Abstract
Social exclusion and risk-taking are both common experiences of concern in adolescence, yet little is known about how the two may be related at behavioral or neural levels. In this fMRI study, adolescents (N=27, 14 male, 14–17 years-old) completed a series of tasks in the scanner assessing risky decision-making before and after an episode of social exclusion. In this particular context, exclusion was associated with greater behavioral risk-taking among adolescents with low self-reported resistance to peer influence (RPI). When making risky decisions after social exclusion, adolescents who had lower RPI exhibited higher levels of activity in right temporoparietal junction (rTPJ), and this response in rTPJ was a significant mediator of the relationship between RPI and greater risk-taking after social exclusion. Lower RPI was also associated with lower levels of activity in lPFC during crashes following social exclusion, but unlike rTPJ this response in lPFC was not a significant mediator of the relationship between RPI and greater risk-taking after social exclusion. The results suggest that mentalizing and/or attentional mechanisms have a unique direct effect on adolescents’ vulnerability to peer influence on risk-taking.
Social interactions take on increased importance in adolescence (Crone & Dahl, 2012) and often provide the context in which teens make decisions to engage in risky behaviors such as substance use, health-risking sexual behavior, and reckless driving (Dishion & Owen, 2002; La Greca et al., 2001; Simons-Morton et al., 2005). One particularly powerful and distressing form of social interaction is exclusion or rejection by peers (Williams, 2007), which can negatively affect individual and interpersonal behavior through decreased self-regulation (Baumeister et al., 2005), aggression (Ayduk et al., 2008), and self-defeating actions (Twenge et al., 2002). Although previous neuroimaging studies have examined risky decisions and associated neural processes in adolescence (Bjork et al., 2007; Burnett et al., 2010; van Leijenhorst et al., 2010) little is currently known about the neural mechanisms relating social exclusion and subsequent risk-taking behavior. This study employed functional magnetic resonance imaging (fMRI) to examine the behavioral and neural consequences of social exclusion on risky decision-making.
1.1 Neuroimaging and adolescent risk-taking
Biological factors contributing to adolescent risky decision-making have been explored using neuroimaging methods in combination with tasks that examine reward processing and cognitive control. For example, reward sensitivity (specifically, reactivity during anticipation of rewards) typically exhibits a non-linear trajectory that peaks in adolescence relative to childhood and adulthood (Ernst et al., 2005; Galvan et al., 2006; Galvan et al., 2010; Geier et al., 2010; Somerville et al., 2011; Van Leijenhorst et al., 2010), although there are some exceptions to this pattern (e.g., Bjork et al., 2004). Further, greater neural responses in the ventral striatum (VS) during reward anticipation is associated with more drug use (Bjork et al., 2011), and responses in nucleus accumbens to reward outcomes are stronger in teens with externalizing disorders that are often associated with risk behavior (Bjork et al., 2010). In addition, adolescents can show decreased neural activity in cognitive regulatory structures such as lateral prefrontal cortex (lPFC) during risk decisions compared to adults (e.g., Eshel et al., 2007), but the evidence for this pattern is mixed (Crone & Dahl, 2012).
Based on these results and findings from animal models of adolescence (Spear, 2011), imbalances between the maturation rates of cortical and subcortical regions respectively associated with cognitive control and reward have been proposed as an explanation for heightened adolescent risk-taking (Casey et al., 2011; Ernst et al., 2009; Somerville et al., 2010; Steinberg, 2008; Steinberg, 2010). These models suggest that approach or reward-seeking systems develop earlier in adolescence than avoidance or control systems, resulting in an imbalance that leads to heightened sensitivity to reward cues and insufficient cognitive control. However, outstanding methodological questions and results inconsistent with these models (Blakemore & Robbins, 2012; Johnson et al., 2009; Pfeifer & Allen, 2012; Romer et al., 2010) suggest that additional factors play a role in adolescent risky decision-making.
1.2 Social exclusion and adolescent risk-taking
Rejection, exclusion, and ostracism are all associated with various forms of negative behavior (Williams, 2007). However, these terms can refer to different types of experiences, ranging from chronic rejection over time to a single episode of exclusion (Leary et al., 2005). Within the developmental literature on peer relations, the term peer rejection generally refers to the social status of a child based on sociometric methods (i.e., peer nominations of children that are least liked), and represents cumulative effects of negative social treatment by peers (Dodge et al., 2003; Coie et al., 1992). Longitudinal studies link chronic peer rejection in childhood with increased risk-taking during adolescence in forms such as externalizing behavior, truancy, substance use, and association with deviant peers (Dishion, Capaldi, Spracklen, & Li, 1995; Prinstein & La Greca, 2004). Other approaches alternately use the terms rejection, exclusion, or ostracism to refer to an event in which an individual is left out of a group or denied participation in some activity (Williams, 2007). In this manuscript, we use the term social exclusion to denote a single event or episode, and we use the term rejection in the more general sense of a person or group indicating that they do not value a personal relationship (Leary et al., 2005). Repeated experiences of rejection by peers, such as those experienced by youth receiving a “rejected” sociometric status using peer nomination techniques, are referred to here by the term chronic peer rejection.
Single experiences of social exclusion have been linked to decreased self-regulation (Baumeister et al., 2005; DeWall et al., 2008), poor health choices (Oaten et al., 2008), and taking irrational, self-defeating risks (Twenge et al., 2002). In addition, reactions to social exclusion can include aggression (Ayduk et al., 2008; Twenge et al., 2007), attempts to affiliate (Maner et al., 2007), or negative social actions (Carter-Sowell et al., 2008; Mallott et al., 2009), any of which could conceivably take the form of risk-taking behavior in peer contexts. That is, teens with threatened or unmet social needs might engage in risky activities or behaviors as a way to interact with or gain the recognition of peers. It is also possible that adolescents might respond to exclusion with risky behavior as a way of establishing a non-conforming identity, in effect “rejecting the rejectors” (Sampson & Laub, 1997). Factors affecting the emotional magnitude and specific behavioral reactions to exclusion can vary by individual and context (Molden et al., 2009; Smart Richman & Leary, 2009). Adolescents with poor social skills or low self-esteem may be more likely to experience rejection (Leary et al., 1995) and may be more emotionally affected than less vulnerable teens (Prinstein & Aikins, 2004). More generally, susceptibility to peer influence varies by individual, and differences in the ability to resist peer influence are significant predictors of real-world risk behavior (Monahan et al., 2009; Steinberg & Monahan, 2007).
1.3 Neuroimaging of mentalizing in adolescence
A growing body of neuroimaging research examines the systems supporting various facets of adolescent social cognition that are relevant to peer relationships and social influences on decision-making (Pfeifer & Blakemore, 2012). One particularly relevant facet of adolescent social cognition that has been examined using fMRI is mentalizing, or the ability to apprehend others’ mental states such as thoughts or feelings, and to use this information to understand others’ behavior (Frith & Frith, 2007). According to a recent meta-analysis (Van Overwalle & Baetens, 2009), multiple complementary neural systems have been implicated in mentalizing. One set of regions, composed of temporoparietal junction (TPJ) and the cortical midline structures (CMS) including medial prefrontal and posterior parietal cortices (mPFC, mPPC) is responsive to more abstract representations of the thoughts and perspectives of others (Uddin et al., 2007).
A relatively consistent pattern in this line of inquiry is that adolescents exhibit enhanced reactivity in mPFC during mentalizing, relative to adults (Blakemore, 2008; Blakemore et al., 2007; Blakemore et al., 2010; Burnett et al., 2008; Gunther Moor et al., 2012; Pfeifer et al., 2009; Pfeifer & Blakemore, 2012; van den Bos et al., 2011; Wang et al., 2006). Another emerging developmental pattern is a linear increase in TPJ responses during mentalizing (Gweon et al., 2012; van den Bos et al., 2011). Prior work in our laboratory suggests that adolescents and adults also utilize TPJ to ascertain what others (parents, friends, and peers) think specifically about one’s self (Pfeifer et al., 2009), and that adolescents engage in this reflective perspective-taking even when they are not prompted to do so. Taken together, this research suggests that when exploring peer influences on adolescent decision-making, it may be profitable to consider not only the VS and lPFC responses that are associated with risk decisions, but also the potential contribution of mentalizing responses in TPJ and CMS (mPFC and mPPC).
A recent and highly relevant study examining peer influence on decision-making, for example, concluded that the presence of peers during risk decisions heightened responses in VS and orbitofrontal cortex (OFC) more for adolescents than adults (Chein et al., 2011). The degree to which responses in VS increased under peer influence was inversely related to self-reported resistance to peer influence. Adults engaged lPFC more than adolescents, but this was not impacted by peer presence. One interpretation of these findings is in that particular context, peer influences on risk-taking in adolescence might be mediated by heightened VS and OFC responses that represent enhanced reward sensitization, rather than diminished cognitive control. Complicating this view, however, are studies in which adolescents exhibit decreased VS response to some reward conditions (Geier et al., 2010; van Leijenhorst et al., 2010; and Bjork et al., 2010), or increased VS activity is associated with more adaptive functioning such as increased resistance to peer influence and decreased risky behavior (Pfeifer et al., 2011). Collectively, these results suggest the existence of additional mechanisms for peer influence on decision-making during adolescence that vary according to the kind of social context experienced.
1.4 Current study
To examine the neural mechanisms underlying the effects of social exclusion on risk decisions in adolescence, the current study combines a behavioral measure of risk-taking (the Stoplight task; Gardner & Steinberg, 2005) with a manipulation producing an experience of social exclusion (the Cyberball game; Williams et al., 2000). The Stoplight task features a series of intersections at which subjects must decide whether to stop for a yellow traffic light (safe option) or try to make it through the intersection (risk option). While the risk option often results in a faster time, it is accompanied by the possibility of crashing and losing time if another car crosses the intersection. The social aspect of the study comes from the presence and actions of two hypothetical peers (implied to be watching the participant via Internet connection). After being trained on the task by playing five rounds alone (to eliminate learning effects), subjects first complete the Stoplight task while the peers are watching, then play the Stoplight task again after an experience of being excluded from a different game by the peers. During the second Stoplight task, the subject is being watched by the same peers that just excluded them. This manipulation creates an additional layer of risk decision factors representing the subject’s expected social evaluation of his or her performance by the peers, above and beyond the risk decisions of the task.
Although previous work has examined the influence of peers on risk decisions using neuroimaging techniques (Chein et al., 2011), to our knowledge, no prior study has examined the effect of social exclusion on risk-taking. We predicted that social exclusion would have a significant behavioral effect on risk-taking, and would also be associated with patterns of brain activity (during risk decisions and/or when receiving feedback about decision outcomes) that significantly differ from those associated with simple peer presence. These differences may be expressed in our a priori regions of interest derived from the above literature review: VS, OFC, lateral PFC, CMS (mPFC and mPPC), and TPJ. In addition, we suspected the effects of exclusion on risk-taking behavior and associated brain activity might be influenced by individual differences in factors relating to adolescent responses to interpersonal relationships, specifically resistance to peer influence.
Methods
2.1 Participants
Twenty-seven adolescents participated in the experiment. Seven subjects were excluded, for various reasons: five for behavioral data lost due to software error, one for excessive movement that resulted in an unusable structural scan, and one due to scanner mechanical malfunction. Therefore, a total of 20 participants (10 girls, 10 boys, 14.0–16.8 years old, M = 15.3, SD = 0.8) were included in the following analyses. All subjects were right-handed and reported no history of neurological or psychiatric disorders and no MRI contraindications. All parents of participants provided written consent, and adolescent participants provided written assent, approved by the University of Oregon Institutional Review Board. Following the study, all adolescent participants were debriefed and received monetary compensation.
2.2 Task descriptions
The Stoplight task is a computerized driving task in which subjects must cross a series of traffic intersections (Chein et al., 2011; Gardner & Steinberg, 2005) as quickly as possible to reach a party being held in the distance by their friends. At each cross-street, subjects were presented with a traffic light that turned yellow at varying distances from the intersection and had to decide to either stop the car or try to make it through the intersection before the light turned red (see Figure 1). No steering or accelerating options were possible. A Stop decision resulted in a 3 second delay while the subject’s car waited for the light to turn from red to green, resulting in a relatively slow overall time for the trial. A Go decision yielded a faster time for the trial with no stopping or waiting, but also carried the risk of crashing if a car was approaching from the cross street. Crashing resulted in a 6 second delay, making the time slower than if the subject had stopped for the red light. The subjects were presented with their overall time and the number of crashes at the end of each round. The probability of crashing was kept constant at 30% (i.e., three intersections out of every ten had cars approaching on the cross street, resulting in a crash if the subject made a Go decision), but this was not explicitly revealed to subjects. Timing of the onset of the yellow and red lights and the presence of a car on the cross street varied; parameters were randomized within five canonical round sequences and the order of rounds was randomized for each subject. Pilot sessions for this study, and previous studies using the Stoplight task (Chein et al., 2011), found learning effects in the form of higher behavioral risk performance during initial rounds of the task followed by decreased and more stable risk patterns thereafter. To reduce these presumed learning effects, five practice rounds were included to allow risk performance to reach stable levels. These rounds were completed with no peers or experimental personnel present to establish a measure of baseline risk prior to the social exclusion manipulation. Practice rounds featured ten trials, each of which required a Stop or Go decision. Rounds in subsequent Peer and Exclusion conditions (see descriptions below) featured 30 trials of the same duration, light timing, and crash probability parameters as the practice rounds. The increased number of trials in Peer and Exclusion conditions was chosen to ensure adequate numbers of Stop and Go decision data points for statistical modeling. Total round time varied by subject decisions and outcomes, but was approximately 6 minutes per round for peer and exclusion conditions.
Figure 1.
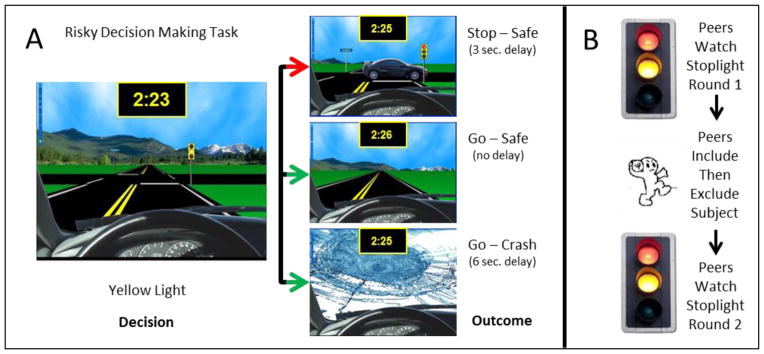
Task design and protocol sequence.
Panel A depicts the Stoplight task and the possible decisions and outcomes. Panel B depicts the overall protocol sequence.
The social exclusion manipulation employed the Cyberball game (Williams et al., 2000) which creates the subjective experience of being excluded using a computerized ball-toss game played by the subject and two peers ostensibly connected via the Internet. The subject viewed three computer figures representing the subject and the two peers (which were portrayed to be the same two individuals throughout all aspects of the experiment), and played a virtual game involving tossing a ball to each other. Unknown to the subject, the first round was programmed to result in equal throws, creating an inclusion condition in which every player receives the ball approximately 1/3 of the 25 total throws. The second round was programmed to include the subject for the first 1–2 throws, after which the peers throw only to each other, creating an exclusion condition in which the subject feels left out of the game.
2.3 Task sequence
Participants were informed that they would be playing several online games with two other adolescents who were located in different states (i.e., not peers that they would likely know from their city). In order to “meet” the other teens, the subjects recorded a brief video profile that consisted of the subject stating his or her name and an activity that they enjoyed. Typical profile statements were “Hi, my name is Julie and I like to play volleyball” or “Hey, I’m Matt and I like skateboarding.” Video recordings were then uploaded to a shared Internet file folder while subjects watched and were told that each participant would meet the other two by viewing the profiles during the fMRI session. Subjects then received instructions and demonstration of the two computer tasks (Stoplight task and Cyberball game).
After completing five practice rounds of the Stoplight task with no peers watching, subjects viewed the video profiles of two same-age adolescent peers (one male and one female), and were told that they would be interacting with these peers via computer desktop sharing software connected over the Internet. Subjects were then told that each participant would complete the Stoplight task while the other two participants watched. To facilitate the cover story, the subject was asked to confirm that they could see and hear the remote connection of each peer and was required to wait to begin their session until similar confirmation was received from the remote peers. The driving behavior of the peers was programmed to represent average to slightly above average performance. One peer completed the course with average risk (number of go decisions) and average performance (number of crashes and course time), while the other peer completed the course with slightly above-average risk and performance. Both performance levels were selected based on pilot testing results to be within the normal range of participant performance. After viewing each of the remote peer sessions, the subject completed the Stoplight task while being watched by these same remote peers (pre-exclusion condition). Next, subjects completed separate inclusion and exclusion rounds of the Cyberball game, ostensibly with the same peers. Following the Cyberball game, subjects completed the Stoplight task again and were told that their performance was still being watched, again by the same peers that previously excluded them from the Cyberball game (post-exclusion condition). All tasks except initial creation of the video profile were conducted in the scanner, but only fMRI data from the pre and post exclusion Stoplight tasks are analyzed or discussed here. Manipulation checks following the scanning procedure indicated that only 1 of the 20 subjects explicitly expressed disbelief that they were interacting with real peers, while 90% either believed they were interacting with real peers or were not certain (16 believed completely, 2 indicated they “weren’t sure,” 1 participant did not believe the peers were real, and 1 participant declined to respond to the question).
2.4 Questionnaires and surveys
Immediately following the scanner session, subjects completed the Need-Threat Scale (NTS), a 12-item self-report measure that assesses the extent to which social needs are satisfied, including items that measure subjects’ feelings of reduced self-esteem, belongingness, social control, and meaningful existence (Williams et al., 2000). Items such as “I felt rejected” were measured on a 5-point scale ranging from 1 = “not at all” to 5 = “very much so” with half of the items reverse-scored. The total NTS score is the average of all item scores, with lower scores reflecting less social need satisfaction (i.e., more threat to social needs). NTS scores ranged from 1.33 to 3.92 (M = 2.91, SD = 0.68).
Subjects also completed the 10-item Resistance to Peer Influence (RPI) scale, which measures the degree to which adolescents are influenced by the views and opinions of peers (Steinberg & Monahan, 2007). The items present subjects with two statements reflecting contradictory positions on aspects of peer influence (e.g., Some people take more risks when they are with their friends than they do when they are alone - BUT - other people act just as risky when they are alone as when they are with their friends). Subjects first choose which statement describes the person they are most like then decide whether the statement is “sort of true for them” or “really true for them.” Items are scored on a scale of 1 to 4, with lower scores representing less resistance to peer influence (i.e., more susceptibility to the opinions of peers), and some items are reverse-coded. The total RPI score is the computed average of all item scores. RPI scores ranged from 1.80 to 3.50 (M = 2.99, SD = 0.47).
Finally, real-world risk-taking behavior was assessed by a self-report measure of substance use, affiliation with deviant peers, and antisocial behavior originally developed and used by the Oregon Research Institute (Metzler et al., 2001) and subsequently employed in several longitudinal studies tracking adolescent risk behavior (e.g., Stormshak et al., 2011). Substance use was defined by 8 items for cigarette, alcohol, marijuana, and other drug use in the last 30 days. Deviant peer affiliation was gauged by 19 items for the number of times in the last month the subject was with friends who engaged in antisocial behavior, for example carrying a weapon, stealing, illegal substance use, fighting, or who got arrested. Antisocial behavior was defined using 11 items that assessed the number of times in the last month that the subject lied to their parents, damaged property, stole, panhandled, or got in fights. Item response scales are similar to those used in the Youth Risk Behavior Surveillance System questionnaire, which monitors health-risk behaviors in a national sample of adolescents and young adults (Eaton et al., 2010). Scales of risk behaviors engaged in by the subject during the last 30 days range from “never” to “more than 20 times.” Individual item scores were aggregated to create a composite of risk-taking (CRT) across substance use, deviant peer affiliation, and antisocial behavior. CRT scores ranged from 0 to 2.36 (M = 0.43, SD = 0.58).
2.5 fMRI data acquisition
MRI data were acquired on a 3.0 Tesla Allegra head-only scanner (Siemens, Erlangen, Germany) at the Robert and Beverly Lewis Center for NeuroImaging at the University of Oregon. Blood oxygen-level dependent, echo-planar images (BOLD-EPI) were acquired with T2*-weighted gradient echo sequence (TE = 30 ms, TR = 2000 ms, flip angle = 80°, 64 × 64 voxel matrix, 200 mm field of view, bandwidth = 2605 Hz/pixel, 32 contiguous axial slices with interleaved acquisition, slice thickness = 4 mm, and in-plane resolution of 3.125 × 3.125 mm). This sequence also prospectively corrected for motion during acquisition using PACE (Thesen et al., 2000). The first 2 scans were discarded to allow scanner magnetization to reach equilibrium. A total of 224 scans were collected during each of two functional runs. High-resolution structural scans were acquired using an inversion recovery T1-weighted 3D MP-RAGE pulse sequence (TE = 4.38 ms, TR = 2500 ms, TI = 1100 ms, flip angle = 8°, 256 × 192 voxel matrix, 256 × 192 rectangular field of view, bandwidth = 130 Hz/pixel, 160 contiguous axial slices coplanar to the functional scans, slice thickness = 1 mm, and in-plane resolution of 1 × 1 mm). Prior to each run, field map scans were acquired to obtain magnetization values used to correct for field inhomogeneity (TE = 4.99 ms, TR = 500 ms, flip angle = 55°, 64 × 64 voxel matrix, 200 mm field of view, bandwidth = 1530 Hz/pixel, 32 contiguous axial slices with interleaved acquisition, slice thickness = 4 mm, and in-plane resolution of 3.125 × 3.125 mm). Computer images for the tasks were projected from an LCD display onto a mirror above the subject’s eyes. Behavioral responses were acquired using a button box interfaced with task software.
2.6 fMRI data analysis
DICOM images were converted to NIfTI format via MRIConvert (http://lcni.uoregon.edu/~jolinda/MRIConvert/) and non-brain tissue was removed using FSL’s Brain Extraction Tool (Smith, 2002). Voxel displacement maps were generated to correct for field inhomogeneities using the FieldMap toolkit, and then used to unwarp and realign functional images in SPM8 (Wellcome Department of Cognitive Neurology, London, United Kingdom). Anatomical images were coregistered to the mean functional image, then segmented into six tissue types using the unified segmentation approach (Ashburner & Friston, 2005). DARTEL was used to create a group anatomical template, transformations from which were applied to warp functional data to the ICBM-152 template supplied with SPM8 (Ashburner, 2007). Normalized data were smoothed using a 6-mm FWHM Gaussian kernel.
In each participant’s fixed-effects analysis, a general linear model (GLM) was created with four regressors of interest, modeled as events: two decision regressors (Stop and Go) and two outcome regressors (Crash and NoCrash). The yellow light preceding a given decision (Stop or Go) served as the onset for that decision. The onset of the Crash event corresponded to another car crashing into the participant’s car. The NoCrash events had no obvious onset time. However, because the Crash events happened at most 2 seconds after the yellow light, we modeled the NoCrash event as being 2 seconds after the yellow light as well, the point at which the outcome of the risky decision was clear. A fifth regressor was used for the “Game Over” period at the end of each run, lasting from its onset until the end of the run. This was done to remove possible feedback effects from viewing the game score from the implicit baseline condition, which could alter contrasts comparing our conditions of interest with the implicit baseline. Six additional motion parameters, derived from the online parameters compiled during acquisition correction, were also used as regressors of no interest. A high-pass filter of 128 seconds was applied to eliminate low-frequency fluctuations in the signal, and AR(1) was used to correct for serial autocorrelations. The model was convolved with the canonical hemodynamic response function, and the parameter estimates resulting from the GLM were used to create linear contrast images for each of four event conditions (decisions: Go, Stop and outcomes: Crash, NoCrash), in which each event was compared to an implicit baseline during which participants were “driving”, but not making any decisions or receiving any feedback, representing a high-level control condition. These fixed-effects contrast images were then entered into subsequent random-effects analyses. Monte Carlo simulations were conducted using AlphaSim (implemented in Neuroelf) to determine the minimum cluster size needed for an FWE rate of .05, given a voxel-wise threshold of p = .005. As such, results are reported at p < .005, uncorrected for magnitude, and an extent threshold of 19–23 or more contiguous voxels (depending on the analysis; precise thresholds are noted in figures and tables). We also used MarsBaR (http://marsbar.sourceforge.net/) to extract parameter estimates for significant clusters in the group-level analyses, for correlations with questionnaires and survey measures.
Results
3.1 Behavioral data
Risk-taking was defined as making a Go decision, expressed as a percentage of trials in the run. Risky decisions were found to increase following the social exclusion manipulation, but this effect failed to reach statistical significance (t(19) = 1.84, two-tailed p = .08). The degree of increase in risk-taking was negatively correlated with resistance to peer influence (RPI) scores (r(18) = −.55, p = .01; see Figure 2), revealing that adolescents with greater susceptibility to peer influence displayed larger increases in risky decisions after being socially excluded by peers. Neither social need satisfaction as assessed by the Need-Threat Scale (NTS), nor the composite of real-world risk-taking behavior (CRT), were significantly related to changes in risk decisions after social exclusion (rs(18) = −.14 and .21, respectively, ns). As expected, the RPI was significantly negatively correlated with the CRT (r(18) = −.50, p = .024). The NTS was also significantly correlated with the RPI (r(18) = .51, p = .021), indicating that adolescents with less resistance to peer influence also experienced lower levels of social need satisfaction after social exclusion. NTS was not significantly correlated with the CRT (r(18) = −.27, ns).
Figure 2.
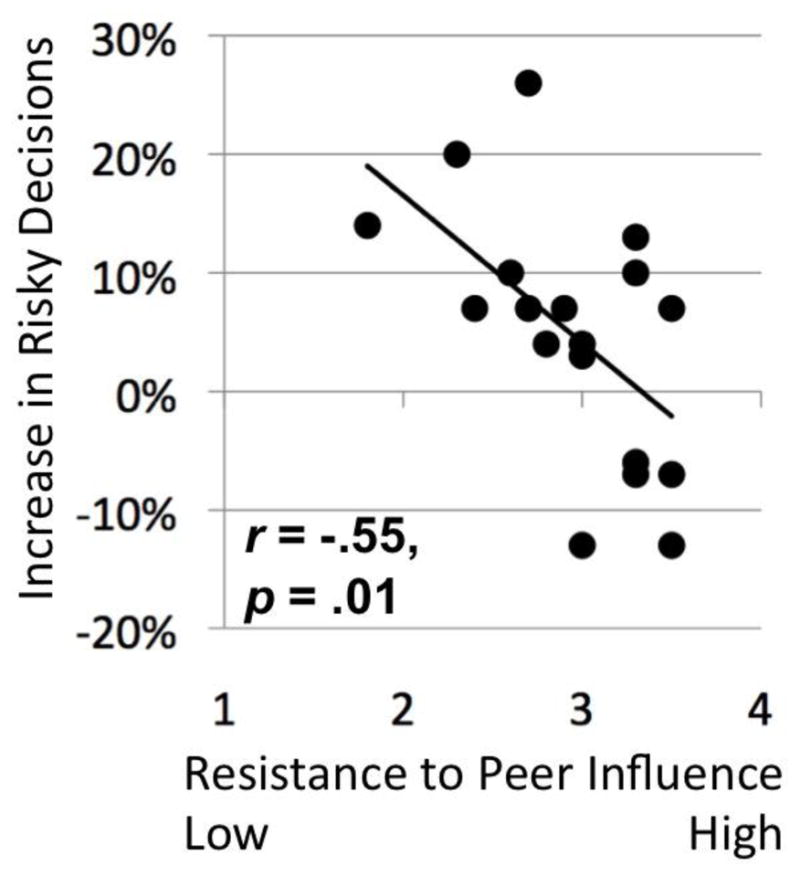
Resistance to peer influence and behavioral risk-taking after social exclusion.
Adolescents with low resistance to peer influence took significantly more risks following social exclusion than adolescents with high resistance to peer influence.
3.2 fMRI data: Decisions and outcomes
To confirm that the neural responses elicited by the Stoplight task were consistent with those observed in prior decision-making studies, we first examined main effects of decision type (collapsed across pre- and post-exclusion conditions), using one-sample t-tests. As expected, making a safe decision (Stop > Go) was associated with greater activity in right lateral PFC (see Table 1), specifically in the inferior frontal gyrus – a region frequently associated with self-regulation (Aron et al., 2004; Cohen, et al., 2013). In addition, making a risky decision (Go > Stop) elicited responses in the ventral striatum (VS), a region consistently implicated in reward motivation (Knutson et al., 2008) and specifically responsive during Go decisions in a prior study using this paradigm (Chein et al., 2011). A robust response during Stop decisions was also found in primary sensory regions including temporal lobe (bilateral superior temporal gyrus) and occipital lobe (lingual gyrus), which likely reflect the auditory and visual effects of the car coming to a stop (i.e., the sound of tires squealing, the light turning red, and sudden cessation of movement). A similar but more pronounced pattern of activation was found during Crash outcomes, most likely due to the auditory and visual effects of the car crash (see Table 1). Note that all events in a given condition were compared to a baseline within that same run during which participants were “driving”, but not making any decisions or receiving any feedback about outcomes, representing a high-level control condition.
Table 1.
Main Effects of Decisions and Outcomes
| MNI
|
|||||
|---|---|---|---|---|---|
| Area | t | k | x | y | z |
| Go Decisions > Stop Decisions | |||||
| Right superior frontal gyrus | 5.01 | 67 | 18 | −3 | 51 |
| Right ventral striatum (caudate) | 4.48 | 145 | 6 | 9 | −3 |
| Left ventral striatum (lentiform nucleus) | 4.25 | 27 | −12 | 0 | −9 |
| Left insula | 4.23 | 30 | −36 | 12 | 9 |
| Left superior frontal gyrus | 4.18 | 43 | −12 | 0 | 63 |
|
| |||||
| Stop Decisions > Go Decisions | |||||
| Right superior temporal gyrus | 9.81 | 2004 | 48 | −48 | 15 |
| Right middle temporal gyrus | 8.98 | 469* | 54 | −3 | −18 |
| Left inferior frontal gyrus | 8.30 | 421* | −48 | 36 | 6 |
| Left postcentral gyrus | 7.89 | 127* | −57 | −6 | 18 |
| Left superior temporal gyrus | 7.66 | 656* | −60 | −21 | 3 |
| Left declive | 7.53 | 925* | −36 | −54 | −18 |
| Left culmen | 7.44 | 123* | −21 | −45 | −21 |
| Left middle temporal gyrus | 7.31 | 543* | −51 | −45 | 6 |
| Left middle frontal gyrus | 7.29 | 90* | −42 | 36 | −6 |
| Precuneus | 6.55 | 982* | −3 | −54 | 36 |
| Left fusiform gyrus | 6.39 | 104* | −21 | −63 | −9 |
| Left dorsomedial prefrontal cortex | 6.25 | 102* | −12 | 30 | 42 |
| Left inferior temporal gyrus | 5.93 | 45* | −60 | −24 | −15 |
| Right culmen | 5.90 | 138* | 24 | −33 | −27 |
| Left middle frontal gyrus | 5.60 | 99* | −27 | 21 | 54 |
| Right lingual gyrus | 5.35 | 74* | 15 | −48 | 0 |
| Right declive | 5.33 | 23* | 36 | −60 | −18 |
| Left temporoparietal junction (supramarginal gyrus) | 5.04 | 66* | −51 | −54 | 39 |
| Cuneus | 4.77 | 29* | −6 | −84 | 18 |
| Right inferior frontal gyrus | 6.30 | 803 | 45 | 27 | 15 |
| Left medial prefrontal cortex | 5.45 | 159 | −12 | 60 | 15 |
| Right medial frontal gyrus | 5.44 | 124 | 6 | −18 | 57 |
| Left medial frontal gyrus | 5.14 | 60* | −6 | −27 | 60 |
| Right dorsomedial prefrontal cortex | 4.30 | 37 | 15 | 30 | 42 |
| Right inferior semi-lunar lobule | 3.92 | 54 | 15 | −78 | 36 |
| Right postcentral gyrus | 3.90 | 38 | 51 | −21 | 51 |
| Orbitofrontal cortex | 3.66 | 29 | 0 | 48 | −18 |
| Right inferior parietal lobule | 3.35 | 27 | 33 | −48 | 39 |
|
| |||||
| Crash Outcomes > NoCrash Outcomes | |||||
| Left superior temporal gyrus | 11.90 | 788 | −45 | −24 | 3 |
| Right superior temporal gyrus | 9.84 | 1103 | 48 | −18 | 6 |
| Right inferior frontal gyrus | 7.01 | 130* | 39 | 24 | 9 |
| Right culmen | 9.03 | 1384 | 24 | −51 | −12 |
| Left declive | 8.29 | 1174* | −27 | −60 | −12 |
| Lingual gyrus | 8.25 | 355* | 0 | −87 | −6 |
| Right parahippocampal gyrus | 6.33 | 35* | 18 | −30 | −6 |
| Cuneus | 5.39 | 28* | 15 | −93 | 18 |
| Left inferior frontal gyrus | 4.82 | 37 | −36 | 24 | 6 |
|
| |||||
| NoCrash Outcomes > Crash Outcomes | |||||
| Medial cerebellum (pyramis) | 9.15 | 493 | 0 | −72 | −27 |
| Right anterior lobe | 6.51 | 94* | 12 | −48 | −27 |
| Left middle frontal gyrus | 7.91 | 524 | −27 | −12 | 45 |
| Left ventral striatum (lentiform nucleus) | 6.95 | 284* | −15 | 9 | −12 |
| Right middle frontal gyrus | 6.87 | 221* | 39 | 30 | 27 |
| Left inferior frontal gyrus | 5.94 | 97* | −42 | 45 | 0 |
| Right middle frontal gyrus | 5.90 | 90* | 30 | 6 | 54 |
| Left inferior parietal lobule | 5.89 | 299* | −48 | −36 | 54 |
| Left precentral gyrus | 5.80 | 78* | −18 | −21 | 60 |
| Left dorsal striatum (caudate) | 5.67 | 115* | −21 | 0 | 24 |
| Left thalamus | 5.61 | 100* | −15 | −36 | 12 |
| Left superior frontal gyrus | 5.60 | 83* | −9 | 15 | 51 |
| Right ventral striatum (lentiform nucleus) | 7.15 | 239 | 15 | 15 | −12 |
| Right dorsal striatum (caudate) | 6.97 | 269* | 18 | 9 | 18 |
| Perigenual anterior cingulate cortex | 6.62 | 56* | 12 | 33 | −6 |
| Left inferior semi-lunar lobule | 6.56 | 63 | −36 | −66 | −39 |
| Right middle frontal gyrus | 6.49 | 114 | 33 | 54 | −3 |
| Right inferior parietal lobule | 6.22 | 138 | 45 | −39 | 51 |
| Left parahippocampal gyrus | 5.97 | 78 | −36 | −45 | −3 |
| Right thalamus | 5.56 | 24 | 15 | −30 | 12 |
| Bilateral thalamus | 5.21 | 29 | 0 | −9 | 9 |
| Posterior cingulate cortex | 3.80 | 40 | 6 | −33 | 30 |
Note. MNI = Montreal Neurological Institute; x, y, and z refer to the left-right, anterior-posterior, and superior-inferior dimensions, respectively; t refers to the t statistic at those coordinates; k refers to cluster extent in voxels (3×3×3 mm);
denotes a local submaximum (and local extent) derived from a larger cluster, the peak maximum (and local, not total, extent) of which is noted in the uppermost line above the line(s) demarcated as submaxima. Results thresholded at p < .005, k = 23.
3.3 fMRI data: Effects of social exclusion on decisions and outcomes
To isolate the effects of social exclusion, each of the four events (decisions: Go, Stop and outcomes: Crash, NoCrash) were compared directly between pre- and post-exclusion (while controlling for baseline responses to “driving” within each run; see Table 2 for a complete list of increases and decreases associated with social exclusion). Stop decisions after social exclusion compared to before (post Stop > pre Stop) were associated with greater activation in right dorsolateral PFC (dlPFC) and a number of regions implicated in social cognition, including posterior cingulate cortex in mPPC, mPFC, bilateral TPJ, and medial OFC (see Figure 3A). In contrast, there were no significant increases in neural responses during Go decisions following social exclusion versus prior to it (post Go > pre Go). Meanwhile, crash outcomes following social exclusion compared to before (post Crash > pre Crash) were associated with increased activation in subgenual anterior cingulate cortex (sACC), left ventrolateral PFC (vlPFC), and mPFC (see Figure 3B). We also made comparisons between decision events (Go > Stop; Stop > Go) as well as between outcome events (Crash > NoCrash; NoCrash > Crash) across conditions (pre > post exclusion; post > pre-exclusion), but none of these contrasts produced any significant interactions between exclusion conditions and decision events or outcome events.
Table 2.
Impact of Social Exclusion on Processing Decisions and Outcomes
| MNI
|
|||||
|---|---|---|---|---|---|
| Area | t | k | x | y | z |
| Go Decision Post-Exclusion > Go Decision Pre-Exclusion | |||||
| No areas above threshold | |||||
|
| |||||
| Stop Decision Post-Exclusion > Stop Decision Pre-Exclusion | |||||
| Left dorsolateral prefrontal cortex | 4.48 | 42 | −18 | 18 | 54 |
| Right precentral gyrus | 4.38 | 24 | 15 | −18 | 63 |
| Right medial prefrontal cortex | 4.23 | 43 | 18 | 51 | −3 |
| Orbitofrontal cortex | 3.88 | 23 | 0 | 39 | −15 |
| Left temporoparietal junction (angular gyrus) | 3.56 | 28 | −39 | −63 | 39 |
| Right dorsolateral prefrontal cortex | 3.51 | 81 | 30 | 24 | 42 |
| Posterior cingulate cortex | 3.45 | 55 | 3 | −30 | 30 |
| Right temporoparietal junction (angular gyrus) | 3.37 | 19 | 42 | −51 | 39 |
|
| |||||
| Crash Outcome Post-Exclusion > Crash Outcome Pre-Exclusion | |||||
| Subgenual anterior cingulate cortex | 3.88 | 20 | −9 | 33 | −3 |
| Left ventrolateral prefrontal cortex | 3.68 | 23 | −39 | 39 | −9 |
| Right medial prefrontal cortex | 3.52 | 27 | 15 | 45 | −6 |
|
| |||||
| No Crash Outcome Post-Exclusion > NoCrash Outcome Pre-Exclusion | |||||
| Left middle frontal gyrus | 3.85 | 23 | −24 | 39 | 15 |
| Right putamen | 3.37 | 24 | 21 | −3 | 0 |
| Precuneus | 3.29 | 25 | 9 | −72 | 42 |
|
| |||||
| Go Decisions Pre-Exclusion > Go Decisions Post-Exclusion | |||||
| Left middle temporal gyrus | 4.17 | 24 | −33 | −57 | 6 |
| Right claustrum | 3.38 | 31 | 27 | 12 | 15 |
|
| |||||
| Stop Decisions Pre-Exclusion > Stop Decisions Post-Exclusion | |||||
| Left thalamus | 3.87 | 27 | −9 | −27 | 9 |
|
| |||||
| Crash Outcomes Pre-Exclusion > Crash Outcomes Post-Exclusion | |||||
| No areas above threshold | |||||
|
| |||||
| NoCrash Outcomes Pre-Exclusion > NoCrash Outcomes Post-Exclusion | |||||
| Right midbrain | 3.44 | 19 | 15 | −24 | −18 |
Note. MNI = Montreal Neurological Institute; x, y, and z refer to the left-right, anterior-posterior, and superior-inferior dimensions, respectively; t refers to the t statistic at those coordinates (local maxima or submaxima); k refers to cluster extent in voxels (3×3×3 mm).
Results thresholded at p < .005, k = 19.
Figure 3.
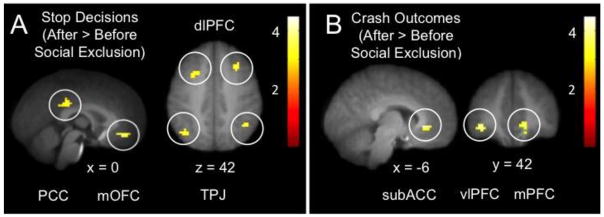
Effects of social exclusion on decisions and outcomes.
Panel A depicts regional increases in posterior cingulate cortex (PCC), medial orbitofrontal cortex (OFC), dorsolateral prefrontal cortex (dlPFC), and temporoparietal junction (TPJ) during decisions to stop following social exclusion. Panel B depicts regional increases in subgenual anterior cingulate cortex (ACC), lateral prefrontal cortex (lPFC), and medial prefrontal cortex (mPFC) during negative outcomes (crashes) following social exclusion. x, y, and z refer to the left-right, anterior-posterior, and superior-inferior dimensions in MNI space, respectively. Results thresholded at p < .005, k = 19, and displayed on an average group structural.
3.4 fMRI data: Regression and mediation analyses focused on resistance to peer influence
Next, regression analyses were conducted to determine whether neural responses during risky decisions following exclusion (while being watched by the same peers that instigated the exclusion) were related to individual differences in susceptibility to peer influence (see Table 3 for a complete listing of brain-behavior relationships based on resistance to peer influence). During risky decisions after social exclusion (Go post > Stop post), RPI was negatively correlated with responses in several structures including the right TPJ (see Figure 4A), a region associated with mentalizing and attention-shifting (Mitchell, 2008; Scholz et al., 2009), and positively correlated with responses in dorsal anterior cingulate cortex (ACC), a region associated with error detection and conflict resolution (Botvinick et al., 2001; Botvinick et al., 2004). In other words, adolescents with less resistance (more susceptibility) to peer influence had higher responses in right TPJ and lower responses in dorsal ACC during risky decisions. During negative outcomes after social exclusion (Crash post > NoCrash post), RPI was positively correlated with responses in both right and left lPFC (see Figure 4B). Similarly, RPI was positively correlated with responses in right lPFC during Crash outcomes after social exclusion relative to before (Crash post > Crash pre). In other words, adolescents with more resistance (less susceptibility) to peer influence had higher responses in lPFC during negative outcomes after social exclusion (both in comparison to positive outcomes after social exclusion, and negative outcomes prior to social exclusion).
Table 3.
Areas of Significant Correlation with Resistance to Peer Influence
| MNI
|
|||||
|---|---|---|---|---|---|
| Area | t | k | x | y | z |
| Go Decisions Post-Exclusion > Stop Decisions Post-Exclusion | |||||
| Negative Correlation with Resistance to Peer Influence | |||||
| Right temporoparietal junction (posterior superior temporal sulcus) | 4.57 | 30 | 45 | −57 | 24 |
| Left tuber/uvula | 4.41 | 42 | −39 | −60 | −36 |
| Left middle temporal gyrus | 4.27 | 36 | −51 | −36 | −9 |
| Left declive | 3.95 | 23 | −9 | −75 | −24 |
| Right culmen | 3.37 | 19 | 9 | −54 | −6 |
| Positive Correlation with Resistance to Peer Influence | |||||
| Dorsal anterior cingulate cortex | 5.50 | 25 | −12 | −3 | 42 |
|
| |||||
| Crash Outcomes Post-Exclusion > NoCrash Outcomes Post-Exclusion | |||||
| Negative Correlation with Resistance to Peer Influence | |||||
| Left lingual gyrus | 4.35 | 39 | −27 | −69 | 3 |
| Positive Correlation with Resistance to Peer Influence | |||||
| Left lateral prefrontal cortex | 5.50 | 37 | −54 | 27 | 6 |
| Right lateral prefrontal cortex | 4.53 | 49 | 48 | 33 | 9 |
| Left dorsolateral prefrontal cortex | 4.27 | 48 | −45 | 3 | 24 |
|
| |||||
| Go Decisions Pre-Exclusion > Stop Decisions Pre-Exclusion | |||||
| Negative Correlation with Resistance to Peer Influence | |||||
| Left middle frontal gyrus | 4.73 | 63 | −39 | 24 | 21 |
| Right parahippocampal gyrus | 4.30 | 21 | 12 | −39 | 3 |
| Medial prefrontal cortex | 4.25 | 70 | 3 | 57 | −3 |
| Right lateral prefrontal cortex | 4.12 | 43 | 42 | 39 | 9 |
| Right lateral orbitofrontal cortex | 3.82 | 20 | 21 | 39 | −18 |
| Positive Correlation with Resistance to Peer Influence | |||||
| No areas above threshold | |||||
|
| |||||
| Crash Outcomes Pre-Exclusion > NoCrash Outcomes Pre-Exclusion | |||||
| Negative Correlation with Resistance to Peer Influence | |||||
| Right parahippocampal gyrus | 4.39 | 24 | 21 | −36 | −18 |
| Bilateral thalamus | 3.83 | 22 | 0 | −24 | 3 |
| Positive Correlation with Resistance to Peer Influence | |||||
| No areas above threshold | |||||
|
| |||||
| Go Decisions Post-Exclusion > Go Decisions Pre-Exclusion | |||||
| Negative Correlation with Resistance to Peer Influence | |||||
| No areas above threshold | |||||
| Positive Correlation with Resistance to Peer Influence | |||||
| Left precentral gyrus | 5.03 | 20 | −30 | −15 | 51 |
| Left postcentral gyrus | 4.14 | 25 | −57 | −18 | 30 |
|
| |||||
| Stop Decisions Post-Exclusion > Stop Decisions Pre-Exclusion | |||||
| Negative Correlation with Resistance to Peer Influence | |||||
| No areas above threshold | |||||
| Positive Correlation with Resistance to Peer Influence | |||||
| Right occipital gyrus | 4.35 | 21 | 27 | −90 | 15 |
|
| |||||
| Crash Outcomes Post-Exclusion > Crash Outcomes Pre-Exclusion | |||||
| Negative Correlation with Resistance to Peer Influence | |||||
| No areas above threshold | |||||
| Positive Correlation with Resistance to Peer Influence | |||||
| Right lateral prefrontal cortex | 4.41 | 24 | 48 | 36 | 15 |
|
| |||||
| No Crash Outcomes Post-Exclusion > NoCrash Outcomes Pre-Exclusion | |||||
| Negative Correlation with Resistance to Peer Influence | |||||
| Left temporoparietal junction (angular gyrus) | 5.07 | 32 | −45 | −66 | 36 |
| Precuneus | 4.82 | 37 | −12 | −54 | 33 |
| Left precentral gyrus | 4.52 | 23 | −51 | 0 | 27 |
| Positive Correlation with Resistance to Peer Influence | |||||
| No areas above threshold | |||||
Note. MNI = Montreal Neurological Institute; x, y, and z refer to the left-right, anterior-posterior, and superior-inferior dimensions, respectively; t refers to the t statistic at those coordinates (local maxima or submaxima); k refers to cluster extent in voxels (3×3×3 mm).
Results thresholded at p < .005, k = 19.
Figure 4.
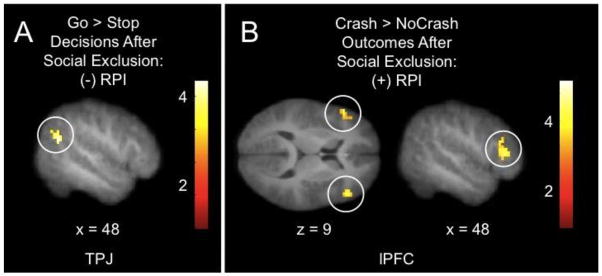
Relationship between brain activity following social exclusion and resistance to peer influence during risky decisions and negative outcomes.
Panel A depicts the negative correlation between resistance to peer influence (RPI) and activity in right temporoparietal junction (TPJ) during risky decisions (go > stop) after social exclusion.
Panel B depicts the positive correlation between RPI and activity in both left and right lateral prefrontal cortex (lPFC) during negative outcomes (crash > nocrash) after social exclusion. x and z refer to the left-right and superior-inferior dimensions in MNI space. Results thresholded at p < .005, k = 19, and displayed on an average group structural.
Finally, we explored whether brain activity after social exclusion (in right TPJ during risky decisions, or in right lPFC during negative outcomes) mediated the observed significant relationship between susceptibility to peer influence and change in risk-taking following social exclusion. To do so, we first extracted parameter estimates from the clusters identified in the prior regression analyses (right TPJ during negative correlation with RPI in Go post > Stop post; right lPFC during positive correlation with RPI in Crash post > NoCrash post) using MarsBaR (Brett et al., 2002). Next, we utilized the “INDIRECT” SPSS Macro for Multiple Mediation (Preacher & Hayes, 2008). The results showed there was a significant indirect effect of resistance to peer influence through only one proposed mediating ROI, right TPJ, on the dependent variable, change in risk-taking behavior after social exclusion (unstandardized coefficient = 0.979, se = .405, Zmed effect = 2.42, p = .016; see Inline Supplementary Figure 1). INDIRECT also uses bootstrapping to calculate bias-corrected estimates of indirect effects (in this case, 5,000 sample draws, with replacement). Estimation of the indirect effect using a nonparametric sampling procedure such as the INDIRECT bootstrapping method increases power while maintaining control over Type I error rate and does not require the assumption of normality of the sampling distribution, which is rarely the case in smaller samples (MacKinnon et al., 2004; Preacher & Hayes, 2008). This analysis demonstrated that the bias-corrected 95% confidence interval for the indirect effect of RPI on change in risk-taking after social exclusion through right TPJ did not include zero (.1407–2.0224), providing additional support for the assertion that right TPJ mediated the effect of resistance to peer influence on change in risk-taking behavior after social exclusion. To further explore the functional role of right TPJ during risky (relative to safe) decisions after social exclusion, psychophysiological interactions (PPIs) were computed for each subject to examine differences in neural activity between Go and Stop trials during the run following social exclusion (for details of this exploratory PPI analysis, see Inline Supplementary Figure 2).
Discussion
This study explored the effects of social exclusion on behavioral risk-taking and associated brain function. Subjects performed a simulated driving task during neuroimaging, while in the implied presence of two online peers, and after an event where the peers socially included, then explicitly excluded the subjects from an online game. Increased risky behavior following social exclusion correlated with low resistance to peer influence. On average, after social exclusion relative to before, safe decisions elicited greater responses in regions associated with social cognition, including bilateral TPJ and cortical midline structures. Furthermore, after social exclusion relative to before, negative outcomes elicited greater responses in regions associated with self-regulation and negative affect, including lPFC and sACC. Finally, there were significant associations between individual differences in resistance to peer influence and post-exclusion neural responses during decisions or outcomes. In general, adolescents with low resistance to peer influence exhibited stronger activity during risky decisions in regions associated with social cognition including right TPJ, suggesting attention to the presence of peers and possible consideration of peer evaluations of adolescent decisions. Meanwhile, adolescents with high resistance to peer influence showed stronger activity during negative outcomes in regions associated with self-regulation like lPFC, which may influence their ability to regulate affective reactions to disappointing results. However, only right TPJ activity during risky decisions after social exclusion significantly mediated some degree of the relationship between resistance to peer influence and behavioral risk-taking after social exclusion.
4.1 Behavioral effects of social exclusion on risk-taking
As a group, adolescents took more risks following social inclusion and subsequent exclusion in the Cyberball task, although the increase did not reach statistical significance in this small sample. However, teens with greater susceptibility to peer influence did take significantly more risks after being excluded by peers, suggesting that individual differences may shape the strategies adolescents employ for risk decisions in social contexts (Monahan et al., 2009; Steinberg & Monahan, 2007). The low correlation between risk after exclusion and self-reported social need satisfaction (Need Threat Scale) suggests that some individuals with decreased social need satisfaction may respond with increased risk while others may respond with decreased risk. This divergence may represent different motivational strategies to rejection (Smart Richman & Leary, 2009), which can include attempts to gain acceptance (DeWall et al., 2008; Maner et al., 2007), to harm others (Twenge et al., 2002), or to withdraw from the rejecting situation (Molden et al., 2009). Previous studies have also shown that responses to rejection and exclusion vary according to how the excluded individual perceives the intentions of the excluder (Molden et al., 2009), and by their estimate of whether reaffiliating with the excluding group is possible (Maner et al., 2007). Given the range of possible self-report and behavioral responses to rejection, a primary goal of this study was therefore to combine those methods with neuroimaging to identify common and individual factors affecting adolescent risk-taking.
4.2 Reward processing and cognitive control
Neuroimaging results of the main effect of decision type collapsed across pre- and postexclusion conditions confirmed activation in expected regions (Cohen et al., 2012; Aron et al., 2004; Knutson et al., 2008), with safe decisions (Stop > Go) producing more activation in lPFC and risky decisions (Go > Stop) producing more activation in VS. Although the Stoplight task features elements of classic reward learning paradigms, the purpose of this study was to isolate the neural mechanisms underlying any effects of social exclusion on risk. To achieve this, each decision (Go, Stop) and outcome (Crash No Crash) following exclusion was contrasted with the same condition prior to exclusion. This controlled for the basic effects of expected value and reward prediction error, enabling the examination of effects specifically attributable to social contextual aspects of decisions.
4.3 Mentalizing and decision-making
Our novel finding that safe decisions elicited greater responses in bilateral TPJ, mPPC, and mPFC following social exclusion suggests that social cognitive processes play a role in decision-making for adolescents. All three regions are associated with mentalizing and Theory of Mind (Scholz et al., 2009; Van Overwalle & Baetens, 2009), suggesting adolescents may be considering potential social evaluation by the peers who just excluded them and are currently watching their performance. All three regions are also centrally implicated in self-referential processing (D’Argembeau et al., 2007; Northoff et al., 2011; Pfeifer et al., 2007; Pfeifer et al., 2009; Pfeifer & Peake, 2011), raising the possibility that making safe decisions in such an amplified social-evaluative context may be more salient to adolescents’ social self-concept, although perhaps in a sample of high-risk teens the pattern would be reversed, as suggested by the brain-behavior correlations between RPI and right TPJ during risky decisions after social exclusion.
Taken together, the enhanced response of regions involved in social cognition suggests that typically-developing adolescents may be considering the thoughts and potential evaluation of peers when deciding not to take risks after being socially excluded. This illuminates an important additional mechanism for peer influences on decision-making during adolescence. The current dominant perspective is that peer influences are exerted on risk-taking primarily via heightened reward sensitivity (e.g., Chein et al., 2011). These results illustrate the value of considering multiple pathways for social influence that may vary according to the risk behaviors and interpersonal contexts being assessed, especially the role of mentalizing regions in social influences on risk-taking (Blakemore & Robbins, 2012; Pfeifer & Allen, 2012).
4.4 Resistance to peer influence, mentalizing during risky decisions, and regulation during negative outcomes
One of two particularly notable findings from the regression analyses is that while making risky decisions following social exclusion (Go post > Stop post), teens with lower resistance to peer influence demonstrated greater neural activation in right TPJ, a region associated with mentalizing (Table 3; Scholz et al., 2009; Van Overwalle & Baetens, 2009). Furthermore, bootstrapping analyses indicated there was a significant indirect effect of right TPJ responses, whereby it mediated the relationship between lower resistance to peer influence and higher behavioral risk-taking after social exclusion. These results suggest that, in contrast to teens with higher resistance to peer influence, adolescents with lower resistance to peer influence may be experiencing divided attention – which may include thinking more about the thoughts of the peers who just excluded them – and these processes of attention-shifting and mentalizing may affect their risk decisions. From a reward-learning perspective, decisions are assumed to track increased expected value (Niv & Montague, 2008; Schultz, 2010) and social reward can influence value calculations (Evans et al., 2011; Fehr, 2009).
In the context of the current study, it may be that the expected value of high-risk rewards becomes greater due to the increased motivational currency of social approval or social status, particularly following an episode of social exclusion for adolescents with low resistance to peer influence. This is consistent with a recent study showing that the effects of emotional preferences on decision-making are mediated by mentalizing networks (Evans et al., 2011), including the TPJ regions found in the current study. We suggest that mentalizing is a reasonable cognitive response to social exclusion in our paradigm, not only because mentalizing during decisions may reflect a concern with the peers’ evaluations of one’s decisions, but also because reactions to exclusion or rejection depend on the perceived intentions of the excluder (Molden et al., 2009; Smart Richman & Leary, 2009).
The second notable set of findings from the regression analyses is that during processing of Crash outcomes following social exclusion (Crash post > NoCrash post), teens with higher resistance to peer influence exhibited greater activation in right and left lPFC, regions associated with cognitive control in general (Hampshire et al., 2010) and with regulation or suppression of emotional responses in particular (Lieberman et al., 2007; Wager et al., 2008). Similarly, greater activation in right lPFC during Crash outcomes after social exclusion relative to before social exclusion (Crash post > Crash pre) was positively correlated with higher resistance to peer influence. Based on the aforementioned studies showing lPFC involvement in emotion regulation and suppression, this suggests that teens who are less susceptible to peer influence (i.e., those with higher RPI) show more activity potentially related to self-regulation when experiencing negative outcomes in a peer context. The ability to better regulate the effect of negative emotions may be related to the ability to make less emotional (more rational) decisions in peer contexts (Figner et al., 2009). Although this finding is suggestive that lPFC plays a role in decreased risk after exclusion, the mediation analysis failed to find a significant indirect effect of RPI on post-exclusion risk through lPFC. This indicates that lPFC activity (considered as a possible proxy for self-regulation) is not sufficient to explain the difference in post-exclusion risk related to RPI.
Taken together, neural response profiles during processing of risky decisions and negative outcomes suggest that some teenagers are better able to regulate their actions and reactions following social exclusion, while other teenagers may experience an increase in the salience of the thoughts of peers after exclusion, or shift their attention from the choices at hand. These varying neural profiles represent different strategies or approaches to decision-making following social exclusion that may help explain why some adolescents engage in risky behavior. Once again, the results of this study reveal additional possible mechanisms of peer influence on adolescent decision-making. Beyond the contributions of reward sensitivity illustrated in prior research (Chein et al., 2011), the brain-behavior relationships illustrated here suggest that mentalizing and regulation processes also serve to impact decision-making and outcome processing in ways that may amplify or buffer against risk-taking in adolescence.
4.5 Negative affect, regulation, and outcome processing
It is worth noting that increased responses during Crash outcomes following social exclusion were observed in subgenual ACC and lPFC, regions that have been implicated in negative affect and emotion regulation. Subgenual ACC activation is common in tasks involving distress associated with social exclusion (Masten et al., 2009; Sebastian et al., 2009), negative self-referential processing (Yoshimura et al., 2009), and reappraisal of negative emotion (Wager et al., 2008). Similarly, ventral lPFC has also been identified in studies of negative affect following social exclusion (Sebastian et al., 2009), emotion regulation in labeling negative affect (Lieberman et al., 2007), and negative emotion reappraisal (Wager et al., 2008). Collectively, the pattern of increased activity in this network of regions points to the possibility that poor performance in the presence of peers (crashing) following exclusion generates even greater negative emotion and subsequent emotional regulation (which may include labeling or reappraisal).
4.6 Limitations and future directions
This study begins to explore the neural mechanisms underlying the effect of social exclusion on adolescent risk-taking. Due to our concern that the potential affective, motivational, and regulatory consequences of social exclusion might linger through multiple rounds of the Stoplight task, we chose to run a fixed sequence which could not counterbalance the order of pre and post exclusion rounds. The post-exclusion round was always performed after the pre-exclusion round, and as such any changes observed in neural responses may be due simply to boredom, fatigue, or adaptation (repetition suppression) – particularly significant decreases. However, this explanation seems less likely to account for significant increases from pre to post exclusion, such as those observed in social cognition and regulatory regions. An ideal follow-up study could utilize a between-subjects design that compares neural responses during decisionmaking and outcome processing across two conditions: post social exclusion, and post social inclusion. This would help to illuminate whether the differences between this study and the effects reported in a prior study of peer influence on adolescent risk-taking (Chein et al., 2011), are due to the social exclusion manipulation per se, or some other aspect of our design such as the use of virtual rather than familiar peers. Any changes may also be due to other aspects of the intervening game of Cyberball, which featured both an inclusion and exclusion round. It is possible that interaction with peers during Cyberball could result in increased awareness of peers that influenced risk without being due specifically to exclusion. While this possibility exists, we feel that any potential additive effect of increased interaction would be a small contributor to the change in risk when compared to the effect of exclusion. This view is informed by the considerable number of Cyberball studies that have found strong exclusion effects in between-subjects designs where one group received only the inclusion condition and another group received both inclusion and exclusion rounds (Boyes & French, 2009; Carter-Sowell et al., 2008; Geniole et al., 2011; van Beest & Williams, 2006).
Another future direction to pursue is the use of this protocol in a higher-risk sample of adolescents. The typically-developing adolescents who participated in this study tended to engage in low rates of risky behavior such as substance use or affiliation with deviant peers, although there was meaningful variability in this measure that could provide clear a priori hypotheses about neural and behavioral differences. Comparing the reactions and underlying neurocognitive mechanisms of high-risk versus typically-developing adolescents to social exclusion and peer influence could reveal unique activation patterns that define the difference between adaptive and maladaptive response profiles. Studies could also manipulate the performance and behavior of the peer observers, to compare between reinforcement for risky decisions and reinforcement for safe decisions, which would be directly relevant to existing research on peer contagion effects in high-risk adolescents (Gardner & Steinberg, 2005; Steinberg et al., 2008). In addition, it is unclear whether these neural mechanisms of peer influence on decision-making in this context are unique to adolescents, or would also be observed in children and adults. Future research should undertake comparisons across these age groups to examine developmental trajectories and age-invariant patterns.
4.7 Conclusion
In conclusion, this study begins to illuminate some possible neural mechanisms of peer influence on decision-making (especially when taking risks and processing negative outcomes), in the specific context of social exclusion. Beyond a pathway previously identified in VS suggesting reward sensitivity may support some forms of peer influence on adolescent risk-taking (Chein et al., 2011), this study suggests two additional relevant pathways. First, at the point of making a decision, engagement of TPJ in adolescents following social exclusion may be associated with mentalizing processes and/or shifting attention away from the decision, which mediates relations between resistance to peer influence and behavioral risk-taking after social exclusion. Thus, in normative development, this may actually be associated with playing it safe, but in youth with low self-reported resistance to peer influence it may instead facilitate more risky behavior. Second, when the outcome of a risky decision is known, engagement of lPFC in adolescents following social exclusion may indicate regulatory processes likewise play a critical role in supporting healthy decisions, perhaps especially when negative affect is already high. These findings may ultimately pave the way for new interventions to reduce adolescent risk-taking that focus not only on reward sensitivity, but also regulatory abilities, attentional control, and mentalizing processes.
Supplementary Material
Highlights.
Adolescents completed a driving risk task before and after social exclusion by peers.
Teens with low resistance to peer influence (RPI) took more risks after exclusion.
Low RPI teens showed increased neural activity in regions of mentalizing during risk.
Signal in temporoparietal junction mediated the effect of RPI on post-exclusion risk.
Thinking about evaluation by peers may affect risk decisions for susceptible teens.
Acknowledgments
Support for this project was provided by the National Institute on Drug Abuse to the Center on Early Adolescence (DA018760, Tony Biglan, Oregon Research Institute, PI) and the NIMH Development, Emotion, Ecology, and Psychopathology Research Training grant to S.A.P. (5T32-MH20012, Elizabeth A. Stormshak, University of Oregon, Child and Family Center and College of Education, PI). Special thanks are due to Lauren Kahn and the University of Oregon Developmental Social Neuroscience lab. Magnetic resonance imaging was performed at the Robert and Beverly Lewis Center for Neuroimaging at the University of Oregon.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Ayduk O, Gyurak A, Luerssen A. Individual differences in the rejection-aggression link in the hot sauce paradigm: The case of Rejection Sensitivity. J Exp Soc Psychol. 2008;44:775–782. doi: 10.1016/j.jesp.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeister RF, DeWall CN, Ciarocco NJ, Twenge JM. Social exclusion impairs self-regulation. J Pers Soc Psychol. 2005;88:589. doi: 10.1037/0022-3514.88.4.589. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Chen G, Smith AR, Hommer DW. Incentive-elicited mesolimbic activation and externalizing symptomatology in adolescents. J Child Psychol Psyc. 2010;51:827–837. doi: 10.1111/j.1469-7610.2009.02201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Knutson B, Fong GW, Caggiano DM, Bennett SM, Hommer DW. Incentive-elicited brain activation in adolescents: similarities and differences from young adults. J Neurosci. 2004;24:1793–1802. doi: 10.1523/JNEUROSCI.4862-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Smith AR, Chen G, Hommer DW. Psychosocial problems and recruitment of incentive neurocircuitry: Exploring individual differences in healthy adolescents. Dev Cognitive Neurosci. 2011;27:4839–4849. doi: 10.1016/j.dcn.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Smith AR, Danube CL, Hommer DW. Developmental differences in posterior mesofrontal cortex recruitment by risky rewards. J Neurosci. 2007;27:4839–49. doi: 10.1523/JNEUROSCI.5469-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ. The social brain in adolescence. Nat Rev Neurosci. 2008;9:267–277. doi: 10.1038/nrn2353. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ. Imaging brain development: The adolescent brain. Neuroimage. 2011;61:397–406. doi: 10.1016/j.neuroimage.2011.11.080. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Robbins TW. Decision-making in the adolescent brain. Nat Neurosci. 2012;15:1184–1191. doi: 10.1038/nn.3177. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Burnett S, Dahl RE. The role of puberty in the developing adolescent brain. Hum Brain Mapp. 2010;31:926–933. doi: 10.1002/hbm.21052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ, den Ouden H, Choudhury S, Frith C. Adolescent development of the neural circuitry for thinking about intentions. Soc Cogn Affect Neurosci. 2007;2:130–139. doi: 10.1093/scan/nsm009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyes ME, French DJ. Having a cyberball: Using a ball-throwing game as an experimental social stressor to examine the relationship between neuroticism and coping. Pers Indiv Differ. 2009;47:396–401. [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108:624. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton J, Valabregue R, Poline J. Region of interest analysis using an SPM toolbox [abstract]. Presented at the 8th International Conference on Functional Mapping of the Human Brain; June 2–6, 2002; Sendai, Japan. 2002. [Google Scholar]
- Burnett S, Bault N, Coricelli G, Blakemore SJ. Adolescents’ heightened risk-seeking in a probabilistic gambling task. Cognitive Dev. 2010;25:183–196. doi: 10.1016/j.cogdev.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett S, Bird, Moll J, Frith C, Blakemore SJ. Development during Adolescence of the Neural Processing of Social Emotion. J Cogn Neurosci. 2008;21:1736–1750. doi: 10.1162/jocn.2009.21121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter-Sowell AR, Chen Z, Williams KD. Ostracism increases social susceptibility. Social Influence. 2008;3:143–153. [Google Scholar]
- Casey BJ, Jones RM, Somerville LH. Braking and Accelerating of the Adolescent Brain. Journal of Research on Adolescence. 2011;21:21–33. doi: 10.1111/j.1532-7795.2010.00712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chein J, Albert D, O’Brien L, Uckert K, Steinberg L. Peers increase adolescent risk taking by enhancing activity in the brain’s reward circuitry. Dev Sci. 2011;14:F1–F10. doi: 10.1111/j.1467-7687.2010.01035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JR, Berkman ET, Lieberman MD. Principles of Frontal Lobe Functions. 2. Oxford University Press; USA: 2012. Intentional and incidental self-control in ventrolateral PFC. [Google Scholar]
- Coie JD, Lochman JE, Terry R, Hyman C. Predicting early adolescent disorder from childhood aggression and peer rejection. J Consult Clin Psych. 1992;60:783. doi: 10.1037//0022-006x.60.5.783. [DOI] [PubMed] [Google Scholar]
- Crone EA, Dahl RE. Understanding adolescence as a period of social-affective engagement and goal flexibility. Nat Rev Neurosci. 2012;13:636–650. doi: 10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- D’Argembeau A, Ruby P, Collette F, Degueldre C, Balteau E, Luxen A, Maquet P, Salmon E. Distinct regions of the medial prefrontal cortex are associated with self-referential processing and perspective taking. J Cogn Neurosci. 2007;19:935–944. doi: 10.1162/jocn.2007.19.6.935. [DOI] [PubMed] [Google Scholar]
- DeWall CN, Baumeister RF, Vohs KD. Satiated with belongingness? Effects of acceptance, rejection, and task framing on self-regulatory performance. J Pers Soc Psychol. 2008;95:1367. doi: 10.1037/a0012632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dishion TJ, Capaldi DM, Spracklen KM, Li F. Peer ecology of male adolescent drug use. Dev Psychopathol. 1995;7:803–824. [Google Scholar]
- Dishion TJ, Owen LD. A longitudinal analysis of friendships and substance use: Bidirectional influence from adolescence to adulthood. Dev Psychol. 2002;38:480. doi: 10.1037//0012-1649.38.4.480. [DOI] [PubMed] [Google Scholar]
- Dodge KA, Lansford JE, Burks VS, Bates JE, Pettit GS, Fontaine R, Price JM. Peer rejection and social information-processing factors in the development of aggressive behavior problems in children. Child Dev. 2003;74:374–393. doi: 10.1111/1467-8624.7402004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton DK, Kann L, Kinchen S, Shanklin S, Ross J, Hawkins J, Harris WA, Lowry R, McManus T, Chyen D. Youth risk behavior surveillance-United States, 2009. MMWR Surveill Summ. 2010;59:1–142. [PubMed] [Google Scholar]
- Ernst M, Nelson EE, Jazbec S, McClure EB, Monk CS, Leibenluft E, Blair J, Pine DS. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. Neuroimage. 2005;25:1279–1291. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Ernst M, Romeo RD, Andersen SL. Neurobiology of the development of motivated behaviors in adolescence: a window into a neural systems model. Pharmacol Biochem Behav. 2009;93:199–211. doi: 10.1016/j.pbb.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Eshel N, Nelson EE, Blair RJ, Pine DS, Ernst M. Neural substrates of choice selection in adults and adolescents: development of the ventrolateral prefrontal and anterior cingulate cortices. Neuropsychologia. 2007;45:1270–1279. doi: 10.1016/j.neuropsychologia.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans S, Fleming SM, Dolan RJ, Averbeck BB. Effects of Emotional Preferences on Value-based Decision-making Are Mediated by Mentalizing and Not Reward Networks. J Cogn Neurosci. 2011;23:2197–2210. doi: 10.1162/jocn.2010.21584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr E. Neuroeconomics: Decision making and the brain. Academic Press; London: 2009. Social preferences and the brain. [Google Scholar]
- Figner B, Mackinlay RJ, Wilkening F, Weber EU. Affective and deliberative processes in risky choice: age differences in risk taking in the Columbia card task. J Exp Psychol Learn. 2009;35:709–730. doi: 10.1037/a0014983. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Frith CD, Frith U. Social cognition in humans. Current Biology. 2007;17:R724–R732. doi: 10.1016/j.cub.2007.05.068. [DOI] [PubMed] [Google Scholar]
- Galvan A. Adolescent development of the reward system. Front Hum Neurosci. 2010;4:6. doi: 10.3389/neuro.09.006.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, Casey BJ. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. J Neurosci. 2006;26:6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner M, Steinberg L. Peer influence on risk taking, risk preference, and risky decision making in adolescence and adulthood: An experimental study. Dev Psychol. 2005;41:625–634. doi: 10.1037/0012-1649.41.4.625. [DOI] [PubMed] [Google Scholar]
- Geier CF, Terwilliger R, Teslovich T, Velanova K, Luna B. Immaturities in reward processing and its influence on inhibitory control in adolescence. Cereb Cortex. 2010;20:1613–1629. doi: 10.1093/cercor/bhp225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geniole SN, Carre JM, McCormick CM. State, not trait, neuroendocrine function predicts costly reactive aggression in men after social exclusion and inclusion. Biol Psychol. 2011;87:137–145. doi: 10.1016/j.biopsycho.2011.02.020. [DOI] [PubMed] [Google Scholar]
- Gunther Moor B, Güroğlu B, Op de Macks ZA, Rombouts SA, Van der Molen MW, Crone EA. Social exclusion and punishment of excluders: Neural correlates and developmental trajectories. Neuroimage. 2012;59:708–717. doi: 10.1016/j.neuroimage.2011.07.028. [DOI] [PubMed] [Google Scholar]
- Gweon H, Dodell-Feder D, Bedny M, Saxe R. Theory of Mind performance in children correlates with functional specialization of a brain region for thinking about thoughts. Child Dev. 2012;83:1853–1868. doi: 10.1111/j.1467-8624.2012.01829.x. [DOI] [PubMed] [Google Scholar]
- Hampshire A, Chamberlain SR, Monti MM, Duncan J, Owen AM. The role of the right inferior frontal gyrus: inhibition and attentional control. Neuroimage. 2010;50:1313–1319. doi: 10.1016/j.neuroimage.2009.12.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SB, Blum RW, Giedd JN. Adolescent maturity and the brain: the promise and pitfalls of neuroscience research in adolescent health policy. J Adolescent Health. 2009;45:216–221. doi: 10.1016/j.jadohealth.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Delgado MR, Phillips PEM. Representation of subjective value in the striatum. Academic Press; London: 2008. [Google Scholar]
- La Greca AM, Prinstein MJ, Fetter MD. Adolescent peer crowd affiliation: Linkages with health-risk behaviors and close friendships. J Pediatr Psychol. 2001;26:131. doi: 10.1093/jpepsy/26.3.131. [DOI] [PubMed] [Google Scholar]
- Leary MR. Varieties of interpersonal rejection. Cambridge University Press; New York: 2005. [Google Scholar]
- Leary MR, Schreindorfer LS, Haupt AL. The role of low self-esteem in emotional and behavioral problems: Why is low self-esteem dysfunctional? J Soc Clin Psychol. 1995;14:297–314. [Google Scholar]
- Lieberman MD, Eisenberger NI, Crockett MJ, Tom SM, Pfeifer JH, Way BM. Putting feelings into words: affect labeling disrupts amygdala activity in response to affective stimuli. Psychol Sci. 2007;18:421–428. doi: 10.1111/j.1467-9280.2007.01916.x. [DOI] [PubMed] [Google Scholar]
- MacKinnon DP, Fairchild AJ, Fritz MS. Mediation analysis. Annu Rev Psychol. 2007;58:593–614. doi: 10.1146/annurev.psych.58.110405.085542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallott MA, Maner JK, DeWall N, Schmidt NB. Compensatory deficits following rejection: The role of social anxiety in disrupting affiliative behavior. Depress Anxiety. 2009;26:438–446. doi: 10.1002/da.20555. [DOI] [PubMed] [Google Scholar]
- Maner JK, DeWall CN, Baumeister RF, Schaller M. Does Social Exclusion Motivate Interpersonal Reconnection? Resolving the “Porcupine Problem”. J Pers Soc Psychol. 2007;92:42. doi: 10.1037/0022-3514.92.1.42. [DOI] [PubMed] [Google Scholar]
- Masten CL, Eisenberger NI, Borofsky LA, Pfeifer JH, McNealy K, Mazziotta JC, Dapretto M. Neural correlates of social exclusion during adolescence: understanding the distress of peer rejection. Soc Cogn Affect Neurosci. 2009;4:143–157. doi: 10.1093/scan/nsp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzler CW, Biglan A, Rusby JC, Sprague JR. Evaluation of a comprehensive behavior management program to improve school-wide positive behavior support. Education & Treatment of Children. 2001;24:448–479. [Google Scholar]
- Mitchell JP. Activity in right temporo-parietal junction is not selective for theory-of-mind. Cereb Cortex. 2008;18:262–271. doi: 10.1093/cercor/bhm051. [DOI] [PubMed] [Google Scholar]
- Molden DC, Lucas GM, Gardner WL, Dean K, Knowles ML. Motivations for prevention or promotion following social exclusion: Being rejected versus being ignored. J Pers Soc Psychol. 2009;96:415. doi: 10.1037/a0012958. [DOI] [PubMed] [Google Scholar]
- Monahan KC, Steinberg L, Cauffman E. Affiliation with antisocial peers, susceptibility to peer influence, and antisocial behavior during the transition to adulthood. Dev Psychol. 2009;45:1520–1530. doi: 10.1037/a0017417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niv Y, Montague PR. Theoretical and empirical studies of learning. Academic Press; London: 2008. [Google Scholar]
- Northoff G, Qin P, Feinberg TE. Brain imaging of the self-Conceptual, anatomical and methodological issues. Conscious Cogn. 2010;20:52–63. doi: 10.1016/j.concog.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Oaten M, Williams KD, Jones A, Zadro L. The effects of ostracism on self-regulation in the socially anxious. J Soc Clin Psychol. 2008;27:471–504. [Google Scholar]
- Pfeifer JH, Allen NB. Arrested development? Reconsidering dual-systems models of brain function in adolescence and disorders. Trends Cogn Sci. 2012;16:322–329. doi: 10.1016/j.tics.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer JH, Blakemore SJ. Adolescent social cognitive and affective neuroscience: past, present, and future. Soc Cogn Affect Neurosci. 2012;7:1–10. doi: 10.1093/scan/nsr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer JH, Peake SJ. Self-Development: integrating cognitive, socioemotional, and neuroimaging perspectives. Dev Cognitive Neurosci. 2011;2:55–69. doi: 10.1016/j.dcn.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer JH, Lieberman MD, Dapretto M. “I know you are but what am I?!”: neural bases of self- and social knowledge retrieval in children and adults. J Cogn Neurosci. 2007;19:1323–1337. doi: 10.1162/jocn.2007.19.8.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer JH, Masten CL, Borofsky LA, Dapretto M, Fuligni AJ, Lieberman MD. Neural correlates of direct and reflected self-appraisals in adolescents and adults: when social perspective-taking informs self-perception. Child Dev. 2009;80:1016–1038. doi: 10.1111/j.1467-8624.2009.01314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer JH, Masten CL, Moore WE, III, Oswald TM, Mazziotta JC, Iacoboni M, Dapretto M. Entering adolescence: resistance to peer influence, risky behavior, and neural changes in emotion reactivity. Neuron. 2011;69:1029–1036. doi: 10.1016/j.neuron.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;40:879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- Prinstein MJ, Aikins JW. Cognitive moderators of the longitudinal association between peer rejection and adolescent depressive symptoms. J Abnorm Child Psychol. 2004;32:147–158. doi: 10.1023/b:jacp.0000019767.55592.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinstein MJ, La Greca AM. Childhood peer rejection and aggression as predictors of adolescent girls’ externalizing and health risk behaviors: A 6-year longitudinal study. J Consult Clin Psych. 2004;72:103–112. doi: 10.1037/0022-006X.72.1.103. [DOI] [PubMed] [Google Scholar]
- Romer D. Adolescent risk taking, impulsivity, and brain development: Implications for prevention. Dev Psychobio. 2010;52:263–276. doi: 10.1002/dev.20442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson RJ, Laub JH. A Life-Course Theory of Cumulative Disadvantage and the Stability of Delinquency. Transaction; New Brunswick, NJ: 1997. [Google Scholar]
- Scholz J, Triantafyllou C, Whitfield-Gabrieli S, Brown EN, Saxe R. Distinct regions of right temporo-parietal junction are selective for theory of mind and exogenous attention. PLoS One. 2009;4:e4869. doi: 10.1371/journal.pone.0004869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Dopamine signals for reward value and risk: basic and recent data. Behav Brain Funct. 2010;6:24. doi: 10.1186/1744-9081-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian C, Blakemore SJ, Charman T. Reactions to Ostracism in Adolescents with Autism Spectrum Conditions. J Autism Dev Disord. 2009;39:1122–1130. doi: 10.1007/s10803-009-0725-4. [DOI] [PubMed] [Google Scholar]
- Simons-Morton B, Lerner N, Singer J. The observed effects of teenage passengers on the risky driving behavior of teenage drivers. Accid Anal Prev. 2005;37:973–982. doi: 10.1016/j.aap.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Smart Richman L, Leary MR. Reactions to discrimination, stigmatization, ostracism, and other forms of interpersonal rejection: A multimotive model. Psychol Rev. 2009;116:365. doi: 10.1037/a0015250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Hare T, Casey BJ. Frontostriatal maturation predicts cognitive control failure to appetitive cues in adolescents. J Cogn Neurosci. 2011;23:2123–2134. doi: 10.1162/jocn.2010.21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Jones RM, Casey BJ. A time of change: behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain Cogn. 2010;72:124–133. doi: 10.1016/j.bandc.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. Rewards, aversions and affect in adolescence: Emerging convergences across laboratory animal and human data. Dev Cogn Neurosci. 2011;1:390–403. doi: 10.1016/j.dcn.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. A Social Neuroscience Perspective on Adolescent Risk-Taking. Dev Rev. 2008;28:78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. A dual systems model of adolescent risk-taking. Dev Psychobiol. 2010;52:216–224. doi: 10.1002/dev.20445. [DOI] [PubMed] [Google Scholar]
- Steinberg L, Albert D, Cauffman E, Banich M, Graham S, Woolard J. Age differences in sensation seeking and impulsivity as indexed by behavior and self-report: evidence for a dual systems model. Dev Psychol. 2008;44:1764–1778. doi: 10.1037/a0012955. [DOI] [PubMed] [Google Scholar]
- Steinberg L, Monahan KC. Age differences in resistance to peer influence. Dev Psychol. 2007;43:1531–1543. doi: 10.1037/0012-1649.43.6.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stormshak EA, Connell AM, Véronneau MH, Myers MW, Dishion TJ, Kavanagh K, Caruthers AS. An ecological approach to promoting early adolescent mental health and social adaptation: family-centered intervention in public middle schools. Child Dev. 2011;82:209–225. doi: 10.1111/j.1467-8624.2010.01551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thesen S, Heid O, Mueller E, Schad LR. Prospective acquisition correction for head motion with image-based tracking for real-time fMRI. Magnet Reson Med. 2000;44:457–465. doi: 10.1002/1522-2594(200009)44:3<457::aid-mrm17>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Twenge JM, Baumeister RF, DeWall CN, Ciarocco NJ, Bartels JM. Social exclusion decreases prosocial behavior. J Pers Soc Psychol. 2007;92:56–66. doi: 10.1037/0022-3514.92.1.56. [DOI] [PubMed] [Google Scholar]
- Twenge JM, Catanese KR, Baumeister RF. Social exclusion causes self-defeating behavior. J Pers Soc Psychol. 2002;83:606. doi: 10.1037//0022-3514.83.3.606. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Iacoboni M, Lange C, Keenan JP. The self and social cognition: the role of cortical midline structures and mirror neurons. Trends Cogn Sci. 2007;11:153–157. doi: 10.1016/j.tics.2007.01.001. [DOI] [PubMed] [Google Scholar]
- van Beest I, Williams KD. When inclusion costs and ostracism pays, ostracism still hurts. J Pers Soc Psychol. 2006;91:918–928. doi: 10.1037/0022-3514.91.5.918. [DOI] [PubMed] [Google Scholar]
- van den Bos W, van Dijk E, Westenberg M, Rombouts SARB, Crone EA. Changing Brains, Changing Perspectives The Neurocognitive Development of Reciprocity. Psych Sci. 2011;22:60–70. doi: 10.1177/0956797610391102. [DOI] [PubMed] [Google Scholar]
- Van Leijenhorst L, Moor BG, Op de Macks ZA, Rombouts SA, Westenberg PM, Crone EA. Adolescent risky decision-making: neurocognitive development of reward and control regions. Neuroimage. 2010;51:345–355. doi: 10.1016/j.neuroimage.2010.02.038. [DOI] [PubMed] [Google Scholar]
- Van Overwalle F, Baetens K. Understanding others’ actions and goals by mirror and mentalizing systems: a meta-analysis. Neuroimage. 2009;48:564–584. doi: 10.1016/j.neuroimage.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang AT, Lee SS, Sigman M, Dapretto M. Developmental changes in the neural basis of interpreting communicative intent. Soc Cogn Affect Neurosci. 2006;1:107–121. doi: 10.1093/scan/nsl018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KD. Ostracism. Psychology. 2007;58:425. doi: 10.1146/annurev.psych.58.110405.085641. [DOI] [PubMed] [Google Scholar]
- Williams KD, Cheung CK, Choi W. Cyberostracism: effects of being ignored over the Internet. J Pers Soc Psychol. 2000;79:748–762. doi: 10.1037//0022-3514.79.5.748. [DOI] [PubMed] [Google Scholar]
- Yoshimura S, Ueda K, Suzuki S, Onoda K, Okamoto Y, Yamawaki S. Self-referential processing of negative stimuli within the ventral anterior cingulate gyrus and right amygdala. Brain Cogn. 2009;69:218–225. doi: 10.1016/j.bandc.2008.07.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


