Abstract
A chemical mutagenesis technique was employed for development of mutant strains of Sparassis crispa targeting the shortened cultivation time and the high β-glucan content. The homogenized mycelial fragments of S. crispa IUM4010 strain were treated with 0.2 vol% methyl methanesulfonate, an alkylating agent, yielding 199 mutant strains. Subsequent screening in terms of growth and β-glucan content yielded two mutant strains, B4 and S7. Both mutants exhibited a significant increase in β-glucan productivity by producing 0.254 and 0.236 mg soluble β-glucan/mg dry cell weight for the B4 and S7 strains, respectively, whereas the wild type strain produced 0.102 mg soluble β-glucan/mg dry cell weight. The results demonstrate the usefulness of chemical mutagenesis for generation of mutant mushroom strains.
Keywords: Beta-glucan, Chemical mutagenesis, Growth rate, Sparassis crispa
Sparassis crispa is an edible mushroom that grows on the root or base of conifer trees during the summer and autumn seasons. It has been regarded as pathogenic to trees. Immunomodulating activities of the fruiting body of S. crispa have been demonstrated, including enhancement of cytokine synthesis [1, 2] and hematopoietic response [3], suppression of mast cell-mediated allergic inflammation [4], induction of dendritic cell maturation [5], and anticancer activities [6, 7]. The medicinal effects of S. crispa are largely derived from β-1,3-glucan (β-glucan in short) [1, 2, 6]. The dried fruiting body of S. crispa has been reported to contain more than 40 wt% of water-soluble β-glucan [6, 8]. β-Glucan is an essential component of the fungal cell wall. The polysaccharide β-glucan is synthesized by β-glucan synthase (EC 2.4.1.34.) using UDP-glucose and a precursor β-1,3-glucan as a substrate [9-11].
However, despite its commercial potential, artificial cultivation of S. crispa has been limited to a few high-tech commercial farms because of its slow mycelial propagation into solid medium, which makes the cultivation vulnerable to pathogenic bacterial and fungal infections [12, 13]. In addition, sophisticated control of environmental conditions is required for primordia formation and growth of fruiting bodies, which is hardly achievable on general commercial farms. Because the mycelia of S. crispa also contain a moderately high amount of β-glucan, liquid culture of mycelia has been sought as an alternative approach for its use as a producer of β-glucan. A similar attempt was made for production of mycelium of Agaricus blazei, another well-known β-glucan producer, by a submerged fermentation [14].
In order to use the mycelia of S. crispa as a β-glucan producer, development of a fast-growing strain with high β-glucan content became essential. Our previous work provided evidence indicating that chemical mutagenesis was an effective method for generation of mutant strains with desirable properties [15]. Treatment of basidiospores with an alkylating agent and subsequent mating resulted in generation of a mushroom with diverse morphological characteristics. Accordingly, chemical mutagenesis was employed in this study for generation of a fast-growing mutant strain of S. crispa. Screening and evaluation of the mutants yielded two promising mutant strains that produced ~2.5 folds more β-glucan than the wild strain.
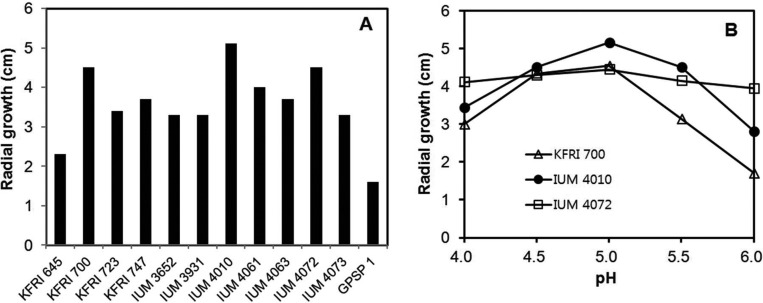
Although S. crispa is a good source of β-glucan, its slow rate of growth is a major obstacle to its employment as a producer of β-glucan [12]. For employment of chemical mutagenesis for development of fast-growing mutant strains, the growth of S. crispa strains obtained from culture collections was evaluated. The strains studied in this experiment were obtained from the Culture Collection of the DNA bank of Mushrooms (strains with IUM numbers, University of Incheon, Korea), Korea Forest Research Institute (strains with KFRI numbers), and Greenpeace Mushroom Institute (GPSP1). Growth evaluation of the strains was performed by measuring the radial diameter of the propagated mycelia on a potato dextrose agar medium (PDA; Ventech Bio Co., Eumseong, Korea) containing potato extract (4 g/L), glucose (20 g/L), and agar (15 g/L) for 15 days at 25℃.
Among the tested strains, KFRI700, IUM4010, and IUM4072 showed better growth than the other strains (Fig. 1A). Because the growth of S. crispa has been reported to show sensitivity to medium pH [13], the effect of pH on growth of the selected strains was examined on the same medium using different pH values. The IUM4010 and IUM4072 strains exhibited optimal growth at pH 5.0, while the KFRI700 strain was relatively insensitive to pH (Fig. 1B). The effect of pH on S. crispa appears to be strain-dependent. Cheong et al. [12] reported a broad optimal pH range (5.0~8.0) for the S. crispa ASI150010 strain, while an independent study by Shim et al. [13] reported pH 4.0 as the optimal pH for a S. crispa strain. Since the growth of IUM4010 was significantly faster than that of the other two strains at pH 5.0 (Fig. 1B), this strain was selected for the chemical mutagenesis.
Fig. 1.
Growth characteristics of various strains of Sparassis crispa. A, Mycelial growth of S. crispa. Growth was measured by the diameter of the propagated mycelia on potato dextrose agar medium for 15 days at 25℃; B, Effect of pH on the selected strains.
Chemical mutagenesis was performed using the broken mycelia of the IUM4010 strain. To obtain the mycelia, five agar blocks (1 cm in diameter) from a PDA plate were inoculated into a potato dextrose broth (PDB) and incubated at 25℃ with gentle shaking (120 rpm) for more than 20 days. The mushroom mycelia were collected and disrupted with glass beads using the Mini-Beadbeater (Biospec Products, Bartlesville, OK, USA) in PDB. The disruption was continued until the mycelia were fragmented into small pieces consisting of fewer than 10 cells. The disrupted mycelia were incubated for 24 hr at 25℃ in order to allow recovery from the disruption. The mycelial fragments were collected by centrifugation (5 min, 10,000 rpm) and subjected to chemical mutagenesis using methyl methanesulfonate (MMS; Sigma-Aldrich, St. Louis, MO, USA) as previously described [15]. Briefly, the mycelial fragments were suspended in 0.5 mL of distilled water. MMS was applied at various concentrations for 1 hr at 25℃. MMS-treated mycelia were collected and washed three times with water. The mycelia were resuspended in distilled water and the suspension (100mL) was spread on PDA. The agar plate was incubated at 25℃ for 20 days.
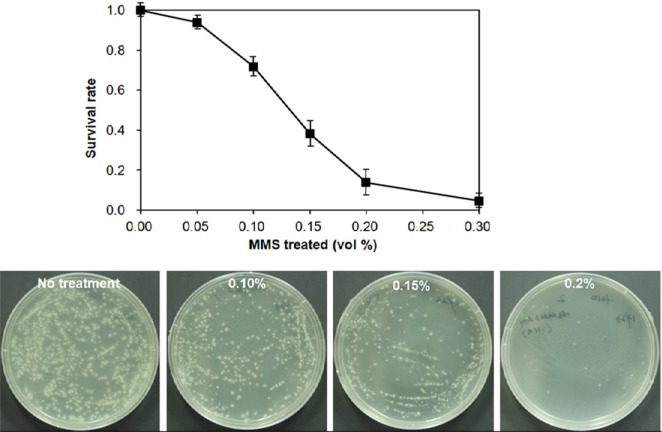
The survival rate of the mycelia showed a sharp decrease with increase of MMS concentration (Fig. 2). The rate reached 14% at 0.20 vol% of MMS, resulting in production of 199 independent fast-growing mycelial colonies. Mushroom mycelia appear to have much greater sensitivity to alkylating agents than basidiospores since a similar survival rate was achieved at 0.65 vol% of MMS for basidiospores of Hypsizigous marmoreus [15] and 33% of Volvariella volvacea basidiospores survived at 0.75 vol% ethylmethane sulfonate [16]. The difference may be due to the elevated susceptibility of mycelia to alkylating agents. The fast-growing mycelial colonies derived from treatment with 0.20 vol% MMS were transferred to new PDA plates for further evaluation.
Fig. 2.
Effect of methyl methanesulfonate (MMS) concentration on the survival of homogenized mycelia fragments. The homogenized mycelia of IUM4010 were treated with different concentrations of MMS for 1 hr at 25℃. The plates showed the surviving mycelial colonies at different concentrations of MMS. The data points were the mean values of triplicate experimental sets.
The MMS-treated mycelial colonies (199 colonies) were individually grown on PDA (pH 5.0). Twenty nine colonies showing comparable growth to the wild strain (IUM4010) with normal mycelial morphology were collected and inoculated to PDB for determination of β-glucan content. The culture broth (50 mL) was collected by centrifugation at 3,500 rpm for 30 min. The collected mycelia were washed three times with water and dried in a drying oven at 70℃ for 24 hr. The dried mycelia were ground using a mortar and pestle. The powder (10 mg) was suspended in 1 mL of 50 mM sodium acetate buffer (pH 5.0). The suspension was incubated at 25℃ with vigorous shaking for 24 hr. The supernatant of the suspension was obtained by centrifugation for 10 min at 10,000 rpm.
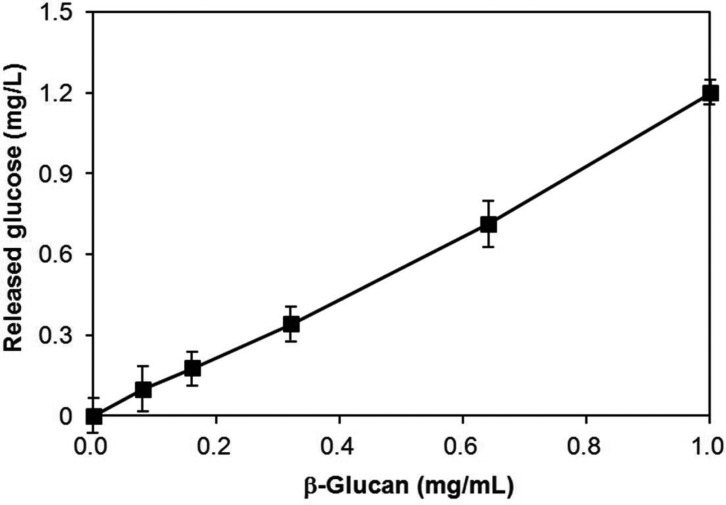
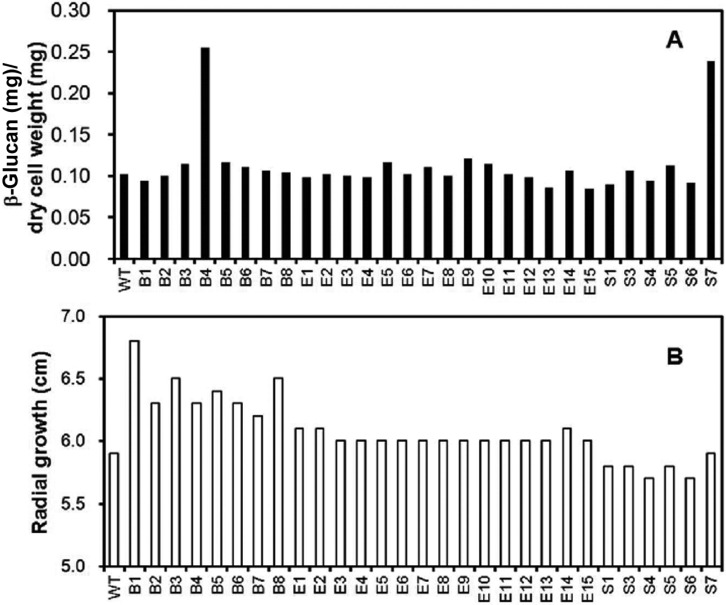
For determination of the β-glucan content, the soluble fraction of dried mycelial powder was treated with β-glucanase in order to release glucose from β-glucan. The released glucose was determined using the 2,3-dinitrosalicylic acid (DNS) method [17]. In brief, the supernatant (100 mL) was mixed with 400 mL of 50 mM sodium acetate buffer (pH 5.0) containing 30 units of β-glucanase (Sigma Co.). The reaction mixture was incubated at 55℃ for 2 hr. Upon completion of the reaction, the released glucose was measured using the DNS assay method. The cellular β-glucan content was determined by comparison of the amount of released glucose with the amount of released glucose from the purified β-glucan in the β-glucanase reaction. Validity of this method was confirmed by the tight correlation between the released glucose and the amount of purified β-glucan standard (Fig. 3). This enabled simple determination of the mycelial β-glucan content of any fungal mycelia. Using this method, the β-glucan content of the wild strain was determined to be 10.2% (0.102 mg soluble β-glucan/mg dry mycelial weight) (Fig. 4A). Most mutant strains were found to contain ~10% soluble β-glucan, similar to the wild strain, regardless of their growth rate (Fig. 4B). Interestingly, however, two mutant strains, B4 and S7, showed remarkable increases in β-glucan content, reaching ~25% of the dried mycelial weight (0.25 mg soluble β-glucan/mg dry mycelial weight) for both strains.
Fig. 3.
Standard curve for β-glucan assay. Various concentrations of the purified β-glucan standard were treated with β-glucanase and the released glucose was measured using the DNS method. The correlation coefficient was more than 0.99.
Fig. 4.
β-Glucan contents and growth of mutant strains. A, β-Glucan contents of the selected mutant strains; B, Mycelial growth of the mutant strains.
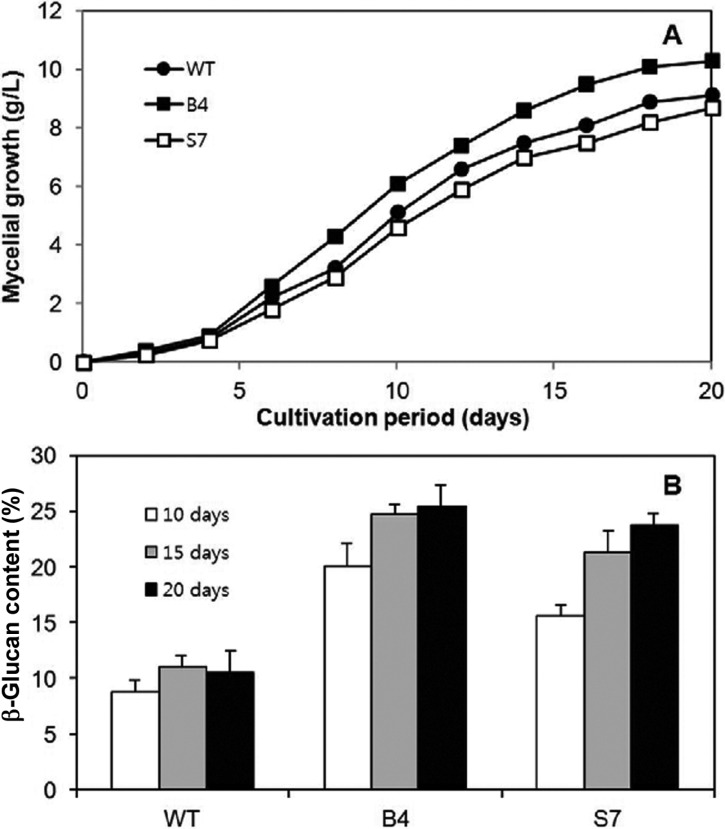
Growth characteristics of the mutant strains were further evaluated while growing in PDB at 25℃. The growth approached the stationary phase at around 15 days of cultivation, which was slightly slower than that of the previous report (Fig. 5A) [12]. Growth of the mutant strain B4 was more rapid than that of the wild strain, whereas the mutant strain S7 showed slightly retarded growth. The final mycelia productivities were 9.1, 10.3, and 8.7 g dry mycelial weight/L/20 days for the wild type, B4, and S7 strains, respectively. Next, the β-glucan contents in the mycelia were determined from culture samples taken at 10, 15, and 20 days. The content showed a gradual increase when the culture approached the stationary phase (Fig. 5B), suggesting that the synthetic activity for β-glucan is more active in this phase.
Fig. 5.
Growth characteristics of the mutant B4 and S7 strains in liquid culture. A, Growth curve. Dried mycelial mass was measured from 50 mL culture samples; B, β-Glucan contents of the selected samples.
In conclusion, this work demonstrated generation of high β-glucan producing mutant strains of S. crispa. Additional culture optimization will further increase β-glucan productivity of the mutant strains. Preliminary analysis of a gene encoding β-glucan synthase revealed that the B4 and S7 strains contained various mutations (data not shown). Biochemical studies on β-glucan biosynthetic enzymes, including β-glucan synthase in mutant strains, will provide detailed explanations on elevated β-glucan production.
ACKNOWLEDGEMENTS
This work was supported by the GyeongNam Technopark program (#70011463), the Ministry of Knowledge Economy, Korea.
References
- 1.Nameda S, Harada T, Miura NN, Adachi Y, Yadomae T, Nakajima M, Ohno N. Enhanced cytokine synthesis of leukocytes by a beta-glucan preparation, SCG, extracted from a medicinal mushroom, Sparassis crispa. Immunopharmacol Immunotoxicol. 2003;25:321–335. doi: 10.1081/iph-120024500. [DOI] [PubMed] [Google Scholar]
- 2.Harada T, Miura NN, Adachi Y, Nakajima M, Yadomae T, Ohno N. Granulocyte-macrophage colony-stimulating factor (GM-CSF) regulates cytokine induction by 1,3-beta-D-glucan SCG in DBA/2 mice in vitro. J Interferon Cytokine Res. 2004;24:478–489. doi: 10.1089/1079990041689656. [DOI] [PubMed] [Google Scholar]
- 3.Harada T, Kawaminami H, Miura NN, Adachi Y, Nakajima M, Yadomae T, Ohno N. Mechanism of enhanced hematopoietic response by soluble beta-glucan SCG in cyclophosphamide-treated mice. Microbiol Immunol. 2006;50:687–700. doi: 10.1111/j.1348-0421.2006.tb03841.x. [DOI] [PubMed] [Google Scholar]
- 4.Kim HH, Lee S, Singh TS, Choi JK, Shin TY, Kim SH. Sparassis crispa suppresses mast cell-mediated allergic inflammation: role of calcium, mitogen-activated protein kinase and nuclear factor-κB. Int J Mol Med. 2012;30:344–350. doi: 10.3892/ijmm.2012.1000. [DOI] [PubMed] [Google Scholar]
- 5.Kim HS, Kim JY, Ryu HS, Park HG, Kim YO, Kang JS, Kim HM, Hong JT, Kim Y, Han SB. Induction of dendritic cell maturation by β-glucan isolated from Sparassis crispa. Int Immunopharmacol. 2010;10:1284–1294. doi: 10.1016/j.intimp.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 6.Ohno N, Miura NN, Nakajima M, Yadomae T. Antitumor 1,3-beta-glucan from cultured fruit body of Sparassis crispa. Biol Pharm Bull. 2000;23:866–872. doi: 10.1248/bpb.23.866. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto K, Kimura T, Sugitachi A, Matsuura N. Antiangiogenic and anti-metastatic effects of beta-1,3-D-glucan purified from hanabiratake, Sparassis crispa. Biol Pharm Bull. 2009;32:259–263. doi: 10.1248/bpb.32.259. [DOI] [PubMed] [Google Scholar]
- 8.Park HG, Shim YY, Choi SO, Park WM. New method development for nanoparticle extraction of water-soluble beta-(1-->3)-D-glucan from edible mushrooms, Sparassis crispa and Phellinus linteus. J Agric Food Chem. 2009;57:2147–2154. doi: 10.1021/jf802940x. [DOI] [PubMed] [Google Scholar]
- 9.Douglas CM. Fungal beta(1,3)-D-glucan synthesis. Med Mycol. 2001;39(Suppl 1):55–66. doi: 10.1080/mmy.39.1.55.66. [DOI] [PubMed] [Google Scholar]
- 10.Kelly R, Register E, Hsu MJ, Kurtz M, Nielsen J. Isolation of a gene involved in 1,3-beta-glucan synthesis in Aspergillus nidulans and purification of the corresponding protein. J Bacteriol. 1996;178:4381–4391. doi: 10.1128/jb.178.15.4381-4391.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mio T, Adachi-Shimizu M, Tachibana Y, Tabuchi H, Inoue SB, Yabe T, Yamada-Okabe T, Arisawa M, Watanabe T, Yamada-Okabe H. Cloning of the Candida albicans homolog of Saccharomyces cerevisiae GSC1/FKS1 and its involvement in beta-1,3-glucan synthesis. J Bacteriol. 1997;179:4096–4105. doi: 10.1128/jb.179.13.4096-4105.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheong JC, Park JS, Hong IP, Seok SJ, Jhune CS, Lee CJ. Cultural characteristics of cauliflower mushroom, Sparassis crispa. Korean J Mycol. 2008;36:16–21. [Google Scholar]
- 13.Shim JO, Son SG, Yoon SO, Lee YS, Lee TS, Lee SS, Lee KD, Lee MW. The optimal factors for the mycelial growth of Sparassis crispa. Korean J Mycol. 1998;26:39–46. [Google Scholar]
- 14.Lin JH, Yang SS. Mycelium and polysaccharide production of Agaricus blazei Murrill by submerged fermentation. J Microbiol Immunol Infect. 2006;39:98–108. [PubMed] [Google Scholar]
- 15.Lee J, Kang HW, Kim SW, Lee CY, Ro HS. Breeding of new strains of mushroom by basidiospore chemical mutagenesis. Mycobiology. 2011;39:272–277. doi: 10.5941/MYCO.2011.39.4.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Z, Zhang K, Lin JF, Guo LQ. Breeding cold tolerance strain by chemical mutagenesis in Volvariella volvacea. Sci Horticult. 2011;130:18–24. [Google Scholar]
- 17.Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959;31:426–428. [Google Scholar]