Abstract
The von Willebrand factor (vWF) is an acute stroke response protein involved in platelet aggregation, adhesion, inflammation, and thrombus formation, responses that occur following an ischemic stroke. We hypothesize that administration of an anti-vWF antibody (anti-vWF-Ab) may be used as adjunctive therapy with tissue plasminogen activator (tPA) to promote behavioral improvement following an embolic stroke.
In this proof-of-concept study, which used a blinded and randomized design, we studied delayed treatment with the anti-vWF-Ab, AJW200 (0.30 mg/kg), alone or in combination with a rabbit low-dose of tPA (0.9 mg/kg) using the rabbit small clot embolic stroke model (RSCEM) with behavioral function as the primary clinically relevant endpoint. To evaluate the quantitative relationship between clot burden in brain and clinical scores, so that an effective stroke dose (P50) could be calculated, logistic sigmoidal quantal analysis curves were constructed. A beneficial treatment significantly increases P50 compared to control. The effect of antibody administration, either alone or with low dose tPA was compared to a “positive control”, a standard rabbit optimized dose of tPA (3.3 mg/kg), as a measure of the maximum improvement potential in the RSCEM.
The anti-vWF-Ab, AJW200, or control IgG were administered IV 1 hour following embolization, and behavior was measured 48 hours later. AJW200 plus low-dose tPA significantly increased the P50 value by 74% (p<0.05, t=2.612) and 81% (p<0.05, t=2.519) compared to low dose tPA or IgG, respectively, but not the AJW200 group (p>0.05). AJW200 increased the P50 value by 28%, (p>0.05) compared to the control IgG-treated group. Standard dose tPA increased the P50 value by 154% (p<0.05). Statistically, the combination response for AJW200 plus low-dose tPA was not significantly different from standard dose tPA (p=0.26).
This study shows that the concomitant administration of the anti-vWF-Ab AJW200 with low dose tPA is synergistic and results in significantly improved behavioral function following embolic stroke. We postulate that neutralization of vWF may suppress or attenuate one or more aspects of the acute phase stroke cascade response including suppression of inflammatory response and reduced leukocyte adhesion.
Keywords: Neuroprotection, Clinical, Thrombus, Behavioral, Thrombolytic, Acute treatment
Introduction
In acute ischemic stroke (AIS) patients, the activation of the blood coagulation cascade due to atherosclerotic plaque formation (i.e.: thrombotic stroke) is intrinsically involved in platelet adhesion and blood clot formation leading to stroke [1]. Alternatively, the formation of a distal clot or embolus (i.e.: cardiac) can travel through the blood stream and move into the brain causing vascular obstruction. Taken together, thrombotic and embolic strokes commonly referred to as thromboembolic strokes result in approximately 80% of all stroke cases [2–4].
Tissue plasminogen activator (tPA), which results in embolus lysis and cerebral reperfusion remains the only approved thrombolytic therapy for AIS [1]. Extensive clinical research continues to support the use of tPA within an extended therapeutic window of up to 4.5 hours [5]. However, even with prompt reperfusion, a secondary wave of emboli and inflammatory events may lead to reperfusion injury or progressive strokes [6–8]. Despite the key role of platelets and the coagulation cascade in the pathophysiology of stroke, antiplatelet therapies such as anti-glycoprotein IIb/IIIa (GPIIb/IIIa or integrin αIIbβ3) inhibitors (i.e: abciximab, eptifibatide and tirofiban), or anticoagulants such as heparin and coumadin have shown limited if any efficacy and been associated with a high risk of intracranial hemorrhage [9].
The von Willebrand factor (vWF) has emerged as a promising target in stroke therapy [10–12] because it is involved in the acute phase response following a stroke [13,14]. vWf is a large multimeric protein that plays a key role in several facets of primary hemostasis [10,15]. While vWf contains multiple modular internal repeat domains with binding sites for multiple ligands, it is the interaction of the A1 domain with platelet GPIb-α that plays a critical role in two key aspects of the acute phase response; thrombosis under high shear as well as platelet tethering to activated endothelium under low shear [16–19]. Inhibitors of the vWF-GPIbα interaction prevent arterial occlusion in animal models of arterial thrombosis without the increased bleeding risk associated with other classes of anti-thrombotic agents [12] and also exhibit efficacy in murine transient middle cerebral artery occlusion (tMCAO) models [20,21]. Similarly, mice lacking vWF maintain vessel patency in models of arterial occlusion [22] and exhibit reduced infarct size in tMCAO models of stroke [23]. Thus, vWF-GPIbα inhibitors may be useful as a monotherapy or in combination with tPA to prevent recruitment and subsequent aggregation of platelets following tPAmediated thrombolysis [10,20]. Moreover, since other detrimental roles have been associated with vWF, such as mediating the inflammatory response and promoting leukocyte adhesion [10,13,18,19] vWF is a rational target for intervention as a possible stroke therapy.
We used a standard translational embolic stroke model, the rabbit small clot embolic stroke model (RSCEM) [24,25], to test the hypothesis that administration of an anti-vWF-Ab would be useful to attenuate embolism-induced behavioral deficits, either alone or in combination with low-dose (0.9mg/kg) intravenous (IV) tPA therapy, which by itself does not improve behavior in embolized rabbits [26]. The RSCEM is a useful translational tool previously used in the development of tPA [26,27] and possibly a model predictive of effective treatments that may eventually translate into efficacy in clinical trials [24,25,28], once the new therapy has been optimized. Moreover, the primary endpoint used when assessing the efficacy of a novel treatment regimen in the RSCEM is behavioral functional, which is primarily based upon motor function components of the National Institutes of Health Stroke Scale (NIHSS) [1,5].
Methods
This study was in compliance with RIGOR guidelines regarding study design, disclosures, blinding and randomization [29]. All surgical, behavioral and tissue handling procedures have been described in extensive detail in previous publications [26–28, 30–32].
Animals and animal welfare
Male New Zealand white rabbits weighing 2–2.5 Kg were supplied food (alfalfa cubes) and water ad libitum while under quarantine in an enriched environment for at least 3 days prior to experimental use. Institutional Animal Care and Use Committee (IACUC) approved the surgical and treatment procedures used in this study and veterinary staff monitored the health status of rabbits throughout the study. Rabbits were humanely euthanized if they were in pain, showed extreme discomfort, or if they were unable to reach food or water after embolization.
Surgical procedures
Surgical procedures were done as described previously [26,28,30], using isoflurane by mask (5% induction/3% maintenance) in a controlled environment with a room temperature between 22.4–23.2°C, with the following minor modifications. After placement of the carotid catheter, the catheter was filled with 0.2 ml of heparinized saline (33 units/ml) and plugged with an injection cap. The skin around the catheter was then sealed tightly using sutures.
In this study, rabbits were allowed to recover from the effects of anesthesia for at least 2 hours before embolization. The absence of volatile anesthetics during the embolization procedure is beneficial when studying neuroprotection, since anesthetics have been shown to be neuroprotective, and can interfere with the ischemic cascade [28,33] which can confound the interpretation of valuable data due to drug-drug interactions and alteration of multiple components of the ischemic cascade. Analgesics are also not administered because of the potential to interfere with the ischemic stroke cascade [28], possibly giving false positive efficacy.
Embolization
Blood is drawn from one or more donor rabbits (non-autologous blood clots) and allowed to clot for 3h at 37°C. The large blood clots are then suspended in phosphate-buffered saline pH 7.4 (PBS) with 0.1% bovine serum albumin (BSA) and Polytron-generated fragments are sequentially passed through sizing screens. Small-sized blood clots (100–250 µm) were suspended in PBS containing 1% bovine serum albumin and were then spiked with 57Co-labeled microspheres (Perkin-Elmer Inc.) to allow for tracking into the brain. An aliquot of the solution was removed for the determination of specific activity.
For embolization, rabbits are placed in a restrainer and 1 ml of a clot particle suspension is injected through carotid catheter, which is then flushed with 5 ml of sterile saline. Rabbits are fully awake during the embolization procedure and they are self-maintaining (i.e.: they do not require artificial respiration or other external support). This allows for immediate observation of the effects of embolization on behavior at the time of embolus injection and thereafter. After the embolization process was completed, the catheter was heat-sealed close to the neck leaving a small portion protruding from the skin.
Behavioral function and quantal dose-response analysis
The use of clinical rating scores and quantal analysis is a powerful statistical method to determine how a heterogeneous stroke population will respond to a treatment [26–28, 30–32]. To construct a quantal analysis curve, a wide range of clot doses were injected in this study in order to produce a spectrum of behaviorally normal to abnormal animals, which included death on the continuum of embolization-induced effects. In the absence of a neuroprotective treatment regimen, small numbers of microclots cause no grossly apparent neurologic dysfunction. However, when large numbers of microclots are injected, they invariably caused encephalopathy. In this study, less than 5% of animals died due to embolization, but in the event that embolization did result in a rapid death, there was a positive correlation with a high clot dose measured in brain and the animal was represented as an abnormal (or dead) on the quantal analysis curve if the rabbit received treatment. Abnormal rabbits with encephalopathy include those with one or more of the following symptoms: ataxia, leaning, circling, lethargy, nystagmus, loss of balance, loss of limb/facial sensation and occasionally, hind-limb paraplegia. Using a simple dichotomous rating system, with a reproducible composite result and low inter-rater variability (<5%), each animal was rated as either behaviorally normal or abnormal by an observer naive to the study design and/or treatment groups [26–28, 30–32].
To evaluate the quantitative relationship between clot burden in brain and clinical scores, logistic sigmoidal quantal analysis curves were constructed as originally described by Waud [32,34] and thereafter [26,28,30]. In this study, all animals were randomized to treatment groups before embolization using http://www.randomizer.org/ design templates. Following embolization, rabbits were administered vehicle or drug and then at the termination of the study, each animal was rated for clinical function by treatment naive-observers (SD and/or technician on duty Mark Wu). The clot burden in brain was measured, and a separate quantal curve was generated for each treatment condition resulting in P50 values are presented as Mean ± SEM (n) in mg clot. A statistically significant increase in the P50 value or clot burden in brain that produce neurologic dysfunction in 50% of a group of animals compared to control is indicative of clinical improvement [26–28, 30–32]. In this model that uses clinical function as the primary measure, the use of radioactive material precludes extensive postmortem analysis other than direct quantitation of clot burden in brain [24,28].
Production and characterization of the anti-vWF antibody, AJW200
AJW200 was chosen for this proof-of-concept study since, although it is directed against human vWF it cross-reacts with rabbit vWF and blocks its interaction with GPIbα [12]. Genes encoding the heavy and light chain of AJW200 were custom synthesized and cloned into a mammalian expression vector, pAb. After confirming by sequencing, the vector was transfected into HEK293 cells and stable cell lines selected using G418 selection for 3 weeks. Antibody was purified from the culture supernatant using protein A Affinity chromatography. The purified antibody was analyzed for binding to the A1 domain of both rabbit and human vWf by ELISA. The antibody preparations were also tested for endotoxin content using a chromogenic LAL endotoxin kit (ToxinSensor; GenScript, NJ).
The purified AJW200 antibody exhibited KD values for human and rat vWf A1 domain of A1 of 0.43 nM and 0.24 nM, respectively, confirming the cloned antibody exhibited the expected binding properties. The endotoxin levels were 2.5U/mg, well within acceptable limits for in vivo studies. The control IgG was purified from normal serum by immobilized Protein A using low endotoxin methodology produced an IgG (Innovative Research, MI). The antibodies were filter sterilized and endotoxin level was determined to be less than 2.5EU/mg.
Drug treatment
For test substance administration, rabbits were placed in a Plexiglas restrainer (Plaslabs Inc.) for the duration of the treatment. For all experiments in this study, rabbits were randomly allocated into treatment groups before the embolization procedure, with concealment of the randomization guaranteed by using an independent party. The randomization sequence was not revealed until all postmortem analyses were complete.. All treatments were given 1 hour post embolization with behavioral analysis done 48 hours after treatment.
VWF antibody administration
Rabbits were given a bolus IV injection of control IgG (0.30 mg/kg) or AJW200 (0.30 mg/kg) over 1 minute using the marginal ear vein at a dose volume of 0.30 ml/kg. The dose was based on the EC50 dose in a rabbit arterial thrombosis model [12]. AJW200 has previously been shown to have a half-life of 23.5–27.2 hours after an IV dose, and had biological effects lasting 12 hours after a single dose [35]. For low-dose tPA (0.9 mg/kg) [26] combination studies, clinical grade tPA (rt-PA; Genentech Inc. Alteplase) was given IV, with 20% bolus/80% infused over 30 min and IV anti-vWF-Ab was given concomitantly. For the positive control group, standard dose tPA (3.3 mg/kg) was given IV with 20% as a bolus/80% infused over 30 min [26].
Power and statistical analysis
Power analysis of historical quantal analysis curves indicates that, assuming α=0.05 and β=0.90, a coefficient of variation of 15% and a difference between means of 20%, a sample size of 14 animals are required per group. P50 values were analyzed for significance using ANOVA with a post-hoc t-test including the Bonferroni correction for multiple group analysis, where appropriate (Figures 1A and B combination studies).
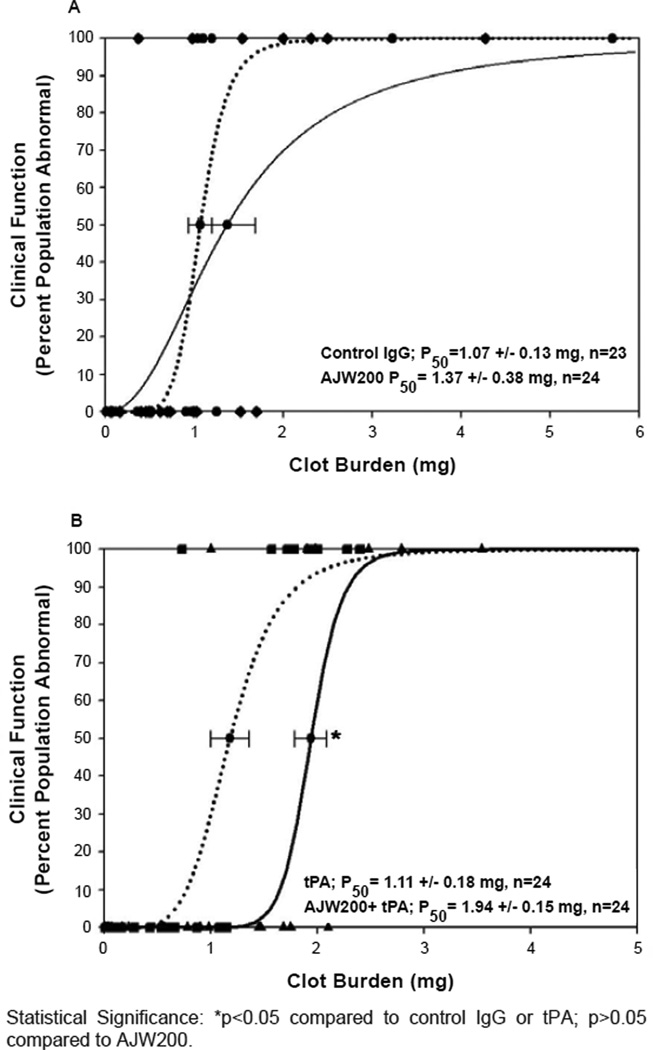
Figure 1. Quantal curves: anti-vwf-ab/tpa combination analysis.
A: Effect of AJW200 alone or in combination with low-dose tPA on clinical function. Control IgG (0.3mg/kg); Black Circles- Black Dotted LINE; AJW200 Ab (0.3 mg/kg) Black DIAMONDS- Black Solid LINE. B: Effect of AJW200 in combination with low-dose tPA on clinical function compared to low dose tPA. tPA (0.9mg/kg)- Black Squares- Black Dotted LINE; AJW200 (0.3mg/kg) + tPA (0.90 mg/kg IV- Black Triangles UP- Black Solid LINE.
Statistical Significance: *p<0.05 compared to control IgG or tPA; p>0.05 compared to AJW200.
Results
Behavioral analysis
Anti-vWF-Ab efficacy analysis
Initial studies (graph not shown) using 0.15 mg/kg anti-VWF antibody showed no behavioral benefit (98.6% of control, n=23 per group, p>0.05). An increased dose of AJW200 (0.3 mg/kg) also did not have a significant effect (p>0.05) on behavioral function following embolization (Figure 1A). For Figure 1A, all raw data points are presented as symbols for normal (y-axis at 0) and abnormal (y-axis at 100).
Anti-vWF-Ab combination efficacy analysis
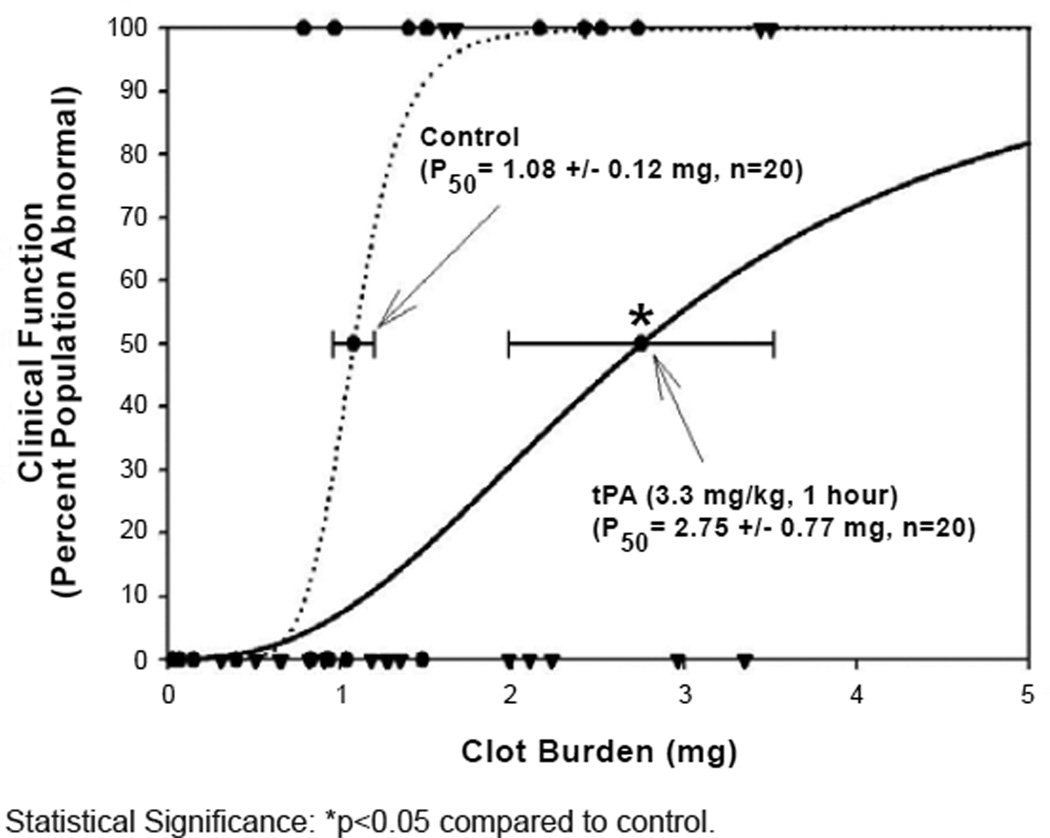
AJW200 in combination with low-dose tPA produced a significant 74–81% increase in P50 (p<0.05) (Figure 1B), compared to either the IgG or tPA groups. The combination response (Figure 1B) was sub-maximal, and did not approach the 154% increase in P50 that was achieved by standard dose tPA treatment (p<0.05) (Figure 2). Statistically, the combination response (Figure 1B) was not significantly different from standard dose tPA in Figure 2 (p=0.26).
Figure 2. Quantal Curves: tPA Positive Control.
Effect of standard dose (3.3 mg/kg) tPA (n=20, Black Triangles DOWN- Black Solid LINE) on clinical function. The vehicle control curve for this study design is shown by Black Circles- Black Dotted LINE (n=20).
Statistical Significance: *p<0.05 compared to control.
Anti-vWF-Ab safety profile
Neither the control IgG, nor AJW200 at the dose used, had any adverse events in embolized rabbits, consistent with [36] With both standard dose tPA and the AJW200/ tPA group, we observed hematomas surrounding the ear vein injection site and an increase in bleeding from peripheral incision sites, which was controlled by pressure using sterile gauze and cleaned using 3% hydrogen peroxide solution.
Discussion
In this proof-of-concept study, delayed administration of an anti-vWF-Ab in combination with low-dose tPA produced a significant behavioral improvement as measured by an increase in P50 values (74–81%), compared to control. The positive control group, standard dose tPA treatment (3.3 mg/kg) increased P50 values by 154% compared to control. This study demonstrates the usefulness of the RSCEM to study novel therapies aimed at interrupting one or more components of the coagulation or inflammatory cascade and acute phase stroke response [10,11,13]. The study shows that therapeutic intervention with attenuation of vWF-mediated processes, results in a synergistic effect when administered with low-dose thrombolytic therapy. In this study, we did not test AJW200 with standard dose tPA at an optimal administration time, because we have previously found that there is a ceiling effect that precludes measurement of additional behavioral benefit above that observed by tPA alone. Nevertheless, additional studies of AJW200 with tPA may be useful to further determine the safety profile of combination therapy using a variation of the embolic stroke model used here [37] and efficacy of combination treatment with tPA administration windows beyond 1–1.5 hours. Moreover, since this is a proof of concept study, additional AJW200 dose optimization studies will be required to determine the optimal safe combination for maximal behavioral improvement, as well as therapeutic window studies to establish the delay (i.e. 3–12 hours) post embolization when an efficacious effect can still be measured.
Two major pools of vWf exist; plasma vWf and vWf stored in Weible-Palade bodies of platelets and vascular endothelial cells [16,38]. The binding site for platelet GP1b-α on the A1 domain of circulating vWf remains cryptic but upon high shear or following binding to collagen, the A1 domain undergoes a conformational change that exposes the GP1b-α binding site [19,39] which is critical in the attachment of platelets to a growing thrombus or endothelial wall under flow. Inhibitors of the vWF-GPIbα interaction prevent arterial occlusion in animal models of arterial thrombosis without the increased bleeding risk associated with other classes of anti-thrombotic agents [12,40] and likewise mice lacking vWf also show diminished vessel occlusion in models of arterial thrombosis [19]. The endothelial pool of vWf, stored in Wiebel-Palade bodies, is released upon activation of the endothelium as ultralarge vWF which, in contrast to plasma vWF, has the A1 binding site constitutively exposed and release results in platelet and platelet-leukocyte complex tethering to the activated endothelium under conditions of low-flow [38]. Studies of 111In-labelled platelets in a baboon model of MCAO revealed that extensive platelet deposition occurs in the surrounding microvasculature together with activated leukocytes during reperfusion suggesting a secondary wave of thrombotic and inflammatory events exacerbates the injury induced by the original infarct [41,42]. vWf knock-out mice or mice treated with vWf-GPIbα inhibitors exhibit reduced infarct size compared to controls in MCAO models of stroke [20,21]. Thus, the vWf-GPIbα interaction appears to play a critical role in two important aspects of the pathophysiology of stroke: vessel occlusion under high shear following an initial arterial embolic event and the subsequent acute phase response.
Since numerous roles have now been attributed to vWF [10,13], including platelet aggregation and thrombus formation, and involvement in the acute phase response following a stroke [13], it is possible that the beneficial effects of the anti-vWF-Ab, AJW200, observed in this study with the RSCEM are related to prevention of platelet and platelet-leukocyte complex adhesion to activated vessel endothelium under low-flow conditions [19] and thrombus formation under high shear [16]. Moreover, neutralization of vWF may suppress or attenuate one or more aspects of the acute phase stroke response [8,43]. Since this study demonstrated a benefit on behavior, further studies will elucidate the mechanisms responsible for the benefit so that this class of therapeutic agents can be further developed.
In conclusion, we have demonstrated significant efficacy for the combination of the anti-vWF-Ab, AJW200, when administered concomitantly with low dose tPA. Based upon evolving information on the roles of vWF in cardiovascular disease and stroke [10–15], we postulate that neutralization of vWF may suppress or attenuate one or more aspects of the acute phase stroke cascade response including suppression of inflammatory response and reduced leukocyte adhesion, promoting reperfusion and providing neuroprotection.
However, it should be noted that the combination therapy also increased the appearance of hematomas at the injection site and peripheral bleeding at incision sites. Our studies suggest that the combination therapy may be useful to improve clinical outcome in AIS patients, but the administration of the drug combination may require specifically trained personnel similar to standard of care therapy with tPA.
Acknowledgments
PAL was CSMC site PI for this study conducted under a subcontract from AvantGen Inc. PAL has no financial interest in AvantGen Inc. and was not compensated to write this scientific manuscript.
Sources of Funding
This article was supported by a U01 Translational research grant NS060685 to PAL and an R43 NS061374 grant to XF.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. No authors listed. [DOI] [PubMed] [Google Scholar]
- 2.Muir KW. Heterogeneity of stroke pathophysiology and neuroprotective clinical trial design. Stroke. 2002;33:1545–1550. doi: 10.1161/01.str.0000018684.86293.ab. [DOI] [PubMed] [Google Scholar]
- 3.Petty GW, Brown RD, Jr, Whisnant JP, Sicks JD, O’Fallon WM, et al. Ischemic stroke subtypes: a population-based study of incidence and risk factors. Stroke. 1999;30:2513–2516. doi: 10.1161/01.str.30.12.2513. [DOI] [PubMed] [Google Scholar]
- 4.Petty GW, Brown RD, Jr, Whisnant JP, Sicks JD, O’Fallon WM, et al. Ischemic stroke subtypes : a population-based study of functional outcome, survival, and recurrence. Stroke. 2000;31:1062–1068. doi: 10.1161/01.str.31.5.1062. [DOI] [PubMed] [Google Scholar]
- 5.Hacke W, Kaste M, Bluhmki E, Brozman M, Dávalos A, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 6.Molina CA. Reperfusion therapies for acute ischemic stroke: current pharmacological and mechanical approaches. Stroke. 2011;42:S16–S19. doi: 10.1161/STROKEAHA.110.598763. [DOI] [PubMed] [Google Scholar]
- 7.Lapchak PA, Araujo DM, Song D, Zivin JA. The nonpeptide glycoprotein IIb/IIIa platelet receptor antagonist SM-20302 reduces tissue plasminogen activator-induced intracerebral hemorrhage after thromboembolic stroke. Stroke. 2002;33:147–152. doi: 10.1161/hs0102.100530. [DOI] [PubMed] [Google Scholar]
- 8.Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010;67:181–198. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lapchak PA, Araujo DM. Therapeutic potential of platelet glycoprotein IIb/IIIa receptor antagonists in the management of ischemic stroke. Am J Cardiovasc Drugs. 2003;3:87–94. doi: 10.2165/00129784-200303020-00002. [DOI] [PubMed] [Google Scholar]
- 10.De Meyer SF, Stoll G, Wagner DD, Kleinschnitz C. von Willebrand factor: an emerging target in stroke therapy. Stroke. 2012;43:599–606. doi: 10.1161/STROKEAHA.111.628867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Markus HS, McCollum C, Imray C, Goulder MA, Gilbert J, et al. The von Willebrand inhibitor ARC1779 reduces cerebral embolization after carotid endarterectomy: a randomized trial. Stroke. 2011;42:2149–2153. doi: 10.1161/STROKEAHA.111.616649. [DOI] [PubMed] [Google Scholar]
- 12.Mannucci PM. Platelet/von Willebrand factor inhibitors to the rescue of ischemic stroke. Arterioscler Thromb Vasc Biol. 2010;30:1882–1884. doi: 10.1161/ATVBAHA.110.212316. [DOI] [PubMed] [Google Scholar]
- 13.Bath PM, Blann A, Smith N, Butterworth RJ. Von Willebrand factor, P-selectin and fibrinogen levels in patients with acute ischaemic and haemorrhagic stroke, and their relationship with stroke sub-type and functional outcome. Platelets. 1998;9:155–159. doi: 10.1080/09537109876618. [DOI] [PubMed] [Google Scholar]
- 14.Liu Y, Jacobowitz DM, Barone F, McCarron R, Spatz M, et al. Quantitation of perivascular monocytes and macrophages around cerebral blood vessels of hypertensive and aged rats. J Cereb Blood Flow Metab. 1994;14:348–352. doi: 10.1038/jcbfm.1994.43. [DOI] [PubMed] [Google Scholar]
- 15.Montgomery R. Structure and Function of von Willebrand factor, in Hemostasis and Thrombosis : Basic Principles and Clinical Practice. USA: H.J. Lipponcott Williams and Wilkins press; 2001. [Google Scholar]
- 16.Savage B, Almus-Jacobs F, Ruggeri ZM. Specific synergy of multiple substrate-receptor interactions in platelet thrombus formation under flow. Cell. 1998;94:657–666. doi: 10.1016/s0092-8674(00)81607-4. [DOI] [PubMed] [Google Scholar]
- 17.Dong JF, Moake JL, Nolasco L, Bernardo A, Arceneaux W, et al. ADAMTS-13 rapidly cleaves newly secreted ultralarge von Willebrand factor multimers on the endothelial surface under flowing conditions. Blood. 2002;100:4033–4039. doi: 10.1182/blood-2002-05-1401. [DOI] [PubMed] [Google Scholar]
- 18.André P, Denis CV, Ware J, Saffaripour S, Hynes RO, et al. Platelets adhere to and translocate on von Willebrand factor presented by endothelium in stimulated veins. Blood. 2000;96:3322–3328. [PubMed] [Google Scholar]
- 19.Siedlecki CA, Lestini BJ, Kottke-Marchant KK, Eppell SJ, Wilson DL, et al. Shear-dependent changes in the three-dimensional structure of human von Willebrand factor. Blood. 1996;88:2939–2950. [PubMed] [Google Scholar]
- 20.Stoll G, Kleinschnitz C, Nieswandt B. Molecular mechanisms of thrombus formation in ischemic stroke: novel insights and targets for treatment. Blood. 2008;112:3555–3562. doi: 10.1182/blood-2008-04-144758. [DOI] [PubMed] [Google Scholar]
- 21.Kleinschnitz C, Pozgajova M, Pham M, Bendszus M, Nieswandt B, et al. Targeting platelets in acute experimental stroke: impact of glycoprotein Ib, VI, and IIb/IIIa blockade on infarct size, functional outcome, and intracranial bleeding. Circulation. 2007;115:2323–2330. doi: 10.1161/CIRCULATIONAHA.107.691279. [DOI] [PubMed] [Google Scholar]
- 22.Ni H, Denis CV, Subbarao S, Degen JL, Sato TN, et al. Persistence of platelet thrombus formation in arterioles of mice lacking both von Willebrand factor and fibrinogen. J Clin Invest. 2000;106:385–392. doi: 10.1172/JCI9896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kleinschnitz C, De Meyer SF, Schwarz T, Austinat M, Vanhoorelbeke K, et al. Deficiency of von Willebrand factor protects mice from ischemic stroke. Blood. 2009;113:3600–3603. doi: 10.1182/blood-2008-09-180695. [DOI] [PubMed] [Google Scholar]
- 24.Lapchak PA. Translational stroke research using a rabbit embolic stroke model: a correlative analysis hypothesis for novel therapy development. Transl Stroke Res. 2010;1:96–107. doi: 10.1007/s12975-010-0018-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turner RJ, Jickling GC, Sharp FR. Are underlying assumptions of current animal models of human stroke correct: from STAIRS to high hurdles? Transl Stroke Res. 2011;2:138–143. doi: 10.1007/s12975-011-0067-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lapchak PA, Araujo DM, Zivin JA. Comparison of Tenecteplase with Alteplase on clinical rating scores following small clot embolic strokes in rabbits. Exp Neurol. 2004;185:154–159. doi: 10.1016/j.expneurol.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 27.Zivin JA, Fisher M, DeGirolami U, Hemenway CC, Stashak JA. Tissue plasminogen activator reduces neurological damage after cerebral embolism. Science. 1985;230:1289–1292. doi: 10.1126/science.3934754. [DOI] [PubMed] [Google Scholar]
- 28.Lapchak PA. A clinically relevant rabbit embolic stroke model for acute ischemic stroke therapy development: mechanisms & targets, in Translational Stroke Research: From Target Selection to Clinical Trials. 2012:541–584. [Google Scholar]
- 29.Lapchak PA, Zhang JH, Noble-Haeusslein LJ. RIGOR Guidelines: Escalating STAIRS And STEPS For Effective Translational Research. Translational Stroke Research 2012. 2012 doi: 10.1007/s12975-012-0209-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lapchak PA, Wei J, Zivin JA. Transcranial infrared laser therapy improves clinical rating scores after embolic strokes in rabbits. Stroke. 2004;35:1985–1988. doi: 10.1161/01.STR.0000131808.69640.b7. [DOI] [PubMed] [Google Scholar]
- 31.Zivin JA, Lyden PD, DeGirolami U, Kochhar A, Mazzarella V, et al. Tissue plasminogen activator. Reduction of neurologic damage after experimental embolic stroke. Arch Neurol. 1988;45:387–391. doi: 10.1001/archneur.1988.00520280033012. [DOI] [PubMed] [Google Scholar]
- 32.Zivin JA, Waud DR. Quantal bioassay and stroke. Stroke. 1992;23:767–773. doi: 10.1161/01.str.23.5.767. [DOI] [PubMed] [Google Scholar]
- 33.Kawaguchi M, Furuya H, Patel PM. Neuroprotective effects of anesthetic agents. J Anesth. 2005;19:150–156. doi: 10.1007/s00540-005-0305-5. [DOI] [PubMed] [Google Scholar]
- 34.Waud DR. On biological assays involving quantal responses. J Pharmacol Exp Ther. 1972;183:577–607. [PubMed] [Google Scholar]
- 35.Frerichs KU, Kennedy C, Sokoloff L, Hallenbeck JM. Local cerebral blood flow during hibernation, a model of natural tolerance to "cerebral ischemia”. J Cereb Blood Flow Metab. 1994;14:193–205. doi: 10.1038/jcbfm.1994.26. [DOI] [PubMed] [Google Scholar]
- 36.Kageyama S, Yamamoto H, Nakazawa H, Matsushita J, Kouyama T, et al. Pharmacokinetics and pharmacodynamics of AJW200, a humanized monoclonal antibody to von Willebrand factor, in monkeys. Arterioscler Thromb Vasc Biol. 2002;22:187–192. doi: 10.1161/hq0102.101520. [DOI] [PubMed] [Google Scholar]
- 37.Lapchak PA, Han MK, Salgado KF, Streeter J, Zivin JA. Safety profile of transcranial near-infrared laser therapy administered in combination with thrombolytic therapy to embolized rabbits. Stroke. 2008;39:3073–3078. doi: 10.1161/STROKEAHA.108.516393. [DOI] [PubMed] [Google Scholar]
- 38.Arya M, Anvari B, Romo GM, Cruz MA, Dong JF, et al. Ultralarge multimers of von Willebrand factor form spontaneous high-strength bonds with the platelet glycoprotein Ib-IX complex: studies using optical tweezers. Blood. 2002;99:3971–3977. doi: 10.1182/blood-2001-11-0060. [DOI] [PubMed] [Google Scholar]
- 39.Hallenbeck JM. Blood-damaged tissue interaction in experimental brain ischemia. Acta Neurochir Suppl (Wien) 1994;60:233–237. doi: 10.1007/978-3-7091-9334-1_62. [DOI] [PubMed] [Google Scholar]
- 40.Montgomery RR, Kroner PA. von Willebrand disease: a common pediatric disorder. Pediatr Ann. 2001;30:534–540. doi: 10.3928/0090-4481-20010901-08. [DOI] [PubMed] [Google Scholar]
- 41.Okada Y, Copeland BR, Fitridge R, Koziol JA, del Zoppo GJ. Fibrin contributes to microvascular obstructions and parenchymal changes during early focal cerebral ischemia and reperfusion. Stroke. 1994;25:1847–1853. doi: 10.1161/01.str.25.9.1847. [DOI] [PubMed] [Google Scholar]
- 42.del Zoppo GJ, Mabuchi T. Cerebral microvessel responses to focal ischemia. J Cereb Blood Flow Metab. 2003;23:879–894. doi: 10.1097/01.WCB.0000078322.96027.78. [DOI] [PubMed] [Google Scholar]
- 43.Fisher M. Injuries to the vascular endothelium: vascular wall and endothelial dysfunction. Rev Neurol Dis. 2008;1:S4–S11. [PubMed] [Google Scholar]