Abstract
Although the axial D-periodic spacing is a well-recognized nanomorphological feature of type I collagen fibrils, the existence of a distribution of values has been largely overlooked since its discovery seven decades ago. Studies based on single fibril measurements occasionally noted variation in D-spacing values, but accredited it with no biological significance. Recent quantitative characterizations supported that a 10-nm collagen D-spacing distribution is intrinsic to collagen fibrils in various tissues as well as in vitro self-assembly of reconstituted collagen. In addition, the distribution is altered in Osteogenesis Imperfecta and long-term estrogen deprivation. Bone collagen is organized into lamellar sheets of bundles at the micro-scale, and D-spacings within a bundle of a lamella are mostly identical, whereas variations among different bundles contribute to the full-scale distribution. This seems to be a very general phenomenon for the protein as the same type of D-spacing/bundle organization is observed for dermal and tendon collagen. More research investigation of collagen nanomorphology in connection to bone biology is required to fully understand these new observations. Here we review the data demonstrating the existence of a D-spacing distribution, the impact of disease on the distribution and possible explanations for the origin of D-spacing variations based on various collagen fibrillogenesis models.
Introduction
Type I collagen, composing 90% of the organic matrix of bone, has a crucial role in maintaining the structural integrity and functional properties of the bone. It modulates signal transduction of bone cells,1 provides the framework for mineral nucleation and growth,2 and contributes to the toughness and resilience of the bone.3 Compositional and conformational changes of bone collagen have profound influence on the bone properties, particularly the mechanical performance. Some of the well-known examples are genetic mutations on type I collagen sequence resulting in Osteogenesis Imperfecta (OI) phenotypes with brittle bones;4 nonenzymatic crosslinking of collagen resulting in accumulation of advanced glycation products and compromised bone strength.5 Our understanding of bone collagen structure–property relationships at the micron to submicron scale is still sparse. Many questions remain to be answered regarding how microstructural organization, fibril orientation and fine details of collagen fibril nanomorphology influence bone properties.
Important aspects of collagen nanomorphological features have been extensively studied in tissues other than the bone. For example, tendon collagen fibrils exhibit a distribution of diameters, and fibril diameter was shown to influence the mechanical properties.6,7 Fibril length in general can reach millimeters and tip-to-tip fusion further extends the length.8 In this review we will focus on one of the most recognized and functionally important aspects of collagen nanomorphology, the axial gap/overlap D-periodic spacing. Bone collagen D-spacing provides open sites for mineral nucleation, proteoglycan binding and crosslinks to occur.3 It also is an effective indicator of fibril strain during bone deformation.9 Evidence from recent research has demonstrated the close relationship of collagen fibril D-spacing with bone micro-organization,10 and significant nanomorphological changes in D-spacing related to bone diseases.11,12 These findings highlight the significance of bone collagen D-spacing variations, and provide insight into the potential mechanisms for the variations in type I collagen fibril D-spacing. Some common features in collagen nano- and microstructures are shared among bone and other biological tissues and connections amongst these tissues will be elucidated.
The significance of bone collagen nanomorphology
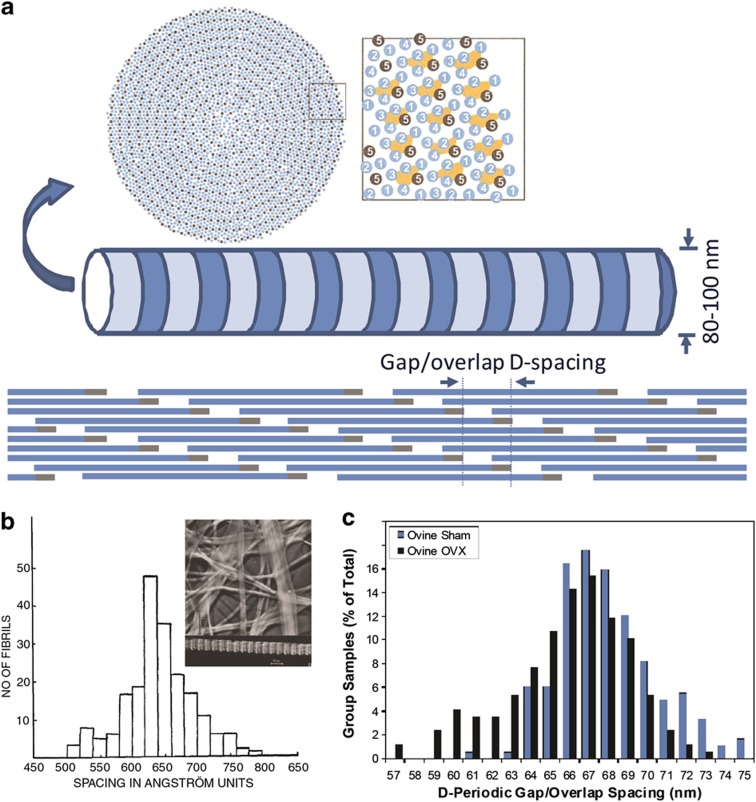
As a characteristic feature of collagen nanomorphology, the D-periodic spacing of collagen fibrils extracted from nerve, skin and cornea was shown in early electron micrographs,13 and an estimated 64.6±5.3 nm distribution of D-spacing was reported for human skin (Figure 1b).14 The use of X-ray diffraction (XRD) in studying subfibrillar packing of collagen fibrils soon gained popularity and D-spacing values between 64 and 67 nm were reported.15 In order to explain the periodic D-spacing, Hodge and Petruska proposed a parallel staggered fibril model.16 A theoretical analysis by Hulmes et al.17 demonstrated that maximal ionic and hydrophobic interactions occur when collagen monomers are offset by 234±1 residues along the fibril axis, which roughly equals to 67 nm. In the following decades, XRD studies provided stunning structural details including 5-strand quasi-hexagonal lateral arrangement of microfibrils (Figure 1a), molecular kinking in the gap zone and longitudinal supertwist.18,19,20 Nevertheless, techniques based on measurements of individual fibril's D-spacing continued to report a distribution of D-spacing values (Table 1); however, the physical and biological importance of D-spacing variation was not recognized. Textbooks and literature commonly introduce D-spacing as a single value of 67 nm.3 Recently, the importance of the collagen D-spacing distribution was brought to light; significant alterations in bone collagen D-spacing distribution are shown in OI and long-term estrogen depletion.11,12
Figure 1.
Type I collagen fibril structure and nanomorphological heterogeneity. (a) The proposed quasi-hexagonal packing at fibril cross-section, adapted from Hulmes et al.20 with permission. (b) TEM studies in the 1940s showing nanomorphological variations result in a D-spacing distribution, adapted from Gross and Schmitt14 with permission. (c) Significant alteration of D-spacing distribution in the OVX ovine bone, adapted from Wallace et al.12 with permission.
Table 1. D-spacing variations reported in literature.
| Variations in D-spacing | Tissue and technique | Results and discussion | Reference |
|---|---|---|---|
| 55–80 nm distribution64.6±5.3 nma | Human skinTEM | The 55–80 nm range of spacing is not unique to collagen, but also shared by neurotubules | Gross and Schmitt14 |
| 64.6±0.8 nm in cornea67.7±0.8 nm in tendon | Cornea and tendonXRD | An 18° axial inclination in the cornea explains the D-spacing difference between the cornea and the tendon (cosα=Dc/Dt) | Marchini et al.57 |
| 67.7±0.9 nm in central zone71.3±0.4 nm in distal zone | Vitrified predentinTEM | D-spacing differences in the two zones may be due to the presence of proteoglycans and ions that bind to collagen | Beniash et al.72 |
| 54–75 nm distribution (predominantly 67–68 nm hydrated; 57, 62, 67 nm dehydrated) | Partially demineralized dentinAFM | Reduced D-spacing may be due to dehydration-induced structure disorder and loss of crystallinity | Habelitz et al.43 |
| 69.6±2.9 nm | Rat digital tendonAFM | Fibril D-spacing is preserved independent of the fibril diameter | Bozec et al.70 |
| 63–73 nm distribution | Mice bone, dentin and tendonAFM | A distribution of D-spacing exist in the bone, dentin and tendon, regardless of the presence of mineral, cellular origin, anatomical location or mechanical function of the tissue | Wallace et al.39 |
| 68.0±2.6 nm in sham; 65.9±3.1 nm in OVX | Sham and OVX ovine radius boneAFM | Estrogen depletion induces changes in type I collagen nanomorphology of bone (P<0.001) | Wallace et al.12 |
| 63–74 nm distribution in WT;56–75 nm distribution in brtl/+ | WT and brtl/+ mice femur boneAFM | D-spacing means between WT and brtl/+ are not different (67.6 nm vs 67.4 nm); D-spacing distributions between the phenotypes are statistically different (P=0.001) | Wallace et al.11 |
| 59–66 nm distribution in sham; 56–67 nm distribution in OVX | Sham and OVX ovine dermisAFM | Estrogen depletion induces changes in type I collagen nanomorphology of dermis (P<0.001) | Fang et al.37 |
| 57–69 nm distribution | Ovine boneAFM | Fibrils from one D-bundle share similar D-spacing; a distribution of values arises at the bundle level | Fang et al.10 |
Abbreviations: AFM, atomic force microscopy; OVX, ovariectomized TEM, transmission electron microscopy; WT, wild type; XRD, X-ray diffraction.
aEstimated from the distribution histogram (Figure 1b).
OI phenotypes are most commonly associated with mutations in genes encoding type I collagen or proteins involved in type I collagen posttranslational modification and intracellular trafficking.21 On the molecular level, the detrimental effects of OI collagen mutations include slower folding of the triple helices, delayed intracellular trafficking and thus over modification,22 destabilized tropocollagen molecules23,24 and decreased mechanical stiffness of tropocollagen molecules25 as well as the fibrils.26 A recent study by Wallace et al.12 showed changes in bone collagen nanomorphology as a result of Glycine 349 to Cysteine substitution in one col1α1 allele. They used a heterozygous brtl/+ mouse model of type IV OI, hence a heterogeneous mixture of mutated and non-mutated collagen monomers. Interestingly, the resulting collagen fibril D-spacing is also more heterogeneous compared with the wild type (WT) animals. Although there was no significant difference between D-spacing means of WT and brtl/+, larger variations along the axial length of brtl/+ bones were noted, and the brtl/+ group contains only 55% of fibrils with D-spacings in 66–70 nm range, versus 75% in WT (P=0.001).11 In a subsequent study, Kemp et al.27 demonstrated correlations between collagen fibril D-spacings and indentation-type nanomechanical properties with tendon fibrils of the brtl/+ OI mouse model. They found that modulus and indentation depth were correlated with brtl/+ fibril D-spacing in dried tendon fibrils, whereas energy dissipation was correlated with WT fibril D-spacing in hydrated tendon fibrils. Tensile stretching of an individual collagen fibril indicated correlation between fibril D-spacing and fibril mechanical properties; the nonlinear stress–strain curve suggests increased fibril modulus accompanying D-spacing elongation induced by tensile force.28,29 In addition, higher elastic modulus in the overlap zone over gap zone has been demonstrated by atomic force microscopy (AFM) nanoindentation experiments.30,31
Alteration in collagen fibril nanomorphology has also been shown in long-term estrogen depletion. Estrogen deficiency in postmenopausal women results in increased bone resorption,32 reduced bone quantity and mineral density,33 changes in the micro-architecture and other material deterioration of the bone.34 Unlike OI, which has a genetic origin directly linked to collagen, the knowledge on how estrogen deficiency may impact collagen is limited. Collagen crosslink content has been shown to decrease with osteoporosis.35,36 The nanomorphology of collagen has been systematically compared between sham and ovariectomized (OVX) ovine bone and dermis.12,37 In both tissue types, a higher percentage of fibrils with D-spacing values below mean minus one s.d. was associated with estrogen depletion (Figure 1c). Specifically, 28% of OVX bone collagen fibrils had D-spacings lower than 64 nm, whereas only 7% of such fibrils were found in the sham group.12 Similar results were found in OVX dermis, further supporting the notion that estrogen deprivation affects collagen nanomorphology, regardless of the presence of mineral or not.37
The studies of collagen nanomorphology in OI and long-term estrogen depletion corroborated the important roles of collagen and the need for an effective method to evaluate the morphology of collagen in bone diseases. Note that in both cases only a subportion of fibrils exhibited abnormal nanomorphological values, indicating the importance of methods capable of a fibril-by-fibril analysis. Connecting collagen fibril nanomorphology with biochemical and mechanical properties of collagen fibrils will require combined techniques capable of nanometer-scale resolution such as AFM/nanoindentation and AFM/Raman. These methods will provide useful information pointing to the biological origin of the disease-induced collagen nanomorphology variations and the functional consequences on bone physiochemical and mechanical properties.
A few practical issues must be taken into consideration when studying bone collagen nanomorphology. Surface demineralization is required to reveal the underlying collagen matrix.38 Gentle demineralization seems to have a minimal effect on collagen D-spacing, as non-mineralized tissues such as the tendon and skin showed similar D-spacing distributions compared with the bone (see Table 1).37,39 In addition, samples measured in air, and thus subject to some dehydration, showed minimal changes on D-spacing distribution of healthy and normal collagen fibrils.40 Dehydration significantly impacts the mechanical behavior of bone tissue,41 and the loss of water has been shown to affect the molecular packing of collagens within fibrils.42 Dehydration could also have a role in varying D-spacing. For example, Kemp et al.27 reported that air drying caused artifacts by removing low D-spacing values in the Brtl/+ phenotype, contrary to the conventional belief that dehydration causes fibril D-spacing shrinkage. Habelitz showed that in partially demineralized dentin, air drying changed the fibril D-spacing distribution from a unimodal distribution with a center at 67–68 nm and a range of 54–75 nm, to a distribution divided into three groups, centered at 57, 62 and 67 nm.43 In another AFM-based investigation, where D-spacings of an identical set of 20 fibrils were measured in water vs air, no correlation in the small D-spacing shifts as a function of water vs air imaging was noted.40 Although surface dehydration may have an impact on the D-spacing, it is unlikely that the 10-nm range distribution is an artifact of surface dehydration.
Collagen nanomorphology associated with tissue hierarchy
Organization of collagen fibrils into various highly hierarchical structures in the bone matrix is a fascinating biological phenomenon. At the ultrastructural level, bone trabeculae and osteons are built by planar or cylindrical lamellar layers of collagen fibrils with different angular orientations between adjacent layers, known as the twisted plywood model.44 Fibrils within one layer are aligned with each other as a bundle, similar to fibril bundles observed in the skin, tendon, cornea and aorta.45 The birefringence of collagen bundles allows optical techniques such as polarized-light microscopy to visualize the different orientations of bone lamellae as alternating dark and bright bands.46 Collagen fibril orientation is influenced by mechanical strain distribution and in turn enhances the mechanical property of bone.47,48
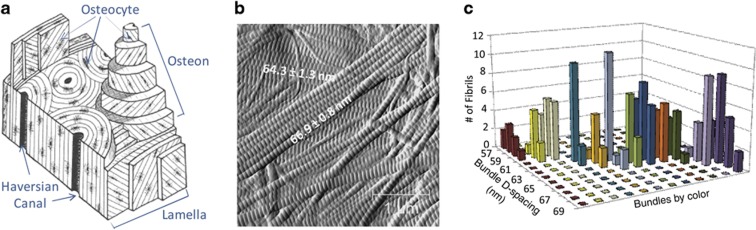
Collagen nanomorphology has a close connection with the aligned fibril bundle unit structure. Although a distribution of values ranging from 60 to 70 nm is frequently found in tissues, within a single collagen fibril bundle, the variation of D-spacing values can be within ±1 nm, suggesting uniform axial packing of collagen monomers within a bundle.10 Such fibril bundles with uniform D-spacing were named D-bundle. Different D-bundles, presumably belonging to different lamellar layers (Figure 2), could differ in D-spacing by up to 10 nm, which give rise to the full-scale distribution. A nested analysis of variance analysis partitioned the variance components at the animal, bundle and fibril level, and found that indeed the bundle-level variance accounted for 76% of the total variance.10 In other words, fibril D-spacing variance nested within one bundle and variance of different animals are small compared with variance of different D-bundles. It should be noted that this characteristic of the D-bundle was also present in the dermis and the tendon.
Figure 2.
Collagen nanomorphology in connection to micro-scale bone lamellar structure. (a) A schematic of bone micro-architecture, showing lamellae with collagen fibril bundles at different orientations. (b) AFM error images showing two layers of collagen bundles presumably from two lamellae, and different D-spacing values are associated with the two bundles. (c) Three-dimentional bar plot showing that bundle D-spacings occupy the full spectrum of distribution ranging from 57 to 69 nm, whereas fibril D-spacings within one bundle are narrow (±1 nm). (a–c) are adapted from Fang et al.10
The observation of narrow D-spacing values within a bundle and large differences across different bundles has important implications in current fibrillogenesis models. Studies carried out in the tendon clearly favor the hypothesis that cells have a dominating role in directing the alignment of fibrils.49,50,51 Using transverse-sectioned transmission electron microscopy (TEM) imaging, Canty et al.50 were able to trace collagen fibrils from extracellular bundles to deep within a fibroblast cell. The membrane protrusions of fibroblasts, also called fibripositors, were proposed as nucleation sites of collagen fibrillogenesis, and responsible for projecting collagen fibrils into parallel alignment. By this theory, a collagen bundle is formed by lateral association of fibrils excreted by one osteoblast and its orientation is determined by the direction in which the cell migrates. In this case, the bundle-to-bundle D-spacing difference could be due to cell-to-cell difference, such as the varying amount of minor collagens and posttranslational modifications. Currently it is unclear to what extent osteoblasts influence bone collagen orientation. Unlike the tendon, bone fibrils are orthogonally stacked in the twisted plywood spatial arrangement. In vitro osteoblast culture reproduces the orthogonal spatial arrangement of secreted collagen fibrils, however no evidence of osteoblast cells dominating the orthogonal fibril orientation was shown.52 A different theory emphasizes the importance of the intrinsic liquid crystallinity of collagen.53,54,55 Highly concentrated acid-soluble collagen has the characteristics of a cholesteric liquid crystal, which exhibits a striking resemblance to the twisted plywood structures in bone.56 It is therefore plausible that high concentrations of procollagen or tropocollagen are pre-aligned before fibrillogenesis at the bone resorption pocket, leading to the formation of a fibril bundle with uniform packing of individual collagen monomers, hence the narrow D-spacing within a bundle. To date, no one has observed the motion of osteoblasts in registration with the collagen fibrils they secrete or directly studied the liquid crystallinity of collagen during the in vivo process of fibrillogenesis.
The origin of collagen D-spacing distribution
For decades collagen D-spacing has been thought as a single value, either 64 nm in the skin and cornea, or 67 nm in the tendon and bone, based on X-ray diffraction data. Some have proposed a helicoidal fibril model to explain the discrepancy based on the observation of a 18 ° axial tilting of microfibrils in cornea using freeze etching technique. The 64 nm D-spacing in the cornea was rationalized as 67 cos(18°).57,58 A distribution of D-spacings has only recently been reported with significant connections to bone diseases and bone tissue micro-architecture.10,11,12,27,37,39,40,59 For now, our understanding of the origin and functions of a collagen D-spacing distribution is limited, potential biological and molecular bases for the D-spacing distribution are discussed in this section.
Many collagen-constituted tissues are also load-bearing tissues. Bone formation and resorption are stimulated by increased mechanical loading and disuse, respectively, as a part of bone functional adaptation.60 It is plausible that variation in D-spacing is a reflection of changing local mechanical stresses. Although this hypothesis could explain the formation of different bundle D-spacings and narrow D-spacing within a bundle, experimental data to date suggest against the possibility of differences in mechanical loading causing a 10-nm distribution of D-spacings. Gupta et al.9 studied the behavior of fibril strain over tissue strain of bone using small-angle X-ray scattering (SAXS) and noted that 0.7% macroscopic tissue strain corresponds with a 0.5% increase in D-spacing average as measured using SAXS. Puxkandl et al.61 and Sasaki et al.62 have demonstrated a similar effect in tendon. At the molecular level, the fibril strain is the direct result of triple-helix stretching; kinks in the gap zone are straightened causing the gap zone to move apart (see more details in the review by Fratzl et al.63). Intermolecular slippage and telopeptide unfolding also accompany mechanical loading.64 In the post-yield regime, bone fibril strain does not increase with tissue strain, which Gupta et al.9,65 suggest is to due to the interfibrillar shearing/sliding of noncollagenous components of the bone. Nevertheless, 0.5% fibril strain corresponds to 0.3 nm D-spacing increase, indicating that mechanical loading alone does not cause the 10-nm D-spacing variations. Another possibility is that the D-bundle variations could be due to different bundle relaxations induced by partial demineralization, depending on their initial levels of mechanical constraints within the bone matrix. However, the fact that similar bundle-to-bundle variations were also found in the dermis, which bears minimal mechanical loading, argues against such a possibility. Nevertheless, it does not rule out the possibility of fibril bundles with different D-spacings being formed by osteoblast cells that are under different mechanical stresses.
In addition to responses to mechanical cues, other potential cell-based factors include differential expression level of homotrimeric collagens and minor collagen type V/XI, and varying amount of posttranslational modification. Homotrimeric collagen is structurally more flexible than heterotrimers, which could have a profound influence on the fibril packing and properties.66,67 For example, an oim mouse model, which uses homotrimeric type I collagen isoform (α1(I)3) instead of the normal heterotrimeric type I collagen (α1(I)2α2(I)) as the organic building block of tissues, exhibits severe OI phenotypic properties. Full-scale atomistic simulation showed that homotrimeric collagen has a higher tendency of forming kinks, which leads to larger lateral intermolecular spacing.67 This molecular level structural alteration could cause reduced intermolecular crosslinking and, consequently, weakened mechanical strength at the organ level. No change in the axial D-spacing was observed in the oim mice tendon, however, this observation was based on XRD measurement that only provides D-spacing average.68 In addition to intracellular factors, extracellular factors such as crosslinks and proteoglycan binding are also potential factors that could lead to different packing density and thus D-spacing variations.
A recent study has shown that self-assembly of type I collagen in vitro produces similar distributions with those found in biological tissues.69 Both mica-surface-mediated fibril assembly and fibrillar gel formed in confined glass capillary tubes exhibited a D-spacing distribution in the range of 60–70 nm. It suggests that D-spacing variation is intrinsic to type I collagen and its self-assembly, and it does not necessarily require cells, interfibrillar crosslinking and proteoglycan binding. The variations in fibril D-spacing may be a result of variant intrafibrillar interactions including hydrophobic interactions, electrostatic interactions, hydrogen bonding and crosslinks on hydroxylysines and hydroxyprolines. As an offset of 234 amino acids between collagen monomers maximizes the sum of these interactions, it is possible that D-spacing variations arise from various degrees of tilting or super coiling within a fibril, similar to the idea used to explain the 64 vs 67 nm D-spacing in different tissues. In addition, Bozec et al.70 observed spiral and twisted rope features in digital tendon fibrils and proposed a classic n-plies rope model, which could also explain fibril D-spacing variation. More refined theoretical models incorporating collagen monomer and/or microfibril assembly should also be able to predict a D-spacing distribution.
Summary and perspectives
From the 2D Hodge-Petruska model in the 1960s16 to the modern computer-simulated 3D model,71 D-spacing has been a key aspect of collagen nanomorphology, yet the intrinsic heterogeneity of collagen D-spacing has rarely been emphasized. An axial D-spacing distribution arises at the bundle level and it is universal among bone and other tissues including the skin, tendon and dentin; it can also be reproduced by the self-assembly of type I collagen alone. The alteration of D-spacing distributions in bone diseases underscores the need to better understand the origin of D-spacing distribution and the biochemical/mechanical consequence of such nanomorphological changes. Different fibril D-spacings may have an impact on mineral nucleation and growth, binding with proteoglycans and fibril stiffness. Experimental investigations using combined instrumental analyses and theoretical modeling are required to elucidate the details of collagen structure–property relationship at nano- to micro-scale.
Acknowledgments
Aspects of the authors' work have been funded by Merck Inc.
Footnotes
Aspects of the authors work have been funded by Merck Inc.
References
- Green J, Schotland S, Stauber DJ, Kleeman CR, Clemens TL. Cell-matrix interaction in bone: type I collagen modulates signal transduction in osteoblast-like cells. Am J Physiol 1995;268:C1090–C1103. [DOI] [PubMed] [Google Scholar]
- Wang Y, Azaïs T, Robin M, Vallée A, Catania C, Legriel P et al. The predominant role of collagen in the nucleation, growth, structure and orientation of bone apatite. Nature Mater 2012;11:724–733. [DOI] [PubMed] [Google Scholar]
- Fratzl P. Collagen Structure and Mechanics Springer Science+Business Media, LLC: Boston, MA, USA, 2008;. [Google Scholar]
- Byers PH. Brittle bones - fragile molecules: disorders of collagen gene structure and expression. Trends Genet 1990;6:293–300. [DOI] [PubMed] [Google Scholar]
- Vashishth D. The role of the collagen matrix in skeletal fragility. Curr Osteoporos Rep 2007;5:62–66. [DOI] [PubMed] [Google Scholar]
- Parry DAD, Barnes GRG, Craig AS. A comparison of the size distribution of collagen fibrils in connective tissues as a function of age and a possible relation between fibril size distribution and mechanical properties. Proc R Soc Lond B 1978;203:305–321. [DOI] [PubMed] [Google Scholar]
- Christiansen DL, Huang EK, Silver FH. Assembly of type I collagen: fusion of fibril subunits and the influence of fibril diameter on mechanical properties. Matrix Biol 2000;19:409–420. [DOI] [PubMed] [Google Scholar]
- Graham HK, Holmes DF, Watson RB, Kadler KE. Identification of collagen fibril fusion during vertebrate tendon morphogenesis. The process relies on unipolar fibrils and is regulated by collagen-proteoglycan interaction. J Mol Biol 2000;295:891–902. [DOI] [PubMed] [Google Scholar]
- Gupta HS, Wagermaier W, Zickler GA, Raz-Ben Aroush D, Funari SS, Roschger P et al. Nanoscale deformation mechanisms in bone. Nano Lett 2005;5:2108–2111. [DOI] [PubMed] [Google Scholar]
- Fang M, Goldstein EL, Turner AS, Les CM, Orr BG, Fisher GJ et al. Type I collagen D-Spacing in fibril bundles of dermis, tendon, and bone: bridging between nano- and micro-level tissue hierarchy. ACS NANO 2012;6:9503–9514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace JM, Orr BG, Marini JC, Holl MMB. Nanoscale morphology of Type I collagen is altered in the Brtl mouse model of Osteogenesis Imperfecta. J Struct Biol 2011;173:146–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace JM, Erickson B, Les CM, Orr BG, Banaszak Holl MM. Distribution of type I collagen morphologies in bone: relation to estrogen depletion. Bone 2010;46:1349–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt FO, Hall CE, Jakus MA. Electron microscope investigations of the structure of collagen. J Cell Comp Physiol 1942;20:11–33. [Google Scholar]
- Gross J, Schmitt FO. The structure of human skin collagen as studied with the electron microscope. J Exp Med 1948;88:555–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear RS. X-ray diffraction studies on protein fibers. I. The large fiber-axis period of collagen. J Am Chem Soc 1944;66:1297–1305. [Google Scholar]
- Hodge AJ, Petruska JA (eds) Recent studies with the electron microscope on ordered aggregates of the tropocollagen molecule. Academic Press: New York, USA, 1963. [Google Scholar]
- Hulmes DJS, Miller A, Parry DAD, Piez KA, Woodhead-Galloway J. Analysis of the primary structure of collagen for the origins of molecular packing. J Mol Biol 1973;79:137–148. [DOI] [PubMed] [Google Scholar]
- Orgel JPRO Miller A, Irving TC, Fischetti RF, Hammersley AP, Wess TJ. The in situ supermolecular structure of type I collagen. Structure 2001;9:1061–1069. [DOI] [PubMed] [Google Scholar]
- Orgel JPRO, Irving TC, Miller A, Wess TJ. Microfibrillar structure of type I collagen in situ. Proc Natl Acad Sci USA 2006;103:9001–9005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulmes DJS, Wess TJ, Prockop DJ, Fratzl P. Radial packing, order, and disorder in collagen fibrils. Biophys J 1995;68:1661–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cundy T. Recent advances in osteogenesis imperfecta. Calcif Tissue Int 2012;90:439–449. [DOI] [PubMed] [Google Scholar]
- Forlino A, Kuznetsova NV, Marini JC, Leikin S. Selective retention and degradation of molecules with a single mutant α1(I) chain in the Brtl IV mouse model of OI. Matrix Biol 2007;26:604–614. [DOI] [PubMed] [Google Scholar]
- Lee KH, Kuczera K, Banaszak Holl MM. The severity of osteogenesis imperfecta: A comparison to the relative free energy differences of collagen model peptides. Biopolymers 2011;95:182–193. [DOI] [PubMed] [Google Scholar]
- Lee KH, Kuczera K, Holl MMB. Effect of osteogenesis imperfecta mutations on free energy of collagen model peptides: a molecular dynamics simulation. Biophys Chem 2011;156:146–152. [DOI] [PubMed] [Google Scholar]
- Gautieri A, Vesentini S, Redaelli A, Buehler MJ. Single molecule effects of osteogenesis imperfecta mutations in tropocollagen protein domains. Protein Sci 2009;18:161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautieri A, Uzel S, Vesentini S, Redaelli A, Buehler MJ. Molecular and mesoscale mechanisms of osteogenesis imperfecta disease in collagen fibrils. Biophys J 2009;97:857–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp AD, Harding CC, Cabral WA, Marini JC, Wallace JM. Effects of tissue hydration on nanoscale structural morphology and mechanics of individual type I collagen fibrils in the Brtl mouse model of Osteogenesis Imperfecta. J Struct Biol 2012;180:428–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutsmann T, Fantner GE, Kindt JH, Venturoni M, Danielsen S, Hansma PK. Force spectroscopy of collagen fibers to investigate their mechanical properties and structural organization. Biophys J 2004;86:3186–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Rijt JAJ, van der Werf KO, Bennink ML, Dijkstra PJ, Feijen J. Micromechanical testing of individual collagen fibrils. Macromol Biosci 2006;6:697–702. [DOI] [PubMed] [Google Scholar]
- Minary-Jolandan M, Yu MF. Nanomechanical heterogeneity in the gap and overlap regions of type I collagen fibrils with implications for bone heterogeneity. Biomacromolecules 2009;10:2565–2570. [DOI] [PubMed] [Google Scholar]
- Grant CA, Phillips MA, Thomson NH. Dynamic mechanical analysis of collagen fibrils at the nanoscale. J Mech Behav Biomed Mater 2012;5:165–170. [DOI] [PubMed] [Google Scholar]
- Rodan GA. Perspectives: mechanical loading, estrogen deficiency, and the coupling of bone formation to bone resorption. J Bone Miner Res 1991;6:527–530. [DOI] [PubMed] [Google Scholar]
- Stone KL, Seeley DG, Lui L-Y, Cauley JA, Ensrud K, Browner WS et al. BMD at multiple sites and risk of fracture of multiple types: long-term results from the study of osteoporotic fractures. J Bone Miner Res 2003;18:1947–1954. [DOI] [PubMed] [Google Scholar]
- Burket JC, Brooks DJ, MacLeay JM, Baker SP, Boskey AL, Van Der Meulen MCH. Variations in nanomechanical properties and tissue composition within trabeculae from an ovine model of osteoporosis and treatment. Bone 2013;52:326–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkoula MG, Vardaki MZ, Kontoyannis CG. Study of bone matrix changes induced by osteoporosis in rat tibia using Raman spectroscopy. Vib Spectrosc 2012;63:404–408. [Google Scholar]
- Oxlund H, Sekilde L, Ørtoft G. Reduced concentration of collagen reducible cross links in human trabecular bone with respect to age and osteoporosis. Bone 1996;19:479–484. [DOI] [PubMed] [Google Scholar]
- Fang M, Liroff KG, Turner AS, Les CM, Orr BG, Holl MMB. Estrogen depletion results in nanoscale morphology changes in dermal collagen. J Invest Dermatol 2012;132:1791–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindt JH, Thurner PJ, Lauer ME, Bosma BL, Schitter G, Fantner GE et al. In situ observation of fluoride-ion-induced hydroxyapatite-collagen detachment on bone fracture surfaces by atomic force microscopy. Nanotechnology 2007;18:135102. [DOI] [PubMed] [Google Scholar]
- Wallace JM, Chen Q, Fang M, Erickson B, Orr BG, Banaszak Holl MM et al. Collagen exists as a distribution of nanoscale morphologies in teeth, bones, and tendons. Langmuir 2010;26:7349–7354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson B, Fang M, Wallace JM, Orr BG, Les CM, Banaszak Holl MM. Nanoscale structure of type I collagen fibrils: quantitative measurement of D-spacing. Biotechnol J 2013;8:117–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rho J-Y, Pharr GM. Effects of drying on the mechanical properties of bovine femur measured by nanoindentation. J Mater Sci Mater Med 1999;10:485–488. [DOI] [PubMed] [Google Scholar]
- Wess TJ, Orgel JP. Changes in collagen structure: drying, dehydrothermal treatment and relation to long term deterioration. Thermochim Acta 2000;365:119–128. [Google Scholar]
- Habelitz S, Balooch M, Marshall SJ, Balooch G, Marshall GW Jr. In situ atomic force microscopy of partially demineralized human dentin collagen fibrils. J Struct Biol 2002;138:227–236. [DOI] [PubMed] [Google Scholar]
- Giraud-Guille MM. Twisted plywood architecture of collagen fibrils in human compact bone osteons. Calcif Tissue Int 1988;42:167–180. [DOI] [PubMed] [Google Scholar]
- Graham HK, Hodson NW, Hoyland JA, Millward-Sadler SJ, Garrod D, Scothern A et al. Tissue section AFM: In situ ultrastructural imaging of native biomolecules. Matrix Biol 2010;29:254–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromage TG, Goldman HM, McFarlin SC, Warshaw J, Boyde A, Riggs CM. Circularly polarized light standards for investigations of collagen fiber orientation in bone. Anat Rec B New Anat 2003;274:157–168. [DOI] [PubMed] [Google Scholar]
- Takano Y, Turner CH, Owan I, Martin RB, Lau ST, Forwood MR et al. Elastic anisotropy and collagen orientation of osteonal bone are dependent on the mechanical strain distribution. J Orth Res 1999;17:59–66. [DOI] [PubMed] [Google Scholar]
- Skedros JG, Dayton MR, Sybrowsky CL, Bloebaum RD, Bachus KN. The influence of collagen fiber orientation and other histocompositional characteristics on the mechanical properties of equine cortical bone. J Exp Biol 2006;209:3025–3042. [DOI] [PubMed] [Google Scholar]
- Trelstad RL, Hayashi K. Tendon collagen fibrillogenesis: intracellular subassemblies and cell surface changes associated with fibril growth. Dev Biol 1979;71:228–242. [DOI] [PubMed] [Google Scholar]
- Canty EG, Lu Y, Meadows RS, Shaw MK, Holmes DF, Kadler KE. Coalignment of plasma membrane channels and protrusions (fibripositors) specifies the parallelism of tendon. J Cell Biol 2004;165:553–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canty EG, Kadler KE. Procollagen trafficking, processing and fibrillogenesis. J Cell Sci 2005;118:1341–1353. [DOI] [PubMed] [Google Scholar]
- Gerstenfeld LC, Riva A, Hodgens K, Eyre DR, Landis WJ. Post-translational control of collagen fibrillogenesis in mineralizing cultures of chick osteoblasts. J Bone Miner Res 1993;8:1031–1043. [DOI] [PubMed] [Google Scholar]
- Giraud-Guille MM. Liquid crystallinity in condensed type I collagen solutions. A clue to the packing of collagen in extracellular matrices. J Mol Biol 1992;224:861–873. [DOI] [PubMed] [Google Scholar]
- Giraud-Guille MM, Mosser G, Belamie E. Liquid crystallinity in collagen systems in vitro and in vivo. Curr Opin Colloid Interface Sci 2008;13:303–313. [Google Scholar]
- Gobeaux F, Mosser G, Anglo A, Panine P, Davidson P, Giraud-Guille MM et al. Fibrillogenesis in Dense Collagen Solutions: A Physicochemical Study. J Mol Biol 2008;376:1509–1522. [DOI] [PubMed] [Google Scholar]
- Giraud-Guille MM, Belamie E, Mosser G, Helary C, Gobeaux F, Vigier S. Liquid crystalline properties of type I collagen: Perspectives in tissue morphogenesis. Comptes Rendus Chimie 2008;11:245–252. [Google Scholar]
- Marchini M, Morocutti M, Ruggeri A, Koch MH, Bigi A, Roveri N. Differences in the fibril structure of corneal and tendon collagen. An electron microscopy and X-ray diffraction investigation. Connect Tissue Res 1986;15:269–281. [DOI] [PubMed] [Google Scholar]
- Wess TJ. Collagen fibril form and function. Adv Protein Chem 2005;70:341–374. [DOI] [PubMed] [Google Scholar]
- Wallace JM. Applications of atomic force microscopy for the assessment of nanoscale morphological and mechanical properties of bone. Bone 2012;50:420–427. [DOI] [PubMed] [Google Scholar]
- Skerry TM. The response of bone to mechanical loading and disuse: fundamental principles and influences on osteoblast/osteocyte homeostasis. Arch Biochem Biophys 2008;473:117–123. [DOI] [PubMed] [Google Scholar]
- Puxkandl R, Zizak I, Paris O, Keckes J, Tesch W, Bernstorff S et al. Viscoelastic properties of collagen: synchrotron radiation investigations and structural model. Phil Trans R Soc Lond B 2002;357:191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki N, Shukunami N, Matsushima N, Izumi Y. Time-resolved X-ray diffraction from tendon collagen during creep using synchrotron radiation. J Biomech 1999;32:285–292. [DOI] [PubMed] [Google Scholar]
- Fratzl P, Misof K, Zizak I, Rapp G, Amenitsch H, Bernstorff S. Fibrillar structure and mechanical properties of collagen. J Struct Biol 1998;122:119–122. [DOI] [PubMed] [Google Scholar]
- Gautieri A, Pate MI, Vesentini S, Redaelli A, Buehler MJ. Hydration and distance dependence of intermolecular shearing between collagen molecules in a model microfibril. J Biomech 2012;45:2079–2083. [DOI] [PubMed] [Google Scholar]
- Gupta HS, Zioupos P. Fracture of bone tissue: the ‘hows' and the ‘whys'. Med Eng Phys 2008;30:1209–1226. [DOI] [PubMed] [Google Scholar]
- Silver FH, Horvath I, Foran DJ. Mechanical implications of the domain structure of fiber-forming collagens: comparison of the molecular and fibrillar flexibilities of the α1-chains found in types I-III collagen. J Theor Biol 2002;216:243–254. [DOI] [PubMed] [Google Scholar]
- Chang S-W, Shefelbine Sandra J, Buehler Markus J. Structural and mechanical differences between collagen homo- and heterotrimers: relevance for the molecular origin of brittle bone disease. Biophys J 2012;102:640–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride DJ Jr, Choe V, Shapiro JR, Brodsky B. Altered collagen structure in mouse tail tendon lacking the α2(I) chain. J Mol Biol 1997;270:275–284. [DOI] [PubMed] [Google Scholar]
- Fang M, Goldstein EL, Matich EK, Orr BG, Banaszak Holl MM, Type I. Collagen self-assembly: the roles of substrate and concentration. Langmuir 2013;29:2330–2338. [DOI] [PubMed] [Google Scholar]
- Bozec L, Van Der Heijden G, Horton M. Collagen fibrils: nanoscale ropes. Biophys J. 2007;92:70–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautieri A, Vesentini S, Redaelli A, Buehler MJ. Hierarchical structure and nanomechanics of collagen microfibrils from the atomistic scale up. Nano Lett 2011;11:757–766. [DOI] [PubMed] [Google Scholar]
- Beniash E, Traub W, Veis A, Weiner S. A transmission electron microscope study using vitrified ice sections of predentin: structural changes in the dentin collagenous matrix prior to mineralization. J Struct Biol 2000;132:212–225. [DOI] [PubMed] [Google Scholar]