Dear Editor,
Abnormal oocytes are one of the major causes of reproductive failure, and also limit the efficiency of assisted reproduction1. The generation of functional oocytes either by differentiation of pluripotent stem cells2 or in vitro culturing of the embryonic genital ridges3 has not yet been achieved. Recently, we and other groups have reported that live fertile mice could be successfully produced by injecting androgenetic haploid embryonic stem (ahES) cells that carry some paternal imprints into oocytes in place of sperm4,5. Considering their parthenogenetic origin, it would be interesting to know whether parthenogenetic haploid ES (phES) cells6,7,8 could also support embryonic development when substituted for the maternal genome, and whether they could directly deliver their genomes at animal level. Here we established several phES cell lines and characterized their pluripotency and diploidization progression in vivo. We also demonstrated that phES cells could reconstruct embryos and produce fertile mice when injected into oocytes in place of maternal genome.
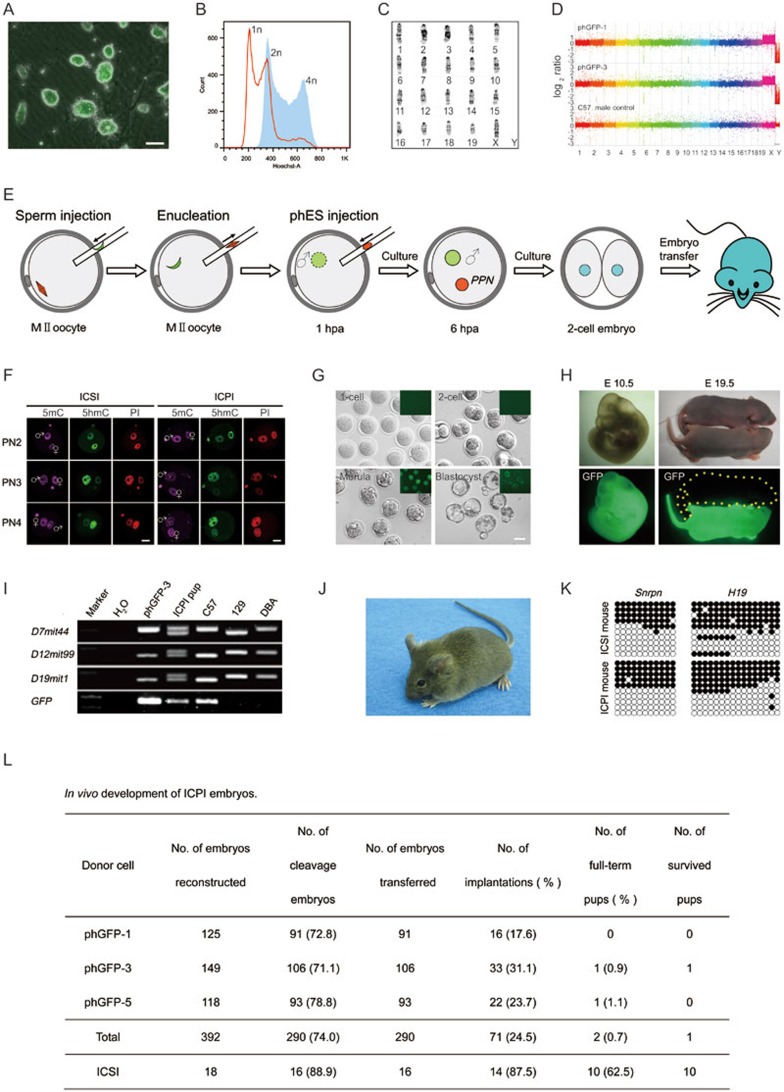
Haploid parthenogenetic embryos were generated from oocytes carrying a chicken β-actin4 or Oct49 gene promoter-driven enhanced green fluorescent protein transgene (EGFP or Oct4-EGFP in short). We generated a total of 13 ES cell lines (indicated as phGFP cell lines, Supplementary information, Figure S1 and Table S1) from 40 haploid parthenogenetic morulas (from EGFP oocytes) and all of the cell lines inherited the EGFP expression (Figure 1A). In addition, nine cell lines were maintained in a haploid state after three or more rounds of purification via fluorescence-activated cell sorting (FACS) and subsequent expansion of the haploid cells (Figure 1B). Interestingly, these cell lines retained their haploid genome even after being passaged for more than 30 times (Figure 1C). We also obtained two Oct4-EGFP haploid cell lines from 30 activated oocytes of Oct4-EGFP mice (indicated as phES OG cell lines, Supplementary information, Table S1). We assessed the genomic integrity of all the phES cell lines by comparative genomic hybridization (CGH) analysis, and did not observe any copy-number variation between the phES cell lines (Figure 1D). These results indicated that the phES cells could stably maintain an intact haploid genome as previously reported8.
Figure 1.
Parthenogenetic haploid ES cells produce fertile mice. (A) Fluorescence detection of phES cells that carry an EGFP transgene. Scale bar, 50 μm. (B) DNA content of phES cells (phGFP-3, passage 11, red) analyzed by FACS. Cells were purified three times by FACS selection of G0/G1 phase haploid cells, followed by culturing for three more passages. Diploid ES cells (blue) with 2n chromosome sets were used as control. (C) Karyotype analysis of phES cell lines. Shown is the standard G-binding karyotype of phGFP-3 cells (passage 12) with 19 + X chromosomal set. (D) Comparative genomic hybridization (CGH) analysis of two phES cell lines (phGFP-1 Passage 16, and phGFP-3 Passage 16). Comparative results of the genomic DNA of the phES cells and the control C57BL/6 male mouse kidney were shown as the y axis, on a log2 base scale. (E) Diagram showing the generation of phES pups. hpa, hours post activation. (F) 5mC and 5hmC staining of the reconstructed embryos (6-7 hpa) with pronucleus after sperm injection. The ICSI embryos were shown in the left panel, whereas reconstructed ICPI embryos were shown in the right panel. (G) Pre-implantation development of ICPI embryos produced by phES cell injection. Scale bar, 50 μm. (H) Left panel shows an E10.5 ICPI fetus with EGFP expression that was produced by phGFP-3. Right panel shows one P2 ICPI pup (phGFP-3) with EGFP expression, and one P2 wild-type ICSI pup. (I) Genetic background analysis of ICPI pup. Three simple sequence length polymorphism (SSLP) DNA markers from different chromosomes and the EGFP positive analysis showed that the pup was originated from hybrid genetic background, i.e., the C57BL/6 strain (phGFP-3 origin) and the 129Sv strain (sperm origin). Primer sequences were cited from the Mouse Genome Informatics website (http://www.informatics.jax.org/). (J) An adult male mouse produced by phES cell (phGFP-3) injection. (K) Bisulfite sequencing analysis of DMRs of imprinted genes Snrpn and H19. Mouse tail DNAs isolated from ICSI and phES adult off-springs were used. Filled circles represent methylated CpG sites, whereas open circles represent unmethylated CpG sites. Primer sequences were listed in Supplementary information, Table S3. (L) In vivo development of ICPI embryos.
Pluripotency is an important feature of haploid ES cells. Interestingly, the phES cells showed expression of key pluripotent marker genes such as Oct4, Sox2, Nanog, SSEA-1 and Klf4 (Supplementary information, Figure S2A and S2B), very similar to diploid ES cells. Furthermore, the global gene expression profile of phES cells (phGFP-3) showed high correlation (r = 0.955) with diploid ES cells, but less correlation (r = 0.779 or 0.799) with mouse embryonic fibroblasts (MEFs) (Supplementary information, Figure S2C). Interestingly, the phES cells formed teratomas with all 3 germ layers when injected subcutaneously into SCID (severe combined immune deficiency) mice (Supplementary information, Figure S2D). We further used chimera formation assay to analyze the differentiation potential and the progression of the diploidization process of the phES cells in vivo. FACS-sorted EGFP-carrying G0- or G1-phase phES cells were obtained from mice with black-colored coats and injected into CD-1 blastocysts (obtained from mice with white-colored coats). We found that these cells properly chimerized the embryos at the E6.5, E8.5, E10.5 and E13.5 stages as reflected by their EGFP expression (Supplementary information, Figure S3). However, only 1.8% of the haploid EGFP-positive cell population was detected at the E6.5 embryonic stage (Supplementary information, Figure S3A), suggesting that the phES cells rapidly diploidize after differentiation, which was similar to the previous results7. Out of 38 E13.5-chimeric embryos generated from Oct4-EGFP-transgenic phES cells (Supplementary information, Table S2), 2 embryos expressed EGFP in their genital ridges (Supplementary information, Figure S2E), indicating successful germline transmission of the phES cells (phES OG-1). Finally, a total of six full-term chimeric pups (Supplementary information, Table S2) were obtained. However, only four survived to adulthood (Supplementary information, Figure S2F). The adult chimeric mouse produced from the phGFP cell line showed EGFP expression in multiple organs including kidney (7.7%), spleen (3.9%), heart (14.6%) and liver (12.6%), which reflected the broad differentiation ability of phES cells in vivo (Supplementary information, Figure S4). Furthermore, all the EGFP-positive cells were found to be diploid. Taken together, our results confirmed that the phES cells were pluripotent, although they were in diploid status.
To test whether the genome of a phES cell could take place of the maternal genome, first we performed intracytoplasmic sperm injection (ICSI), followed by removal of spindle to generate an androgenetic haploid embryo4. One h later, we performed intracytoplasmic phES cell (at G0/G1 stage) injection (ICPI) to generate diploid reconstructed embryos (Figure 1E). The phES cells (phGFP cell lines) formed 'maternal' pseudo-pronuclei and exhibited the genomic methylation pattern very similar to the ICSI embryos (Figure 1F). The ICPI embryos further developed to morula and blastocyst in vitro (Figure 1G), and inherited the EGFP transgene derived from phES cells. To determine the capacity for in vivo development of ICPI embryos, a total of 290 2-cell stage embryos were transferred into pseudo-pregnant mice. From this, two full-term pups were finally obtained. Both EGFP expression (Figure 1H) and simple sequence length polymorphism (SSLP) analysis (Figure 1I) confirmed that the pups were indeed produced from phES cells. Interestingly, one of these two pups survived to adulthood (Figure 1J), and showed normal methylation pattern of differentially methylated regions (DMRs) of Snrpn and H19 (Figure 1K). This adult male mouse was fertile and delivered normal pups that inherited its EGFP transgene (Figure 1L). This indicated the Mendelian separation theory (Supplementary information, Figure S5). Taken together, our results demonstrate that phES cells could functionally replace the maternal genome and support full-term embryonic development, albeit with low efficiency.
Our data showed that phES cells were pluripotent, contributing to the germline of chimeric mice, and could produce a live mouse by ICPI. However, the efficiency was very low, which may have been caused by two reasons: 1) the ICPI embryo manipulations, especially the spindle removal procedure may have harmed the embryo's subsequent development10; 2) the epigenetic state of the phES cells may different from that of the spindle and thereby compromised the development. Future efforts in modification of the manipulation process, utilization of the small compounds to reduce the negative effects caused by the spindle removal, and stabilization of the epigenetic status of the phES cells, especially the maternal imprinting, may improve the developmental efficiency of ICPI embryos. When women survive conditions such as ovarian cancer, phES cells could provide an alternative means of maintaining their fertility, and with ICPI procedure, a woman could give birth to a child who inherited her DNA. Compared to oocyte cryopreservation, cryopreservation of phES cells is easier and more convenient, and these cells are known to survive much longer in liquid nitrogen. Administration of phES cells also provides a novel way of gene therapy for the treatment of severe human diseases caused by cells with mitochondrial defects inherited through the egg's cytoplasm. ICPI injection could transfer the maternal DNA and fewer mtDNA than spindle transfer procedure, and thus provide the best clear mtDNA replacement in oocytes. Nevertheless, our study demonstrated that the phES cells could support embryo development via substitution of maternal genome, which further provides a new model for studying epigenetic regulation of embryonic development, and may shed new light on assisted reproduction.
Acknowledgments
We thank all the members of Reproductive Engineering Group for discussion and help. This study was supported by the National Natural Science Foundation of China (90919060), the National Basic Research Program of China (2012CBA01300), and the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA01020100) to QZ. We thank Fluidigm Corporation for their support in the utilization of BioMark HD system. We also thank Leica and Eppendorf for supporting the facility.
Footnotes
(Supplementary information is linked to the online version of the paper on the Cell Research website.)
Supplementary Information
A schematic representation of parthenogenetic haploid ES cells derivation.
Derivation of parthenogenetic haploid ES cell lines.
The pluripotency of parthenogenetic haploid ES cells.
Pluripotency and diploidization of mouse phES cells (phGFP-3).
Pluripotency and diploidization of mouse phES cells.
Flowcytometry analysis of DNA content of different tissues dissected from adult chimaeric mice.
The ICPI mouse generated by intracytoplasmic phES cells (phGFP-3) injection and its progeny.
Primers used in PCR analysis.
References
- Serhal PF, Ranieri DM, Kinis A, et al. Hum Reprod. 1997. pp. 1267–1270. [DOI] [PubMed]
- Hayashi K, Ogushi S, Kurimoto K, et al. Science. 2012. pp. 971–975. [DOI] [PubMed]
- Obata Y, Kono T, Hatada I. Nature. 2002. p. 497. [DOI] [PubMed]
- Li W, Shuai L, Wan H, et al. Nature. 2012. pp. 407–411. [DOI] [PubMed]
- Yang H, Shi L, Wang BA, et al. Cell. 2012. pp. 605–617. [DOI] [PubMed]
- Leeb M, Walker R, Mansfield B, et al. Development. 2012. pp. 3301–3305. [DOI] [PMC free article] [PubMed]
- Elling U, Taubenschmid J, Wirnsberger G, et al. Cell Stem Cell. 2011. pp. 563–574. [DOI] [PMC free article] [PubMed]
- Leeb M, Wutz A. Nature. 2011. pp. 131–134. [DOI] [PMC free article] [PubMed]
- Yoshimizu T, Sugiyama N, De Felice M, et al. Dev Growth Differ. 1999. pp. 675–684. [DOI] [PubMed]
- Noggle S, Fung HL, Gore A, et al. Nature. 2011. pp. 70–75. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A schematic representation of parthenogenetic haploid ES cells derivation.
Derivation of parthenogenetic haploid ES cell lines.
The pluripotency of parthenogenetic haploid ES cells.
Pluripotency and diploidization of mouse phES cells (phGFP-3).
Pluripotency and diploidization of mouse phES cells.
Flowcytometry analysis of DNA content of different tissues dissected from adult chimaeric mice.
The ICPI mouse generated by intracytoplasmic phES cells (phGFP-3) injection and its progeny.
Primers used in PCR analysis.