified on cross sections of vein grafts (6/group) stained with elastica van Gieson (EvG) and Mayer’s hematoxylin and eosin (H&E). For randomization, vessels were longitudinally divided into 6 regions, and from each region the first cross section was picked for analysis. Intima, media, internal and external elastic lamina were identified and traced on digital images of sections cut at five spaced intervals using Image-Pro Plus (Version 4.1.5, Cybernetics Inc., Silver Spring, MD). The investigator was blinded since labeling of cross sections did not allow identification of experimental group. Three parameters were calculated: stenosis degree (intima - lumen/lumen), intima/media ratio (intima - lumen/media - lumen) and lesion thickness (radius of the media - radius of the intima).
Statistical Analysis
Statistical analysis was performed by One-way ANOVA combined with post-hoc-test LSD using SPSS (Version 12.0, Chicago). p < 0.05 values were considered statistically significant.
RESULTS
HAoSMC Transduced with Ad.MMP-3 Overexpress Functional MMP-3
To study the role of MMP-3 in the generation of intimal hyperplasia we used adenovirus mediated gene transfer to upregulate MMP-3 expression in aortic smooth muscle cells. By Northern blotting, Ad.hMMP-3-transduced hAoSMC had high levels of MMP-3 mRNA, whereas control, non-transduced or Ad.βgal-transduced cells showed no such transcript (Fig. 1a). Western blotting with MMP-3 antibody showed expression of a 57-kDa band consistent with the Mr of pro-MMP-3 (Fig. 1b). This band was undetectable in the CM of control cells, showing that non-transduced hAoSMC expressed no MMP-3. A similar amount of MMP-3 was present in the CM of hAoSMC grown on the amniotic membrane (Fig. 1b, SMC transduced/a). In vitro experiments with different adenoviral vector concentrations showed the highest transduction efficiency of 24.1 % and the lowest cytotoxicity with 2 × 108 pfu/ml (100 pfu/cell). Dose response experiments showed no relevant increase of transduction efficiency when vector concentration was higher. The growth rate of virus-transduced cells was slightly but not significantly higher that of non-transduced cells (data not shown), and no difference in viability was observed between transduced and non-transduced hAoSMC. Consistent with the Western blotting result, casein zymography of medium conditioned by Ad.hMMP-3-transduced hAoSMC showed a lysis band of 57 kDa (Fig. 1c). This caseinolytic band was converted to 45 kDa following incubation of the conditioned medium with 1 mmol/l p-aminophenylmercuric acetate (APMA) at 37°C for 2 h, indicating activation of pro-hMMP-3 by cleavage of the pro-domain (data not shown). Thus, transduction of hAoSMC with Ad.hMMP-3 resulted in overexpression of functional hMMP-3.
Figure 1. Characterization of hMMP-3 overexpression in hAoSMC transduced with Ad.hMMP-3.
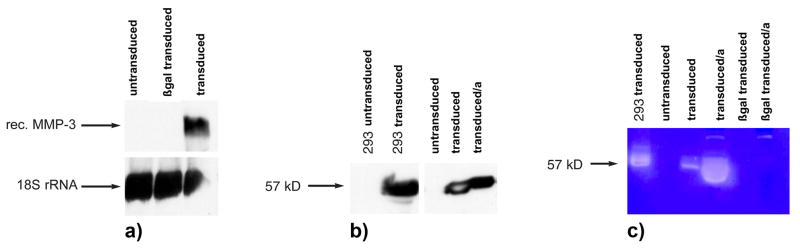
a) Northern blotting analysis of total RNA from non-transduced, Ad.βgal- or Ad.hMMP-3-transduced hAoSMC. Only cells transduced with Ad.hMMP-3 express the 2.2 Kb MMP-3 mRNA (rec. hMMP-3). 18 s rRNA is shown as a control. b) Western blotting. Expression of an immunoreactive 57 kDa band consistent with the Mr of pro-MMP-3 is present in Ad.hMMP-3-transduced hAoSMC or 293 cells. HAoSMC grown on the amniotic membrane (transfected/a) or in a culture dish (transfected) produced comparable levels of MMP-3. c) Casein zymography. Medium conditioned by Ad.hMMP-3-transduced 293 cells, or by non-transduced, Ad.βgal- or Ad.hMMP-3-transduced hAoSMC was used. A lysis band of 57 kDa consistent with the Mr of pro-MMP-3 was present only in Ad.MMP-3 transduced cells. Transduced hAoSMC grown on the amniotic membrane (transfected/a) showed higher MMP-3 caseinolytic activity than cells grown on plastic.
Overexpression of MMP-3 Inhibits hAoSMC Migration
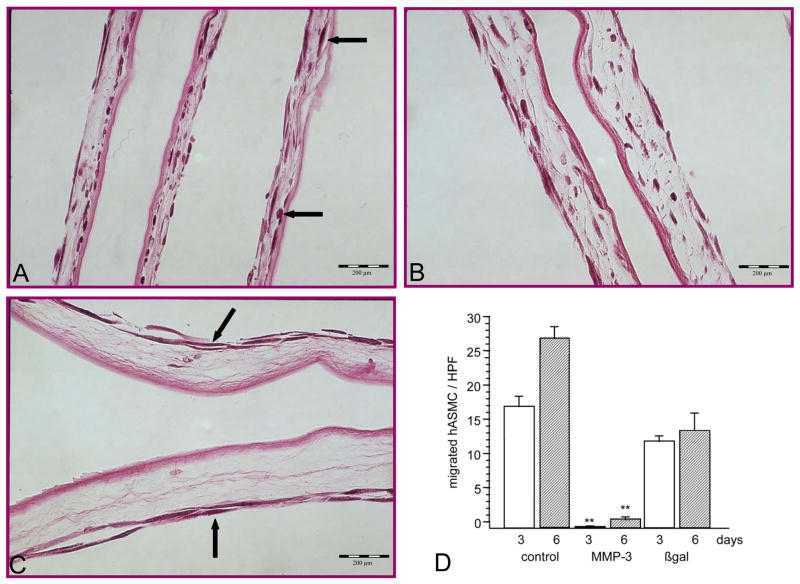
To evaluate the effect of MMP-3 gene transfer on SMC invasion of the extracellular matrix, MMP-3 transduced hAoSMC were seeded onto the stromal aspect of the amniotic membrane and SMC-migration was measured. In the presence of 10 ng/ml of PDGF, non-transduced or Ad.βgal-transduced hAoSMC migrated into the collagenous stroma to the same extent. Cells were found at varying depth in the stromal meshwork; some cells in close contact with the stromal side of the basement membrane (Fig. 2a, b). In contrast, only occasional Ad.MMP-3-transduced hAoSMC were detected in the superficial layers of the stroma (Fig. 2c). Counting of the cells in 10 random high-power (400x) microscopic fields showed only single MMP-3-transduced hAoSMC in the stroma after 3 or 6 days of incubation (Fig. 2d/graph). In contrast, up to 20-fold more non-transduced or Ad.βgal-transduced hAoSMC were counted under the same conditions (p< 0.001). These results indicated that high levels of MMP-3 expression inhibit SMC invasion of a collagenous stroma.
Figure 2.
Migration of human aortic smooth muscle cells (hAoSMC) through the amniotic membrane mimicking the vascular extracellular matrix, stimulated with 10 ng/ml of PDGF. A: Untreated hAoSMC migrated from the surface into the stroma and were found in various depths of the matrix (arrows). B: HAoSMC were transduced with Ad.βgal before seeding. Cells migrated in comparable numbers into the stroma as untreated hAoSMC. C: When transduced with Ad.hMMP-3 before seeding, hAoSMC rarely invade the stroma and remain on the surface (arrows). D: Number of migrated hAoSMC were counted by 10 random microscopic high power fields (HPF) per histologic sections. Difference between MMP-3-overexpressing hAoSMC and controls was highly significant (** p<0.001), but not between controls. Differences in thickness of the membrane (C) relate to natural variability of amnion membrane. H&E, magnification 400x for all photographs. Mean ± standard deviation is shown (D).
Ex vivo Transduction of Autologous Vein Grafts with Ad.hMMP-3 Inhibits the Development of Intimal Hyperplasia in vivo
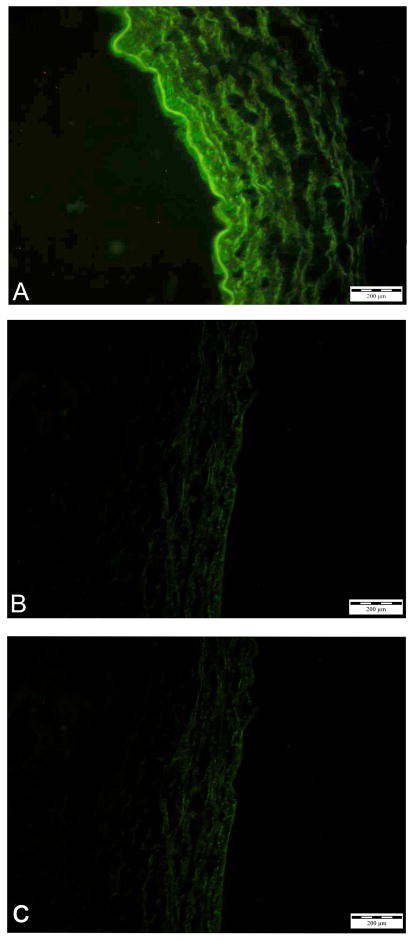
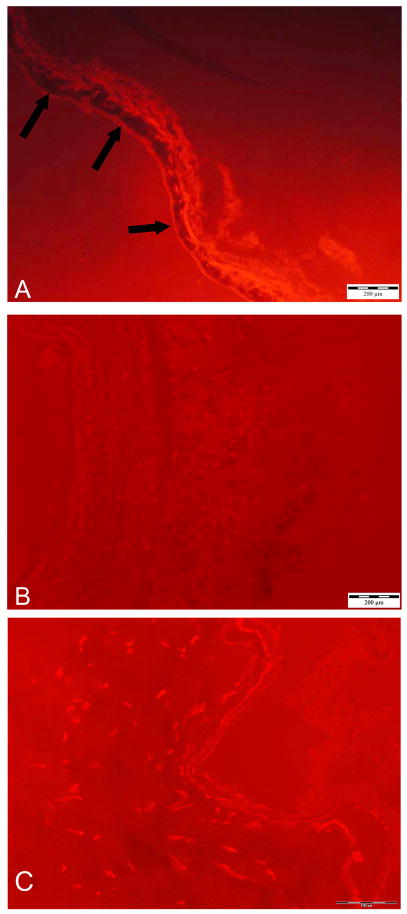
To investigate the role of MMP-3 in the development of intimal hyperplasia we transduced jugular vein explants ex vivo and grafted them into the carotid artery as described under Materials and Methods. Analysis of the venous grafts by immunocytochemistry and in situ zymography 7 days after implantation showed that adenovirus-mediated gene transfer of hMMP-3 resulted in sustained overexpression of active hMMP-3. By immunocytochemistry MMP-3-transduced grafts showed prominent staining in the intima and faint staining in the media (Fig. 3a). Virtually the whole luminal lining of the vessel was stained, indicating high transduction efficiency. Conversely, non-transduced control grafts showed no specific staining. Similarly, in situ casein zymography showed high proteolytic activity localized to the intima of MMP-3-transduced grafts (Fig. 4a) and no caseinolytic activity in control grafts incubated with PBS alone or transduced with Ad.βgal (Fig. 4b + c). Thus, ex vivo transduction of vein explants resulted in high and sustained levels of expression of active MMP-3.
Figure 3.
Immunocytochemical analysis of MMP-3 in cross-sections of vein grafts 7 days after implantation. A, vein graft transduced with Ad.hMMP-3. B, non- transduced control. C, vein graft transduced with Ad.hMMP-3 stained with secondary antibody alone.
Figure 4.
In situ casein zymography of vein grafts 7 days after implantation. A, vein graft transduced with Ad.hMMP-3. Dark areas in the intima (arrows) indicate digestion of red-labelled casein. B, non-transduced control. C, Ad.βgal transduced control. No caseinolytic activity is visible in the two controls.
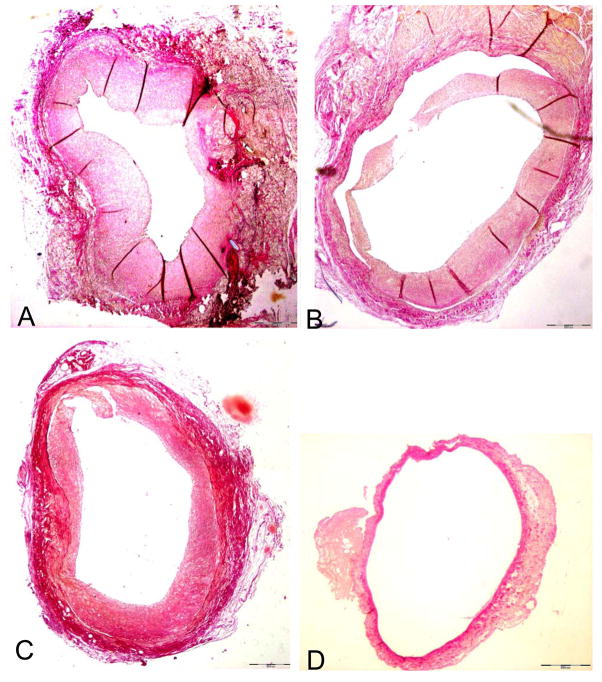
To analyze the development of intimal hyperplasia vein grafts were harvested 28 days after implantation, and cross sections were characterized histologically. Significant intimal hyperplasia was detected in control vein grafts incubated with PBS or Poloxamer 407 (Fig. 5a and b; Fig. 6). Similarly, control vein grafts transduced with β-gal showed development of intimal hyperplasia comparable to the other controls (Fig. 5c). Conversely, intimal hyperplasia was significantly reduced in vein grafts transduced with MMP-3 (Fig. 5d), as assessed by measurement of stenosis degree (p=0,001), intima/media-ratio (p=0,023) and lesion thickness (p=0,003) (Fig. 6). Therefore, these results showed that overexpression of MMP-3 inhibits the development of intimal hyperplasia in arterialized vein grafts.
Figure 5.
Development of intimal hyperplasia in vein grafts 28 after interposition into the carotid artery. Elastica van Gieson, magnification 20x. A: Graft incubated with PBS before implantation, showing severe neointima formation. Comparable amounts of intimal hyperplasia were found in vein grafts incubated with Poloxamer 407 (B) or transduced ex vivo with Ad.βgal (C) before implantation. Vein grafts transduced with Ad.hMMP-3 showed almost no intimal hyperplasia 28 days after implantation (D).
Figure 6.
Degree of intimal hyperplasia in vein grafts 28 day after implantation. N=6 animals per group were investigated. Lesion thickening, intima/media ratio and stenosis degree were measured as detailed under Materials and Methods. Mean ± standard deviation is shown.
DISCUSSION
The data presented show that adenovirus-mediated overexpression of Stromelysin-1 (MMP-3) reduces migration of hAoSMC in an in vitro model of the vascular wall, and causes reduced development of intimal hyperplasia in vivo. These findings contrast with the understanding of the role of MMP-3 in the development of intimal hyperplasia. The broad substrate specificity and strong proteolytic activity against components of the extracellular matrix led to the assumption that – like other proteinases - MMP-3 promotes SMC migration and development of intimal hyperplasia. High levels of MMP-3 have been described in atherosclerotic plaques and in the SMC of atherosclerotic coronary arteries [10,11]. Mechanical injury of rabbit VSMCs was associated with induction of stromelysin-1 mRNA expression, migration and proliferation, and neointima formation after vessel wall injury of rat carotid arteries was substantially inhibited by antisense oligonucleotides to stromelysin-1 mRNA [21,22]. All these findings supported the hypothesis that MMP-3 promotes SMC migration and development of intimal hyperplasia.
However, other studies have shown that reduced expression of MMP-3 in patients with a common 6A/6A polymorphism leads to increased progression of coronary atherosclerosis [12], whereas the 5A/6A polymorphism with higher MMP-3 levels is associated with reduced restenosis after PTCA and stenting compared to patients with 6A/6A alleles [13]. It has been speculated that MMP-3 has a dominant protective effect from lumen loss associated with the 5A allele and consecutive higher MMP-3 levels. Consistent with this hypothesis healthy male subjects homozygous for the 6A allele (i.e. with low MMP-3 expression) show increased wall thickness, enlarged arterial lumen, and local reduction of wall shear stress, which might predispose them to atherosclerotic plaque formation [23]. Studies with apoE−/− MMP-3−/− double knock out mice also support the hypothesis of a protective function of MMP-3 in vascular remodelling. Atherosclerotic plaques in these animals are four times as large as in apoE−/−: MMP-3+/+ mice [14,24]. These findings support the hypothesis that high levels of MMP-3 have a protective role in vascular remodelling.
In our study, both migration of hAoSMC and development of intimal hyperplasia were reduced by overexpression of MMP-3. Initially, we observed reduced migration of hAoSMC overexpressing MMP-3 in a Boyden chamber assay (data not shown). Therefore, to study SMC migration in a more physiological setting we used a cell migration model based on the amniotic membrane [16]. The composition and structure of the amnion extracellular matrix is more similar to that of vessels than is matrigel. The amniotic membrane has a dense basement membrane and abundant collagenous stroma with elastin fibers, which confer great resistance to traction and resilience on the tissue, making it more similar to a vessel than matrigel. Because of the harsh treatment used to remove the epithelial layer (incubation in 0.25 M NH4Cl for 1 h), the occasional fibroblasts present in the amnion stroma are destroyed, leaving an acellular collagenous matrix. Therefore, in this model the cells that are found in the stroma are the viable cells that invaded from the stromal surface. We found that stimulation with PDGF induced SMC migration and invasion into the amnion stroma, a process blocked by MMP-3 overexpression. For practical reasons, we used arterial instead of venous SMC for our studies. Venous SMC tend to exhibit increased proliferation and migration compared to arterial SMC [25–27]. However, although we used less motile hAoSMC, migration was upregulated by PDGF both in untreated and Ad.βgal-transduced cells but not in MMP-3-transduced cells.
The vein graft model of intimal hyperplasia that we used showed significant reduction of intimal hyperplasia in grafts transduced with Ad.hMMP-3 relative to non-transduced or Ad.βGal-transduced controls. Immunocytochemical and in situ zymography analysis showed that MMP-3 overexpression in transduced grafts lasted at least 7 days. We did not characterize MMP-3 expression at 28 days after grafting, the time when intimal hyperplasia was analyzed. Since adenoviral expression is transient, overexpression is unlikely to last longer than 4 weeks after transduction. However, the early development of IH occurs in the first two weeks after venous graft arterialization [28,29]; whereas the late remodelling of the arterialized venous graft is essential for adaptation of the vessel wall to the high pressure system. Experience with drug eluting stents in percutaneous catheter interventions (PCI) for coronary artery disease shows that the benefit of early inhibition of intimal hyperplasia lasts longer than the drug eluting effect for prevention of stent stenosis. However, from our experiments we have no information if longer overexpression of MMP-3 is required for long-term reduction of intimal hyperplasia in arterilized vein grafts.
Gene transfer with adenoviral vectors is a potentially usefully technique for this clinical application. For the treatment of vein grafts the adenoviral vectors are used only ex vivo, and grafts are flushed several times after transduction for removal of free adenoviral vectors. Therefore, only trace amounts of viral vectors are expected to remain in the vein graft. As long as large numbers of infectious adenoviral vectors are not injected systemically, the risk for the patient is extremely low. There are no reports that topical ex vivo administration of adenoviral vectors results in systemic complications. Furthermore, to improve the safety for possible clinical application, we used a relatively low number of viral infectious units and a cell-system for vector production that makes the development of replication-competent adenoviral vectors (RCA) almost impossible. However, a perfect delivery vehicle for gene tranfer still remains unavailable.
The mechanism by which MMP-3 reduces SMC-migration and intimal hyperplasia remains unclear. The observation that relatively low transduction efficiency resulted in almost complete reduction of migration in vitro, presumably including uninfected cells, suggest that the underlying effect is not cell specific. It is possible that MMP-3 degrades PDGF, thus depriving vascular smooth muscle cells of the major inducer of cell migration. However, this hypothesis has not been tested. Based on existing reports we speculate that excess stromal degradation by high levels of MMP-3 deprive SMC of anchorage sites required for migration. This hypothesis is consistent with previous findings that inhibition of cell attachment to matrigel inhibits cell migration [30]. Fibronectin (FN) mediates cell adhesion, migration and cytodifferentiation [31], and SMC use FN to bind to type I and III collagens [32]. Intact FN (and laminin) is therefore essential for SMC migration. Because FN and laminin are substrates for MMP-3, it is possible that excess degradation of these (and other) extracellular matrix proteins by MMP-3 results in impaired SMC migration. The theory of “no grip, no invasion” has been discussed elsewhere [33] and is supported by findings that excessive proteolysis can cause damage to the tissue and dissolve the matrix needed for anchoring the migrating cells [34,35]. This view is also supported by a recent study showing that MMP-3 knockdown skeletal muscle satellite cells migrated faster than controls [36]. MMP-3 can promote extracellular matrix degradation directly and indirectly by activating other MMPs, including MMP-9 and MMP-7 [8,18]. The collective action of these MMPs in the graft may therefore cause excessive degradation of the extracellular matrix, generating microenvironmental conditions that are not permissive for SMC migration.
In addition, MMP-3 may play a role in the chemotactic recruitment of a number of inflammatory cell types directly [37,38] or through the chemotactic action of protein (e.g. laminin) degradation products [39]. Additional secretion of MMPs (e.g. MMP-9) from attracted macrophages will result in increased proteolytic activity.
Thus, a variety of observations support the concept that MMP-3 has beneficial effects on venous vascular remodeling in the setting of venous grafting. Further studies are warranted to clarify the role of MMP-3 in plaque rupture, restenosis and narrowing of arterialized autologous vein grafts, and to investigate the therapeutic potential of MMP-3 overexpression in the vascular wall.
Acknowledgments
The authors thank Stefanie Geveke and Christiane Mörike for technical assistance, and Antje Bog and Serghei Cebotari for scientific support of this study.
FUNDING
This project was supported by a grant of the “Deutsche Forschungsgemeinschaft” (DFG), KA-1309/2-1/2 to K.K, by funds from the Cardiovascular Surgery Department of NYU School of Medicine and partly by grant R01 HL070203 to P.M.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Klinkert P, Post PN, Breslau PJ, van Bockel JH. Saphenous vein versus PTFE for above – knee femoropolpliteal bypass. A review of the literature. Eur J Vasc Endovasc Surg. 2004;27:357–62. doi: 10.1016/j.ejvs.2003.12.027. [DOI] [PubMed] [Google Scholar]
- 2.Taylor LM, Edwards JM, Porter JM. Present status of reversed vein bypass grafting: Five-year results of a modern series. J Vasc Surg. 1990;11:193–206. doi: 10.1067/mva.1990.17235. [DOI] [PubMed] [Google Scholar]
- 3.Shah DM, Darling C, 3rd, Chang BB, Fitzgerald KM, Paty PSK, Leather RP. Long-term results of in situ saphenous vein grafts. Analysis of 2058 cases. Ann Surg. 1995;222:438–48. doi: 10.1097/00000658-199510000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldman S, Zadina K, Moritz T, Ovitt T, Sethi G, Copeland JG, et al. VA Cooperative Study Group #207/297/364. Long-term patency of saphenous vein and left internal mammary artery grafts after coronary artery bypass surgery: results from a Department of Veterans Affairs Cooperative Study. J Am Coll Cardiol. 2004 Dec 7;44(11):2149–56. doi: 10.1016/j.jacc.2004.08.064. [DOI] [PubMed] [Google Scholar]
- 5.Fitzgibbon GM, Kafka HP, Leach AJ, Keon WJ, Hooper GD, Burton JR. Coronary bypass graft fate and patient outcome: angiographic follow-up of 5,065 grafts related to survival and reoperation in 1,388 patients during 25 years. J Am Coll Cardiol. 1996 Sep;28(3):616–26. doi: 10.1016/0735-1097(96)00206-9. [DOI] [PubMed] [Google Scholar]
- 6.Gibbons GH, Dzau VJ. The emerging concept of vascular remodeling. N Engl J Med. 1994;330:1431–8. doi: 10.1056/NEJM199405193302008. [DOI] [PubMed] [Google Scholar]
- 7.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–9. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 8.Newby AC. Dual Role of Matrix Metalloproteinases (Matrixins) in Intimal Hyperplasia and Atherosclerosis Plaque Rupture. Physiol Rev. 2005;85:1–31. doi: 10.1152/physrev.00048.2003. [DOI] [PubMed] [Google Scholar]
- 9.Mignatti P, Rifkin D, Welgus H, Parks W. Proteinases and Tissue Remodeling. In: Clark RAF, editor. The Molecular and Cellular Biology of Wound Repair. Vol. 14. Plenum Press; New York: 1996. pp. 427–474. [Google Scholar]
- 10.Henney AM, Wakeley PR, Davies MJ, Foster K, Hembry R, Murphy G, Humphries S. Localization of stromelysin gene expression in atherosclerotic plaques by in situ hybridization. Proc Natl Acad Sci USA. 1991;88:8154–8. doi: 10.1073/pnas.88.18.8154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galis ZS, Sukhova GK, Lark MW, Libby P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest. 1994;94:2493–503. doi: 10.1172/JCI117619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ye S, Eriksson P, Hamsten A, Kurkinen M, Humphries SE, Henney AM. Progression of coronary atherosclerosis is associated with a common genetic variant of the human stromelysin-1 promoter which results in reduced gene expression. J Biol Chem. 1996;271:13055–60. doi: 10.1074/jbc.271.22.13055. [DOI] [PubMed] [Google Scholar]
- 13.Humphries S, Bauters C, Meirhaeghe A, Luong L, Bertrand M, Amouyel P. The 5A6A polymorphism in the promoter of the stromelysin-1 (MMP3) gene as a risk factor for restenosis. Eur Heart J. 2002 May;23(9):721–5. doi: 10.1053/euhj.2001.2895. [DOI] [PubMed] [Google Scholar]
- 14.Johnson JL, George SJ, Newby AC, Jackson CL. Divergent effects of matrix metalloproteinases 3, 7, 9, and 12 on atherosclerotic plaque stability in mouse brachiocephalic arteries. Proc Natl Acad Sci U S A. 2005 Oct 25;102(43):15575–80. doi: 10.1073/pnas.0506201102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Terashima M, Akita H, Kanazawa K, Inoue N, Yamada S, Ito K, et al. Stromelysin promoter 5A/6A polymorphism is associated with acute myocardial infarction. Circulation. 1999 Jun 1;99(21):2717–9. doi: 10.1161/01.cir.99.21.2717. [DOI] [PubMed] [Google Scholar]
- 16.Kallenbach K, Fernandez HA, Baumann FG, Seghezzi G, Patel S, Grossi EA, et al. A quantitative in vitro model of smooth muscle cell migration through the arterial wall using the human amniotic membrane. Arterioscler Thromb Vasc Biol. 2003 Jun 1;23(6):1008–13. doi: 10.1161/01.ATV.0000069880.81136.38. [DOI] [PubMed] [Google Scholar]
- 17.Fernandez HA, Kallenbach K, Seghezzi G, Mehrara B, Apazidis A, Baumann FG, et al. Modulation of matrix metalloproteinase activity in human saphenous vein grafts using adenovirus-mediated gene transfer. Surgery. 1998 Aug;124(2):129–36. [PubMed] [Google Scholar]
- 18.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003 May 2;92(8):827–39. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 19.Pintucci G, Yu PJ, Sharony R, Baumann FG, Saponara F, Frasca A, et al. Induction of stromelysin-1 (MMP-3) by fibroblast growth factor-2 (FGF-2) in FGF-2−/− microvascular endothelial cells requires prolonged activation of extracellular signal-regulated kinases-1 and -2 (ERK-1/2) J Cell Biochem. 2003;90(5):1015–25. doi: 10.1002/jcb.10721. [DOI] [PubMed] [Google Scholar]
- 20.March KL, Madison JE, Trapnell BC. Pharmacokinetics of adenoviral vector-mediated gene delivery to vascular smooth muscle cells: modulation by poloxamer 407 and implications for cardiovascular gene therapy. Hum Gene Ther. 1995 Jan;6(1):41–53. doi: 10.1089/hum.1995.6.1-41. [DOI] [PubMed] [Google Scholar]
- 21.James TW, Wagner R, White LA, Zwolak RM, Brinckerhoff CE. Induction of collagenase and stromelysin gene expression by mechanical injury in a vascular smooth muscle-derived cell line. J Cell Physiol. 1993 Nov;157(2):426–37. doi: 10.1002/jcp.1041570227. [DOI] [PubMed] [Google Scholar]
- 22.Lovdahl C, Thyberg J, Cercek B, Blomgren K, Dimayuga P, Kallin B, et al. Antisense oligonucleotides to stromelysin mRNA inhibit injury-induced proliferation of arterial smooth muscle cells. Histol Histopathol. 1999 Oct;14(4):1101–12. doi: 10.14670/HH-14.1101. [DOI] [PubMed] [Google Scholar]
- 23.Gnasso A, Motti C, Irace C, Carallo C, Liberatoscioli L, Bernardini S, et al. Genetic variation in human stromelysin gene promoter and common carotid geometry in healthy male subjects. Arterioscler Thromb Vasc Biol. 2000 Jun;20(6):1600–5. doi: 10.1161/01.atv.20.6.1600. [DOI] [PubMed] [Google Scholar]
- 24.Silence J, Lupu F, Collen D, Lijnen HR. Persistence of atherosclerotic plaque but reduced aneurysm formation in mice with stromelysin-1 (MMP-3) gene inactivation. Arterioscler Thromb Vasc Biol. 2001 Sep;21(9):1440–5. doi: 10.1161/hq0901.097004. [DOI] [PubMed] [Google Scholar]
- 25.Turner NA, Ho S, Warburton P, O’Regan DJ, Porter KE. Smooth muscle cells cultured from human saphenous vein exhibit increased proliferation, invasion, and mitogen-activated protein kinase activation in vitro compared with paired internal mammary artery cells. J Vasc Surg. 2007 May;45(5):1022–8. doi: 10.1016/j.jvs.2007.01.061. [DOI] [PubMed] [Google Scholar]
- 26.Mekontso-Dessap A, Kirsch M, Guignambert C, Zadigue P, Adnot S, Loisance D, et al. Vascular-wall remodeling of 3 human bypass vessels: organ culture and smooth muscle cell properties. J Thorac Cardiovasc Surg. 2006 Mar;131(3):651–8. doi: 10.1016/j.jtcvs.2005.08.048. [DOI] [PubMed] [Google Scholar]
- 27.Deng DX, Spin JM, Tsalenko A, Vailaya A, Ben-Dor A, Yakhini Z, et al. Molecular signatures determining coronary artery and saphenous vein smooth muscle cell phenotypes: distinct responses to stimuli. Arterioscler Thromb Vasc Biol. 2006 May;26(5):1058–65. doi: 10.1161/01.ATV.0000208185.16371.97. [DOI] [PubMed] [Google Scholar]
- 28.Davies MG, Hagen PO. Pathophysiology of vein graft failure: a review. Eur J Vasc Endovasc Surg. 1995;9(1):7–18. doi: 10.1016/s1078-5884(05)80218-7. [DOI] [PubMed] [Google Scholar]
- 29.Angelini GD, Bryan AJ, Williams HM, Soyombo AA, Williams A, Tovey J, et al. Time-course of medial and intimal thickening in pig venous arterial grafts: relationship to endothelial injury and cholesterol accumulation. J Thorac Cardiovasc Surg. 1992;103(6):1093–1103. [PubMed] [Google Scholar]
- 30.Santos MF, Viar MJ, McCormack SA, Johnson LR. Polyamines are important for attachment of IEC-6 cells to extracellular matrix. Am J Physiol. 1997;273:G175–83. doi: 10.1152/ajpgi.1997.273.1.G175. [DOI] [PubMed] [Google Scholar]
- 31.Everitt EA, Malik AB, Hendey B. Fibronectin enhances the migration rate of human neutrophils in vitro. J Leukoc Biol. 1996;60:199–206. doi: 10.1002/jlb.60.2.199. [DOI] [PubMed] [Google Scholar]
- 32.Grotendorst GR, Seppa HE, Kleinman HK, Martin GR. Attachment of smooth muscle cells to collagen and their migration toward platelet-derived growth factor. Proc Natl Acad Sci USA. 1981;78:3669–72. doi: 10.1073/pnas.78.6.3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Hinsbergh VW, Engelse MA, Quax PH. Pericellular proteases in angiogenesis and vasculogenesis. Arterioscler Thromb Vasc Biol. 2006 Apr;26(4):716–28. doi: 10.1161/01.ATV.0000209518.58252.17. [DOI] [PubMed] [Google Scholar]
- 34.Bajou K, Noël A, Gerard RD, Masson V, Brunner N, Holst-Hansen C, et al. Absence of host plasminogen activator inhibitor 1 prevents cancer invasion and vascularization. Nat Med. 1998 Aug;4(8):923–8. doi: 10.1038/nm0898-923. [DOI] [PubMed] [Google Scholar]
- 35.Bajou K, Masson V, Gerard RD, Schmitt PM, Albert V, Praus M, et al. The plasminogen activator inhibitor PAI-1 controls in vivo tumor vascularization by interaction with proteases, not vitronectin. Implications for antiangiogenic strategies. J Cell Biol. 2001 Feb 19;152(4):777–84. doi: 10.1083/jcb.152.4.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nishimura T, Nakamura K, Kishioka Y, Kato-Mori Y, Wakamatsu J, Hattori A. Inhibition of matrix metalloproteinases suppresses the migration of skeletal muscle cells. J Muscle Res Cell Motil. 2008;29(1):37–44. doi: 10.1007/s10974-008-9140-2. [DOI] [PubMed] [Google Scholar]
- 37.Haro H, Crawford HC, Fingleton B, MacDougall JR, Shinomiya K, Spengler DM, et al. Matrix metalloproteinase-3-dependent generation of a macrophage chemoattractant in a model of herniated disc resorption. J Clin Invest. 2000 Jan;105(2):133–41. doi: 10.1172/JCI7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haro H, Crawford HC, Fingleton B, Shinomiya K, Spengler DM, Matrisian LM. Matrix metalloproteinase-7-dependent release of tumor necrosis factor-alpha in a model of herniated disc resorption. J Clin Invest. 2000 Jan;105(2):143–50. doi: 10.1172/JCI7091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adair-Kirk TL, Atkinson JJ, Broekelmann TJ, Doi M, Tryggvason K, Miner JH, et al. A site on laminin alpha 5, AQARSAASKVKVSMKF, induces inflammatory cell production of matrix metalloproteinase-9 and chemotaxis. J Immunol. 2003 Jul 1;171(1):398–406. doi: 10.4049/jimmunol.171.1.398. [DOI] [PubMed] [Google Scholar]