Abstract
Background:
Functional electrical stimulation (FES) therapy has been shown to be one of the most promising approaches for improving voluntary grasping function in individuals with subacute cervical spinal cord injury (SCI).
Objective:
To determine the effectiveness of FES therapy, as compared to conventional occupational therapy (COT), in improving voluntary hand function in individuals with chronic (≥24 months post injury), incomplete (American Spinal Injury Association Impairment Scale [AIS] B-D), C4 to C7 SCI.
Methods:
Eight participants were randomized to the intervention group (FES therapy; n = 5) or the control group (COT; n = 3). Both groups received 39 hours of therapy over 13 to 16 weeks. The primary outcome measure was the Toronto Rehabilitation Institute-Hand Function Test (TRI-HFT), and the secondary outcome measures were Graded Redefined Assessment of Strength Sensibility and Prehension (GRASSP), Functional Independence Measure (FIM) self-care subscore, and Spinal Cord Independence Measure (SCIM) self-care subscore. Outcome assessments were performed at baseline, after 39 sessions of therapy, and at 6 months following the baseline assessment.
Results:
After 39 sessions of therapy, the intervention group improved by 5.8 points on the TRI-HFT’s Object Manipulation Task, whereas the control group changed by only 1.17 points. Similarly, after 39 sessions of therapy, the intervention group improved by 4.6 points on the FIM self-care subscore, whereas the control group did not change at all.
Conclusion:
The results of the pilot data justify a clinical trial to compare FES therapy and COT alone to improve voluntary hand function in individuals with chronic incomplete tetraplegia.
Key words: chronic patients, functional electrical stimulation, grasping, therapy, upper limb
In the United States and Canada, there is a steady rate of incidence and an increasing rate of prevalence of individuals living with spinal cord injury (SCI). For individuals with tetraplegia, hand function is essential for achieving a high level of independence in activities of daily living.1–5 For the majority of individuals with tetraplegia, the recovery of hand function has been rated as their highest priority.5
Traditionally, functional electrical stimulation (FES) has been used as a permanent neuroprosthesis to achieve this goal.6–14 More recently, researchers have worked toward development of surface FES technologies that are meant to be used as shortterm therapies rather than permanent prosthesis. This therapy is frequently called FES therapy or FET. Most of the studies published to date, where FES therapy was used to help improve upper limb function, have been done in both the subacute and chronic stroke populations15–23 and 2 have been done in the subacute SCI population.1–3 With respect to the chronic SCI population, there are no studies to date that have looked at use of FES therapy for retraining upper limb function. In a review by Kloosterman et al,24 the authors have discussed studies that have used various combinations of therapies for improving upper extremity function in chronic SCI individuals; however, the authors found that the only study that showed significant improvements before and after was the study published by Needham-Shropshire et al.25 This study examined the effectiveness of neuromuscular stimulation (NMS)–assisted arm ergometry for strengthening triceps brachii. In this study, electrical stimulation was used to facilitate arm ergometry, and it was not used in the context of retraining reaching, grasping, and/or object manipulation.
Since 2002, our team has been investigating whether FES therapy has the capacity to improve voluntary hand function in complete and incomplete subacute cervical SCI patients who are less than 180 days post injury at the time of recruitment in the study.1–3 In randomized controlled trials (RCTs) conducted by our team, we found that FES therapy is able to restore voluntary reaching and grasping functions in individuals with subacute C4 to C7 incomplete SCI.1–3 The changes observed were transformational; individuals who were unable to grasp at all were able to do so after only 40 one-hour sessions of the FES therapy, whereas the control group showed significantly less improvement. Inspired by these results, we decided to conduct a pilot RCT with chronic (≥24 months following injury) C4 to C7 SCI patients (American Spinal Injury Association Impairment Scale [AIS] B-D), which is presented in this article. The purpose of this pilot study was to determine whether the FES therapy is able to restore voluntary hand function in chronic tetraplegic individuals. Based on the results of our prior phase I1 and phase II2,3 RCTs in the subacute SCI population, we hypothesized that individuals with chronic tetraplegia who underwent the FES therapy (intervention group) may have greater improvements in voluntary hand function, especially in their ability to grasp and manipulate objects, and perform activities of daily living when compared to individuals who receive similar volume and duration of conventional occupational therapy (COT: control group).
Methods
The study was designed to be a single center, open-label RCT. Participants recruited to the study had their injury at least 24 months prior to enrollment in the study (chronic SCI). However, upon completion of the study, we had 3 complete data sets for the control group and 5 complete data sets for the intervention group. Due to this small sample size, we present the results of the current study as a pilot clinical trial looking at benefits of FES versus COT. Toronto Rehabilitation Institute’s Ethics Board approval was obtained for the study (TRI-REB-09-008). Participants were recruited through advertisements posted within Toronto Rehab Hospital–Lyndhurst Centre and through physician referrals. The study was also registered on www.clinicaltrials.gov (NCT01208688).
Individuals who consented to participant in the study were screened for eligibility, after which baseline assessments were conducted for eligible individuals. Participants were then randomized to either the control or the intervention group. Participants randomized to the control group received 1 hour of COT and those randomized to the intervention group received 1 hour of FES therapy. All participants in both groups received therapy for both hands within the allocated 1 hour of therapy. Individuals in the control and intervention groups received 39 therapy sessions. The original plan was to deliver these 39 sessions as 3 sessions per week, each session lasting 1 hour over a 13-week time period. However, due to illness or other reasons not under the participants’ control or our control, some participants required up to 16 weeks to complete all 39 sessions. Therefore, all participants received 39 sessions delivered during a 13- to 16-week time period.
Participants
Individuals were included who (a) had sustained a traumatic, motor or sensory incomplete SCI (AIS B-D) between C4 and C7, at least 24 months prior to enrollment in the study; (b) were 18 years old or older; and (c) were unable to grasp and manipulate various objects either unilaterally or bilaterally to allow independent performance of activities of daily living (ie, eating, dressing, grooming, etc).
Individuals were excluded who (a) had contraindications for FES, such as a cardiac pacemaker, skin lesions, or a rash at a potential electrode site; (b) had cardiovascular conditions such as uncontrolled hypertension or autonomic dysreflexia requiring medication; or (c) had denervated muscles (ie, individuals, who in addition to SCI, also sustained partial or complete damage of the peripheral nerves that were innervating muscles of interest).
Interventions
COT included (a) muscle facilitation exercises emphasizing the neuro-developmental treatment approach; (b) task-specific, repetitive functional training; (c) strengthening and motor control training using resistance to available arm motion to increase strength; (d) stretching exercises; (e) electrical stimulation applied primarily for muscle strengthening (this was neither FES nor FES therapy, but electro-muscular stimulation); (f) practice of activities of daily living (ADLs), including self-care where the upper extremities were used as appropriate; and (g) caregiver training.
FES therapy used in this study was delivered with the Compex Motion stimulator.8 This stimulator uses surface self-adhesive stimulation electrodes. Prior to initiation of training, based on the individual participant’s abilities and needs, FES stimulation protocols were designed for power grasp and precision grip. Muscles that were stimulated during therapy were wrist flexors – flexor carpi radialis and flexor carpi ulnaris; wrist extensors – extensor carpi radialis longus and brevis and extensor carpi ulnaris; finger flexors – flexor digitorum superficialis and flexor digitorum profundus; finger extensors – extensor digitorum; thumb abductors – median nerve or abductor pollicis brevis and abductor pollicis longus; thumb flexors – flexor pollicis brevis and flexor pollicis longus; and thumb oppositors – opponens pollicis. Each subject had customized stimulation protocols that were applied to these muscles on a per need basis. During therapy, the command for activating the stimulation sequence was issued with a push button by the treating therapist.
FES in this study was used solely for therapeutic purposes. The idea was to train individuals using FES over a period of 13 to 16 weeks; the expectation was that the participants would maintain the gains or improve further once the therapy was stopped. A detailed account of the methods used for FES application can be found in Popovic et al.2 In this study, FES was used for re-training the neuromuscular system. The stimulation parameters used were balanced, biphasic, current-regulated electrical pulses; pulse amplitude from 8 to 50 mA (typical values 15-30 mA); pulse width of 250 μs; and pulse frequency of 40 Hz. During intervention, the occupational therapist adjusted the placement of electrodes and guided the hand movements. The participants were encouraged to attempt the movement voluntarily through the time that they were being stimulated. The occupational therapist ensured that all movements were functional and efficient and used normal movement patterns. An independent hand strengthening and stretching program was provided as needed to facilitate normal hand function.
Primary outcome measure
Toronto Rehabilitation Institute–Hand Function Test (TRI-HFT) is an evaluation tool used to assess improvement in hand function. The reliability and validity of the TRI-HFT has been established in the SCI population.26 The objective of the test is to capture improvement in unilateral gross motor hand function. Hand functions tested are reach, grasp, and manipulation of various objects. There are 2 components of the test: evaluation of object manipulation for lateral or pulp pinch grasp and palmar grasp graded on a 0 to 7 scoring system,26 and evaluation of strength of lateral or pulp pinch grasp and palmar grasp.
Secondary outcome measures
Graded Redefined Assessment of Strength, Sensibility and Prehension (GRASSP) test combines the features of several other tests that have been used to assess hand and upper limb function in people with SCI.27,28 The various components of the GRASSP are strength testing, sensory testing, and qualitative and quantitative prehension testing.27,28
In addition to the GRASSP, Functional Independence Measure (FIM)29 and Spinal Cord Independence Measure (SCIM)30 self-care subscores were used as secondary outcome measures.
Statistical analysis
The participants’ impairment and demographic characteristics are shown in Table 1. Owing to the small sample size, especially of the control group, statistical analysis was not performed. We present raw data for each of the participants before therapy, after completion of 39 sessions of therapy, and at 6 months follow-up relative to baseline on the primary outcome measure and on all of the secondary outcome measures (Tables 2–4).
Table 1. Individual participant neurological data.
| Subject | Age, years | Cause of injury | Intervention start date years after SCI | AIS level |
|---|---|---|---|---|
| Control group: Received one dose of COT | ||||
| AAJU | 61-90 | MVA | 6 ≤ start date < 8 | B |
| AAKL | 61-90 | Sports | 6 ≤ start date < 8 | B |
| AAKC | 15-30 | Sports | 2 ≤ start date < 4 | B |
| Intervention group: Received one dose of FES | ||||
| AAKN | 31-60 | Fall | 4 ≤ start date < 6 | N/A |
| AAKR | 31-60 | MVA | 8 ≤ start date | N/A |
| AALF | 61-90 | Gun shot | 8 ≤ start date | N/A |
| AAMH | 15-30 | MVA | 2 ≤ start date < 4 | B |
| AAMI | 15-30 | Diving accident | 2 ≤ start date < 4 | B |
Note: All participants in the study were male, and they had a neurological level at baseline in the range from C4 to C6. AIS = American Spinal Injury Association (ASIA) Impairment Scale; COT = conventional occupational therapy; FES = functional electrical stimulation; MVA = motor vehicle accident; N/A = not available.
Table 2. Summary of the mean test results for the control and intervention groups at baseline, after 39 sessions of therapy, and at the 6-month follow-up.
| Control group (Mean scores) | Intervention group (Mean scores) | |||||
|---|---|---|---|---|---|---|
| Test | Before | After | 6-month follow-up | Before | After | 6-month follow-up |
| FIM self-care subscores | 16.6 | 16.6 | 18 | 19 | 23.6 | 27.25 |
| SCIM self-care subscore | 5.6 | 6.3 | 6.3 | 7 | 9.2 | 10.5 |
| TRI-HFT components | ||||||
| Object Manipulation Test | 31.99 | 33.16 | 32.33 | 40.2 | 46 | 47.25 |
| Wooden Blocks Test | 30 | 31.16 | 26 | 40.8 | 41.1 | 42.87 |
| Instrumented Cylinder Test, torque values (Nm) | 3.08 | 3.08 | 6.16 | 1.7 | 5.02 | 7.56 |
| Credit Card Test, force values (N) | 13.3 | 16.5 | 15 | 8.6 | 15.35 | 14.25 |
| Eccentric Loading Test – thumb direction, length values (cm) | 10 | 10 | 10 | 0.9 | 3.6 | 2.88 |
| Eccentric Loading Test – little finger direction, length values (cm) | 10 | 10 | 10 | 6 | 18 | 11.25 |
| GRASSP components | ||||||
| Strength | 23.5 | 25 | 23.6 | 17.8 | 21.2 | 21.62 |
| Sensibility | 14.16 | 12.6 | 13 | 12.8 | 13.5 | 13.87 |
| Qualitative grasp | 2.83 | 4.66 | 3.33 | 3.2 | 4.3 | 4.12 |
| Quantitative grasp | 8 | 6.33 | 9.6 | 9.9 | 11.1 | 12.87 |
Note: FIM = Functional Independence Measure; GRASSP = Graded Redefined Assessment of Strength, Sensibility, and Prehension; SCIM = Spinal Cord Independence Measure; TRI-HFT = Toronto Rehabilitation Institute–Hand Function Test.
Table 3. Individual participant right and left hand scores pre and post therapy and at 6-month follow-up on some of the components of the Toronto Rehabilitation Institute–Hand Function Test.
| Object Manipulation test | Instrumented Cylinder test | Credit Card test | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Subject | Pre | Post | 6-month follow-up | Pre | Post | 6-month follow-up | Pre | Post | 6 month follow-up |
| Control group (right hand scores) | |||||||||
| AAJU | 28 | 25 | 27 | 0 | 0 | 0 | 0 | 0 | 0 |
| AAKL | 60 | 60 | 60 | 3.5 | 3.5 | 20 | 40 | 45 | 42.5 |
| AAKC | 7 | 15 | 10 | 0 | 0 | 0 | 0 | 0 | 0 |
| Intervention group (right hand scores) | |||||||||
| AAKN | 22 | 40 | 31 | 0.3 | 7.5 | 5 | 7 | 28.5 | 7 |
| AAKR | 28 | 27 | 34 | 0 | 0 | 0 | 0 | 0 | 0 |
| AALF | 60 | 60 | NA | 5 | 5 | NA | 5 | 23 | NA |
| AAMH | 49 | 56 | 58 | 0 | 0.5 | 0 | 0 | 1.5 | 0.5 |
| AAMI | 38 | 60 | 46 | 2.5 | 8.5 | 17 | 16 | 31.5 | 25 |
| Control group (left hand scores) | |||||||||
| AAJU | 16 | 16 | 13 | 0 | 0 | 0 | 0 | 0 | 0 |
| AAKL | 60 | 60 | 60 | 15 | 15 | 17 | 40 | 50 | 42.5 |
| AAKC | 21 | 23 | 24 | 0 | 0 | 0 | 0 | 4 | 5 |
| Intervention group (left hand scores) | |||||||||
| AAKN | 30 | 50 | 45 | 0.7 | 0.7 | 0 | 20 | 27 | 39 |
| AAKR | 37 | 41 | 44 | 0 | 0 | 0 | 0 | 0 | 0 |
| AALF | 24 | 22 | NA | 0 | 0 | NA | 0 | 0 | NA |
| AAMH | 54 | 58 | 60 | 0 | 1 | 0 | 0.5 | 0 | 0 |
| AAMI | 60 | 46 | 60 | 8.5 | 27 | 38.5 | 37.5 | 42 | 42.5 |
Note: NA = not available.
Table 4. Individual participant scores pre and post therapy and at 6-month follow-up on the FIM and SCIM self-care subscores.
| FIM self-care subscore |
SCIM self-care subscore |
|||||
|---|---|---|---|---|---|---|
| Subject | Pre | Post | 6-month follow-up | Pre | Post | 6-month follow-up |
| Control group: Received one dose of COT | ||||||
| AAJU | 20 | 19 | 22 | 6 | 8 | 8 |
| AAKL | 24 | 24 | 23 | 11 | 10 | 10 |
| AAKC | 6 | 7 | 9 | 0 | 1 | 1 |
| Intervention group: Received one dose of FES | ||||||
| AAKN | 11 | 13 | 14 | 2 | 3 | 3 |
| AAKR | 16 | 15 | 16 | 4 | 4 | 4 |
| AALF | 11 | 15 | NA | 3 | 4 | NA |
| AAMH | 24 | 36 | 40 | 11 | 18 | 18 |
| AAMI | 33 | 39 | 39 | 15 | 17 | 17 |
Note: COT = conventional occupational therapy; FES = functional electrical stimulation; FIM = functional independence measure; NA = not available; SCIM = Spinal Cord Independence Measure.
Results
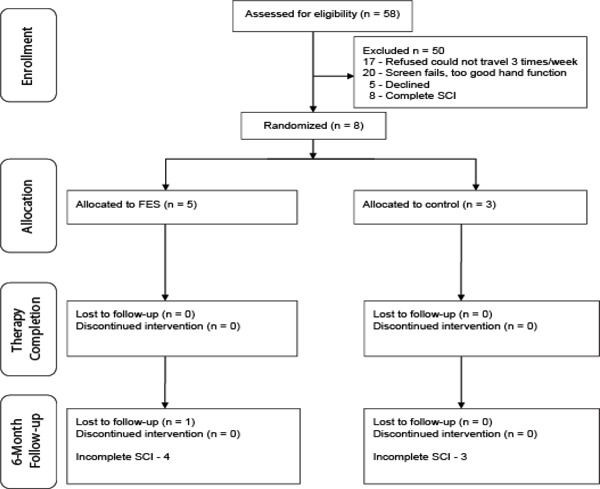
We were able to recruit 8 male, incomplete SCI individuals. All 8 individuals completed baseline and discharge assessments. However, only 7 individuals completed the 6-month follow-up assessment. Of the 8 individuals who completed the discharge assessment, 5 were allocated to the intervention group and 3 were allocated to the control group. One individual in the control group was 15 years old at the time of recruitment and was brought into the study at the investigator’s discretion. At 6-month follow-up, we lost 1 individual from the intervention group, which left 4 individuals in the intervention group and 3 in the control group. Please refer to the CONSORT diagram for details (Figure 1).
Figure 1. CONSORT diagram. FES = functional electrical stimulation.
Primary outcome measure results
TRI-HFT was the primary outcome measure. On the Object Manipulation Test, only 1 participant in the control group showed improvements in both the right and left hands, whereas 3 participants in the intervention group showed improvement in both the right and left hands. On the Wooden Blocks Test, 1 participant in each group showed improvement on both the right and left hands. On the Instrumented Cylinder Test, none of the participants in the control group demonstrated any change, whereas 3 participants in the intervention group showed improvement in right hand scores and 2 showed improvement in left hand scores. On the Instrumented Credit Card Test for the right hand, 1 participant in the control group showed improvement whereas 4 participants in the intervention group showed improvement; for the left hand, 2 participants in both groups improved.
On the Instrumented Credit Card Test, in the control group 1 participant showed improvement in the right hand and 2 in the left hand, whereas in the intervention group 4 participants showed improvement in the right hand and 2 in the left hand. On the Eccentric Loading Test, no change was seen in the right and left hand scores for all 3 control group participants, whereas 2 of the 5 participants of the intervention group improved on the left side and 3 of the 5 improved on the right side. Table 2 shows the mean scores of all participants on various components of the TRI-HFT test, and Table 3 shows individual participant scores pre and post therapy and at 6-month follow-up.
Secondary outcome measures results
Similar results for the 2 groups were observed on the secondary outcome measures (ie, FIM self-care subscore, SCIM self-care subscore, and GRASSP). The control group showed no improvement on FIM self-care subscore and minimal and clinically irrelevant improvement on SCIM self-care subscore. With respect to the GRASSP test, the control group had minimal improvements on the strength and qualitative subcomponents of the test and a slight decrease on the sensibility subcomponent of the test. In case of the intervention group, we saw considerable improvements on both the FIM and SCIM self-care subscores (Table 4) as well as FIM and SCIM total scores pre and post therapy. Similarly, we saw improvements on all components of the GRASSP test, where most notable improvements have been recorded on the strength and quantitative subcomponents of the test. Table 2 shows the mean scores pre therapy, post therapy, and at 6-month follow-up for the control and intervention groups.
Discussion
The results of the present pilot data collection suggest that a treatment consisting of repetitive FES therapy designed to improve hand function may promote recovery of voluntary grasping function in persons with chronic incomplete SCI. Moreover these pilot data suggest that these improvements may precipitate subsequent enhancement in the quality and complexity of tasks that individuals were able to execute with their hands. However, because of the limited number of participants, we are unable to test for statistical significance, and therefore these data may only serve to demonstrate the potential value of a clinical trial.
The mechanism of recovery in the individuals with incomplete chronic SCI is identical to the one observed in individuals with incomplete subacute SCI in our previous studies. It could be attributed to neurological recovery such as (a) an increase in muscle controllability, (b) better synchronization of different muscle groups, and (c) reactivation of previously inactive muscle groups. We have evidence from previous research that the repetitive application of FES therapy promotes neural plasticity in the spinal cord and allows neural signals from the periphery to bridge the site of injury and reach the cortex.31
The results of our research to date indicate that the following FES therapy practices are useful to maximize recovery: (a) applying individualized FES protocols that are monitored and adjusted by an occupational therapist on regular basis; (b) combining FES therapy with regular occupational therapy; and (c) incorporating FES therapy with functional tasks typically performed in activities of daily living. Our long-term follow-ups with chronic incomplete SCI individuals, presented in this article, and with subacute incomplete SCI individuals presented in our previous publication3 suggest that the results of the FES therapy are long lasting and persist undiminished for months following discharge.
Among SCI clinicians, it is commonly believed that motor improvement plateaus at 12 to 18 months after SCI32 and that one should not expect significant improvements thereafter. Our present pilot study begins to show some preliminary evidence against this belief, however it does not have sufficient cases to demonstrate statistical significance and, therefore, scientific validity. This article begins to establish a foundation for future larger scale rehabilitation trials in the chronic SCI population. We believe that the presented pilot data warrant that further trials should be carried out with this patient population using our FES therapy and a larger pool of participants. The larger sample size will provide a scientifically valid test of the ability of the FES therapy to improve voluntary hand function in chronic incomplete SCI individuals.
The pilot data trends are consistent with our previous findings with subacute SCI individuals2 and with the findings obtained in both subacute and chronic severe stroke individuals.15,16
Limitations
At the start of the study, our hope was to recruit a sufficient number of participants so that the data could provide solid evidence regarding benefits of one type of therapy over the other. However, because of challenges with recruitment, the study ended as a pilot clinical trial with a small sample size prohibiting any statistical analysis. Therefore, the data represent only pilot data of insufficient quantity to test scientific hypotheses.
Conclusion
The pilot data presented represent a foundation for a clinical trial of repetitive FES therapy in combination with functional and task-specific training to determine whether it promotes recovery of voluntary grasping function in persons with chronic incomplete SCI and improves independence in performance of ADLs.
Acknowledgments
The work presented in this article was supported by a grant from the Rick Hansen Institute (SCISN grant 2009-36).
All authors, except for Dr. Popovic, declare no potential conflicts of interest with respect to the authorship and/or publication of this article. Dr. Popovic is a shareholder in the company, MyndTec Inc., that intends to develop and manufacture FESbased devices in the near future.
Additional contributions: The authors would also like to thank Shaghayegh Bagher, Jennifer Holmes, and Sylvia Haycock for providing therapy for the study participants.
References
- 1.Popovic MR, Thrasher TA, Adams ME, Takes V, Zivanovic V, Tonack MI. Functional electrical therapy: Retraining grasping in spinal cord injury. Spinal Cord. 2006;44(3):143–151 [DOI] [PubMed] [Google Scholar]
- 2.Popovic MR, Kapadia N, Zivanovic V, Furlan JC, Craven BC, McGillivray C. Functional electrical stimulation therapy of voluntary grasping versus only conventional rehabilitation for patients with subacute incomplete tetraplegia: A randomized clinical trial. Neurorehabil Neural Repair. 2011;25(5):433–443 [DOI] [PubMed] [Google Scholar]
- 3.Kapadia N, Zivanovic V, Furlan J, Craven BC, McGillivray C, Popovic MR. Functional electrical stimulation therapy for grasping in traumatic incomplete spinal cord injury: Randomized control trial. Artificial Organs. 2011;35(3):212–216 [DOI] [PubMed] [Google Scholar]
- 4.Popovic MR, Popovic DB, Keller T. Neuroprostheses for grasping. Neurol Res. 2002;24:443–452 [DOI] [PubMed] [Google Scholar]
- 5.Anderson KD. Targeting recovery: Priorities of the spinal cord-injured population. J Neurotrauma. 2004;21(10):1371–1383 [DOI] [PubMed] [Google Scholar]
- 6.Peckham PH, Knutson JS. Functional electrical stimulation for neuromuscular application. Ann Rev Biomed Eng. 2005;7:327–360 [DOI] [PubMed] [Google Scholar]
- 7.Smith B, Peckham PH, Michael MW, Roscoe DD. An externally powered, multichannel, implantable stimulator for versatile control of paralyzed muscle. IEEE Trans Biomech Eng. 1987;44:781–790 [DOI] [PubMed] [Google Scholar]
- 8.Popovic MR, Keller T. Modular transcutaneous functional electrical stimulation system. Med Eng Phys. 2005;27(1):81–92 [DOI] [PubMed] [Google Scholar]
- 9.Popovic MR, Curt A, Keller T, Dietz V. Functional electrical stimulation for grasping and walking: Indications and limitations. Spinal Cord. 2001;39(8):403–412 [DOI] [PubMed] [Google Scholar]
- 10.Popovic D, Stojanovic A, Pjanovic A, et al. Clinical evaluation of the bionic glove. Arch Phys Med Rehabil. 1999;80(3):299–304 [DOI] [PubMed] [Google Scholar]
- 11.Gan LS, Prochazka A, Bornes TD, Denington AA, Chan KM. A new means of transcutaneous coupling for neural prostheses. IEEE Trans Biomed Eng. 2007;54(3):509–517 [DOI] [PubMed] [Google Scholar]
- 12.Snoek GJ, IJzerman MJ, in ‘t Groen FA, Stoffers TS, Zilvold G. Use of the NESS Handmaster to restore handfunction in tetraplegia: Clinical experiences in ten patients. Spinal Cord. 2000;38:244–249 [DOI] [PubMed] [Google Scholar]
- 13.Prochazka A, Gauthier M, Wieler M, Kenwell Z. The bionic glove: An electrical stimulator garment that provides controlled grasp and hand opening in quadriplegia. Arch Phys Med Rehabil. 1997;78(6):608–614 [DOI] [PubMed] [Google Scholar]
- 14.Mangold S, Keller T, Curt A, Dietz V. Transcutaneous functional electrical stimulation for grasping in subjects with cervical spinal cord injury. Spinal Cord. 2004;43(1):1–13 [DOI] [PubMed] [Google Scholar]
- 15.Popovic MR, Thrasher TA, Zivanovic V, Takaki J, Hajek V. Neuroprosthesis for restoring reaching and grasping functions in severe hemiplegic patients. Neuromodulation. 2005;8(1):60–74 [DOI] [PubMed] [Google Scholar]
- 16.Thrasher TA, Zivanovic V, McIlroy W, Popovic MR. Rehabilitation of reaching and grasping function in severe hemiplegic patients using functional electrical stimulation therapy. Neurorehabil Neural Repair. 2008;22(6):706–714 [DOI] [PubMed] [Google Scholar]
- 17.Knutson JS, Hisel TZ, Harley MY, Chae J. A novel functional electrical stimulation treatment for recovery of hand function in hemiplegia: 12-week pilot study. Neurorehabil Neural Repair. 2009;23(1):17–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Popovic DB, Sinkaer T, Popovic MB. Electrical stimulation as a means for achieving recovery of function in stroke patients. NeuroRehabilitation. 2009;25(1):45–58 [DOI] [PubMed] [Google Scholar]
- 19.Hendricks HT, IJzerman MJ, de Kroon JR, in ‘t Groen FA, Zilvold G. Functional electrical stimulation by means of the “Ness Handmaster Orthosis” in chronic stroke patients: An exploratory study. Clin Rehabil. 2001;15(2):217–220 [DOI] [PubMed] [Google Scholar]
- 20.Chae J, Harley MY, Hisel TZ, et al. Intramuscular electrical stimulation for upper limb recovery in chronic hemiparesis: An exploratory randomized clinical trial. Neurorehabil Neural Repair. 2009;23(6):569–578 [DOI] [PubMed] [Google Scholar]
- 21.Mangold S, Schuster C, Keller T, Zimmermann-Schlatter A, Ettlin T. Motor training of upper extremity with functional electrical stimulation in early stroke rehabilitation. Neurorehabil Neural Repair. 2009;23(2):184–190 [DOI] [PubMed] [Google Scholar]
- 22.Popovic MB, Popovic DB, Sinkjaer T, Stefanovic A, Schwirtlich L. Restitution of reaching and grasping promoted by functional electrical therapy. Artific Organs. 2002;26(3):271–275 [DOI] [PubMed] [Google Scholar]
- 23.Popovic MB, Popovic DB, Sinkjaer T, Stefanovic A, Schwirtlich L. Therapy of paretic arm in hemiplegic subjects augmented with a neural prosthesis: A cross-over study. Can J Physiol Pharmacol. 2004;82(8):749–756 [DOI] [PubMed] [Google Scholar]
- 24.Kloosterman MG, Snoek GJ, Jannink MJ. Systemic review of the effects of exercise therapy on the upper extremity of patients with spinal cord injury. Spinal Cord. 2009;47(3):196–203 [DOI] [PubMed] [Google Scholar]
- 25.Needham-Shropshire BM, Broton JG, Cameron TL, Klose KJ. Improved motor function in tetraplegics following neuromuscular stimulation-assisted arm ergometry. J Spinal Cord Med. 1997;20(1):49–55 [DOI] [PubMed] [Google Scholar]
- 26.Kapadia N, Zivanovic V, Verrier M, Popovic MR. Toronto Rehabilitation Institute-Hand Function Test: Assessment of gross motor function in individuals with spinal cord injury. Top Spinal Cord Inj Rehabil. 2012;18(2):167–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalsi-Ryan S, Beaton D, McIlroy W, Fehlings M, Verrier M. Quantification of multimodality sensation of the hand in cervical spinal cord injury. J Spinal Cord Med. 2006;29(3):311 [Google Scholar]
- 28.Kalsi-Ryan S, Duff S, Rudhe C, Curt A, Fehlings M, Verrier M. Reliability and validity of the graded and redefined assessment of sensibility strength and prehension (GRASSP): A measure for hand impairment/function in tetraplegia. J Spinal Cord Med. 2008;31(2):226 [Google Scholar]
- 29.Dodds TA, Martin DP, Stolov WC, Deyo RA. A validation of the functional independence measurement and its performance among rehabilitation inpatients. Arch Phys Med Rehabil. 1993;74:531–536 [DOI] [PubMed] [Google Scholar]
- 30.Catz A, Itzkovich M, Agranov E, Ring H, Tamir A. SCIM. – Spinal Cord Independence Measure: A new disability scale for patients with spinal cord lesions. Spinal Cord. 1997;35:850–856 [DOI] [PubMed] [Google Scholar]
- 31.Beaumont E, Codina EG, Dubeau S, Lesage F, Nagai M, Popovic MR. Restoring locomotion after spinal cord injury by optimizing the afferent neuronal circuitry with functional electrical stimulation. J Spinal Cord Med. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fawcett JW, Curt A, Steeves JD, et al. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: Spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord. 2007; 45(3):190–205 [DOI] [PubMed] [Google Scholar]


