Abstract
The goal of this study was to extend our previous research that reported a significant association between Attention Deficit Hyperactivity Disorder (ADHD)-relevant childhood behaviors and the frequency of methamphetamine (MA)-induced psychotic symptoms in an expanded sample. 190 participants who met DSM-IV criteria for MA dependence were administered the Methamphetamine Experience Questionnaire that assessed MA-induced psychosis. Data related to MA exposure, comorbid drug use, education, familial psychiatric history and assessments of ADHD-relevant childhood behaviors as measured by the Wender Utah Rating Scale (WURS) were collected. Although WURS scores did not differ between 145 MAP+ and 45 MAP-subjects, MAP+ subjects with higher WURS scores were significantly more likely to report more frequent psychosis. Although mean daily MA dosage did not differ between the MAP+ and MAP- subjects, MAP+ who consumed larger doses of MA were significantly more likely to experience frequent psychosis. These data suggest that ADHD-relevant childhood behaviors may interact with MA exposure to reflect a neurobiological vulnerability related to the emergence of frequent MA-induced psychotic symptoms. These results may elucidate factors that contribute to the psychiatric sequelae of MA abuse.
Keywords: methamphetamine, psychosis, attention, ADHD, prefrontal cortex, substance abuse, predictors
1. Introduction
Worldwide use of methamphetamine (MA) is now estimated to be at 51 million users (Roehr, 2005; Degenhardt et al.,2008; Nations, 2008, 2009), with global abuse of amph/methamphetamines now surpassing that of cocaine and opiates combined (United Nations, 2009).Approximately 5% of the adult population in the United States has used MA on at least one occasion and Emergency Department admissions related to MA use have doubled during the period of 1994 to 2002 (SAMSHA, 2004). A subset of individuals who chronically abuse MA also develop severe psychotic symptoms, which are often associated with high levels of psychiatric hospitalization and serious social dysfunction (Sato, 1992; Chen et al., 2003; Dore and Sweeting, 2006; McKetin et al., 2006; Pasic et al., 2007). In 2010, the nation's Drug Abuse Warning Network estimated that 66,308 drug-related Emergency Department visits involved amphetamines and methamphetamine use (SAMSHA, 2010) and that patients with MA-related psychotic disorders were 33% more likely than patients with cocaine disorders to be transferred to the inpatient ward (Leamon et al., 2002). The highly addictive nature of MA, as well as its ability to produce psychotic symptoms, makes this drug a major public health concern in the 21st century (United Nations, 2009).
Approximately 60% of MA abusers report a history of paranoia, delusions and hallucinations while under the influence of the drug (Dore and Sweeting, 2006; McKetin et al., 2006; Schuckit, 2006; Degenhardt et al., 2008; Salo et al., 2008). Early symptom descriptions of psychosis induced by amphetamines that are still relevant today include the following: 1) paranoid delusions with ideas of reference; 2) persecutory delusions; and 3) auditory and visual hallucinations that occur in a state of clear consciousness (Connell, 1958). Although negative symptoms and thought disorder have also been reported following long-term MA and amphetamine use, they are rare (Siomopoulos, 1976; Srisurapanont et al., 2003). One large study of 309 MA users reported that after adjusting for comorbid psychiatric disorders (e.g., schizophrenia), MA abusers were 13 times more likely to develop psychotic symptoms compared to the general population (McKetin et al., 2006). Two other studies also reported high prevalence rates of psychosis in MA abusers, with one study reporting psychotic symptoms in 75% of their sample (Sato, 1992) and another reporting auditory hallucinations and persecutory delusions in 44.6 and 22.8%, respectively (Srisurapanont et al., 2003). Pre-morbid risk factors for MA psychosis include a familial history of psychiatric illness and age of first MA use (Chen et al., 2005) as well as genetic risk factors (Harano et al., 2004; Suzuki et al., 2006; Bousman et al., 2009).
Although it is well known that MA is highly neurotoxic to the brain, causing damage to multiple transmitter systems (Ricaurte et al., 1984; Schmidt et al., 1985; Seiden and Ricaurte, 1987; Axt and Molliver, 1991; Bowyer and Holson, 1995; Seiden and Sabol, 1996; Davidson et al., 2001; Thompson et al., 2004; Meredith et al., 2005; Yamamoto and Bankson, 2005; Quinton and Yamamoto, 2006), less is known about why MA abusers are at increased risk for psychotic episodes.
Similarities between amph/methamphetamine induced psychosis and psychotic symptoms associated with schizophrenia have long been observed (Angrist and Gershon, 1970; Angrist et al., 1974). Studies that have administered large doses of amphetamine to non-schizophrenia individuals reported symptoms similar to those observed in schizophrenia patients including: delusions, hallucinations and to a lesser degree thought disorder (Angrist et al., 1974). Acute amphetamine administration has also been reported to produce or enhance psychotic symptoms in schizophrenia patients that are ineffective in non-substance-using controls (Curran et al., 2004). Following amphetamine challenge in acutely ill schizophrenia patients, increased dopamine release was observed within the striatum (Laruelle et al., 1996; Laruelle et al., 1999; Abi-Dargham et al., 2003), with levels of dopamine release correlating both with positive symptoms (Abi-Dargham et al., 2000) as well as with response to dopamine blockers (Laruelle et al., 1999). Collectively these findings suggest substantial overlap between schizophrenia psychosis and amph/methamphetamine-induced psychosis.
Different models have emerged to describe the mechanisms underlying drug-induced psychoses (Buckley, 1998; Chambers et al., 2001). One model is that drug use alone can cause psychosis. If this were true then one would expect 100% of MA users to experience drug-induced psychosis after using this powerful stimulant drug and the facts simply do not support this. Statistics across studies report that on average only about 60% of MA abusers report a history of paranoia, delusions and hallucinations while under the influence of the drug (Dore and Sweeting, 2006; McKetin et al., 2006; Schuckit, 2006; Degenhardt et al., 2008; Salo et al., 2008). A second widely held model is a version of the “self medication hypothesis”, which proposes that individuals with psychiatric disorders abuse substances to alleviate adverse disease symptoms or medication side-effects” (Dalack et al., 1998). This hypothesis has been disputed in recent years due to numerous factors: 1) the prevalence rate of major psychiatric disorders (e.g., schizophrenia) has remained constant while the incidence of substance abuse in these patients has increased exponentially (Chambers et al., 2001); 2) drugs of abuse have wide ranging effects in psychiatric patients, some of which alleviate symptoms and some that exacerbate them (DeQuardo et al., 1994; Addington and Duchak, 1997); and 3) drug abuse often occurs before the onset of the disease (Berti, 1994). A third model is the primary addiction hypothesis that proposes the existence of common neural circuits underlying the neuropathology of both schizophrenia and addiction (Chambers et al., 2001). These neural circuits include a dysregulation of a wide neural network that involves mesolimbic dopamine in the nucleus accumbens along with cortical and hippocampal inputs (Jentsch and Taylor, 1999). And finally, there is the stress-vulnerability model that proposes that individuals who are at pre-existing risk for developing psychosis, develop psychosis after using drugs (Bramness et al., 2012).
In the current study we sought to replicate and extend our previous research that reported a significant association between Attention Deficit Hyperactivity Disorder (ADHD) relevant childhood behaviors as measured by the Wender Utah Rating Scale (WURS) and the frequency of MA-induced psychotic symptoms in a small sample of 39 MA abusers (Salo et al., 2008). Recent studies have documented a high-level of ADHD symptomatology in stimulant-dependent individuals (Kaye et al., 2012), while other studies have also reported increased WURS scores in chronic substance abusers (Clure et al., 1999; Lynskey and Hall, 2001; Matsumoto et al., 2005a; Matsumoto et al., 2005b), including individuals dependent on methamphetamine (Simon et al., 2000) and cocaine (Boutros et al., 2002). However, this is one of the first studies to link measures of ADHD-relevant childhood behaviors to MA-induced psychoses.
The main goal of the current study was to examine whether the presence of MA psychosis was associated with any risk factors (MAP+ vs. MAP- comparison) and conditional on having MA psychosis, whether or not increased frequency of psychosis was associated with any predictors. Furthermore, as cannabis use has been linked to non-substance induced psychosis (Broome et al., 2005), we wanted to examine cannabis use patterns as well as the prevalence of family history of psychiatric disorders in our sample.
2. Method
2.1. Participants
The MA-dependent subjects comprised 99 men and 91 women meeting criteria for lifetime MA dependence according to DSM-IV criteria. Data from thirty nine of the subjects were reported as part of our previous study (Salo et al., 2008). The MA abusers were recruited from substance abuse treatment centers and residential housing programs in the Sacramento area. Our study criteria required that our sample was/had: 1) MA abstinent at the time of study; 2) no current or lifetime comorbid non-substance induced Axis I disorder; 3) free of any neurological medical issues. 3) no substance dependence (other than MA and nicotine) within the past five years; and 4) no self-reported history of a seropositive test for HIV.
Among the 190 subjects, 91 were currently in treatment and 99 were in non-treatment settings. It should be emphasized however that most of our sample had received structured substance abuse treatment at some point in their addiction. All subjects had been drug abstinent for a minimum period of three weeks (range 3 weeks to 10 years) by self report and random urine drug screens performed at referring sites. All subjects were literate and completed a standardized measure of verbal IQ (National Adult Reading Test; NART) (Nelson, 1982). All subjects signed informed consent approved by the University of California Davis Institutional Review Board and were paid a modest stipend for study participation.
2.2. Assessments and semi-structured interviews
2.2.1. Structured clinical interview for DSM-IV
All participants were interviewed by PhD level research personnel using the Structured Clinical Interview for DSM-IV (SCID) and a consensus diagnosis was obtained for each participant. The SCID was used as an adjunctive measurement to assess the presence and frequency of psychotic episodes associated with MA use.
2.2.2. Methamphetamine Experience Questionnaire
All participants were interviewed using the Methamphetamine Experience Questionnaire (MEQ) which is an interview based on the Cocaine Experience Questionnaire (Gelernter et al., 1994; Leamon et al., 2010). The MEQ is designed to assess the lifetime frequency of psychotic episodes associated with MA use, conditions in which psychotic episodes occur, as well as the persistence of these symptoms.
Sample MEQ questions include:
How often have you had paranoid experiences while using methamphetamine, using a 0 to 5 Likert Scale? (Where 0 is never and 5 is always)
Were you more likely to get paranoid when you used greater amounts of methamphetamine?
2.2.3. Wender Utah Rating Scale (WURS)
All participants completed the WURS which retrospectively assesses Attention Deficit Hyperactivity Disorder (ADHD)-relevant childhood behaviors and symptoms in adults (Wender, 1985; Ward et al., 1993). The version of the WURS used in the current study contained 25 items that assessed symptoms which occurred between five and twelve years of age.
2.3. Statistical analyses
Statistical analyses were conducted using the SAS Institute SAS Version 9.3 (SAS Institute, 2002-2010) and included descriptive statistics for all categorical and continuous variables. First, differences between MA users with (MAP+) and without (MAP-) a history of psychosis were assessed using chi-squared test for the categorical variables, two sample t-tests for the continuous variables that were normally distributed and nonparametric Wilcoxon two-sample tests for those continuous variables that violated the assumption of normality. Second, for the MAP+ users, proportional-odds models (McCullagh, 1980) were used to examine the association between demographic and clinical characteristics and the frequency of psychosis. These models can be thought of as an extension of the logistic regression model for dichotomous dependent variables, allowing for more than two ordered response categories and have the advantage of being able to capture information from the entire spectrum of psychosis (measured on an ordinal Likert scale). The odds ratio (OR) is calculated for each cut-off across the frequency psychosis (for example, 1 versus 2-5, then 1-2 versus 2-5, and so on), and then a summary OR is calculated from the individual ORs, under the assumption that the individual OR are constant for all categories. When the testable proportional odds assumption is met, the odds ratio provided by the model does not reflect a simple odds ratio, but rather an average across multiple thresholds of classification of the outcome and can be interpreted as the odds of having “more frequent” psychosis across the whole range of the psychosis. We first examined unadjusted models (with one predictor at the time), followed by adjusted models to assess if the significant effect of the predictors from the univariate analyses could be an artifact of confounding. The validity of the proportional odds assumption was tested using Score tests and was deemed adequate for all models.
3. Results
Table 1 presents summary measures (means and standard deviations for the continuous variables and frequencies and percentages for the categorical ones) for all demographic and clinical characteristics, stratified on the history of paranoia. Out of the 190 participants in the sample, 145 (76%: 79 men and 66 women) reported symptoms associated with MA psychosis that included paranoid delusions as well as visual, auditory and tactile hallucinations (MAP+), and 45 (24%: 20 men, 25 women) did not (MAP-). The mean age in the entire sample was 38 years (standard deviation = 8, range 19 – 55 years), with the average participant having completed high school. The two groups did not differ significantly in age, gender composition, education, years of parental education, estimates of pre-morbid intelligence as assessed by the NART (Nelson, 1982), or WURS scores (see Table 1). An analysis of the methamphetamine use patterns revealed that on average the MAP+ and MAP- subjects had similar ages of first use, duration of drug use, mean daily MA dose consumed and months drug abstinent (see Table 1).
Table 1. Summary of the demographic and clinical characteristics of the methamphetamine abusers withpsychosis (MAP+) and without psychosis (MAP-).
| MAP+ (n = 145) |
MAP- (n = 45) |
Test Statistic | p-value | |
|---|---|---|---|---|
| Demographic Characteristics | ||||
|
| ||||
| Age | 37.6 (8.2) | 37.7 (8.4) | z = -0.06 | 0.95 |
| Females | 66 (46%) | 25 (56%) | χ2 = 1.39 | 0.24 |
| Education (years) | 12.2 (2.0) | 12.6 (1.8) | z = 1.30 | 0.20 |
| Parental Education (years)1 | 13.0 (2.6) | 13.2 (2.9) | z = 0.46 | 0.65 |
| NART2 | 105.6 (5.6) | 107.3 (6.4) | t183 = 1.71 | 0.09 |
| Family History of Psychiatric Disorder3 | 45 (31%) | 9 (21%) | χ2 = 1.78 | 0.18 |
|
| ||||
| Clinical Characteristics | ||||
|
| ||||
| WURS score1 | 64.9 (21.8) | 59.4 (20.7) | z = -1.41 | 0.16 |
| Methamphetamine use | ||||
| Age of first use | 18.8 (6.0) | 18.8 (6.1) | z = -0.11 | 0.91 |
| Years of use | 14.1 (6.7) | 14.2 (7.2) | z = 0.06 | 0.95 |
| Mean Dose (g) | 1.3 (1.0) | 1.2 (0.9) | z = -1.17 | 0.24 |
| Months Abstinent | 18.2 (24.2) | 14.5 (23.0) | z = -1.72 | 0.09 |
| Alcohol use | ||||
| Alcohol Abuse/Dependence | 81 (56%) | 26 (58%) | χ2 = 0.05 | 0.82 |
| Years Alcohol clear | 10.1 (7.9) | 10.2 (8.3) | z = 0.09 | 0.93 |
| Tobacco use | ||||
| Tobacco smokers | 121 (83%) | 37 (82%) | χ2 = 0.04 | 0.85 |
| Pack years | 14.8 (11.3) | 16.0 (9.9) | z = 0.89 | 0.37 |
| Cocaine use | ||||
| Cocaine Abuse/Dependence | 41 (28%) | 8 (18%) | χ2 = 1.98 | 0.16 |
| Years Cocaine clear | 15.7 (8.0) | 18.3 (7.6) | t46 = 0.97 | 0.34 |
| Cannabis use | ||||
| Cannabis Abuse/Dependence | 113 (78%) | 32 (71%) | χ2 = 0.88 | 0.35 |
| Age of first use | 14.2 (2.6) | 14.7 (4.7) | z = -0.77 | 0.44 |
| Years of Cannabis use | 12.2 (9.0) | 15.1 (9.9) | z = 1.55 | 0.12 |
| Years Cannabis clear | 9.7 (9.6) | 6.5 (8.5) | z = -2.04 | 0.04 |
Note: continuous variables are summarized as mean (SD) and categorical ones as frequency (percent). Group differences were assessed using Chi-squared (χ2) tests for categorical variables, two-sample t-test the continuous variables whose distribution was normal, and Wilcoxon two-sample (z) tests for the continuous variables who violated the assumption of normality.
Frequency missing = 3 in MAP+ sample
Frequency missing = 3 in MAP+ sample and 2 in the MAP- sample
Frequency missing = 2 in MAP+ sample and 2 in the MAP- sample
We had information on history of psychiatric illness in first-degree relatives from 98% of our sample. Four subjects were adopted at an early age and thus were unable to provide family history. In the whole MA abusers sample, 54 (28%) reported a family history of psychiatric illness. The proportion of participants with a history of psychiatric illness was 31% in the MAP+ group and 21% in the MAP- group, but this difference did not reach statistical significance (see Table 1).
3.1. Comorbid drug use
In any sample of illicit drug users it is common that most abusers will have used/abused more than one drug during their lifetime. Among the 190 MA abusers, several met criteria for lifetime, but not current, abuse/dependence for the following substances: Alcohol: 56%, Cocaine: 26%; Cannabis: 76%, Tobacco: 83%. Of those who had lifetime comorbid disorders, inclusion criteria required any that comorbid dependence on any substance (other than tobacco) must have occurred more than 5 years prior to study. Comorbid abuse was allowed if it did not occur within the past year. Median length of abstinence from the comorbid drugs was: Alcohol: 8 years, Cocaine: 15 years, Cannabis: 5 years.
Comorbid drug use patterns were similar in the two groups, for alcohol and cocaine, as well as tobacco use (see Table 1). Planned analysis between the MAP+ and the MAP-groups revealed no difference in the proportion of abuse/dependence users, age of first cannabis use, or years of cannabis use (see Table 1). There was however a difference in the abstinence times, with MAP+ participants having longer cannabis abstinence than the MAP- participants (p = 0.04, see Table 1).
73% of the MAP+ subjects experienced both auditory and visual hallucinations, 17% reported hallucinations in the visual modality only, 8% in the auditory modality only, and 2% reported delusions with no experience of hallucinations in either sensory modality. None of the MAP- subjects had experienced delusions or hallucinations. Among the 145 participants with reported history of psychosis, 23 (16%) had the lowest frequency (1), 27 (19%) had frequency 2, 38 (26%) frequency 3, 33 (23%) frequency 4 and 24 (17%) reported the highest levels (5).
Table 2 summarizes the results of the univariate unadjusted analyses (each predictor entered the model by itself, without any adjustment for other variables) examining the associations between demographic and clinical characteristics and the frequency of psychosis. Neither gender nor any of the other demographic characteristics were associated with frequency of psychosis in univariate analyses. Although WURS scores did not differ between the MAP+ and the MAP- subjects, MAP+ subjects with higher WURS scores were more likely to report being in a category with more frequent psychosis (OR = 1.04, 95% CI 1.02 – 1.05, p < 0.0001). There was a 4 percent increase in the odds of a more frequent psychosis per 1 point increase in WURS score. This interpretation holds across the entire range of psychosis (from 1 to 5). Among the MA use variables, the mean daily MA dosage was associated with frequency of psychosis. MA abusers who consumed larger quantities of MA on a daily basis had higher likelihood of being in a more frequent psychosis category (OR = 1.61, 95% CI 1.17 – 2.20, p = 0.003). None of the comorbid drug use variables were associated with frequency of psychosis, although there was a trend for the tobacco use and cannabis abuse/dependence to be associated with higher frequency of psychosis (p = 0.08 and 0.06, respectively) in unadjusted analyses. We then fitted an adjusted model in which the two main predictors associated with frequency of psychosis in univariate analysis (WURS and mean daily MA dosage) were included as predictors of the frequency of psychosis simultaneously, to examine if they would be significant after controlling for the effect of the other. The significance of WURS remained unchanged, but the coefficient for daily MA dosage decreased (OR = 1.46, 95% CI 1.06 – 2.00, p = 0.02).
Table 2. Results of the unadjusted proportional odds models examining the association between demographic and clinical characteristics of the 145 MAP+ methamphetamine abusers with the frequency of psychotic episodes.
| OR (95% CI) | p-value | |||
|---|---|---|---|---|
| Demographic Characteristics | ||||
|
| ||||
| Age (years) | 1.02 (0.98 – 1.06) | 0.28 | ||
| Female Gender | 0.93 (0.52 – 1.66) | 0.81 | ||
| Education (years) | 0.99 (0.86 – 1.15) | 0.94 | ||
| Parental Education (years)1 | 0.93 (0.83 – 1.05) | 0.24 | ||
| NART | 0.99 (0.94 – 1.05) | 0.82 | ||
| Family History of Psychiatric Disorder2 | 1.16 (0.62 – 2.17) | 0.64 | ||
|
| ||||
| Clinical Characteristics | ||||
|
| ||||
| WURS score1 | 1.04 (1.02 – 1.05) | < 0.0001 | ||
| Methamphetamine use | ||||
| Age of first use | 1.01 (0.96 – 1.05) | 0.84 | ||
| Years of use | 1.02 (0.98 – 1.06) | 0.40 | ||
| Mean Dose (g) | 1.61 (1.17 – 2.20) | 0.003 | ||
| Months Abstinent | 1.00 (0.99 – 1.01) | 0.61 | ||
| Alcohol Abuse/Dependence | 1.01 (0.57 – 1.80) | 0.98 | ||
| Tobacco use | 2.00 (0.92 – 4.39) | 0.08 | ||
| Cocaine Abuse/Dependence | 0.94 (0.49 – 1.78) | 0.84 | ||
| Cannabis Abuse/Dependence | 2.00 (0.99 – 4.04) | 0.06 | ||
Note: unadjusted models with frequency of psychosis as a dependent variable were fit for each of the demographic and clinical characteristics
Frequency missing = 3
Frequency missing = 2
3.2. Gender differences
This dataset was well balanced on gender (males = 99; females = 91), thus we were in a position to examine gender as a moderator of MA psychosis. Across the entire sample of 190 MA abusers, the males and females did not differ in age (Wilcoxon two-sample z = 1.26, p = 0.21), years of education (z = -0.37, p = 0.71), years of parental education (z = -0.55, p = 0.58), or estimates of pre-morbid intelligence as assessed by the NART (t183 = -0.01, p = 0.99). An examination of the drug use patterns revealed that male and female MA abusers did not differ in duration of drug use (z = 0.96, p = 0.34), age of first use (z = 0.46, p = 0.64), mean daily dose of MA consumed (z = -0.81, p = 0.42), or the number of months for which they were MA abstinent at the time of study (z = -1.16, p = 0.25). No gender differences among the MAP+ participants reached significance.
4. Discussion
The data in the current study extend our previous findings of a significant positive correlation between frequency of MA-related psychotic episodes in the MAP+ subjects and scores on the WURS, a scale that retrospectively measures childhood attention function and hyperactivity (Salo et al., 2008). Although WURS scores did not differ between the MAP+ and the MAP- subjects, those MAP+ subjects who reported a higher lifetime frequency of MA induced psychotic episodes were those with the highest WURS scores reflecting ADHD-relevant childhood behaviors. In this new study we also examined mean daily MA dose consumed and found that although the mean daily dose consumed did not differ between the MAP+ and the MAP- subjects, among the MAP+ subjects there was a correlation between the amount of MA consumed and the frequency of MA psychotic episodes. These results appear to be consistent with other published studies (Batki and Harris, 2004; McKetin et al., 2013).
We did not replicate our previous finding that a familial history of psychiatric illness may further increase the risk of developing MA-induced psychotic symptoms (Chen et al., 2005; Salo et al., 2008). In this dataset, there was no statistically significant difference in either the prevalence or the frequency of MA induced psychotic episodes between those 54 MA Abusers with a family history of psychiatric illness, versus those who had no family history. We also failed to detect gender differences related to psychosis in this sample. These findings are inconsistent with others who have reported that MA dependent women were more likely than their male counterparts to report experiencing various psychotic symptoms (Mahoney et al., 2010). Additional studies in a larger community based sample are needed to explore these issues further.
Within the context of existing models that propose frameworks relating drug use to psychosis (Buckley, 1998; Chambers et al., 2001; Bramness et al., 2012), our results are perhaps the most consistent with the stress-vulnerability model. Our data suggest there is a degree of dose-response relationship between meth exposure and frequency of psychotic symptoms among individuals vulnerable to psychosis and that a pre-existing history of ADHD-relevant childhood behaviors is yet another contributing factor. If one accepts this model that preexisting vulnerabilities put one at risk for developing MA-induced psychosis then this can also explain why some individuals develop psychotic symptoms when exposed to methamphetamine and others do not. However, the one issue that remains unresolved is why WURS scores do not differ between the MAP+ and MAP- subjects. One explanation might be that early attention problems represent only one of several factors that predispose individuals to MAP and that although ADHD-relevant childhood behaviors may contribute to the severity (i.e., frequency) of psychotic episodes experienced, it is not a sufficient factor on its own to produce the symptoms.
As recent epidemiological studies have shown that early substance use (i.e., cannabis) among individuals who are at genetic risk may increase the likelihood of developing psychotic symptoms in adulthood (Luzi et al., 2008; Di Forti et al., 2009; Fisher et al., 2009; Smith et al., 2009; Barkus and Murray, 2010; Mazzoncini et al., 2010; Stilo and Murray, 2010; Casadio et al., 2011; Paparelli et al., 2011; Donoghue et al., 2012), we also examined the contribution of comorbid drug use to MA psychosis, specifically cannabis. In this data set the prevalence of cannabis use did not differ between the MAP+ and MAP-subjects and there was no significant relationship between the frequency of MA psychosis and the age at which they first used cannabis. There was however a statistical trend towards increased frequency of MA-induced psychotic episodes among those MAP+ subjects with a history of cannabis use. However, as the MAP+ participants had longer cannabis abstinence than the MAP- participants, it is unclear how strong the relationship is between cannabis use and psychosis in this sample. A recent meta-analysis of studies that examined the role of cannabis use on psychosis suggests that the reported link between cannabis use and psychotic symptoms is inconsistent (Zammit et al., 2008).
Although MA exposure does not appear to differ between the MAP+ and the MAP- subjects, mean daily lifetime dosage does appear to correlate with the frequency of which the psychotic symptom occurs. This might suggest that pharmacological exposure alone may not be the sole factor that produces MA psychosis, but might interact with other factors that contribute to an increased frequency of psychotic symptoms. If MA exposure alone were sufficient to produce MAP, then why do approximately 40% of chronic MA abusers never experience MA-induced psychotic episodes? This paradox points to the need to search for other factors.
Although genetic risk factors clearly play a role (Bousman et al., 2009), further studies are needed to determine if the development and recurrence of MA psychosis is linked to abnormal brain function in regions such as the prefrontal and temporal cortices, both of which are often implicated in the emergence of non-substance induced psychotic symptoms (e.g., schizophrenia) (Fusar-Poli et al., 2011a; Fusar-Poli et al., 2011b; Fusar-Poli et al., 2012). ADHD is associated with dysfunction in cortical circuits supporting attention and executive function including frontal parietal attentional networks and the anterior cingulate cortex. These circuits are also disrupted in MA abusing individuals. In rodent models (Pycock et al., 1980), as well as in individuals with schizophrenia, impaired prefrontal function is associated with increased dopamine release in the striatum (Tost et al., 2010) and excessive DA release in the striatum in schizophrenia is associated with clinical symptoms of psychosis (Laruelle et al., 1999; Abi-Dargham et al., 2000). In the context of this literature the present results suggest that childhood ADHD, with its associated deficits on cortical attention circuitry that persists throughout adolescence and into adulthood, may render MA abusing individuals more vulnerable to psychosis by limiting their ability to modulate excessive sub-cortical dopamine release. Future studies comparing the function of the neural circuitry modulating attention and the function of sub-cortical dopamine system will enable a test of these hypotheses and inform potential treatments (Grelotti et al., 2010).
4.1. Limitations
There are several limitations with the use of retrospective measures, especially in clinical populations. They include but are not limited to: 1) memory impairments; 2) report bias; and 3) subjective nature of the instrument. It is possible that a subgroup of MA abusers may have overestimated both psychotic symptoms and ADHD-relevant childhood behaviors in childhood, and others may underestimated them. Such response patterns could explain the correlations observed in the present study. It is also possible that substance abusers may be limited in their ability to accurately recollect childhood attention behaviors. Although we believe that such a recollection bias is unlikely to have played a role in our results, such concerns can be addressed by future studies designed specifically to control for such confounds. If such a bias would be present, it is unclear why it would manifest in a unique correlation with severity of psychosis and not with the other self-report variables that were measured.
As our participants were recruited from treatment and residential housing programs, it may limit the generalizability of our findings to the entire community of individuals with methamphetamine dependence. Finally, although the correlational patterns reported within the current study cannot imply causality, the data nonetheless reveal an interesting relationship between both the quantity of MA consumed and measures of early attention/hyperactivity and the emergence of frequent psychotic episodes in individuals who abuse MA heavily in adulthood and reach criteria for dependence.
4.2. Conclusion
The results suggest the existence of possible behavioral markers reflecting an early cognitive vulnerability to the development of frequent MA-induced psychotic symptoms. These behavioral markers may interact with drug exposure in individuals with other pre-existing genetic and cortical vulnerabilities. Understanding and identifying these cortical risk factors for MA psychosis will provide insight into the general mechanisms of psychosis as well as serve as a foundation to detect MAP susceptibility and the development of targeted clinical interventions.
Figure 1.
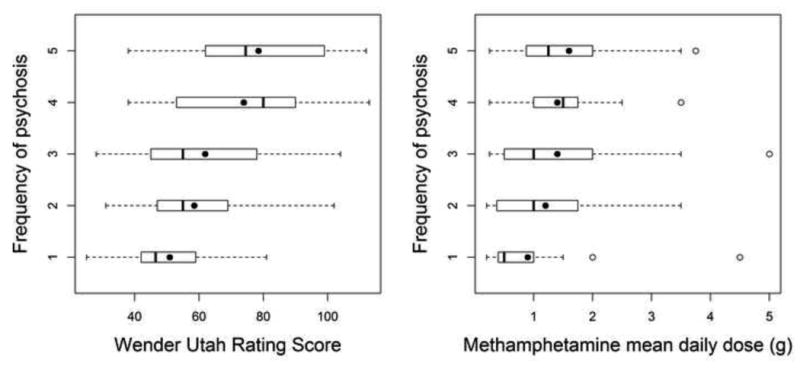
Association between Wender Utah Rating Scale of childhood attention and methamphetamine daily dose with frequency of psychosis measured on a Likert scale. Box plots define the values for median, range, 25th and 75th percentiles. Means are represented by filled circles. The proportional odds model was used to calculate the odds (OR) of developing more frequent psychosis for higher Wender Utah scores (OR = 1.04, p < 0.0001) and larger methamphetamine daily dose (OR = 1.61, p = 0.003), respectively.
Table 3. Frequency of Psychosis measured on a Likert Scale, mean Wender Utah score and mean daily methamphetamine (MA) dose across the range of frequency of psychosis.
| Frequency of Psychosis in Likert Units | n | Wender-Utah (WURS) | Mean daily MA dose |
|---|---|---|---|
| 1 | 23 | 50.9 | 0.88 |
| 2 | 27 | 58.5 | 1.16 |
| 3 | 38 | 61.9 | 1.43 |
| 4 | 33 | 73.9 | 1.40 |
| 5 | 24 | 78.5 | 1.64 |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abi-Dargham A, Kegeles LS, Martinez D, Innis RB, Laruelle M. Dopamine mediation of positive reinforcing effects of amphetamine in stimulant naive healthy volunteers: Results from a large cohort. European Neuropsychopharmacology. 2003;13:459–468. doi: 10.1016/j.euroneuro.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Abi-Dargham A, Rodenhiser J, Printz D, Zea-Ponce Y, Gil R, Kegeles LS, Weiss R, Cooper TB, Mann JJ, Van Heertum RL, Gorman JM, Laruelle M. Increased baseline occupancy of d2 receptors by dopamine in schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:8104–8109. doi: 10.1073/pnas.97.14.8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addington J, Duchak V. Reasons for substance use in schizophrenia. Acta Psychiatrica Scandinavica. 1997;96:329–333. doi: 10.1111/j.1600-0447.1997.tb09925.x. [DOI] [PubMed] [Google Scholar]
- Angrist B, Sathananthan G, Wilk S, Gershon S. Amphetamine psychosis: behavioral and biochemical aspects. Journal of Psychiatric Research. 1974;11:13–23. doi: 10.1016/0022-3956(74)90064-8. [DOI] [PubMed] [Google Scholar]
- Angrist BM, Gershon S. The phenomenology of experimentally induced amphetamine psychosis--preliminary observations. Biological Psychiatry. 1970;2:95–107. [PubMed] [Google Scholar]
- Axt KJ, Molliver ME. Immunocytochemical evidence for methamphetamine-induced serotonergic axon loss in the rat brain. Vol. 9. Synapse; New York, N.Y: 1991. pp. 302–313. [DOI] [PubMed] [Google Scholar]
- Barkus E, Murray RM. Substance use in adolescence and psychosis: clarifying the relationship. Annual Review of Clinical Psychology. 2010;6:365–389. doi: 10.1146/annurev.clinpsy.121208.131220. [DOI] [PubMed] [Google Scholar]
- Batki SL, Harris DS. Quantitative drug levels in stimulant psychosis: Relationship to symptom severity, catecholamines and hyperkinesia. The American Journal on Addictions. 2004;13:461–470. doi: 10.1080/10550490490512834. [DOI] [PubMed] [Google Scholar]
- Berti A. Schizophrenia and substance abuse: The interface. Progress in Neuro-psychopharmacology and Biological Psychiatry. 1994;18:279–284. doi: 10.1016/0278-5846(94)90059-0. [DOI] [PubMed] [Google Scholar]
- Bousman CA, Glatt SJ, Everall IP, Tsuang MT. Genetic association studies of methamphetamine use disorders: a systematic review and synthesis. American Journal on Medical Genetics Part B Neuropsychiatric Genetics. 2009;150B:1025–1049. doi: 10.1002/ajmg.b.30936. [DOI] [PubMed] [Google Scholar]
- Boutros NN, Gelernter J, Gooding DC, Cubells J, Young A, Krystal JH, Kosten T. Sensory gating and psychosis vulnerability in cocaine-dependent individuals: preliminary data. Biological Psychiatry. 2002;51:683–686. doi: 10.1016/s0006-3223(01)01237-9. [DOI] [PubMed] [Google Scholar]
- Bowyer JF, Holson RR. Methamphetamine and amphetamine neurotoxicity. In: Chang LW, Dyer RS, editors. Handbook of neurotoxicity. Marcel Dekker; New York: 1995. pp. 45–870. [Google Scholar]
- Bramness JG, Gundersen OH, Guterstam J, Rognli EB, Konstenius M, Loberg EM, Medhus S, Tanum L, Franck J. Amphetamine-induced psychosis--a separate diagnostic entity or primary psychosis triggered in the vulnerable? BMC Psychiatry. 2012;12:221. doi: 10.1186/1471-244X-12-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broome MR, Woolley JB, Tabraham P, Johns LC, Bramon E, Murray GK, Pariante C, McGuire PK, Murray RM. What causes the onset of psychosis? Schizophrenia Research. 2005;79:23–34. doi: 10.1016/j.schres.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Buckley PF. Substance abuse in schizophrenia: a review. Journal of Clinical Psychiatry. 1998;59(Suppl 3):26–30. [PubMed] [Google Scholar]
- Callaghan RC, Cunningham JK, Allebeck P, Arenovich T, Sajeev G, Remington G, Boileau I, Kish SJ. Methamphetamine use and schizophrenia: a population-based cohort study in california. The American Journal of Psychiatry. 2012;169:389–396. doi: 10.1176/appi.ajp.2011.10070937. [DOI] [PubMed] [Google Scholar]
- Casadio P, Fernandes C, Murray RM, Di Forti M. Cannabis use in young people: the risk for schizophrenia. Neuroscience and Biobehavioral Reviews. 2011;35:1779–1787. doi: 10.1016/j.neubiorev.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Krystal JH, Self DW. A neurobiological basis for substance abuse comorbidity in schizophrenia. Biological Psychiatry. 2001;50:71–83. doi: 10.1016/s0006-3223(01)01134-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CK, Lin SK, Sham PC, Ball D, Loh el W, Murray RM. Morbid risk for psychiatric disorder among the relatives of methamphetamine users with and without psychosis. American Journal on Medical Genetics Part B Neuropsychiatric Genetics. 2005;136B:87–91. doi: 10.1002/ajmg.b.30187. [DOI] [PubMed] [Google Scholar]
- Chen CK, Lin SK, Sham PC, Ball D, Loh EW, Hsiao CC, Chiang YL, Ree SC, Lee CH, Murray RM. Pre-morbid characteristics and comorbidity of methamphetamine users with and without psychosis. Psychological Medicine. 2003;33:1407–1414. doi: 10.1017/s0033291703008353. [DOI] [PubMed] [Google Scholar]
- Clure C, Brady KT, Saladin ME, Johnson D, Waid R, Rittenbury M. Attention-deficit/hyperactivity disorder and substance use: symptom pattern and drug choice. The American Journal of Drug and Alcohol Abuse. 1999;25:441–448. doi: 10.1081/ada-100101871. [DOI] [PubMed] [Google Scholar]
- Connell PH. Amphetamine psychosis Oxford University Press. Maudsley Institute of Psychiatry Monographs; London: 1958. [Google Scholar]
- Curran C, Byrappa N, McBride A. Stimulant psychosis: systematic review. The British Journal of Psychiatry. 2004;185:196–204. doi: 10.1192/bjp.185.3.196. [DOI] [PubMed] [Google Scholar]
- Dalack GW, Healy DJ, Meador-Woodruff JH. Nicotine dependence in schizophrenia: clinical phenomena and laboratory findings. The American Journal of Psychiatry. 1998;155:1490–1501. doi: 10.1176/ajp.155.11.1490. [DOI] [PubMed] [Google Scholar]
- Davidson C, Gow AJ, Lee TH, Ellinwood EH. Methamphetamine neurotoxicity: necrotic and apoptotic mechanisms and relevance to human abuse and treatment. Brain Research and Brain Research Reviews. 2001;36:1–22. doi: 10.1016/s0165-0173(01)00054-6. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Baker A, Maher L. Methamphetamine: geographic areas and populations at risk, and emerging evidence for effective interventions. Drug and Alcohol Review. 2008;27:217–219. doi: 10.1080/09595230801956538. [DOI] [PubMed] [Google Scholar]
- DeQuardo JR, Carpenter CF, Tandon R. Patterns of substance abuse in schizophrenia: nature and significance. Journal of Psychiatric Research. 1994;28:267–275. doi: 10.1016/0022-3956(94)90010-8. [DOI] [PubMed] [Google Scholar]
- Di Forti M, Morgan C, Dazzan P, Pariante C, Mondelli V, Marques TR, Handley R, Luzi S, Russo M, Paparelli A, Butt A, Stilo SA, Wiffen B, Powell J, Murray RM. High-potency cannabis and the risk of psychosis. The British Journal of Psychiatry. 2009;195:488–491. doi: 10.1192/bjp.bp.109.064220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue K, Mazzoncini R, Hart J, Zanelli J, Morgan C, Dazzan P, Morgan KD, Murray RM, Jones PB, Doody GA. The differential effect of illicit drug use on cognitive function in first-episode psychosis and healthy controls. Acta Psychiatrica Scandinavica. 2012;125:400–411. doi: 10.1111/j.1600-0447.2011.01803.x. [DOI] [PubMed] [Google Scholar]
- Dore G, Sweeting M. Drug-induced psychosis associated with crystalline methamphetamine. Australas Psychiatry. 2006;14:86–89. doi: 10.1080/j.1440-1665.2006.02252.x. [DOI] [PubMed] [Google Scholar]
- Fisher H, Morgan C, Dazzan P, Craig TK, Morgan K, Hutchinson G, Jones PB, Doody GA, Pariante C, McGuffin P, Murray RM, Leff J, Fearon P. Gender differences in the association between childhood abuse and psychosis. The British Journal of Psychiatry. 2009;194:319–325. doi: 10.1192/bjp.bp.107.047985. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Broome MR, Matthiasson P, Woolley JB, Mechelli A, Johns LC, Tabraham P, Bramon E, Valmaggia L, Williams SC, McGuire P. Prefrontal function at presentation directly related to clinical outcome in people at ultrahigh risk of psychosis. Schizophrenia Bulletin. 2011a;37:189–198. doi: 10.1093/schbul/sbp074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Broome MR, Woolley JB, Johns LC, Tabraham P, Bramon E, Valmaggia L, Williams SC, McGuire P. Altered brain function directly related to structural abnormalities in people at ultra high risk of psychosis: longitudinal vbm-fmri study. Journal of Psychiatry Research. 2011b;45:190–198. doi: 10.1016/j.jpsychires.2010.05.012. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, McGuire P, Borgwardt S. Mapping prodromal psychosis: a critical review of neuroimaging studies. European Psychiatry. 2012;27:181–191. doi: 10.1016/j.eurpsy.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Gelernter J, Kranzler HR, Satel SL, Rao PA. Genetic association between dopamine transporter protein alleles and cocaine-induced paranoia. Neuropsychopharmacology. 1994;11:195–200. doi: 10.1038/sj.npp.1380106. [DOI] [PubMed] [Google Scholar]
- Grelotti DJ, Kanayama G, Pope HG., Jr Remission of persistent methamphetamine-induced psychosis after electroconvulsive therapy: presentation of a case and review of the literature. The American Journal of Psychiatry. 2010;167:17–23. doi: 10.1176/appi.ajp.2009.08111695. [DOI] [PubMed] [Google Scholar]
- Harano M, Uchimura N, Abe H, Ishibashi M, Iida N, Yanagimoto K, Tanaka T, Maeda H, Sora I, Iyo M, Komiyama T, Yamada M, Sekine Y, Inada T, Ozaki N, Ujike H. A polymorphism of drd2 gene and brain atrophy in methamphetamine psychosis. Annals of the New York Academy of Sciences. 2004;1025:307–315. doi: 10.1196/annals.1316.038. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology. 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Kaye S, Darke S, Torok M. Attention deficit hyperactivity disorder (ADHD) among illicit psychostimulant users: a hidden disorder? Addiction (Abingdon, England) 2012 doi: 10.1111/add.12086. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Abi-Dargham A, Gil R, Kegeles L, Innis R. Increased dopamine transmission in schizophrenia: relationship to illness phases. Biological Psychiatry. 1999;46:56–72. doi: 10.1016/s0006-3223(99)00067-0. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Abi-Dargham A, van Dyck CH, Gil R, D'Souza CD, Erdos J, McCance E, Rosenblatt W, Fingado C, Zoghbi SS, Baldwin RM, Seibyl JP, Krystal JH, Charney DS, Innis RB. Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:9235–9240. doi: 10.1073/pnas.93.17.9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leamon MH, Flower K, Salo RE, Nordahl TE, Kranzler HR, Galloway GP. Methamphetamine and paranoia: The methamphetamine experience questionnaire. The American Journal on Addictions. 2010;19:155–168. doi: 10.1111/j.1521-0391.2009.00014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leamon MH, Gibson DR, Canning RD, Benjamin L. Hospitalization of patients with cocaine and amphetamine use disorders from a psychiatric emergency service. Psychiatric Services (Washington, DC) 2002;53:1461–1466. doi: 10.1176/appi.ps.53.11.1461. [DOI] [PubMed] [Google Scholar]
- Luzi S, Morrison PD, Powell J, di Forti M, Murray RM. What is the mechanism whereby cannabis use increases risk of psychosis? Neurotoxicology Research. 2008;14:105–112. doi: 10.1007/BF03033802. [DOI] [PubMed] [Google Scholar]
- Lynskey MT, Hall W. Attention deficit hyperactivity disorder and substance use disorders: is there a causal link? Addiction (Abingdon, England) 2001;96:815–822. doi: 10.1046/j.1360-0443.2001.9668153.x. [DOI] [PubMed] [Google Scholar]
- Mahoney JJ, 3rd, Hawkins RY, De La Garza R, 2nd, Kalechstein AD, Newton TF. Relationship between gender and psychotic symptoms in cocaine-dependent and methamphetamine-dependent participants. Gender Medicine. 2010;7:414–421. doi: 10.1016/j.genm.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto T, Kamijo A, Yamaguchi A, Iseki E, Hirayasu Y. Childhood histories of attention-deficit hyperactivity disorders in japanese methamphetamine and inhalant abusers: Preliminary report. Psychiatry and Clinical Neurosciences. 2005a;59:102–105. doi: 10.1111/j.1440-1819.2005.01340.x. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Yamaguchi A, Asami T, Kamijo A, Iseki E, Hirayasu Y, Wada K. Drug preferences in illicit drug abusers with a childhood tendency of attention deficit/hyperactivity disorder: a study using the wender utah rating scale in a japanese prison. Psychiatry and Clinical Neurosciences. 2005b;59:311–318. doi: 10.1111/j.1440-1819.2005.01376.x. [DOI] [PubMed] [Google Scholar]
- Mazzoncini R, Donoghue K, Hart J, Morgan C, Doody GA, Dazzan P, Jones PB, Morgan K, Murray RM, Fearon P. Illicit substance use and its correlates in first episode psychosis. Acta Psychiatrica Scandinavica. 2010;121:351–358. doi: 10.1111/j.1600-0447.2009.01483.x. [DOI] [PubMed] [Google Scholar]
- McCullagh P. Regression models for ordinal data (with discussion) Journal of Royal Statistics Society B. 1980;42:109–142. [Google Scholar]
- McKetin R, Lubman DI, Baker AL, Dawe S. Psychotic symptoms are dose-related in chronic methamphetamine users: evidence from a prospective longitudinal study. JAMA Psychiatry. 2013;70:319–324. doi: 10.1001/jamapsychiatry.2013.283. [DOI] [PubMed] [Google Scholar]
- McKetin R, McLaren J, Lubman DI, Hides L. The prevalence of psychotic symptoms among methamphetamine users. Addiction (Abingdon, England) 2006;101:1473–1478. doi: 10.1111/j.1360-0443.2006.01496.x. [DOI] [PubMed] [Google Scholar]
- Meredith CW, Jaffe C, Ang-Lee K, Saxon AJ. Implications of chronic methamphetamine use: a literature review. Harvard Review of Psychiatry. 2005;13:141–154. doi: 10.1080/10673220591003605. [DOI] [PubMed] [Google Scholar]
- Nelson HE. The national adult reading test (nart) Nelson Publishing Company; Windsor, Canada: 1982. [Google Scholar]
- Paparelli A, Di Forti M, Morrison PD, Murray RM. Drug-induced psychosis: how to avoid star gazing in schizophrenia research by lookingat more obvious sources of light. Frontiers in Behavioral Neuroscience. 2011;5:1. doi: 10.3389/fnbeh.2011.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasic J, Russo JE, Ries RK, Roy-Byrne PP. Methamphetamine users in the psychiatric emergency services: a case-control study. The American Journal of Drug and Alcohol Abuse. 2007;33:675–686. doi: 10.1080/00952990701522732. [DOI] [PubMed] [Google Scholar]
- Pycock CJ, Kerwin RW, Carter CJ. Effect of lesion of cortical dopamine terminals on subcortical dopamine receptors in rats. Nature. 1980;286:74–76. doi: 10.1038/286074a0. [DOI] [PubMed] [Google Scholar]
- Quinton MS, Yamamoto BK. Causes and consequences of methamphetamine and mdma toxicity. The AAPS Journal. 2006;8:E337–347. doi: 10.1007/BF02854904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricaurte GA, Seiden LS, Schuster CR. Further evidence that amphetamines produce long-lasting dopamine neurochemical deficits by destroying dopamine nerve fibers. Brain Research. 1984;303:359–364. doi: 10.1016/0006-8993(84)91221-6. [DOI] [PubMed] [Google Scholar]
- Roehr B. BMJ. Clinical Research; 2005. Half a million americans use methamphetamine every week; p. 476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salo R, Nordahl TE, Leamon MH, Natsuaki Y, Moore CD, Waters C, Carter CS. Preliminary evidence of behavioral predictors of recurrent drug-induced psychosis in methamphetamine abuse. Psychiatry Research. 2008;157:273–277. doi: 10.1016/j.psychres.2007.04.018. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (), Office of Applied Studies. DASIS Report. Arlington, Virginia: 2004. Primary methamphetamine/amphetamine treatment admissions: 1992-2002. [Google Scholar]
- Substance Abuse and Mental Health Services Administration(SAMSHA) Office of Applied Studies. Rockville, MD: Aug 24, 2010. The DAWN Report: Emergency department visits involving methamphetamine: 2004 to2008. [PubMed] [Google Scholar]
- SAS Institute I. Sas/stat version 9.3. Cary; NC, USA: 2002-2010. [Google Scholar]
- Sato M. A lasting vulnerability to psychosis in patients with previous methamphetamine psychosis. Annals of the New York Academy of Sciences. 1992;654:160–170. doi: 10.1111/j.1749-6632.1992.tb25965.x. [DOI] [PubMed] [Google Scholar]
- Schmidt CJ, Ritter JK, Sonsalla PK, Hanson GR, Gibb JW. Role of dopamine in the neurotoxic effects of methamphetamine. The Journal of Pharmacology and Experimental Therapeutics. 1985;233:539–544. [PubMed] [Google Scholar]
- Schuckit MA. Comorbidity between substance use disorders and psychiatric conditions. Addiction (Abingdon, England) 2006;101(Suppl 1):76–88. doi: 10.1111/j.1360-0443.2006.01592.x. [DOI] [PubMed] [Google Scholar]
- Seiden LS, Ricaurte GA. Neurotoxicity of methamphetamine and related drugs. In: Meltzer HY, editor. Psychopharmacology: The third generation of progress. Raven Press; New York: 1987. pp. 359–366. [Google Scholar]
- Seiden LS, Sabol KE. NIDA. 1996. Methamphetamine and methyl-enedioxymethamphetamine neurotoxicity:possible mechanismsof cell destruction, NIDA Research Monograph; pp. 251–276. [PubMed] [Google Scholar]
- Simon SL, Domier C, Carnell J, Brethen P, Rawson R, Ling W. Cognitive impairment in individuals currently using methamphetamine. The American Journal on Addictions. 2000;9:222–231. doi: 10.1080/10550490050148053. [DOI] [PubMed] [Google Scholar]
- Siomopoulos V. Thought disorder in amphetamine psychosis: a case report. Psychosomatics. 1976;17:42–44. doi: 10.1016/S0033-3182(76)71172-1. [DOI] [PubMed] [Google Scholar]
- Smith MJ, Thirthalli J, Abdallah AB, Murray RM, Cottler LB. Prevalence of psychotic symptoms in substance users: a comparison across substances. Comprehensive Psychiatry. 2009;50:245–250. doi: 10.1016/j.comppsych.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srisurapanont M, Ali R, Marsden J, Sunga A, Wada K, Monteiro M. Psychotic symptoms in methamphetamine psychotic in-patients. International Journal of Neuropsychopharmacology. 2003;6:347–352. doi: 10.1017/S1461145703003675. [DOI] [PubMed] [Google Scholar]
- Stilo SA, Murray RM. The epidemiology of schizophrenia: replacing dogma with knowledge. Dialogues Clinical Neuroscience. 2010;12:305–315. doi: 10.31887/DCNS.2010.12.3/sstilo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Nakamura K, Sekine Y, Minabe Y, Takei N, Suzuki K, Iwata Y, Kawai M, Takebayashi K, Matsuzaki H, Iyo M, Ozaki N, Inada T, Iwata N, Harano M, Komiyama T, Yamada M, Sora I, Ujike H, Mori N. An association study between catechol-o-methyl transferase gene polymorphism and methamphetamine psychotic disorder. Psychiatric Genetics. 2006;16:133–138. doi: 10.1097/01.ypg.0000218613.35139.cd. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, Simon SL, Geaga JA, Hong MS, Sui Y, Lee JY, Toga AW, Ling W, London ED. Structural abnormalities in the brains of human subjects who use methamphetamine. Journal of Neuroscience. 2004;24:6028–6036. doi: 10.1523/JNEUROSCI.0713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tost H, Alam T, Meyer-Lindenberg A. Dopamine and psychosis: theory, pathomechanisms and intermediate phenotypes. Neuroscience and Biobehavioral Reviews. 2010;34:689–700. doi: 10.1016/j.neubiorev.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuang MT, Stone WS, Faraone SV. Genes, environment and schizophrenia. The British Journal of Psychiatry. 2001;40:s18–24. doi: 10.1192/bjp.178.40.s18. [DOI] [PubMed] [Google Scholar]
- United Nations. Amphetamines and ecstasy- 2008. In: Crime, U.N.O.D.C., editor. Global ATS Assessment. United Nations, Vienna: 2008. [Google Scholar]
- Crime, U.N.O.D.C., editor. World Drug Report 2009. United Nations; United Nations, Vienna: 2009. The world drug problem: A status report. [Google Scholar]
- Ward MF, Wender PH, Reimherr FW. The Wender Utah rating scale: an aid in the retrospective diagnosis of childhood attention deficit hyperactivity disorder. The American Journal of Psychiatry. 1993;150:885–890. doi: 10.1176/ajp.150.6.885. [DOI] [PubMed] [Google Scholar]
- Wender P. Wender aqcc (adult-questionnaire-childhood characteristics) scale. Psychopharmacology Bulletin. 1985;21:927–928. [Google Scholar]
- Yamamoto BK, Bankson MG. Amphetamine neurotoxicity: cause and consequence of oxidative stress. Critical Reviews in Neurobiology. 2005;17:87–117. doi: 10.1615/critrevneurobiol.v17.i2.30. [DOI] [PubMed] [Google Scholar]
- Zammit S, Moore TH, Lingford-Hughes A, Barnes TR, Jones PB, Burke M, Lewis G. Effects of cannabis use on outcomes of psychotic disorders: systematic review. The British Journal of Psychiatry. 2008;193:357–363. doi: 10.1192/bjp.bp.107.046375. [DOI] [PubMed] [Google Scholar]


