Abstract
OBJECTIVE
This study investigated the safety and efficacy of sitagliptin (Januvia) for the inpatient management of type 2 diabetes (T2D) in general medicine and surgery patients.
RESEARCH DESIGN AND METHODS
In this pilot, multicenter, open-label, randomized study, patients (n = 90) with a known history of T2D treated with diet, oral antidiabetic agents, or low total daily dose of insulin (≤0.4 units/kg/day) were randomized to receive sitagliptin alone or in combination with glargine insulin (glargine) or to a basal bolus insulin regimen (glargine and lispro) plus supplemental (correction) doses of lispro. Major study outcomes included differences in daily blood glucose (BG), frequency of treatment failures (defined as three or more consecutive BG >240 mg/dL or a mean daily BG >240 mg/dL), and hypoglycemia between groups.
RESULTS
Glycemic control improved similarly in all treatment groups. There were no differences in the mean daily BG after the 1st day of treatment (P = 0.23), number of readings within a BG target of 70 and 140 mg/dL (P = 0.53), number of BG readings >200 mg/dL (P = 0.23), and number of treatment failures (P > 0.99). The total daily insulin dose and number of insulin injections were significantly less in the sitagliptin groups compared with the basal bolus group (both P < 0.001). There were no differences in length of hospital stay (P = 0.78) or in the number of hypoglycemic events between groups (P = 0.86).
CONCLUSIONS
Results of this pilot indicate that treatment with sitagliptin alone or in combination with basal insulin is safe and effective for the management of hyperglycemia in general medicine and surgery patients with T2D.
Increasing evidence from observational and randomized controlled studies in general medicine and surgery patients show that type 2 diabetes (T2D) is associated with prolonged hospital stay and increased incidence of infections and hospital complications (1–6). Recent guidelines from professional organizations (7–10) recommend the use of subcutaneous insulin as the preferred therapy for glycemic control in hospitalized patients in a non–intensive-care unit (non-ICU) setting. Scheduled basal bolus insulin therapy using long- or intermediate-acting insulin preparations in combination with short- (regular) or rapid-acting insulin analogs has been proven to be safe and effective for glycemic management in patients with diabetes or hyperglycemia (10–12). Recent studies in general medicine and surgery patients with T2D have reported both improved glycemic control and reductions in a composite of hospital complications, including wound infections, pneumonia, bacteremia, and acute renal and respiratory failure, using basal bolus insulin regimens when compared with sliding scale insulin alone (11–14). Basal bolus regimens, however, are labor intensive, require multiple insulin injections, and are associated with a significant risk of hypoglycemia. The rate of hypoglycemia in non-ICU patients with T2D treated with basal bolus insulin regimens has been reported to be up to 32% (12,14–16).
Current practice guidelines recommend against inpatient use of oral antidiabetic drugs and noninsulin injectable medications in part due to the absence of efficacy studies as well as safety concerns (7,8,10). A major limitation to using oral antidiabetic agents in the inpatient setting relates to the delay and unpredictable onset of action of these drugs, which can prevent rapid attainment of glycemic control or dose adjustments to meet the changing needs of the acutely ill patient. There is also concern regarding the potential for adverse cardiovascular effects with the use of sulfonylureas in patients with cardiac and cerebral ischemia (17) and with the safety of metformin in patients with renal or liver dysfunction, heart failure, and intravenous iodine contrast and after surgical procedures (7,8,10). In addition, the use of thiazolidinediones is limited by their lag time to active glucose control and their tendency to increase intravascular volume and precipitate or worsen congestive heart failure and peripheral edema (18).
Since the U.S. approval of incretin mimetic agents in 2005–2006, dipeptidyl peptidase-4 (DPP-4) inhibitors have been rapidly incorporated into the outpatient management of T2D (19). These agents improve metabolic control by enhancing endogenous prandial insulin secretion and inhibiting glucagon secretion, thereby reducing postprandial glucose excursions (20). The low risk of hypoglycemia and good tolerability of the DPP-4 inhibitors (21–23) make them attractive considerations for use in hospitalized patients. At this time, however, no previous studies have investigated the use of these agents in the hospital setting. Accordingly, we conducted a prospective, randomized clinical trial to determine the safety and efficacy of sitagliptin alone or in combination with basal insulin in the management of general medicine and surgery patients with T2D.
RESEARCH DESIGN AND METHODS
In this pilot, multicenter, prospective, open-label, randomized study, we enrolled 90 adult patients admitted to general medicine and surgery services. Recruited patients had a known history of T2D with a blood glucose (BG) prior to randomization of between 140 and 400 mg/dL and a known history of T2D for >3 months, were between 18 and 80 years of age, and were treated at home with diet alone, any combination of oral antidiabetic agents, or low-dose insulin therapy at a daily dose ≤0.4 units/kg prior to admission. On admission, we stopped oral antidiabetic agents and insulin therapy, and BG was measured before meals and bedtime. Patients were recruited when BG was >140 mg/dL. We excluded patients with any BG between admission and randomization of >400 mg/dL or with a prior history of hyperglycemic crises; patients with hyperglycemia but without a known history of diabetes; patients admitted to or expected to require ICU admission or cardiac surgery; patients with a history of pancreatitis or active gallbladder disease, corticosteroid therapy, clinically relevant hepatic disease, or impaired renal function (glomerular filtration rate [GFR] <30 mL/min or serum creatinine ≥3.0 mg/dL); and patients with a history of diabetic ketoacidosis (24), pregnancy, or any mental condition rendering the subject unable to give informed consent.
Patients were randomized according to a 1:1:1 ratio into three regimens: sitagliptin once daily, sitagliptin and basal insulin (glargine Lantus; Sanofi) once daily, and basal bolus insulin with glargine once daily and lispro before meals (Humalog; Eli Lilly and Company). Patients treated with sitagliptin received a single dose of 100 mg/day (at any time of day) if GFR >50 mL/min or 50 mg/day if GFR was between 30 and 50 mL/min. Patients in the sitagliptin and basal group received a starting total daily dose (TDD) of glargine of 0.25 units/kg/day, except for those patients ≥70 years of age and/or with a serum creatinine ≥2.0 mg/dL who received a starting TDD of 0.15 units/kg. Patients in the basal bolus group were started at a TDD of 0.5 units/kg divided half as insulin glargine once daily and half as insulin lispro before meals. In patients ≥70 years of age and/or with a serum creatinine ≥2.0 mg/dL, the starting TDD in the basal bolus group was reduced to 0.3 units/kg in the basal bolus group. Patients in all three groups received supplemental (correction) doses of insulin lispro before meals and bedtime for BG >140 mg/dL. The goal of therapy was to maintain a fasting and premeal glucose concentration between 100 and 140 mg/dL. The doses of insulin were adjusted daily according to protocol (included in Supplementary Table 1). Treatment failure was arbitrarily defined as an average daily BG >240 mg/dL or two consecutive values BG >240 mg/dL (11,14). If this occurred, patients in the sitagliptin and sitagliptin plus glargine groups were switched to basal bolus regimen starting at a TDD of 0.5 units/kg.
BG was measured before each meal and at bedtime (or every 6 h if a patient was not eating) using a point-of-care glucose meter (ACCU-CHECK; Roche, Indianapolis, IN). In addition, BG was measured at any time if a patient experienced symptoms of hypoglycemia or if requested by the treating physician. HbA1c was measured on the first day of hospitalization. The results of BG values are presented as premeal glucose, bedtime glucose, and mean daily BG during the hospital stay after day 1.
This study was conducted at Grady Memorial Hospital (Atlanta, GA), Emory University Hospital, and University of Michigan Health System. The study protocol and consent form were approved by the institutional review board at each participating institution. A research pharmacist at each institution according to a computer-generated randomization table coordinated the randomization and treatment assignment. All patients were managed for medical and surgical problem(s) by their primary care team who received a copy of the assigned treatment protocol.
Outcome measures
The primary outcome of the study was to determine differences in glycemic control as measured by mean daily BG concentration among treatment groups. Secondary outcomes included differences between treatment groups in any of the following measures: number of BG values within range, number of hypoglycemic events (BG <70 and <40 mg/dL), number of episodes of hyperglycemia (BG >200 mg/dL) after the first day of treatment, TTD of insulin, length of hospital stay, hospital complications, and differences in glycemic control between medicine and surgery patients.
Statistical analysis
This was a noninferiority study design based on the hypothesis that the difference in mean daily BG between basal plus sitagliptin and basal bolus regimens would be no greater than 18 mg/dL (1 mmol/L) (11,14). We compared baseline and clinical characteristics and outcomes, such as mean daily BG after day 1, occurrence of hypoglycemia, and occurrence of complications, among treatment groups and between medical and surgical patients. The comparisons were made with the use of one-way ANOVA for continuous variables and χ2 tests (or Fisher exact test) for discrete variables. A P value of <0.05 was considered significant. Multiple comparisons across different days on therapy were adjusted conservatively by using Tukey adjustment. Statistical analyses were performed using SAS (version 9.2; Cary, NC). The data were generally presented as mean ± SD for continuous variables and count (percentage) for discrete variables.
RESULTS
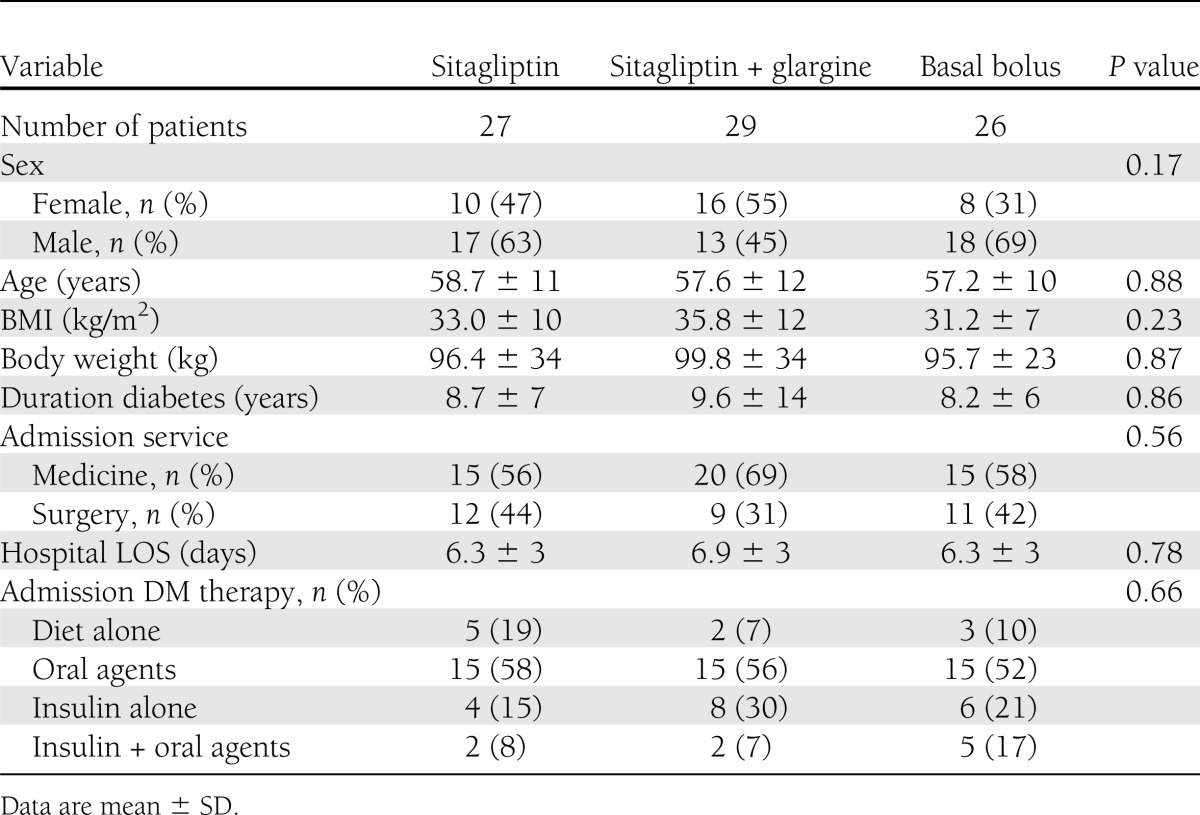
A total of 90 patients with T2D were consented (55 medicine and 35 surgery); 8 patients were excluded from further analysis because they received <24 h of insulin treatment, were transferred to the ICU, or received corticosteroid therapy. A total of 27 patients in the sitagliptin alone group, 29 patients in the sitagliptin and glargine group, and 26 in the basal bolus group were included in the final analysis. The clinical characteristics of study patients are shown in Table 1. There were no significant differences in the mean age, racial distribution, BMI, duration of diabetes, type of treatment prior to admission, or mean hospital length of stay (LOS) among groups. The most common admitting diagnoses in medicine patients were cardiovascular (14%), infectious (28%), and pulmonary (22%) disorders, whereas the most common types of surgery were orthopedic (28%), urologic (19%), thoracic (16%), and abdominal (9%) procedures.
Table 1.
Clinical characteristics of study patients
The admission BG, HbA1c concentration, and changes in glycemic control during the hospital stay are shown in Table 2. The mean admission glucose for the entire cohort was 211.9 ± 63 mg/dL and the mean HbA1c was 8.2 ± 2%. All treatment regimens resulted in prompt and similar improvement in mean daily BG concentration after the 1st day of therapy (Fig. 1). The percentages of glucose readings within target range between 70 and 140 mg/dL were slightly higher in the sitagliptin and glargine (43%) and basal bolus (43%) regimens compared with sitagliptin (36%), but results were not statistically significant (P = 0.53) (Table 2). Similarly, there were fewer BG readings >200 mg/dL in the sitagliptin and glargine group compared with basal bolus and sitagliptin alone (13, 21, and 21%, respectively); however, the difference was not statistically significant (P = 0.23). In addition, there were no differences in the number of treatment failures (8 vs. 11 vs. 10%, respectively, P > 0.99).
Table 2.
Glycemic control, insulin therapy, and hypoglycemic events in patients treated with sitagliptin alone or in combination with basal insulin and basal bolus regimen
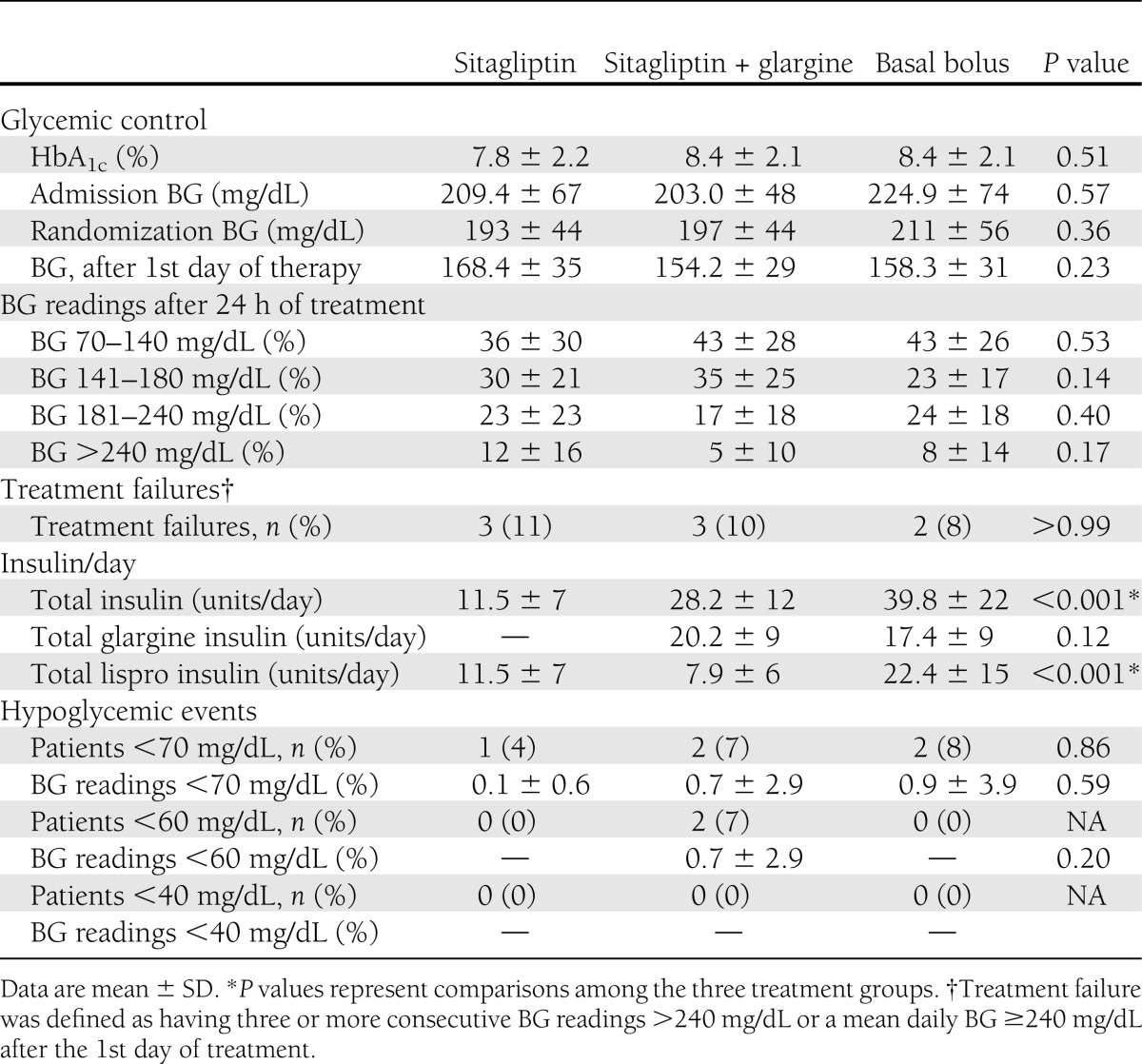
Figure 1.
Differences in glycemic control in medicine and surgery patients with T2D treated with sitagliptin alone or in combination with basal insulin and basal bolus regimen. A: Mean daily glucose levels in patients treated with sitagliptin alone or in combination with basal (glargine) insulin and basal bolus (glargine + lispro) insulin regimens. All groups received supplemental (correction) doses of lispro before meals and bedtime for BG >140 mg/dL. B: Mean BG levels before meals and bedtime during the hospital stay in patients treated with sitagliptin alone or in combination with basal insulin and basal bolus insulin regimens.
The TDD of insulin (units/day) was higher in the basal bolus group (39.8 ± 22 units/day) than in the glargine plus sitagliptin (28.2 ± 12 units/day) and sitagliptin (11.5 ± 7 units/day) groups (P < 0.001). There were no differences in the total dose of basal insulin between basal bolus (17 ± 9 units/day) and sitagliptin and glargine (20 ± 9 units/day) groups, but patients in the basal bolus group received three times the amount of lispro (22.4 ± 15 units/day) before meals compared with the sitagliptin and glargine (7.9 ± 6 units/day) and sitagliptin (11.5 ± 7 units/day) groups (P < 0.001) (Table 2). Most patients received insulin supplements for correction of hyperglycemia during treatment with basal bolus 96%, glargine and sitagliptin 93%, and sitagliptin 100% (P = 0.65). In addition, patients in the basal bolus group received a higher number of insulin injections per day (2.4 ± 0.8) than patients in the sitagliptin and glargine and sitagliptin groups (1.8 ± 0.9 and 1.8 ± 1.1, respectively, P < 0.01) (Table 2).
There were no differences in the frequency of hypoglycemic events between treatment groups. A BG <70 mg/dL was reported in one patient in the sitagliptin group (4%), two patients (8%) in the basal bolus group, and two patients (7%) in the sitagliptin and glargine group (P = 0.86). There were no patients with severe hypoglycemia (<40 mg/dL). In all cases, hypoglycemia was corrected with oral dextrose, and none of these episodes were associated with adverse outcomes.
The level of glucose at admission or at randomization was found to be a good predictor of glycemic control and treatment response during the hospital stay. Compared with patients with glucose ≤180 mg/dL, those with a BG >180 mg/dL had significantly higher mean daily glucose levels after the 1st day of therapy (P < 0.001). There were no differences in mean daily BG concentration or in the number of treatment failures among different treatment groups in patients with a randomization BG <180 mg/dL (Supplementary Fig. 2B); however, patients with a randomization BG >180 mg/dL treated with sitagliptin alone had higher mean daily BG (182.7 ± 30 mg/dL) compared with patients treated with basal bolus (168.1 ± 31 mg/dL) and sitagliptin plus glargine (161.8 ± 31 mg/dL) (P = 0.08).
CONCLUSIONS
This pilot, multicenter, randomized clinical trial compared the efficacy and safety of a daily dose of sitagliptin alone or in combination with glargine insulin to a standard basal bolus regimen in general medicine and surgery patients with T2D. We observed similar improvements in glycemic control in all treatment groups with no differences in the mean daily BG, number of BG readings within target, number of treatment failures, hospital LOS, or number of hypoglycemic events. In addition, the total daily insulin dose and number of insulin injections were significantly less in the sitagliptin groups compared with the basal bolus regimen. The result of this preliminary study suggests that treatment with sitagliptin alone or in combination with basal insulin is safe and effective for the management of general medicine and surgery patients with T2D.
The association between hyperglycemia and increased risk of hospital complications is well established in ICU and non-ICU patients (3–6,25–27). Recent guidelines from professional organizations (18–20) recommend the use of subcutaneous insulin as the preferred therapy for glycemic control in hospitalized patients in a non-ICU setting. The two most common subcutaneous insulin regimens for inpatient glycemic management are sliding scale regular insulin (SSI) and basal bolus insulin therapy in combination with correction insulin scale. The use of basal bolus regimen is preferred as it improves glycemic control and reduces the rate of hospital complications (12,13). The RABBIT 2 medicine trial (11) reported that a BG target of <140 mg/dL was achieved in two-thirds of patients treated with basal bolus regimen, whereas only one-third of those treated with SSI achieved target glycemia. The RABBIT surgery trial also reported a higher percentage of glucose readings <140 mg/dL with basal bolus compared with SSI treatment (53 ± 30 vs. 31 ± 28%) (14). In this study, we report that sitagliptin alone or in combination with basal (glargine) insulin resulted in similar improvements in glycemic control compared with basal bolus regimen.
In agreement with recent reports, the level of glucose at admission or at randomization was found to be a good predictor of glycemic control and treatment response during the hospital stay (25). Compared with patients with glucose >180 mg/dL, those with a BG ≤180 mg/dL had a lower mean daily glucose and less treatment failures, independent of treatment regimen. In patients with an admission or randomization BG ≤180 mg/dL, we observed no differences in mean daily BG concentration or in the number of treatment failures among patients treated with sitagliptin plus supplements compared with patients treated with sitagliptin and glargine or basal bolus regimens (P = 0.63). Patients with a randomization BG >180 mg/dL treated with sitagliptin alone had higher mean daily BG compared with sitagliptin and glargine or basal bolus regimens (P = 0.08). This observation indicates that sitagliptin plus rapid-acting supplements (correction) before meals is useful in patients with mild-to-moderate hyperglycemia, whereas treatment with sitagliptin plus basal insulin or basal bolus regimens should be considered in those with more severe hyperglycemia.
As previously reported (11,14), we show that the use of basal insulin as part of a basal bolus regimen or in combination with sitagliptin is well tolerated with a low rate of hypoglycemia. In the RABBIT medicine trial, 3% of patients in the basal bolus group had a BG <60 mg/dL and no patients had a value <40 mg/dL (11). In the RABBIT surgery trial, 12% of patients treated with basal bolus had a BG <60 mg/dL and 4% had a value <40 mg/dL (14). In the current study, a BG <70 mg/dL was reported in 7% of patients treated with sitagliptin and glargine and in no patients treated with basal bolus or sitagliptin alone. Minimizing hypoglycemic events is of major importance in hospitalized patients because it has been shown to be an independent risk factor of poor outcome (26,27).
We acknowledge the following limitations in this study. We recruited a relatively small number of patients in this pilot study and excluded a large number of patients, which included those admitted to the ICU, with clinically relevant hepatic disease, with pancreatitis, with serum creatinine ≥3.0 mg/dL or GFR <30 mL/min, with severe hyperglycemia (BG >400 mg/dL), and receiving a total dose of insulin >0.4 units/kg/day prior to admission. In such patients, a standard basal bolus approach may be the preferred approach in achieving glycemic control. In addition, our study was not powered to determine differences in hospital complications across the three groups. A large, prospective, randomized, multicenter trial of glycemic control comparing sitagliptin alone or in combination with basal insulin with the basal bolus approach is needed to address these important issues.
In summary, these preliminary results indicate that the inpatient use of sitagliptin alone or in combination with basal insulin resulted in a similar improvement in glycemic control compared with a standard basal bolus insulin regimen. These results indicate that sitagliptin alone or in combination with basal insulin is an effective alternative to the basal bolus insulin regimen for general medicine and surgery patients with T2D.
Supplementary Material
Acknowledgments
G.E.U. is supported in part by research grants from the American Diabetes Association (7-03-CR-35) and PHS Grant UL1-RR-025008 from the Clinical and Translational Science Award program, National Institutes of Health, National Center for Research Resources.
This investigator-initiated study was supported by an unrestricted grant from Merck. No other potential conflicts of interest relevant to this article were reported.
The sponsors of this study were not involved in the study design, data collection, analysis or interpretation of the results, or preparation of the manuscript.
G.E.U. initiated and designed the study and wrote the initial research proposal and manuscript. R.G. initiated and designed the study, reviewed and edited the research proposal and manuscript, and contributed to the discussion. D.S., S.J., D.H.W., C.N., F.F., and L.P. reviewed and edited the research proposal and manuscript and contributed to the discussion. D.R. and S.L.-P. collected the research data. F.P. reviewed and edited the research proposal and manuscript, contributed to the discussion, and collected the research data. G.E.U. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
An abstract of this study was accepted for oral presentation at the 73rd Scientific Sessions of the American Diabetes Association, Chicago, Illinois, 21–25 June 2013.
Footnotes
Clinical trial reg. no. NCT01378117, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc13-0277/-/DC1.
A slide set summarizing this article is available online.
References
- 1.Clement S, Braithwaite SS, Magee MF, et al. American Diabetes Association Diabetes in Hospitals Writing Committee Management of diabetes and hyperglycemia in hospitals. Diabetes Care 2004;27:553–591 [DOI] [PubMed] [Google Scholar]
- 2.Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab 2002;87:978–982 [DOI] [PubMed] [Google Scholar]
- 3.Inzucchi SE. Clinical practice. Management of hyperglycemia in the hospital setting. N Engl J Med 2006;355:1903–1911 [DOI] [PubMed] [Google Scholar]
- 4.Frisch A, Chandra P, Smiley D, et al. Prevalence and clinical outcome of hyperglycemia in the perioperative period in noncardiac surgery. Diabetes Care 2010;33:1783–1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pomposelli JJ, Baxter JK, 3rd, Babineau TJ, et al. Early postoperative glucose control predicts nosocomial infection rate in diabetic patients. JPEN J Parenter Enteral Nutr 1998;22:77–81 [DOI] [PubMed] [Google Scholar]
- 6.Noordzij PG, Boersma E, Schreiner F, et al. Increased preoperative glucose levels are associated with perioperative mortality in patients undergoing noncardiac, nonvascular surgery. Eur J Endocrinol 2007;156:137–142 [DOI] [PubMed] [Google Scholar]
- 7.Moghissi ES, Korytkowski MT, DiNardo M, et al. American Association of Clinical Endocrinologists. American Diabetes Association American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care 2009;32:1119–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schnipper JL, Magee M, Larsen K, Inzucchi SE, Maynard G, Society of Hospital Medicine Glycemic Control Task Force Society of Hospital Medicine Glycemic Control Task Force summary: practical recommendations for assessing the impact of glycemic control efforts. J Hosp Med 2008;3(Suppl.):66–75 [DOI] [PubMed] [Google Scholar]
- 9.Seley JJ, D’Hondt N, Longo R, et al. Position statement: inpatient glycemic control. Diabetes Educ 2009;35:65–69 [Google Scholar]
- 10.Umpierrez GE, Hellman R, Korytkowski MT, et al. Endocrine Society Management of hyperglycemia in hospitalized patients in non-critical care setting: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2012;97:16–38 [DOI] [PubMed] [Google Scholar]
- 11.Umpierrez GE, Smiley D, Zisman A, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial). Diabetes Care 2007;30:2181–2186 [DOI] [PubMed] [Google Scholar]
- 12.Umpierrez GE, Hor T, Smiley D, et al. Comparison of inpatient insulin regimens with detemir plus aspart versus neutral protamine hagedorn plus regular in medical patients with type 2 diabetes. J Clin Endocrinol Metab 2009;94:564–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Korytkowski MT, Salata RJ, Koerbel GL, et al. Insulin therapy and glycemic control in hospitalized patients with diabetes during enteral nutrition therapy: a randomized controlled clinical trial. Diabetes Care 2009;32:594–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Umpierrez GE, Smiley D, Jacobs S, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery). Diabetes Care 2011;34:256–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wexler DJ, Meigs JB, Cagliero E, Nathan DM, Grant RW. Prevalence of hyper- and hypoglycemia among inpatients with diabetes: a national survey of 44 U.S. hospitals. Diabetes Care 2007;30:367–369 [DOI] [PubMed] [Google Scholar]
- 16.Boucai L, Southern WN, Zonszein J. Hypoglycemia-associated mortality is not drug-associated but linked to comorbidities. Am J Med 2011;124:1028–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brady PA, Terzic A. The sulfonylurea controversy: more questions from the heart. J Am Coll Cardiol 1998;31:950–956 [DOI] [PubMed] [Google Scholar]
- 18.Nesto RW, Bell D, Bonow RO, et al. Thiazolidinedione use, fluid retention, and congestive heart failure: a consensus statement from the American Heart Association and American Diabetes Association. Diabetes Care 2004;27:256–263 [DOI] [PubMed] [Google Scholar]
- 19.Lovshin JA, Drucker DJ. Incretin-based therapies for type 2 diabetes mellitus. Nat Rev Endocrinol 2009;5:262–269 [DOI] [PubMed] [Google Scholar]
- 20.Meier JJ. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol 2012;8:728–742 [DOI] [PubMed] [Google Scholar]
- 21.Goldstein BJ, Feinglos MN, Lunceford JK, Johnson J, Williams-Herman DE, Sitagliptin 036 Study Group Effect of initial combination therapy with sitagliptin, a dipeptidyl peptidase-4 inhibitor, and metformin on glycemic control in patients with type 2 diabetes. Diabetes Care 2007;30:1979–1987 [DOI] [PubMed] [Google Scholar]
- 22.Hanefeld M, Herman GA, Wu M, Mickel C, Sanchez M, Stein PP, Sitagliptin Study 014 Investigators Once-daily sitagliptin, a dipeptidyl peptidase-4 inhibitor, for the treatment of patients with type 2 diabetes. Curr Med Res Opin 2007;23:1329–1339 [DOI] [PubMed] [Google Scholar]
- 23.Raz I, Hanefeld M, Xu L, Caria C, Williams-Herman D, Khatami H, Sitagliptin Study 023 Group Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy in patients with type 2 diabetes mellitus. Diabetologia 2006;49:2564–2571 [DOI] [PubMed] [Google Scholar]
- 24.Kitabchi AE, Umpierrez GE, Murphy MB, Kreisberg RA. Hyperglycemic crises in adult patients with diabetes: a consensus statement from the American Diabetes Association. Diabetes Care 2006;29:2739–2748 [DOI] [PubMed] [Google Scholar]
- 25.Umpierrez GE, Smiley D, Hermayer K, et al. Randomized study comparing a basal bolus with a basal plus correction insulin regimen for the hospital management of medical and surgical patients with type 2 diabetes: Basal Plus Trial. Diabetes Care 22 February 2013. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krinsley JS, Grover A. Severe hypoglycemia in critically ill patients: risk factors and outcomes. Crit Care Med 2007;35:2262–2267 [DOI] [PubMed] [Google Scholar]
- 27.Turchin A, Matheny ME, Shubina M, Scanlon JV, Greenwood B, Pendergrass ML. Hypoglycemia and clinical outcomes in patients with diabetes hospitalized in the general ward. Diabetes Care 2009;32:1153–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.