Abstract
The nuclear accumulation and transcriptional activity of NFκB are constitutively increased in cutaneous T-cell lymphoma (CTCL) cells, and are responsible for their increased survival and proliferation. However, in addition to the anti-apoptotic and pro-inflammatory genes, NFκB induces expression of immunosuppressive genes, such as IL-10 and TGFβ, which inhibit the immune responses and are characteristic for the advanced stages of CTCL. While the mechanisms regulating NFκB-dependent transcription of anti-apoptotic and pro-inflammatory genes have been studied extensively, very little is known about the NFκB regulation of immunosuppressive genes. The specificity of NFκB-regulated responses is determined by the subunit composition of NFκB complexes recruited to the individual promoters, post-translational modifications of NFκB proteins, as well as by their interactions with other transcriptional factors and regulators. In this review, we discuss the mechanisms regulating the transcription of NFκB-dependent anti-apoptotic, pro-inflammatory and immunosuppressive genes in CTCL cells, as potential targets for CTCL therapies.
Keywords: Apoptosis, bortezomib, cutaneous T cell lymphoma, IκBα, IL-10, immunosuppression, NFκB, proteasome inhibition, TGFβ
Introduction
Nuclear factor κB (NFκB) is a key transcriptional regulator of genes involved in immune and inflammatory responses, as well as genes regulating cell survival, differentiation, proliferation, angiogenesis and metastasis [1]. Since NFκB activity and transcription of NFκB-dependent genes are increased in many types of cancer and leukemia, inhibition of NFκB-dependent transcription thus represents an important therapeutic target [2-4]. NFκB activity is constitutively increased in cutaneous T-cell lymphoma (CTCL), where it plays a central mediator between malignant cell survival and inflammatory signaling. Recently, studies from our laboratory have indicated that the increased NFκB activity in CTCL is responsible for the increased resistance to apoptosis by up-regulating the anti-apoptotic genes cIAP1, cIAP2 and Bcl-2 [5]. However, in addition to the anti-apoptotic role of NFκB in CTCL, NFκB also regulates the expression of pro-inflammatory and anti-inflammatory genes.
Tumors and leukemia cells often avoid the immune surveillance by expressing anti-inflammatory genes that inhibit expression of pro-inflammatory genes, thus suppressing the immune responses [6]. Indeed, CTCL cells are characterized by the high expression of anti-inflammatory genes, IL-10 and TGFβ [7], which may be involved in the suppression of pro-inflammatory cytokines IL-1β, IL-8, TNFα and IL-17. Thus, NFκB seems to have a complex regulatory role in CTCL, where it regulates expression of anti-apoptotic, pro-inflammatory as well as immunosuppressive genes (Figure 1). However, while the NFκB regulation of anti-apoptotic and pro-inflammatory genes has been extensively studied and documented, relatively very little is known about the NFκB regulation of immunosuppressive genes. Thus, effective therapeutic targeting of NFκB in CTCL should include the anti-apoptotic, pro-inflammatory as well as the immunosuppressive function of NFκB.
Figure 1.
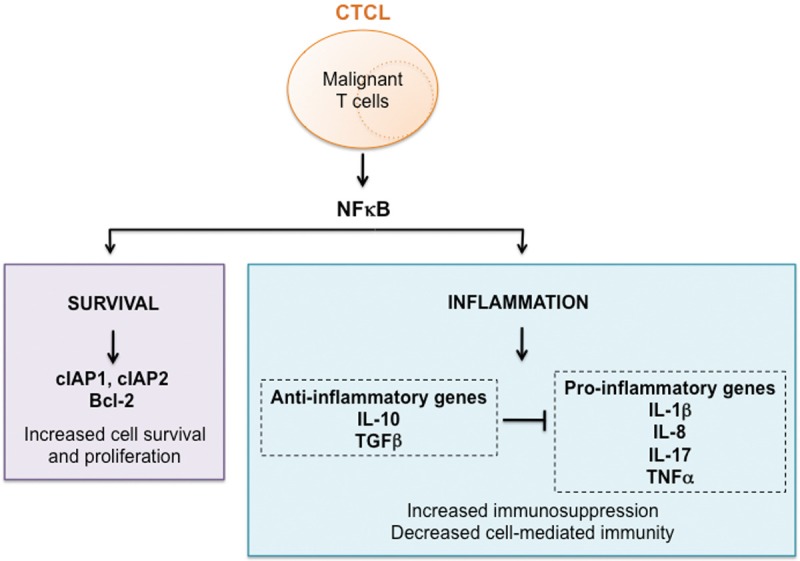
Schematic representation of the NFκB-regulated genes in CTCL. The increased activity of NFκB induces expression of anti-apoptotic genes cIAP1, cIAP2 and Bcl-2 in CTCL cells, resulting in their increased survival. In addition, NFκB also induces expression of pro-inflammatory genes IL-1, IL-8, TNFα and IL-17, and anti-inflammatory genes IL-10 and TGFβ. The increased expression of anti-inflammatory genes in CTCL inhibits expression of pro-inflammatory genes, resulting in the characteristic immuno-suppressory nature of CTCL.
CTCL
Cutaneous T-cell lymphoma (CTCL) encompasses a group of lymphoproliferative disorders characterized by skin invasive neoplastic T cells [8,9]. Mycosis fungoides (MF) and the leukemic variant Sézary syndrome (SS) are the most common clinical forms [10]. MF patients often present with patches and plaques on skin and experience skin symptoms without serious complications. In contrast, patients with SS exhibit a leukemic form of the disease, which is characterized by malignant T cells in the blood. Advanced stages of MF and SS are associated with aggressive course and poor prognosis [11-14].
SS is an erythrodermic leukemic variant of CTCL that is characterized by a high level of constitutive NFκB activity, which is respon- sible for the increased expression of NFκB-dependent anti-apoptotic genes and resistance to apoptosis [15-17]. Patients with SS have high levels of malignant CD4+ T cells expressing IL-4, IL-10 and TGFβ that suppress the immune system and diminish the antitumor responses [18-23]. However, despite the recent advances in elucidating the immune mechanisms responsible for pathogenesis of CTCL, there is no effective strategy to prolong survival in the advanced stages.
NFκB
The NFκB family consists of five distinct transcription factors: p65 (RelA), RelB, c-Rel, p50 (p105/NFκB1) and p52 (p100/NFκB2) [24]. These transcription factors share the N-terminal Rel-homology domain (RHD) that is responsible for dimerization, DNA binding and nuclear translocation [25,26]. The individual NFκB proteins can form homo- and heterodimers, which can bind to promoter κB sites and modulate transcription of NFκB-dependent genes [27-29].
The Rel proteins, including RelA, RelB and c-Rel, contain transcription activation domain (TAD), while p105/50 and p100/52 contain C-terminal ankyrin-repeat domain (ANK), but no TAD. Thus, while p105/p50 and p100/p52 can bind to DNA, they cannot activate transcription. The precursor proteins p105 and p100 can function as IκB proteins, and inhibit nuclear localization and transcriptional activity of NFκB dimers. Removal of the ANK domains produces p50 and p52 subunits that can form homodimers, which can repress transcription by displacing the transcriptionally active heterodimers from κB binding sites [30,31].
Signaling pathways
The signaling pathways that mediate NFκB activation can be broadly classified into canonical and non-canonical pathways [32,33]. The canonical pathway is engaged by ligands for antigen and cytokine receptors, and leads to the nuclear translocation of p50/RelA and p50/c-Rel dimers. The non-canonical pathway is initiated by stimulation of different signaling molecules, and leads to the activation of the p52/RelB dimers [34-36].
In most unstimulated cells, NFκB proteins are bound to the inhibitory IκB proteins, which retain them in an inactive form in the cytoplasm. Upon activation with different stimuli including pro-inflammatory cytokines, oxidative stress and lipopolysaccharide, IκB is phosphorylated by the enzymes of IκB kinase (IKK) complex, ubiquitinated and subsequently degraded by the 26S proteasome. The released NFκB proteins then translocate to the nucleus and bind to the promoter regions of target genes to stimulate their transcription [37,38].
While the cytoplasmic pathways leading to nuclear translocation and activation of NFκB have been studied extensively [28-34], much less is known about the nuclear events regulating NFκB-dependent transcription. This nuclear regulation involves post-translational modifications of NFκB subunits, variations in the DNA sequence of the NFκB binding site, and binding of other transcription factors or coactivators [28-34].
Regulation of NFκB activity
The primary mechanism for regulating NFκB activity is through the inhibitory IκB proteins, which include IκBα, IκBβ, IκBε, IκBζ, Bcl-3, p100, and p105 [39-47]. Phosphorylation of IκB proteins is mediated by the enzymes of IKK complex that include IKKα, IKKβ, and the regulatory subunit IKKγ (NEMO) [48,49]. While the cytoplasmic degradation of IκB, resulting in the nuclear translocation of NFκB subunits, represents a general step in NFκB activation, the specificity of NFκB-regulated responses is mediated by the subunit composition of NFκB dimers and their post-translational modifications [49-54].
The repertoire of pro-inflammatory genes expressed upon NFκB activation includes pro-inflammatory cytokines IL-1β, IL-17 and TNFα, chemokines IL-8, CCL2 and CXCL5, as well as adhesion molecules. In addition, NFκB activates expression of many anti-apoptotic genes that include the cellular inhibitor of apoptosis (cIAP), the TNF receptor-associated factors (TRAF-1 and TRAF-2), and the family of Bcl-2 proteins, A-1/Bfl-1, Bcl-2 and Bcl-xL. By increasing expression of these anti-apoptotic proteins, NFκB activation decreases apoptosis and increases survival of leukemia and cancer cells [1-6]. Accordingly, inhibition of NFκB activity decreases the expression of pro-inflammatory and anti-apoptotic genes, and induces apoptosis.
In majority of human cancers and leukemia, NFκB is constitutively activated due to the increased degradation of IκBα and increased nuclear levels of NFκB subunits. Since the suppression of NFκB activity inhibits pro-inflammatory and anti-apoptotic gene expression, NFκB appears to be one of the most promising targets in the treatment of many inflammatory disorders as well as different types of cancer and leukemia. However, one of the main concerns regarding the NFκB inhibitors is their specificity, since many steps leading to NFκB activation are important for other cellular functions as well. Thus, a better understanding of the mechanisms regulating the specificity of NFκB-regulated responses will ultimately lead to the development of more specific anti-cancer and anti-inflammatory therapies.
Dimerization of NFκB
Dimerization is required for the NFκB binding to promoter regions of target genes [55]. More than 12 different combinations of NFκB homo- and heterodimers have been described [56]. Different dimer combinations have different transcriptional activity and regulate different sets of target genes [57,58]. In addition, the dimer-specific functions are controlled by interactions with other co-regulatory proteins or transcription factors. Thus, depending on these interactions, NFκB dimers can function as activators or repressors. For example, even though p50 homodimers function mainly as transcriptional repressors, since they lack the transactivation domain, their association with Bcl-3 in T cell lymphoma cells increases transcriptional activation [59].
NFκB in CTCL
Increased activation of NFκB promotes cell survival, proliferation, tumorigenesis, angiogenesis and metastasis [60-75]. CTCL cells express all five members of the NFκB family; however, only p65, p50, p52 and Rel-B have been found in patients with MF or SS [76,77]. The increased activity of NFκB induces expression of anti-apoptotic and pro-inflammatory genes in CTCL cells, resulting in their increased proliferation and survival. However, NFκB also induces expression of anti-inflammatory genes, thus contributing to the immunosuppressive nature of CTCL. Therefore, NFκB plays a central regulatory role in the pathogenesis of CTCL, by regulating expression of anti-apoptotic, pro-inflammatory and anti-inflammatory genes (Figure 1).
NFκB rearrangement
Chromosomal amplification, over-expression and rearrangement of genes coding for NFκB subunits have been described in many human hematopoietic and solid tumors [78]. Rearrangements of RelA, c-Rel and NFκB1 genes have been found in human lymphoid tumors, but not in CTCL [79-81]. However, NFκB2 rearrangements have occurred in some cases of CTCL, B-cell chronic lymphocytic leukemia, multiple myeloma and B-cell lymphoma [82], and have been associated with poor prognosis in CTCL [83-87].
Anti-apoptotic role of NFκB
High resistance to apoptosis is a characteristic feature of CTCL. This high resistance to apoptosis is mediated by the high constitutive activity of NFκB, both in CTCL cell lines and in tumor cells from patients with SS [15,88-90]. CTCL cells express constitutive NFκB, c-myc and STAT5 activities that regulate the transcription of anti-apoptotic genes cIAP1, cIAP2 and Bcl-2 [91]. NFκB has been suggested to regulate the apoptotic sensitivity in CTCL through Fas pathway [92]. In addition, the deregulation of Notch1 signaling might be linked to the development of CTCL and several solid malignancies based on the NFκB-mediated cell survival [93].
Several pharmacological agents have been shown to inhibit NFκB activity and induce apoptosis in CTCL. Arsenic trioxide (As2O3) is effective against CTCL by reducing the DNA-binding activity of NFκB and inducing apoptosis [94]. PBOXs (pyrrolo-1,5-benzoxazepines) induces apoptosis in several CTCL cell lines through the NFκB-mediated activation of caspase-3 like proteases, and has the potential use as a novel anticancer drug [95]. The nitric oxide generating compound, sodium nitroprusside (SNP), can induce apoptosis in CTCL Hut-78 cell line by suppressing NFκB activity, and thereby Bcl-xL expression [96]. Non-steroidal anti-inflammatory drugs (NSAIDs), such as acetylsalicylic acid, sodium salicylate, and diclofenac, which have been widely used in the treatment of chronic inflammatory disorders, induce apoptosis in CTCL cells [97]. AraC (cytosine arabinoside) inhibits NFκB activity by dephosphorylating the p65 subunit, resulting in the increased apoptosis in CTCL Hut-78 cells [98].
Curcumin (diferuloylmethane) is the active compound in turmeric, a dietary spice that has been widely consumed for centuries. Curcumin has been found to have anti-proliferative and pro-apoptotic effects in a number of tumor cell lines. In CTCL cells, curcumin induces apoptosis by inhibiting phosphorylation of IκBα and DNA binding activity of NFκB [99]. Curcumin also has an oxidative effect by generating reactive oxygen species (ROS) and inhibiting the constitutive activity of NFκB in CTCL Hut-78 cells [100]. Inhibition of the nuclear accumulation of NFκB p65 and p50 by IKKβ (IKK2) inhibitor (AS6028668) induces a potent apoptotic response in CTCL cell lines and patients with SS [16]. In malignant T-cell lines established from patients with CTCL, the high constitutive activity of NFκB induces expression of the oncogenic B-lymphoid kinase (Blk) that promotes proliferation of malignant CTCL cells [101].
The 26S proteasome inhibitor bortezomib (BZ; Velcade), which has been approved by the FDA for treatment of multiple myeloma and mantel cell lymphoma, acts by targeting the catalytic 20S core of the proteasome and induces apoptosis in cancer cells. One of the mechanisms consists of inhibiting the cytoplasmic degradation of IκBα, resulting in the suppression of NFκB DNA binding activity and decreased expression of NFκB-dependent anti-apoptotic genes. BZ has been also evaluated in CTCL and exhibited promising anti-tumor activity [102-104]. Sors et al. have demonstrated that in CTCL cells, proteasome inhibition by BZ inhibits the in vitro DNA binding activity of NFκB [15]. Interestingly however, a recent study has indicated that in CTCL cell lines, proteasome inhibition actually increases NFκB activity [105].
This seeming discrepancy can be explained by our previous study demonstrating that proteasome inhibition by BZ has a gene specific effect on the regulation of NFκB-dependent anti-apoptotic genes in CTCL Hut-78 cells [5]. Our results have shown that proteasome inhibition suppresses NFκB activity and induces apoptosis by a novel mechanism that consists of the increased nuclear translocation and accumulation of IκBα [5,106,107]. Promoters of the anti-apoptotic genes cIAP1 and cIAP2 are occupied by NFκB p65/50 heterodimers, and the BZ-induced nuclear IκBα inhibits this occupancy, resulting in the decreased cIAP1 and cIAP2 expression. In contrast, Bcl-2 promoter is occupied predominantly by p50/50 homodimers, and this occupancy and Bcl-2 expression are not suppressed by the BZ-induced nuclear IκBα (Figure 2). These data suggest that the regulation of anti-apoptotic genes by NFκB is gene specific, and depends on the subunit composition of NFκB proteins recruited to the promoters.
Figure 2.
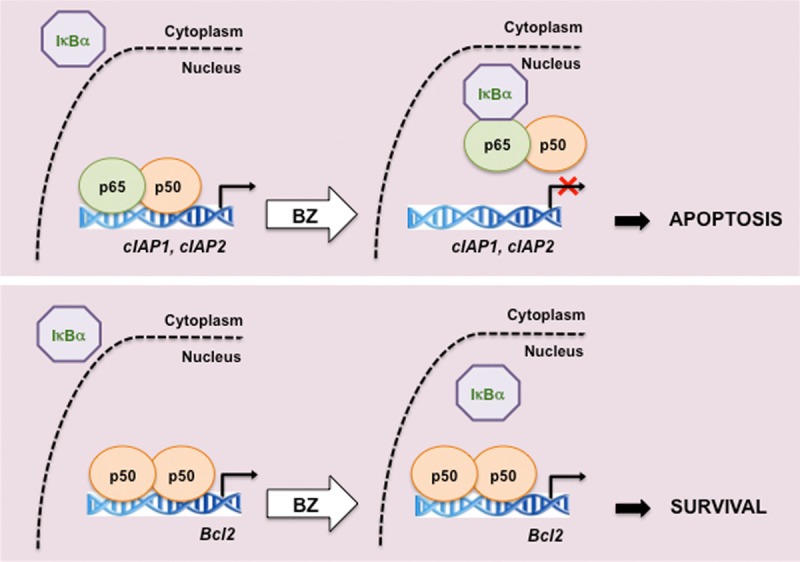
Proposed model of the gene specific regulation of NFκB-dependent anti-apoptotic genes by proteasome inhibition in CTCL cells. In CTCL Hut-78 cells, proteasome inhibition by BZ induces nuclear translocation and accumulation of IκBα. The BZ-induced nuclear IκBα removes NFκB p65/p50 heterodimers from the promoters of cIAP1 and cIAP2 genes, resulting in their suppression. However, the nuclear IκBα does not remove p50/50 homodimers from Bcl-2 promoter; consequently, Bcl-2 expression is not inhibited by BZ [5].
Pro-inflammatory role of NFκB
Inflammatory response is a critical part of innate immunity and involves signaling pathways that regulate both pro-inflammatory and anti-inflammatory genes [108]. Transcription of many of the pro-inflammatory genes is regulated by NFκB [109-112]. In the early stages of CTCL, activation of NFκB and cellular proliferation are induced by the autocrine production of TNFα, resulting in the increased activation of NFκB and resistance to apoptosis [113-116]. In addition to TNFα, epidermis of patients with CTCL displays increased levels of NFκB-dependent cytokines IL-1β and IL-8, suggesting a role of these cytokines in the pathogenesis of CTCL [117-119]. Recent studies have shown that malignant T cells and skin lesions from CTCL patients produce the pro-inflammatory cytokine IL-17 [120-122] that is also regulated by NFκB [123].
Zinc is an essential trace element and plays an important role in the activation of many enzymes involved in normal development and function of the immune system; therefore, zinc deficiency can cause growth retardation and decrease many cellular immune responses [124]. Zinc deficiency decreases Th1 cytokines, resulting in the shift from Th1 to Th2, and causing a severe cell-mediated dysfunction [125,126]. Zinc-deficient CTCL Hut-78 cells displayed decreased phosphorylation of IKK and IκB, resulting in the reduced DNA binding of NFκB [127-129].
Anti-inflammatory role of NFκB
Although the role of NFκB in the transcriptional regulation of pro-inflammatory genes has been well established, recent studies have indicated that NFκB has an important anti-inflammatory function as well [130,131]. In the later stages of CTCL, there is a gradual increase in malignant CD4 cells releasing the immunosuppressive cytokines IL-4, IL-10 and TGFβ [132-134]. Increased expression of these cytokines correlates with disease progression, immunosuppression, and susceptibility to infection [134-138].
Regulation of expression of IL-4, IL-10 and TGFβ is complex, and is controlled by several transcription factors and regulators, including NFκB [139-143]. In vitro study in CTCL Hut-78 cells has indicated that the proximal NFκB binding site in IL-10 promoter is regulated predominantly by p50/50 homodimers that activate IL-10 transcription [141]. The IL-10 regulation by p50/50 homodimers was later confirmed by analysis of NFκB proteins recruited to the IL-10 promoter in murine macrophages [142]. This study showed that p50/50 homodimers activate IL-10 transcription, together with the transcriptional co-activator CREB-binding protein [142]. These data suggest that the p50/p50 homodimers might exert their immuno-suppressory function either by inhibiting transcription of NFκB-dependent pro-inflammatory genes, or by stimulating transcription of anti-inflammatory genes, such as IL-10.
Recent studies from our laboratory have indicated that the human TGFβ promoter is occupied predominantly by p65/p50 heterodimers in Hut-78 cells (Figure 3). In addition, the nuclear IκBα that is induced by proteasome inhibition by BZ significantly decreases this occupancy, resulting in the inhibition of TGFβ expression (Figure 3). These results indicate that proteasome inhibition has two beneficial effects in CTCL cells (Figure 4). It induces nuclear accumulation of IκBα, which inhibits expression of NFκB p65/p50-regulated anti-apoptotic genes, resulting in the increased apoptosis of CTCL cells [5]. In addition, the BZ-induced nuclear IκBα inhibits expression of TGFβ, which may decrease the immunosuppressive phenotype associated with the advanced stages of CTCL (Figure 4).
Figure 3.
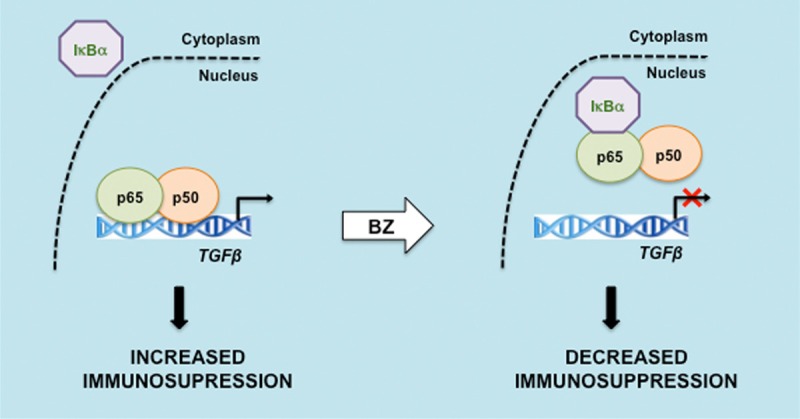
Proposed model of TGFβ regulation by NFκB and proteasome inhibition in CTCL cells. In CTCL Hut-78 cells, the promoter of TGFβ is occupied predominantly by NFκB p65/p50 homodimers. Proteasome inhibition induces the nuclear accumulation of IκBα, resulting in p65/p50 removal from TGFβ promoter, and inhibition of the TGFβ expression [Chang et al, manuscript in preparation].
Figure 4.
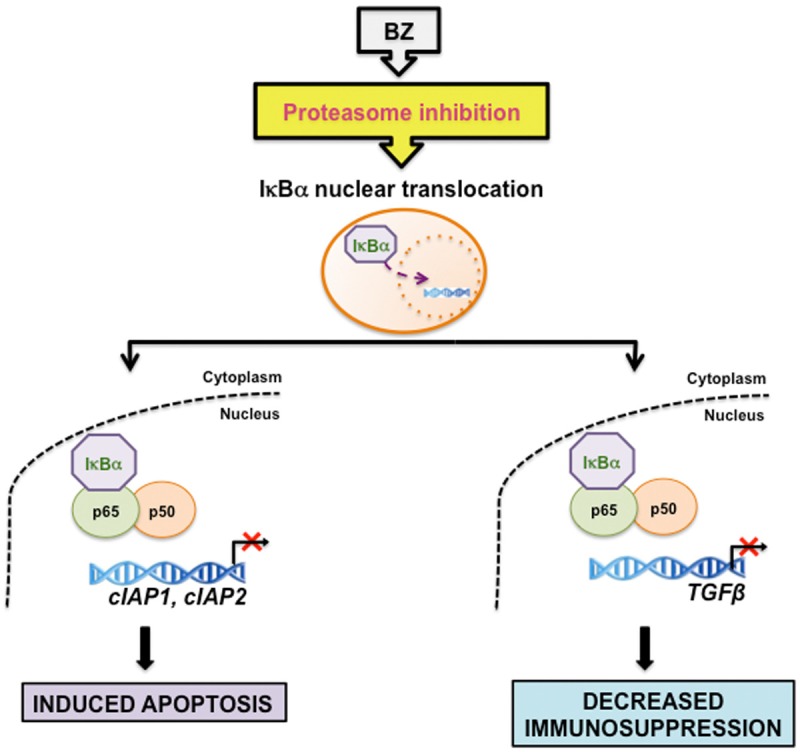
Proposed model of the regulation of NFκB-dependent genes by proteasome inhibition in CTCL cells. Proteasome inhibition by BZ induces the nuclear translocation and accumulation of IκBα, which inhibits expression of NFκB p65/p50-regulated anti-apoptotic genes cIAP1 and cIAP2, resulting in the increased apoptosis of CTCL cells. In addition, the BZ-induced nuclear IκBα inhibits expression of TGFβ, which may decrease the immunosuppressive phenotype associated with advanced stages of CTCL.
Conclusion
The high constitutive NFκB activity in CTCL cells is responsible for their increased survival and proliferation, as well as for the increased expression of NFκB-dependent pro-inflammatory and anti-inflammatory cytokines. However, while the mechanisms regulating NFκB-dependent transcription of anti-apoptotic and pro-inflammatory genes have been studies extensively, the mechanisms of how NFκB regulates transcription of immuno-suppressory genes remain largely elusive. The specificity of NFκB binding to the individual promoters is determined by the subunit composition of NFκB complexes, their post-translational modifications, and interactions with other transcriptional factors and regulators. Understanding the mechanisms responsible for the NFκB regulation of immunosuppressive genes may provide new strategy for the treatment of CTCL and other disorders characterized by high levels of NFκB activity and immunosuppressive gene expression.
Acknowledgements
This work was supported in part by NIH grants AI085497 and CA173452 to I.V.
References
- 1.DiDonato JA, Mercurio F, Karin M. NFκB and the link between inflammation and cancer. Immunol Rev. 2012;246:379–400. doi: 10.1111/j.1600-065X.2012.01099.x. [DOI] [PubMed] [Google Scholar]
- 2.Yamamoto Y, Gaynor RB. Therapeutic potential of inhibition of the NFκB pathway in the treatment of inflammation and cancer. J Clin Invest. 2001;107:135–42. doi: 10.1172/JCI11914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–44. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 4.Chaturvedi MM, Sung B, Yadav VR, Kannappan R, Aggarwal BB. NFκB addiction and its role in cancer: ‘one size does not fit all’. Oncogene. 2011;30:1615–30. doi: 10.1038/onc.2010.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Juvekar A, Manna S, Ramaswami S, Chang TP, Vu HY, Ghosh CC, Celiker MY, Vancurova I. Bortezomib induces nuclear translocation of IκBα resulting in gene-specific suppression of NFΚB-dependent transcription and induction of apoptosis in CTCL. Mol Cancer Res. 2011;9:183–94. doi: 10.1158/1541-7786.MCR-10-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aggarwal BB. NFκB: the enemy within. Cancer Cell. 2004;6:203–8. doi: 10.1016/j.ccr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Berger CL, Edelson R. The life cycle of cutaneous T cell lymphoma reveals opportunities for targeted drug therapy. Curr Cancer Drug Targets. 2004;4:609–19. doi: 10.2174/1568009043332808. [DOI] [PubMed] [Google Scholar]
- 8.Berger CL, Tigelaar R, Cohen J, Mariwalla K, Trinh J, Wang N, Edelson RL. Cutaneous T-cell lymphoma: malignant proliferation of T-regulatory cells. Blood. 2005;105:1640–7. doi: 10.1182/blood-2004-06-2181. [DOI] [PubMed] [Google Scholar]
- 9.Guitart J. What’s new in cutaneous T-cell lymphomas: 2006. Semin Cutan Med Surg. 2006;25:87–90. doi: 10.1016/j.sder.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 10.Diamandidou E, Cohen PR, Kurzrock R. Mycosis fungoides and Sezary syndrome. Blood. 1996;88:2385–409. [PubMed] [Google Scholar]
- 11.Siegel RS, Kuzel TM. Cutaneous T-cell lymphoma/leukemia. Curr Treat Options Oncol. 2000;1:43–50. doi: 10.1007/s11864-000-0014-0. [DOI] [PubMed] [Google Scholar]
- 12.Willemze R, Jaffe ES, Burg G, Cerroni L, Berti E, Swerdlow SH, Ralfkiaer E, Chimenti S, Diaz-Perez JL, Duncan LM, Grange F, Harris NL, Kempf W, Kerl H, Kurrer M, Knobler R, Pimpinelli N, Sander C, Santucci M, Sterry W, Vermeer MH, Wechsler J, Whittaker S, Meijer CJ. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768–85. doi: 10.1182/blood-2004-09-3502. [DOI] [PubMed] [Google Scholar]
- 13.Querfeld C, Rosen ST, Guitart J, Kuzel TM. The spectrum of cutaneous T-cell lymphomas: new insights into biology and therapy. Curr Opin Hematol. 2005;12:273–8. doi: 10.1097/01.moh.0000166498.64515.03. [DOI] [PubMed] [Google Scholar]
- 14.Gardner JM, Evans KG, Musiek A, Rook AH, Kim EJ. Update on treatment of cutaneous T-cell lymphoma. Curr Opin Oncol. 2009;21:131–7. doi: 10.1097/CCO.0b013e3283253190. [DOI] [PubMed] [Google Scholar]
- 15.Sors A, Jean-Louis F, Pellet C, Laroche L, Dubertret L, Courtois G, Bachelez H, Michel L. Down-regulating constitutive activation of the NFκB canonical pathway overcomes the resistance of cutaneous T-cell lymphoma to apoptosis. Blood. 2006;107:2354–63. doi: 10.1182/blood-2005-06-2536. [DOI] [PubMed] [Google Scholar]
- 16.Sors A, Jean-Louis F, Bégué E, Parmentier L, Dubertret L, Dreano M, Courtois G, Bachelez H, Michel L. Inhibition of IκB kinase subunit 2 in cutaneous T-cell lymphoma down-regulates NFκB constitutive activation, induces cell death, and potentiates the apoptotic response to antineoplastic chemotherapeutic agents. Clin Cancer Res. 2008;14:901–11. doi: 10.1158/1078-0432.CCR-07-1419. [DOI] [PubMed] [Google Scholar]
- 17.Kiessling MK, Klemke CD, Kaminski MM, Galani IE, Krammer PH, Gülow K. Inhibition of constitutively activated NFκB induces reactive oxygen species- and iron-dependent cell death in cutaneous T-cell lymphoma. Cancer Res. 2009;69:2365–74. doi: 10.1158/0008-5472.CAN-08-3221. [DOI] [PubMed] [Google Scholar]
- 18.Saed G, Fivenson DP, Naidu Y, Nickoloff BJ. Mycosis fungoides exhibits a Th1-type cell-mediated cytokine profile whereas Sezary syndrome expresses a Th2-type profile. J Invest Dermatol. 1994;103:29–33. doi: 10.1111/1523-1747.ep12388985. [DOI] [PubMed] [Google Scholar]
- 19.Chong BF, Wilson AJ, Gibson HM, Hafner MS, Luo Y, Hedgcock CJ, Wong HK. Immune function abnormalities in peripheral blood mononuclear cell cytokine expression differentiates stages of cutaneous T-cell lymphoma/mycosis fungoides. Clin Cancer Res. 2008;14:646–53. doi: 10.1158/1078-0432.CCR-07-0610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krejsgaard T, Odum N, Geisler C, Wasik MA, Woetmann A. Regulatory T cells and immunodeficiency in mycosis fungoides and Sezary syndrome. Leukemia. 2012;26:424–32. doi: 10.1038/leu.2011.237. [DOI] [PubMed] [Google Scholar]
- 21.Kim EJ, Hess S, Richardson SK, Newton S, Showe LC, Benoit BM, Ubriani R, Vittorio CC, Junkins-Hopkins JM, Wysocka M, Rook AH. Immunopathogenesis and therapy of cutaneous T cell lymphoma. J Clin Invest. 2005;115:798–812. doi: 10.1172/JCI24826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu XS, Lonsdorf AS, Hwang ST. Cutaneous T-cell lymphoma: roles for chemokines and chemokine receptors. J Invest Dermatol. 2009;129:1115–9. doi: 10.1038/jid.2009.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li JY, Horwitz S, Moskowitz A, Myskowski PL, Pulitzer M, Querfeld C. Management of cutaneous T cell lymphoma: new and emerging targets and treatment options. Cancer Manag Res. 2012;4:75–89. doi: 10.2147/CMAR.S9660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oeckinghaus A, Ghosh S. The NFκB family of transcription factors and its regulation. Cold Spring Harb Perspect Biol. 2009;1:a000034. doi: 10.1101/cshperspect.a000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghosh G, van Duyne G, Ghosh S, Sigler PB. Structure of NFκB p50 homodimer bound to a κB site. Nature. 1995;373:303–10. doi: 10.1038/373303a0. [DOI] [PubMed] [Google Scholar]
- 26.Chen FE, Huang DB, Chen YQ, Ghosh G. Crystal structure of p50/p65 heterodimer of transcription factor NFκB bound to DNA. Nature. 1998;391:410–3. doi: 10.1038/34956. [DOI] [PubMed] [Google Scholar]
- 27.Hoffmann A, Leung TH, Baltimore D. Genetic analysis of NFκB/Rel transcription factors defines functional specificities. EMBO J. 2003;22:5530–9. doi: 10.1093/emboj/cdg534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoffmann A, Natoli G, Ghosh G. Transcriptional regulation via the NFκB signaling module. Oncogene. 2006;25:6706–16. doi: 10.1038/sj.onc.1209933. [DOI] [PubMed] [Google Scholar]
- 29.Hayden MS, Ghosh S. NFκB, the first quarter-century: remarkable progress and outstanding questions. Genes Dev. 2012;26:203–34. doi: 10.1101/gad.183434.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fujita T, Nolan GP, Ghosh S, Baltimore D. Independent modes of transcriptional activation by the p50 and p65 subunits of NFκB. Genes Dev. 1992;6:775–87. doi: 10.1101/gad.6.5.775. [DOI] [PubMed] [Google Scholar]
- 31.Wan F, Lenardo MJ. Specification of DNA binding activity of NFκB proteins. Cold Spring Harb Perspect Biol. 2009;1:a000067. doi: 10.1101/cshperspect.a000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hayden MS, Ghosh S. Signaling to NFκB. Genes Dev. 2004;18:2195–224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 33.Bonizzi G, Karin M. The two NFκB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004;25:280–8. doi: 10.1016/j.it.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 34.Hayden MS, Ghosh S. Shared principles in NFκB signaling. Cell. 2008;132:344–62. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 35.Sun SC. Non-canonical NFκB signaling pathway. Cell Res. 2011;21:71–85. doi: 10.1038/cr.2010.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun SC. The non-canonical NFκB pathway. Immunol Rev. 2012;246:125–40. doi: 10.1111/j.1600-065X.2011.01088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Henkel T, Machleidt T, Alkalay I, Krönke M, Ben-Neriah Y, Baeuerle PA. Rapid proteolysis of IκBα is necessary for activation of transcription factor NFκB. Nature. 1993;365:182–5. doi: 10.1038/365182a0. [DOI] [PubMed] [Google Scholar]
- 38.Palombella VJ, Rando OJ, Goldberg AL, Maniatis T. The ubiquitin-proteasome pathway is required for processing the NFκB1 precursor protein and the activation of NFκB. Cell. 1994;78:773–85. doi: 10.1016/s0092-8674(94)90482-0. [DOI] [PubMed] [Google Scholar]
- 39.Wulczyn FG, Naumann M, Scheidereit C. Candidate proto-oncogene bcl-3 encodes a subunit- specific inhibitor of transcription factor NFκB. Nature. 1992;358:597–9. doi: 10.1038/358597a0. [DOI] [PubMed] [Google Scholar]
- 40.Bours V, Franzoso G, Azarenko V, Park S, Kanno T, Brown K, Siebenlist U. The oncoprotein Bcl-3 directly transactivates through κB motifs via association with DNA-binding p50B homodimers. Cell. 1993;72:729–39. doi: 10.1016/0092-8674(93)90401-b. [DOI] [PubMed] [Google Scholar]
- 41.Dobrzanski P, Ryseck RP, Bravo R. Differential interactions of Rel-NFκB complexes with IκB determine pools of constitutive and inducible NFκB activity. EMBO J. 1994;13:4608–16. doi: 10.1002/j.1460-2075.1994.tb06782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baldwin AS Jr. The NFκB and IκB proteins: New discoveries and insights. Annu Rev Immunol. 1996;14:649–83. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 43.Cheng JD, Ryseck RP, Attar RM, Dambach D, Bravo R. Functional redundancy of the NFκB inhibitors IκBβ and IκBα. J Exp Med. 1998;188:1055–62. doi: 10.1084/jem.188.6.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huxford T, Huang DB, Malek S, Ghosh G. The crystal structure of the IκB/NFκB complex reveals mechanisms of NFκB inactivation. Cell. 1998;95:759–70. doi: 10.1016/s0092-8674(00)81699-2. [DOI] [PubMed] [Google Scholar]
- 45.Tam WF, Sen R. IκB family members function by different mechanisms. J Biol Chem. 2001;276:7701–4. doi: 10.1074/jbc.C000916200. [DOI] [PubMed] [Google Scholar]
- 46.Hoffmann A, Levchenko A, Scott ML, Baltimore D. The IκB-NFκB signaling module: Temporal control and selective gene activation. Science. 2001;298:1241–5. doi: 10.1126/science.1071914. [DOI] [PubMed] [Google Scholar]
- 47.Anest V, Hanson JL, Cogswell PC, Steinbrecher KA, Strahl BD, Baldwin AS. A nucleosomal function for IκB kinase-α in NFκB-dependent gene expression. Nature. 2003;423:659–63. doi: 10.1038/nature01648. [DOI] [PubMed] [Google Scholar]
- 48.Schuster M, Annemann M, Plaza-Sirvent C, Schmitz I. Atypical IκB proteins - nuclear modulators of NFκB signaling. Cell Commun Signal. 2013;11:23. doi: 10.1186/1478-811X-11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: The control of NFκB activity. Annu Rev Immunol. 2000;18:621–63. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 50.Chen LF, Mu Y, Greene WC. Acetylation of RelA at discrete sites regulates distinct nuclear functions of NFκB. EMBO J. 2002;21:6539–48. doi: 10.1093/emboj/cdf660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xiao W. Advances in NFκB signaling transduction and transcription. Cell Mol Immunol. 2004;1:425–35. [PubMed] [Google Scholar]
- 52.Chen LF, Williams SA, Mu Y, Nakano H, Duerr JM, Buckbinder L, Greene WC. NFκB RelA phosphorylation regulates RelA acetylation. Mol Cell Biol. 2005;25:7966–75. doi: 10.1128/MCB.25.18.7966-7975.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perkins ND. Post-translational modifications regulating the activity and function of the NFκB pathway. Oncogene. 2006;25:6717–30. doi: 10.1038/sj.onc.1209937. [DOI] [PubMed] [Google Scholar]
- 54.Huang B, Yang XD, Lamb A, Chen LF. Posttranslational modifications of NFκB: another layer of regulation for NFκB signaling pathway. Cell Signal. 2010;2:1282–90. doi: 10.1016/j.cellsig.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thanos D, Maniatis T. NFκB: a lesson in family values. Cell. 1995;80:529–32. doi: 10.1016/0092-8674(95)90506-5. [DOI] [PubMed] [Google Scholar]
- 56.Verma IM, Stevenson JK, Schwarz EM, Van Antwerp D, Miyamoto S. Rel/NFκB/IκB family: intimate tales of association and dissociation. Genes Dev. 1995;9:2723–35. doi: 10.1101/gad.9.22.2723. [DOI] [PubMed] [Google Scholar]
- 57.O’Dea E, Hoffmann A. The regulatory logic of the NFκB signaling system. Cold Spring Harb Perspect Biol. 2010;2:a000216. doi: 10.1101/cshperspect.a000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smale ST. Dimer-specific regulatory mechanisms within the NFκB family of transcription factors. Immunol Rev. 2012;246:193–204. doi: 10.1111/j.1600-065X.2011.01091.x. [DOI] [PubMed] [Google Scholar]
- 59.Mathas S, Jöhrens K, Joos S, Lietz A, Hummel F, Janz M, Jundt F, Anagnostopoulos I, Bommert K, Lichter P, Stein H, Scheidereit C, Dörken B. Elevated NFκB p50 complex formation and Bcl-3 expression in classical Hodgkin, anaplastic large-cell, and other peripheral T-cell lymphomas. Blood. 2005;106:4287–93. doi: 10.1182/blood-2004-09-3620. [DOI] [PubMed] [Google Scholar]
- 60.Beg AA, Baltimore D. An essential role for NFκB in preventing TNF-α-induced cell death. Science. 1996;274:782–4. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 61.Grossmann M, O’Reilly LA, Gugasyan R, Strasser A, Adams JM, Gerondakis S. The anti-apoptotic activities of Rel and RelA required during B-cell maturation involve the regulation of Bcl-2 expression. EMBO J. 2000;19:6351–60. doi: 10.1093/emboj/19.23.6351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Joyce D, Albanese C, Steer J, Fu M, Bouzahzah B, Pestell RG. NFκB and cell-cycle regulation: The cyclin connection. Cytokine Growth Factor Rev. 2001;12:73–90. doi: 10.1016/s1359-6101(00)00018-6. [DOI] [PubMed] [Google Scholar]
- 63.Huang S, Pettaway CA, Uehara H, Bucana CD, Fidler IJ. Blockade of NFκB activity in human prostate cancer cells is associated with suppression of angiogenesis, invasion, and metastasis. Oncogene. 2001;20:4188–97. doi: 10.1038/sj.onc.1204535. [DOI] [PubMed] [Google Scholar]
- 64.Lin A, Karin M. NFκB in cancer: A marked target. Semin Cancer Biol. 2003;3:107–14. doi: 10.1016/s1044-579x(02)00128-1. [DOI] [PubMed] [Google Scholar]
- 65.Gilmore TD. The Rel/NFκB/IκB signal transduction pathway and cancer. Cancer Treat Res. 2003;115:241–65. [PubMed] [Google Scholar]
- 66.Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF, Karin M. IKKβ links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–96. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 67.Karin M. NFκB in cancer development and progression. Nature. 2006;441:431–6. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 68.Ghosh S, May MJ, Kopp EB. NFκB and Rel proteins: Evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–60. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 69.Hayden MS, West AP, Ghosh S. NFκB and the immune response. Oncogene. 2006;25:6758–80. doi: 10.1038/sj.onc.1209943. [DOI] [PubMed] [Google Scholar]
- 70.Karin M. NFκB as a critical link between inflammation and cancer. Cold Spring Harb Perspect Biol. 2009;1:a000141. doi: 10.1101/cshperspect.a000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lawrence T, Fong C. The resolution of inflammation: anti-inflammatory roles for NFκB. Int J Biochem Cell Biol. 2010;42:519–23. doi: 10.1016/j.biocel.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 72.Balkwill F, Mantovani A. Inflammation and cancer: Back to Virchow? Lancet. 2001;357:539–45. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 73.Araki S, Omori Y, Lyn D, Singh RK, Meinbach DM, Sandman Y, Lokeshwar VB, Lokeshwar BL. Interleukin-8 is a molecular determinant of androgen independence and progression in prostate cancer. Cancer Res. 2007;67:6854–62. doi: 10.1158/0008-5472.CAN-07-1162. [DOI] [PubMed] [Google Scholar]
- 74.Lawrence T, Bebien M. IKKα in the regulation of inflammation and adaptive immunity. Biochem Soc Trans. 2007;35:270–2. doi: 10.1042/BST0350270. [DOI] [PubMed] [Google Scholar]
- 75.Lawrence T. The nuclear factor NFκB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1:a001651. doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Qin JZ, Nestle FO, Häffner A, Dummer R, Burg G, Döbbeling U. Cutaneous T cell lymphoma cells contain constitutive NFκB complexes. J Invest Derm. 1997;108:225. [Google Scholar]
- 77.Döbbeling U. Transcription factor profiling shows new ways towards new treatment options of cutaneous T cell lymphomas. Curr Drug Discov Technol. 2007;4:24–30. doi: 10.2174/157016307781115467. [DOI] [PubMed] [Google Scholar]
- 78.Rayet B, Gélinas C. Aberrant rel/NFκB genes and activity in human cancer. Oncogene. 1999;18:6938–47. doi: 10.1038/sj.onc.1203221. [DOI] [PubMed] [Google Scholar]
- 79.Trecca D, Guerrini L, Fracchiolla NS, Pomati M, Baldini L, Maiolo AT, Neri A. Identification of a tumor-associated mutant form of the NFκB RelA gene with reduced DNA-binding and transactivating activities. Oncogene. 1997;14:791–9. doi: 10.1038/sj.onc.1200895. [DOI] [PubMed] [Google Scholar]
- 80.Ferrier R, Nougarede R, Doucet S, Kahn-Perles B, Imbert J, Mathieu-Mahul D. Physical interaction of the bHLH LYL1 protein and NFκB1 p105. Oncogene. 1999;18:995–1005. doi: 10.1038/sj.onc.1202374. [DOI] [PubMed] [Google Scholar]
- 81.Epinat JC, Kazandjian D, Harkness DD, Petros S, Dave J, White DW, Gilmore TD. Mutant envelope residues confer a transactivation function onto N-terminal sequences of the v-Rel oncoprotein. Oncogene. 2000;19:599–607. doi: 10.1038/sj.onc.1203376. [DOI] [PubMed] [Google Scholar]
- 82.Neri A, Fracchiolla NS, Roscetti E, Garatti S, Trecca D, Boletini A, Perletti L, Baldini L, Maiolo AT, Berti E. Molecular analysis of cutaneous B- and T-cell lymphomas. Blood. 1995;86:3160–72. [PubMed] [Google Scholar]
- 83.Migliazza A, Lombardi L, Rocchi M, Trecca D, Chang CC, Antonacci R, Fracchiolla NS, Ciana P, Maiolo AT, Neri A. Heterogeneous chromosomal aberrations generate 3’ truncations of the NFκB2/lyt-10 gene in lymphoid malignancies. Blood. 1994;84:3850–60. [PubMed] [Google Scholar]
- 84.Zhang J, Chang CC, Lombardi L, Dalla-Favera R. Rearranged NFκB2 gene in the HUT78 T-lymphoma cell line codes for a constitutively nuclear factor lacking transcriptional repressor functions. Oncogene. 1994;9:1931–7. [PubMed] [Google Scholar]
- 85.Thakur S, Lin HC, Tseng WT, Kumar S, Bravo R, Foss F, Gélinas C, Rabson AB. Rearrangement and altered expression of the NFκB-2 gene in human cutaneous T-lymphoma cells. Oncogene. 1994;9:2335–44. [PubMed] [Google Scholar]
- 86.Keutgens A, Robert I, Viatour P, Chariot A. Deregulated NFκB activity in haematological malignancies. Biochem Pharmacol. 2006;72:1069–80. doi: 10.1016/j.bcp.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 87.Robert I, Aussems M, Keutgens A, Zhang X, Hennuy B, Viatour P, Vanstraelen G, Merville MP, Chapelle JP, de Leval L, Lambert F, Dejardin E, Gothot A, Chariot A. Matrix metalloproteinase-9 gene induction by a truncated oncogenic NFκB2 protein involves the recruitment of MLL1 and MLL2 H3K4 histone methyltransferase complexes. Oncogene. 2009;28:1626–38. doi: 10.1038/onc.2009.6. [DOI] [PubMed] [Google Scholar]
- 88.Döbbeling U, Qin JZ, Dummer R, Burg G. Suppressors of constitutive NFκB activities in CTCL cells induce apoptosis. Arch Dermatol Res. 1998;290:58. [Google Scholar]
- 89.Izban KF, Ergin M, Qin JZ, Martinez RL, Pooley RJ JR, Saeed S, Alkan S. Constitutive expression of NFκB is a characteristic feature of mycosis fungoides: implications for apoptosis resistance and pathogenesis. Hum Pathol. 2000;31:1482–90. doi: 10.1053/hupa.2000.20370. [DOI] [PubMed] [Google Scholar]
- 90.Van Kester MS, Borg MK, Zoutman WH, Out-Luiting JJ, Jansen PM, Dreef EJ, Vermeer MH, van Doorn R, Willemze R, Tensen CP. A meta-analysis of gene expression data identifies a molecular signature characteristic for tumor-stage mycosis fungoides. J Invest Dermatol. 2012;132:2050–9. doi: 10.1038/jid.2012.117. [DOI] [PubMed] [Google Scholar]
- 91.Garatti SA, Roscetti E, Trecca D, Fracchiolla NS, Neri A, Berti E. bcl-1, bcl-2, p53, c-myc, and lyt-10 analysis in cutaneous lymphomas. Recent Results Cancer Res. 1995;139:249–61. doi: 10.1007/978-3-642-78771-3_19. [DOI] [PubMed] [Google Scholar]
- 92.Wu J, Wood GS. Reduction of Fas/CD95 promoter methylation, upregulation of Fas protein, and enhancement of sensitivity to apoptosis in cutaneous T-cell lymphoma. Arch Dermatol. 2011;147:443–9. doi: 10.1001/archdermatol.2010.376. [DOI] [PubMed] [Google Scholar]
- 93.Kamstrup MR, Gjerdrum LM, Biskup E, Lauenborg BT, Ralfkiaer E, Woetmann A, Ødum N, Gniadecki R. Notch1 as a potential therapeutic target in cutaneous T-cell lymphoma. Blood. 2010;116:2504–12. doi: 10.1182/blood-2009-12-260216. [DOI] [PubMed] [Google Scholar]
- 94.Tun-Kyi A, Qin JZ, Oberholzer PA, Navarini AA, Hassel JC, Dummer R, Döbbeling U. Arsenic trioxide down-regulates antiapoptotic genes and induces cell death in mycosis fungoides tumors in a mouse model. Ann Oncol. 2008;19:1488–94. doi: 10.1093/annonc/mdn056. [DOI] [PubMed] [Google Scholar]
- 95.Zisterer DM, Campiani G, Nacci V, Williams DC. Pyrrolo-1, 5-benzoxazepines induce apoptosis in HL-60, Jurkat, and Hut-78 cells: a new class of apoptotic agents. J Pharmacol Exp Ther. 2000;293:48–59. [PubMed] [Google Scholar]
- 96.Rishi L, Dhiman R, Raje M, Majumdar S. Nitric oxide induces apoptosis in cutaneous T cell lymphoma (HuT-78) by downregulating constitutive NFκB. Biochim Biophys Acta. 2007;1770:1230–9. doi: 10.1016/j.bbagen.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 97.Braun FK, Al-Yacoub N, Plötz M, Möbs M, Sterry W, Eberle J. Nonsteroidal anti-inflammatory drugs induce apoptosis in cutaneous T-cell lymphoma cells and enhance their sensitivity for TNF-related apoptosis-inducing ligand. J Invest Dermatol. 2012;132:429–39. doi: 10.1038/jid.2011.316. [DOI] [PubMed] [Google Scholar]
- 98.Sreenivasan Y, Sarkar A, Manna SK. Mechanism of cytosine arabinoside-mediated apoptosis: role of Rel A (p65) dephosphorylation. Oncogene. 2003;22:4356–69. doi: 10.1038/sj.onc.1206486. [DOI] [PubMed] [Google Scholar]
- 99.Zhang C, Li B, Zhang X, Hazarika P, Aggarwal BB, Duvic M. Curcumin selectively induces apoptosis in cutaneous T-cell lymphoma cell lines and patients’ PBMCs: potential role for STAT-3 and NFκB signaling. J Invest Dermatol. 2010;130:2110–9. doi: 10.1038/jid.2010.86. [DOI] [PubMed] [Google Scholar]
- 100.Khan MA, Gahlot S, Majumdar S. Oxidative stress induced by curcumin promotes the death of cutaneous T-cell lymphoma (HuT-78) by disrupting the function of several molecular targets. Mol Cancer Ther. 2012;11:1873–83. doi: 10.1158/1535-7163.MCT-12-0141. [DOI] [PubMed] [Google Scholar]
- 101.Krejsgaard T, Vetter-Kauczok CS, Woetmann A, Kneitz H, Eriksen KW, Lovato P, Zhang Q, Wasik MA, Geisler C, Ralfkiaer E, Becker JC, Ødum N. Ectopic expression of B-lymphoid kinase in cutaneous T-cell lymphoma. Blood. 2009;113:5896–904. doi: 10.1182/blood-2008-09-181024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zinzani PL, Musuraca G, Tani M, Stefoni V, Marchi E, Fina M, Pellegrini C, Alinari L, Derenzini E, de Vivo A, Sabattini E, Pileri S, Baccarani M. Phase II trial of proteasome inhibitor bortezomib in patients with relapsed or refractory cutaneous T-cell lymphoma. J. Clin. Oncol. 2007;25:4293–7. doi: 10.1200/JCO.2007.11.4207. [DOI] [PubMed] [Google Scholar]
- 103.Horwitz SM. Novel therapies for cutaneous T-cell lymphomas. Clin Lymphoma Myeloma. 2008;8(Suppl 5):S187–92. doi: 10.3816/CLM.2008.s.015. [DOI] [PubMed] [Google Scholar]
- 104.Kim SJ, Yoon DH, Kang HJ, Kim JS, Park SK, Kim HJ, Lee J, Ryoo BY, Ko YH, Huh J, Yang WI, Kim HK, Min SK, Lee SS, Do IG, Suh C, Kim WS Consortium for Improving Survival of Lymphoma (CISL) investigators. Bortezomib in combination with CHOP as first-line treatment for patients with stage III/IV peripheral T-cell lymphomas: a multicentre, single-arm, phase 2 trial. Eur J Cancer. 2012;48:3223–31. doi: 10.1016/j.ejca.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 105.Biskup E, Kamstrup MR, Manfé V, Gniadecki R. Proteasome inhibition as a novel mechanism of the proapoptotic activity of γ-secretase inhibitor I in cutaneous T-cell lymphoma. Br J Dermatol. 2013;168:504–12. doi: 10.1111/bjd.12071. [DOI] [PubMed] [Google Scholar]
- 106.Vu HY, Juvekar A, Ghosh C, Ramaswami S, Le DH, Vancurova I. Proteasome inhibitors induce apoptosis of prostate cancer cells by inducing nuclear translocation of IκBα. Arch Biochem Biophys. 2008;475:156–63. doi: 10.1016/j.abb.2008.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Manna S, Singha B, Phyo SA, Gatla HR, Chang TP, Sanacora S, Ramaswami S, Vancurova I. Proteasome inhibition by bortezomib increases IL-8 expression in androgen-independent prostate cancer cells: The role of IKKα. J Immunol. 2013;191:2837–46. doi: 10.4049/jimmunol.1300895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gasparini C, Feldmann M. NFκB as a target for modulating inflammatory responses. Curr Pharm Des. 2012;18:5735–45. doi: 10.2174/138161212803530763. [DOI] [PubMed] [Google Scholar]
- 109.Vallabhapurapu S, Karin M. Regulation and function of NFκB transcription factors in the immune system. Annu Rev Immunol. 2009;27:693–733. doi: 10.1146/annurev.immunol.021908.132641. [DOI] [PubMed] [Google Scholar]
- 110.Hayden MS, Ghosh S. NFκB in immunobiology. Cell Res. 2011;21:223–44. doi: 10.1038/cr.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ghosh S, Karin M. Missing pieces in the NFκB puzzle. Cell. 2002;109(Suppl):S81–96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- 112.Karin M, Yamamoto Y, Wang QM. The IKK NFκB system: a treasure trove for drug development. Nat Rev Drug Discov. 2004;3:17–26. doi: 10.1038/nrd1279. [DOI] [PubMed] [Google Scholar]
- 113.Cleere R, Long A, Kelleher D, O’Neill LA. Autocrine regulation of the transcription factor NFκB by TNFα in the human T cell lymphoma line Hut 78. Biochem Soc Trans. 1995;23:113S. doi: 10.1042/bst023113s. [DOI] [PubMed] [Google Scholar]
- 114.O’Connell MA, Cleere R, Long A, O’Neill LA, Kelleher D. Cellular proliferation and activation of NFκB are induced by autocrine production of TNFα in the human T lymphoma line HuT 78. J Biol Chem. 1995;270:7399–404. doi: 10.1074/jbc.270.13.7399. [DOI] [PubMed] [Google Scholar]
- 115.Liu RY, Fan C, Liu G, Olashaw NE, Zuckerman KS. Activation of p38 mitogen-activated protein kinase is required for TNFα-supported proliferation of leukemia and lymphoma cell lines. J Biol Chem. 2000;275:21086–93. doi: 10.1074/jbc.M001281200. [DOI] [PubMed] [Google Scholar]
- 116.Giri DK, Aggarwal BB. Constitutive activation of NFκB causes resistance to apoptosis in human cutaneous T cell lymphoma HuT-78 cells. Autocrine role of tumor necrosis factor and reactive oxygen intermediates. J Biol Chem. 1998;273:14008–14. doi: 10.1074/jbc.273.22.14008. [DOI] [PubMed] [Google Scholar]
- 117.Wismer JM, McKenzie RC, Sauder DN. Interleukin-8 immunoreactivity in epidermis of cutaneous T-cell lymphoma patients. Lymphokine Cytokine Res. 1994;13:21–7. [PubMed] [Google Scholar]
- 118.Hansen ER, Vejlsgaard GL, Lisby S, Heidenheim M, Baadsgaard O. Epidermal IL-1 functional activity and IL-8 immunoreactivity are increased in patients with cutaneous T-cell lymphoma. J Invest Dermatol. 1991;97:818–23. doi: 10.1111/1523-1747.ep12489011. [DOI] [PubMed] [Google Scholar]
- 119.Tron VA, Rosenthal D, Sauder DN. Epidermal interleukin-1 is increased in cutaneous T-cell lymphoma. J Invest Dermatol. 1988;90:378–81. doi: 10.1111/1523-1747.ep12456433. [DOI] [PubMed] [Google Scholar]
- 120.Krejsgaard T, Litvinov IV, Wang Y, Xia L, Willerslev-Olsen A, Koralov SB, Kopp KL, Bonefeld CM, Wasik MA, Geisler C, Woetmann A, Zhou Y, Sasseville D, Odum N. Elucidating the role of interleukin-17F in cutaneous T-cell lymphoma. Blood. 2013 Aug 8;122:943–50. doi: 10.1182/blood-2013-01-480889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Krejsgaard T, Ralfkiaer U, Clasen-Linde E, Eriksen KW, Kopp KL, Bonefeld CM, Geisler C, Dabelsteen S, Wasik MA, Ralfkiaer E, Woetmann A, Odum N. Malignant cutaneous T-cell lymphoma cells express IL-17 utilizing the Jak3/Stat3 signaling pathway. J Invest Dermatol. 2011;131:1331–8. doi: 10.1038/jid.2011.27. [DOI] [PubMed] [Google Scholar]
- 122.Chong BF, Wilson AJ, Gibson HM, Hafner MS, Luo Y, Hedgcock CJ, Wong HK. Immune function abnormalities in peripheral blood mononuclear cell cytokine expression differentiates stages of cutaneous T-cell lymphoma/mycosis fungoides. Clin Cancer Res. 2008;14:646–53. doi: 10.1158/1078-0432.CCR-07-0610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cho ML, Kang JW, Moon YM, Nam HJ, Jhun JY, Heo SB, Jin HT, Min SY, Ju JH, Park KS, Cho YG, Yoon CH, Park SH, Sung YC, Kim HY. STAT3 and NFκB signal pathway is required for IL-23-mediated IL-17 production in spontaneous arthritis animal model IL-1 receptor antagonist-deficient mice. J Immunol. 2006;176:5652–61. doi: 10.4049/jimmunol.176.9.5652. [DOI] [PubMed] [Google Scholar]
- 124.Salgueiro MJ, Zubillaga M, Lysionek A, Cremaschi G, Goldman CG, Caro R, De Paoli T, Hager A, Weill R, Boccio J. Zinc status and immune system relationship: a review. Biol Trace Elem Res. 2000;76:193–205. doi: 10.1385/BTER:76:3:193. [DOI] [PubMed] [Google Scholar]
- 125.Prasad AS, Bao B, Beck FW, Sarkar FH. Zinc activates NFκB in HUT-78 cells. J Lab Clin Med. 2001;138:250–6. doi: 10.1067/mlc.2001.118108. [DOI] [PubMed] [Google Scholar]
- 126.Prasad AS. Zinc in human health: effect of zinc on immune cells. Mol Med. 2008;14:353–7. doi: 10.2119/2008-00033.Prasad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Prasad AS, Bao B, Beck FW, Sarkar FH. Zinc enhances the expression of interleukin-2 and interleukin-2 receptors in HUT-78 cells by way of NFκB activation. J Lab Clin Med. 2002;140:272–89. doi: 10.1067/mlc.2002.127908. [DOI] [PubMed] [Google Scholar]
- 128.Prasad AS, Bao B, Beck FW, Sarkar FH. Correction of interleukin-2 gene expression by in vitro zinc addition to mononuclear cells from zinc-deficient human subjects: a specific test for zinc deficiency in humans. Transl Res. 2006;148:325–33. doi: 10.1016/j.trsl.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 129.Bao B, Prasad AS, Beck FW, Sarkar FH. Zinc up-regulates NFκB activation via phosphorylation of IκB in HUT-78 (Th0) cells. FEBS Lett. 2007;581:4507–11. doi: 10.1016/j.febslet.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 130.Greten FR, Arkan MC, Bollrath J, Hsu LC, Goode J, Miething C, Göktuna SI, Neuenhahn M, Fierer J, Paxian S, Van Rooijen N, Xu Y, O’Cain T, Jaffee BB, Busch DH, Duyster J, Schmid RM, Eckmann L, Karin M. NFκB is a negative regulator of IL-1β secretion as revealed by genetic and pharmacological inhibition of IKKβ. Cell. 2007;130:918–31. doi: 10.1016/j.cell.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lawrence T, Fong C. The resolution of inflammation: anti-inflammatory roles for NFκB. Int J Biochem Cell Biol. 2010;42:519–23. doi: 10.1016/j.biocel.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 132.Mosser DM, Zhang X. Interleukin-10: new perspectives on an old cytokine. Immunol Rev. 2008;226:205–18. doi: 10.1111/j.1600-065X.2008.00706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tiffon C, Adams J, van der Fits L, Wen S, Townsend P, Ganesan A, Hodges E, Vermeer M, Packham G. The histone deacetylase inhibitors vorinostat and romidepsin downmodulate IL-10 expression in cutaneous T-cell lymphoma cells. Br J Pharmacol. 2011;162:1590–602. doi: 10.1111/j.1476-5381.2010.01188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Guenova E, Watanabe R, Teague JE, Desimone JA, Jiang Y, Dowlatshahi M, Schlapbach C, Schaekel K, Rook AH, Tawa M, Fisher DC, Kupper TS, Clark RA. TH2 Cytokines from Malignant Cells Suppress TH1 Responses and Enforce a Global TH2 Bias in Leukemic Cutaneous T-cell Lymphoma. Clin Cancer Res. 2013;19:3755–63. doi: 10.1158/1078-0432.CCR-12-3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Abraham RM, Zhang Q, Odum N, Wasik MA. The role of cytokine signaling in the pathogenesis of cutaneous T-cell lymphoma. Cancer Biol Ther. 2011;12:1019–22. doi: 10.4161/cbt.12.12.18144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kasprzycka M, Zhang Q, Witkiewicz A, Marzec M, Potoczek M, Liu X, Wang HY, Milone M, Basu S, Mauger J, Choi JK, Abrams JT, Hou JS, Rook AH, Vonderheid E, Woetmann A, Odum N, Wasik MA. Gamma c-signaling cytokines induce a regulatory T cell phenotype in malignant CD4+ T lymphocytes. J Immunol. 2008;181:2506–12. doi: 10.4049/jimmunol.181.4.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rook AH, Gottlieb SL, Wolfe JT, Vowels BR, Sood SS, Niu Z, Lessin SR, Fox FE. Pathogenesis of cutaneous T-cell lymphoma: implications for the use of recombinant cytokines and photopheresis. Clin Exp Immunol. 1997;107(Suppl 1):16–20. [PubMed] [Google Scholar]
- 138.Vowels BR, Lessin SR, Cassin M, Jaworsky C, Benoit B, Wolfe JT, Rook AH. Th2 cytokine mRNA expression in skin in cutaneous T-cell lymphoma. J Invest Dermatol. 1994;103:669–73. doi: 10.1111/1523-1747.ep12398454. [DOI] [PubMed] [Google Scholar]
- 139.Li-Weber M, Giaisi M, Baumann S, Pálfi K, Krammer PH. NFκB synergizes with NF-AT and NF-IL6 in activation of the IL-4 gene in T cells. Eur J Immunol. 2004;34:1111–8. doi: 10.1002/eji.200324687. [DOI] [PubMed] [Google Scholar]
- 140.Lohoff M, Giaisi M, Köhler R, Casper B, Krammer PH, Li-Weber M. Early growth response protein-1 (Egr-1) is preferentially expressed in T helper type 2 (Th2) cells and is involved in acute transcription of the Th2 cytokine interleukin-4. J Biol Chem. 2010;285:1643–52. doi: 10.1074/jbc.M109.011585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mori N, Prager D. Activation of the interleukin-10 gene in the human T lymphoma line HuT 78: identification and characterization of NFκB binding sites in the regulatory region of the interleukin-10 gene. Eur J Haematol. 1997;59:162–70. doi: 10.1111/j.1600-0609.1997.tb00970.x. [DOI] [PubMed] [Google Scholar]
- 142.Cao S, Zhang X, Edwards JP, Mosser DM. NFκB1 (p50) homodimers differentially regulate pro- and anti-inflammatory cytokines in macrophages. J Biol Chem. 2006;281:26041–50. doi: 10.1074/jbc.M602222200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.De Siervi A, De Luca P, Moiola C, Gueron G, Tongbai R, Chandramouli GV, Haggerty C, Dzekunova I, Petersen D, Kawasaki E, Kil WJ, Camphausen K, Longo D, Gardner K. Identification of new Rel/NFκB regulatory networks by focused genome location analysis. Cell Cycle. 2009;8:2093–100. doi: 10.4161/cc.8.13.8926. [DOI] [PMC free article] [PubMed] [Google Scholar]


