Abstract
No known studies have tested the hypothesis that a blunted pattern of cortisol reactivity to stress, which is often found following exposure to chronic life stressors, is associated with a higher body mass index (BMI) in very young children. Low-income children (n = 218, mean age 56.6 (range: 38.1 to 78.5; SD 7.0) months, 49.1% male, 56.4% white, 16.1% black, 11.5% Hispanic/Latino) participated in a series of behavioral tasks designed to elicit stress. Cortisol was sampled in saliva 5 times during the protocol, and area under the curve (AUC), representing total cortisol output during stress elicitation, was calculated. Children were weighed and height measured and body mass index (BMI) z-score was calculated. Linear regression was used to evaluate the association between cortisol AUC and BMI z-score, controlling for child age, sex, and race/ethnicity (non-Hispanic white vs. not); primary caregiver weight status (overweight, defined as BMI > 25 vs. not); and family income-to-needs ratio. Mean child BMI z-score was 0.88 (SD = 1.03). Mean cortisol AUC was 6.11 μg/dL/min (SD = 10.44). In the fully adjusted model, for each 1-standard deviation unit decrease in cortisol AUC, the child's BMI z-score increased by 0.17 (SE 0.07) standard deviation units (p <.02). A blunted cortisol response to stress, as is often seen following chronic stress exposure, is associated with increased BMI z-score in very young children. Further work is needed to understand how associations between stress, cortisol, and elevated body mass index may develop very early in the lifespan.
Keywords: child, obesity, stress, BMI, cortisol, low-income
Introduction
More than one third of low-income United States preschool-aged children are overweight (Kimbro et al., 2007). Living in poverty can expose children to significant life stressors (Evans and English, 2002), which can be biologically manifested through changes in the hypothalamic-pituitary-adrenal (HPA) axis leading to altered patterns of cortisol secretion (Zalewski, 2012). Chronically stressed children and adults have also been shown to display atypical HPA axis responses to an acute stressor. Typically, stress signals originating in the brain result in the activation of the adrenal glands to secrete cortisol, which rises to a peak in blood or saliva in 20 to 40 minutes after a stressor, followed by a decline to baseline 40 to 60 minutes post-stressor (Dickerson, 2004). Chronically stressed individuals instead can show either a blunted cortisol reactivity response to a stressor, or prolonged elevation with little to no recovery to baseline (Van Ryzin, 2009). Such changes in stress response have been proposed as a pathway through which poverty can “get under the skin” and lead to health problems, specifically obesity (Gundersen et al., 2011). Cortisol increases appetite and shifts food preferences to so-called “comfort foods” (foods high in fat and added sugars) (Pasquali et al., 2010) that may reduce feelings of stress via dampening of HPA axis activity (Dallman et al., 2003). Repeated stressors and associated cortisol release may lead to excessive consumption of foods high in sugar and fat, and ultimately excessive weight gain. Therefore, following exposure to repeated stressors, both a blunted cortisol reactivity response to a stressor and elevated body mass index (BMI) may be observed. In the current study, we examined cortisol responses to an acute stressor in relation to BMI in a sample of low-income preschool-age children.
Six prior studies in adults have examined associations between cortisol reactivity to a stressor and adiposity, with conflicting results. These studies found no association of cortisol reactivity with BMI (Brydon, 2011; Epel et al., 2000; Therrien et al., 2010); high cortisol reactivity (peak response) associated with greater central adiposity (Epel et al., 2000) and BMI (Benson et al., 2009); and less (blunted) cortisol reactivity associated with higher BMI (Jones et al., 2012; Phillips et al., 2012) and greater central adiposity (Jones et al., 2012). Only two studies have evaluated the association of cortisol reactivity with adiposity in children, finding that high cortisol reactivity was associated with a higher BMI in children ages 8-13 years (Dockray et al., 2009) or 8-9 years (Francis et al., 2012), but not in children ages 5-7 years (Francis et al., 2012). Young, preschool-age children are in a unique developmental period both for the establishment of effective responses for coping with stress (Cole, 1986; Cole et al., 2009), as well as childhood obesity, which, once established during the early childhood years, tends to persist into adulthood (Freedman et al., 2001). Documenting the nature of cortisol reactivity-obesity associations at younger ages is important in order to understand the development of such associations over time. In addition, understanding these associations among low-income children is particularly important, since children growing up in poverty are at risk for both self-regulation challenges (Evans and English, 2002) and obesity (Shrewsbury and Wardle, 2008).
Thus, in the context of these conflicting findings and the lack of data in young children, we sought to test the hypothesis that blunted patterns of cortisol reactivity to an immediate stress are associated with a higher BMI z-score (BMIz) among low-income preschool-aged children.
Methods
Study Design and Participants
Participants were children who had attended Head Start, a free, federally-funded preschool program for low-income children, who were participating in a longitudinal study of child eating behavior. Families were invited to participate in the current study, which was described as a study of whether children with different levels of stress hormone eat differently. Exclusion criteria were: parent with ≥ 4 year college degree; parent or child not English-speaking; child in foster care, with food allergies, significant medical problems or perinatal complications, or children who were < 35 weeks gestation at birth. The sample described in this report includes participants with complete data for the predictor, outcome, and all covariates in this analysis (n = 218). Children were 56.6 months old on average (range: 38.1 to 78.5; SD = 7.0). The sample was 49.1% male. Most children (56.4%) were white, 16.1% were black, and 15.1% were biracial; 11.5% were of Hispanic/Latino ethnicity. Family income-to-needs ratio was below 1.0 (M = 0.93, SD = 0.88), confirming that this was a very low-income sample. The majority of children's mothers were overweight (78.9%). The study was approved by the University of Michigan Institutional Review Board.
Procedure and Measures
Children and their primary caregivers attended a study visit at 1:00pm on one afternoon during which children participated in a stress-elicitation challenge protocol and were weighed and measured. The primary caregiver reported child race/ethnicity, family income and number of family members; and for the child on the day of the protocol, any medication use, illness, unusually good or bad events, exact time of morning awakening and if it was the usual time, and the last time the child ate.
Stress-Elicitation Challenge Protocol
The child was brought to a room separate from the parent and was first engaged in calming free play with the examiner for 20 minutes. The child then participated in four challenge tasks. Each task was designed to elicit a mild to moderate level of stress, with tasks including a negative social evaluation component, which is a particularly robust elicitor of cortisol reactivity (Dickerson, 2004; Gunnar et al., 2009).
The child first rated six prizes from most preferred (e.g., toy car or doll) to least preferred (e.g., broken comb or deflated ball) and was told he or she could have the most preferred prize as a gift later. The examiner then removed the prize from the room.
During the first challenge task, Perfect Circles (Goldsmith and Rothbart, 1996), the examiner instructed the child to draw a “perfect circle”. For 3.5 minutes, the examiner critiqued each circle the child drew, explaining that the circle was not quite perfect enough, and telling the child to “keep trying.” The examiner ultimately told the child that the final circle was “pretty good” and moved on to the next task. During the second challenge task, Puzzles, children were instructed to persist at solving a wooden puzzle that, though age-appropriate, contained two incorrect pieces, making it impossible to solve. After persisting for 3 minutes, the child was told, “we're out of time on that one” and the puzzle was removed. Then, children were instructed to solve a puzzle that was designed for older children and therefore too difficult because it was age-inappropriate. After 4 minutes, the child was again told that time was up. No child was able to solve the puzzle. The examiner did not provide help, encouragement, or reassurance, but at the conclusion of the Puzzles task acknowledged that the puzzles were “hard”.
The examiner then told the child that he or she could now have the previously selected prize, but that the examiner first needed to gift wrap it. During this third challenge task, Gift Wrap/Wait (McCabe and Brooks-Gunn, 2002), the child then waited for 1.5 minutes while the examiner pretended to wrap the gift behind a screen by crinkling paper. For the fourth challenge task, Disappointing Gift (Cole, 1986), the examiner then presented the child with the box containing the gift. However, instead of containing the child's preselected, most preferred prize (e.g. toy car or doll), the box instead contained the least preferred prize (e.g. broken comb or deflated ball). The child opened the gift box, and the examiner remained unresponsive for 30 seconds while the child reacted to the disappointing gift. After 30 seconds, the examiner “realized” the mistake, apologized for the “mistake”, and retrieved the “correct” preferred prize for the child, which the child was allowed to take home as a gift. For 40 more minutes, the child was given the choice of engaging in quiet free play with the examiner or watching an age appropriate children's movie.
Cortisol Sampling
Cortisol reactivity to the stress-elicitation challenge protocol was measured in saliva; salivary cortisol is highly correlated with free serum cortisol (Kirschbaum and Hellhammer, 1994). Children provided saliva samples by drooling into a tube or chewing on a piece of cotton, per standard methods. Saliva was sampled at five points during the protocol: (1) 20 minutes after room entry, to reflect cortisol prior to beginning the study session; (2) 30 minutes after room entry, (10 minutes into the free play period) prior to beginning the challenge tasks; (3) at 10 minutes after receipt of the gift; (4) at 20 minutes after receipt of the gift; and (5) at 40 minutes after receipt of the gift. Saliva was sampled at these multiple time points following the stress-elicitation challenge tasks to allow for and capture individual differences in response time for cortisol reactivity and recovery (Dickerson, 2004; Lopez-Duran et al., 2009).
Saliva was stored at -20 degree Celsius until extracted and assayed in duplicate using an Expanded Range High Sensitivity Salivary Cortisol Enzyme Immunoassay Kit (Salimetrics LLC, PA, USA). The sensitivity of the assays was 0.003 μg/dL and the intra and inter-assay coefficients of variation were 4.6% and 5.5%, respectively.
Anthropometry
BMI concurrent with this protocol was the focus of our analysis. At the end of the protocol, the child was weighed and measured without shoes or heavy clothing by trained staff according to standard protocols. Body mass index (BMI) z-score was calculated based on US Centers for Disease Control reference growth curves for age and sex. We excluded from this analysis children with a BMI <5th percentile. Children were categorized as overweight (BMI ≥ 85th percentile for age and sex) or normal weight (BMI < 85th percentile and > 5th percentile for age and sex). Primary caregivers' BMI's were also calculated from measured weights and heights, and categorized as overweight (BMI ≥ 25) versus not. A subset of children in this longitudinal cohort (n = 115) were also weighed and measured in an identical manner an average of 12.4 (SD = 3.3) months following this protocol; these BMI values were used for a supplementary longitudinal analysis.
Statistical Analysis
Data analysis was performed using SAS 9.2 (SAS Institute Inc., Cary, NC). Univariate and bivariate statistics were used to describe the sample. Of the 218 participants, 217 had cortisol results for all 5 timed data points. One participant had valid results for 4 of the 5 data points and this individual was included in the analyses.
We assessed the cortisol response to stress by calculating the area under the curve (AUC). The cortisol AUC was calculated using the trapezoidal rule and reflected the child's total cortisol output from the 2nd sample (prior to starting the challenge tasks) to the 5th and final sample (40 minutes post-challenge tasks). AUC is typically used in this manner as an indicator of overall stress response (Pruessner et al., 2003). We also calculated AUC from the 1st (room entry) to the 2nd sample, and the 3rd sample (10 minutes post-challenge tasks) to the 5th sample to examine the child's cortisol output prior to the start of the challenge tasks, and output after the tasks. AUC units were standardized for analyses. We then considered whether there was an association with cortisol AUC and several variables that could influence the cortisol response to stress. We found that there was no association of the AUC with the child taking a medication known or hypothesized to affect cortisol, the child having experienced an unusual circumstance that day (i.e., an unusually good or bad day), the child being ill (i.e., with a cold or flu-like illness), the exact time of morning awakening, whether the child had awakened at the usual time, and the time the child last ate prior to the protocol (M time since eating = 139 minutes, SD = 102 minutes). Thus, these variables were not included as covariates and all data were included in analyses.
We performed multiple linear regression to assess the association of cortisol AUC with child BMIz at the time of the stress protocol challenge, controlling for child age, child sex, child race/ethnicity (categorized for this analysis as non-Hispanic white vs. not), primary caregiver weight status (overweight versus not), and household income-to-needs ratio (annual gross family income divided by the poverty threshold for a family of the same size). All primary analyses examined cortisol AUC from the 2nd to the 5th sample. We repeated these analyses using cortisol AUC from the 1st to the 2nd sample; cortisol AUC from the 3rd to the 5th sample; and separately for children whose cortisol increased during the protocol (responders) versus children who did not show an increase (non-responders). We also examined cortisol AUC in relation to child weight status (overweight vs. normal weight). For children with a longitudinal BMIz datapoint (n = 115), we performed the same analyses examining change in BMIz over time. For all analyses, we used an alpha level of 0.05 (two-tailed) to determine statistical significance.
Results
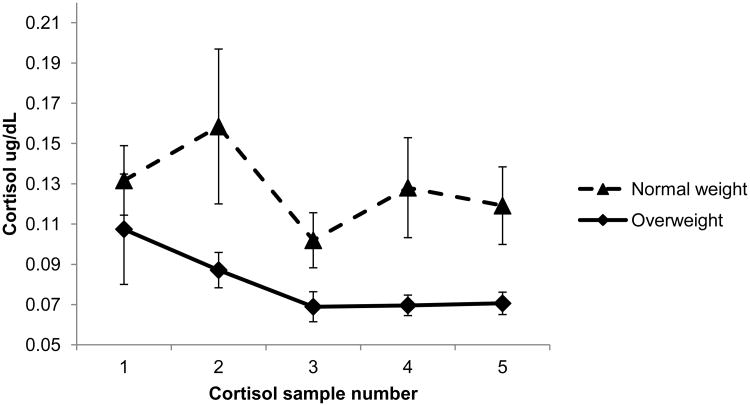
Mean child BMIz was 0.88 (SD = 1.03). Of the children, 40.8% were overweight, and 59.2% normal weight. Mean cortisol AUC was 6.11 μg/dL/min (SD = 10.44). Patterns of cortisol secretion for overweight and normal weight children are illustrated in Figure 1. The overall pattern shows cortisol decreasing from point 1 to the end of the protocol, as is the case in many studies of young children (Gunnar et al., 2009). However, the majority of children had an increase in cortisol at some point following free play (i.e., after point 2). Specifically, 58% showed some cortisol increase and 51% showed an increase of 5% or more after point 2. The majority of children also had an increase in cortisol 10 minutes or more after the stress elicitation protocol ended (i.e., after point 3 or later). Specifically, 66% showed some cortisol increase; 61% showed an increase of 5% or more after point 3. Thus, most children experienced an increase in cortisol at some point following the stress elicitation protocol. In addition, not surprisingly, cortisol at points 1 and 2 predicted response at later points. For example, children with a higher early cortisol level had higher cortisol at time 5 (time 1 standardized β=0.17, SE=0.04, p<.001; time 2 standardized β=0.75, SE=0.04, p<.001), and AUC from points 1-2 predicted AUC from points 3-5 (standardized β=2.55, SE=0.38, p<.001).
Figure 1. Cortisol reactivity to stress in normal weight (n = 129) and overweight children (n = 89).
- Bars represent standard errors.
- Cortisol sample numbers reflect: (1) 20 minutes after room entry; (2) 30 minutes after roomentry (during free play); (3) 10 minutes after disappointing gift receipt; (4) 20 minutes after giftreceipt; (5) 40 minutes after gift receipt.
Concurrent Associations between Cortisol AUC, Child BMIz, and Child Weight Status
In the unadjusted linear regression model, less overall cortisol AUC output (from points 2 to 5) was associated with greater concurrent child BMIz (standardized β = -0.16 (SE 0.07), p =.017). Cortisol AUC from points 3 to 5 was associated with child BMIz (standardized β = -0.14 (SE 0.07), p = .036). The association of cortisol AUC from points 1 to 2 and child BMIz approached significance (standardized β = -0.13 (SE 0.07), p = .070). In unadjusted t-tests, overweight children (n = 89) had lower AUC cortisol output (M AUC = 4.26 μg/dL/min (SD = 3.36), compared to children of normal weight (n=129; M AUC = 7.37 μg/dL/min (SD = 13.15), p = .023). The association between cortisol AUC and concurrent BMIz did not differ by child sex (p = .741 for interaction).
The cortisol AUC-BMIz association persisted even when covarying child sex, age, race/ethnicity, maternal weight status, and income-to-needs ratio (Table 1). The association of cortisol AUC from points 3 to 5 and BMIz (standardized β = -0.15 (SE 0.07), p = .036) persisted, but the association of cortisol AUC from points 1 to 2 and BMIz did not remain significant when covariates were included in the model (standardized β = -0.12 (SE 0.07), p = .105). The association of cortisol AUC (points 2-5) and BMIz persisted even when controlling for the above covariates plus cortisol at point 1 (standardized β = -0.22 (SE 0.10), p = .020). We next sought to determine if the association between cortisol AUC and child BMIz was non-linear and therefore tested the quadratic term in the fully adjusted model. It was not significant (p = .381), indicating that the association was linear. We conducted repeat analyses of the final adjusted model for the subset of responders, in order to examine whether the association between cortisol AUC and BMIz held for children who had increased their cortisol from the free play period (point 2; “responders”, n = 126) or in children who did not show an increase (“non-responders”, n = 92). The association between cortisol AUC and BMIz was present for responders, (standardized β = -0.20 (SE 0.10), p = .044), but not for non-responders, (standardized β = -0.13 (SE 0.09), p = .182). We also found that children who were responders were marginally more likely to be normal weight, compared to overweight (χ2= 3.23, p = .072), than non-responders.
Table 1. Association of cortisol AUC output with child BMIz (n = 218).
| Variable | β (SE) | p-value |
|---|---|---|
| Cortisol AUC | -0.168 (0.070) | 0.018 |
| Child sex female (vs. male) | 0.077 (0.139) | 0.581 |
| Child age (months) | 0.005 (0.010) | 0.623 |
| Mother overweight (vs. not) | 0.241 (0.172) | 0.164 |
| Child race/ethnicity Hispanic or non-white (vs. non-Hispanic white) | 0.170 (0.142) | 0.234 |
| Family income-to-needs ratio | 0.009 (0.082) | 0.911 |
Finally, we tested the interaction of cortisol AUC with age in the fully adjusted model and it was not significant (p = .745), indicating that the association was consistent across the age range of children in our sample.
Prospective Analyses between Cortisol AUC and BMIz 12 months later
Of the 218 children in this sample, 115 had a follow-up BMIz measured a mean of 12.4 (SD 3.3) months later. We repeated the adjusted model with the outcome being change in BMIz per month and the main effect of AUC was not significant (standardized β = 0.004 (SE 0.003), p = .191). Finally, we repeated this model with the outcome being change in BMIz while controlling for baseline BMIz, and the main effect of AUC again was not significant (standardized β = 0.002 (SE 0.003), p = .410).
Discussion
We found that low cortisol reactivity to stress was associated with a higher concurrent BMIz among preschool-aged, low-income children, supporting our hypothesis. This association was not altered after covarying for multiple sociodemographic confounders, was linear and was consistent across age and gender. The BMIz-cortisol association remained robust in the subsample of children whose cortisol values increased during the protocol. In follow-up analyses, it appeared that the association was driven primarily by cortisol reactivity following the stress protocol, and not by differences on arrival to the study center preceding the stress protocol. There was also no association between cortisol reactivity and height z-score (p<121), confirming that the relationship was accounted for by excessive weight and not deficits in linear growth. The association was not present for future changes in BMIz.
Our finding of an inverse association between cortisol reactivity and adiposity differs from six of eight prior studies on this topic. Of these six studies, the four that found a positive association included primarily white women (Epel et al., 2000); (Benson et al., 2009)), or school-age children (Dockray et al., 2009) (Francis et al., 2012)). The two studies that did not detect an association (Brydon, 2011) (Therrien et al., 2010), as well as those with findings similar to ours (Phillips et al., 2012) (Jones et al., 2012) also included primarily white adults. Our study thus adds to the literature by documenting this association for the first time in a moderately large sample of young, low-income children.
Results across studies may have differed for multiple reasons. The pattern of findings in previous studies suggests that there may be an evolution of the association with age., In other populations, an association was not detectable among children ages 5-7 years (Francis et al., 2012), became detectable as a positive association by age 8 years (Dockray et al., 2009; Francis et al., 2012) and in adulthood (Benson et al., 2009; Epel et al., 2000), but was an inverse association by middle age (Jones et al., 2012; Phillips et al., 2012). Factors such as exposure to psychosocial stressors may shape the course and timing of the cortisol response to stress, and the evolution of the cortisol reactivity-adiposity association. Specifically, populations exposed to fewer life stressors may be less likely to develop these associations, or only do so much later in the life course. The fact that this association emerged so early in the life course in our sample may be due to poverty, which is associated with substantial stressors (Evans and English, 2002). Some have noted that blunted responses to stress, under certain chronic stressful conditions (e.g., child maltreatment; (Trickett et al., 2010)) may be adaptive (Susman, 2006), so it is certainly important to understand the development and implications of this profile in high-risk samples. In contrast, the samples in other studies were either overwhelmingly white, well-educated, and middle- to upper-income (Brydon, 2011; Dockray et al., 2009; Epel et al., 2000; Francis et al., 2012; Jones et al., 2012; Phillips et al., 2012), or their sample demographics were not described (Benson et al., 2009; Therrien et al., 2010).
Other differences in sample characteristics may also have contributed to inconsistent findings in the literature. For example, the range of adiposity within a sample is important to consider. Studies that detected an inverse association as we did had, as did we, a substantial proportion (i.e. at least a third) of the sample that was overweight or obese (Jones et al., 2012; Phillips et al., 2012). Other studies had either a small proportion of overweight participants (Brydon, 2011), or small sample sizes overall, limiting power (Benson et al., 2009; Epel et al., 2000; Therrien et al., 2010). Prior studies with children that found a positive association also had smaller proportions of overweight or obesity (Dockray et al., 2009; Francis et al., 2012) and included some children who were underweight (Francis et al., 2012). Puberty may also moderate these associations. No child in our study had entered puberty, but in the prior studies of children with different findings, more than half the sample had entered puberty (Dockray et al., 2009) or pubertal status was not described (Francis et al., 2012). We did not find sex differences in our sample, but Dockray and colleagues (2009) found cortisol-BMI assocations for school-age girls, not boys. Given sex differences in cortisol responses that emerge during adolescence (Stroud et al., 2011), and puberty-related changes in adiposity (Mihalopoulos et al., 2010), this may be particularly important to consider in future longitudinal work on cortisol-BMI associations.
Results across studies may also differ due to protocol variations. Clearly, protocols used to evoke stress in adults must be tailored (as we did here) to be developmentally appropriate for younger children (Gunnar et al., 2009). For this reason, however, it is difficult to know with certainty how the level of stress compares across studies. There are also other methodological differences: cortisol was assayed in serum versus in saliva in some studies (e.g., Benson et al., 2009), and cortisol reactivity was analyzed in different ways (e.g., AUC vs. time-to-peak analyses). For example, two studies with null findings for BMI assessed cortisol change from baseline to peak (Therrien et al., 2010) and to post-stress (Brydon, 2011). Here, we found that cortisol response after the stress was more strongly related to BMIz than were pre-stress cortisol levels. Thus, it is possible that subtle and as-yet not understood variations in the dynamics of cortisol response to stress may explain inconsistencies across studies with regard to associations with BMI.
With regard to mechanism(s) of association, as others have also speculated (Jones et al., 2012; Phillips et al., 2012), we hypothesize that blunted stress reactivity may reflect an adaptation of the stress response as a consequence of down-regulation of stress receptors following chronic stress exposure. In other words, repeated prior stress exposure may have led to substantial stress-eating and obesity; subsequently the obesity persisted, but stress reactivity became blunted due to repeated stimulation. Longitudinal and additional mechanistic work will help to disentangle these associations. Second, it has been hypothesized that the association may be driven by dysregulation of central motivational circuits (Phillips et al., 2012). Specifically, blunted physiological stress responses have been linked with dysfunction of amygdala systems that support motivated behavior (Gianaros et al., 2008). Third, a reduced response to stress or “passive coping style” may underlie both reduced cortisol reactivity and adiposity (Boersma et al., 2010; Jones et al., 2012). Adiposity itself may reduce HPA axis responsivity to stress; indeed, circulating leptin levels, which are higher in obesity, can inhibit HPA axis response to stress (Heiman et al., 1997). The possibility that obesity itself inhibits this response requires investigation, as the implications of cortisol “hypo-responsivity” to a stressor for health and well-being, particularly for very young children, are unknown (Gunnar and Vazquez, 2001; Phillips et al., in press).
Our study has several strengths. Our sample size was substantially larger than the two other extant studies of children (Dockray et al., 2009) (Francis et al., 2012)) and most (Benson et al., 2009; Brydon, 2011; Epel et al., 2000; Jones et al., 2012; Therrien et al., 2010) but not all (Phillips et al., 2012) studies with adults. Our sample also was relatively diverse, high-risk, and had a high proportion of overweight children. Unlike other child studies (Dockray et al., 2009; Francis et al., 2012), we controlled for maternal weight status, a substantial contributor to child BMI. We also included prospective longitudinal data on BMI, which has been done in only one prior study, in adults, and also found no association (Phillips et al., 2012). It may be that BMI-cortisol reactivity associations are present only at certain developmental periods, as noted above, and we do not yet know the timing of how such associations emerge. Alternatively, other contributors to adiposity may, over time, overwhelm the effect of early cortisol reactivity. Future work should investigate such associations longitudinally, as physical growth, physiological stress response system(s) and the development of the capacity to cope with stress are each complex and may follow non-linear developmental trajectories.
Our study also has limitations. The sample was limited to low-income preschool-aged children who had attended Head Start, which limits generalizability to other ages and other socioeconomic circumstances. We evaluated cortisol reactivity during only a single laboratory session, so could not evaluate potential differences in habituation to a stressor, as others have done (Epel et al., 2000). We are also unable to draw conclusions about the stability of the stress response over time or the bidirectional associations of cortisol and BMI. Finally, although the majority of children in our study increased their cortisol level during the protocol, as in other work with young children (Francis, et al., 2012), the greatest mean cortisol reactivity occurred upon arrival to the study center, which may reflect anticipatory stress. Ethical considerations limit our ability to impose even greater stressors on children this young, and of note, the stressors in our protocol resembled events that children encounter in their everyday lives and our protocol occurred in familiar classroom environments, increasing the applicability of the results to real-world situations.
Our findings provide further impetus to examine cortisol-BMI associations at earlier ages, and in populations like ours, who may experience more early life stress than their peers. In addition to the well-known association of stress and low-income environments (Evans, 2012), there is evidence that prenatal stress increases the risk of childhood overweight and obesity, and may play an important role in mediating individual stress reactivity (Dancause, 2012; Therrien et al., 2010). Future research on childhood obesity should pave the way to a better understanding of the influence of early life stress, including prenatal stress, on individual variability of the stress response in children, the coping mechanisms that may moderate the response, and their relation to obesity risk and poor health outcomes in adulthood.
Acknowledgments
Role of the Funding Source: This research was supported by NIH 1R21DK090718 and American Heart Association Midwest Affiliate Grant-in-Aid 10GRNT4460043. Grants were awarded to Alison L. Miller.
Footnotes
Conflict of Interest Statement: The authors have no conflicts of interest to disclose.
Contributors' Statement: Each author has made a substantial contribution to the work as listed below:
Alison Miller, PhD: concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, obtaining funding, supervision
Caitlin Clifford, BA: acquisition of data, analysis and interpretation of data, drafting of the manuscript
Julie Sturza, MPH: analysis and interpretation of data, critical revision of the manuscript for important intellectual content, statistical analysis
Katherine Rosenblum, PhD: concept and design, acquisition of data, analysis and interpretation of data, critical revision of the manuscript for important intellectual content, supervision
Delia M. Vazquez, MD: concept and design, acquisition of data, analysis and interpretation of data, critical revision of the manuscript for important intellectual content, obtaining funding, supervision
Niko Kaciroti, PhD: analysis and interpretation of data, critical revision of the manuscript for important intellectual content, statistical analysis, obtaining funding, supervision
Julie C. Lumeng, MD: concept and design, acquisition of data, analysis and interpretation of data, critical revision of the manuscript for important intellectual content, obtaining funding, supervision
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Benson S, Arck PC, Tan S, Mann K, Hahn S, Janssen OE, Schedlowski M, Elsenbruch S. Effects of obesity on neuroendocrine, cardiovascular, and immune cell responses to acute psychosocial stress in premenopausal women. Psychoneuroendocrinology. 2009;34:181–189. doi: 10.1016/j.psyneuen.2008.08.019. [DOI] [PubMed] [Google Scholar]
- Boersma GJ, Benthem L, van Dijk G, Steimer TJ, Scheurink AJW. Coping style predicts the (in)sensitivity for developing hyperinsulinemia on a high fat diet in rats. Physiology & Behavior. 2010;100:401–407. doi: 10.1016/j.physbeh.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Brydon L. Adiposity, leptin and stress reactivity in humans. Biological Psychology. 2011;86:114–120. doi: 10.1016/j.biopsycho.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole PM. Children's spontaneous control of facial expression. Child Development. 1986:1309. [Google Scholar]
- Cole PM, Dennis TA, Smith-Simon KE, Cohen LH. Preschoolers' emotion regulation strategy understanding: Relations with emotion socialization and child self-regulation. Social Development. 2009;18:324–352. [Google Scholar]
- Dallman M, Pecoraro N, Akana S, et al. Chronic stress and obesity: A new view of “comfort food”. Proceedings of the National Academy of Sciences. 2003;100:11696–11701. doi: 10.1073/pnas.1934666100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dancause KN. Prenatal exposure to a natural disaster increases risk for obesity in 5½-year-old children. Pediatric Research. 2012;71:126–131. doi: 10.1038/pr.2011.18. [DOI] [PubMed] [Google Scholar]
- Dickerson SS. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Dockray S, Susman EJ, Dorn LD. Depression, cortisol reactivity, and obesity in childhood and adolescence. Journal of Adolescent Health. 2009;45:344–350. doi: 10.1016/j.jadohealth.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES, McEwen B, Seeman T, Matthews K, Castellazzo G, Brownell KD, Bell J, Ickovics JR. Stress and body shape: Stress-induced cortisol secretion is consistently greater among women with central fat. Psychosomatic Medicine. 2000;62:623–632. doi: 10.1097/00006842-200009000-00005. [DOI] [PubMed] [Google Scholar]
- Evans GW. Childhood cumulative risk and obesity: The mediating role of self-regulatory ability. Pediatrics (Evanston) 2012;129:e68–e73. doi: 10.1542/peds.2010-3647. [DOI] [PubMed] [Google Scholar]
- Evans GW, English K. The environment of ooverty: Multiple stressor exposure, psychophysiological stress, and socioemotional adjustment. Child Development. 2002;73:1238–1248. doi: 10.1111/1467-8624.00469. [DOI] [PubMed] [Google Scholar]
- Francis LA, Granger DA, Susman EJ. Adrenocortical regulation, eating in the absence of hunger and BMI in young children. Appetite. 2012 doi: 10.1016/j.appet.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman DS, Khan LK, Dietz WH, Srinivasan SR, Berenson GS. Relationship of childhood obesity to coronary heart disease risk factors in adulthood: The Bogalusa Heart Study. Pediatrics. 2001;108:712–718. doi: 10.1542/peds.108.3.712. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Sheu LK, Matthews KA, Jennings JR, Manuck SB, Hariri AR. Individual differences in stressor-evoked blood pressure reactivity vary with activation, volume, and functional connectivity of the amygdala. The Journal of Neuroscience. 2008;28:990–999. doi: 10.1523/JNEUROSCI.3606-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith H, Rothbart M. The Laboratory Temperament Assessment Battery (LabTAB): Locomotor, Version 3 (Technical Manual) Department of Psychology, University of Wisconsin; Madison, WI: 1996. [Google Scholar]
- Gundersen C, Mahatmya D, Garasky S, Lohman B. Linking psychosocial stressors and childhood obesity. Obesity Reviews. 2011;12:e54–e63. doi: 10.1111/j.1467-789X.2010.00813.x. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Talge NM, Herrera A. Stressor paradigms in developmental studies: What does and does not work to produce mean increases in salivary cortisol. Psychoneuroendocrinology. 2009;34:953–967. doi: 10.1016/j.psyneuen.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Vazquez DM. Low cortisol and a flattening of expected daytime rhythm: Potential indices of risk in human development. Developmental Psychopathology. 2001;13:515–538. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- Heiman ML, Ahima RS, Craft LS, Schoner B, Stephens TW, Flier JS. Leptin Inhibition of the hypothalamic-pituitary-adrenal axis in response to stress. Endocrinology. 1997;138:3859–3863. doi: 10.1210/endo.138.9.5366. [DOI] [PubMed] [Google Scholar]
- Jones A, McMillan MR, Jones RW, Kowalik GT, Steeden JA, Deanfield JE, Pruessner JC, Taylor AM, Muthurangu V. Adiposity is associated with blunted cardiovascular, neuroendocrine and cognitive responses to acute mental stress. PLoS ONE. 2012;7:e39143. doi: 10.1371/journal.pone.0039143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimbro RT, Brooks-Gunn J, McLanahan S. Racial and ethnic differentials in overweight and obesity among 3-year-old children. American Journal of Public Health. 2007;97:298–305. doi: 10.2105/AJPH.2005.080812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Hellhammer DH. Salivary cortisol in psychoneuroendocrine research: Recent developments and applications. Psychoneuroendocrinology. 1994;19:313–333. doi: 10.1016/0306-4530(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Lopez-Duran NL, Hajal NJ, Olson SL, Felt BT, Vazquez DM. Individual differences in cortisol responses to fear and frustration during middle childhood. Journal of Experimental Child Psychology. 2009;103:285–295. doi: 10.1016/j.jecp.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe L, Brooks-Gunn J. Unpublished manual for live gift wrap protocol 2002 [Google Scholar]
- Mihalopoulos NL, Holubkov R, Young P, Dai S, Labarthe DR. Expected changes in clinical measures of adiposity during puberty. Journal of Adolescent Health. 2010;47:360–366. doi: 10.1016/j.jadohealth.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquali R, Vicennati V, Agostini A, Pagotto U. Glucocorticoids, stress and obesity. Expert Review of Endocrinology & Metabolism. 2010;5:425–434. doi: 10.1586/eem.10.1. [DOI] [PubMed] [Google Scholar]
- Phillips AC, Ginty AT, Hughes BM. The other side of the coin: Blunted cardiovascular and cortisol reactivity are associated with negative health outcomes. International Journal of Psychophysiology. doi: 10.1016/j.ijpsycho.2013.02.002. in press. [DOI] [PubMed] [Google Scholar]
- Phillips AC, Roseboom TJ, Carroll D, de Rooij SR. Cardiovascular and cortisol reactions to acute psychological stress and adiposity: Cross-sectional and prospective sssociations in the Dutch Famine Birth Cohort Study. Psychosomatic Medicine. 2012;74:699–710. doi: 10.1097/PSY.0b013e31825e3b91. [DOI] [PubMed] [Google Scholar]
- Pruessner J, Kirschbaum C, Meinlschmid G, Hellhammer D. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinol. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Shrewsbury V, Wardle J. Socioeconomic status and adiposity in childhood: A systematic review of cross-sectional studies 1990-2005. Obesity. 2008;16:275–284. doi: 10.1038/oby.2007.35. [DOI] [PubMed] [Google Scholar]
- Stroud LR, Papandonatos GD, Williamson DE, Dahl RE. Sex differences in cortisol response to corticotropin releasing hormone challenge over puberty: Pittsburgh Pediatric Neurobehavioral Studies. Psychoneuroendocrinology. 2011;36:1226–1238. doi: 10.1016/j.psyneuen.2011.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susman EJ. Psychobiology of persistent antisocial behavior: Stress, early vulnerabilities and the attenuation hypothesis. Neuroscience & Biobehavioral Reviews. 2006;30:376–389. doi: 10.1016/j.neubiorev.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Therrien F, Drapeau V, Lalonde J, Lupien SJ, Beaulieu S, Doré J, Tremblay A, Richard D. Cortisol response to the Trier Social Stress Test in obese and reduced obese individuals. Biological Psychology. 2010;84:325–329. doi: 10.1016/j.biopsycho.2010.03.013. [DOI] [PubMed] [Google Scholar]
- Trickett PK, Noll JG, Susman EJ, Shenk CE, Putnam FW. Attenuation of cortisol across development for victims of sexual abuse. Development and psychopathology. 2010;22:165. doi: 10.1017/S0954579409990332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ryzin MJ. Identifying atypical cortisol patterns in young children: The benefits of group-based trajectory modeling. Psychoneuroendocrinology. 2009;34:50. doi: 10.1016/j.psyneuen.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalewski M. Understanding the relation of low income to HPA-Axis Functioning in preschool children: Cumulative family rsk and parenting as pathways to disruptions in cortisol. Child Psychiatry & Human Development. 2012 doi: 10.1007/s10578-012-0304-3. [DOI] [PMC free article] [PubMed] [Google Scholar]