Abstract
Epithelial stem cells are maintained within niches that promote self-renewal by providing signals that specify the stem cell fate. In the Drosophila ovary, epithelial follicle stem cells (FSCs) reside in niches at the anterior tip of the tissue and support continuous growth of the ovarian follicle epithelium. Here, we demonstrate that a neighboring dynamic population of stromal cells, called escort cells, are FSC niche cells. We show that escort cells produce both Wingless and Hedgehog ligands for the FSC lineage, and that Wingless signaling is specific for the FSC niche whereas Hedgehog signaling is active in both FSCs and daughter cells. In addition, we show that multiple escort cells simultaneously encapsulate germ cell cysts and contact FSCs. Thus, FSCs are maintained in a dynamic niche by a non-dedicated population of niche cells.
Keywords: Drosophila, Epithelial stem cells, Escort cells, Follicle stem cells, Niche, Ovary
INTRODUCTION
Adult stem cells divide to self-renew and produce differentiated progeny that replace lost or damaged cells in the tissue. This asymmetric segregation of cell fates is enforced by a specialized microenvironment, or niche, that surrounds the stem cells and preserves their fate while allowing non-stem cells to exit the niche and differentiate (Morrison and Spradling, 2008). Although there is a considerable diversity of niche architecture and composition in different tissues (Nystul and Spradling, 2006), stem cell niches generally consist of a combination of: niche-producing cells that provide self-renewal signals; extracellular components such as the basement membrane; and unique structural features of the tissue, such as the base of the intestinal crypt or the bulge region of the hair follicle (Blanpain and Fuchs, 2006; van der Flier and Clevers, 2009; Wagers, 2012).
Drosophila is a highly tractable model for the study of these and other aspects of stem cell niches in vivo (Losick et al., 2011; Sahai-Hernandez et al., 2012). Indeed, the male and female germline stem cell (GSC) niches are particularly informative examples of a type of static niche, in which a dedicated population of niche cells produces a stable microenvironment that is necessary and sufficient for maintaining the stem cell fate. A second population of stem cells in the ovary, the follicle stem cells (FSCs), offers an opportunity to investigate the composition of a more dynamic type of niche that may be common in epithelial tissues (Margolis and Spradling, 1995; Nystul and Spradling, 2007). Drosophila ovaries are composed of 14-18 ovarioles, and two mitotically active FSCs reside within a structure at the tip of each ovariole called the germarium. During normal adult oogenesis, the FSCs divide asymmetrically to self-renew and produce prefollicle cell daughters that differentiate to form a canonical polarized epithelium (Fig. 1A). Lineage-tracing studies demonstrated that the two FSCs are consistently positioned on opposite sides of the germarium, at the boundary between stromal escort cells in region 2a and the follicle cells in region 2b (Fig. 1A) (Nystul and Spradling, 2007). Notably, when FSCs are lost from the tissue, a new replacement cell colonizes these same locations and acquires the FSC fate. These observations suggest that these locations are FSC niches, but it is unclear which cells within the germarium are contributing to this niche.
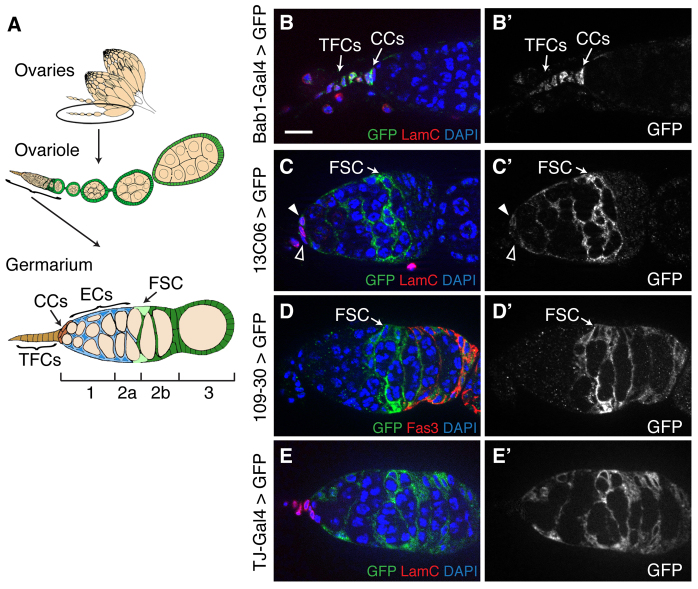
Fig. 1.
The germarium contains multiple types of somatic cells. (A) Diagram of the Drosophila ovary. Each ovary is composed of multiple subunits called ovarioles, and each ovariole has a structure at the anterior tip called the germarium. Each germarium has terminal filament cells (TFCs, orange) and cap cells (CCs, red) at the anterior tip, escort cells (ECs, blue) surrounding germ cell cysts in the anterior half, and two follicle stem cells (FSCs, light green) that produce follicle cells (dark green) that surround germ cell cysts in the posterior half. The germarium is divided into regions (1, 2a, 2b and 3) that are defined by the stages of germ cell development. FSCs are always found at the region 2a/2b border. (B-E) UAS::CD8-GFP; Bab1-Gal4 (B), UAS::CD8-GFP, 13C06 (C), 109-30; UAS::CD8-GFP (D) or UAS::CD8-GFP; Tj-Gal4 (E) stained for GFP (green) to identify cells that express Gal4, Lamin C (B,C,E, red) to identify terminal filament cells and cap cells or Fas3 (D, red) to identify follicle cells, and with DAPI (blue). (B′-E′) GFP channel only. Bab1-Gal4 is expressed strongly in terminal filament and cap cells. 13C06 is expressed strongly in posterior escort cells, FSCs and some early follicle cells, and weakly in anterior escort cells, and occasionally in one to two cap cells (white arrowhead). Cap cells that do not express Gal4 at detectable levels are indicated by the open arrowhead. 109-30 is expressed strongly in FSCs and all follicle cells in the germarium. Tj-Gal4 is expressed in escort cells, FSCs and follicle cells. Anterior is to the left. Scale bar: 5 μm.
Among the many genes required for FSC self-renewal are several key members of the Hedgehog (Hh) (Kirilly et al., 2005; Vied and Kalderon, 2009; Zhang and Kalderon, 2001) and Wingless (Wg) (Song and Xie, 2003) signaling pathways. The only cell types known to express Hh and Wg in the germarium are two somatic cell types, the cap cells and terminal filament cells (Forbes et al., 1996a; Forbes et al., 1996b; Hartman et al., 2010), which reside at the anterior tip of the germarium, several cell diameters away from the FSCs. This led to the suggestion that the Hh and Wg produced by terminal filament cells and cap cells diffuse through the tissue to the region 2a/2b border, where they act in the FSC niche to promote self-renewal (Forbes et al., 1996a; Hartman et al., 2010; Song and Xie, 2003; Vied et al., 2012). However, whether the Hh and Wg ligands produced by the terminal filament cells and cap cells are actually required by FSCs or their daughters has not been tested genetically. A second common function of niche cells is to adhere to stem cells through cell adhesion complexes. Indeed, FSCs require DE-cadherin (Song and Xie, 2002), the core protein in adherens junctions, for maintenance in the tissue. In addition, FSCs are known to form adherens junctions with neighboring stromal cells that are presumed to be a part of the escort cell population (Song and Xie, 2002), but whether these are dedicated FSC niche cells or cells that also associate with germ cells is unclear.
Here we show that escort cells, not the cap cells and terminal filament cells, are the relevant source of Wg for the FSC niche. In addition, we show that Wg signaling is activated specifically in FSCs and not in the surrounding prefollicle cells. By contrast, we find that Hh signaling is active throughout the early FSC lineage, including in both FSCs and prefollicle cells, and that multiple cell types in the germarium are relevant sources of Hh for the FSC lineage. Lastly, we show that multiple escort cells both encapsulate germ cell cysts and contact the FSC niche. Collectively, these results indicate that escort cells are an essential component of the FSC niche.
RESULTS
Tools for the expression of transgenes in subpopulations of somatic cells in the germarium
The germarium contains multiple somatic cell types, including terminal filament cells, cap cells, escort cells and follicle cells. To begin our investigation into the source of FSC niche signals, we collected lines that express Gal4 in each of these different cell types. Bab1-Gal4-5 is commonly used to express transgenes specifically in terminal filament cells and cap cells (Cabrera et al., 2002), which can be identified by their position at the anterior tip of the germarium and by bright Lamin C staining on the nuclear membrane. As expected, when we combined this driver with UAS::CD8-GFP, we observed strong GFP expression specifically in terminal filament cells and cap cells (Fig. 1B).
109-30-Gal4 has been reported to be expressed in the FSC lineage (Hartman et al., 2010), and we indeed observed consistently high levels of expression in the FSCs and all follicle cells in the germarium (Fig. 1D), although we also occasionally observed GFP expression in one to four posterior escort cells adjacent to the FSCs. Likewise, we confirmed that Tj-Gal4 is expressed in escort cells, FSCs and follicle cells (Hayashi et al., 2002; Morris and Spradling, 2011) (Fig. 1E).
Lastly, we identified one line from the Janelia Gal4 collection (Pfeiffer et al., 2008), 13C06-Gal4, that expresses Gal4 throughout the anterior half of the germarium. We found that 13C06-Gal4 drives high levels of expression in: posterior escort cells, which surround cysts in region 2a; FSCs; and prefollicle cells near the region 2a/2b border. In addition, we observed low levels of expression in the escort cells in region 1 and occasionally in one to two cap cells (Fig. 1C).
Escort cells are the predominant source of Wg for the FSC lineage
Wg signaling is required for FSC self-renewal and proliferation (Song and Xie, 2003), and perturbations in Wg signaling lead to severe follicle formation defects (Li et al., 2010; Song and Xie, 2003). For example, when Wgts flies are shifted to the non-permissive temperature, follicle cell production is reduced and germ cell cysts entering into the follicle epithelium fail to bud from the germarium as distinct follicles (Song and Xie, 2003). These phenotypes are likely to be due to a defect in somatic cells because essential genes in the Wg pathway are not required in the germline (Song et al., 2002). To determine which population of cells is the source of Wg for the FSC lineage, we used the Gal4 drivers described above in combination with a temperature-sensitive Gal80 driven by a constitutive tubulin promoter (tub-Gal80ts) to knock down Wg expression by RNAi specifically during adulthood in distinct subsets of somatic cells in the germarium.
First, we examined Wg protein levels by immunofluorescence in each genotype. Consistent with previous studies (Forbes et al., 1996b; Song and Xie, 2003), we found that Wg protein was detectable in the terminal filament cells and cap cells in wild-type germaria (supplementary material Fig. S1A). Likewise, Wg was detectable in the terminal filament cells and cap cells when WgRNAi was driven in escort cells or follicle cells (supplementary material Fig. S1C,D). By contrast, Wg was substantially reduced in the terminal filament cells and cap cells of most germaria when WgRNAi was driven in apical cells (supplementary material Fig. S1B).
Next, we looked for follicle formation defects in each genotype. Surprisingly, we did not observe a significant number of follicle formation defects when WgRNAi was driven in apical cells (Fig. 2A,D). Indeed, we found that only 7.4% of ovarioles had follicle formation defects at 7 days after temperature shift (DATS) compared with 11.3% at 7 DATS in the control, and that this rose to only 15.6% by 21 DATS (versus 9.3% in the control) (Fig. 2G). Likewise, we did not see a statistically significant occurrence of the follicle defect phenotype when WgRNAi was driven by 109-30. Just 2.9% of the germaria had follicle formation defects at 7 DATS, and this increased to only 9.1% by 21 DATS (Fig. 2C,F,G). To determine whether oogenesis was proceeding in these ovarioles, we quantified the rate of follicle cell cycle progression using an EdU incorporation assay. The frequency of EdU+ follicle cells in the germarium was not significantly different from that of the control when WgRNAi was driven in either apical cells or follicle cells (6.4% in the control versus 5.6% or 5.1% in flies with Bab1-Gal4 or 109-30, respectively; Fig. 2J). Collectively, these observations indicate that terminal filament cells, cap cells and follicle cells are not significant sources of Wg for the FSC lineage.
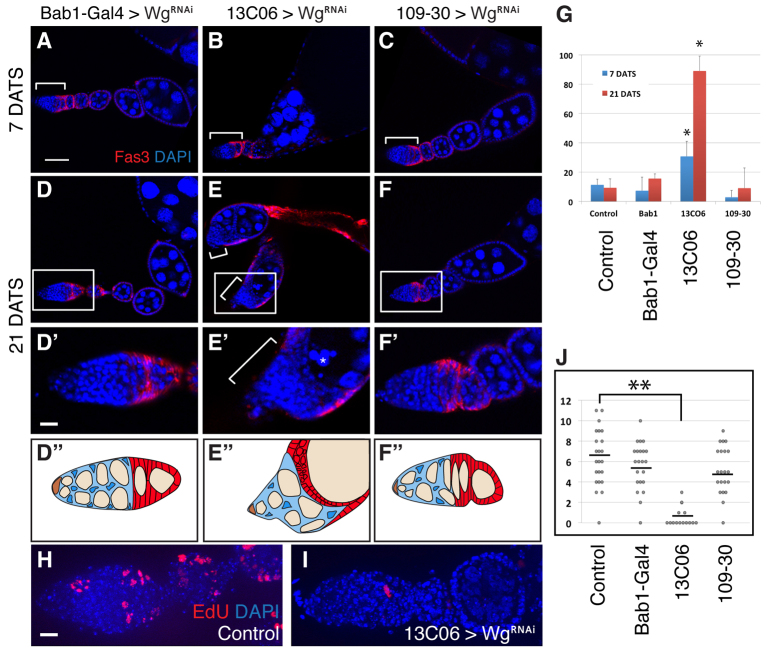
Fig. 2.
Wg produced by escort cells is required for follicle formation. (A-F) Ovarioles in which WgRNAi is expressed in apical cells by Bab1-Gal4 (A,D), escort cells by 13C06 (B,E) or follicle cells by 109-30 (C,F) at 7 (A-C) or 21 (D-F) days after flies were shifted to 29°C to repress tub-Gal80ts and promote Gal4 activity. Tissue is stained for Fas3 (red) and with DAPI (blue). Brackets indicate the extent of the germarium. Boxed regions in D-F are magnified in D′-F′ and schematized in D′-F′ (in the diagrams, cap cells are orange, germ cells are tan, escort cells are blue, and the FSC lineage is red). The asterisk in E′ indicates an apoptotic germ cell. (G) Quantification of follicle formation defects in ovarioles of each genotype. Ovarioles from 13C06, tub-Gal80ts; TM2/+ flies were used as the control. Each data point is the mean of at least three replicates. Error bars represent s.d. *P<0.01 compared with control. Total n values are greater than 200 ovarioles for each data point. (H,I) Ovarioles from 13C06, tub-Gal80ts; TM2/+ (control, H) or 13C06, tub-Gal80ts; WgRNAi/+ (I) that were incubated with EdU for 2 hours, fixed, and stained for EdU (red) and with DAPI. (J) Quantification of the number of EdU+ follicle cells in ovarioles of each genotype. **P<0.001. Anterior is to the left. Scale bars: 25 μm in A-F; 5 μm in D′-F′,H,I.
By contrast, we observed a dramatic phenotype when WgRNAi was driven in escort cells. By 7 DATS, 30.8% of the germaria had severe cyst formation defects (Fig. 2B,G). By 21 DATS, nearly all ovarioles (89.0%) had severe follicle formation defects (Fig. 2E,G). In these ovarioles, regions 1 and 2a were still intact in most germaria, but regions 2b and 3 were no longer identifiable, and these ovarioles lacked the typical chain of developing follicles downstream from the germarium. Instead, a few mid- and late-stage follicles were clustered together and surrounded by a disorganized follicle epithelium (Fig. 2E). In addition, nurse cells frequently had fragmented nuclei (Fig. 2E′, asterisk), suggesting that they were undergoing apoptosis. Moreover, we observed a significant reduction in the frequency of EdU+ follicle cells at 21 DATS (0.6% versus 6.4% in the control; Fig. 2H-J).
These phenotypes suggest that Wg is expressed in escort cells, but Wg expression has not been detected in escort cells by immunofluorescence. However, escort cells have a very large surface area and Wg protein might not normally accumulate in sufficient quantity to be detectable. Therefore, we investigated Wg levels in germaria from shibirets flies, which are defective for Wg secretion at 29°C (Strigini and Cohen, 2000). We found that, by 2 hours after shifting to 29°C, Wg was visible as bright foci in both terminal filament cells and cap cells, and also throughout regions 1 and 2a, often near nuclei of a shape and position characteristic of escort cells (Fig. 3A). Many of these foci co-stained with an antibody against Wntless, which is known to be co-trafficked with Wg in vesicles (Fig. 3A) (Tang et al., 2012). This indicates that Wg protein is indeed present in escort cells.
Fig. 3.
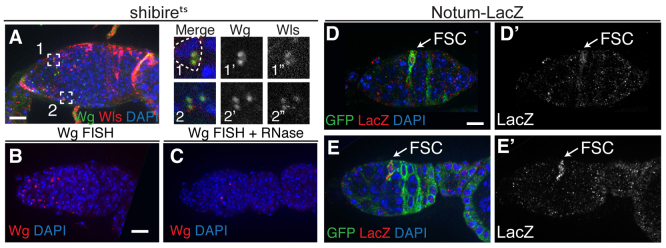
Wg is expressed in escort cells and Wg signaling is active specifically in FSCs. (A) A germarium from a shibirets fly that was shifted to the non-permissive temperature for 2 hours prior to dissection stained for Wg (green), Wntless (red) and with DAPI (blue). Wg+ puncta are visible throughout the region containing escort cells and many colocalize with Wntless. Boxed regions 1 and 2 are magnified to the right and shown as a merged image (1,2), Wg channel only (1′,2′) and Wntless channel only (1′,2′). The dashed line in box 1 shows a nucleus of a shape and position characteristic of escort cells. (B,C) Wild-type germaria stained with a FISH probe for wg transcript (red) and DAPI. Pretreatment of tissue with RNase (C) eliminates the signal, demonstrating that the FISH probe is specific for an RNA target. (D,E) The Wg pathway activity reporter Notum-lacZ is expressed in the anteriormost labeled cell of a GFP+ FSC clone. Tissue is stained for GFP (green), lacZ (β-galactosidase, red) and with DAPI. (D′,E′) The lacZ channel only. Anterior is to the left. Scale bars: 5 μm.
As a complementary approach, we assayed for wg transcript using a highly sensitive single-molecule fluorescence in situ hybridization (FISH) (Batish et al., 2011). We observed puncta throughout the anterior half of the germarium, and the signal was substantially diminished by pretreatment of the fixed tissue with RNase (Fig. 3B,C). Collectively, these data indicate that escort cells are the relevant source of Wg for the FSC lineage.
Wg is received specifically by FSCs and not follicle cells
To determine which cells within the germarium have active Wg signaling, we assayed for lacZ expression (by immunostaining for β-galactosidase) in flies with a sensitive Wg activity reporter, Notum-lacZ (Liu et al., 2008). We noted consistent lacZ expression in terminal filament cells, cap cells and outer muscle sheath cells, and sporadic low levels of lacZ expression in escort cells. Notably, germ cells were never lacZ+, consistent with the observation that Wg signaling is not required in the germline. Lastly, we found that Notum-lacZ is highly expressed in one to three cells at the region 2a/2b border. The shape and position of these cells suggested that they were FSCs and recently produced prefollicle cells. Therefore, we used lineage analysis to investigate this possibility.
FSCs can be reliably identified as the anteriormost labeled cell on the side of the germarium in a mature FSC clone (Nystul and Spradling, 2007). Nearby escort cells sometimes resemble FSCs, so if a labeled escort cell is adjacent to a labeled FSC then it could appear to be part of the FSC clone. However, escort cells rarely divide in adult ovaries (Kirilly et al., 2011; Morris and Spradling, 2011) and are thus unlikely to be labeled by a lineage-tracing system that requires mitosis to activate expression of the lineage marker. To determine whether Wg signaling is active specifically in FSCs, we first combined the Notum-lacZ reporter with a MARCM lineage-tracing system (Lee and Luo, 2001), which labels the lineage of mitotically active cells with GFP. Then, we generated clones in adult flies and assayed for lacZ and GFP expression.
We found that in 65% (n=87/134) of mature GFP+ FSC clones, the FSC at the base of the clone was lacZ+ (Fig. 3D,E), whereas in the remaining 35% of GFP+ FSC clones, all GFP+ cells, including the FSC, were lacZ-. In addition, we found that in most (91.7%) of the clones with a lacZ+ FSC, the FSC was the only lacZ+ cell in the clone. The remaining (8.3%) clones contained just one additional lacZ+ cell, which was always near the FSC (supplementary material Fig. S2A). Given their proximity to the FSC niche, these cells are likely to be recently produced prefollicle cells in which the β-galactosidase protein perdures. As expected, FSCs that were not part of a GFP+ clone could also be lacZ+. Specifically, we observed a GFP- lacZ+ cell that was likely to be an FSC based on its shape and position in 42.0% of the germaria examined (supplementary material Fig. S2B). Interestingly, we also noticed that a subset of Delta+ intestinal stem cells (ISCs) (Ohlstein and Spradling, 2007) were also lacZ+ (supplementary material Fig. S2C,D).
Next, to determine whether Wg pathway activity is sufficient to activate Notum-lacZ expression in the FSC lineage, we assayed for Notum-lacZ expression in Apc1-/-, Apc2-/- FSC clones. Apc1 and Apc2 are essential components of the β-catenin destruction complex, so the Wg pathway should be constitutively active in these cells. Indeed, we found that some FSC daughter cells within these clones ectopically expressed Notum-lacZ (supplementary material Fig. S3D). This indicates that ectopic Wg pathway activation is sufficient to induce activation of the Notum-lacZ reporter in at least a subset of FSC daughter cells within the germarium.
Finally, to determine which cells produce the Wg ligand that activates Notum-lacZ in FSCs, we combined the Notum-lacZ reporter with UAS-WgRNAi and either Bab1-Gal4, 109-30 or 13C06. There was little or no effect on the frequency of lacZ+ cells at the region 2a/2b border when WgRNAi was driven in cap and terminal filament cells or in follicle cells (supplementary material Table S1, Fig. S3A,C). By contrast, we observed a substantial reduction in the frequency of germaria with lacZ+ cells at the region 2a/2b border compared with the control population (35% versus 75%; supplementary material Table S1, Fig. S3B) when WgRNAi was driven in escort cells. Furthermore, we observed a significant correlation between the lack of lacZ+ cells at the region 2a/2b border and the presence of follicle formation defects when WgRNAi was driven in escort cells (supplementary material Table S1). These observations indicate that, within the FSC lineage, Wg signal transduction is active specifically in FSCs, and therefore the defects that we observe when Wg is removed from escort cells are due to a defect specifically in the FSCs.
Hh is produced by multiple cell types
Hh signaling is required for both FSC self-renewal (Vied and Kalderon, 2009; Zhang and Kalderon, 2001) and for the proliferation and differentiation of prefollicle cells. Consistent with previous studies (Forbes et al., 1996a; Zhao et al., 2008), we found that an enhancer trap located in the hh locus (Hh-lacZ) is expressed at high levels in terminal filament cells, cap cells and anterior escort cells, and at lower levels in posterior escort cells (Fig. 4A), but Hh protein is only clearly detectable in terminal filament cells and cap cells by immunofluorescence in wild-type germaria (Fig. 4B) (Forbes et al., 1996a; Hartman et al., 2010). However, as with Wg, Hh protein may be sparse in escort cells and therefore difficult to detect. Therefore, we investigated Hh levels in germaria in which Hh protein trafficking in escort cells was blocked by the ectopic expression of Rab5DN (Callejo et al., 2011). By 2 days after the temperature shift to activate Rab5DN expression, Hh protein was clearly detectable in escort cells as well as in cap cells and terminal filament cells (Fig. 4C). Importantly, this signal was substantially reduced by co-expression of HhRNAi with Rab5DN (Fig. 4D), which confirms that the staining was specific for Hh. Lastly, we assayed for the presence of hh transcript in escort cells and prefollicle cells by FISH, as described above. Again, we observed bright puncta throughout the anterior half of the germarium (Fig. 4E) and found that this signal was eliminated by pretreatment of the tissue with RNase (Fig. 4F). Together, these data are consistent with the pattern of Hh-lacZ expression in the germarium and indicate that Hh is expressed in terminal filament cells, cap cells and escort cells.
Fig. 4.
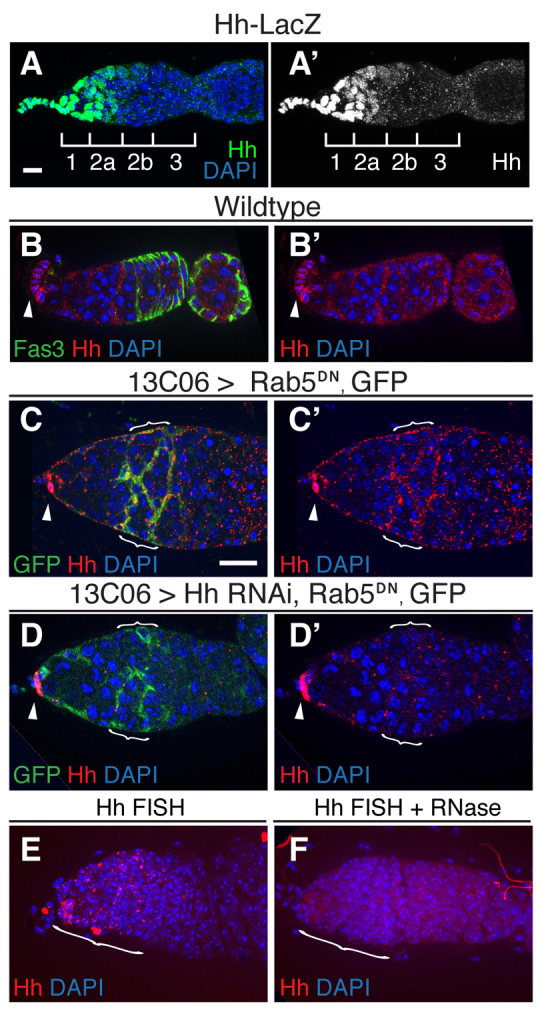
Hh is expressed in escort cells. (A,A′) Hh-lacZ expression in the germarium. Tissue is stained for lacZ (β-galactosidase, green) and with DAPI (blue). Regions 1, 2a, 2b and 3 are indicated. (B,B′) Wild-type germarium stained for Fas3 (green) to highlight follicle cell membranes, Hh (red) and with DAPI. Hh signal is bright in cap cells (arrowheads). (C-D′) Germaria in which GFP and Rab5DN are driven in escort cells by 13C06 2 days after flies were shifted to 29°C to repress tub-Gal80ts and promote Gal4 activity. Tissue is stained for GFP (green) to visualize the extent of Gal4 expression, Hh (red) and with DAPI. (C) Hh puncta are abundant on escort cell membranes in region 2a, where Gal4 expression is high (brackets), and sparse in region 1 where Gal4 expression is lower. In addition, as in wild-type germaria, Hh signal is bright in cap cells (arrowheads). Co-expression of HhRNAi with Rab5DN (D) significantly decreases Hh staining in escort cells (brackets) but not cap cells (arrowhead). (E,F) Wild-type germaria stained with a FISH probe for hh transcript (red) and with DAPI. Pretreatment of tissue with RNase (F) eliminates the signal, demonstrating that the FISH probe is specific for an RNA target. Anterior is to the left. Scale bars: 5 μm.
Multiple cell types respond to Hh signaling in the germarium
To determine which cells are the source of the Hh ligand that acts on the early FSC lineage, we combined UAS-HhRNAi with tub-Gal80ts and either Bab1-Gal4, 13C06 or 109-30, and assayed for follicle formation defects in adult ovaries at 7 and 21 DATS as described above. At 21 DATS, we found a significantly higher frequency of germaria with follicle formation defects as compared with the control population when UAS-HhRNAi was expressed in apical cells or escort cells (32.1% or 53.3% for flies with Bab1-Gal4 or 13C06, respectively, versus 12.4% for control) (Fig. 5A-F). Specifically, we observed ovarioles with disorganized and discontinuous follicle epithelia (Fig. 5B,C), fused cysts (Fig. 5D, arrow) and defective follicle budding from the germarium (Fig. 5F, arrow). Next, we investigated the frequency of follicle formation defects when HhRNAi was driven more broadly throughout the germarium by either Tj-Gal4 alone or by Tj-Gal4 and Bab1-Gal4 in combination, and found that this produced an even higher frequency of follicle formation defects (48.2% and 56.2%, respectively) (Fig. 5G-K).
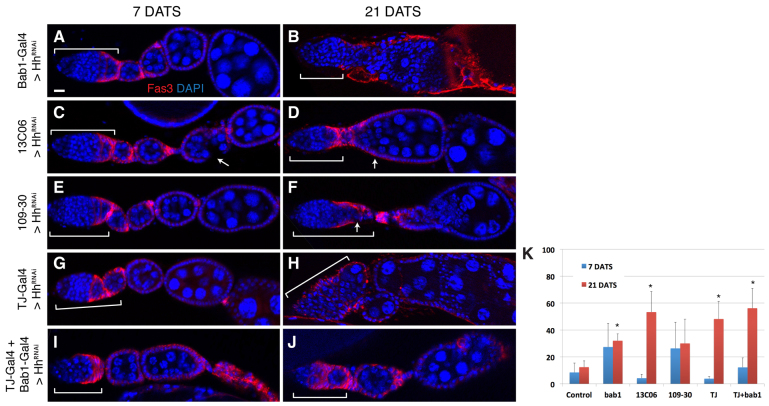
Fig. 5.
Hh produced by multiple sources is required for follicle formation. (A-J) Ovarioles in which HhRNAi is driven by Bab1-Gal4 (A,B), 13C06 (C,D), 109-30 (E,F), Tj-Gal4 (G,H) or both Bab1-Gal4 and Tj-Gal4 (I,J) at 7 (A,C,E,G,I) or 21 (B,D,F,H,J) days after flies were shifted to 29°C to repress tub-Gal80ts and promote Gal4 activity. Tissue is stained for Fas3 (red) and with DAPI (blue). Brackets indicate the extent of the germarium. Arrows indicate follicle cell defects. (K) Quantification of follicle formation defects in ovarioles of each genotype. Each data point is the mean of at least three replicates. Ovarioles from 13C06, tub-Gal80ts; TM2/+ flies were used as the control. *P<0.05 compared with the control; error bars represent s.d. Total n values are greater than 200 ovarioles for each data point. Anterior is to the left. DATS, days after temperature shift. Scale bar: 5 μm.
To determine which cells have active Hh signaling in the germarium, we used a sensitive reporter of Hh pathway activity, Ptc-pelican-GFP(nls). This construct contains multiple Cubitus interruptus binding sites upstream of GFP and activates expression of a nuclear-localized GFP specifically in cells with active Hh signaling (T. Kornberg, personal communication). To verify the fidelity of this reporter, we examined GFP expression in wing discs. As expected (Phillips et al., 1990), we observed a bright stripe of GFP expression along the anteroposterior boundary, with tapered expression toward the anterior (supplementary material Fig. S4A). In germaria, Ptc-pelican-GFP(nls) expression closely resembled Ptc-lacZ expression, but was brighter and more consistent. Specifically, escort cells, FSCs and all follicle cells were GFP+, and the level of GFP expression tapered off in an anterior-to-posterior gradient (Fig. 6A).
Fig. 6.
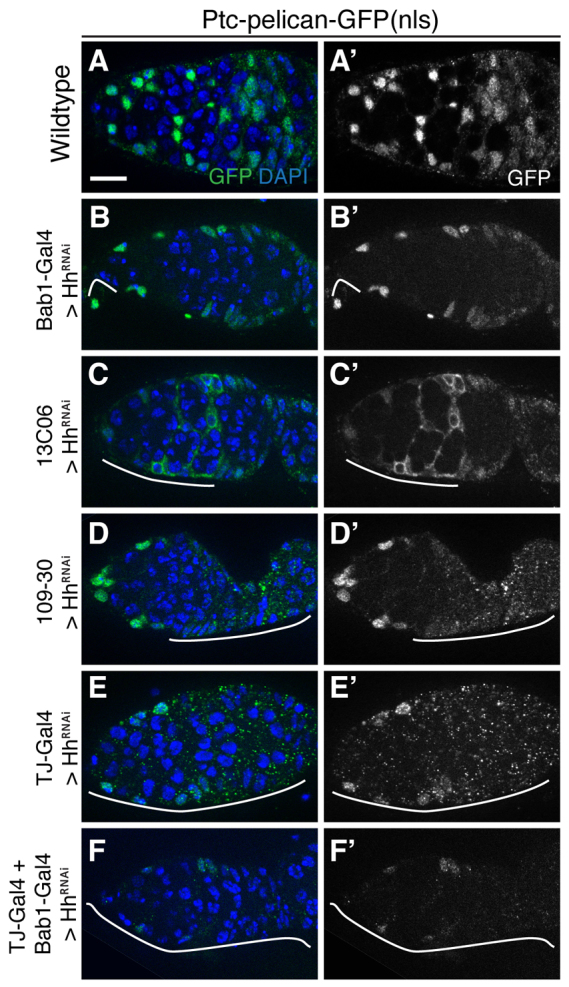
Multiple cell types have active Hh signaling. (A-F) Ovarioles from flies at 7 DATS that contain Ptc-pelican-GFP(nls), UAS-HhRNAi, tub-Gal80ts, and either no Gal4 driver (‘wild type’, A), Bab1-Gal4 (B), 13C06 (C), 109-30 (D), Tj-Gal4 (E) or Bab1-Gal4 and Tj-Gal4 (F) stained for GFP (green) to visualize Ptc-pelican-GFP(nls) expression and with DAPI (blue). (A-F′) GFP channel only. In wild type, GFP expression is bright in escort cells and tapers off toward the posterior in follicle cells. Germ cells are GFP-. In C, the germarium also includes UAS::CD8-GFP, which labels the membranes of cells expressing Gal4. Note the absence of the nuclear Ptc-GFP signal in these cells. White lines indicate the approximate range of Gal4 expression. Anterior is to the left. Scale bar: 5 μm.
Next, we examined Ptc-pelican-GFP(nls) expression in germaria in which HhRNAi expression is controlled by tub-Gal80ts and each of the four Gal4 drivers alone, as well as by Tj-Gal4 and Bab1-Gal4 in combination at 7 DATS. GFP expression was substantially decreased in all cases, particularly within the regions of the germarium in which the HhRNAi was expressed (Fig. 6B-E). Specifically, GFP levels in escort cells were most affected when HhRNAi was expressed in apical cells or escort cells (Fig. 6B,C), whereas GFP levels in follicle cells were affected when HhRNAi was expressed in follicle cells (Fig. 6D). GFP expression was decreased throughout the germarium when HhRNAi was expressed broadly by Tj-Gal4 (Fig. 6E) or by both Tj-Gal4 and Bab1-Gal4 together (Fig. 6F). Taken together, these results suggest that Hh is produced by multiple cell types in the germarium, and that the ligand secreted by these sources acts in an additive manner on escort cells, FSCs and prefollicle cells.
FSCs contact multiple escort cells
The best-studied function of escort cells is to support germ cell development in regions 1 and 2a. To better understand how escort cells interact with both germ cells and the FSC niche, we generated twin-spot MARCM escort cell clones (Yu et al., 2009) by heat shocking flies of the appropriate genotype during pupal development (Fig. 7). Consistent with previous studies (Kirilly et al., 2011), we found that posterior escort cells have long membrane extensions (Fig. 7A,B), and that multiple escort cells encapsulate each germ cell cyst in regions 1 and 2a (Fig. 7D,E). In addition, we frequently observed germaria in which at least two escort cells produced membrane extensions that traversed the region 2a/2b border, immediately adjacent to the follicle cell membranes (Fig. 7C). Lastly, we noticed that multiple escort cells frequently contacted a single FSC niche (Fig. 7F), and that the nuclei of these escort cells could be positioned anywhere throughout region 2a, even as far as a full cyst diameter away (GFP+ escort cell in Fig. 7A,E). Therefore, our data suggest that escort cells throughout region 2a function in aggregate to support both germ cell development and FSC self-renewal.
Fig. 7.
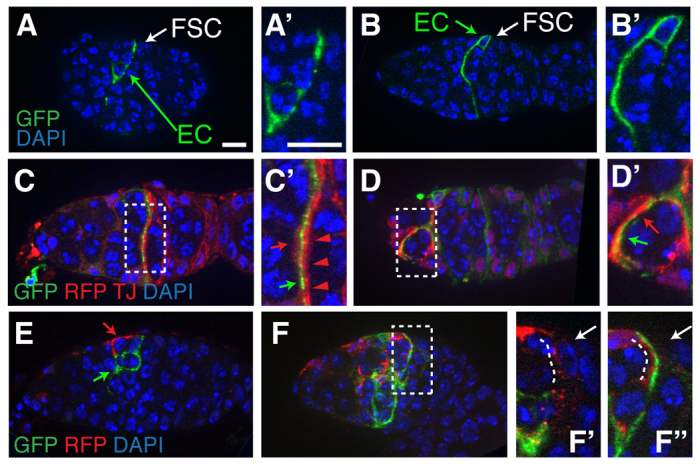
Multiple escort cells surround region 2a cysts and contact the FSC niche. Germaria with twin-spot MARCM escort cell clones. (A-B′) Germaria with a single GFP+ posterior escort cell. Although the escort cell nucleus can be in the center of the germarium (A) or on the edge (B), escort cells in this region always have extensive membranes that wrap around the region 2a cysts. The regions containing the single labeled escort cells are expanded in A′ and B′. (C-E) Germaria with both GFP+ and RFP+ escort cells reveal that multiple escort cell membranes (red and green arrows, C′,D′) traverse the germarium at the region 2a/2b border (C) and contact follicle cell membranes (red arrowheads, C′) and surround each cyst in region 1 (D) and 2a (E). Boxed regions in C and D are magnified in C′ and D′. (F-F′) Multiple escort cell membranes also contact the FSC niche. Boxed region in F is magnified in F′ and F′, which show single optical sections in which the membrane extension from the RFP+ escort cell (F′) or GFP+ escort cell (F′) is adjacent to the FSC niche. White dotted lines in F′ and F′ indicate the escort cell membranes and white arrows indicate the position of the FSC niche. Germaria are imaged for DAPI (blue) and GFP (green), RFP (red) and Traffic jam (Tj) (red, C and D only). All images are single optical sections except D, which is a maximum projection of two optical sections. Anterior is to the left. Scale bars: 5 μm.
DISCUSSION
Taken together, the results of this study challenge the notion that the FSC niche is maintained by gradients of ligands produced solely at distant sites (Vied et al., 2012). Instead, our data indicate that the FSC niche has a more canonical architecture in which at least some key niche signals are produced locally (Amoyel et al., 2013; Losick et al., 2011; Michel et al., 2012), although the FSC niche might also differ from other well-characterized niches in some ways, such as the extent to which it remodels during adulthood. Notably, our results do not contradict the observation that Hh protein relocalizes from apical cells to the FSC niche during changes from a poor to a rich diet (Hartman et al., 2013), as our flies were consistently maintained on nutrient-rich media. It will be interesting to investigate how such distantly produced ligands interact with locally produced niche signals to control FSC behavior during normal homeostasis and in response to stresses.
In addition, our results confirm and extend the conclusion that Wg acts specifically on FSCs (Song and Xie, 2003) and ISCs (Lin et al., 2008), thus highlighting the role of Wg as a specific epithelial stem cell niche factor. As in other types of stem cell niches, this specificity could be achieved through multiple mechanisms, including local delivery of the Wg ligand to the niche and crosstalk with other pathways such as Notch and Hh, which are known to interact with the Wg pathway (DiNardo et al., 1988; Muñoz-Descalzo et al., 2012; van den Brink et al., 2004). Although the precise function(s) of Wg signaling in FSCs is unclear, our observation that a reduction in Wg ligand results in a backup of cysts near the FSC niche at the region 2a/2b border and fused cysts downstream from the FSC niche suggests that one role is to promote FSC proliferation. In addition, the finding that FSC daughter cells with ectopic Wg signaling fail to form into a polarized follicle epithelium (Li et al., 2010; Song and Xie, 2003) suggests that Wg signaling might also promote self-renewal in FSCs by suppressing the follicle cell differentiation program.
By contrast, our observations and published studies indicate that Hh signaling is not specific for the FSC niche but instead constitutes a more general signal that derives from multiple sources and regulates proliferation and differentiation in both FSCs and prefollicle cells. Consistent with this conclusion, Hh signaling is active throughout the germarium (Fig. 6A) (Forbes et al., 1996a) and is required both in FSCs to promote self-renewal (Vied and Kalderon, 2009; Zhang and Kalderon, 2001) and in prefollicle cells to promote development toward the stalk and polar lineages (Forbes et al., 1996a; Tworoger et al., 1999).
Finally, our multicolor labeling of somatic cells in the germarium indicated that multiple densely packed escort cell membranes surround region 2a cysts and contact the FSC niche. Although we cannot rule out the possibility that one or more cells in this region are dedicated FSC niche cells, our observations strongly suggest that at least some escort cells contribute to both germ cell development and the FSC niche. Since these escort cells are dynamic (Morris and Spradling, 2011), constantly changing their shape and position to facilitate the passage of germ cell cysts, it is perhaps somewhat surprising that the FSCs are so stable in the tissue. Indeed, the rate of FSC turnover is comparable to that of female GSCs, which are maintained by a dedicated and more static niche cell population (Margolis and Spradling, 1995). It will be interesting to investigate how this dynamic population of escort cells is able to maintain such a stable microenvironment for the FSCs. One possibility is that redundant sources of niche signals may allow niches of this type to partially break down and reform as needed to rapidly accommodate the changing demands of the tissue.
Our observations reinforce several themes that are emerging from recent studies of stem cell niches in different epithelial tissues. First, as in the FSC niche, the Wnt/Wg signaling pathway is a key stem cell niche signal in many Drosophila and mammalian epithelial tissues. Second, in several epithelial tissues, the stem cell self-renewal signals are also known to be produced by differentiated cells rather than a dedicated niche cell population. For example, Drosophila ISCs receive self-renewal signals from both nearby enterocytes (Jiang et al., 2009) and the surrounding visceral muscle (Lin et al., 2008; Lin et al., 2010). Likewise, mammalian ISCs at the base of the crypt receive self-renewal signals from Paneth cells (Sato et al., 2011), which are adjacent secretory cells with antimicrobial functions. Lastly, several epithelial niches have recently been shown to have a transitory capacity that may resemble the dynamic nature of the FSC niche. For example, stem cell niches can form de novo in the Drosophila intestine to accommodate increased food availability (O’Brien et al., 2011), and in the mammalian skin in response to hyperactive Wnt signaling (Lo Celso et al., 2004). In addition, mammalian ISCs produce niche cells in vivo (Barker et al., 2007) and can spontaneously reform a niche in culture (Sato et al., 2009). In all of these examples, it seems likely that the relationship between the epithelial stem cell and its niche is not static, but instead flexible and dynamic. Further studies of the Drosophila FSC niche and these other experimental models will continue to provide insights into the mechanism by which a dynamic epithelial stem cell niche functions.
MATERIALS AND METHODS
Fly stocks
Stocks were maintained on standard molasses food at 25°C and adults were given fresh wet yeast daily. The following stocks were used: (1) yw, hsflp, UAS::CD8-GFP; FRT40a, tubGal80; tub-Gal4/TM6; (2) y1w, hsflp, UAS::CD8-GFP, tubGal4; FRT82B,tub-Gal80/TM6; (3) yw; Notum-lacZ (from Dr Ken Cadigan, University of Michigan, Ann Arbor, USA); (4) yw; Hh-lacZ (from Allan Spradling, Carnegie Institution for Science, Baltimore, USA); (5) yw; Traffic jam Gal4 (from Guy Tanentzapf, University of British Colombia, Vancouver, Canada); (6) hsFlp, FRT40A, UAS-Cd2::rfp, FRT40A, UAS-Cd8::gfp, UAS-Cd2-Mir/CyO,Y (from Tzumin Lee, Janelia Farm, Ashburn, USA); (7) yw*; Ptc-pelican (from Tom Kornberg, University of California San Francisco, San Francisco, USA); (8) yw*; FRT40a/Cyo, y1, sc, v1; P{TRiP.HMS00844}attP2, y1 sc* v1; P{TRiP.HMS00492}attP2/TM3, Sb1, w[*]; P{w[+mW.hs]=GawB}bab1[Agal4-5]/TM3, Sb[1], y1 w*; P{GawB}109-30/CyO, w[*]; P{w[+mC]=tubP-GAL80[ts]}20; TM2/TM6B, Tb[1], w1118; P{GMR13C06-GAL4}attP2 (from the Bloomington Stock Center, Bloomington, USA); and (9) yw;; FRT82B, Apc1Q8, Apc2G10 (from Mark Peifer, University of North Carolina, Chapel Hill, USA).
Temperature-sensitive regulation of Gal4 activity
To promote Gal4 expression specifically during adulthood, flies bearing a tub-Gal80ts construct were raised at 18°C and shifted to 29°C after at least 2 days post-eclosion. Phenotypes were quantified and significance was determined with a two-tailed t-test or, for supplementary material Table S1, a two-tailed Fisher’s exact test.
Immunostaining
Ovaries were dissected in 1× PBS, fixed in 1× PBS + 4% formaldehyde for 15 minutes, rinsed, and incubated with primary antibodies overnight at 4°C. Next, the tissues were washed three times over the course of an hour, incubated with secondary antibodies for 2 hours, and washed three times again over the course of an hour at room temperature. Finally, tissues were rinsed in 1× PBS, incubated in 1× PBS + 1 μg/ml DAPI for 5 minutes and mounted on glass slides in Vectashield (Vector Labs). 1× PBS with 0.3% Tween 20 (PBST) was used for all rinses and washes and to dilute antibodies. To detect Wg protein, the tissue was incubated with anti-Wg antibody for 30-60 minutes on ice prior to fixation as described previously (Strigini and Cohen, 2000), and then fixed and processed as described above.
The following primary antibodies were used: mouse anti-Wg (1:4; 4D4), mouse anti-Fas3 (1:100; 7G10) and mouse anti-Lamin C (1:100; LC28.26) (all from the Developmental Studies Hybridoma Bank); rabbit anti-Hh (1:500) (Taylor et al., 1993), rabbit anti-Wntless (1:1000) (from Konrad Basler, University of Zurich, Switzerland), rabbit anti-GFP (1:2000; TP401, Torrey Pines Biolabs) and mouse anti-β-galactosidase (1:1000; Z3781, Promega). The following secondary antibodies were used: anti-rabbit and anti-mouse conjugated to Alexa Fluor 488, 546 or 555 (1:1000; A11001, A11008, A11010, A21424, Invitrogen).
FISH
Stellaris RNA FISH probes (Biosearch Technologies) were custom ordered for Drosophila hh and wg transcripts (see supplementary material Table S2 for sequences), and the manufacturer’s protocol was modified for labeling of Drosophila ovaries. Briefly, ovaries were dissected in RNase-free 1× PBS (Invitrogen) and fixed in 1× PBS + 4% paraformaldehyde (Fixation Buffer) at room temperature. Samples were washed three times in 1× PBS, placed in 70% ethanol for 2 hours at 4°C and then rehydrated in 10% formamide in 2× SSC (Wash Buffer) at room temperature for 5 minutes. Next, the probe was diluted to 1 μM in 2× SSC with 100 mg/ml dextran sulfate and 10% formamide (Hybridization Buffer) and incubated with the tissue, first at 37°C for 15 minutes and then at 30°C overnight. Next, the tissue was washed twice in Wash Buffer for 30 minutes each, with the last wash containing 1 μg/ml DAPI. Finally, the tissues were mounted on glass slides in Vectashield and imaged within 12 hours of mounting. For RNase treatment, ovaries were incubated with 50 units of RNase If (New England BioLabs) in NEB Buffer 3 at 37°C for 1 hour during the fixation and wash steps. Then, the tissue was incubated at 70°C for 20 minutes to heat inactivate the RNase, and washed twice with 1× PBS. The hybridization protocol was then continued as described above, starting with the ethanol dehydration step.
EdU incorporation
For EdU incorporation experiments, ovaries were dissected and incubated in Schneider’s Medium supplemented with 15% fetal bovine serum plus 20 μM 5-ethynyl-2′-deoxyuridine (EdU) (Click-it Cell Proliferation Assays, Invitrogen) at room temperature for 2 hours. Ovaries were then fixed in 4% paraformaldehyde for 15 minutes, washed twice in 1× PBS, permeabilized in 1× PBST for 30 minutes, washed twice in 1× PBS, and then incubated in the Click-it Cell Proliferation Assay reaction cocktail for 30 minutes at room temperature. Finally, tissues were rinsed in 1× PBS, incubated in 1× PBS + 1 μg/ml DAPI for 5 minutes and mounted on glass slides in Vectashield.
Clone induction
Clones were generated by culturing flies of the appropriate genotype with fresh wet yeast for at least 2 days to ensure maturity to adulthood and then heat shocking in culture vials without food in a 37°C water bath either once for 15 minutes to achieve labeling of single escort cells, or once for 30 minutes to achieve labeling of multiple escort cells. To generate twin-spot MARCM escort cell clones, progeny were heat shocked once for 1 hour at the onset of pupation, 5-6 days after egg laying. To generate MARCM GFP+ FSC clones, adult flies were heat shocked at 37°C twice for 1 hour, 8 hours apart, and dissected at 3 days after heat shock.
Supplementary Material
Acknowledgments
We thank Tom Kornberg, Zena Werb, Yassi Hafezi and Angela Castanieto for critical comments on the manuscript; Tom Kornberg for Ptc-GFP(nls) (unpublished) and our colleagues in the fly community (cited in the Materials and methods section) for fly stocks and antibodies; and the Bloomington Stock Center and the Developmental Studies Hybridoma Bank for curation of many stocks and reagents used in this study.
Footnotes
Competing interests
The authors declare no competing financial interests.
Author contributions
P.S.-H. conceived of, designed and performed the experiments, analyzed the data and assisted in writing the paper. T.G.N. conceived of the project, designed the experiments, analyzed the data and wrote the paper.
Funding
P.S.-H. is in the UCSF Biomedical Sciences graduate program and is supported by the California Institute for Regenerative Medicine [grant number TG2-01153]; and this work was supported by a National Institutes of Health grant [R01 GM097158] to T.G.N. Deposited in PMC for release after 12 months.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.098558/-/DC1
References
- Amoyel M., Sanny J., Burel M., Bach E. A. (2013). Hedgehog is required for CySC self-renewal but does not contribute to the GSC niche in the Drosophila testis. Development 140, 56–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N., van Es J. H., Kuipers J., Kujala P., van den Born M., Cozijnsen M., Haegebarth A., Korving J., Begthel H., Peters P. J., et al. (2007). Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449, 1003–1007 [DOI] [PubMed] [Google Scholar]
- Batish M., Raj A., Tyagi S. (2011). Single molecule imaging of RNA in situ. Methods Mol. Biol. 714, 3–13 [DOI] [PubMed] [Google Scholar]
- Blanpain C., Fuchs E. (2006). Epidermal stem cells of the skin. Annu. Rev. Cell Dev. Biol. 22, 339–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera G. R., Godt D., Fang P.-Y., Couderc J.-L., Laski F. A. (2002). Expression pattern of Gal4 enhancer trap insertions into the bric à brac locus generated by P element replacement. Genesis 34, 62–65 [DOI] [PubMed] [Google Scholar]
- Callejo A., Bilioni A., Mollica E., Gorfinkiel N., Andrés G., Ibáñez C., Torroja C., Doglio L., Sierra J., Guerrero I. (2011). Dispatched mediates Hedgehog basolateral release to form the long-range morphogenetic gradient in the Drosophila wing disk epithelium. Proc. Natl. Acad. Sci. USA 108, 12591–12598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Celso C., Prowse D. M., Watt F. M. (2004). Transient activation of beta-catenin signalling in adult mouse epidermis is sufficient to induce new hair follicles but continuous activation is required to maintain hair follicle tumours. Development 131, 1787–1799 [DOI] [PubMed] [Google Scholar]
- DiNardo S., Sher E., Heemskerk-Jongens J., Kassis J. A., O’Farrell P. H. (1988). Two-tiered regulation of spatially patterned engrailed gene expression during Drosophila embryogenesis. Nature 332, 604–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes A. J., Lin H., Ingham P. W., Spradling A. C. (1996a). hedgehog is required for the proliferation and specification of ovarian somatic cells prior to egg chamber formation in Drosophila. Development 122, 1125–1135 [DOI] [PubMed] [Google Scholar]
- Forbes A. J., Spradling A. C., Ingham P. W., Lin H. (1996b). The role of segment polarity genes during early oogenesis in Drosophila. Development 122, 3283–3294 [DOI] [PubMed] [Google Scholar]
- Hartman T. R., Zinshteyn D., Schofield H. K., Nicolas E., Okada A., O’Reilly A. M. (2010). Drosophila Boi limits Hedgehog levels to suppress follicle stem cell proliferation. J. Cell Biol. 191, 943–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman T. R., Strochlic T. I., Ji Y., Zinshteyn D., O’Reilly A. M. (2013). Diet controls Drosophila follicle stem cell proliferation via Hedgehog sequestration and release. J. Cell Biol. 201, 741–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi S., Ito K., Sado Y., Taniguchi M., Akimoto A., Takeuchi H., Aigaki T., Matsuzaki F., Nakagoshi H., Tanimura T., et al. (2002). GETDB, a database compiling expression patterns and molecular locations of a collection of Gal4 enhancer traps. Genesis 34, 58–61 [DOI] [PubMed] [Google Scholar]
- Jiang H., Patel P. H., Kohlmaier A., Grenley M. O., McEwen D. G., Edgar B. A. (2009). Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell 137, 1343–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirilly D., Spana E. P., Perrimon N., Padgett R. W., Xie T. (2005). BMP signaling is required for controlling somatic stem cell self-renewal in the Drosophila ovary. Dev. Cell 9, 651–662 [DOI] [PubMed] [Google Scholar]
- Kirilly D., Wang S., Xie T. (2011). Self-maintained escort cells form a germline stem cell differentiation niche. Development 138, 5087–5097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T., Luo L. (2001). Mosaic analysis with a repressible cell marker (MARCM) for Drosophila neural development. Trends Neurosci. 24, 251–254 [DOI] [PubMed] [Google Scholar]
- Li X., Han Y., Xi R. (2010). Polycomb group genes Psc and Su(z)2 restrict follicle stem cell self-renewal and extrusion by controlling canonical and noncanonical Wnt signaling. Genes Dev. 24, 933–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin G., Xu N., Xi R. (2008). Paracrine Wingless signalling controls self-renewal of Drosophila intestinal stem cells. Nature 455, 1119–1123 [DOI] [PubMed] [Google Scholar]
- Lin G., Xu N., Xi R. (2010). Paracrine unpaired signaling through the JAK/STAT pathway controls self-renewal and lineage differentiation of drosophila intestinal stem cells. J. Mol. Cell Biol. 2, 37–49 [DOI] [PubMed] [Google Scholar]
- Liu Y. I., Chang M. V., Li H. E., Barolo S., Chang J. L., Blauwkamp T. A., Cadigan K. M. (2008). The chromatin remodelers ISWI and ACF1 directly repress Wingless transcriptional targets. Dev. Biol. 323, 41–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losick V. P., Morris L. X., Fox D. T., Spradling A. (2011). Drosophila stem cell niches: a decade of discovery suggests a unified view of stem cell regulation. Dev. Cell 21, 159–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis J., Spradling A. (1995). Identification and behavior of epithelial stem cells in the Drosophila ovary. Development 121, 3797–3807 [DOI] [PubMed] [Google Scholar]
- Michel M., Kupinski A. P., Raabe I., Bökel C. (2012). Hh signalling is essential for somatic stem cell maintenance in the Drosophila testis niche. Development 139, 2663–2669 [DOI] [PubMed] [Google Scholar]
- Morris L. X., Spradling A. C. (2011). Long-term live imaging provides new insight into stem cell regulation and germline-soma coordination in the Drosophila ovary. Development 138, 2207–2215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison S. J., Spradling A. C. (2008). Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell 132, 598–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Descalzo S., de Navascues J., Arias A. M. (2012). Wnt-Notch signalling: an integrated mechanism regulating transitions between cell states. BioEssays 34, 110–118 [DOI] [PubMed] [Google Scholar]
- Nystul T. G., Spradling A. C. (2006). Breaking out of the mold: diversity within adult stem cells and their niches. Curr. Opin. Genet. Dev. 16, 463–468 [DOI] [PubMed] [Google Scholar]
- Nystul T. G., Spradling A. (2007). An epithelial niche in the Drosophila ovary undergoes long-range stem cell replacement. Cell Stem Cell 1, 277–285 [DOI] [PubMed] [Google Scholar]
- O’Brien L. E., Soliman S. S., Li X., Bilder D. (2011). Altered modes of stem cell division drive adaptive intestinal growth. Cell 147, 603–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlstein B., Spradling A. (2007). Multipotent Drosophila intestinal stem cells specify daughter cell fates by differential notch signaling. Science 315, 988–992 [DOI] [PubMed] [Google Scholar]
- Pfeiffer B. D., Jenett A., Hammonds A. S., Ngo T.-T. B., Misra S., Murphy C., Scully A., Carlson J. W., Wan K. H., Laverty T. R., et al. (2008). Tools for neuroanatomy and neurogenetics in Drosophila. Proc. Natl. Acad. Sci. USA 105, 9715–9720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips R. G., Roberts I. J., Ingham P. W., Whittle J. R. (1990). The Drosophila segment polarity gene patched is involved in a position-signalling mechanism in imaginal discs. Development 110, 105–114 [DOI] [PubMed] [Google Scholar]
- Sahai-Hernandez P., Castanieto A., Nystul T. G. (2012). Drosophila models of epithelial stem cells and their niches. Wiley Interdisciplinary Reviews: Developmental Biology 1, 447–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T., Vries R. G., Snippert H. J., van de Wetering M., Barker N., Stange D. E., van Es J. H., Abo A., Kujala P., Peters P. J., et al. (2009). Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459, 262–265 [DOI] [PubMed] [Google Scholar]
- Sato T., van Es J. H., Snippert H. J., Stange D. E., Vries R. G., van den Born M., Barker N., Shroyer N. F., van de Wetering M., Clevers H. (2011). Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 469, 415–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X., Xie T. (2002). DE-cadherin-mediated cell adhesion is essential for maintaining somatic stem cells in the Drosophila ovary. Proc. Natl. Acad. Sci. USA 99, 14813–14818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X., Xie T. (2003). Wingless signaling regulates the maintenance of ovarian somatic stem cells in Drosophila. Development 130, 3259–3268 [DOI] [PubMed] [Google Scholar]
- Song X., Zhu C.-H., Doan C., Xie T. (2002). Germline stem cells anchored by adherens junctions in the Drosophila ovary niches. Science 296, 1855–1857 [DOI] [PubMed] [Google Scholar]
- Strigini M., Cohen S. M. (2000). Wingless gradient formation in the Drosophila wing. Curr. Biol. 10, 293–300 [DOI] [PubMed] [Google Scholar]
- Tang X., Wu Y., Belenkaya T. Y., Huang Q., Ray L., Qu J., Lin X. (2012). Roles of N-glycosylation and lipidation in Wg secretion and signaling. Dev. Biol. 364, 32–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. M. A., Nakano Y. Y., Mohler J. J., Ingham P. W. P. (1993). Contrasting distributions of patched and hedgehog proteins in the Drosophila embryo. Mech. Dev. 42, 89–96 [DOI] [PubMed] [Google Scholar]
- Tworoger M., Larkin M. K., Bryant Z., Ruohola-Baker H. (1999). Mosaic analysis in the drosophila ovary reveals a common hedgehog-inducible precursor stage for stalk and polar cells. Genetics 151, 739–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Brink G. R., Bleuming S. A., Hardwick J. C. H., Schepman B. L., Offerhaus G. J., Keller J. J., Nielsen C., Gaffield W., van Deventer S. J. H., Roberts D. J., et al. (2004). Indian Hedgehog is an antagonist of Wnt signaling in colonic epithelial cell differentiation. Nat. Genet. 36, 277–282 [DOI] [PubMed] [Google Scholar]
- van der Flier L. G., Clevers H. (2009). Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu. Rev. Physiol. 71, 241–260 [DOI] [PubMed] [Google Scholar]
- Vied C., Kalderon D. (2009). Hedgehog-stimulated stem cells depend on non-canonical activity of the Notch co-activator Mastermind. Development 136, 2177–2186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vied C., Reilein A., Field N. S., Kalderon D. (2012). Regulation of stem cells by intersecting gradients of long-range niche signals. Dev. Cell 23, 836–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagers A. J. (2012). The stem cell niche in regenerative medicine. Cell Stem Cell 10, 362–369 [DOI] [PubMed] [Google Scholar]
- Yu H.-H., Chen C.-H., Shi L., Huang Y., Lee T. (2009). Twin-spot MARCM to reveal the developmental origin and identity of neurons. Nature Neurosci. 12, 947–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Kalderon D. (2001). Hedgehog acts as a somatic stem cell factor in the Drosophila ovary. Nature 410, 599–604 [DOI] [PubMed] [Google Scholar]
- Zhao R., Xuan Y., Li X., Xi R. (2008). Age-related changes of germline stem cell activity, niche signaling activity and egg production in Drosophila. Aging Cell 7, 344–354 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.