Esophageal adenocarcinoma (EAC) arises from Barrett’s esophagus, a columnar metaplasia of the epithelium caused by chronic acid reflux.1 This disease is growing faster than any other type of cancer in Western countries,2 and despite extensive efforts for prevention, the incidence remains high and the 5- year survival rate is poor. Early detection may allow for more effective interventions, and is critical to improving prognosis. Current methods of surveillance with white light endoscopy are limited in effectiveness because pre-malignant lesions (dysplasia) are flat in appearance and difficult to visualize.3 Molecular imaging is an emerging technology that can help physicians guide biopsy by generating image contrast based on unique biological properties tissue.4 Imaging agents that are specific for overexpressed molecular targets can improve diagnosis, staging, intervention, and management of this disease. Early imaging validation is needed to establish safety and evidence of efficacy.5
Description of Technology
A fluorescently labeled peptide specific for esophageal neoplasia was selected using phage display technology.6 The sequence ASYNYDA was conjugated to FITC by a GGGSK linker. An apparent dissociation constant (kd) of 32 nM and an association rate constant (k) of 0.2 min-1 (~5 minutes for binding onset) was measured. We isolated cyclophilin A (CypA) as a possible target. The fluorophore used is compatible with a fiber-based (2.6 mm diameter) confocal laser endomicroscope (Cellvizio) that delivers <2 mW of excitation at λex = 488 nm.7 This level of power has been determined to be nonsignificant risk by the U.S. Food and Drug Administration.8 Images are collected at 8 frames/second over a field-of-view of 240×240 µm2 with a resolution of 1.4 and 7 µm in the lateral and axial dimensions, respectively. Optical sections are collected at a depth of 50 µm below the mucosal surface with 1000× magnification.
Video description
On conventional white light endoscopy, Barrett’s esophagus (arrow) appears with a “salmon pink” color, and can be several centimeters in length Fig. 1A. By comparison, adjacent squamous epithelium (normal) appears pink. No gross lesions or architectural changes are apparent to suggest the presence of dysplasia. The aims of this video journal are to 1) demonstrate safe topical administration of fluorescently labeled peptides to the mucosal surface of the distal esophagus, 2) show that peptides have rapid binding onset in vivo; and 3) validate specific binding of peptides to Barrett’s neoplasia. IRB approval was obtained from the University of Michigan. Patients previously scheduled for endoscopy per routine clinical care were recruited. Informed consent was obtained from each subject prior to the procedure.
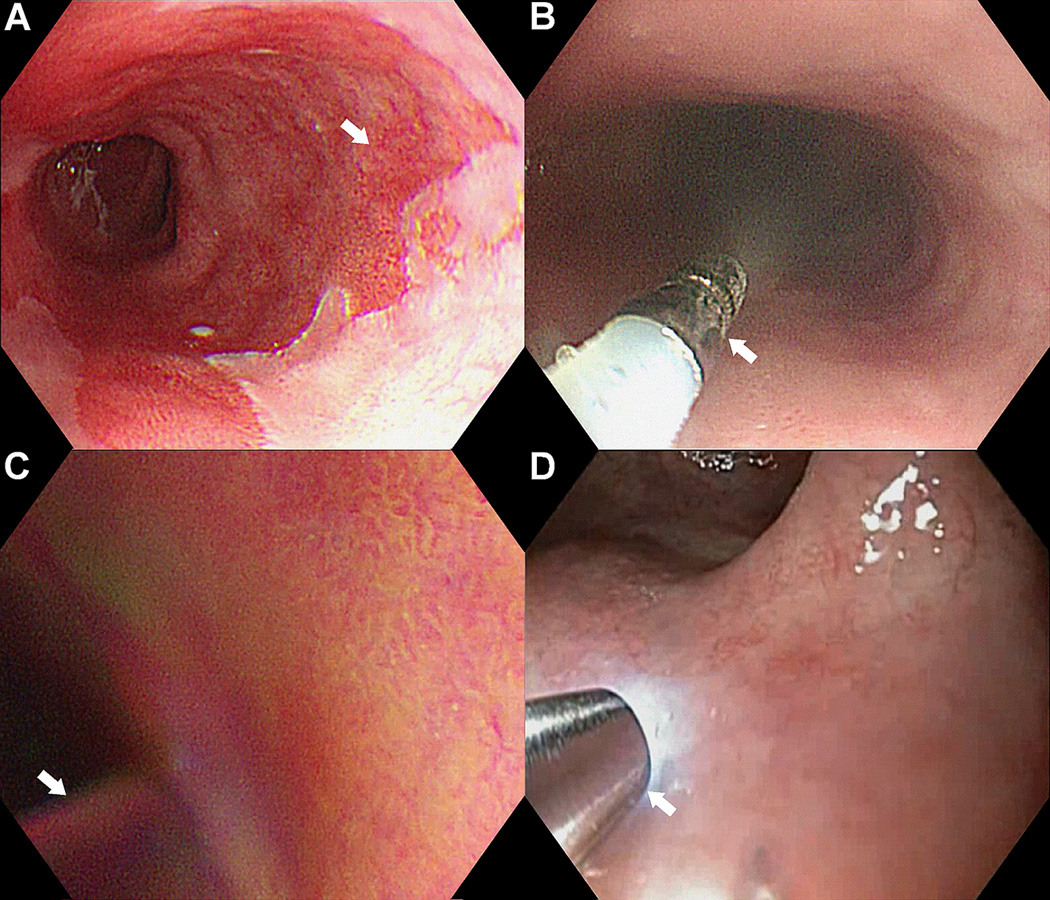
Fig 1. Targeted imaging.
A) Barrett’s esophagus. B) Topical peptide administration. C) Rinsing of unbound peptides. D) Confocal endomicroscopy.
The upper endoscopy was performed first with conventional white light. The distal esophagus was rinsed thoroughly with saline to remove mucous. Lyophilized ASY*-FITC (100 µM) was reconstituted in 5 ml of saline (IND# 110,444, Sponsor DKT), and delivered via a circumferential spray catheter (arrow) passed through the instrument channel, Fig. 1B. This method provides comprehensive coverage of the peptide over the mucosal surface of the distal esophagus where the molecular targets are overexpressed. A high peptide concentration maximizes binding interactions to achieve a large fluorescence signal needed for real time imaging with little risk of toxicity. After 5 minutes for incubation, the unbound peptides are rinsed off (arrow) using the irrigator, Fig. 1C. This rapid onset of binding distinguishes peptides from other types of molecular probes that typically require hours for peak effect. The endomicroscope (arrow) was passed through the instrument channel and placed into contact with the mucosa, Fig. 1D. The locations of the images collected were recorded using landmarks defined by Prague criteria for the Barrett’s segment. Endoscopic mucosal resection was performed, and a gastrointestinal pathologist (H.A.) evaluated the histology from the epithelial surface (≤50 µm depth) where the peptides were applied. The average time required to perform the experimental portion (peptide spray and imaging) of the procedure was <15 minutes.
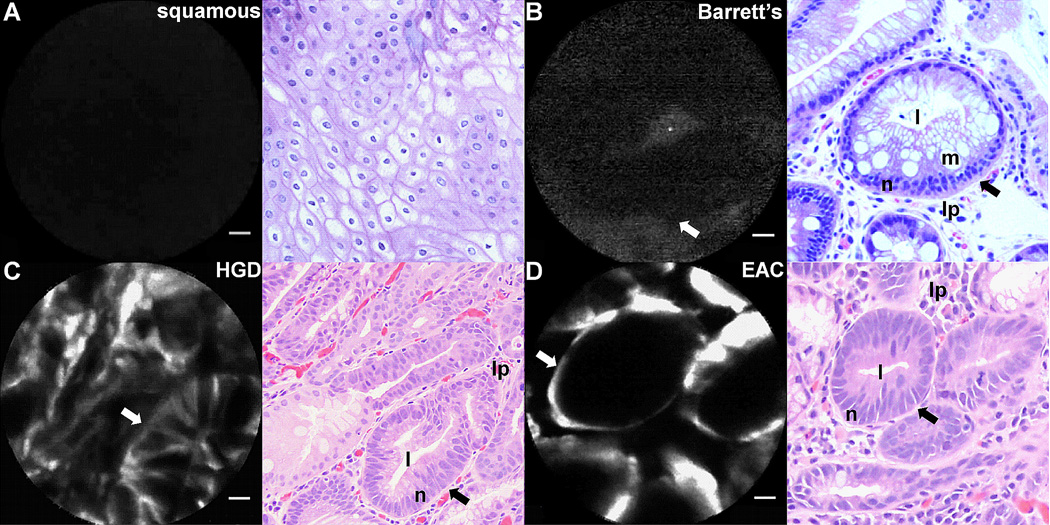
The real time confocal video of squamous epithelium shows no peptide binding in most frames, scale bar 20 µm, Fig. 2A. Some non-specific signal was seen in some locations. Representative histology (H&E) shows a horizontal section of squamous cells. For Barrett’s, faint signal can be seen that may represent non-specific pooling of contrast, and representative histology shows the lumen (l) of a benign-appearing, circular crypt (c) with mucin (m) filled goblet cells and round, basal nuclei (n) surrounded by lamina propria (lp), Fig. 2B. By comparison, video of high-grade dysplasia (HGD) shows strong fluorescence from the cell surface within transformed crypts that have distorted architecture, and the corresponding histology shows elongated nuclei with extensive stratification and reduced cytoplasmic mucin, Fig. 2C. Images of EAC shows intense fluorescence around discrete tubular structures, and the histology shows epithelium lined tubules with fully stratified nuclei and absence of mucin, Fig. 2D.
Fig. 2. In vivo validation.
A) Squamous epithelium. B) Barrett’s. C) High-grade dysplasia (HGD). D) esophageal adenocarcinoma (EAC), scale bar 20 µm
The peptides were found to bind specifically to overexpressed targets to provide positive contrast, resulting in improved dynamic range for imaging. Topical rather than systemic administration reduces the risk of toxicity by limiting the biodistribution of the molecular probe to the region of disease. The images collected on endomicroscopy have a microscopic field-of-view, and the in situ pathology examined has a superficial tissue depth. These images can be used to guide biopsy or endoscopic mucosal resection of tissue to provide conventional pathology. In the future, a wide-area endoscope will be used to collect images that can rapidly visualize fluorescence from the entire mucosal surface of the esophagus. Confocal laser endomicroscopy can still be used as an adjunct to validate specific peptide binding to Barrett’s neoplasia.
Take home message
We have demonstrated a fluorescently labeled peptide can be topically and safely administered during endoscopy to comprehensively cover the mucosal surface of the distal esophagus. The onset of binding occurs in <5 minutes to facilitate practical use in a busy clinical unit. Specific binding of the peptide to Barrett’s neoplasia has been shown in real time using confocal laser endomicroscopy in vivo.
Supplementary Material
Acknowledgments
Funding Support: NIH U54 CA163059, U54 CA136429 (TDW)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions: Study concept and design: MBS, BPJ, DKT, TDW. Acquisition of data: MBS, CP, BJE, RSK. Analysis and interpretation of data: MBS, BPJ, HA, DKT, TDW. Drafting of the manuscript: MBS, BPJ, TDW. Technical support: MBS, BPJ, SL, SK. Obtained funding: TDW.
Conflicts of Interest: U. S. Patent No. 8,247,529 has been filed on behalf of TDW on the peptides presented in this study. All other authors report no conflicts.
References
- 1.Sharma P. Clinical practice. Barrett's esophagus. N Engl J Med. 2009;361:2548–2556. doi: 10.1056/NEJMcp0902173. [DOI] [PubMed] [Google Scholar]
- 2.Pohl H, Welch HG. The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst. 2005;97:142–146. doi: 10.1093/jnci/dji024. [DOI] [PubMed] [Google Scholar]
- 3.Kariv R, Plesec TP, Goldblum JR, Bronner M, Oldenburgh M, Rice TW, Falk GW. The Seattle protocol does not more reliably predict the detection of cancer at the time of esophagectomy than a less intensive surveillance protocol. Clin Gastroenterol Hepatol. 2009;7:653–658. doi: 10.1016/j.cgh.2008.11.024. [DOI] [PubMed] [Google Scholar]
- 4.Bird-Lieberman EL, Neves AA, Lao-Sirieix P, O'Donovan M, Novelli M, Lovat LB, Eng WS, Mahal LK, Brindle KM, Fitzgerald RC. Molecular imaging using fluorescent lectins permits rapid endoscopic identification of dysplasia in Barrett's esophagus. Nat Med. 2012;18:315–321. doi: 10.1038/nm.2616. [DOI] [PubMed] [Google Scholar]
- 5.Aldrich MB, Marshall MV, Sevick-Muraca EM, Lanza G, Kotyk J, Culver J, Wang LV, Uddin J, Crews BC, Marnett LJ, Liao JC, Contag C, Crawford JM, Wang K, Reisdorph B, Appelman H, Turgeon DK, Meyer C, Wang T. Seeing it through: translational validation of new medical imaging modalities. Biomed Opt Express. 2012;3:764–776. doi: 10.1364/BOE.3.000764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sturm MB, Joshi BP, Lu S, Piraka C, Khondee S, Elmunzer BJ, Kwon RS, Beer DG, Appelman HD, Turgeon DK, Wang TD. Targeted endoscopic imaging of Barrett’s neoplasia with specific fluorescent-labeled peptide: first in-humans results. Sci Transl Med. 2013:5. doi: 10.1126/scitranslmed.3004733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang TD, Friedland S, Sahbaie P, Soetikno R, Hsiung PL, Liu JT, Crawford JM, Contag CH. Functional imaging of colonic mucosa with a fibered confocal microscope for real-time in vivo pathology. Clin Gastroenterol Hepatol. 2007;5:1300–1305. doi: 10.1016/j.cgh.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holbein ME, Berglund JP. Understanding Food and Drug Administration regulatory requirements for an investigational device exemption for sponsor-investigators. J Investig Med. 2012;60:987–994. doi: 10.231/JIM.0b013e318262df40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.