Summary
Objective
To compare the population prevalence, inter-relationships, risk factor profiles and clinical characteristics of subsets of symptomatic hand osteoarthritis (OA) with a view to understanding their relative frequency and distinctiveness.
Method
1076 community-dwelling adults with hand symptoms (60% women, mean age 64.7 years) were recruited and classified into pre-defined subsets using physical examination and standardised hand radiographs, scored with the Kellgren & Lawrence (K&L) and Verbruggen–Veys grading systems. Detailed information on selected risk factors was obtained from direct measurement (Body Mass Index (BMI)), self-complete questionnaires (excessive use of hands, previous hand injury) and medical record review (hypertension, dyslipidaemia, type 2 diabetes). Hand pain and disability were self-reported at baseline and 3-year follow-up using Australian/Canadian Osteoarthritis Hand Index (AUSCAN).
Results
Crude population prevalence estimates for symptomatic hand OA subsets in the adult population aged 50 years and over were: thumb base OA (22.4%), nodal interphalangeal joint (IPJ) OA (15.5%), generalised hand OA (10.4%), non-nodal IPJ OA (4.9%), erosive OA (1.0%). Apart from thumb base OA, there was considerable overlap between the subsets. Erosive OA appeared the most distinctive with the highest female: male ratio, and the most disability at baseline and 3-years. A higher frequency of obesity, hypertension, dyslipidaemia, and metabolic syndrome was observed in this subset.
Conclusion
Overlap in the occurrence of hand OA subsets poses conceptual and practical challenges to the pursuit of distinct phenotypes. Erosive OA may nevertheless provide particular insight into the role of metabolic and cardiovascular risk factors in the pathogenesis of OA.
Keywords: Hand osteoarthritis, Epidemiology, Subsets, Erosive OA, Thumb base OA, Nodal OA
Introduction
Symptomatic hand osteoarthritis (OA) occurs in 13–26% of older adults1. Far from being ‘just a part of growing old’, individuals often perceive it as a serious condition, affecting their everyday lives, capable of causing persistent pain, interference with activities, and considerable frustration2.
Hand OA may not be a single disease but a number of subsets that include erosive OA, thumb base OA, interphalangeal joint (IPJ) OA (with or without nodes) and a widespread form involving all joint groups in the hands (generalised hand OA)3, 4. A EULAR Task Force called for further research to determine whether such possible subsets of hand OA are separate phenotypes with different risk factors and clinical outcomes3. Such evidence of discrete phenotypes could help advance our understanding of causal mechanisms, of the heterogeneous prognosis of hand OA5, 6, and of differential treatment response with a view to developing targeted interventions. Epidemiological studies can contribute evidence on the distinctiveness of hand OA subsets in the form of the relative frequencies, patterns of co-occurrence, risk factor profiles and clinical outcomes of posited subsets. Such evidence has already started to emerge.
Several studies sampling different populations have provided detailed prevalence estimates stratified by age and gender for erosive OA and thumb base OA7, 8, 9, 10, 11, 12, 13. Comprehensive comparisons of the relative frequencies of all hand OA subsets, including nodal and non-nodal IPJ OA, in a single population are rarer, with the recent publication of a range of prevalence estimates from the Framingham cohorts being exceptional7.
A number of studies have examined patterning of hand OA11, 14, 15, 16, 17, 18 showing patterns of co-occurrence to be polyarticular with clustering by row and symmetry14, 16. The co-occurrence of nodes in erosive OA has also been previously reported19, 20. A comprehensive examination of the inter-relationships across all the hand OA subsets would be beneficial to explore further the overlap between subsets.
Risk factors that have conclusively been associated with hand OA include older age, female gender and inheritance through a genetic component3. There is also mixed evidence for a number of possible exposures that include obesity, biomechanical forces through occupational and sporting activities and muscle strength as well ethnic background3 (though this may be related to different cultural practices that may be mechanical such as chopstick use21). There have been recent reports of an association between metabolic risk factors and OA22, 23 but the association with hand OA is not clear24, 25, 26. Conflicting evidence on risk factors for hand OA may partly reflect the use of case definitions for hand OA that combine different subsets with different risk factor profiles. To date, some risk factors are known to be associated with specific hand OA subsets: hypermobility and previous hysterectomy with thumb base OA vs IPJ OA27, 28; specific genetic factors with erosive OA vs non-erosive OA20, 29.
The clinical burden of erosive OA has been shown to be greater than non-erosive OA in a number of studies8, 19, 30, 31, 32 but the composition of the comparison non-erosive OA groups has been mixed. Additionally, the clinical burden of combined thumb base and IPJ OA has been found to be greater than thumb base or IPJ OA alone33. Thus far, no comprehensive comparison of clinical outcomes at baseline or over time has been made across all the possible hand OA subsets proposed by EULAR.
The present work sought to contribute to the aforementioned epidemiological studies on the distinctiveness of proposed EULAR hand OA subsets by estimating the prevalence, co-occurrence, risk factor profiles on selected mechanical and metabolic risk factors, clinical characteristics at baseline and 3-years for five symptomatic hand OA subsets (erosive OA, generalised hand OA, thumb base OA, nodal and non-nodal IPJ OA) in two samples of community-dwelling older adults drawn from comparable sampling frames in a single geographical region.
Methods
Study population
Participants were recruited from a prospective observational cohort study undertaken in North Staffordshire, UK between February 2004 and April 2005: the Clinical Assessment Study of the Hand (CASHA). All adults aged ≥50 years registered with two general practices were invited to participate at baseline in a two-stage self-report questionnaire survey34. In the UK over 95% of people are registered with general practices; thus providing convenient general population sampling frames35. Participants were not required to have consulted about their hand pain or hand problem. Those with hand pain or hand problems (e.g., stiffness or knobbly swellings) in the last 12 months were invited to research clinics that included an interview, physical examination and radiographs.
Participants reported the frequency of hand pain, aching or stiffness in the last month (no days, few days, some days, most days or all days). Those who reported symptoms on a few days or more were deemed symptomatic and eligible for inclusion in the analyses. Participants were excluded if General Practitioners or local Rheumatology hospital medical records or a musculoskeletal radiologist identified them as having inflammatory arthritis (rheumatoid arthritis, psoriatic arthritis). Those with no hand radiographs or missing radiographic scoring data were also excluded.
While population prevalence estimates of hand OA subsets were determined from the above CASHA participants this sample alone was not expected to provide sufficient numbers in some of the subsets to examine the inter-relationships, risk factor profiles and clinical characteristics. Therefore, the sample was enriched, a priori, from an identically performed survey in a similar population.
In the Clinical Assessment Study of the Knee (CASK) all adults aged ≥50 years registered with three general practices in North Staffordshire were invited to participate at baseline in the same two-stage self-report questionnaire survey36 between July 2002 and October 2003. All persons reporting knee pain were invited to attend research clinics however they all underwent an identical hand assessment and hand radiographs to those in the CASHA study. All individuals included in the analysis had hand pain on few days or more in the previous month. UK Local Research Ethics Committees approved these studies (LREC Project Nos: 1430, 05/Q2604/72, 06/Q2801/90). All participants provided written informed consent.
Data collection
Radiographic assessment and scoring
Posterior–anterior (PA) radiographs of the hands were taken with separate exposures for each hand according to a standardised protocol34, 36. The Kellgren & Lawrence (K&L) grading system37 was used by two trained readers (MM, JH) to grade OA in the IPJs and first carpometacarpal joints (CMCJ) in each hand. Intra-rater reliability for the presence of OA (K&L ≥ 2) in an individual joint was excellent (unweighted mean kappa = 0.92 & 0.85, mean percentage agreement = 98% & 98% for reader 1 & 2 respectively) and inter-rater reliability was moderate (unweighted mean kappa = 0.5, mean percentage agreement = 90%). The presence of erosive OA in the IPJ was determined using the Verbruggen–Veys Anatomical Phase Progression Score38 by an additional reader (WYK), intra-rater reliability was excellent (unweighted mean kappa 0.94, mean percentage agreement 98%).
Descriptive data
Demographic and socioeconomic data (age, gender, occupation, education) were collected in the baseline survey. At the research clinics the second and third distal and proximal IPJs were observed and palpated for the presence of nodes. Height and weight, from which Body Mass Index (BMI) was calculated, were also measured. In both studies knee radiographs (PA semi-flexed metatarsophalangeal, lateral and skyline views) were taken and scored for the presence of OA (K&L ≥ 2 on the PA and/or skyline views and/or definite osteophytes (Burnett grade ≥ 1) on the lateral view)34, 36.
Risk factor profiles
Participants reported previous hand injuries and excessive use of hands in employment or pastimes in the baseline survey34. A review of general practice consultations was undertaken for a 2-year period prior to clinic attendance for participants who gave permission (n = 1007, 94%). Participants with diagnoses or consultations for hypertension, type 2 diabetes or Impaired Fasting Glucose (IFG) and dyslipidaemia (raised cholesterol or triglycerides) or prescription of a lipid-regulating drug were identified. Metabolic syndrome was defined as the presence of three or more of the following: BMI >30 kg/m2, hypertension, dyslipidaemia and type 2 diabetes or IFG.
Clinical characteristics at baseline and 3-years
In the baseline survey participants completed the Australian/Canadian Osteoarthritis Hand Index (AUSCAN) subscales for Pain (0–20) and Function (0–36) on a five-point Likert scale39 and the aesthetics scale of the Michigan Hand Outcomes Questionnaire (MHQ) (0–100)40. Higher scores on the AUSCAN indicate more pain and functional limitation, whereas higher scores on the MHQ represented greater satisfaction with hand aesthetics. At research clinics power grip strength (Jamar dynamometer) and pinch strength (B&L pinch gauge) were obtained from a maximum trial in a standardised position. Consenting participants in both studies were followed-up at 3-years by postal questionnaire. Clinical outcomes at 3-years were the AUSCAN Pain and Function subscales.
OA definitions
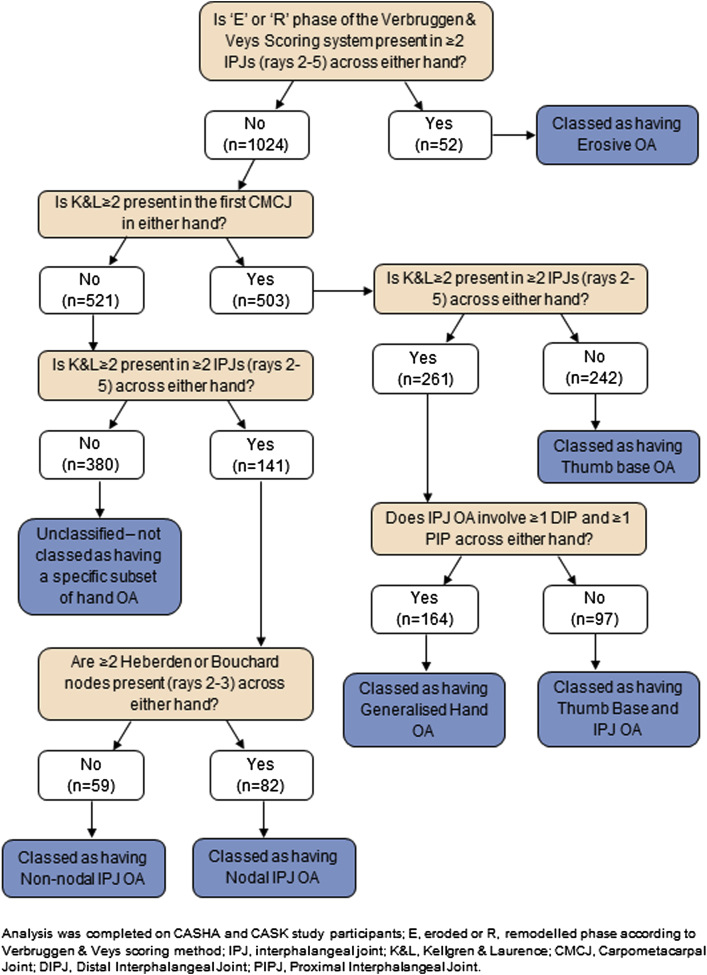
All individuals included in the analyses were symptomatic: defined as hand pain, aching or stiffness on a few days or more on the last month. The definitions used to define the hand OA subsets are displayed in Table I. For prevalence estimates, participants could belong in more than one subset if they satisfied the criteria. To permit comparisons between subsets of descriptive characteristics, risk factors and clinical characteristics, participants were then allocated to one subset based on an algorithm devised by the authors (Fig. 1). Some participants had IPJ and thumb base OA but did not satisfy the criteria for generalised hand OA (i.e., their IPJ OA was confined to only proximal or distal joints and not both). Individuals who did not satisfy the criteria for any hand OA subset were labelled “Unclassified” (n = 380); all were symptomatic but over half (n = 201) did not have either any radiographic OA (K&L ≥ 2) or any nodes on rays 2 & 3. The remaining unclassified participants had nodes without any radiographic OA (n = 108) and radiographic OA affecting a single IPJ with or without the presence of nodes (n = 71). There was no evidence that selected non-OA conditions (positive Phalen's test for carpal tunnel syndrome, Dupuytren's contracture, or trigger finger) were more common in the unclassified group supporting the interpretation that this group comprised predominantly sub-threshold hand OA.
Table I.
Population prevalence estimates of symptomatic hand OA subsets stratified by gender and age
| Phenotypes |
N | Erosive OA |
Generalised hand OA |
Thumb base OA |
IPJ OA∗ |
Nodal IPJ OA |
Non-nodal IPJ OA |
|---|---|---|---|---|---|---|---|
| Definition | E/R phase ≥2 IPJs (rays 2–5) across either hand | K&L ≥ 2 in ≥1 DIPJ & ≥1 PIPJ & ≥1 first CMCJ across either hand | K&L ≥ 2 in first CMCJ in either hand | K&L ≥ 2 in ≥2 IPJs (rays 2–5) across either hand | K&L ≥ 2 in ≥2 IPJs (rays 2–5) & ≥2 HN or BN (rays 2–3) across either hand | K&L ≥ 2 in ≥2 IPJs (rays 2–5) & <2 HN or BN (rays 2–3) across either hand | |
| Overall | 6306 | 1.0 (0.6, 1.5) | 10.4 (9.1, 11.7) | 22.4 (20.6, 24.1) | 20.4 (18.6, 22.2) | 15.5 (13.9, 17.1) | 4.9 (3.9, 5.9) |
| Men: | |||||||
| All ages (50–97) | 2973 | 0.3 (−0.1, 0.6)† | 6.5 (4.8, 8.3) | 16.7 (14.2, 19.2) | 15.2 (12.6, 17.7) | 9.6 (7.3, 11.8) | 5.6 (4.1, 7.0) |
| 50–54 | 506 | ‡ | ‡ | 9.8 (4.5, 15.2) | 8.1 (4.1, 12.2) | 4.7 (1.2, 8.2) | 3.4 (0.3, 6.6) |
| 55–59 | 695 | ‡ | 2.9 (0.4, 5.4) | 11.3 (7.3, 15.4) | 9.6 (5.4, 13.7) | 6.1 (2.8, 9.4) | 3.5 (0.8, 6.3) |
| 60–64 | 566 | ‡ | 5.1 (2.4, 7.9) | 16.8 (12.6, 21.0) | 15.9 (10.8, 21.0) | 9.1 (5.2, 13.0) | 6.8 (3.4, 10.1) |
| 65–69 | 434 | 0.6 (−0.5, 1.7)† | 8.5 (4.5, 12.6) | 18.8 (13.1, 24.6) | 18.7 (13.6, 23.7) | 11.2 (7.2, 15.2) | 7.5 (4.0, 11.0) |
| 70–74 | 323 | ‡ | 8.8 (3.4, 14.3) | 21.6 (15.0, 28.2) | 18.3 (11.9, 24.8) | 11.3 (4.7, 17.8) | 7.1 (3.0, 11.2) |
| 75–79 | 238 | ‡ | 10.9 (4.6, 17.1) | 21.8 (13.7, 30.0) | 20.1 (12.5, 27.6) | 14.0 (7.0, 21.0) | 6.1 (1.9, 10.3) |
| 80+ | 211 | ‡ | 16.2 (8.2, 24.2) | 27.4 (18.3, 36.4) | 25.4 (16.0, 34.7) | 19.8 (9.4, 30.3) | 5.5 (−0.3, 11.4)† |
| Women: | |||||||
| All ages (50–98) | 3333 | 1.7 (1.0, 2.4) | 13.8 (11.8, 15.9) | 27.4 (24.9, 29.8) | 25.1 (22.6, 27.5) | 20.8 (18.5, 23.1) | 4.3 (2.9, 5.6) |
| 50–54 | 539 | 0.9 (−0.3, 2.1)† | 4.4 (1.3, 7.5) | 16.2 (10.9, 21.6) | 12.9 (7.4, 18.4) | 9.8 (4.9, 14.7) | 3.1 (0.3, 5.9) |
| 55–59 | 694 | 1.1 (−0.1, 2.2)† | 8.6 (5.2, 12.1) | 21.7 (16.7, 26.6) | 18.9 (14.0, 23.8) | 14.9 (10.3, 19.6) | 4.0 (1.4, 6.5) |
| 60–64 | 517 | 1.5 (−0.0, 3.0)† | 9.5 (5.6, 13.5) | 24.7 (18.8, 30.5) | 22.8 (17.8, 27.8) | 18.9 (14.1, 23.6) | 3.9 (1.6, 6.2) |
| 65–69 | 442 | 1.8 (0.2, 3.4) | 15.1 (10.4, 19.7) | 30.0 (24.4, 35.6) | 29.5 (23.8, 35.3) | 24.7 (19.3, 30.1) | 4.8 (1.8, 7.8) |
| 70–74 | 402 | 3.2 (0.8, 5.6) | 20.8 (14.7, 26.8) | 34.4 (28.2, 40.5) | 32.4 (25.5, 39.2) | 27.4 (20.9, 33.8) | 5.0 (1.9, 8.0) |
| 75–79 | 319 | 2.4 (0.0, 4.8) | 22.0 (14.7, 29.4) | 36.7 (29.5, 44.0) | 34.1 (26.8, 41.4) | 28.9 (21.6, 36.2) | 5.2 (1.1, 9.2) |
| 80+ | 420 | 2.2 (−0.2, 4.6)† | 28.1 (20.0, 36.2) | 39.8 (31.7, 47.9) | 36.9 (29.2, 44.6) | 32.0 (23.8, 40.2) | 4.9 (−0.1, 9.9)† |
Population prevalence estimates were calculated from CASHA study participants. Figures are percentages (95% CI) unless otherwise specified.
IPJ OA (includes nodal IPJ OA and non-nodal IPJ OA).
Negative lower CIs are due to imputation of data.
Estimate could not be calculated as no participants were classified as having the subset in one or more imputed dataset; E/R, eroded or remodelled phase according to Verbruggen & Veys scoring method; DIPJ, Distal Interphalangeal Joint; PIPJ, Proximal Interphalangeal Joint; HN, Heberden node; BN, Bouchard node.
Fig. 1.
Classification of mutually exclusive groups (n = 1076).
Statistical analysis
The population prevalence of each subset was calculated from baseline CASHA study participants using a combined approach of multiple imputation and weighted logistic regression. Multiple imputations (dataset N = 20 generated using multivariate normal approach in STATA version 11.0 (Stata Corporation, TX, USA)) were used to estimate the prevalence of each subset in those participants who completed the survey but did not attend the research clinics. Variables in the imputation model were age, gender, general practice, social class, marital status, number of days in last year with hand pain, AUSCAN subscales, MHQ score, Short Form 12 scores41, Hospital Anxiety and Depression Scale42 and hand OA subset. Weighted logistic regression was used to adjust prevalence for participants' likelihood to return the initial postal questionnaire with weights based on age, gender and participants' general practice.
For the following analyses, the CASHA sample was enriched with CASK study participants who had hand symptoms as the combined sample would provide more precise estimates for the risk factors and clinical characteristics, particularly in rarer subsets. The inter-relationships between the different subsets were examined using an area-proportional Venn diagram. Descriptive statistics were used to compare the risk factor profiles and clinical characteristics at baseline and 3-years for each mutually exclusive subset. Additionally, Analysis of Variance (ANOVA) was used to test whether the mean clinical characteristics at baseline and 3-years varied by hand OA subset. Multinomial logistic regression was used to explore the association of each risk factor with hand OA subset after adjustment for age and gender (outcome = hand OA subset; reference category = the unclassified group). Results from the multinomial logistic regression are presented as adjusted relative risk ratios (RRR) and represent a ratio of the relative risk of being in each hand OA subset (compared to the reference category) for those with the risk factor of interest (numerator) compared to those without (denominator). Analyses were performed using IBM SPSS Statistics version 20 (SPSS, IL, USA) and STATA version 11.0 (Stata Corporation, TX, USA). All tests were two tailed and a P value of <0.05 was considered statistically significant.
Results
Study population
In total 15,396 adults aged 50 years and over from five general practices in North Staffordshire were mailed a two-stage survey from which CASHA and CASK study participants were recruited (see Supplementary Fig. 1 for full details of the recruitment process). In total 1167 participants with hand pain attended research clinics (CASHA n = 578, CASK n = 589). Even though only a small proportion of those surveyed attended research clinics the CASK sample has been shown to be representative of the symptomatic general population43 and similar analyses in CASHA resulted in the same conclusions (Supplementary Table I).
Following exclusions for inflammatory arthritis (n = 44), no hand X-rays (n = 6) and missing X-ray data (n = 41), 1076 participants were included in the analysis (CASHA n = 521, CASK n = 555), 60% female, mean age 64.8 years (SD 8.3, range 50–93). 963 participants were followed-up at 3-years (response rate 90%). Reasons for loss to follow-up included deaths (n = 32), exclusions by GP due to severe/terminal illness or dementia (n = 8), changed GP/address not traceable (n = 5), refusal to take part (n = 37) and non-response (n = 31). Loss to follow-up was slightly higher (19%) in those with erosive OA at baseline compared to the other subsets (9–12%) mainly due to more deaths (8% cf. 2–3%).
Population prevalence
Prevalence estimates were derived from CASHA participants (n = 521) and extrapolated to the wider survey population (n = 6306) through multiple imputation and weighted logistic regression. Thumb base OA (22.4%) was the most prevalent form of symptomatic hand OA, followed by nodal IPJ OA (15.5%), with erosive OA (1.0%) being least prevalent (Table I). The majority of IPJ OA was nodal. After adjusting for age, the prevalence of each hand OA subset (except non-nodal IPJ OA) was higher in women than in men (OR = 1.8–2.5) and was greatest for erosive OA (age-adjusted OR for female vs male 7.7; 95% Confidence Interval (95% CI): 1.3, 45.5). With the exception of non-nodal IPJ OA, the age-related rate of increase in prevalence of each subset was broadly linear, with little apparent difference between subsets or between men and women (data not shown).
Combining of CASHA and CASK participants
The CASHA sample was enriched with CASK study participants with hand symptoms. Supplementary Table II compares the characteristics of both samples. CASHA participants had a higher percentage of women, reported pain on most days and had radiographic OA affecting more than two hand joints slightly more often than CASK participants. The frequency of hand OA subsets was largely similar with CASHA participants having slightly more generalised hand OA, IPJ and thumb base OA, and slightly less who remained unclassified.
The following analyses were undertaken using data from combined CASHA and CASK cohorts.
Inter-relationships
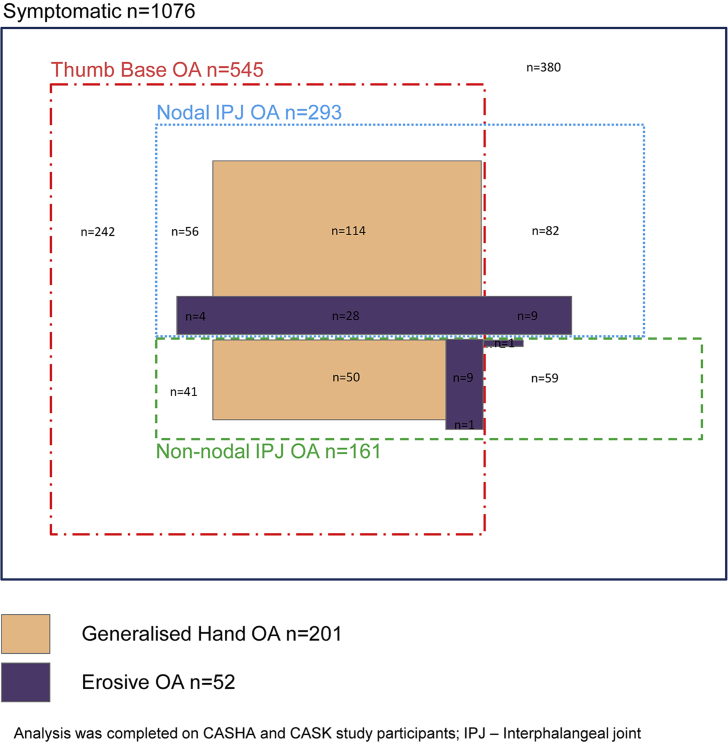
Isolated thumb base OA (n = 242, 22.5%) occurred more often than isolated nodal IPJ OA (n = 82, 7.6%) and non-nodal IPJ OA (n = 59, 5.5%) (Fig. 2). Generalised hand OA comprised a higher proportion of nodal IPJ OA (48%, n = 142/293) than non-nodal IPJ OA (37%, n = 59/161) or thumb base OA (37%, n = 201/545). The same pattern was evident for erosive OA (14% cf. 7% cf. 8% respectively).
Fig. 2.
Area-proportional Venn diagram showing the relative frequency and overlap of hand OA subsets in a symptomatic population (n = 1076).
Descriptive characteristics
Erosive OA and generalised hand OA were older, with higher proportions of females (Table II). Those with erosive OA had substantially more hand joints with radiographic OA than any other subset but interestingly did not have a higher rate of concurrent radiographic knee OA.
Table II.
Descriptive characteristics of the symptomatic hand OA subsets (n = 1076)
| Overall | Unclassified∗ | Erosive OA | Generalised hand OA | IPJ and thumb base OA | Thumb base OA | Nodal IPJ OA | Non-nodal IPJ OA | |
|---|---|---|---|---|---|---|---|---|
| Frequency in study population % (no.) | 100% (1076) | 35.3% (380) | 4.8% (52) | 15.2% (164) | 9.0% (97) | 22.5% (242) | 7.6% (82) | 5.5% (59) |
| Age, mean (s.d.) | 64.8 (8.3) | 60.9 (7.4) | 70.1 (8.1) | 69.6 (8.0) | 67.2 (7.0) | 65.1 (8.2) | 65.3 (7.1) | 65.5 (8.1) |
| % Female (no.) | 60.4% (650) | 52.9% (201) | 82.7% (43) | 73.2% (120) | 67.0% (65) | 55.0% (133) | 63.4% (52) | 61.0% (36) |
| BMI, mean (s.d.) | 29.1 (5.1) | 29.2 (4.9) | 28.7 (4.9) | 29.2 (5.2) | 29.5 (5.3) | 28.8 (5.0) | 30.0 (6.1) | 28.6 (4.4) |
| % Manual occupational class (no.) | 53.5% (540) | 58.0% (206) | 54.3% (25) | 51.6% (81) | 48.9% (44) | 48.2% (109) | 51.2% (41) | 61.8% (34) |
| % Attended higher education (no.) | 15.9% (167) | 14.0% (52) | 17.6% (9) | 14.6% (23) | 23.4% (22) | 16.0% (38) | 14.6% (12) | 18.6% (11) |
| % Pain on most or all days in the last month (no.) | 43.6% (469) | 35.8% (136) | 55.8% (29) | 45.7% (75) | 44.3% (43) | 49.6% (120) | 50.0% (41) | 42.4% (25) |
| % Radiographic hand OA (K&L ≥ 2 in ≥2 joints) (no.) | 61.9% (666) | 7.4% (28) | 100.0% (52) | 100.0% (164) | 100.0% (97) | 76.0% (184) | 100.0% (82) | 100.0% (59) |
| Number of joints K&L ≥ 2 (0–20), mean (s.d.) | 3.7 (4.1) | 0.4 (0.7) | 12.2 (4.1) | 9.0 (3.7) | 5.5 (1.6) | 2.3 (1.1) | 5.0 (3.1) | 4.3 (2.2) |
| Number of joints K&L ≥ 3 (0–20), mean (s.d.) | 1.4 (2.6) | <0.1 (0.2) | 8.8 (4.1) | 2.8 (3.0) | 2.0 (2.0) | 0.8 (0.9) | 1.1 (1.6) | 0.8 (1.4) |
| Number of joints K&L = 4 (0–20), mean (s.d.) | 0.6 (1.6) | <0.1 (0.1) | 5.3 (3.4) | 1.1 (1.7) | 0.7 (1.3) | 0.3 (0.6) | 0.4 (0.8) | 0.1 (0.4) |
| Total K&L score (0–80), mean (s.d.) | 10.8 (11.8) | 1.8 (2.2) | 39.8 (14.0) | 23.8 (10.3) | 15.4 (5.8) | 7.0 (3.4) | 13.4 (7.2) | 11.4 (6.3) |
| % Symptomatic radiographic knee OA (no.)† | 58.3% (595) | 56.4% (207) | 54.2% (26) | 65.6% (103) | 66.7% (60) | 54.6% (125) | 60.0% (45) | 52.7% (29) |
| % Symptomatic moderate–severe radiographic knee OA (no.)‡ | 29.3% (302) | 20.2% (74) | 37.5% (18) | 41.4% (65) | 40.0% (36) | 31.4% (72) | 30.7% (23) | 25.5% (14) |
Analysis was completed on CASHA and CASK study participants using mutually exclusive subsets as determined in Fig. 1.
Individuals in this sub-group are symptomatic with pain in the last month and may have some radiographic changes, but they do not meet the criteria for any of the hand OA subsets.
Symptomatic radiographic knee OA is knee pain in the last month and radiographic OA of tibiofemoral or patella–femoral joints of either knee (K&L ≥ 2 on PA and/or skyline views and/or posterior or lateral osteophytes ≥1 on lateral view).
Symptomatic moderate–severe radiographic knee OA is knee pain in the last month and moderate to severe radiographic OA of tibiofemoral or patella–femoral joints of either knee (K&L ≥ 3 on PA and/or skyline views and/or posterior or lateral osteophytes ≥3 on lateral view); s.d., standard deviation; data was missing for the following characteristics: BMI n = 2 (0.3%), manual occupational class n = 67 (6.2%), attended higher education n = 23 (2.1%), symptomatic radiographic knee OA n = 55 (5.1%) and symptomatic moderate–severe radiographic knee OA n = 55 (5.1%).
Risk factor profiles
No statistically significant differences between subsets were found in any of risk factors included in this study (Table III). Participants with erosive OA had the highest crude rates of obesity, hypertension, dyslipidaemia, and metabolic syndrome (Table III). However, due to small numbers within this subset, the strength of these associations could not be estimated precisely. In comparison to the unclassified group, and adjusted for age and gender, the rates for these metabolic factors were still elevated but statistically non-significant (Table III).
Table III.
Risk of selected exposures for subsets of symptomatic hand OA (n = 1076)
| Unclassified∗ | Erosive OA | Generalised hand OA | IPJ and thumb base OA | Thumb base OA | Nodal IPJ OA | Non-nodal IPJ OA | Likelihood ratio chi-square test | ||
|---|---|---|---|---|---|---|---|---|---|
| Previous hand injury | % (no.) | 28.8% (88) | 32.0% (16) | 24.8% (36) | 37.8% (31) | 34.1% (71) | 31.6% (24) | 31.4% (16) | |
| aRRR (95% CI) | 1 | 1.5 (0.7, 2.9) | 0.9 (0.6, 1.5) | 1.7 (1.0, 2.9) | 1.3 (0.9, 1.9) | 1.2 (0.7, 2.1) | 1.2 (0.6, 2.2) | χ2 = 6.4; P = 0.385 | |
| Excessive use of hands in employment or pastimes | % (no.) | 81.1% (258) | 84.0% (42) | 79.0% (120) | 86.9% (73) | 82.7% (177) | 85.5% (65) | 80.8% (42) | |
| aRRR (95% CI) | 1 | 1.7 (0.7, 4.0) | 1.1 (0.7, 2.0) | 1.9 (0.9, 3.9) | 1.2 (0.8, 2.0) | 1.6 (0.8, 3.2) | 1.1 (0.5, 2.3) | χ2 = 5.0; P = 0.540 | |
| BMI >30 kg/m2 | % (no.) | 37.5% (142) | 42.3% (22) | 39.0% (64) | 34.0% (33) | 33.9% (82) | 40.7% (33) | 37.3% (22) | |
| aRRR (95% CI) | 1 | 1.2 (0.7, 2.3) | 1.1 (0.7, 1.6) | 0.9 (0.5, 1.4) | 0.9 (0.6, 1.2) | 1.1 (0.7, 1.9) | 1.0 (0.6, 1.8) | χ2 = 2.7; P = 0.850 | |
| Diabetes/IFG | % (no.) | 6.3% (24) | 9.6% (5) | 13.4% (22) | 9.3% (9) | 7.0% (17) | 11.0% (9) | 5.1% (3) | |
| aRRR (95% CI) | 1 | 1.3 (0.4, 3.6) | 1.7 (0.9, 3.4) | 1.2 (0.5, 2.7) | 0.9 (0.5, 1.7) | 1.5 (0.7, 3.4) | 0.6 (0.2, 2.2) | χ2 = 6.1; P = 0.414 | |
| Hypertension | % (no.) | 26.3% (100) | 48.1% (25) | 40.9% (67) | 28.9% (28) | 35.1% (85) | 25.6% (21) | 32.2% (19) | |
| aRRR (95% CI) | 1 | 1.7 (0.9, 3.2) | 1.3 (0.9, 2.0) | 0.9 (0.5, 1.4) | 1.3 (0.9, 1.8) | 0.8 (0.5, 1.4) | 1.1 (0.6, 2.0) | χ2 = 8.2; P = 0.223 | |
| Dyslipidaemia | % (no.) | 21.1% (80) | 30.8% (16) | 20.1% (33) | 22.7% (22) | 25.2% (61) | 14.6% (12) | 23.7% (14) | |
| aRRR (95% CI) | 1 | 1.8 (0.9, 3.6) | 1.0 (0.6, 1.6) | 1.1 (0.6, 1.8) | 1.1 (0.8, 1.7) | 0.6 (0.3, 1.2) | 1.1 (0.6, 2.1) | χ2 = 7.0; P = 0.317 | |
| Metabolic syndrome† | % (no.) | 7.4% (28) | 15.4% (8) | 10.4% (17) | 7.2% (7) | 8.3% (20) | 6.2% (5) | 5.1% (3) | |
| aRRR (95% CI) | 1 | 1.7 (0.7, 4.1) | 1.1 (0.5, 2.2) | 0.8 (0.3, 1.8) | 0.9 (0.5, 1.7) | 0.7 (0.2, 1.8) | 0.5 (0.2, 1.9) | χ2 = 4.2; P = 0.650 |
Analysis was completed on CASHA and CASK study participants using mutually exclusive subsets as determined in Fig. 1; Likelihood ratio chi-square test indicates (overall) whether model fit was improved by having the risk factor in the model, statistical significance all of models were calculated to six degrees of freedom.
Individuals in this sub-group are symptomatic with pain in the last month and may have some radiographic changes, but they do not meet the criteria for any of the hand OA subsets.
Defined as three or more of the following factors: i) BMI >30 kg/m2, ii) diagnosis by general practitioner of diabetes or IFG, iii) hypertension, iv) dyslipidaemia (elevated triglycerides or cholesterol) or prescription of a lipid-regulating drug; aRRR, age/sex adjusted relative risk ratios; data was missing for the following exposures: previous hand injury n = 158 (14.7%), excessive use of hand in employment or pastimes n = 130 (12.1%), BMI >30 kg/m2n = 2 (0.2%) and metabolic syndrome n = 2 (0.2%).
Clinical characteristics at baseline and 3-years
At baseline, there were statistically significant differences between subsets in all clinical characteristics except AUSCAN Pain (Table IV). Individuals with erosive OA had more pain, disability, weaker grip and pinch strength and less satisfaction with hand appearance than those with other hand OA subsets. In contrast to the unclassified group who showed little or no change in pain and disability over 3-years, all symptomatic hand OA subsets showed small average increases in pain (0.5–1.5 points on AUSCAN Pain) and disability (1–3 points on AUSCAN Function). After adjusting for baseline scores, those with erosive OA still had the highest levels of disability at 3-years (Table V).
Table IV.
Clinical characteristics at baseline of the symptomatic hand OA subsets (n = 1076)
| Unclassified∗ | Erosive OA | Generalised hand OA | IPJ and thumb base OA | Thumb base OA | Nodal IPJ OA | Non-nodal IPJ OA | Difference between the groups (ANOVA) significance | |
|---|---|---|---|---|---|---|---|---|
| AUSCAN Pain (0–20), mean (95% CI) | 6.3 (5.9, 6.8) | 7.6 (6.4, 8.7) | 7.0 (6.4, 7.7) | 6.6 (5.7, 7.5) | 6.7 (6.1, 7.3) | 6.9 (6.0, 7.8) | 6.7 (5.6, 7.8) | P = 0.408 |
| AUSCAN Function (0–36), mean (95% CI) | 9.4 (8.5, 10.3) | 13.3 (11.1, 15.5) | 11.7 (10.4, 12.9) | 10.4 (8.6, 12.1) | 10.8 (9.7, 11.9) | 10.1 (8.4, 11.9) | 9.9 (7.8, 12.0) | P = 0.013 |
| Grip strength in lbs., mean (95% CI) | 59.1 (56.5, 61.8) | 35.6 (28.6, 42.6) | 45.0 (41.1, 49.0) | 47.6 (42.4, 52.8) | 52.3 (49.1, 55.6) | 49.3 (43.7, 54.9) | 54.5 (47.9, 61.1) | P < 0.001 |
| Pinch strength in lbs., mean (95% CI) | 11.8 (11.7, 12.2) | 8.2 (7.1, 9.3) | 9.6 (8.9, 10.2) | 9.7 (8.9, 10.5) | 10.4 (9.9, 10.9) | 10.5 (9.6, 11.4) | 10.3 (9.3, 11.4) | P < 0.001 |
| MHQ Appearance subscale (0–100), mean (95% CI) | 75.3 (73.0, 77.6) | 51.2 (45.4, 57.1) | 68.2 (64.9, 71.6) | 70.2 (65.7, 74.6) | 75.3 (72.5, 78.1) | 69.1 (64.4, 73.8) | 66.4 (60.7, 72.1) | P < 0.001 |
Analysis was completed on CASHA and CASK study participants using mutually exclusive subsets as determined in Fig. 1; Data are marginal means and 95% CIs estimated from the ANOVA model.
Individuals in this sub-group are symptomatic with pain in the last month and may have some radiographic changes, but they do not meet the criteria for any of the hand OA subsets. AUSCAN, Australian/Canadian Osteoarthritis Hand Index; data was missing for the following characteristics: AUSCAN Pain n = 130 (12.1%), AUSCAN Function n = 121 (11.2%), grip strength n = 10 (0.9%), pinch strength n = 20 (1.9%) and MHQ Appearance subscale n = 164 (15.2%).
Table V.
Clinical characteristics at 3-years for subsets of symptomatic hand OA (n = 963)
| Unclassified∗ | Erosive OA | Generalised hand OA | IPJ and thumb base OA | Thumb base OA | Nodal IPJ OA | Non-nodal IPJ OA | Difference between the groups (ANCOVA) significance | |
|---|---|---|---|---|---|---|---|---|
| AUSCAN Pain (0–20) | ||||||||
| Crude baseline mean score (95% CI) | 6.3 (5.8, 6.8) | 8.0 (6.7, 9.2) | 6.8 (6.2, 7.5) | 6.3 (5.4, 7.2) | 6.6 (6.0, 7.2) | 7.2 (6.2, 8.2) | 6.8 (5.6, 7.9) | P = 0.215 |
| Crude 3-years mean score (95% CI) | 6.2 (5.7, 6.7) | 8.9 (7.4, 10.3) | 7.6 (6.9, 8.4) | 7.5 (6.5, 8.5) | 7.1 (6.5, 7.8) | 8.3 (7.2, 9.4) | 7.2 (5.9, 8.5) | P < 0.001 |
| Adjusted 3-years mean score (95% CI)† | 6.6 (6.1, 7.0) | 8.0 (6.8, 9.2) | 7.7 (7.0, 8.3) | 8.0 (7.1, 8.8) | 7.1 (6.6, 7.7) | 8.0 (7.1, 8.9) | 7.4 (6.3, 8.5) | P = 0.006 |
| AUSCAN Function (0–36) | ||||||||
| Crude baseline mean score (95% CI) | 9.3 (8.4, 10.2) | 13.5 (11.0, 15.9) | 11.1 (9.8, 12.5) | 9.9 (8.1, 11.7) | 10.6 (9.5, 11.8) | 10.8 (8.9, 12.7) | 10.0 (7.8, 12.2) | P = 0.039 |
| Crude 3-years mean score (95% CI) | 9.5 (8.5, 10.4) | 16.5 (13.8, 19.2) | 12.2 (10.7, 13.6) | 11.6 (9.7, 13.5) | 11.5 (10.3, 12.8) | 12.5 (10.5, 14.5) | 10.8 (7.8, 12.2) | P < 0.001 |
| Adjusted 3-years mean score (95% CI)† | 10.6 (10.0, 11.3) | 13.9 (12.1, 15.6) | 11.9 (10.9, 12.9) | 12.6 (11.2, 13.9) | 11.3 (10.5, 12.2) | 12.3 (10.9, 13.6) | 11.4 (9.7, 13.0) | P = 0.007 |
Analysis was completed on CASHA and CASK study participants using mutually exclusive subsets as determined in Fig. 1; Data are marginal means and 95% CI estimated from the ANCOVA model, baseline mean scores in this table differ from those in Table IV as these are for the individuals who were followed-up at 3-years.
Individuals in this sub-group are symptomatic with pain in the last month and may have some radiographic changes, but they do not meet the criteria for any of the hand OA subsets.
Adjusted for baseline score; ANCOVA, Analysis of covariance; Data was missing for the following characteristics: AUSCAN Pain at baseline n = 117 (12.1%), AUSCAN Pain at 3-years n = 156 (16.2%), AUSCAN Function at baseline n = 1078 (11.1%) and AUSCAN Function at 3-years n = 146 (15.2%).
Discussion
Thumb base OA and IPJ OA, particularly the nodal form, were the most commonly occurring symptomatic hand OA subsets in a general population with the least frequent being erosive OA. The prevalence of each subset was higher in women than in men and the age-related rate of increase in prevalence was broadly linear. In addition, we have demonstrated the overlapping occurrence of these subsets among older adults with symptoms, and presented exploratory findings that appear to point to the potential distinctiveness of erosive OA, including its relation to metabolic risk factors, and greater functional decline.
Previous population prevalence estimates for specific hand OA subsets are heterogeneous due to variation in case definitions and genetic and environmental differences between populations44, 45. Accepting this, our estimates are mostly within the published ranges8, 9, 10, 11, 12, 13. For example, our age–sex stratified estimates of thumb base OA are similar to those reported in the national Mini-Finland study12, lower than those reported in the large Rotterdam and Zoetermeer studies9, 11, but higher than those in the Hizen-Oshima study10 (Table VI). The prevalence of symptomatic erosive OA in the current study was lower than that reported in the Rotterdam and Framingham general population cohorts7, 8 and two clinic based studies32, 46. This may be due to the setting as well as our decision to use a more stringent case definition that required the involvement of at least two IPJs and the presence of symptoms19, 47. With the exception of non-nodal IPJ OA, the prevalence of all hand OA subsets generally increased linearly with age and was similar for all subsets. This is consistent with Kalichman and Kobyliansky's finding of a cumulative linear increase with age in the number of joints affected by OA48. The higher preponderance among women of nodal IPJ OA, generalised hand OA, and, most strikingly, erosive OA, is in keeping with previous population studies7, 8, 11.
Table VI.
Comparison of population prevalence estimates of hand OA subsets from different studies
| Population | Erosive OA |
Thumb base OA |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| North Staffordshire, UK | Rotterdam, Netherlands8 | Framingham, USA7 | North Staffordshire, UK | Rotterdam, Netherlands11 | Copenhagen, Denmark13 | Zoetermeer, Netherlands9 | National, Finland12 | Hizen-Oshima, Japan10 | |
| Overall: | |||||||||
| 55–64 | – | 1.8 | – | – | 23.5 | – | – | – | – |
| 65–74 | – | 3.8 | – | – | 45.2 | – | – | – | – |
| 75–84 | – | 3.4 | – | – | 55.1 | – | – | – | – |
| 85+ | – | 3.6 | – | – | 51.9 | – | – | – | – |
| All ages | 1.0% (50+) | 2.8% (55+) | – | 22.4% (50+) | 35.8% (55+) | – | – | – | – |
| Men: | |||||||||
| 50–54 | – | – | 0∗ | 9.8% | – | 0.8 | 11.5 | – | – |
| 55–59 | – | – | 2.0∗ | 11.3% | – | 1.3 | 15.5 | 10.5∗ | |
| 60–64 | – | – | 2.0∗ | 16.8% | – | 2.6 | 20.8 | – | |
| 65–69 | 0.6% | – | 4.0∗ | 18.8% | – | 5.8 | 18.1 | 13.0∗ | |
| 70–74 | – | – | 10.0∗ | 21.6% | – | 9.6 | 23.5 | – | |
| 75–79 | – | – | 8.0∗ | 21.8% | – | 11.4 | 42.4 | 26.5∗ | |
| 80+ | – | – | 28.0∗ | 27.4% | – | 22.7 | 25.9 | – | |
| All ages | 0.3% (50+) | – | 3.6 (28+) | 16.7% (50+) | – | 4.0 (40+) | – | 7.0 (30+) | – |
| Women: | |||||||||
| 50–54 | 0.9% | – | 1.0∗ | 16.2% | – | 0 | 16.4 | – | 3.4 |
| 55–59 | 1.1% | – | 4.0∗ | 21.7% | – | 0 | 24.5 | 16.0∗ | |
| 60–64 | 1.5% | – | 11.0∗ | 24.7% | – | 4.5 | 34.5 | 7.0 | |
| 65–69 | 1.8% | – | 12.0∗ | 30.0% | – | 9.3 | 42.1 | 31.0∗ | |
| 70–74 | 3.2% | – | 30.0∗ | 34.4% | – | 15.2 | 46.7 | 20.5 | |
| 75–79 | 2.4% | – | 28.0∗ | 36.7% | – | 33.0 | 53.0 | 38.0∗ | |
| 80+ | 2.2% | – | 51.0∗ | 39.8% | – | 36.9 | 57.0 | 25.0 | |
| All ages | 1.7% (50+) | – | 9.8 (28+) | 27.4% (50+) | – | 7.4 (40+) | – | 15.0 (30+) | 10.2 (40+) |
Figures in brackets for all ages are the minimum age for each of the study populations.
Estimated from graphs provided in the publication.
By simultaneously describing the occurrence of different subsets, the degree of overlap becomes apparent. Although thumb base OA was the most prevalent subset to occur in isolation, as previously reported49, the majority of individuals with thumb base OA had IPJ OA and vice versa. Erosive OA occurs most often in those satisfying criteria for generalised hand OA and nodal IPJ OA, the latter of which is consistent with previous findings19, 20.
Erosive OA had a greater burden radiographic hand OA and moderate to severe radiographic hand OA which is consistent with the findings of previous studies29, 31, 50. Erosive OA has previously been found to be associated with subchondral bone attrition in the knees in comparison to individuals with no IPJ OA51. This feature is thought to be similar in appearance to central finger joint erosions51, has been shown to increase the risk of future cartilage loss52 and could be regarded as a relatively severe feature. In our analysis while the presence of any symptomatic radiographic knee OA was not increased in the erosive OA subset those with erosive OA did have one of the higher percentages of moderate to severe knee OA, particularly in comparison to the unclassified group.
Erosive OA had the poorest clinical characteristics and least satisfaction with their appearance at baseline and the most disability over a 3-year period compared to other subsets, even after adjustment for an already more severe baseline score. Our findings are consistent with previous studies, which reported more hand pain, disability and less satisfaction with aesthetics in individuals with erosive OA when compared to non-erosive or nodal OA8, 19, 30, 32, 47. While differences between erosive OA and other hand OA subsets exist, they may still form a common sequence of development with erosive OA marking the severe end of the continuum, rather than being a separate form of hand OA. Further exploration using longitudinal data is required to determine this.
The association of obesity with OA in non-weight-bearing joints may be indicative of a metabolic mechanism25, 26, 53. It has been postulated this may be through altered lipid metabolism and chronic inflammatory responses54, 55. These in turn are related to the pathogeneses of hypertension, dyslipidaemia and diabetes22, 56. In our study no differences were seen between the hand OA subsets for obesity, possibly because we measured BMI rather than more accurate measures of body fat like waist circumference57. A relatively higher frequency of dyslipidaemia, hypertension and metabolic syndrome was observed in those with erosive OA. Previous studies investigating associations between dyslipidaemia, hypertension and metabolic syndrome and OA have been based in a range of settings23, 58, 59, on very different case definitions22, 23, 59 and produced inconsistent findings. Two other population-based studies have found no association between hand OA and dyslipidaemia or hypertension when each risk factor was examined separately24, 26 however, the Rotterdam Study did find that the presence of multiple metabolic factors (BMI >27.4 kg/m2, diabetes and hypertension) was associated with hand OA26. We have extended the findings of Dahaghin et al.26 by investigating the association of metabolic syndrome with specific hand OA subsets. One further study reported increased frequency of obesity in individuals with erosive OA8. More severe hand OA, which may include those with erosive OA60 has also been associated in women with increased risk of carotid and coronary atherosclerosis61. Further longitudinal studies with careful control of potential confounding are needed.
A few methodological limitations should be considered when interpreting the findings in this paper. Although all individuals included in the current analysis had to have recent hand symptoms those from CASK who were recruited on the basis on having knee pain and may have a different, more widespread, form of OA. To determine if this affected the frequency of hand OA subsets we compared the proportion of each subset in CASHA and CASK participants and no excess was seen in the CASK participants. Hand OA subsets in this analysis were defined by the presence of radiographic OA alongside symptoms however, it should be noted that symptoms were classified as any hand pain, aching or stiffness in the last month and were not joint specific. Only nodes on rays 2 and 3 were included in the definition of nodal IPJ OA, as nodes on rays 4 and 5 were not assessed in the CASK study. Sensitivity analyses were carried out in the CASHA participants with the inclusion of nodes on rays 4 and 5. Population prevalence estimates of nodal IPJ OA slightly increased from 15.5 (13.9, 17.1) to 17.9 (16.2, 19.5) and non-nodal IPJ OA decreased from 4.9 (3.9, 5.9) to 2.8 (2.0, 3.6). The precision of estimates was reduced for the co-occurrence, risk factors and clinical characteristics but the findings were qualitatively unchanged. Certain potentially important risk factors were not examined, in particular family history, which have been associated with erosive OA62 and thumb base OA63 and hypermobility and subluxation have previously been associated with thumb base OA27, 64. Information on excessive use was not related to particular activities or specific hand joints and so provides limited insight into the relative contribution of local mechanical factors to different subsets of hand OA. This may explain the lack of differences observed in our study that would have been anticipated from previous findings65, 66, 67.
Our cross-sectional findings provide a population snapshot of the frequency, co-occurrence, and descriptive characteristics of symptomatic hand OA subsets. Additional information on retrospective exposures and prospective follow-up data was used to explore comparative risk profiles and clinical outcomes. Erosive OA, a relatively rare but severe form of hand OA, with poorer prognosis appears to provide the most distinctive phenotype. The possible association with dyslipidaemia, hypertension and metabolic syndrome, together with previous studies reporting a stronger association with obesity, suggest further studies of this subset may help disentangle the role of exposure to, and control of, metabolic and cardiovascular risk factors in the development and progression of hand OA.
Author contributions
Conception and design: MM, GP, EN, DvdW, HM, KD. Data acquisition: MM, GP, EN, HM, KD. Analysis and interpretation of the data: MM, GP, EN, DvdW. Drafting of the article: MM, GP, EN, DvdW, HM, KD. Final approval of the article: MM, GP, EN, DvdW, HM, KD.
Funding source
This work was supported by Programme Grants awarded by the Medical Research Council, UK (Grant Code: G9900220) and Arthritis Research UK (Grant Code: 18174) and NHS service support costs were provided by Support for Sciences funding secured from North Staffordshire Primary Care Consortium.
Role of funding source
The funders did not contribute to data collection, analysis or interpretation of the data, manuscript preparation or submission.
Conflict of interest
The authors have no conflicts of interest to declare.
Acknowledgements
The authors would like to acknowledge the contributions of Professor Peter Croft, Professor Elaine Hay, Dr Laurence Wood, Dr Elaine Thomas, June Handy, Charlotte Purcell, Catherine Tyson, Professor Chris Buckland-Wright and Professor Iain McCall for aspects of the conception and design of the study and the acquisition of data. Dr Jacqueline Saklatvala, Carole Jackson, Julia Matheson, Janet Wisher, Sandra Yates, Krystina Wallbank and Jean Bamford from the Department of Radiography, Haywood Hospital have contributed specifically to the acquisition of radiographs. We also wish to acknowledge June Handy for grading the CASK study radiographs for OA and Dr Wing-Yee Kwok, with the support of Dr Margreet Kloppenburg, who examined all the radiographs for presence of erosive OA. The authors would also like to thank the administrative and health informatics staff at the Arthritis Research UK Primary Care Centre at Keele University, as well as the staff and patients of the participating general practices.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.joca.2013.08.004.
Contributor Information
M. Marshall, Email: m.marshall@keele.ac.uk.
G. Peat, Email: g.m.peat@keele.ac.uk.
E. Nicholls, Email: e.nicholls@keele.ac.uk.
D. van der Windt, Email: d.van.der.windt@keele.ac.uk.
H. Myers, Email: h.l.myers@keele.ac.uk.
K. Dziedzic, Email: k.s.dziedzic@keele.ac.uk.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
Flow diagram showing recruitment of CASHA and CASK study participants.
References
- 1.Zhang Y., Niu J., Kelly-Hayes M., Chaisson C.E., Aliabadi P., Felson D.T. Prevalence of symptomatic hand osteoarthritis and its impact on functional status among the elderly: the Framingham Study. Am J Epidemiol. 2002;156:1021–1027. doi: 10.1093/aje/kwf141. [DOI] [PubMed] [Google Scholar]
- 2.Dziedzic K., Thomas E., Hill S., Wilkie R., Peat G., Croft P.R. The impact of musculoskeletal hand problems in older adults: findings from the North Staffordshire Osteoarthritis Project (NorStOP) Rheumatology (Oxford) 2007;46:963–967. doi: 10.1093/rheumatology/kem005. [DOI] [PubMed] [Google Scholar]
- 3.Zhang W., Doherty M., Leeb B.F., Alekseeva L., Arden N.K., Bijlsma J.W. EULAR evidence-based recommendations for the diagnosis of hand osteoarthritis: report of a task force of ESCISIT. Ann Rheum Dis. 2009;68:8–17. doi: 10.1136/ard.2007.084772. [DOI] [PubMed] [Google Scholar]
- 4.Kloppenburg M., Kwok W.Y. Hand osteoarthritis – a heterogeneous disorder. Nat Rev Rheumatol. 2011;8:22–31. doi: 10.1038/nrrheum.2011.170. [DOI] [PubMed] [Google Scholar]
- 5.Allen K.D., Jordan J.M., Renner J.B., Kraus V.B. Relationship of global assessment of change to AUSCAN and pinch and grip strength among individuals with hand osteoarthritis. Osteoarthritis Cartilage. 2006;14:1281–1287. doi: 10.1016/j.joca.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Botha-Scheepers S., Riyazi N., Watt I., Rosendaal F.R., Slagboom E., Bellamy N. Progression of hand osteoarthritis over 2 years: a clinical and radiological follow-up study. Ann Rheum Dis. 2009;68:1260–1264. doi: 10.1136/ard.2008.087981. [DOI] [PubMed] [Google Scholar]
- 7.Haugen I.K., Englund M., Aliabadi P., Niu J., Clancy M., Kvien T.K. Prevalence, incidence and progression of hand osteoarthritis in the general population: the Framingham Osteoarthritis Study. Ann Rheum Dis. 2011;70:1581–1586. doi: 10.1136/ard.2011.150078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwok W.Y., Kloppenburg M., Rosendaal F.R., van Meurs J.B., Hofman A., Bierma-Zeinstra S.M. Erosive hand osteoarthritis: its prevalence and clinical impact in the general population and symptomatic hand osteoarthritis. Ann Rheum Dis. 2011;70:1238–1242. doi: 10.1136/ard.2010.143016. [DOI] [PubMed] [Google Scholar]
- 9.van Saase J.L., van Romunde L.K., Cats A., Vandenbroucke J.P., Valkenburg H.A. Epidemiology of osteoarthritis: Zoetermeer survey. Comparison of radiological osteoarthritis in a Dutch population with that in 10 other populations. Ann Rheum Dis. 1989;48:271–280. doi: 10.1136/ard.48.4.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toba N., Sakai A., Aoyagi K., Yoshida S., Honda S., Nakamura T. Prevalence and involvement patterns of radiographic hand osteoarthritis in Japanese women: the Hizen-Oshima Study. J Bone Miner Metab. 2006;24:344–348. doi: 10.1007/s00774-006-0693-0. [DOI] [PubMed] [Google Scholar]
- 11.Dahaghin S., Bierma-Zeinstra S.M., Ginai A.Z., Pols H.A., Hazes J.M., Koes B.W. Prevalence and pattern of radiographic hand osteoarthritis and association with pain and disability (the Rotterdam Study) Ann Rheum Dis. 2005;64:682–687. doi: 10.1136/ard.2004.023564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haara M.M., Heliovaara M., Kroger H., Arokoski J.P., Manninen P., Karkkainen A. Osteoarthritis in the carpometacarpal joint of the thumb. Prevalence and associations with disability and mortality. J Bone Joint Surg Am. 2004;86:1452–1457. doi: 10.2106/00004623-200407000-00013. [DOI] [PubMed] [Google Scholar]
- 13.Sonne-Holm S., Jacobsen S. Osteoarthritis of the first carpometacarpal joint: a study of radiology and clinical epidemiology. Results from the Copenhagen Osteoarthritis Study. Osteoarthritis Cartilage. 2006;14:496–500. doi: 10.1016/j.joca.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 14.Egger P., Cooper C., Hart D.J., Coggon D., Spector T.D. Patterns of joint involvement in osteoarthritis of the hand: the Chingford Study. J Rheumatol. 1995;22:1509–1513. [PubMed] [Google Scholar]
- 15.Hirsch R., Guralnik J.M., Ling S.M., Fried L.P., Hochberg M.C. The patterns and prevalence of hand osteoarthritis in a population of disabled older women: the Women's Health and Aging Study. Osteoarthritis Cartilage. 2000;8:S16–S21. doi: 10.1053/joca.2000.0330. [DOI] [PubMed] [Google Scholar]
- 16.Niu J., Zhang Y., LaValley M., Chaisson C.E., Aliabadi P., Felson D.T. Symmetry and clustering of symptomatic hand osteoarthritis in elderly men and women: the Framingham Study. Rheumatology (Oxford) 2003;42:343–348. doi: 10.1093/rheumatology/keg110. [DOI] [PubMed] [Google Scholar]
- 17.Poole J., Sayer A.A., Hardy R., Wadsworth M., Kuh D., Cooper C. Patterns of interphalangeal hand joint involvement of osteoarthritis among men and women: a British cohort study. Arthritis Rheum. 2003;48:3371–3376. doi: 10.1002/art.11339. [DOI] [PubMed] [Google Scholar]
- 18.Marshall M., van der Windt D., Nicholls E., Myers H., Dziedzic K. Radiographic thumb osteoarthritis: frequency, patterns and associations with pain and clinical assessment findings in a community-dwelling population. Rheumatology (Oxford) 2011;50:735–739. doi: 10.1093/rheumatology/keq371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bijsterbosch J., Watt I., Meulenbelt I., Rosendaal F.R., Huizinga T.W., Kloppenburg M. Clinical burden of erosive hand osteoarthritis and its relationship to nodes. Ann Rheum Dis. 2010;69:1784–1788. doi: 10.1136/ard.2009.125435. [DOI] [PubMed] [Google Scholar]
- 20.Stern A.G., de Carvalho M.R., Buck G.A., Adler R.A., Rao T.P., Disler D. Association of erosive hand osteoarthritis with a single nucleotide polymorphism on the gene encoding interleukin-1 beta. Osteoarthritis Cartilage. 2003;11:394–402. doi: 10.1016/s1063-4584(03)00054-2. [DOI] [PubMed] [Google Scholar]
- 21.Hunter D.J., Zhang Y., Nevitt M.C., Xu L., Niu J., Lui L.Y. Chopstick arthropathy: the Beijing Osteoarthritis Study. Arthritis Rheum. 2004;50:1495–1500. doi: 10.1002/art.20145. [DOI] [PubMed] [Google Scholar]
- 22.Puenpatom R.A., Victor T.W. Increased prevalence of metabolic syndrome in individuals with osteoarthritis: an analysis of NHANES III data. Postgrad Med. 2009;121:9–20. doi: 10.3810/pgm.2009.11.2073. [DOI] [PubMed] [Google Scholar]
- 23.Yoshimura N., Muraki S., Oka H., Kawaguchi H., Nakamura K., Akune T. Association of knee osteoarthritis with the accumulation of metabolic risk factors such as overweight, hypertension, dyslipidemia, and impaired glucose tolerance in Japanese men and women: the ROAD study. J Rheumatol. 2011;38:921–930. doi: 10.3899/jrheum.100569. [DOI] [PubMed] [Google Scholar]
- 24.Bagge E., Bjelle A., Eden S., Svanborg A. Factors associated with radiographic osteoarthritis: results from the population study 70-year-old people in Göteborg. J Rheumatol. 1991;18:1218–1222. [PubMed] [Google Scholar]
- 25.Oliveria S.A., Felson D.T., Cirillo P.A., Reed J.I., Walker A.M. Body weight, body mass index, and incident symptomatic osteoarthritis of the hand, hip, and knee. Epidemiology. 1999;10:161–166. [PubMed] [Google Scholar]
- 26.Dahaghin S., Bierma-Zeinstra S.M., Koes B.W., Hazes J.M., Pols H.A. Do metabolic factors add to the effect of overweight on hand osteoarthritis? The Rotterdam Study. Ann Rheum Dis. 2007;66:916–920. doi: 10.1136/ard.2005.045724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jonsson H., Valtysdottir S.T., Kjartansson O., Brekkan A. Hypermobility associated with osteoarthritis of the thumb base: a clinical and radiological subset of hand osteoarthritis. Ann Rheum Dis. 1996;55:540–543. doi: 10.1136/ard.55.8.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spector T.D., Hart D.J., Brown P., Almeyda J., Dacre J.E., Doyle D.V. Frequency of osteoarthritis in hysterectomized women. J Rheumatol. 1991;18:1877–1883. [PubMed] [Google Scholar]
- 29.Ramonda R., Musacchio E., Campana C., Frigato M., Barbieri V., Piccoli A. Immunogenetic aspects of erosive osteoarthritis of the hand in patients from northern Italy. Scand J Rheumatol. 2011;40:139–144. doi: 10.3109/03009742.2010.507216. [DOI] [PubMed] [Google Scholar]
- 30.Pattrick M., Aldridge S., Hamilton E., Manhire A., Doherty M. A controlled study of hand function in nodal and erosive osteoarthritis. Ann Rheum Dis. 1989;48:978–982. doi: 10.1136/ard.48.12.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olejarova M., Kupka K., Pavelka K., Gatterova J., Stolfa J. Comparison of clinical, laboratory, radiographic, and scintigraphic findings in erosive and nonerosive hand osteoarthritis. Results of a two-year study. Joint Bone Spine. 2000;67:107–112. [PubMed] [Google Scholar]
- 32.Wittoek R., Cruyssen B.V., Verbruggen G. Predictors of functional impairment and pain in erosive osteoarthritis of the interphalangeal joins: comparison with controlled inflammatory arthritis. Arthritis Rheum. 2012;64:1430–1436. doi: 10.1002/art.33502. [DOI] [PubMed] [Google Scholar]
- 33.Bijsterbosch J., Visser W., Kroon H.M., Stamm T., Meulenbelt I., Huizinga T.W. Thumb base involvement in symptomatic hand osteoarthritis is associated with more pain and functional disability. Ann Rheum Dis. 2010;69:585–587. doi: 10.1136/ard.2009.104562. [DOI] [PubMed] [Google Scholar]
- 34.Myers H., Nicholls E., Handy J., Peat G., Thomas E., Duncan R. The Clinical Assessment Study of the Hand (CAS-HA): a prospective study of musculoskeletal hand problems in the general population. BMC Musculoskelet Disord. 2007;8:85. doi: 10.1186/1471-2474-8-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bowling A. 3rd edn. Open University Press; Maidenhead: 2009. Research Methods in Health: Investigating Health and Health Sciences; pp. 194–196. [Google Scholar]
- 36.Peat G., Thomas E., Handy J., Wood L., Dziedzic K., Myers H. The Knee Clinical Assessment Study – CAS(K). A prospective study of knee pain and knee osteoarthritis in the general population. BMC Musculoskelet Disord. 2004;5:4. doi: 10.1186/1471-2474-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lawrence J.S. In: Rheumatism in Populations. Lawrence J.S., editor. William Heinemann Medical Books; London: 1977. Osteo-arthrosis; pp. 98–155. [Google Scholar]
- 38.Verbruggen G., Veys E.M. Numerical scoring systems for the anatomic evolution of osteoarthritis of the finger joints. Arthritis Rheum. 1996;39:308–320. doi: 10.1002/art.1780390221. [DOI] [PubMed] [Google Scholar]
- 39.Bellamy N., Campbell J., Haraoui B., Buchbinder R., Hobby K., Roth J.H. Dimensionality and clinical importance of pain and disability in hand osteoarthritis: development of the Australian/Canadian (AUSCAN) Osteoarthritis Hand Index. Osteoarthritis Cartilage. 2002;10:855–862. doi: 10.1053/joca.2002.0837. [DOI] [PubMed] [Google Scholar]
- 40.Chung K.C., Pillsbury M.S., Walters M.R., Hayward R.A. Reliability and validity testing of the Michigan Hand Outcomes Questionnaire. J Hand Surg Am. 1998;23:575–587. doi: 10.1016/S0363-5023(98)80042-7. [DOI] [PubMed] [Google Scholar]
- 41.Ware J., Jr., Kosinski M., Keller S.D. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 42.Zigmond A.S., Snaith R.P. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 43.Peat G., Thomas E., Handy J., Wood L., Dziedzic K., Myers H. The Knee Clinical Assessment Study – CAS(K). A prospective study of knee pain and knee osteoarthritis in the general population: baseline recruitment and retention at 18-months. BMC Musculoskelet Disord. 2006;7:30. doi: 10.1186/1471-2474-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pereira D., Peleteiro B., Araujo J., Branco J., Santos R.A., Ramos E. The effect of osteoarthritis definition on prevalence and incidence estimates: a systematic review. Osteoarthritis Cartilage. 2011;19:1270–1285. doi: 10.1016/j.joca.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 45.Yoshida S., Aoyagi K., Felson D.T., Aliabadi P., Shindo H., Takemoto T. Comparison of the prevalence of radiographic osteoarthritis of the knee and hand between Japan and the United States. J Rheumatol. 2002;29:1454–1458. [PubMed] [Google Scholar]
- 46.Maheu E., Michon M., Carrat F., Berenbaum F. Erosive versus non-erosive hand OA: prospective cross-sectional comparison of clinical data. Ann Rheum Dis. 2008;67:94. [Google Scholar]
- 47.Hodkinson B., Maheu E., Michon M., Carrat F., Berenbaum F. Assessment and determinants of aesthetic discomfort in hand osteoarthritis. Ann Rheum Dis. 2012;71:45–49. doi: 10.1136/ard.2011.153965. [DOI] [PubMed] [Google Scholar]
- 48.Kalichman L., Li L., Kobyliansky E. Prevalence, pattern and determinants of radiographic hand osteoarthritis in Turkmen community-based sample. Rheumatol Int. 2009;29:1143–1149. doi: 10.1007/s00296-008-0815-1. [DOI] [PubMed] [Google Scholar]
- 49.Mannoni A., Briganti M.P., Di Bari M., Ferrucci L., Serni U., Masotti G. Prevalence of symptomatic hand osteoarthritis in community-dwelling older persons: the ICARe Dicomano study. Osteoarthritis Cartilage. 2000;8:S11–S13. doi: 10.1053/joca.2000.0328. [DOI] [PubMed] [Google Scholar]
- 50.Smith D., Braunstein E.M., Brandt K.D., Katz B.P. A radiographic comparison of erosive osteoarthritis and idiopathic nodal osteoarthritis. J Rheumatol. 1992;19:896–904. [PubMed] [Google Scholar]
- 51.Haugen I.K., Felson D.T., Englund M., Wang K., Aliabadi P., Guermazi A. The association between erosive hand osteoarthritis and subchondral bone attrition of the knee: the Framingham Osteoarthritis Study. Ann Rheum Dis. 2012;71:1698–1701. doi: 10.1136/annrheumdis-2012-201659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Neogi T., Felson D., Niu J., Lynch J., Nevitt M., Guermazi A. Cartilage loss occurs in the same subregions as subchondral bone attrition: a within-knee subregion-matched approach from the Multicenter Osteoarthritis Study. Arthritis Rheum. 2009;61:1539–1544. doi: 10.1002/art.24824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grotle M., Hagen K.B., Natvig B., Dahl F.A., Kvien T.K. Obesity and osteoarthritis in knee, hip and/or hand: an epidemiological study in the general population with 10 years follow-up. BMC Musculoskelet Disord. 2008;9:132. doi: 10.1186/1471-2474-9-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Loeser R.F. Systemic and local regulation of articular cartilage metabolism: where does leptin fit in the puzzle? Arthritis Rheum. 2003;48:3009–3012. doi: 10.1002/art.11315. [DOI] [PubMed] [Google Scholar]
- 55.Metcalfe A., Harte A.L., Aletrari M.O., Al Daghri N.M., Al Disi D., Tripathi G. Does endotoxemia contribute to osteoarthritis in obese patients? Clin Sci (Lond) 2012;123:627–634. doi: 10.1042/CS20120073. [DOI] [PubMed] [Google Scholar]
- 56.Bastard J.P., Maachi M., Lagathu C., Kim M.J., Caron M., Vidal H. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006;17:4–12. [PubMed] [Google Scholar]
- 57.van Dijk S.B., Takken T., Prinsen E.C., Wittink H. Different anthropometric adiposity measures and their association with cardiovascular disease risk factors: a meta-analysis. Neth Heart J. 2012;20:208–218. doi: 10.1007/s12471-011-0237-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lawrence J.S. Hypertension in relation to musculoskeletal disorders. Ann Rheum Dis. 1975;34:451–456. doi: 10.1136/ard.34.5.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sturmer T., Sun Y., Sauerland S., Zeissig I., Gunther K.P., Puhl W. Serum cholesterol and osteoarthritis. The baseline examination of the Ulm Osteoarthritis Study. J Rheumatol. 1998;25:1827–1832. [PubMed] [Google Scholar]
- 60.Cobby M., Cushnaghan J., Creamer P., Dieppe P., Watt I. Erosive osteoarthritis: is it a separate disease entity? Clin Radiol. 1990;42:258–263. doi: 10.1016/s0009-9260(05)82114-2. [DOI] [PubMed] [Google Scholar]
- 61.Jonsson H., Helgadottir G.P., Aspelund T., Eiriksdottir G., Sigurdsson S., Ingvarsson T. Hand osteoarthritis in older women is associated with carotid and coronary atherosclerosis: the AGES Reykjavik study. Ann Rheum Dis. 2009;68:1696–1700. doi: 10.1136/ard.2008.096289. [DOI] [PubMed] [Google Scholar]
- 62.Bijsterbosch J., van Bemmel J.M., Watt I., Meulenbelt I., Rosendaal F.R., Huizinga T.W. Systemic and local factors are involved in the evolution of erosions in hand osteoarthritis. Ann Rheum Dis. 2011;70:326–330. doi: 10.1136/ard.2010.138230. [DOI] [PubMed] [Google Scholar]
- 63.Min J.L., Meulenbelt I., Riyazi N., Kloppenburg M., Houwing-Duistermaat J.J., Seymour A.B. Association of matrilin-3 polymorphisms with spinal disc degeneration and osteoarthritis of the first carpometacarpal joint of the hand. Ann Rheum Dis. 2006;65:1060–1066. doi: 10.1136/ard.2005.045153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hunter D.J., Zhang Y., Sokolove J., Niu J., Aliabadi P., Felson D.T. Trapeziometacarpal subluxation predisposes to incident trapeziometacarpal osteoarthritis (OA): the Framingham study. Osteoarthritis Cartilage. 2005;13:953–957. doi: 10.1016/j.joca.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 65.Lawrence J.S. Rheumatism in cotton operatives. Br J Ind Med. 1961;18:270–276. doi: 10.1136/oem.18.4.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Solovieva S., Vehmas T., Riihimӓki H., Takala E.P., Murtomaa H., Luoma K. Finger osteoarthritis and differences in dental work tasks. J Dent Res. 2006;85:344–348. doi: 10.1177/154405910608500412. [DOI] [PubMed] [Google Scholar]
- 67.Fontana L., Neel S., Claise J.M., Ughetto S., Catilina P. Osteoarthritis of the thumb carpometacarpal joint in women and occupational risk factors: a case-control study. J Hand Surg Am. 2007;32:459–465. doi: 10.1016/j.jhsa.2007.01.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Flow diagram showing recruitment of CASHA and CASK study participants.