Abstract
Hypoxia-inducible factor 1-alpha (HIF-1α) is a subunit of HIF-l and thought to be able to protect hypoxic cells from apoptosis or necrosis under ischemic and anoxic conditions. This study aimed to investigated whether recombinant adenovirus vector over-expressing HIF-lα could affect apoptosis-related proteins (Bcl-2 and Bax) and vascular endothelial growth factor (VEGF) in a rat spinal cord injury (SCI) model. A total of 60 male SD rats were divided into 4 groups: Sham, Control, Ad-Blank and Ad-HIF-1α groups. 1, 3, 7, 14, 28 days after surgery, the behavioral recovery was evaluated with BBB scales. Then, rats were sacrificed and the spinal cord was collected for detection of Bcl-2, Bax and VEGF expressions by immunohistochemistry. Results showed the Bcl-2, Bax, VEGF and HIF-lα expressions increased in animals with SCI, but the increase in Bcl-2, VEGF and HIF-lα expressions were higher in Ad-HIF-1α group when compared with other groups, but Bax expression decreased significantly. In addition, administration of Ad-HIF-1α significantly reduced apoptotic cells and promoted the recovery of neurological function. In conclusion, administration of Ad-HIF-1α after SCI could ameliorate neuronal apoptosis and promote angiogenesis in rats. Our study provides a basis for further exploration of the relationship between HIF1α and SCI.
Keywords: HIF-1α, spinal cord injury, apoptosis, vascular endothelial growth factor
Introduction
Spinal cord injury (SCI) is a complex assault with pathological procedures of original injury, secondary lesion and spinal restoration [1]. Consequently, how to relieve the secondary insult and how to accelerate spinal functional restoration are always the focuses and difficulties in neurosurgical researches. Numerous studies have shown that the pathogenesis of SCI involves the disordered blood circulation [2], abnormal distribution of bioactive substances [3] and production of free radicals [4], etc. However, anoxemia is a key problem accounting for the tissue destruction following SCI. Thus, this study focused on how to improve the oxygen supply to the injured spinal injury, promote angiogenesis and improve microenvironment for the restoration of spinal function in a rat SCI model.
Hypoxia-inducible factor 1 (HIF-1) is a basic-helix-loop-helix transcription factor playing essential roles in the mammalian development and physiology. HIF-1 alpha (HIF-1α) expression and HIF-1 transcriptional activity increase exponentially as cellular oxygen concentration is decreased [5]. HIF-1 is a heterodimer composed of HIF-1α and HIF-1β subunits. The expression and activity of HIF-1α subunit are tightly regulated by the cellular oxygen concentration. Under hypoxic conditions, HIF-1α activates the transcription of genes encoding erythropoietin, glucose transporters, glycolytic enzymes, vascular endothelial growth factor and others, and these proteins may increase oxygen delivery or facilitate metabolic adaptation to hypoxia [6]. A study shows HIF-1α is closely correlated with bcl2 and Bax, two proteins regulating cell apoptosis [7]. Meanwhile, adeno-associated virus can infect cells in division or non-division stages, and integrate the target gene into the genome of host cells leading to the persistent expression of target genes, which is hardly neurotoxic [8]. Due to the disability of spinal tissues to endure hypoxia and reconstruct microcirculation, it is undoubtedly attractive to investigate the relationship between HIF1α and development of SCI.
In order to ascertain whether HIF1α can attenuate secondary SCI and promote its recovery, the expressions of HIF-1α, vascular endothelial growth factor (VEGF), Bax, and Bcl-2 and the changes in apoptosis and hind limb function were determined at different time points before and after recombinant adenovirus vector administration in rats with SCI. Our findings may provide a basis for further exploration of the relationship between HIF1α and development of SCI.
Materials and methods
Materials
A total of 60 adult Sprague-Dawley (SD) rats, weighting 250±20 g, were provided by Animal Center of Wenzhou Medical College. Streptavidin/Peroxidase (SP) kit was purchased from Z-med Company (USA), DAB kit from Sigma Company (USA), TritonX-100 from Huamei Biotec Company (Shanghai, China), and Bcl-2 monoclonal antibody, Bax monoclonal antibody, and VEGF monoclonal antibody from Santa Cruz Company (USA). Adenovirus vectors with (Ad-HIF-1α) or without (Ad-Blank) HIF-1α expression were kindly provided by Professor Yu Ru-Tong in Xuzhou Medical College.
Establishment of rat SCI model with modified Allen method
SD rats were randomly assigned into 4 groups. 1. sham group: rats received laminotomy alone; 2. SCI group: rats received laminotomy and microinjection of 2 μl of 1×PBS; 3. Ad-HIF-1α group: rats received laminotomy and microinjection of virus vectors expressing HIF-1α in 2 μl of 1×PBS into the spinal cord; 4. Ad-Blank group: rats received laminotomy and microinjection of blank virus vectors in 2 μl of 1×PBS into the spinal cord.
Based on the modified Allen method reported by Basso et al [9], a rat SCI model was established. Animals were given ad libitum access to food and water before surgery. Rats were anesthetized by intraperitoneal (i.p.) injection of pentobarbital sodium at 65 mg/kg. Briefly, after anesthesia, rats were fixed on a stereotaxic apparatus (Second Military Medical University, China). After preparing the skin, the an incision was made at the 13th rib which cut apart for about 3 cm, and muscles around the spinous process were separated. The T13-L1 vertebral plate was removed. The canalis spinalis was opened until the vertebral arch root was present. The spinal cord was exposed without impairing dura mater spinalis. The T12 and L2 centrums were fixed by a forceps to ensure the precise position for injury. A contusion was induced by a self-made electromagnetic programmed weight-drop device in the spinal cord corresponding to the T13 spinous process, centering at the posterior median spinal vessels. The striking force was 20×2.5 g•cm: the iron stick was 20 g in weight and 2.5 cm in bottom diameter, the dropping distance was 2.5 cm, and the time of contact with the dura mater spinalis was 0.1 s. During the surgery, the body temperature was maintained at 36.0-37.0°C by continuous rectal temperature monitoring and incandescent lamp exposure. After injury, the wound was closed.
Rats with following conditions were assigned into the recombinant adenovirus transfection groups (with or without HIF-1α). (1) spastic swing of tail, retraction and fluttering of hind limbs and body, and spastic paralysis of hind limbs; (2) stable body temperature at 36-37°C. After surgery, the animals were housed in individual cages at 25-30°C. All rats were injected with penicillin (25000 U) twice daily for 3 days. The bladder was pressed thrice daily until emptying reflex was established, or the bladder was punctured for emptying. The body weight was monitored everyday within the first week and then once weekly.
Microinjection of adenovirus vector particles into spinal cord
Based on the method reported by Miura et al [10-12], gene transferring was done by intraspinal injection. After anesthesia, animals were fixed on a stereotaxic apparatus, and a small longitudinal incision was made at 1 mm away from the median vessels posterior to the dura mater spinalis at the level of T13. The adenovirus vectors, with (Ad-HIF-1α) or without (1×PBS) HIF-1α expression, were injected into the spinal cord with a microinjector (4×1010 PFU/ml, injection time: 2 min, retaining time: 5 min). Animals in SCI group were injected with 2 μl of PBS. After the wound was closed, rats were housed individually.
Perfusion, fixation and sample collection
1, 3, 7, 14, and 28 days after surgery, rats were sacrificed (n=6) for immunohistochemistry. The remaining rats were used for detection of apoptosis (without perfusion). Briefly, after anesthesia with 0.7% pentobarbital, rats were fixed on an operation table, and perfused rapidly through the ascending aorta with 200 ml of 0.9% NaCl solution, and then with 500 ml of 4% paraformaldehyde in 0.1 M phosphate buffer (pH7.4) for 30 min. Subsequently, the injured spinal cord (contusion area-centered and about 1.5 cm in length) were collected and post-fixed in 4% paraformaldehyde for 12 h, and then dehydrated in 15% sucrose solution and 30% sucrose solution until the tissues sank down.
Sectioning
Spinal cords were sectioned consecutively at the coronal plane using a freezing microtome. The 20-μm sections were collected into plates with 0.01 M PBS. Five wells were numbered A, B, C, D and E, and every well contained 10 sections. The sections in A, B, C and D wells were used for immunohistochemistry for HIF-1α, VEGF, Bax and Bcl-2, respectively; and the sections in E well were used for H-E staining.
H-E staining
H-E staining was done as follows. The frozen sections were dried in air after coating, and then washed by distilled water for 1-2 min; immersed in hematoxylin solution for 1 min, and then washed. After treatment with 1% hydrochloric acid for several seconds, sections were washed and treated with saturated lithium carbonate solution for 1 min, followed by washing. Then, these sections were dehydrated in 80% alcohol for 1-2 min, stained in eosin for 1-2 min, and then washed. After dehydration in gradient alcohol and transparentizing in xylene, sections were mounted with neutral gum.
Immunohistochemistry
Sections were incubated in 3% H2O2 (diluted with 0.3% TritonX-100) for 30 min at room temperature after washing in 0.01 M PBS thrice (5 min for each), followed by washing in 0.01 M PBS thrice (5 min for each) at room temperature. After incubation with goat serum for 30 min, sections were incubated with primary antibody HIF-1α 1:80, Bcl-2 1:200, Bax 1:150, VEGF 1:200) for 48 h at 4°C. After washing in 0.01 M PBS, sections were incubated with secondary antibody (biotin-labeled goat anti-mouse IgG, 1:200) for 1 h at 4°C, washed thrice (5 min for each) with 0.01 M PBS and incubated with horseradish peroxidase conjugated streptavidin (1:200) for 1 h at room temperature, and then with diaminobenzidine (DAB)/H2O2 for 15 min at room temperature. After dehydration in gradient alcohol, and transparentizing in xylene, sections were mounted with neutral gum and observed under a microscope. In control sections, the primary antibody was replaced with 1% calf serum.
Evaluation of spinal function after spinal injury in rats
Behavioral scoring was done at different time points (n=6 at each time point) after surgery. The recovery of hind limb motor function was observed and scored using BBB (Basso, Beattie, and Bresnahan open field locomotion rating score) [9] scoring system. To ensure the accuracy of scoring, two hind limbs were evaluated by two investigators, respectively, and the mean score was obtained. The scoring was done as follows: 0, no movement; 1, slight movement of one or two joints (hip joint or/and knee joint); 2, extensive movement of a joint with or without slight movement of other joints; 3, extensive movement of two joints; 4, slight movement of 3 joints; 5, slight movement of 2 of 3 joints and extensive movement of another joint; 6, extensive movement of 2 joints from 3 joints and slight movement of another joint; 7, extensive movement of 3 joints; 8, movement without weight loading, or touching the ground with paws without weight loading. All behavioral observations were done at 20:00 to avoid the variation of locomotion of animals between day and night.
Image analysis
H-E stained sections were observed under a light microscope. Image analysis of sections after immunohistochemistry was done with a LEICA QWin system (Leika, Germany). At each time point, data from 4 groups (Sham, SCI, Ad-HIF-1α and Ad-Blank) were analyzed, and the optical density (OD) of positive cells was assayed (4 slides in each group). We selected 3 measuring points from each cell with identical image element and measured 5 positive cells from each section in the corresponding region. The opposite number of the mean of (measured value - corresponding negative control value) was taken as the final OD.
Statistical analysis
All data were presented as mean±S.E. and analyzed with SPSS version 13.0 for Windows. One-way analysis of variance (ANONA) was used for comparison among groups. A value of P<0.05 was considered statistically significant.
Results
Morphology of spinal cord after H-E staining
In sham group, the motor neurons and glial cells in the spinal cord were clear, had erythrotic plasm, and clear and blue nuclei. In some cells, the nucleoli were visible, and bird’s eye-like, especially in anterior horn motor neurons, with a diameter of 50-70 μm.
In SCI group, an injured region was observed at the dorsal spinal cord 1 day after SCI, with disorganization of grey matter. There were degenerative and dead neurons in the gray matter, as well as swollen axons and numerous vacuoles in the white matter. The injury of adjacent segments aggravated at 3 and 7 days after injury, with altered cell morphology, decreased neurites, proliferation of glial cells and infiltration of inflammatory cells in the white/gray matter. From day 14 to day 28, the lesion was clearer, with porosis and glia ulosis in the thinner injured segment.
In Ad-HIF-1α group, there were hemorrhagic foci and disorganization of grey matter in the spinal cord, with died neurons, decreased Nissl body and reduced cell volume. From day 3 to day 7 after injury, the injury reached a peak, but less severe than that in Ad-Blank group and SCI group, with increased amount of residual cells and proliferation of glial cells around the dead cells. From day 14 to day 28, remaining cells had almost normal morphology in the injured spinal cord, and increased Nissl body (less than that in sham group; see Figure 1).
Figure 1.
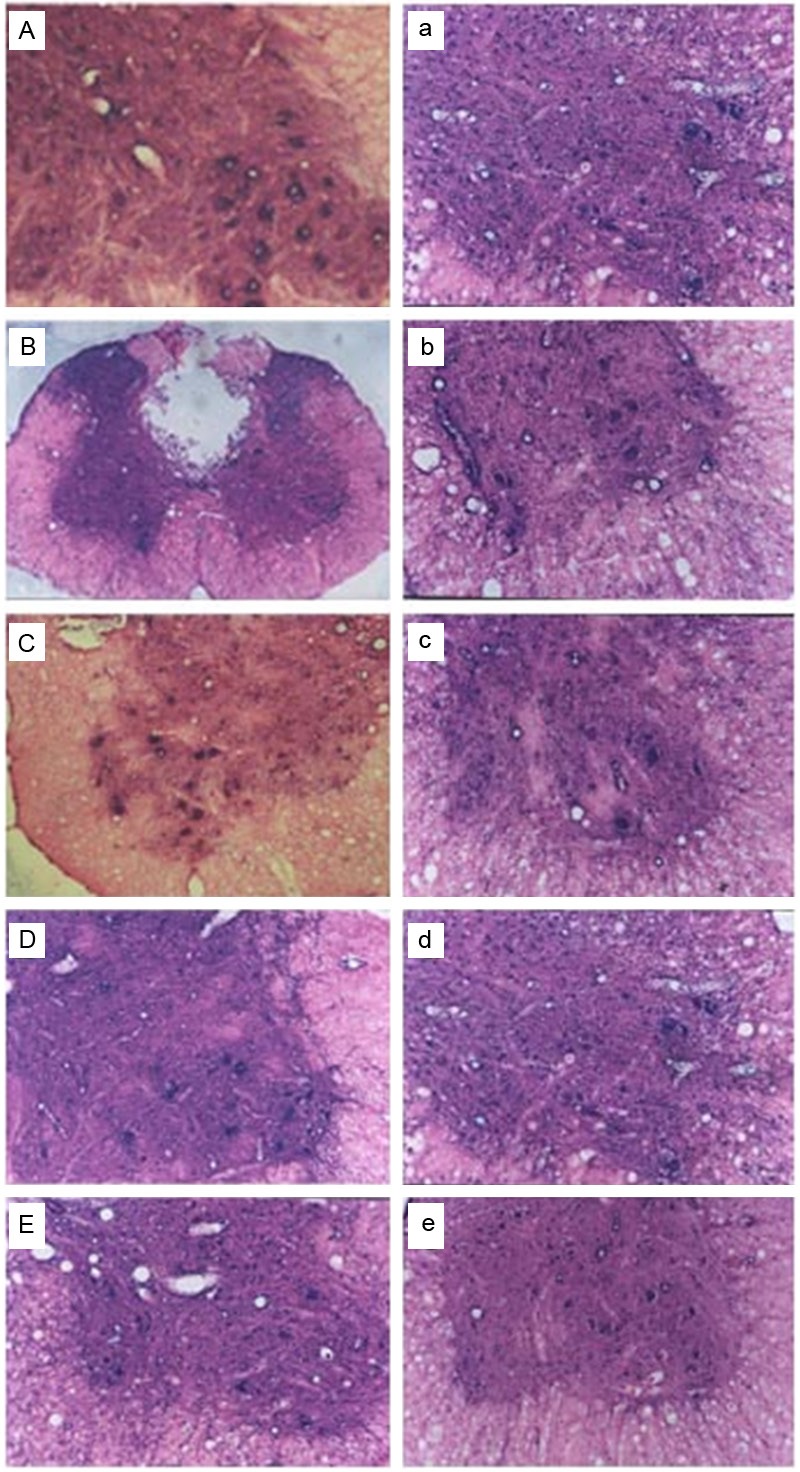
HE staining of spinal cord: A: Sham group; B, C: Spinal cord 3 days after SCI; D, E: Spinal cord 3 and 14 days after SCI, respectively and the treatment of Ad-Blank. a-e: Spinal cord 1, 3, 7, 14, and 28 days after SCI respectively and the treatment of Ad-HIF-1α. B: 40×; remaining figures: 100×.
Immunohistochemistry for HIF-1, VEGF, Bax and Bcl-2 in rat spinal cord
In SCI rats, cells positive for HIF-1α, VEGF, Bax or Bcl-2 were not observed when the primary antibody was replaced with PBS, which indicates the specificity of primary antibody. Cells positive specific antibody had brown granules in the cytoplasm (Figure 2).
Figure 2.
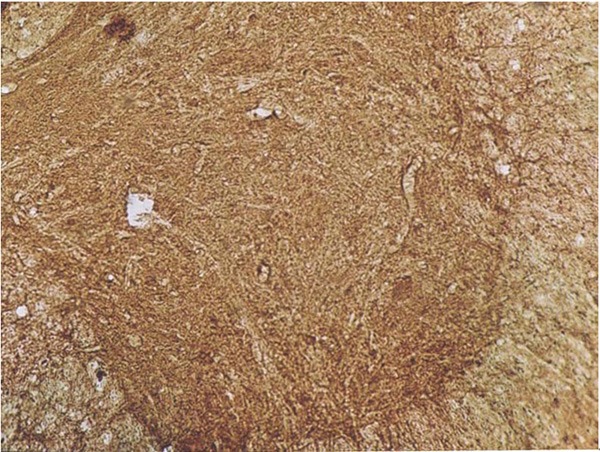
Control spinal cord in immunohistochemistry (100×).
Immunohistochemistry for HIF-1α
There were no HIF-1α positive neurons in the anterior horn and dorsal horn in sham group. 1 day after SCI, the expression of HIF-1α increased significantly in the spinal anterior horn and dorsal horn (especially the motor neurons in the anterior horn) of SCI group and Ad-Blank group. The HIF-1α expression increased significantly on day 3, with the highest OD of 84.1, which was significantly different from that on day 1 (P<0.05). Since 7 days after surgery, the HIF-1α expression decreased, and reached a minimal level on day 28, which was significantly different from that on day 1 (P<0.05). The HIF-1α protein expression in Ad-Blank group was similar to that in SCI group at the same time points (P>0.05).
At 1 day after SCI, a lot of HIF-1α positive cells were observed in the dorsal horn, anterior horn and dorsal funiculus of Ad-HIF-1α group. Microscopy showed brown granules in the cytoplasm of neurons and neurites. In the motor neurons of anterior horn, the HIF-1α positive granules were mainly distributed in cell bodies and neurites. At 3 days after injury, the HIF-1α expression increased significantly, reaching a maximal level on day 7 (DO: 112.5±7.6). On day 14, the HIF-1α expression began to decrease gradually, and reached a minimal level on day 28. The OD on day 1 was significantly different from that at other time points (P<0.05). The HIF-1α expression in Ad-HIF-1α group was significantly higher than that in Ad-Blank group and SCI group (P<0.05), with exception on day 28. Our results showed, in Ad-HIF-1α group, the increased HIF-1α expression last for a longer period and reached a maximal level on day 7. However, the peak of HIF-1α expression in Ad-Blank group and SCI group was observed at 3 days after injury (Figure 3).
Figure 3.
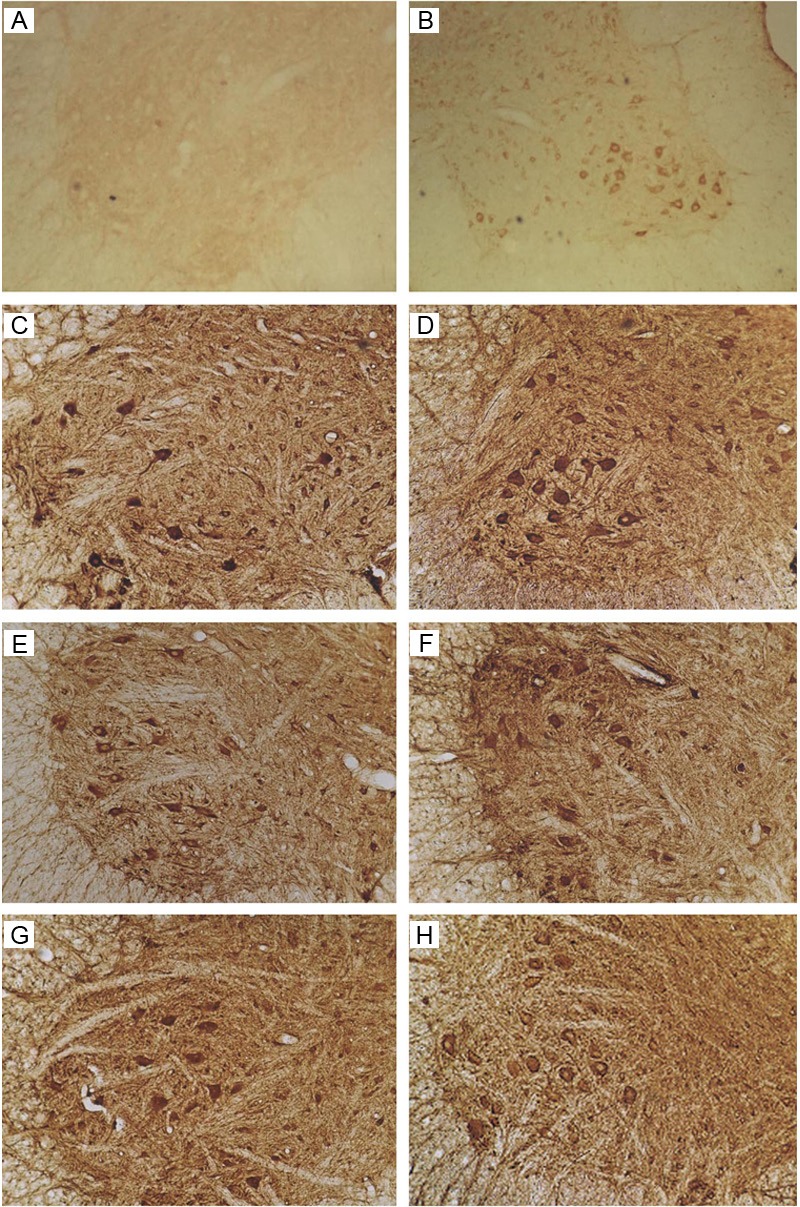
Immunohistochemistry for HIF-1α. A: Sham group. No HIF-1α positive neurons were found. B: SCI group: 7 days after SCI. C: Ad-Blank group: 7 days after SCI. D-H: Ad-HIF-1α group: 1, 3, 7, 14, and 28 days after ACI (100×).
Immunohistochemistry for VEGF
In sham group, the VEGF expression was at a low level in the cytoplasm and predominantly distributed in the anterior horn, especially in motor neurons. Occasionally, positive neurites were visible and the OD was 31.3±6.2. One day after SCI, the VEGF expression in SCI group and Ad-Blank group increased, especially in the motor neurons of dorsal horn. The OD reached a peak (80.0) on day 3, which was significantly different from that on day 1 (P<0.05). Since 7 days after surgery, the OD decreased and reached a minimal level on day 28. The VEGF expression in Ad-Blank group was similar with that in SCI group (P>0.05).
One day after SCI, the VEGF positive cells were found in the dorsal horn and anterior horn of spinal cord in Ad-HIF-1α group. Microscopy showed that the brown granules were mainly found in the cytoplasm and neurites. In motor neurons of anterior horn, the VEGF expression was obvious and predominantly distributed in cell bodies and neurites, with identical staining intensity. At 7 days after SCI, the VEGF expression reached a peak, but decreased gradually since day 14 and reached a minimal level on day 28. The VEGF expression on day 1 was significantly different from that at other time points (P<0.05). VEGF expression in Ad-HIF-1α group was markedly higher than that in Ad-Blank group and SCI group (P<0.05), but only the OD value of the Ad-Blank group on day 28 was significantly different from that of the Ad-HIF-1α group (P<0.05). Our results indicated that the increased VEGF expression in Ad-HIF-1α group prolonged, and reached a maximal level on day 7. However, the VEGF expression reached a maximal level on day 3 in SCI group and Ad-Blank group (Figure 4).
Figure 4.
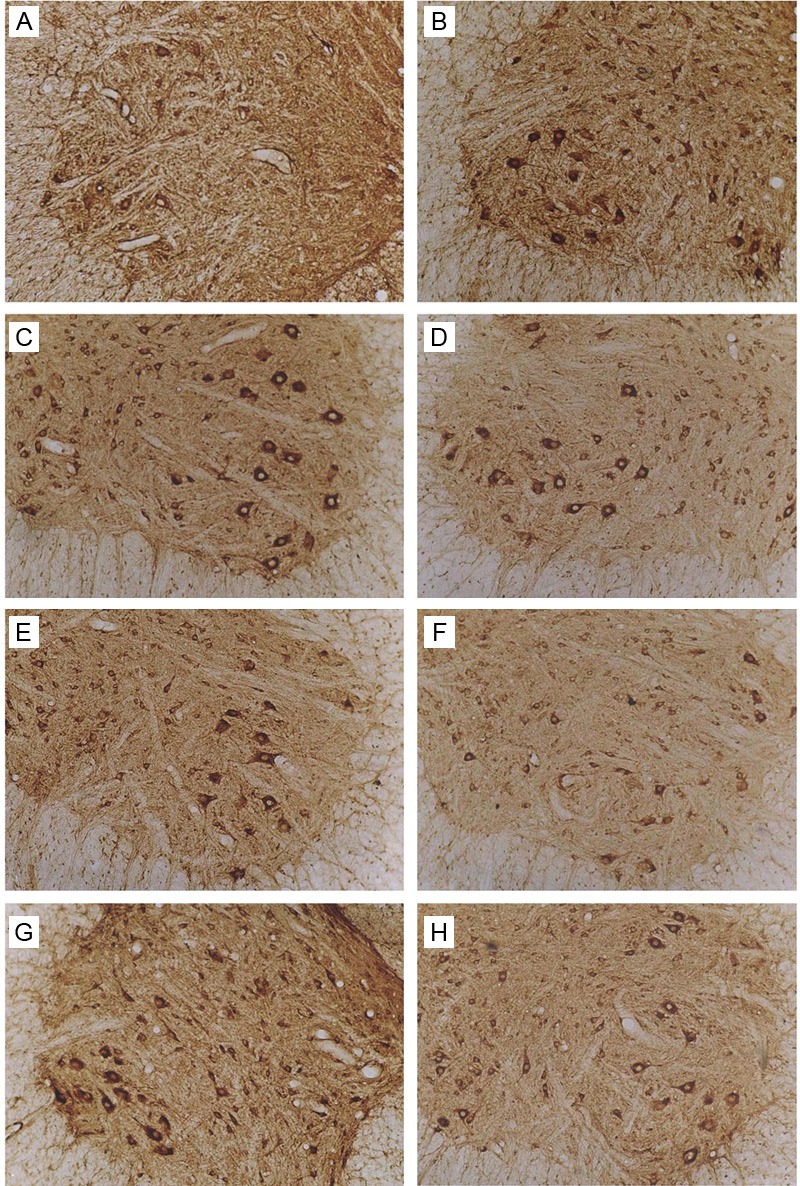
Immunohistochemistry for VEGF. A: Sham group. B: SCI group: At 7 days after SCI. C: Ad-Blank group. At 7 days after SCI. D-H: Ad-HIF-1α group: At 1, 3, 7, 14, and 28 days after SCI (100×).
Immunohistochemistry for Bax results
In Sham group, Bax was weakly expressed in the cytoplasm of neurons of anterior horn, dorsal horn and intermediate zone. Occasionally, positive neurites were observed.
One day after SCI in SCI group, the Bax positive neurons increased when compared with sham group (P<0.05). From day 3 to day 7, the Bax expression reached a maximal level and was significantly different from that on day 1 (P<0.05). Then, the Bax expression decreased gradually and reached a minimal level on day 28. The Bax expression in Ad-Blank group was similar to that in SCI group (P>0.05).
One day after SCI in Ad-HIF-1α group, Bax positive neurons were detectable in the anterior horn and dorsal horn, and positive granules were mainly found on the cell membrane and in the cytoplasm. Neurites were not clear and neurons varied in shape. The Bax expression reached a maximal level on day 2, and then decreased gradually, reaching a minimal level on day 28, which was significantly different from that on day 1 (P<0.05). The Bax expression in Ad-HIF-1α group was significantly lower than that in Ad-Blank group and SCI group (P<0.05) at each time point (except on day 1). The Bax expression in Ad-HIF-1α group on day 1 was similar to that in Ad-Blank group and SCI group. The Bax expression in Ad-HIF-1α group decreased and reached a maximal level on day 3, which was earlier than that in Ad-Blank group and SCI group (3-7 days after SCI) (Figure 5).
Figure 5.
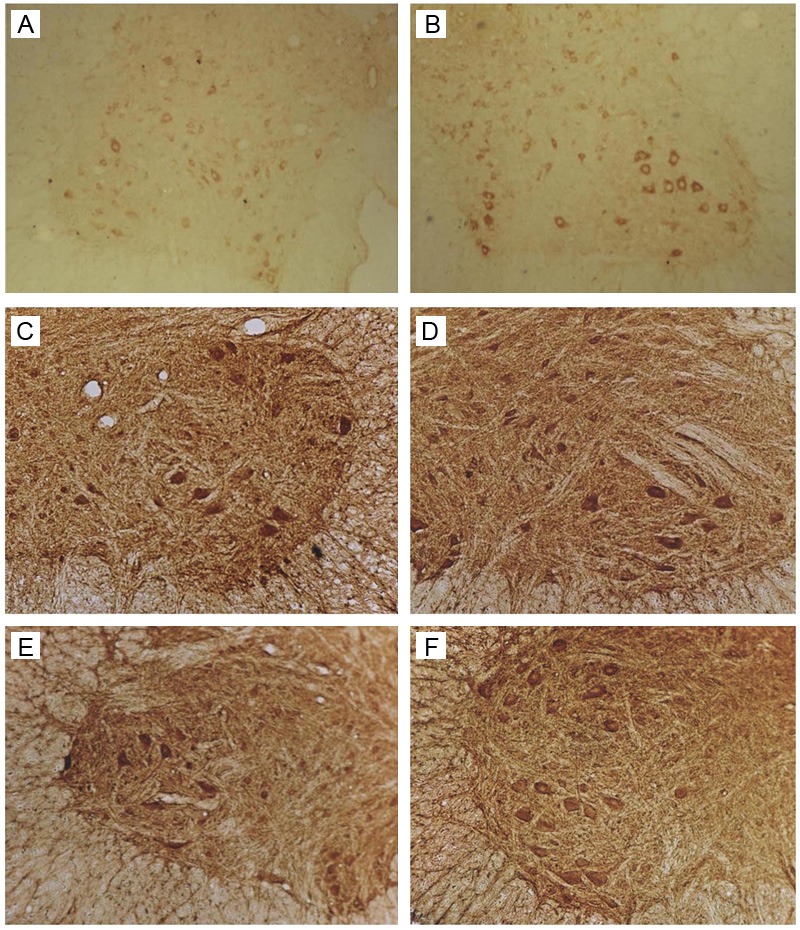
Immunohistochemistry for Bcl-2. A: Sham group. B: SCI group: 7 days after SCI. C: Ad-Blank group: 7 days after SCI. D-F: Ad-HIF-1α group: 3, 7, and 14 days after SCI (100×).
Immunohistochemistry for Bcl-2
In sham group, Bcl-2 was weakly expressed in the cytoplasm of motor neurons of anterior horn, and dorsal horn. Occasionally, positive neurites were observed.
One day after SCI in SCI group, the number of Bcl-2 positive neurons and Bcl-2 expression increased significantly when compared with Sham group (P<0.05). On day 3, the Bcl-2 expression reached a maximal level and then decreased gradually, reaching a minimal level on day 28 with no significant difference from that on day 1. The Bcl-2 expression in Ad-Blank group was similar to that in SCI group (P>0.05).
One day after SCI in Ad-HIF-1α group, Bcl-2 positive neurons were detectable in the anterior horn and dorsal horn, and positive granules were observed on the cell membrane and in the cytoplasm. Positive neurites were obvious. The Bcl-2 expression reached a maximal level from day 3 to day 7 and then decreased gradually, reaching a minimal level on day 28 without significant difference from that on day 1. The Bcl-2 expression in Ad-HIF-1α group was markedly higher than that in Ad-Blank group and SCI group (P<0.05) at each time point (except on days 1 and 28). The Bcl-2 expression in Ad-HIF-1α group was comparable to that in Ad-Blank group and SCI group on days 1 and 28. The Bcl-2 expression reached a maximal level on days 3-7 in Ad-HIF-1α group but on day 3 in Ad-Blank group and SCI group (Figure 5).
Relationship between Bax and Bcl-2, and between HIF-1α and VEGF
In Ad-HIF-1α group, there was significant correlation between Bax expression and Bcl-2 expression (r=0.50, P<0.05) (Figure 6). The Bax/Bcl-2 ratio was >1 1 and 3 days after injury, but <1 a 7, 14 and 28 days after surgery. Significant correlation was noted between HIF-1α expression and VEGF expression (r=0.93, P<0.05).
Figure 6.
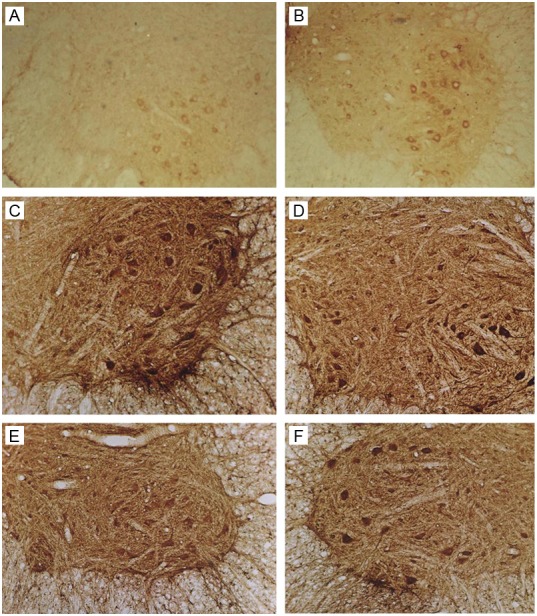
Immunohistochemistry for Bax. A: Sham group. B: SCI group: 7 days after SCI. C: Ad-Blank group: 3 days after SCI. D-F: Ad-HIF-1α group: 3, 7, and 14 days after SCI (100×).
BBB scores of hind limbs
The score of hind limb function was 21 in sham group. Paralysis was observed in the hind limbs of all rats after injury. One day after injury, the BBB scores of all rats after SCI were 0, except one was scored 0.5 in Ad-HIF-1α group. At 3 days after injury, the BBB score increased and reached a maximal level on day 28. The BBB score in Ad-HIF-1α group was significantly higher than that in Ad-Blank group and SCI group at each time points (P<0.05), except at 1 day after SCI. Although the BBB score in Ad-HIF-1α group was higher (mean BBB score: 7.2 on day 28), the whole body could not be supported by hind limbs.
Discussion
In the present study, the expressions of Bcl-2, Bax, VEGF and HIF1α were up-regulated after SCI. However, the expressions of Bcl-2, VEGF and HIF1α in Ad-HIF-1α group were significantly increased when compared with SCI group and Ad-Blank group. Meanwhile, after Ad-HIF-1α treatment, the Bax expression was suppressed, the apoptotic cell death ameliorated, and the recovery of neurological functions was promoted significantly. These findings suggest that Ad-HIF-1α attenuates the SCI induced apoptosis of neurons and alleviates secondary spinal cord lesion after primary injury in SCI rats.
It is estimated that there are 2.5 million people worldwide living with SCI, and 130,000 new cases occur each year [13]. Despite advances in medical and surgical care, devastating loss of motor, sensory and autonomic functions cannot be reversed, and current clinical therapies for SCI have limited effectiveness [14,15]. A cascade of cellular and molecular events constitute the secondary injury, and ultimately result in necrosis or apoptosis of neurons after SCI [16]. The apoptosis-promoting factors increase after SCI including intracellular free Ca2+, free radicals and caspase-3 [17,18]. In thhhe present study, our results showed the expressions of Bcl-2 and Bax changes significantly after SCI. Both proteins belong to Bcl-2 family which plays essential roles in the apoptosis. In addition, our findings also revealed that Ad-HIF-1α could attenuate apoptosis of neurons secondary to SCI.
HIF is an oxygen-sensitive transcriptional factor, a heterodimer composed of HIF-1α and HIF-1β, and a well characterized mediator of the hypoxic response in mammalian cells [19]. HIF-1α is specifically stabilized under hypoxic conditions and degraded rapidly under normoxia [6]. The regulation of HIF-1α plays an important role in the expression of hypoxia-specific genes, such as VEGF and EPO, via binding to the HREs at 5’-regulatory regions. HIF-1α binds to the specific cis-regulatory elements in the promoters and facilitates the transcription under hypoxia conditions. These factors involve in the cellar and systemic reactions to hypoxia and can maintain cell survival and promote the restoration of normal oxygen level [20]. In our study, the HIF1α expression in the anterior horn of spinal cord was enhanced after SCI in Ad-HIF-1α group as compared to control group and SCI group, which might be attributed to the relief of neuronal injury and apoptosis following SCI. Previous studies have shown the neuroprotective effect of HIF1α. Tae et al. found that fluoxetine and sertraline would attenuate brain injury and facilitate functional recovery via the enhancement of HIF-1α proteins expression in a permanent focal ischemia mice model [21]. The expressions of HIF-1α and its target genes, (erythropoietin EPO and VEGF) increased rapidly significantly when animals were pretreated with normobaric hypoxia 24 hours before ischemia; and they also investigated that the infarct volume reduced by approximately 30% when compared with controls [22]. As mentioned by in the study of Juan et al. in their literature [23], they analyzed results showed increased HIF-1α expression may persisted for at least 7 days in the rat the cerebral cortex of rats after transient global ischemia induced due to cardiac arrest and resuscitation and it could promote cell survival after cerebral ischemia. In contrast to prosurvival effect, the role of HIF-1α in responses of developing brain to injury come up with other studies. Middle cerebral artery occlusion-induced focal cerebral ischemia was associated with increases in HIF1α, inducible nitric oxide synthase, and active caspase-3 protein expressions as well as the mRNA expression of tumor necrosis factor-αa in ischemic region [24]. Data Evidence also supports a role of HIF-1α in the matrix metalloproteinase regulatory cascade in the synapse loss after traumatic brain injury [25]. Prodeath or prosurvival effect of HIF-1α might depend on the time window and the extent of HIF-1α expression; however, further studies are required to determine the exact role of HIF1α in brain damage.
Furthermore, the relationship between HIF-1α expression and VEGF expression was determined in rats with SCI. Results showed significant correlation between them (r=0.93). This was consistent with previous studies. HIF-1α is a protein accumulating in tissues under hypoxic/ischemia conditions [26]. When activated, HIF-1α binds to the HRE and up-regulates the VEGF expression [27], which leads to the angiogenesis after cerebral ischemia [28].
In conclusion, our result showed SCI could up-regulate the expressions of Bcl-2, Bax, VEGF and HIF-1α, which were influenced after Ad-HIF-1α treatment. The therapeutic effect of Ad-HIF-1α may be attributed to the promotive effect on angiogenesis and suppression of neuronal apoptosis after SCI.
Acknowledgements
This study was supported by the Public Technology Research Program of Zhejiang Province (NO. 2010C33015) and Fund of Medicine and Health Program Co-constructed by Wenzhou City and Zhejiang Province (2010SSA004).
Disclosure of conflict of interest
None.
References
- 1.Amar AP, Levy ML. Pathogenesis and pharmacological strategies for mitigating secondary damage in acute spinal cord injury. Neurosurgery. 1999;44:1027–1039. doi: 10.1097/00006123-199905000-00052. discussion 1039-1040. [DOI] [PubMed] [Google Scholar]
- 2.Dolan EJ, Tator CH. The effect of blood transfusion, dopamine, and gamma hydroxybutyrate on posttraumatic ischemia of the spinal cord. J Neurosurg. 1982;56:350–358. doi: 10.3171/jns.1982.56.3.0350. [DOI] [PubMed] [Google Scholar]
- 3.Schwab ME, Bartholdi D. Degeneration and regeneration of axons in the lesioned spinal cord. Physiol Rev. 1996;76:319–370. doi: 10.1152/physrev.1996.76.2.319. [DOI] [PubMed] [Google Scholar]
- 4.Webb AA, Ngan S, Fowler JD. Spinal cord injury I: A synopsis of the basic science. Can Vet J. 2010;51:485–492. [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang BH, Semenza GL, Bauer C, Marti HH. Hypoxia-inducible factor 1 levels vary exponentially over a physiologically relevant range of O2 tension. Am J Physiol. 1996;271:C1172–1180. doi: 10.1152/ajpcell.1996.271.4.C1172. [DOI] [PubMed] [Google Scholar]
- 6.Ratcliffe PJ, O'Rourke JF, Maxwell PH, Pugh CW. Oxygen sensing, hypoxia-inducible factor-1 and the regulation of mammalian gene expression. J Exp Biol. 1998;201:1153–1162. doi: 10.1242/jeb.201.8.1153. [DOI] [PubMed] [Google Scholar]
- 7.Carmeliet P, Dor Y, Herbert JM, Fukumura D, Brusselmans K, Dewerchin M, Neeman M, Bono F, Abramovitch R, Maxwell P, Koch CJ, Ratcliffe P, Moons L, Jain RK, Collen D, Keshert E. Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature. 1998;394:485–490. doi: 10.1038/28867. [DOI] [PubMed] [Google Scholar]
- 8.Xiao X, Li J, McCown TJ, Samulski RJ. Gene transfer by adeno-associated virus vectors into the central nervous system. Exp Neurol. 1997;144:113–124. doi: 10.1006/exnr.1996.6396. [DOI] [PubMed] [Google Scholar]
- 9.Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 10.Zlokovic BV, Apuzzo ML. Cellular and molecular neurosurgery: pathways from concept to reality--part II: vector systems and delivery methodologies for gene therapy of the central nervous system. Neurosurgery. 1997;40:805–812. doi: 10.1097/00006123-199704000-00028. discussion 812-803. [DOI] [PubMed] [Google Scholar]
- 11.Miura T, Tanaka S, Seichi A, Arai M, Goto T, Katagiri H, Asano T, Oda H, Nakamura K. Partial functional recovery of paraplegic rat by adenovirus-mediated gene delivery of constitutively active MEK1. Exp Neurol. 2000;166:115–126. doi: 10.1006/exnr.2000.7493. [DOI] [PubMed] [Google Scholar]
- 12.Wilson JM. Adenoviruses as gene-delivery vehicles. N Engl J Med. 1996;334:1185–1187. doi: 10.1056/NEJM199605023341809. [DOI] [PubMed] [Google Scholar]
- 13.Thuret S, Moon LD, Gage FH. Therapeutic interventions after spinal cord injury. Nat Rev Neurosci. 2006;7:628–643. doi: 10.1038/nrn1955. [DOI] [PubMed] [Google Scholar]
- 14.Hawryluk GW, Rowland J, Kwon BK, Fehlings MG. Protection and repair of the injured spinal cord: a review of completed, ongoing, and planned clinical trials for acute spinal cord injury. Neurosurg Focus. 2008;25:E14. doi: 10.3171/FOC.2008.25.11.E14. [DOI] [PubMed] [Google Scholar]
- 15.Rowland JW, Hawryluk GW, Kwon B, Fehlings MG. Current status of acute spinal cord injury pathophysiology and emerging therapies: promise on the horizon. Neurosurg Focus. 2008;25:E2. doi: 10.3171/FOC.2008.25.11.E2. [DOI] [PubMed] [Google Scholar]
- 16.Baptiste DC, Fehlings MG. Emerging drugs for spinal cord injury. Expert Opin Emerg Drugs. 2008;13:63–80. doi: 10.1517/14728214.13.1.63. [DOI] [PubMed] [Google Scholar]
- 17.Springer JE, Azbill RD, Knapp PE. Activation of the caspase-3 apoptotic cascade in traumatic spinal cord injury. Nat Med. 1999;5:943–946. doi: 10.1038/11387. [DOI] [PubMed] [Google Scholar]
- 18.Ray SK, Wilford GG, Matzelle DC, Hogan EL, Banik NL. Calpeptin and methylprednisolone inhibit apoptosis in rat spinal cord injury. Ann N Y Acad Sci. 1999;890:261–269. doi: 10.1111/j.1749-6632.1999.tb08001.x. [DOI] [PubMed] [Google Scholar]
- 19.Bruick RK. Expression of the gene encoding the proapoptotic Nip3 protein is induced by hypoxia. Proc Natl Acad Sci U S A. 2000;97:9082–9087. doi: 10.1073/pnas.97.16.9082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Semenza GL, Agani F, Booth G, Forsythe J, Iyer N, Jiang BH, Leung S, Roe R, Wiener C, Yu A. Structural and functional analysis of hypoxia-inducible factor 1. Kidney Int. 1997;51:553–555. doi: 10.1038/ki.1997.77. [DOI] [PubMed] [Google Scholar]
- 21.Shin TK, Kang MS, Lee HY, Seo MS, Kim SG, Kim CD, Lee WS. Fluoxetine and sertraline attenuate postischemic brain injury in mice. Korean J Physiol Pharmacol. 2009;13:257–263. doi: 10.4196/kjpp.2009.13.3.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bernaudin M, Nedelec AS, Divoux D, MacKenzie ET, Petit E, Schumann-Bard P. Normobaric hypoxia induces tolerance to focal permanent cerebral ischemia in association with an increased expression of hypoxia-inducible factor-1 and its target genes, erythropoietin and VEGF, in the adult mouse brain. J Cereb Blood Flow Metab. 2002;22:393–403. doi: 10.1097/00004647-200204000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Chavez JC, LaManna JC. Activation of hypoxia-inducible factor-1 in the rat cerebral cortex after transient global ischemia: potential role of insulin-like growth factor-1. J Neurosci. 2002;22:8922–8931. doi: 10.1523/JNEUROSCI.22-20-08922.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang Y, Hsieh CY, Peng ZA, Yen TL, Hsiao G, Chou DS, Chen CM, Sheu JR. Neuroprotective mechanisms of puerarin in middle cerebral artery occlusion-induced brain infarction in rats. J Biomed Sci. 2009;16:9. doi: 10.1186/1423-0127-16-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ding JY, Kreipke CW, Schafer P, Schafer S, Speirs SL, Rafols JA. Synapse loss regulated by matrix metalloproteinases in traumatic brain injury is associated with hypoxia inducible factor-1alpha expression. Brain Res. 2009;1268:125–134. doi: 10.1016/j.brainres.2009.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Post DE, Van Meir EG. Generation of bidirectional hypoxia/HIF-responsive expression vectors to target gene expression to hypoxic cells. Gene Ther. 2001;8:1801–1807. doi: 10.1038/sj.gt.3301605. [DOI] [PubMed] [Google Scholar]
- 28.Marti HJ, Bernaudin M, Bellail A, Schoch H, Euler M, Petit E, Risau W. Hypoxia-induced vascular endothelial growth factor expression precedes neovascularization after cerebral ischemia. Am J Pathol. 2000;156:965–976. doi: 10.1016/S0002-9440(10)64964-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


