Abstract
Disulfiram is a cocaine addiction pharmacotherapy that inhibits dopamine β-hydroxylase (DβH) and reduces norepinephrine production. We examined whether a functional variant of the ADRA1A gene (Cys to Arg at codon 347 in exon 2, Cys347Arg) may enhance treatment response through decreased stimulation of this α1A-adrenoceptor, since antagonists of this receptor show promise in reducing cocaine use. Sixty-nine cocaine and opioid co-dependent (DSM-IV) subjects were stabilized on methadone for two weeks and subsequently randomized into disulfiram (250 mg/day, N = 32) and placebo groups (N = 37) for 10 weeks. We genotyped the ADRA1A gene polymorphism (rs1048101) and evaluated its role for increasing cocaine free urines in those subjects treated with disulfiram using repeated measures analysis of variance, corrected for population structure. The 47 patients who carried at least one T allele of rs1048101 (TT or TC genotype) reduced their cocaine positive urines from 84% to 56% on disulfiram (p = .0001), while the 22 patients with the major allele CC genotype showed no disulfiram effect. This study indicates that a patient’s ADRA1A genotype could be used to identify a subset of individuals for which disulfiram and, perhaps, other α1-adrenoceptor blockers may be an effective pharmacotherapy for cocaine dependence.
Keywords: Gene, polymorphism, disulfiram, α1A-adrenoceptor, cocaine dependence
1. Introduction
Cocaine dependence (CD) is widely recognized as a chronic medical illness for which there is currently no US Food and Drug Administration (FDA) approved pharmacotherapy (McLellan et al., 2000). CD frequently co-occurs with opioid dependence, particularly in methadone maintenance programs, and contributes to worsened psychosocial outcomes (Kosten et al., 1988). In this population, rates of cocaine use may range from 30% to 80% (Grella et al., 1997) and contribute to increases in HIV risk behaviors and/or transmission, continued illicit opioid use, increased number of hospitalizations for drug and alcohol problems, and increased emergency department utilization (Meandzija et. al., 1994; Magura et al., 1998; Bovasso and Cacciola, 2003). Cocaine is also a common co-intoxicant in cases of fatal methadone overdose (Wolf et al., 2004). Various pharmacological approaches have been evaluated to reduce CD, but with only moderate success (Shorter and Kosten, 2012).
The psychostimulant properties of cocaine stem from its ability to inhibit reuptake at the dopamine, serotonin, and norepinephrine transporters, producing an increase in synaptic levels of these neurotransmitters (Rothman and Baumann, 2003). In persons abusing cocaine, evidence suggests that stimulation of the noradrenergic system contributes to reward and reinforcement from the drug. Dopamine transporter (DAT) knockout (KO) mice continue to self-administer cocaine, suggesting that blockage of DAT alone is not sufficient to account for the reinforcing effects of cocaine and that other neurotransmitter systems must contribute (Carboni et al., 2001). Additionally, norepinephrine transporter (NET) KO mice display a reduced response to acute cocaine administration, when compared to wild-type controls, although behavioral sensitization to cocaine remained unchanged (Mead et al., 2002). A functional coupling of the noradrenergic system to the dopaminergic system may be mediated through the activation of α1A-adrenoceptors, contributing to a cocaine-induced increase in synaptic levels of norepinephrine (NE) and subsequent increase in firing of dopamine (DA) neurons in the ventral tegmental area and prefrontal cortex (Paladini and Williams, 2004). In addition to its role in the acute effects of cocaine, the noradrenergic system underlies the neurobiology for stress-induced reinstatement of drug seeking behavior. Preclinical evidence has demonstrated that pharmacologic blockade of this system attenuates reinstatement of cocaine-seeking behavior in rats (Leri et al., 2002). These findings suggest that decreasing noradrenergic stimulation of α1A-adrenoceptors may represent an exciting potential pharmacotherapeutic target for treatment of CD.
Disulfiram (Antabuse, Antabus), which acts on multiple enzymes through copper chelation, inhibits dopamine β-hydroxylase (DβH), the enzyme responsible for transformation of DA to NE (Gaval-Cruz and Weinshenker, 2009). Disulfiram has shown initial promise in treating CD among opioid-dependent patients (George et al., 2000; Petrakis et al., 2000; Carroll et al., 2004; Schroeder et al., 2010) as well as CD in the context of abuse of other substances (i.e., alcohol) (Carroll et al., 1998). Inhibition of DβH decreases brain NE levels, leading to a subsequent reduction in stimulation of α1A-adrenoceptors. In a series of recent studies, our group found that pharmacologic antagonism of α1A-adrenoceptors with prazosin reduced cocaine-induced reinstatement of cocaine-seeking in rats (Zhang and Kosten, 2005; Zhang and Kosten, 2007), and doxazosin reduced cocaine use in humans (Shorter et al., 2013, in press).
The strong genetic basis of CD, estimated at up to 72% (Goldman et al., 2005) encourages a molecular genetics approach to understanding disulfiram’s mechanism of action in the treatment of this illness. In a previous study, our group showed that a DBH genetic polymorphism associated with relatively low DβH levels (rs1611115), (CT/TT) identified CD patients who do not reduce their cocaine use in response to treatment with disulfiram, perhaps reflecting the relatively small reduction in NE levels observed in this group and the subsequently small reduction in noradrenergic stimulation (Kosten et al., 2013). As part of this larger pharmacogenetics study, and in order to further identify clinical subpopulations in which the efficacy of disulfiram may be improved, in this present study we examined selected CD patients based upon ADRA1A genotype. The gene ADRA1A that codes for the α1A-adrenoceptor has a functional polymorphism rs1048101 in exon 2 coding for the substitution of an arginine (ARG) for a cytosine (CYS) at codon 347 of the C-terminus (Lei et al., 2005), that may alter the activity of this receptor and impact cognition. More specifically, this polymorphism may impact activation of α1A-adrenoceptors reported to influence critical functions for prevention of relapse in CD including vigilance, impulsivity, and working memory (Puumala et al., 1997; Arnstein et al., 1999).
The first aim of this study is to determine whether CD patients who were carriers of the T allele (TT/TC) which codes for the Cys347 form of the α1A-adrenoceptor have a different response to disulfiram than patients who are homozygous (CC) for the Arg347 form of the receptor. We hypothesize that individuals who are T allele carriers (Cys form) will respond preferentially to disulfiram, displaying a greater reduction in cocaine use when compared to individuals who are Arg347Arg homozygous. If one of these genotype groups is associated with a preferential response to disulfiram, a second aim is to examine the impact of this “preferred” ADRA1A genotype in the context of DBH activity. We hypothesize that patients with a genotype pattern consisting of those carrying the preferred ADRA1A allele (i.e., the Cys form) with the DBH -1021C/T CC genotype that is associated with normal DβH levels will have a better response to disulfiram, in contrast to a poorer response to the medication in the group of patients with lower DβH levels (CT/TT) or the ADRA1A Arg347Arg CC genotype.
2. Experimental procedures
Patients
From 2005 to 2006 at Yale University (N =40) and then from 2006 to 2008 at the Baylor College of Medicine (N= 53), 93 patients entered into a clinical trial to evaluate disulfiram for cocaine dependence. At the time of screening, patients underwent physical examination and psychiatric evaluation as well as assessment of laboratory values. During intake, each participant was interviewed using the Mini International Neuropsychiatric Interview (MINI (English Version 5.0.0., 1 July 2006); Sheehan et al., 1997) and completed the Addiction Severity Index (ASI-Lite; McLellan et al., 1992). Initially, patients entered a two-week screening period during which they were stabilization on methadone maintenance. Patients were selected based on thrice weekly urine toxicology being positive for both opiates and cocaine metabolites during this screening and were retained if they had at least one cocaine positive urine samples leading to 11 patients being excluded. Eight additional patients dropped out during the screening period (i.e., lost to follow-up). Five additional patients were excluded from the remaining 74 cocaine and opioid dependent patients due to lack of genotypic data (Kosten et al. 2013). All patients met DSM-IV criteria for opioid and cocaine dependence. Other exclusions included a current diagnosis of other drug or alcohol dependence (other than tobacco), current major medical illness that was not stabilized on medications, a history of major psychiatric disorder (psychosis, schizophrenia, bipolar), current suicidality, and an inability to read and understand the consent form. Women of childbearing age were included provided they had a negative urine pregnancy test, agreed to use adequate contraception to prevent pregnancy during the study, and agreed to monthly pregnancy tests. All signed an informed consent approved by Yale University and the Baylor College of Medicine Institutional Review Boards that gave specific consent for genetic studies. Ethnicity was based self-report of ethnic/cultural background of the patients.
Study Design and Medications
The remaining 69 patients were stabilized on methadone maintenance at 60 mg daily and were assigned randomly to placebo or disulfiram 250 mg daily. Methadone dose increased from an initially 25 mg by 5 mg per day until patients reached a 60 mg maintenance dose. Individual manual-driven cognitive behavioral therapy (Carroll, 1997) was provided weekly to all patients. Supervised urine samples were obtained thrice weekly and tested for the presence of cocaine metabolite (benzoylecgonine) and other drugs using an Olympus AU 640 Emit system (Olympus America Inc., Melville, NY) with a cut-off concentration of 300 ng/ml. We obtained saliva samples for genotyping.
Genotyping
The DNA was purified as previously described (Kosten et al., 2013). Briefly, DNA was isolated from pelleted buccal cells that were obtained by centrifugation of 10 ml Scope mouthwash that was used to rinse the subject’s mouth for 60 second using the Gentra Puregene Buccal Cell Kit (Qiagen, Valencia, CA) following the manufacturer’s recommendations.
ADRA1A genotype was determined using a5’-fluorogenic exonuclease assays (TaqMan®, Applied Biosystems, Foster City, CA). The ADRA1A Cys347Arg genetic variant was genotyped using the TaqMan® primer-probe set (Applied Biosystems) ADRA1A rs1048101, Assay ID C_ 2696454_30. Genotyping was conducted using the condition as described previously (Kosten et al., 2012) All genotype analyses were performed by an individual unaware of the clinical status of the subjects. Sex was determined using an SRY rs11575897 (C_32310143_10, Applied Biosystems) TaqMan® assay and ten ancestry informative markers were genotyped as described (Kosten et al., 2012). All TaqMan® assays were performed in duplicate.
Statistical Analysis
Our sample size of 69 with at least 32 patients in each treatment group had a power of 0.8 with alpha 0.05 based on effect sizes from three previous Yale studies of disulfiram for cocaine. We compared baseline differences in demographics and drug use history using chi squared or t-test. A repeated measures analysis of variance (ANOVA) used the number of cocaine positive urines over the total number of samples (six) for each two week period to compare disulfiram to placebo over time and to determine if the effect of disulfiram is modulated by the ARDA1A genotype using R version 2.9.1 (R_Development_Core_Team, 2009). We compared condition (disulfiram or placebo), ARDA1A genotype (0 = TT/TC genotype, 1 = CC genotype), time (each two week period), and interactions between condition and time, and between condition and ARDA1A genotype. We analyzed all individuals who had complete data (N = 56) and unbalanced repeated measures ANOVA for all individuals (N = 69). The two analyses yielded similar results.
We used genotype patterns of the ADRA1A and the DBH genes and a similar statistical approach to analyze disulfiram’s mechanism of action using ADRA1A genotypes by dividing the cohort in those with both an ADRA1A TT or TC genotype and a DBH CC genotype and then comparing this group to those with all other genotype patterns combined to test the hypotheses about the effects of baseline norepinephrine (NE) supply on the efficacy of disulfiram for cocaine dependence. We expected disulfiram to maintain its efficacy in the responder ADRA1A group when paired with normal DBH level, but disulfiram to be ineffective in those patients without this genotype pattern.
To determine population structure, our cohort was compared against CEPH-HGDP samples (1,035 subjects of 51 populations) as described (Kosten et al., 2013). For all analyses we corrected for any possible confounding effects, by including the proportion of each subject from the founder populations as well as gender and site effects as covariates in the model. The obtained p-values were very similar to those obtained when we did not correct for these covariates. Furthermore, analyses were performed with the total group then within the two ARDA1A subgroups.
3. Results
Baseline characteristics by treatment and ARDA1A genetics
We were unable to genotype five patients for the α1A -adrenergic receptor gene (ARDA1A) thereby providing a sample of 69 patients who were randomized, 32 patients to disulfiram and 37 to placebo. Of this group, we found that 14 patients possessed the TT genotype, while 33 patients carried the CT genotype and 22 patients carried the CC genotype. The patients were mostly Caucasian males with a mean age of 39 years and 13 years of opiate abuse. They used cocaine for a mean of 13 years and for 19 days in the month before entering the study. Forty (54%) patients had been previously treated with methadone maintenance. Only 29 patients (39%) reported any alcohol abuse history reflecting our exclusion criteria, and 39 patients (53%) reported marijuana use. As shown in Table 1, we found no significant baseline differences among the four treatment by genotype groups in any clinical characteristics (p >.05), with the exception of percentage with a past history of marijuana abuse (p = 0.05).
Table 1.
Demographic and clinical characteristics by treatment and ADRA1A genotype
| Characteristic | Placebo | Disulfiram | |||
|---|---|---|---|---|---|
| CC | TT/TC | CC | TT/TC | p-value | |
| N | 15 | 22 | 7 | 25 | |
| % Male | 73 | 63 | 86 | 56 | 0.4 |
| % Caucasian | 53 | 82 | 71 | 80 | 0.2 |
| % Employed | 40 | 68 | 71 | 68 | 0.2 |
| Age years (s.d.) | 41 (13) | 39 (9) | 38 (12) | 38 (10) | 0.9 |
| Cocaine last 30 days (s.d.) | 19 (7) | 20 (8) | 11 (8) | 20 (8) | 0.97 |
| Cocaine years (s.d.) | 15 (10) | 12 (7) | 9 (8) | 13 (7) | 0.13 |
| Heroin years (s.d.) | 15 (11) | 12 (9) | 12 (7) | 14 (8) | 0.98 |
| % Alcohol abuse | 53 | 32 | 57 | 40 | 0.5 |
| % Marijuana abuse | 67 | 32 | 71 | 56 | 0.05* |
| % past Methadone | 53 | 59 | 57 | 48 | 0.9 |
statistically significant difference
Cocaine Treatment Outcomes by ARDA1A Genotype
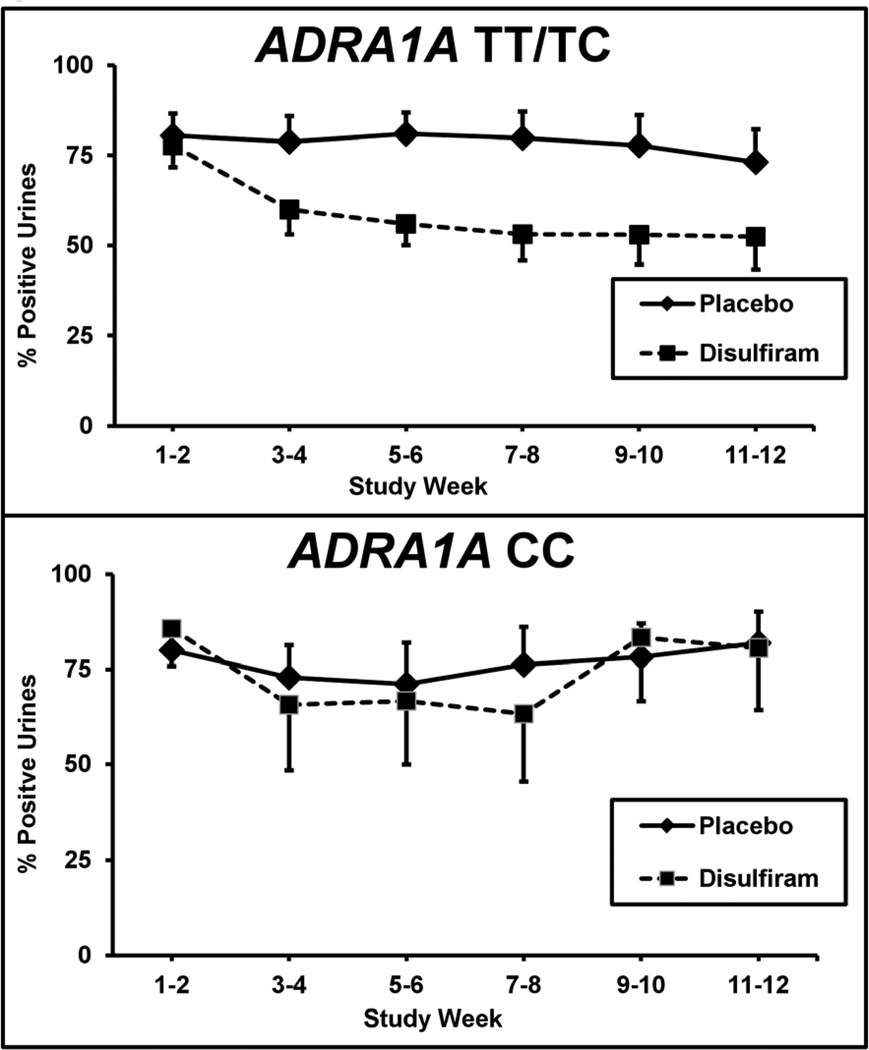
Similar to our previous report, cocaine positive urine screens showed a significant difference between treatment groups. The positive cocaine urine rates decreased from 80% during the baseline two weeks to 59% for disulfiram and to 77% for placebo during the last two weeks of treatment (F = 13.0; df = 1,550; p <.0005) (Kosten et al., 2013). For the current analyses, we divided the 69 patients who had been genotyped at the ARDA1A gene into two groups based upon whether or not they carried the rs1048101 T allele. When separated into these two genotype groups (CC versus TT/TC), cocaine positive urine rates differed between the treatment groups for patients carrying the T allele (F = 17.1; df = 1,358; p <.00005), but did not differ for those with the CC genotype (F = 0.86; df = 1,158; p >.05). As shown in Figure 1, cocaine positive urines for the TT/TC genotype group during the two baseline weeks were 78% for disulfiram and 80% for placebo. These rates dropped during the last two weeks of treatment to 52% for disulfiram and to 73% for placebo. In comparison, cocaine urines for the CC genotype group during the two baseline weeks were 86% for disulfiram and 80% for placebo. These rates were relatively unchanged during the last two weeks of treatment at 81% for disulfiram and to 82% for placebo. When we only included the 56 subjects who completed the study, the disulfiram treatment effect remained highly significant only among the TT/TC genotype patients (F = 16.9; df = 1,446; p <.00005).
Figure 1.
Percentage of cocaine positive urine toxicology screens for two-week time blocks across the 12-week trial for the placebo (solid line) versus disulfiram (250 mg/day) (dashed line) treatment groups. Top figure is the TT/TC genotype patients (N=47; disulfiram = 25; placebo = 22) and bottom figure is the CC genotype patients (N=22; disulfiram = 7; placebo = 15). Standard error bars are shown at each time point.
Genotype pattern of DBH and ARDA1A: Mechanism of Action for Cocaine
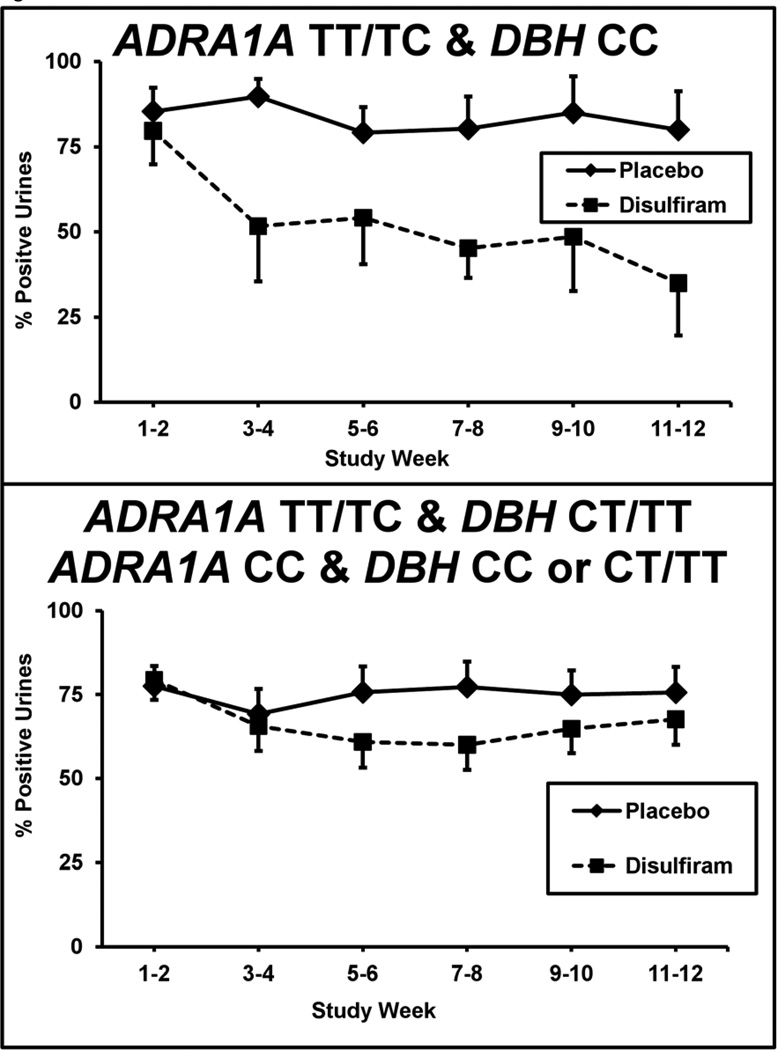
Those patients having both the responsive ARDA1A (TT/TC) and the normal DBH (CC) genotypes (n=23) were compared to the remaining 46 patients not carrying an ADRA1A T allele (i.e., ADRA1 CC homozygotes) combined with those T carriers without DBH CC genotype. As shown in Figure 2, those patients with both responsive genotypes (i.e., ARDA1A –TT/TC and DBH CC genotypes) (n=23) showed a significant reduction in cocaine urines from 80% to 35% on disulfiram (n=10) compared to a minimal change from 85% to 80% on placebo (n=13) (F= 24.6; df= 1,550; p = .000003). The remaining 46 patients showed no disulfiram effect compared to placebo (n=22 disulfiram; n=24 placebo). When we included only the 56 subjects who completed the study, the disulfiram treatment effect remained highly significant only among the patients with the responsive genotype pattern (F = 19.9; df = 1,446; p <.00002).
Figure 2. Interaction of the ADRA1 and DBH genotypes with percentage of cocaine positive urines.
Urine toxicology screens are shown for each 2-week time period across the 12-week trial for the placebo (solid line) versus disulfiram (250mg/day) (dashed line) treatment groups. Patients with the responsive ADRA1A TT/TC and DBH CC genotype combination (N=23; disulfiram = 10, placebo = 13) are shown in the top graph. Patients with the ADRA1A CC genotype and those with DBH CT/TT genotype (N=46; disulfiram = 22; placebo = 24) are shown in the bottom graph. Standard error bars are shown at each time point.
Opioid Treatment Outcomes by ARDA1A Genotype
Similar to our previous report, opioid positive urine screens decreased over time, but did not significantly differ between treatment groups (Kosten et al, 2013). When separated into the two genotype groups, patients in neither group showed a difference between the treatment regimens.
4. Discussion
We found that disulfiram significantly reduced the percentage of cocaine positive urines among individuals with the CYS conformation of the ADRA1 receptor, but not in those with the ARG substitution. Thus, we interpret these findings to be consistent with our hypothesis that the CYS polymorphism of the alpha-1 adrenergic receptor confers preferential response to the medication. In regards to the second aim of this study, examination of the combined effect of the preferred, or responsive, ADRA1 allele (CYS) with the normal DBH polymorphism (CC), we found that disulfiram significantly reduced cocaine-positive urines among this group, while the remaining subjects did not demonstrate a treatment effect. This finding is consistent with our previous finding that disulfiram appears to be most effective in those individuals with normal, rather than low, DBH levels at baseline.
The underlying neurobiology of the noradrenergic system provides clues as to the mechanism behind our observations. As stated previously, decreasing noradrenergic stimulation in those abusing cocaine has several likely downstream effects: (1) attenuation of cocaine reward (due to NE and DA coupling), (2) decrease in stress-induced reinstatement (i.e., craving), given the noradrenergic basis of this phenomenon, and (3) increased ability to resist craving and/or utilize CBT techniques for relapse prevention, since NE stimulation has been linked to impairment in cognitive function (distractibility, impulsivity, poor filtering).
Despite disulfiram’s reduction of NE activity, however, there appears to be no observed clinical benefit in those with the ARG polymorphism. The differential responses of the receptor subtypes are unlikely to be due to changes in the physical conformation of ADRA1, since there are no substantial changes to the structure of the protein based on this polymorphism (Lei et al., 2005). Additionally, there are no statistically significant differences between the CYS and ARG receptor polymorphisms in regards to binding affinity for agonists (such as NE or epinephrine) or antagonists (prazosin) (Lei et al., 2005). Further, other studies found there were no differences between the CYS and ARG polymorphisms in terms of (a) signal transduction – the potency of NE-stimulated inositol triphosphate formation, or (b) NE-induced sensitization. Thus, given that NE is likely to bind and activate both genetic variants of this receptor in an equivalent manner, we cannot attribute the reduction in cocaine use in the CYS group to the combination of reduced NE levels (from disulfiram) with an adrenergic receptor that has reduced binding.
One possible explanation of our finding is that the ARG polymorphism of the alpha-1 adrenergic receptor experiences enhanced, or “tight,” coupling with NE, meaning that it maintains its level of activity, even in the context of reduced NE levels from disulfiram. Conversely, the CYS polymorphism displays “loose” coupling with NE, which can be further attenuated with reduction in NE levels from disulfiram. This coupling theory is supported by the effect of this polymorphism in the cardiovascular system (Snapir et al., 2003). In Chinese subjects with hypertension, a greater reduction in diastolic blood pressure (DBP) in response to the anti-hypertensive medication, irbesartan, was observed in the group with the CYS polymorphism when compared to those with the ARG polymorphism (Jiang et al., 2005). Irbesartan, an angiotensin-converting enzyme inhibitor, reduces circulating levels of NE (Remme, 1998). The enhanced response in DBP to irbesartan in those with the CYS polymorphism is similar to the reduction in cocaine use seen in our study, in that, there may be continued elevated activity in the ARG polymorphism ADRA1 receptor despite reduced NE levels (“tight” coupling).
In a separate study, Herlyn et. al. found the CYS to ARG polymorphism results in genetic predisposition to the development of complex regional pain syndrome (CPRS) type I after distal radial fracture (Herlyn et al., 2010). CPRS is thought to be due to adrenergic hypersensitivity, and one treatment is local adrenergic blockade. As in the aforementioned example, the ARG polymorphism of the receptor appears to be associated with “tight” coupling, in that it is associated with increased, or persistent, activity (despite presumably normal NE levels). Ultimately, we believe that persistent activity of the ADRA1 receptor in those with the ARG polymorphism can account for the continued use of cocaine observed in this group.
We also found a contribution from the DBH polymorphism to disulfiram response. In a study conducted in mice, Bourdelat-Parks et al. found the number of DBH alleles was proportional to the level of DBH protein, and by extension, affected the levels of dopamine and NE in the prefrontal cortex (PFC) (Bourdelat-Parks et al., 2005). More specifically, disulfiram increased dopamine and decreased NE levels in the PFC of mice with two normal alleles; however, in mice with null alleles, disulfiram showed relatively little effect on neurotransmitter levels. Our findings are consistent with this previous trial, since participants with high DBH activity demonstrated a favorable response to the medication, while those with low DBH appeared to be less affected by disulfiram-induced inhibition of the enzyme.
An alternative mechanism that may explain the efficacy of disulfiram relates to the creation of aversive symptoms during acute cocaine intoxication as a result of reduced DBH activity. In previous trials, disulfiram produced increased cocaine-associated negative effects (i.e., anxiety, paranoia) and reduced positive subjective effects during acute cocaine administration in human laboratory studies (McCance-Katz et al. 1998b; Baker et al., 2007). Additionally, in a recent clinical trial of alcohol-dependent patients treated with disulfiram, those individuals carrying the low activity DBH polymorphism (T allele) were found to have an increased risk of disulfiram-related adverse events (Mutschler et al., 2012). Although none of the participants in our study reported such negative experiences related to cocaine use, examination of adverse effects in relation to DBH allele may represent an important area of future study.
It is important to note that there are many examples, independent of disulfiram use, in which reduced DBH level or activity was associated with acute exacerbation of psychiatric symptoms and/or development of psychosis (see review, Cubells and Zabetian 2004). For instance, schizophrenic or depressed patients with low plasma or cerebrospinal fluid levels of DBH exhibit more positive psychotic symptoms when compared to those with higher levels of DBH (Sternberg et al. 1983; Mod et al 1986; van Kammen et al. 1994). Moreover, patients diagnosed with unipolar depression with psychotic features have lower DBH levels than those without psychotic features (Cubells et al 2002). Further, the genetic predisposition for lower levels of DBH protein is associated with cocaine-induced paranoia (Cubells et al., 2000). Thus, although not reported by our participants, a component of subclinical discomfort and/or reduced euphoria may have contributed to the reduction of cocaine use in the genetic groups with a preferential response.
This trial has several limitations. First, the sample size is small and makes it difficult to generalize these results; thus, replication in genetic association studies with a larger number of participants is needed. Second, the genetic associations reflect only a modest reduction in cocaine use (to a mean proportion of 0.56 cocaine positive urines), although it is important to note that individuals with normal DBH levels (CC genotype) treated with disulfiram alone experienced a 33% reduction in cocaine use, in comparison to no change with placebo. Additionally, low DBH patients showed only a 13% reduction in cocaine use, similar to the 13% reduction seen in the placebo group. Thus, we observed a significant increase in efficacy with this genetic selection. Third, most cocaine abusers are not also opiate dependent, further limiting the generalizability of our findings. Fourth, alcohol abuse can be common among cocaine abusers and our rates of alcohol abuse were low, reflecting our exclusion criteria. Overall, disulfiram may not be the optimal medication for attaining DBH inhibition and may reduce cocaine use through a combination of mechanisms, including disinhibition of aldehyde dehydrogenase (Weinshenker, 2010). Another DBH inhibitor, nepicastat, is currently under investigation and does not inhibit aldehyde dehydrogenase or produce aversive interactions with alcohol (Stanley et al., 1997). Nepicastat represents a promising alternative, given its selectivity for the DBH enzyme, and warrants further study in clinical trials.
Acknowledgements
We would like to kindly thank NIH/NIDA, the Veterans Health Administration, and the Toomin Family Fund for their support.
Role of Funding Source:
Funding for this study was provided by NIH/NIDA Grant 5 P50 DA018197-05 (TRK); the NIH/NIDA had no further role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors:
Drs. Nielsen and Kosten designed the study and wrote protocol. Drs. Shorter, Kosten, Nielsen managed the literature searches. Drs. Huang, Harding, and Nielsen managed to genetic analysis. Dr. Hamon undertook the statistical analysis. Drs. Shorter and Kosten wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Conflict of Interest:
The authors declare that they have no biomedical financial interests or potential conflicts of interest.
References
- Arnstein AF, Mathew R, Ubriani R, Taylor JR, Li BM. Alpha-1 noradrenergic receptor stimulation impairs prefrontal cortical cognitive function. Biol. Psychiatry. 45:26–31. doi: 10.1016/s0006-3223(98)00296-0. [DOI] [PubMed] [Google Scholar]
- Baker JR, Jatlow P, McCance-Katz EF. Disulfiram effects on responses to intravenous cocaine administration. Drug Alcohol Depend. 2007;87:202–209. doi: 10.1016/j.drugalcdep.2006.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdelat-Parks BN, Anderson GM, Donaldson ZR, Weiss JM, Bonsall RW, Emery MS, Liles LC, Weinshenker D. Effects of dopamine beta-hydroxylase genotype and disulfiram inhibition on catecholamine homeostasis in mice. Psychopharmacology (Berl) 2005;183:72–80. doi: 10.1007/s00213-005-0139-8. [DOI] [PubMed] [Google Scholar]
- Bovasso G, Cacciola J. The long-term outcomes of drug use by methadone maintenance patients. J. Behav. Health Serv. Res. 2003;30:290–303. doi: 10.1007/BF02287318. [DOI] [PubMed] [Google Scholar]
- Carboni E, Tanda GL, Frau R, DiChiara G. Blockade of the noradrenaline carrier increases extracellular dopamine concentrations in the prefrontal cortex: evidence that dopamine is taken up in vivo by noradrenergic terminals. J. Neurochem. 1990;55:1067–1070. doi: 10.1111/j.1471-4159.1990.tb04599.x. [DOI] [PubMed] [Google Scholar]
- Carroll KM. Psychotherapeutic treatment of cocaine abuse: models for its evaluation alone and in combination with pharmacotherapy. NIDA Res. Monogr. 1993;135:116–132. [PubMed] [Google Scholar]
- Carroll KM. Manual-guided psychosocial treatment. A new virtual requirement for pharmacotherapy trials? Arch. Gen. Psychiatry. 1997;54:923–928. doi: 10.1001/archpsyc.1997.01830220041007. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Nich C, Ball SA, McCance E, Rounsaville BJ. Treatment of cocaine and alcohol dependence with psychotherapy and disulfiram. Addiction. 1998;93:713–727. doi: 10.1046/j.1360-0443.1998.9357137.x. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Fenton LR, Ball SA, Nich C, Frankforter TL, Shi J, Rounsaville BJ. Efficacy of disulfiram and cognitive behavior therapy in cocaine-dependent outpatients. Arch. Gen. Psychiatry. 2004;61:264–272. doi: 10.1001/archpsyc.61.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubells JF, Kranzler HR, McCance-Katz E, Anderson GM, Malison RT, Price LH, Gerlenter J. A haplotype at the DBH locus, associated with low plasma dopamine beta-hydroxylase activity, also associates with cocaine-induced paranoia. Mol. Psychiatry. 2000;5:56–63. doi: 10.1038/sj.mp.4000657. [DOI] [PubMed] [Google Scholar]
- Cubells JF, Price LH, Meyers BS, Anderson GM, Zabetian CP, Alexopoulos GS, Nelson JC, Sanacora G, Kirwin P, Carpenter L, Malison RT, Gerlenter J. Genotype-controlled analysis of plasma dopamine beta-hydroxylase activity in psychotic unipolar major depression. Biol. Psychiatry. 2002;51:358–364. doi: 10.1016/s0006-3223(01)01349-x. [DOI] [PubMed] [Google Scholar]
- Cubells JF, Zabetian CP. Human genetics of plasma dopamine beta-hydroxylase activity: applications to research in psychiatry and neurology. Psychopharmacology (Berl) 2004;174:463–476. doi: 10.1007/s00213-004-1840-8. [DOI] [PubMed] [Google Scholar]
- Gaval-Cruz M, Weinshenker D. Mechanisms of disulfiram-induced cocaine abstinence: Antabuse and cocaine relapse. Mol. Interv. 2009;9:175–187. doi: 10.1124/mi.9.4.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawin F, Kleber H. Pharmacologic treatments of cocaine abuse. Psych. Clin. North Am. 1986;9:573–583. [PubMed] [Google Scholar]
- George TP, Chawarski MP, Pakes J, Carroll KM, Kosten TR, Schottenfeld RS. Disulfiram versus placebo for cocaine dependence in buprenorphine-maintained subjects: a preliminary trial. Biol. Psychiatry. 2000;47:1080–1086. doi: 10.1016/s0006-3223(99)00310-8. [DOI] [PubMed] [Google Scholar]
- Goldman D, Oroszi G, Ducci F. The genetics of addictions: uncovering the genes. Nat. Rev. Genet. 2005;6:521–532. doi: 10.1038/nrg1635. [DOI] [PubMed] [Google Scholar]
- Grella CE, Anglin MD, Wugalter SE. Patterns and predictors of cocaine and crack use by clients in standard and enhanced methadone maintenance treatment. Am. J. Drug Alcohol Abuse. 1997;23:15–42. doi: 10.3109/00952999709001685. [DOI] [PubMed] [Google Scholar]
- Herlyn P, Muller-Hilke B, Wendt M, Hecker M, Mittlmeier T, Gradl G. Frequencies of polymorphisms in cytokines, neurotransmitters and adrenergic receptors in patients with complex regional pain syndrome type I after distal radial fracture. Clin. J. Pain. 2010;26:175–181. doi: 10.1097/AJP.0b013e3181bff8b9. [DOI] [PubMed] [Google Scholar]
- Jiang S, Mao G, Zhang S, Hong X, Tang G, Li Z, Liu X, Zhang Y, Wang B, Xu X, Wang X. Individual and joint association of alpha1A-adrenergic receptor Arg347Cys polymorphism and plasma irbesartan concentration with blood pressure therapeutic response in Chinese hypertensive subjects. Clin. Pharmacol. Ther. 2005;78:239–248. doi: 10.1016/j.clpt.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Kosten TR, George TP, Kosten TA. The potential of dopamine agonists in drug addiction. Expert Opin. Investig. Drugs. 2002;11:491–499. doi: 10.1517/13543784.11.4.491. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Rounsaville BJ, Kleber HD. Antecedents and consequences of cocaine abuse among opioid addicts. A 2.5-year follow-up. J. Nerv. Ment. Dis. 1988;176:176–181. doi: 10.1097/00005053-198803000-00006. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Wu G, Huang W, Harding MJ, Hamon SC, Lappalainen J, Nielsen DA. Pharmacogenetic randomized trial for cocaine abuse: disulfiram and dopamine beta-hydroxylase. Biol. Psychiatry. 2013;73:219–224. doi: 10.1016/j.biopsych.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei B, Morris DP, Smith MP, Svetkey LP, Newman MF, Rotter JI, Buchanan TA, Beckstrom-Sternberg SM, Green ED, Schwinn DA. Novel human alpha-1a-adrenocepter single nucleotide polymorphisms alter receptor pharmacology and biological function. Naunyn Schmiedebergs Arc Pharmacol. 2005;371:229–239. doi: 10.1007/s00210-005-1019-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leri F, Flores J, Rodaros D, Stewart J. Blockade of stress-induced but not cocaine-induced reinstatement by infusion of noradrenergic antagonists into the bed nucleus of the stria terminalis or the nentral nucleus of the amygdala. J. Neurosci. 2002;22:5713–5718. doi: 10.1523/JNEUROSCI.22-13-05713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magura S, Rosenblum A, Rodriguez AM. Changes in HIV risk behaviors among cocaine-using methadone patients. J. Addict. Dis. 1998;17:71–90. doi: 10.1300/J069v17n04_07. [DOI] [PubMed] [Google Scholar]
- McCance-Katz EF, Kosten TR, Jatlow P. Disulfiram effects on acute cocaine administration. Drug Alcohol Depend. 1998;52:27–39. doi: 10.1016/s0376-8716(98)00050-7. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M. The fifth edition of the Addiction Severity Index. J. Subst. Abust Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Lewis DC, O'Brien CP, Kleber HD. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284:1689–1695. doi: 10.1001/jama.284.13.1689. [DOI] [PubMed] [Google Scholar]
- Mead AN, Rocha BA, Donovan DM, Katz JL. Intravenous cocaine induced-activity and behavioural sensitization in norepinephrine-, but not dopamine-transporter knockout mice. Eur. J. Neurosci. 2002;16:514–520. doi: 10.1046/j.1460-9568.2002.02104.x. [DOI] [PubMed] [Google Scholar]
- Meandzija B, O'Connor PG, Fitzgerald B, Rounsaville BJ, Kosten TR. HIV infection and cocaine use in methadone maintained and untreated intravenous drug users. Drug Alcohol Depend. 1994;36:109–113. doi: 10.1016/0376-8716(94)90092-2. [DOI] [PubMed] [Google Scholar]
- Mod L, Rihmer Z, Magyar I, Arato M, Alfoldi A, Bagdy G. Serum DBH activity in psychotic vs. nonpsychotic unipolar and bipolar depression. Psychiatry Res. 1986;19:331–333. doi: 10.1016/0165-1781(86)90127-7. [DOI] [PubMed] [Google Scholar]
- Mutschler J, Abbruzzese E, Witt SH, Dirican G, Nieratschker V, Frank J, Grosshans M, Rietschel M, Kiefer F. Functional polymorphism of the dopamine beta-hydroxylase gene is associated with increased risk of disulfiram-induced adverse effects in alcohol-dependent patients. J. Clin. Psychopharm. 2012;32:578–580. doi: 10.1097/JCP.0b013e31825ddbe6. [DOI] [PubMed] [Google Scholar]
- Paladini CA, Williams JT. Noradrenergic inhibition of midbrain dopamine neurons. J. Neurosci. 2004;24:4568–4575. doi: 10.1523/JNEUROSCI.5735-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrakis IL, Carroll KM, Nich C, Gordon LT, McCance-Katz EF, Frankforter T, Rounsaville BJ. Disulfiram treatment for cocaine dependence in methadone-maintained opioid addicts. Addiction. 2000;95:219–228. doi: 10.1046/j.1360-0443.2000.9522198.x. [DOI] [PubMed] [Google Scholar]
- Puumalu T, Riekkinen P, Sirvio J. Modulation of vigilance and behavioral activation by alpha-1 adrenoceptors in the rat. Pharmacol. Biochem. Behav. 1997;56:705–712. doi: 10.1016/s0091-3057(96)00408-x. [DOI] [PubMed] [Google Scholar]
- R_Development_Core_Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2009. [Google Scholar]
- Remme WJ. Effect of ACE inhibition on neurohormones. Eur. Heart J. 1998;19:J16–J23. [PubMed] [Google Scholar]
- Rothman R, Baumann MH. Monoamine transporters and psychostimulant drugs. Eur. J. Pharm. 2003;479:23–40. doi: 10.1016/j.ejphar.2003.08.054. [DOI] [PubMed] [Google Scholar]
- Schroeder JP, Cooper DA, Schank JR, Lyle MA, Gaval-Cruz M, Ogbonmwan YE, Pozdeyev N, Freeman KG, Iuvone PM, Edwards GL, Holmes PV, Weinshenker D. Disulfiram attenuates drug-primed reinstatement of cocaine seeking via inhibition of dopamine beta-hydroxylase. Neuropsychopharmacology. 2010;35:2440–2449. doi: 10.1038/npp.2010.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- Shorter D, Kosten TR. Novel pharmacotherapeutic treatments for cocaine addiction. BMC Med. 2012;2011:119. doi: 10.1186/1741-7015-9-119. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorter D, Lindsay JA, Kosten TR. The alpha-1 adrenergic antagonist doxazosin for treatment of cocaine dependence: a pilot study. Drug Alcohol Depend. 2013 doi: 10.1016/j.drugalcdep.2012.11.021. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snapir A, Koskenvuo J, Toikka J, Orho-Melander M, Hinkka S, Saraste M, Hartiala J, Scheinin M. Effects of common polymorphisms in the alpha1A-, alpha2B-, beta1- and beta2-adrenoceptors on haemodynamic responses to adrenaline. Clinic. Sci. (Lond) 2003;204:509–520. doi: 10.1042/CS20020299. [DOI] [PubMed] [Google Scholar]
- Stanley WC, Li B, Bonhaus DW, Johnson LG, Lee K, Porter S, Walker K, Martinez G, Eglen RM, Whiting RL, Hedge SS. Catecholamine modulatory effects of nepicastat (RS-25560-197), a novel, potent and selective inhibitor of dopamine-beta-hydroxylase. Br. J. Pharmacol. 1997;121:1803–1809. doi: 10.1038/sj.bjp.0701315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg DE, van Kammen DP, Lerner P, Ballenger JC, Marder SR, Post RM, Bunney WE., Jr CSF dopamine beta-hydroxylase in schizophrenia. Arch. Gen. Psychiatry. 1983;40:743–747. doi: 10.1001/archpsyc.1983.01790060041005. [DOI] [PubMed] [Google Scholar]
- van Kammen DP, Kelley ME, Gilbertson MW, Gurklis J, O'Connor DT. CSF dopamine beta-hydroxylase in schizophrenia: associations with premorbid functioning and brain computerized tomography scan measures. Am. J. Psychiatry. 1994;151:372–378. doi: 10.1176/ajp.151.3.372. [DOI] [PubMed] [Google Scholar]
- Weinshenker D. Cocaine sobers up. Nat. Med. 2010;16:969–970. doi: 10.1038/nm0910-969. [DOI] [PubMed] [Google Scholar]
- Wolf BC, Lavezzi WA, Sullivan LM, Middleberg RA, Flannagan LM. Methadone-related deaths in Palm Beach County. J. Forensic Sci. 2004;49:375–378. [PubMed] [Google Scholar]
- Zhang XY, Kosten TA. Prazosin, an alpha-1 adrenergic antagonist, reduces cocaine-induced reinstatement of drug-seeking. Biol. Psychiatry. 2005;57:1202–1204. doi: 10.1016/j.biopsych.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Zhang XY, Kosten TA. Previous exposure to cocaine enhances cocaine self-administration in an alpha-1 adrenergic receptor dependent manner. Neuropsychopharmacology. 2007;32:638–645. doi: 10.1038/sj.npp.1301120. [DOI] [PubMed] [Google Scholar]