Abstract
Epstein-Barr virus (EBV) is a widespread human herpes virus that immortalizes cells as part of its latent infection and is a causative agent in the development of several types of lymphomas and carcinomas. Replication and stable persistence of the EBV genomes in latent infection require the viral EBNA1 protein, which binds specific DNA sequences in the viral DNA. While the roles of EBNA1 were initially thought to be limited to effects on the viral genomes, more recently EBNA1 has been found to have multiple effects on cellular proteins and pathways that may also be important for viral persistence. In addition, a role for EBNA1 in lytic infection has been recently identified. The multiple roles of EBNA1 in EBV infection are the subject of this paper.
1. Introduction
Epstein-Barr nuclear antigen 1 (EBNA1) was the first Epstein-Barr virus (EBV) protein detected and is the most widely studied [1]. EBNA1 is expressed in both latent and lytic modes of EBV infection, although it has mainly been studied in latency, where it plays multiple important roles. The importance of EBNA1 in EBV latency is reflected in the fact that EBNA1 is the only viral protein expressed in all forms of latency in proliferating cells and in all EBV-associated tumours. EBNA1 is required for the persistence of EBV genomes due to its contributions to both the replication and mitotic segregation of EBV episomes. EBNA1 also activates the expression of other EBV latency genes important for cell immortalization. All of these functions involve EBNA1 binding to specific DNA recognition sites in the EBV latent origin of DNA replication (oriP). In addition, EBNA1 has been shown to alter the cellular environment in multiple ways that might facilitate viral infection and contribute to cell immortalization and survival. This paper will review the known functions, cellular effects, and mechanisms of action of EBNA1.
2. EBNA1 Functions at the EBV Genomes
2.1. DNA Replication
The origin of latent DNA replication, termed oriP (for plasmid origin), was initially identified by screening EBV DNA fragments for the ability to enable the replication and stable maintenance of plasmids in human cells that were latently infected with EBV [2]. Subsequent studies showed that the only viral protein required for the replication of oriP plasmids was EBNA1 [3]. Both EBV episomes and oriP plasmids were found to replicate once per cell cycle, mimicking cellular replication and providing a good model system for human DNA replication [4, 5].
OriP is comprised of two functional components, the dyad symmetry (DS) element and the family of repeats (FRs) [6] (Figure 1(a)). The DS element contains four EBNA1 recognition sites, two of which are located within a 65 bp dyad symmetry sequence [6, 7]. Three copies of a 9 bp sequence, referred to as nonamers, were also identified at the ends and in the middle of the DS element (Figure 1(a)), [8] and were later shown to be binding sites for telomeric repeat binding factor 2 (TRF2) [9]. The FR element consists of 20 tandem copies of a 30 bp sequence, each of which contains an 18 bp palindromic EBNA1 binding site, followed by a 12 bp AT-rich sequence [6, 7]. The primary function of the FR is not in DNA replication but rather in the mitotic segregation and transcriptional activation functions of EBNA1 as discussed later. However the EBNA1-bound FR element can affect DNA replication by inhibiting the passage of replication forks, forming a major pause site [10–14].
Figure 1.
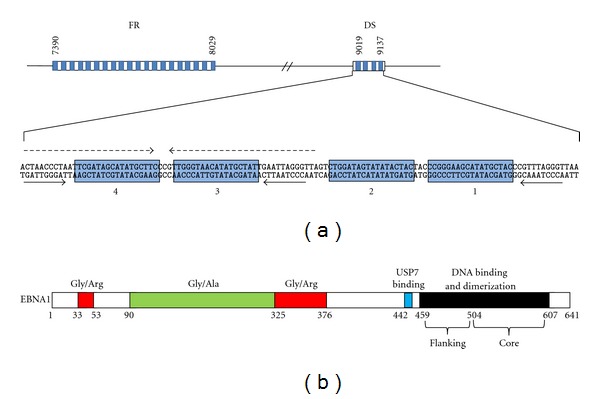
Schematic representation of oriP and the EBNA1 protein. (a) Organization of the oriP DS and FR elements showing genome nucleotide coordinates and EBNA1 binding sites (blue boxes). For the DS element, the positions of the four EBNA1-binding sites (numbered blue boxes), 65 bp dyad symmetry sequence (broken arrows), and nonamer repeats (solid arrows) are indicated. (b) Organization of the EBNA1 protein showing the two Gly-Arg-rich regions (red), Gly-Ala repeat (green), USP7-binding site (blue), and DNA binding and dimerization domain (black). Amino acids numbers are indicated below.
The DS element is the origin of replication within oriP [10] and has been shown to be both essential and sufficient for plasmid replication in the presence of EBNA1 [15–17]. Efficient replication from the DS element requires all four EBNA1 binding sites as well as the nonamer repeats that flank the EBNA1 sites [18, 19]. A low level of DNA replication can be achieved with only two of the adjacent EBNA1 sites (either site 1 + 2 or site 3 + 4) but the 3 bp spacing between these sites is critical [16–18, 20, 21].
The EBNA1 DNA binding domain is essential for the replication function of EBNA1; however it is not sufficient for this activity, as the N-terminal half of EBNA1 is also required [22–29]. The replication function has not been disrupted by any single localized deletion or mutation and appears to involve redundant contributions of at least two EBNA1 regions (amino acids 8–67 and 325–376; Figure 1(b)). However, deletion of EBNA1 residues 61–83 or 395–450 has been observed to increase replication efficiency [29, 30], and, consistent with these results, a point mutation within each of these regions (G81 and G425) causes a similar enhancement of EBNA1-dependent DNA replication [31]. These point mutations disrupt EBNA1 binding to tankyrase, suggesting that tankyrase negatively regulates replication by EBNA1, possibly through the poly-ADP ribosylation of EBNA1 [31]. The EBNA1 395–450 region mediates an interaction with the host ubiquitin specific protease, USP7, suggesting that USP7 may negatively regulate replication [30].
EBNA1 is the only EBV protein involved in latent-phase DNA replication, but lacks any enzymatic activities such as DNA helicase or origin melting activities possessed by some viral origin binding proteins [32]. Therefore EBV depends heavily on host cellular proteins to replicate its episomes. Several studies have shown that the cellular origin recognition complex (ORC) and minichromosome maintenance (MCM) complex are associated with the DS element of oriP, implicating them in the initiation and licensing of EBV DNA replication [33–35]. In addition, a functional role for ORC in oriP plasmid replication was indicated by the failure of these plasmids to stably replicate in a cell line containing a hypomorphic ORC2 mutation [35]. EBV replication was also found to be inhibited by geminin, a protein that inhibits rereplication from cellular origins by interacting with Cdt1 [35]. This suggests that Cdt1 loads the MCM complexes on EBV origins, as it does on cellular origins.
EBNA1 has been shown to be important for ORC recruitment to the DS [33, 35, 36]. In addition, EBNA1 was recently found to interact with Cdc6, and this interaction increased ORC recruitment to the DS in vitro [37]. Interestingly, ORC is not recruited by EBNA1 bound to the FR element, suggesting that the DS DNA sequence or arrangement of the EBNA1 binding sites is important for ORC recruitment [33–35, 37]. ORC recruitment by EBNA1 requires EBNA1 N-terminal sequences including the Gly-Arg-rich regions (Figure 1(b)), [37, 38]. In vitro, these regions were found to interact with ORC through RNA molecules [38], although a second study suggested that, in the presence of Cdc6, EBNA1 could recruit ORC to the DS in an RNA-independent manner [37]. EBNA1 may also facilitate the recruitment of telomere repeat binding factor 2 (TRF2) to the three nonamer repeats of the DS, which contributes to origin activation [9, 37, 39]. TRF2 then appears to contribute to ORC recruitment to the DS in conjunction with EBNA1 [20, 36] and also affects the timing of replication in S phase through recruitment of additional proteins [40, 41].
In addition, EBNA1 has been found to recruit template activating factor Iβ (TAF-Iβ also called SET) to both the DS and FR elements, through a direct interaction with the 325–376 Gly-Arg-rich region of EBNA1 [42, 43]. TAF-Iβ negatively regulates replication from oriP as TAF-Iβ depletion was found to increase oriP plasmid replication while TAF-Iβ overexpression inhibited it [43]. Since TAF-Iβ is a nucleosome-associated protein that can recruit either histone acetylases or deacetylases [44, 45], TAF-Iβ may negatively regulate replication from oriP by affecting the chromatin structure.
2.2. Mitotic Segregation
EBV episomes are present at low copy numbers and replicate only once per cell cycle. Therefore their maintenance at a stable copy number in dividing cells requires a mechanism to ensure even segregation during cell division. The mitotic segregation or partitioning of the EBV episomes requires two viral components, EBNA1 and the oriP FR element [46–48]. In fact, EBNA1 and the FR element confer stability on a variety of constructs even when combined with heterologous origin sequences [47, 49, 50]. EBNA1 binding to its multiple recognition sites in the FR is crucial for its segregation function, as is the central Gly-Arg-rich region of EBNA1 (325–376; Figure 1(b)), [27].
EBNA1 functions in segregation by tethering the EBV episomes to the cellular chromosomes in mitosis. Accordingly, EBNA1 and EBV episomes and oriP-containing constructs have all been found to associate with mitotic chromosomes [50–54], and the association of oriP plasmids with mitotic chromosomes was shown to depend on the EBNA1-chromosome association [55, 56]. In addition, EBNA1 mutants that are nuclear but defective in mitotic chromosome attachment fail to partition oriP plasmids [27, 57, 58]. EBNA1 and EBV episomes are not localized to particular regions of mitotic chromosomes, but rather are widely distributed over the chromosomes, leading to the initial suggestion that EBNA1 and EBV episomes interact randomly with chromosomes [51]. However, subsequent studies have indicated that initial pairing of EBV episomes on sister chromatids may ensure their equal distribution to the daughter cells and that this pairing may stem from the catenation of the newly replicated EBV plasmids [52, 59–61]. In addition, the FR element has been found to direct EBV genomes to chromatin regions with histone modifications typical of active chromatin [62].
Studies with EBNA1 deletion mutants showed that the central Gly-Arg-rich region of EBNA1 (amino acids 325–376) was critical for chromosome attachment and that N-terminal sequences (8–67) also contribute to this interaction [27, 29, 57, 58, 63, 64]. Interestingly, fusion proteins in which these EBNA1 regions have been replaced by other chromosome binding sequences are also able to support oriP plasmid maintenance [57, 65]. Both the central Gly-Arg repeat of EBNA1 and sequences spanning the smaller Gly-Arg-rich N-terminal sequence (amino acids 33–53; Figure 1(b)) can cause proteins to associate with mitotic chromosomes when fused to them [57, 63, 66]. However, deletion of the N-terminal Gly-Arg sequence within EBNA1 does not affect EBNA1's ability to maintain oriP plasmids or to associate with mitotic chromosomes, indicating that it is the central Gly-Arg-rich region that is normally used by EBNA1 for chromosome interactions and segregation [29, 67]. This region contains a repeated GGRGRGGS sequences that is phosphorylated on the serines and methylated by PRMT1 or PRMT5 on the arginine residues [64, 68].
The segregation of viral genomes by attachment to cellular chromosomes is not unique to EBV but is a strategy also used by Kaposi sarcoma-associated herpesvirus (KSHV) and papillomavirus. In each case the viral origin binding (LANA for KSHV and E2 for papillomavirus) tethers the viral plasmid to the cellular chromosome through interactions with one or more cellular proteins [69–72]. For EBNA1, interactions with the cellular protein, EBP2, appears to be important for metaphase chromosome attachment and segregation function [27, 58, 73, 74]. The EBNA1 325–376 region critical for chromosome attachment also mediates EBP2 binding, and there is a close correspondence between the effect of EBNA1 mutations on EBP2 and metaphase chromosome interactions [27, 29, 58, 64, 67]. In addition, EBP2 depletion in various cell lines, including the EBV-positive C666-1 NPC cells, resulted in redistribution of EBNA1 from the metaphase chromosomes to the soluble cell fraction and a corresponding release of oriP plasmids from the chromosomes [56]. EBP2 was also found to enable EBNA1-mediated plasmid segregation in budding yeast by facilitating EBNA1 attachment to the yeast mitotic chromosomes [49, 73].
However, examination of the timing of chromosome association in human cells showed that EBNA1 associates with the chromosomes earlier in mitosis than EBP2 and that EBNA1 and EBP2 only associate on the chromosomes in metaphase to telophase [67]. This suggests that EBNA1 initially contacts the chromosomes by an EBP2-independent mechanism and that subsequent interactions with EBP2 in mid-to-late mitosis might be important to maintain EBNA1 on chromosomes. The initial chromosome contact might involve direct DNA binding or interactions with chromosome-associated RNA molecules, since the 325–376 and N-terminal arginine-rich regions have been found to have some capacity to interact with DNA and RNA in vitro, and drugs that bind G-quadruplex RNA have been reported to decrease the mitotic chromosome association of EBNA1 [38, 66, 75, 76]. Recently FRET analysis identified an interaction between EBNA1 and EBP2 in the nucleoplasm and nucleolus in interphase suggesting additional roles for this interaction, including the possibility that the EBNA1-EBP2 interaction in interphase is important for EBNA1-chromosome interactions in mitosis [77]. This is intriguing in light of the findings that the E2 papillomavirus protein requires an interphase interaction with host ChlR1 in order to associate with mitotic chromosomes to segregate papillomavirus genomes [72, 78].
2.3. EBV Transcriptional Activation
EBNA1 can also act as a transcriptional activator when bound to the oriP FR element, enhancing the expression of reporter genes on FR-containing plasmids in a distance-independent manner [46, 79]. The EBNA1-bound FR was also shown to activate expression from the viral Cp and LMP promoters, suggesting a role for EBNA1 in inducing the expression of the EBNA and LMP EBV latency genes in latent infection [80, 81]. The EBNA1 residues required for transcriptional activation have been mapped to the central Gly-Arg-rich region (residues 325–376) also required for segregation function [22, 23, 28, 82] and to the 61–89 N-terminal sequence [29, 83]. EBNA1 requires both of these regions to activate transcription as deletion of either one abrogates the transcriptional activation function of EBNA1 [28, 29]. A Δ61–83 EBNA1 mutant was found to be fully active for replication and segregation functions, indicating that transcriptional activation is a distinct EBNA1 function [29]. Similar conclusions were reached with a Δ65–89 EBNA1 mutant in the context of an infectious EBV; where EBNA1 Δ65–89 was shown to be defective in activating expression of the EBNA genes from the Cp promoter, but still supported stable plasmid replication [84]. EBV containing the Δ65–89 EBNA1 was also shown to be severely impaired in the ability to transform cells, indicating the importance of EBNA1-mediated transcriptional activation for EBV infection [84].
The fact that two transcriptional activation sequences are required for efficient transcriptional activation by EBNA1 suggests that they make unique contributions to this process, likely by mediating different cellular protein interactions. The 61–83 region was found to mediate an interaction with Brd4 [85], a cellular bromodomain protein that interacts with chromatin to regulate transcription [86]. In addition, Brd4 preferentially localized to the EBNA1-bound FR enhancer element in EBV genomes and Brd4 depletion inhibited EBNA1-mediated transcriptional activation [85]. The results suggest that EBNA1 uses Brd4 to activate transcription. Interestingly, an interaction between Brd4 and papillomavirus E2 proteins (the functional equivalent to EBNA1) has been shown to be important for transcriptional activation by E2 [87–89], suggesting that EBNA1 and E2 may use common mechanisms to activate transcription.
The EBNA1 325–376 region mediates interactions with several cellular proteins, some of which have been implicated in the transcriptional activity of EBNA1. For example, P32/TAP, which interacts with Arg-rich sequences, has been detected at oriP by chromatin immunoprecipitation, and its C-terminal region has some ability to activate a reporter gene when fused to the GAL4 DNA-binding domain [23, 82]. However, it is not clear whether P32/TAP is important for EBNA1-mediated transcriptional activation. The related nucleosome assembly proteins, NAP1 and TAF-Iβ (also called SET), also interact with the EBNA1 325–376 sequence and are known to affect transcription in multiple ways [30, 43, 90]. A role for NAP1 and TAF-Iβ in EBNA1-mediated transcriptional activation is supported by the finding that both proteins are recruited to the FR element by EBNA1 and that EBNA1 transactivation activity is decreased upon depleting either protein [43]. Interestingly, NAP1 has been shown to bind and enhance the transcriptional activation activity of the papillomavirus E2 protein, through recruitment of the p300 histone acetyltransferase [91]. This suggests that, like Brd4, NAP1 is used by both EBNA1 and E2 for transcriptional activation.
Transcriptional activation by EBNA1 may not only be influenced by histone acetylation but also by ubiquitylation of histone H2B. The latter is suggested by the finding that EBNA1 binds to a complex of USP7 and GMP synthetase, that functions to deubiquitylate H2B and recruits it to the FR [92]. USP7 depletion results in increased levels of monoubiquitylated H2B at the FR and decreased transcriptional activation, suggesting that monoubiquitylation of H2B inhibits EBNA1-mediated transcriptional activation. In keeping with this result, an EBNA1 mutant defective in USP7 binding was found to have decreased transcriptional activation activity [30].
2.4. Autoregulation
In addition to interactions with the oriP FR and DS elements, EBNA1 was found to bind a third region of the EBV genome near the Qp promoter that is used to express EBNA1 in the absence of other EBNAs [93–95]. EBNA1 binding to two recognition sites located downstream of Qp was reported to repress EBNA1 expression from Qp [94]. Since EBNA1 has lower affinity for these sites than either the DS or FR elements, EBNA1 would only bind the Qp sites when its levels are high enough to saturate the FR and DS elements, providing a feedback mechanism to shut off EBNA1 expression when EBNA1 levels are high [93, 96]. While EBNA1 was initially thought to inhibit expression from Qp by repressing transcription, a more recent study found that EBNA1 acts post- or cotranscriptionally to inhibit the processing of primary transcripts [97].
3. EBNA1-DNA Interactions
3.1. Interactions with the EBV Genome
EBNA1 is a DNA binding protein that specifically interacts with three regions of the EBV genome, the oriP FR and DS elements, and the BamHI-Q fragment containing the Qp promoter [7, 93]. All of the EBNA1-bound EBV fragments contain multiple copies of an 18 bp palindromic sequence that was protected by EBNA1 in DNA footprints [7, 96]. The multiple copies of this sequence in the EBV genome contain some sequence variations that account for the different affinities of EBNA1 for the FR, DS, and BamHI-Q regions and for individual sites within these regions [96, 98]. EBNA1 has highest affinity for the FR and DS regions and remains bound to these sites throughout the cell cycle [8, 99, 100].
EBNA1 interacts with its recognition sites through its C-terminal domain (amino acids 459 and 607; Figure 1(b)), which also mediates the dimerization of EBNA1 [98, 101–103]. EBNA1 forms very stable homodimers both in solution and when bound to its recognition sites [32, 101, 103]. The crystal structure of the DNA binding and dimerization domain was determined both in solution and bound to the EBNA1 consensus binding site [104, 105]. The structure revealed that dimerization was mediated by residues 504–604 (referred to as the core domain), which form an eight-stranded antiparallel β-barrel, comprised of four strands from each monomer and two α-helices per monomer. This core domain is strikingly similar to the structure of the DNA-binding domain of the E2 protein of papillomavirus, despite a complete lack of sequence homology [106, 107]. Residues 461–503 flank the core domain (flanking domain) and are comprised of an α-helix oriented perpendicular to the DNA and an extended chain that tunnels along the base of the minor groove of the DNA. Both the helix and the extended chain make sequence-specific DNA contacts. In addition, a direct role of the core domain in DNA recognition was suggested by analogy to the E2 DNA-binding domain and later confirmed by mutational analyses [108]. Combined, the structural and biochemical studies indicate that the core and flanking domains of EBNA1 work together to load EBNA1 on its recognition site, likely through a two-step DNA-binding mechanism. In keeping with this model, thermodynamic and kinetic analyses of the EBNA1 DNA-binding domain-DNA interaction revealed two DNA association and dissociation events [109]. In addition, the ability of EBNA1 to bind its recognition sites, both in vitro and in vivo, was found to be greatly stimulated by USP7 through its interaction with EBNA1 amino acids close to the flanking domain (442–448; Figure 1(b)), [92], suggesting that this USP7 interaction may facilitate the DNA loading of the flanking domain.
The interaction of the EBNA1 DNA-binding and dimerization domain with a single recognition site causes the DNA to be smoothly bent and causes localized regions of helical overwinding and underwinding [105]. The overwinding is caused by the EBNA1 flanking domain residues that traverse along the minor groove (amino acids 463–468) [110, 111], and this results in the increased sensitivity of one T residue within the DS sites to permanganate oxidation [99, 111–113]. EBNA1 dimers assemble cooperatively on adjacent sites in the DS [16, 98], and this is predicted to induce additional changes in the DNA structure (such as unwinding), in order to accommodate the closely packed dimers [105]. The strict requirement for the 3 bp spacing that separates neighboring sites in the DS for origin function suggests that the proper interaction between the EBNA1 dimers bound to these sites is crucial for the initiation of DNA replication, possibly because of the DNA structural changes that it imparts [16, 21]. Interactions of EBNA1 dimers on the multiple sites within the DS and FR elements likely also contribute to the pronounced bending of these elements that have been observed and to the appearance of EBNA1 as a large single complex on each element [21, 114, 115].
EBNA1 complexes bound to the DS and FR elements of oriP can also interact with each other cause the looping out of the intervening DNA (when interactions occur within an oriP molecule) and the linking of multiple oriP molecules (when interactions occur between oriP molecules) [114–117]. The DNA looping and linking interactions stabilize EBNA1 binding to the DS and involve homotypic interactions mediated by two different regions of EBNA1: a stable interaction mediated by amino acids 327–377 and a less stable interaction mediated by residues 40–89 [26, 68, 118–120]. The looping/linking interactions of EBNA1 are not restricted to EBNA1 complexes formed on the DS or FR elements but also occur between single EBNA1 dimers bound to distant recognition sites [115]. The contribution of DNA looping and linking to EBNA1 functions remains unclear but the amino acids required for these interactions overlap with those required for EBNA1 replication, segregation, and transcriptional activation functions [26, 27, 29].
In vivo EBV genomes are assembled into nucleosomes with a spacing similar to that in cellular chromatin [121]. Since nucleosomes tend to inhibit sequence-specific DNA interactions, the ability of EBNA1 to bind its site in the DS in the context of a nucleosome was examined. Surprisingly, EBNA1 was able to access its recognition sites within the nucleosome and destabilized the nucleosome structure such that the histones could be displaced from the DNA [122]. Efficient assembly of EBNA1 on the FR and DS elements was also observed on larger oriP templates containing physiologically spaced nucleosomes [123]. The disruption of the DS nucleosome by EBNA1 required all four recognition sites in the DS and was intrinsic to the DNA binding and dimerization domain of EBNA1 [122]. The ability of EBNA1 to destabilize nucleosomes might be important for initiating DNA replication, a process known to be sensitive to nucleosome positioning. In addition, the ability of EBNA1 to access its sites within a nucleosome is likely to be important at times when chromatin is established prior to EBNA1 expression, for example, when latently infected resting cells (which do not express EBNA1) switch to proliferating forms of latency in which EBNA1 is expressed.
3.2. Interactions with Cellular DNA Sequences
The fact that EBNA1 can activate transcription, when bound to the EBV FR element, has prompted several studies to determine if EBNA1 might also interact with specific sequences in cellular DNA to affect cellular gene expression. Chromatin IP (ChIP) experiments performed for EBNA1 from EBV-positive lymphoblastoid cell lines, followed by promoter array analysis, identified several EBNA1-associated DNA fragments, some of which were confirmed to be directly bound by EBNA1 in vitro [124]. While this approach identified a new EBNA1 recognition sequence (distinct from those in oriP), EBNA1 binding to this sequence did not activate reporter gene expression so the significance of these EBNA1-cellular DNA interactions is not clear. ChIP combined with deep sequencing was also used to determine EBNA1-binding sites in B cells, identifying many EBNA1-associated sites, several of which were close to transcriptional start sites for cellular genes [125]. The expression of some of these cellular genes was decreased upon EBNA1 depletion and induced by EBNA1 expression, suggesting that EBNA1 may affect their transcription. Like the previous study, these EBNA1 sites differed from those in oriP, but some were similar in sequence to those identified by Dresang et al. [124]. In addition, a cluster of high-affinity EBNA1 binding sites was identified on chromosome 11 between the divergent FAM55D and FAM55B genes, although the expression of these genes was not affected by EBNA1 [125]. Canaan et al. [126] conducted microarray experiments to compare cellular transcripts in B cells and 293 cells with and without EBNA1 and identified a small percentage of transcripts that were affected by EBNA1. In addition, EBNA1 was found to ChIP to most of these gene promoters, suggesting it directly regulated them. However, whether or not EBNA1 bound directly to these promoters was not determined, and it is not yet clear how the array of genes affected might contribute to EBV infection or associated cancers.
The transcriptional activation function of EBNA1 on the EBV genome requires EBNA1 binding to multiple tandem recognition sites in the FR [15], and therefore it seems unlikely that EBNA1 binding to any single recognition site would be sufficient to activate cellular transcription. To increase the probability of identifying functionally relevant EBNA1 interactions with cellular DNA, d'Herouel et al. [127] used nearest neighbor position weight matrices to identify repeated EBNA1-binding sites in the human genome. The sites they identified had considerable overlap with those found by Dresang et al. [124]. Although the significance of the repeated EBNA1 sites that they identified remains to be determined, it is interesting that they include weak binding sites near the c-Jun and ATF promoters, which were previously shown to be activated by and associated with EBNA1 in NPC cells [128].
Finally, Lu et al. [129] identified survivin as an EBNA1 target gene by comparing cell cycle-specific transcripts from EBV-negative B cells with and without EBNA1 expression. EBNA1 increased the levels of survivin transcripts and protein and was subsequently shown to associate with the survivin promoter. Induction of survivin protein and transcripts required EBNA1 amino acids 65–89, which are known to be important for transcriptional activation of EBV genes [29, 83], suggesting that EBNA1 was activating the transcription of the survivin gene. However, EBNA1 may interact with the promoter through the Sp1-host protein, since activation of the survivin promoter by EBNA1 involves the Sp1-binding sites.
Presumably any of the above direct interactions of EBNA1 with specific DNA sites would be mediated by the EBNA1 DNA-binding domain. However, it has been suggested that EBNA1 might also mediate less specific cellular DNA interactions through its Gly-Arg-rich regions, which resemble AT hooks [66]. Indeed, these regions have been shown to have some ability to interact with AT-rich DNA in vitro. However, these regions also interact with several cellular proteins, and it remains to be determined whether the Gly-Arg-rich regions directly contact DNA in cells.
4. Cellular Effects of EBNA1
In addition to the roles of EBNA1 at the EBV genome, numerous reports suggest that EBNA1 directly contributes to cell proliferation and survival typical of latent EBV infection. The first implications came from the observations that EBNA1 is the only EBV protein expressed in all EBV-positive tumours and latency types in proliferating cells and is sometimes the only EBV protein expressed. EBNA1 was subsequently shown to be important for efficient B-cell immortalization by EBV [84, 130] and for the continued proliferation of some EBV-positive tumour cells [131–133]. EBNA1 expression in various EBV-negative cancer cells has also been found to increase tumorigenicity [134–137]. In addition, EBNA1 expression in the B-cell compartment of a transgenic mouse has been reported to be sufficient to induce B cell lymphomas [138, 139]. However, these results were not reiterated in a second independent transgenic mouse study, suggesting that secondary events might contribute to the development of EBNA1-induced lymphomas [140, 141]. Nonetheless, the body of evidence indicates that EBNA1 contributes to oncogenesis, likely due to multiple effects on cellular proteins as discussed in the following.
4.1. USP7 Interaction
Proteomics methods (affinity column profiling and TAP tagging) identified several cellular proteins that are bound by EBNA1, including an interaction with the cellular ubiquitin-specific protease USP7 (also called HAUSP [30, 142]). USP7 was originally discovered as a binding partner of the ICP0 protein from herpes simplex virus type 1 and has since been shown to be targeted by proteins from several different herpes viruses [143–146]. USP7 has been reported to bind and regulate several cellular proteins including p53 and Mdm2 (an E3 ubiquitin ligase for p53), which USP7 stabilizes by removing the polyubiquitin chains that normally signal degradation [147–151]. EBNA1, p53, and Mdm2 compete for the same binding pocket in the N-terminal TRAF domain of USP7; however EBNA1 was found to outcompete p53 or Mdm2 due to its higher affinity for USP7 [152–154]. The EBNA1 region just N-terminal to the DNA binding domain was identified as the USP7-binding site, and a subsequent crystal structure of this EBNA1 peptide bound to the USP7 TRAF domain showed that EBNA1 amino acids 442–448 contact USP7 (Figure 1(b)) and bind USP7 residues in addition to those contacted by p53 or Mdm2 [152–155].
In theory, EBNA1 could destabilize either p53 or Mdm2 by blocking their interaction with USP7, resulting in opposite effects on p53 levels. In vivo EBNA1 has not been reported to lower Mdm2 levels, but has been confirmed to lower p53 levels at least in some cell backgrounds. For example, expression of EBNA1 but not a USP7-binding mutant of EBNA1 in U2OS cells was shown to reduce the accumulation of p53 in response to DNA damage and subsequent apoptosis [153]. Similarly, EBNA1 expression in CNE2 nasopharyngeal carcinoma (NPC) cells decreased the accumulation of p53 in response to DNA damage [156], and the presence of EBNA1 or EBV in AGS or SCM1 gastric carcinoma cells decreased the steady-state levels of p53 [135, 157]. This suggests that EBNA1 could promote cell survival by modulating p53 in EBV-infected epithelial cells.
4.2. Effects on PML Nuclear Bodies
Promyelocytic leukemia (PML) nuclear bodies (also called ND10s) are nuclear foci for which PML tumour suppressor proteins form the structural basis. PML bodies are important for several cellular processes, including apoptosis, DNA repair, senescence, and p53 activation by acetylation [158–163], and their loss has been associated with the development and/or progression of several tumours [158, 164]. In addition, PML nuclear bodies suppress lytic viral infection as part of the innate antiviral response [165–167]. To counter this defense, many viruses encode proteins that disrupt PML nuclear bodies either by interfering with the interactions of PML proteins to form the bodies or by inducing the degradation of the PML proteins [168].
EBNA1 was found to induce the loss of PML nuclear bodies in both NPC and gastric carcinoma cells, by inducing the degradation of the PML proteins [156, 157]. Consistent with the known PML functions, EBNA1 expression in these cells was also found to decrease DNA repair efficiency, p53 acetylation, and apoptosis in response to DNA damaging agents [156, 157]. The results suggest that, as a result of EBNA1-induced PML loss, cells expressing EBNA1 are more likely to survive with DNA damage, which would be expected to contribute to the development of carcinomas. Importantly, these observations in cell lines appear to hold true in vivo, as a comparison of EBV-positive and EBV-negative gastric carcinoma tumour biopsies showed that PML levels were greatly reduced by the presence of EBV, presumably due to the action of EBNA1 [157].
The mechanism by which EBNA1 induces the degradation of PML proteins involves EBNA1 binding to both USP7 and the host casein kinase 2 (CK2) and recruitment of these proteins to the PML nuclear bodies [156, 169]. EBNA1 was found to preferentially interact with PML isoform IV over the other five nuclear PML isoforms, and therefore EBNA1 may localize to PML nuclear bodies through interactions with PML IV [156, 170]. EBNA1 mutants that fail to bind either USP7 or CK2 can still associate with PML bodies but do not induce their loss [156, 169]. Similarly, wildtype EBNA1 does not affect PML nuclear bodies when USP7 or CK2 is depleted. In keeping with these observations, USP7 was subsequently shown to negatively regulate PML proteins (even in the absence of EBV or EBNA1), by a mechanism that is independent of its ubiquitin cleavage activity [171].
The interaction of EBNA1 with CK2 involves a direct interaction with the β regulatory subunit of CK2 through EBNA1 amino acids 387–394 [169]. CK2 was previously identified as a negative regulator of PML and was shown to phosphorylate PML proteins at a particular serine residue that triggers polyubiquitylation and subsequent degradation [172, 173]. Through its interaction with CK2, EBNA1 was shown to increase CK2-mediated phosphorylation of PML, which would increase PML polyubiquitylation [169]. Since CK2 is involved in many cellular processes, it is possible that the interaction of EBNA1with CK2 also affects additional pathways.
4.3. Modulation of Signaling Pathways
EBNA1 has been reported to affect several signaling pathways. First, EBNA1 expression in three different carcinoma cell lines was found to increase the expression of STAT1 [174, 175]. EBNA1 was subsequently shown to enhance STAT1 phosphorylation and nuclear localization in response to IFNγ [174]. Second, EBNA1 expression was found to decrease the expression of TGF-β1-responsive genes suggesting that EBNA1 interferes with TGF-β signaling [174]. This effect may be due to increased turnover of SMAD2 in the presence of EBNA1, resulting in decreased levels of SMAD complexes needed for TGF-β1-induced transcription [174, 176]. Third, using NF-κB reporter plasmids in carcinoma cell lines, EBNA1 was found to inhibit NF-κB activity and DNA binding [177]. Additional experiments showed that the levels, nuclear localization, and phosphorylation of the p65 NF-κB subunit were all reduced in the presence of EBNA1 as was the phosphorylation of the p65 kinase, IKKα/β [177]. How EBNA1 elicits any of the above effects is presently unclear as no physical interaction has been detected between EBNA1 and STAT1, SMAD2, p65, or IKKα/β.
4.4. Induction of Oxidative Stress
EBV infection is associated with increased oxidative stress [178, 179], and this may be at least partly due to EBNA1 expression. Stable or transient EBNA1 expression in B-cell lines was found to increase levels of reactive oxygen species (ROS), DNA damage foci, and dysfunctional, uncapped telomeres, and these EBNA1 effects were decreased by ROS scavengers [180, 181]. In addition, EBNA1 was found to increase the expression of the NOX2 NADPH oxidase which might account for the ROS induction [180]. Similarly, a comparison of the nuclear proteome in NPC cells with and without EBNA expression showed that EBNA1 increased the levels of several oxidative stress response proteins including the antioxidants superoxide dismutase 1 and peroxiredoxin 1, known to be induced by ROS [182]. Further studies confirmed that, in the presence of EBNA1, ROS levels were elevated and that NOX1 and NOX2 transcripts were increased [182]. Therefore EBNA1 appears to have multiple effects on the oxidative stress response, although the mechanisms of these effects are not yet known.
4.5. Effects on Metastatic Potential
A comparison of the nuclear proteomes of NPC cells with and without EBNA1 expression found that EBNA1 increased the nuclear levels of Nm23-H1, stathmin 1, and maspin, all of which have been found to be contribute to metastases [182]. This effect on Nm23-H1 corroborated a previous study that showed that EBNA1 coimmunoprecipitated with Nm23-H1 from lymphoid cells and caused it to relocalize to the nucleus [183]. This interaction required EBNA1 amino acids 65–89, which are also important for transcriptional activation [183]. Nm23-H1 is a known suppressor of metastasis and cell migration, and EBNA1 was shown to counteract the ability of Nm23-H1 to suppress cell migration both in vitro and in a nude mouse model [137, 183]. The results suggest that EBNA1 contributes to the metastatic potential of EBV tumours and are supported by a report by Sheu et al. [134] that EBNA1 expression in HONE-1 NPC cells increased tumour metastases in nude mice. In addition, the relocalization of Nm23-H1 by EBNA1 may be another way that EBNA1 decreases apoptosis and increases cell proliferation, as pathway-specific microarray analysis recently suggested roles for Nm23-H1 in promoting apoptosis and inhibiting cell proliferation [184].
5. Immune Evasion
While most of the EBV latency proteins elicit a strong immune response, cells that only express EBNA1 (referred to as latency I or EBNA1-only program) largely avoid immune detection [185–187]. This is due to inefficient presentation of EBNA1 peptides on MHC class I molecules [188]. Reduced EBNA1 presentation has been attributed to the central Gly-Ala repeat of EBNA1 which varies in size in different EBV isolates (~230 amino acid long in the B95-8 strain; Figure 1(b)). Removal of this repeat has been shown to restore EBNA1 presentation while the addition of the Gly-Ala repeat to EBNA4 inhibits its recognition by cytotoxic T lymphocytes [188, 189].
Initially it was thought that the Gly-Ala repeat inhibited EBNA1 presentation by interfering with its proteasomal processing. This was based on studies in which insertion of the Gly-Ala repeat in other proteins inhibited their degradation [190–194]. However, the deletion of the Gly-Ala repeat from EBNA1 was not found to affect EBNA1 turnover, as EBNA1 is extremely stable with or without the Gly-Ala repeat [195]. In addition, Tellam et al. [196] found that EBNA1 turnover varied considerably in different cell backgrounds but that these rates did not correspond to the level of MHC I-restricted presentation of EBNA1 peptides. Moreover, EBNA1 presentation was shown to derive from newly synthesized protein, and the primary contribution of the Gly-Ala repeat on the presentation of EBNA1 peptides was found to be due to inhibition of its own translation [196, 197]. This supported a model where the MHC I-restricted presentation of EBNA1 occurs through the generation of defective ribosomal products (DRiPs) which are reduced by the presence of the Gly-Ala repeat [198]. Further studies confirmed that MHC class I presentation and CTL recognition corresponded to the rate of EBNA1 translation and the levels of DRiPs, and that all were inhibited by the Gly-Ala repeat [199–201]. The Gly-Ala repeat was further shown to interfere with the initiation stage of translation [200]. In addition, EBNA1 presentation was shown to depend on the rate at which the DriPs were generated, rather than on their degradation [199], a phenomenon that involves purine loading of the EBNA1 mRNA [202]. Exactly how the Gly-Ala repeat regulates translation initiation is not yet clear but Hsp90 has been reported to be a contributing factor [203].
6. EBNA1 in Lytic EBV Infection
The above roles of EBNA1 all pertain to latent EBV infection. However, EBNA1 is also expressed in lytic infection from a lytic cycle-specific promoter (Fp), suggesting that it also contributes to productive infection [204–206]. Recently the contributions of EBNA1 to viral reactivation to the lytic cycle were examined in EBV-positive AGS gastric carcinoma cells [170]. EBNA1 silencing was found to increase the frequency with which EBV spontaneously entered the lytic cycle, suggesting that EBNA1 can suppress reactivation. In contrast, when the lytic cycle was chemically induced, EBNA1 silencing inhibited lytic gene expression and viral genome amplification indicating that EBNA1 can promote lytic infection once the lytic switch has occurred. However EBNA1 did not positively contribute to lytic infection when the PML proteins were silenced, suggesting that the role of EBNA1 in lytic infection was in overcoming suppression by PML proteins. In keeping with this interpretation, PML proteins and nuclear bodies were found to suppress lytic infection by EBV [170, 207].
7. Conclusion
In summary, EBNA1 makes multiple contributions to EBV infection due to its ability to interact with specific DNA sequences and multiple cellular proteins. Latency contributions include the replication and mitotic segregation of EBV episomes, contributions to viral transcription, and multiple effects on cellular proteins and pathways that promote cell survival and proliferation. Several of the cellular changes also result in increased DNA damage which could contribute to the development of EBV-associated tumours. Finally, EBNA1 is now known to have a role in lytic infection in overcoming suppression by PML nuclear bodies, further emphasizing its importance in EBV infection.
References
- 1.Reedman BM, Klein G. Cellular localization of an Epstein Barr barr virus (EBV) associated complement fixing antigen in producer and non producer lymphoblastoid cell lines. International Journal of Cancer. 1973;11(3):499–520. doi: 10.1002/ijc.2910110302. [DOI] [PubMed] [Google Scholar]
- 2.Yates J, Warren N, Reisman D, Sugden B. A cis-acting element from the Epstein-Barr viral genome that permits stable replication of recombinant plasmids in latently infected cells. Proceedings of the National Academy of Sciences of the United States of America. 1984;81(12):3806–3810. doi: 10.1073/pnas.81.12.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yates JL, Warren N, Sugden B. Stable replication of plasmids derived from Epstein-Barr virus in various mammalian cells. Nature. 1985;313(6005):812–815. doi: 10.1038/313812a0. [DOI] [PubMed] [Google Scholar]
- 4.Yates JL, Guan N. Epstein-Barr virus-derived plasmids replicate only once per cell cycle and are not amplified after entry into cells. Journal of Virology. 1991;65(1):483–488. doi: 10.1128/jvi.65.1.483-488.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sternas L, Middleton T, Sugden B. The average number of molecules of Epstein-Barr nuclear antigen 1 per cell does not correlate with the average number of Epstein-Barr virus (EBV) DNA molecules per cell among different clones of EBV-immortalized cells. Journal of Virology. 1990;64(5):2407–2410. doi: 10.1128/jvi.64.5.2407-2410.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reisman D, Yates J, Sugden B. A putative origin of replication of plasmids derived from Epstein-Barr virus is composed of two cis-acting components. Molecular and Cellular Biology. 1985;5(8):1822–1832. doi: 10.1128/mcb.5.8.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rawlins DR, Milman G, Hayward SD, Hayward GS. Sequence-specific DNA binding of the Epstein-Barr virus nuclear antigen (EBNA-1) to clustered sites in the plasmid maintenance region. Cell. 1985;42(3):859–868. doi: 10.1016/0092-8674(85)90282-x. [DOI] [PubMed] [Google Scholar]
- 8.Niller HH, Glaser G, Knuchel R, Wolf H. Nucleoprotein complexes and DNA 5’-ends at oriP of Epstein-Barr virus. Journal of Biological Chemistry. 1995;270(21):12864–12868. doi: 10.1074/jbc.270.21.12864. [DOI] [PubMed] [Google Scholar]
- 9.Deng Z, Lezina L, Chen CJ, Shtivelband S, So W, Lieberman PM. Telomeric proteins regulate episomal maintenance of epstein-barr virus origin of plasmid replication. Molecular Cell. 2002;9(3):493–503. doi: 10.1016/s1097-2765(02)00476-8. [DOI] [PubMed] [Google Scholar]
- 10.Gahn TA, Schildkraut CL. The Epstein-Barr virus origin of plasmid replication, oriP, contains both the initiation and termination sites of DNA replication. Cell. 1989;58(3):527–535. doi: 10.1016/0092-8674(89)90433-9. [DOI] [PubMed] [Google Scholar]
- 11.Dhar V, Schildkraut CL. Role of EBNA-1 in arresting replication forks at the Epstein-Barr virus oriP family of tandem repeats. Molecular and Cellular Biology. 1991;11(12):6268–6278. doi: 10.1128/mcb.11.12.6268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Norio P, Schildkraut CL. Plasticity of DNA replication initiation in Epstein-Barr virus episomes. PLoS Biology. 2004;2(6, article e152) doi: 10.1371/journal.pbio.0020152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Norio P, Schildkraut CL. Visualization of DNA replication on individual Epstein-Barr virus episomes. Science. 2001;294(5550):2361–2364. doi: 10.1126/science.1064603. [DOI] [PubMed] [Google Scholar]
- 14.Ermakova OV, Frappier L, Schildkraut CL. Role of the EBNA-1 protein in pausing of replication forks in the Epstein-Barr virus genome. Journal of Biological Chemistry. 1996;271(51):33009–33017. doi: 10.1074/jbc.271.51.33009. [DOI] [PubMed] [Google Scholar]
- 15.Wysokenski DA, Yates JL. Multiple EBNA1-binding sites are required to form an EBNA1-dependent enhancer and to activate a minimal replicative origin within oriP of Epstein-Barr virus. Journal of Virology. 1989;63(6):2657–2666. doi: 10.1128/jvi.63.6.2657-2666.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrison S, Fisenne K, Hearing J. Sequence requirements of the Epstein-Barr virus latent origin of DNA replication. Journal of Virology. 1994;68(3):1913–1925. doi: 10.1128/jvi.68.3.1913-1925.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yates JL, Camiolo SM, Bashaw JM. The minimal replicator of Epstein-Barr virus oriP . Journal of Virology. 2000;74(10):4512–4522. doi: 10.1128/jvi.74.10.4512-4522.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koons MD, Van Scoy S, Hearing J. The replicator of the Epstein-Barr virus latent cycle origin of DNA replication, oriP, is composed of multiple functional elements. Journal of Virology. 2001;75(22):10582–10592. doi: 10.1128/JVI.75.22.10582-10592.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindner SE, Zeller K, Schepers A, Sugden B. The affinity of EBNA1 for its origin of DNA synthesis is a determinant of the origin’s replicative efficiency. Journal of Virology. 2008;82(12):5693–5702. doi: 10.1128/JVI.00332-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Atanasiu C, Deng Z, Wiedmer A, Norseen J, Lieberman PM. ORC binding to TRF2 stimulates oriP replication. EMBO Reports. 2006;7(7):716–721. doi: 10.1038/sj.embor.7400730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bashaw JM, Yates JL. Replication from oriP of Epstein-Barr virus requires exact spacing of two bound dimers of EBNA1 which bend DNA. Journal of Virology. 2001;75(22):10603–10611. doi: 10.1128/JVI.75.22.10603-10611.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yates JL, Camiolo SM. Dissection of DNA replication and enhancer activation functions of Epstein-Barr virus nuclear antigen 1. Cancer Cells. 1988;6:197–205. [Google Scholar]
- 23.Van Scoy S, Watakabe I, Krainer AR, Hearing J. Human p32: a coactivator for Epstein-Barr virus nuclear antigen-1-mediated transcriptional activation and possible role in viral latent cycle DNA replication. Virology. 2000;275(1):145–157. doi: 10.1006/viro.2000.0508. [DOI] [PubMed] [Google Scholar]
- 24.Kim AL, Maher M, Hayman JB, et al. An imperfect correlation between DNA replication activity of Epstein- Barr virus nuclear antigen 1 (EBNA1) and binding to the nuclear import receptor, Rch1/importin α . Virology. 1997;239(2):340–351. doi: 10.1006/viro.1997.8874. [DOI] [PubMed] [Google Scholar]
- 25.Kirchmaier AL, Sugden B. Dominant-negative inhibitors of EBNA-1 of Epstein-Barr virus. Journal of Virology. 1997;71(3):1766–1775. doi: 10.1128/jvi.71.3.1766-1775.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mackey D, Sugden B. The linking regions of EBNA1 are essential for its support of replication and transcription. Molecular and Cellular Biology. 1999;19(5):3349–3359. doi: 10.1128/mcb.19.5.3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shire K, Ceccarelli DFJ, Avolio-Hunter TM, Frappier L. EBP2, a human protein that interacts with sequences of the Epstein-Barr virus nuclear antigen 1 important for plasmid maintenance. Journal of Virology. 1999;73(4):2587–2595. doi: 10.1128/jvi.73.4.2587-2595.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ceccarelli DFJ, Frappier L. Functional analyses of the EBNA1 origin DNA binding protein of Epstein- Barr virus. Journal of Virology. 2000;74(11):4939–4948. doi: 10.1128/jvi.74.11.4939-4948.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu H, Kapoor P, Frappier L. Separation of the DNA replication, segregation, and transcriptional activation functions of Epstein-Barr nuclear antigen 1. Journal of Virology. 2002;76(5):2480–2490. doi: 10.1128/jvi.76.5.2480-2490.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holowaty MN, Zeghouf M, Wu H, et al. Protein profiling with Epstein-Barr nuclear antigen-1 reveals an interaction with the herpesvirus-associated ubiquitin-specific protease HAUSP/USP7. Journal of Biological Chemistry. 2003;278(32):29987–29994. doi: 10.1074/jbc.M303977200. [DOI] [PubMed] [Google Scholar]
- 31.Deng Z, Atanasiu C, Zhao K, et al. Inhibition of Epstein-Barr virus oriP function by tankyrase, a telomere-associated poly-ADP ribose polymerase that binds and modifies EBNA1. Journal of Virology. 2005;79(8):4640–4650. doi: 10.1128/JVI.79.8.4640-4650.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frappier L, O’Donnell M. Overproduction, purification, and characterization of EBNA1, the origin binding protein of Epstein-Barr virus. Journal of Biological Chemistry. 1991;266(12):7819–7826. [PubMed] [Google Scholar]
- 33.Schepers A, Ritzi M, Bousset K, et al. Human origin recognition complex binds to the region of the latent origin of DNA replication of Epstein-Barr virus. EMBO Journal. 2001;20(16):4588–4602. doi: 10.1093/emboj/20.16.4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chaudhuri B, Xu H, Todorov I, Dutta A, Yates JL. Human DNA replication initiation factors, ORC and MCM, associate with oriP of Epstein-Barr virus. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(18):10085–10089. doi: 10.1073/pnas.181347998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dhar SK, Yoshida K, Machida Y, et al. Replication from oriP of Epstein-Barr virus requires human ORC and is inhibited by Geminin. Cell. 2001;106(3):287–296. doi: 10.1016/s0092-8674(01)00458-5. [DOI] [PubMed] [Google Scholar]
- 36.Julien MD, Polonskaya Z, Hearing J. Protein and sequence requirements for the recruitment of the human origin recognition complex to the latent cycle origin of DNA replication of Epstein-Barr virus oriP . Virology. 2004;326(2):317–328. doi: 10.1016/j.virol.2004.05.026. [DOI] [PubMed] [Google Scholar]
- 37.Moriyama K, Yoshizawa-Sugata N, Obuse C, Tsurimoto T, Masai H. Epstein-barr nuclear antigen 1 (EBNA1)-dependent recruitment of origin recognition complex (Orc) on oriP of epstein-barr virus with purified proteins: stimulation by Cdc6 through its direct interaction with EBNA1. Journal of Biological Chemistry. 2012;287:23977–23994. doi: 10.1074/jbc.M112.368456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Norseen J, Thomae A, Sridharan V, Aiyar A, Schepers A, Lieberman PM. RNA-dependent recruitment of the origin recognition complex. EMBO Journal. 2008;27(22):3024–3035. doi: 10.1038/emboj.2008.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deng Z, Atanasiu C, Burg JS, Broccoli D, Lieberman PM. Telomere repeat binding factors TRF1, TRF2, and hRAP1 modulate replication of Epstein-Barr virus oriP . Journal of Virology. 2003;77(22):11992–12001. doi: 10.1128/JVI.77.22.11992-12001.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou J, Snyder AR, Lieberman PM. Epstein-barr virus episome stability is coupled to a delay in replication timing. Journal of Virology. 2009;83(5):2154–2162. doi: 10.1128/JVI.02115-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou J, Deng Z, Norseen J, Lieberman PM. Regulation of Epstein-Barr virus origin of plasmid replication (oriP) by the S-phase checkpoint kinase Chk2. Journal of Virology. 2010;84(10):4979–4987. doi: 10.1128/JVI.01300-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Holowaty MN, Zeghouf M, Wu H, et al. Protein profiling with Epstein-Barr nuclear antigen-1 reveals an interaction with the herpesvirus-associated ubiquitin-specific protease HAUSP/USP7. Journal of Biological Chemistry. 2003;278(32):29987–29994. doi: 10.1074/jbc.M303977200. [DOI] [PubMed] [Google Scholar]
- 43.Wang S, Frappier L. Nucleosome assembly proteins bind to Epstein-Barr virus nuclear antigen 1 and affect its functions in DNA replication and transcriptional activation. Journal of Virology. 2009;83(22):11704–11714. doi: 10.1128/JVI.00931-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seo SB, McNamara P, Heo S, Turner A, Lane WS, Chakravarti D. Regulation of histone acetylation and transcription by INHAT, a human cellular complex containing the set oncoprotein. Cell. 2001;104(1):119–130. doi: 10.1016/s0092-8674(01)00196-9. [DOI] [PubMed] [Google Scholar]
- 45.Shikama N, Chan HM, Krstic-Demonacos M, et al. Functional interaction between nucleosome assembly proteins and p300/CREB-binding protein family coactivators. Molecular and Cellular Biology. 2000;20(23):8933–8943. doi: 10.1128/mcb.20.23.8933-8943.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lupton S, Levine AJ. Mapping genetic elements of Epstein-Barr virus that facilitate extrachromosomal persistence of Epstein-Barr virus-derived plasmids in human cells. Molecular and Cellular Biology. 1985;5(10):2533–2542. doi: 10.1128/mcb.5.10.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krysan PJ, Haase SB, Calos MP. Isolation of human sequences that replicate autonomously in human cells. Molecular and Cellular Biology. 1989;9(3):1026–1033. doi: 10.1128/mcb.9.3.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee MA, Diamond ME, Yates JL. Genetic evidence that EBNA-1 is needed for efficient, stable latent infection by Epstein-Barr virus. Journal of Virology. 1999;73(4):2974–2982. doi: 10.1128/jvi.73.4.2974-2982.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kapoor P, Shire K, Frappier L. Reconstitution of Epstein-Barr virus-based plasmid partitioning in budding yeast. EMBO Journal. 2001;20(1-2):222–230. doi: 10.1093/emboj/20.1.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Simpson K, McGuigan A, Huxley C. Stable episomal maintenance of yeast artificial chromosomes in human cells. Molecular and Cellular Biology. 1996;16(9):5117–5126. doi: 10.1128/mcb.16.9.5117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harris A, Young BD, Griffin BE. Random associated of Epstein-Barr virus genomes with host cell metaphase chromosome in Burkitt’s lymphoma-derived cell lines. Journal of Virology. 1985;56(1):328–332. doi: 10.1128/jvi.56.1.328-332.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Delecluse HJ, Bartnizke S, Hammerschmidt W, Bullerdiek J, Bornkamm GW. Episomal and integrated copies of Epstein-Barr virus coexist in Burkitt lymphoma cell lines. Journal of Virology. 1993;67(3):1292–1299. doi: 10.1128/jvi.67.3.1292-1299.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grogan EA, Summers WP, Dowling S. Two Epstein-Barr viral nuclear neoantigens distinguished by gene transfer, serology, and chromosome binding. Proceedings of the National Academy of Sciences of the United States of America. 1983;80(24):7650–7653. doi: 10.1073/pnas.80.24.7650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Petti L, Sample C, Kieff E. Subnuclear localization and phosphorylation of Epstein-Barr virus latent infection nuclear proteins. Virology. 1990;176(2):563–574. doi: 10.1016/0042-6822(90)90027-o. [DOI] [PubMed] [Google Scholar]
- 55.Kanda T, Otter M, Wahl GM. Coupling of mitotic chromosome tethering and replication competence in Epstein-Barr virus-based plasmids. Molecular and Cellular Biology. 2001;21(10):3576–3588. doi: 10.1128/MCB.21.10.3576-3588.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kapoor P, Lavoie BD, Frappier L. EBP2 plays a key role in Epstein-Barr virus mitotic segregation and is regulated by Aurora family kinases. Molecular and Cellular Biology. 2005;25(12):4934–4945. doi: 10.1128/MCB.25.12.4934-4945.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hung SC, Kang MS, Kieff E. Maintenance of Epstein-Barr virus (EBV) oriP-based episomes requires EBV-encoded nuclear antigen-1 chromosome-binding domains, which can be replaced by high-mobility group-I or histone H1. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(4):1865–1870. doi: 10.1073/pnas.031584698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu H, Ceccarelli DFJ, Frappier L. The DNA segregation mechanism of Epstein-Barr virus nuclear antigen 1. EMBO Reports. 2000;1(2):140–144. doi: 10.1093/embo-reports/kvd026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kanda T, Kamiya M, Maruo S, Iwakiri D, Takada K. Symmetrical localization of extrachromosomally replicating viral genomes on sister chromatids. Journal of Cell Science. 2007;120(9):1529–1539. doi: 10.1242/jcs.03434. [DOI] [PubMed] [Google Scholar]
- 60.Dheekollu J, Deng Z, Wiedmer A, Weitzman MD, Lieberman PM. A role for MRE11, NBS1, and recombination junctions in replication and stable maintenance of EBV episomes. PLoS ONE. 2007;2(12, artcle 1257) doi: 10.1371/journal.pone.0001257.e1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nanbo A, Sugden A, Sugden B. The coupling of synthesis and partitioning of EBV’s plasmid replicon is revealed in live cells. EMBO Journal. 2007;26(19):4252–4262. doi: 10.1038/sj.emboj.7601853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Deutsch MJ, Ott E, Papior P, Schepers A. The latent origin of replication of Epstein-Barr virus directs viral genomes to active regions of the nucleus. Journal of Virology. 2010;84(5):2533–2546. doi: 10.1128/JVI.01909-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marechal V, Dehee A, Chikhi-Brachet R, Piolot T, Coppey-Moisan M, Nicolas JC. Mapping EBNA-1 domains involved in binding to metaphase chromosomes. Journal of Virology. 1999;73(5):4385–4392. doi: 10.1128/jvi.73.5.4385-4392.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shire K, Kapoor P, Jiang K, et al. Regulation of the EBNA1 epstein-barr virus protein by serine phosphorylation and arginine methylation. Journal of Virology. 2006;80(11):5261–5272. doi: 10.1128/JVI.02682-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sears J, Kolman J, Wahl GM, Aiyar A. Metaphase chromosome tethering is necessary for the DNA synthesis and maintenance of oriP plasmids but is insufficient for transcription activation by Epstein-Barr nuclear antigen 1. Journal of Virology. 2003;77(21):11767–11780. doi: 10.1128/JVI.77.21.11767-11780.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sears J, Ujihara M, Wong S, Ott C, Middeldorp J, Aiyar A. The amino terminus of Epstein-Barr virus (EBV) nuclear antigen 1 contains at hooks that facilitate the replication and partitioning of latent EBV genomes by tethering them to cellular chromosomes. Journal of Virology. 2004;78(21):11487–11505. doi: 10.1128/JVI.78.21.11487-11505.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nayyar VK, Shire K, Frappier L. Mitotic chromosome interactions of Epstein-Barr nuclear antigen 1 (EBNA1) and human EBNA1-bindingprotein 2 (EBP2) Journal of Cell Science. 2009;122(23):4341–4350. doi: 10.1242/jcs.060913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Laine A, Frappier L. Identification of Epstein-Barr virus nuclear antigen 1 protein domains that direct interactions at a distance between DNA-bound proteins. Journal of Biological Chemistry. 1995;270(52):30914–30918. doi: 10.1074/jbc.270.52.30914. [DOI] [PubMed] [Google Scholar]
- 69.You J. Papillomavirus interaction with cellular chromatin. Biochimica et Biophysica Acta. 2010;1799(3-4):192–199. doi: 10.1016/j.bbagrm.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Krithivas A, Fujimuro M, Weidner M, Young DB, Hayward SD. Protein interactions targeting the latency-associated nuclear antigen of Kaposi’s sarcoma-associated herpesvirus to cell chromosomes. Journal of Virology. 2002;76(22):11596–11604. doi: 10.1128/JVI.76.22.11596-11604.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Barbera AJ, Chodaparambil JV, Kelley-Clarke B, et al. The nucleosomal surface as a docking station for Kaposi’s sarcoma herpesvirus LANA. Science. 2006;311(5762):856–861. doi: 10.1126/science.1120541. [DOI] [PubMed] [Google Scholar]
- 72.Parish JL, Bean AM, Park RB, Androphy EJ. ChlR1 Is Required for Loading Papillomavirus E2 onto Mitotic Chromosomes and Viral Genome Maintenance. Molecular Cell. 2006;24(6):867–876. doi: 10.1016/j.molcel.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 73.Kapoor P, Frappier L. EBNA1 partitions Epstein-Barr virus plasmids in yeast cells by attaching to human EBNA1-binding protein 2 on mitotic chromosomes. Journal of Virology. 2003;77(12):6946–6956. doi: 10.1128/JVI.77.12.6946-6956.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kapoor P, Frappier L. Methods for measuring the replication and segregation of Epstein-Barr virus-based plasmids. Methods in molecular biology (Clifton, N.J.) 2005;292:247–266. doi: 10.1385/1-59259-848-x:247. [DOI] [PubMed] [Google Scholar]
- 75.Norseen J, Johnson FB, Lieberman PM. Role for G-quadruplex RNA binding by Epstein-Barr virus nuclear antigen 1 in DNA replication and metaphase chromosome attachment. Journal of Virology. 2009;83(20):10336–10346. doi: 10.1128/JVI.00747-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Snudden DK, Hearing J, Smith PR, Grasser FA, Griffin BE. EBNA-1, the major nuclear antigen of Epstein-Barr virus, resembles ’RGG’ RNA binding proteins. EMBO Journal. 1994;13(20):4840–4847. doi: 10.1002/j.1460-2075.1994.tb06810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jourdan N, Jobart-Malfait A, Dos Reis G, Quignon F, Piolot T, et al. Live-cell imaging reveals multiple interactions between Epstein-Barr virus nuclear antigen 1 and cellular chromatin during interphase and mitosis. Journal of Virology. 2012;86:5314–5329. doi: 10.1128/JVI.06303-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Feeney KM, Saade A, Okrasa K, Parish JL. In vivo analysis of the cell cycle dependent association of the bovine papillomavirus E2 protein and ChlR1. Virology. 2011;414(1):1–9. doi: 10.1016/j.virol.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 79.Reisman D, Sugden B. trans activation of an Epstein-Barr viral transcriptional enhancer by the Epstein-Barr viral nuclear antigen 1. Molecular and Cellular Biology. 1986;6(11):3838–3846. doi: 10.1128/mcb.6.11.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sugden B, Warren N. A promotor of Epstein-Barr virus that can function during latent infection can be transactivated by EBNA-1, a viral protein required for viral DNA replication during latent infection. Journal of Virology. 1989;63(6):2644–2649. doi: 10.1128/jvi.63.6.2644-2649.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gahn TA, Sugden B. An EBNA-1-dependent enhancer acts from a distance of 10 kilobase pairs to increase expression of the Epstein-Barr virus LMP gene. Journal of Virology. 1995;69(4):2633–2636. doi: 10.1128/jvi.69.4.2633-2636.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang Y, Finan JE, Middeldorp JM, Hayward SD. P32/TAP, a cellular protein that interacts with EBNA-1 of Epstein-Barr virus. Virology. 1997;236(1):18–29. doi: 10.1006/viro.1997.8739. [DOI] [PubMed] [Google Scholar]
- 83.Kennedy G, Sugden B. EBNA-1, a bifunctional transcriptional activator. Molecular and Cellular Biology. 2003;23(19):6901–6908. doi: 10.1128/MCB.23.19.6901-6908.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Altmann M, Pich D, Ruiss R, Wang J, Sugden B, Hammerschmidt W. Transcriptional activation by EBV nuclear antigen 1 is essential for the expression of EBV’s transforming genes. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(38):14188–14193. doi: 10.1073/pnas.0605985103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lin A, Wang S, Nguyen T, Shire K, Frappier L. The EBNA1 protein of Epstein-Barr virus functionally interacts with Brd4. Journal of Virology. 2008;82(24):12009–12019. doi: 10.1128/JVI.01680-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wu SY, Chiang CM. The double bromodomain-containing chromatin adaptor Brd4 and transcriptional regulation. Journal of Biological Chemistry. 2007;282(18):13141–13145. doi: 10.1074/jbc.R700001200. [DOI] [PubMed] [Google Scholar]
- 87.Schweiger MR, You J, Howley PM. Bromodomain protein 4 mediates the papillomavirus E2 transcriptional activation function. Journal of Virology. 2006;80(9):4276–4285. doi: 10.1128/JVI.80.9.4276-4285.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.McPhillips MG, Oliveira JG, Spindler JE, Mitra R, McBride AA. Brd4 is required for E2-mediated transcriptional activation but not genome partitioning of all papillomaviruses. Journal of Virology. 2006;80(19):9530–9543. doi: 10.1128/JVI.01105-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ilves I, Mäemets K, Silla T, Janikson K, Ustav M. Brd4 is involved in multiple processes of the bovine papillomavirus type 1 life cycle. Journal of Virology. 2006;80(7):3660–3665. doi: 10.1128/JVI.80.7.3660-3665.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Park YJ, Luger K. Structure and function of nucleosome assembly proteins. Biochemistry and Cell Biology. 2006;84(4):549–558. doi: 10.1139/o06-088. [DOI] [PubMed] [Google Scholar]
- 91.Rehtanz M, Schmidt HM, Warthorst U, Steger G. Direct Interaction between nucleosome assembly protein 1 and the papillomavirus E2 proteins involved in activation of transcription. Molecular and Cellular Biology. 2004;24(5):2153–2168. doi: 10.1128/MCB.24.5.2153-2168.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sarkari F, Sanchez-Alcaraz T, Wang S, Holowaty MN, Sheng Y, Frappier L. EBNA1-mediated recruitment of a histone H2B deubiquitylating complex to the Epstein-Barr virus latent origin of DNA replication. PLoS Pathogens. 2009;5(10, article e1000624) doi: 10.1371/journal.ppat.1000624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jones CH, Hayward SD, Rawlins DR. Interaction of the lymphocyte-derived Epstein-Barr virus nuclear antigen EBNA-1 with its DNA-binding sites. Journal of Virology. 1989;63(1):101–110. doi: 10.1128/jvi.63.1.101-110.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sample J, Henson EBD, Sample C. The Epstein-Barr virus nuclear protein 1 promoter active in type I latency is autoregulated. Journal of Virology. 1992;66(8):4654–4661. doi: 10.1128/jvi.66.8.4654-4661.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nonkwelo C, Skinner J, Bell A, Rickinson A, Sample J. Transcription start sites downstream of the Epstein-Barr virus (EBV) Fp promoter in early-passage Burkitt lymphoma cells define a fourth promoter for expression of the EBV EBNA-1 protein. Journal of Virology. 1996;70(1):623–627. doi: 10.1128/jvi.70.1.623-627.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ambinder RF, Shah WA, Rawlins DR, Hayward GS, Hayward SD. Definition of the sequence requirements for binding of the EBNA-1 protein to its palindromic target sites in Epstein-Barr virus DNA. Journal of Virology. 1990;64(5):2369–2379. doi: 10.1128/jvi.64.5.2369-2379.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yoshioka M, Crum MM, Sample JT. Autorepression of Epstein-Barr virus nuclear antigen 1 expression by inhibition of pre-mRNA processing. Journal of Virology. 2008;82(4):1679–1687. doi: 10.1128/JVI.02142-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Summers H, Barwell JA, Pfuetzner RA, Edwards AM, Frappier L. Cooperative assembly of EBNA1 on the Epstein-Barr virus latent origin of replication. Journal of Virology. 1996;70(2):1228–1231. doi: 10.1128/jvi.70.2.1228-1231.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hsieh DJ, Camiolo SM, Yates JL. Constitutive binding of EBNA 1 protein to the Epstein-Barr virus replication origin, oriP, with distortion of DNA structure during latent infection. EMBO Journal. 1993;12(13):4933–4944. doi: 10.1002/j.1460-2075.1993.tb06187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ritzi M, Tillack K, Gerhardt J, et al. Complex protein-DNA dynamics at the latent origin of DNA replication of Epstein-Barr virus. Journal of Cell Science. 2003;116(19):3971–3984. doi: 10.1242/jcs.00708. [DOI] [PubMed] [Google Scholar]
- 101.Ambinder RF, Mullen M, Chang YN, Hayward GS, Hayward SD. Functional domains of Epstein-Barr virus nuclear antigen EBNA-1. Journal of Virology. 1991;65(3):1466–1478. doi: 10.1128/jvi.65.3.1466-1478.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen MR, Middeldorp JM, Hayward SD. Separation of the complex DNA binding domain of EBNA-1 into DNA recognition and dimerization subdomains of novel structure. Journal of Virology. 1993;67(8):4875–4885. doi: 10.1128/jvi.67.8.4875-4885.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shah WA, Ambinder RF, Hayward GS, Hayward SD. Binding of EBNA-1 to DNA creates a protease-resistant domain that encompasses the DNA recognition and dimerization functions. Journal of Virology. 1992;66(6):3355–3362. doi: 10.1128/jvi.66.6.3355-3362.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bochkarev A, Barwell JA, Pfuetzner RA, Furey W, Edwards AM, Frappier L. Crystal structure of the DNA-binding domain of the epstein-barr virus origin-binding protein EBNA. Cell. 1995;83(1):39–46. doi: 10.1016/0092-8674(95)90232-5. [DOI] [PubMed] [Google Scholar]
- 105.Bochkarev A, Barawell JA, Pfuetzner RA, Bochkareva E, Frappier L, Edwards AM. Crystal structure of the DNA-binding domain of the Epstein-Barr virus origin-binding protein, EBNA1, bound to DNA. Cell. 1996;84(5):791–800. doi: 10.1016/s0092-8674(00)81056-9. [DOI] [PubMed] [Google Scholar]
- 106.Edwards AM, Bochkarev A, Frappier L. Origin DNA-binding proteins. Current Opinion in Structural Biology. 1998;8(1):49–53. doi: 10.1016/s0959-440x(98)80009-2. [DOI] [PubMed] [Google Scholar]
- 107.Hegde RS, Grossman SR, Laimins LA, Sigler PB. Crystal structure at 1.7 Å of the bovine papillomavirus-1 E2 DNA-binding domain bound to its DNA target. Nature. 1992;359(6395):505–512. doi: 10.1038/359505a0. [DOI] [PubMed] [Google Scholar]
- 108.Cruickshank J, Shire K, Davidson AR, Edwards AM, Frappier L. Two domains of the Epstein-Barr virus origin DNA-binding protein, EBNA1, orchestrate sequence-specific DNA binding. Journal of Biological Chemistry. 2000;275(29):22273–22277. doi: 10.1074/jbc.M001414200. [DOI] [PubMed] [Google Scholar]
- 109.Oddo C, Freire E, Frappier L, De Prat-Gay G. Mechanism of DNA recognition at a viral replication origin. Journal of Biological Chemistry. 2006;281(37):26893–26903. doi: 10.1074/jbc.M602083200. [DOI] [PubMed] [Google Scholar]
- 110.Bochkarev A, Bochkareva E, Frappier L, Edwards AM. The 2.2 Å structure of a permanganate-sensitive DNA site bound by the Epstein-Barr virus origin binding protein, EBNA1. Journal of Molecular Biology. 1998;284(5):1273–1278. doi: 10.1006/jmbi.1998.2247. [DOI] [PubMed] [Google Scholar]
- 111.Summers H, Fleming A, Frappier L. Requirements for Epstein-Barr nuclear antigen 1 (EBNA1)-induced permanganate sensitivity of the Epstein-Barr virus latent origin of DNA replication. Journal of Biological Chemistry. 1997;272(42):26434–26440. doi: 10.1074/jbc.272.42.26434. [DOI] [PubMed] [Google Scholar]
- 112.Frappier L, O’Donnell M. EBNA1 distorts oriP, the Epstein-Barr virus latent replication origin. Journal of Virology. 1992;66(3):1786–1790. doi: 10.1128/jvi.66.3.1786-1790.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hearing J, Mulhaupt Y, Harper S. Interaction of Epstein-Barr virus nuclear antigen 1 with the viral latent origin of replication. Journal of Virology. 1992;66(2):694–705. doi: 10.1128/jvi.66.2.694-705.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Frappier L, O’Donnell M. Epstein-Barr nuclear antigen 1 mediates a DNA loop within the latent replication origin of Epstein-Barr virus. Proceedings of the National Academy of Sciences of the United States of America. 1991;88(23):10875–10879. doi: 10.1073/pnas.88.23.10875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Goldsmith K, Bendell L, Frappier L. Identification of EBNA1 amino acid sequences required for the interaction of the functional elements of the Epstein-Barr virus latent origin of DNA replication. Journal of Virology. 1993;67(6):3418–3426. doi: 10.1128/jvi.67.6.3418-3426.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Su W, Middleton T, Sugden B, Echols H. DNA looping between the origin of replication of Epstein-Barr virus and its enhancer site: Stabilization of an origin complex with Epstein-Barr nuclear antigen 1. Proceedings of the National Academy of Sciences of the United States of America. 1991;88(23):10870–10874. doi: 10.1073/pnas.88.23.10870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Middleton T, Sugden B. EBNA1 can link the enhancer element to the initiator element of the Epstein-Barr virus plasmid origin of DNA replication. Journal of Virology. 1992;66(1):489–495. doi: 10.1128/jvi.66.1.489-495.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Frappier L, Goldsmith K, Bendell L. Stabilization of the EBNA1 protein on the Epstein-Barr virus latent origin of DNA replication by a DNA looping mechanism. Journal of Biological Chemistry. 1994;269(2):1057–1062. [PubMed] [Google Scholar]
- 119.Mackey D, Middleton T, Sugden B. Multiple regions within EBNA1 can link DNAs. Journal of Virology. 1995;69(10):6199–6208. doi: 10.1128/jvi.69.10.6199-6208.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Avolio-Hunter TM, Frappier L. Mechanistic studies on the DNA linking activity of Epstein-Barr nuclear antigen 1. Nucleic Acids Research. 1998;26(19):4462–4470. doi: 10.1093/nar/26.19.4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shaw JE, Levinger LF, Carter CW. Nucleosomal structure of Epstein-Barr virus DNA in transformed cell lines. Journal of Virology. 1979;29(2):657–665. doi: 10.1128/jvi.29.2.657-665.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Avolio-Hunter TM, Lewis PN, Frappier L. Epstein-Barr nuclear antigen 1 binds and destabilizes nucleosomes at the viral origin of latent DNA replication. Nucleic Acids Research. 2001;29(17):3520–3528. doi: 10.1093/nar/29.17.3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Avolio-Hunter TM, Frappier L. EBNA1 efficiently assembles on chromatin containing the Epstein-Barr virus latent origin of replication. Virology. 2003;315(2):398–408. doi: 10.1016/s0042-6822(03)00561-0. [DOI] [PubMed] [Google Scholar]
- 124.Dresang LR, Vereide DT, Sugden B. Identifying sites bound by Epstein-Barr Virus nuclear antigen 1 (EBNA1) in the human genome: defining a position-weighted matrix to predict sites bound by EBNA1 in viral genomes. Journal of Virology. 2009;83(7):2930–2940. doi: 10.1128/JVI.01974-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lu F, Wikramasinghe P, Norseen J, et al. Genome-wide analysis of host-chromosome binding sites for Epstein-Barr Virus Nuclear Antigen 1 (EBNA1) Virology Journal. 2010;7, article 262 doi: 10.1186/1743-422X-7-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Canaan A, Haviv I, Urban AE, et al. EBNA1 regulates cellular gene expression by binding cellular promoters. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(52):22421–22426. doi: 10.1073/pnas.0911676106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.d'Herouel AF, Birgersdotter A, Werner M. FR-like EBNA1 binding repeats in the human genome. Virology. 2010;405(2):524–529. doi: 10.1016/j.virol.2010.06.040. [DOI] [PubMed] [Google Scholar]
- 128.O’Neil JD, Owen TJ, Wood VHJ, et al. Epstein-Barr virus-encoded EBNA1 modulates the AP-1 transcription factor pathway in nasopharyngeal carcinoma cells and enhances angiogenesis in vitro. Journal of General Virology. 2008;89(11):2833–2842. doi: 10.1099/vir.0.2008/003392-0. [DOI] [PubMed] [Google Scholar]
- 129.Lu J, Murakami M, Verma SC, et al. Epstein-Barr Virus nuclear antigen 1 (EBNA1) confers resistance to apoptosis in EBV-positive B-lymphoma cells through up-regulation of survivin. Virology. 2011;410(1):64–75. doi: 10.1016/j.virol.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Humme S, Reisbach G, Feederle R, et al. The EBV nuclear antigen 1 (EBNA1) enhances B cell immortalization several thousandfold. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(19):10989–10994. doi: 10.1073/pnas.1832776100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kennedy G, Komano J, Sugden B. Epstein-Barr virus provides a survival factor to Burkitt’s lymphomas. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(2):14269–14274. doi: 10.1073/pnas.2336099100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hong M, Murai Y, Kutsuna T, et al. Suppression of Epstein-Barr nuclear antigen 1 (EBNA1) by RNA interference inhibits proliferation of EBV-positive Burkitt’s lymphoma cells. Journal of Cancer Research and Clinical Oncology. 2006;132(1):1–8. doi: 10.1007/s00432-005-0036-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yin Q, Flemington EK. siRNAs against the Epstein Barr virus latency replication factor, EBNA1, inhibit its function and growth of EBV-dependent tumor cells. Virology. 2006;346(2):385–393. doi: 10.1016/j.virol.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 134.Sheu LF, Chen A, Meng CL, Ho KC, Lee WH, et al. Enhanced malignant progression of nasopharyngeal carcinoma cells mediated by the expression of Epstein-Barr nuclear antigen 1 in vivo. Journal of Pathology. 1996;180:243–248. doi: 10.1002/(SICI)1096-9896(199611)180:3<243::AID-PATH655>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 135.Cheng TC, Hsieh SS, Hsu WL, Chen YF, Ho HH, Sheu LFA. Expression of Epstein-Barr nuclear antigen 1 in gastric carcinoma cells is associated with enhanced tumorigenicity and reduced cisplatin sensitivity. International Journal of Oncology. 2010;36(1):151–160. [PubMed] [Google Scholar]
- 136.Kube D, Vockerodt M, Weber O, et al. Expression of Epstein-Barr virus nuclear antigen 1 is associated with enhanced expression of CD25 in the Hodgkin cell line L428. Journal of Virology. 1999;73(2):1630–1636. doi: 10.1128/jvi.73.2.1630-1636.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kaul R, Murakami M, Choudhuri T, Robertson ES. Epstein-Barr virus latent nuclear antigens can induce metastasis in a nude mouse model. Journal of Virology. 2007;81(19):10352–10361. doi: 10.1128/JVI.00886-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wilson JB, Bell JL, Levine AJ. Expression of Epstein-Barr virus nuclear antigen-1 induces B cell neoplasia in transgenic mice. EMBO Journal. 1996;15(12):3117–3126. [PMC free article] [PubMed] [Google Scholar]
- 139.Tsimbouri P, Drotar ME, Coy JL, Wilson JB. bcl-xL and RAG genes are induced and the response to IL-2 enhanced in EμEBNA-1 transgenic mouse lymphocytes. Oncogene. 2002;21(33):5182–5187. doi: 10.1038/sj.onc.1205490. [DOI] [PubMed] [Google Scholar]
- 140.Kang MS, Lu H, Yasui T, et al. Epstein-Barr virus nuclear antigen 1 does not induce lymphoma in transgenic FVB mice. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(3):820–825. doi: 10.1073/pnas.0408774102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kang MS, Soni V, Bronson R, Kieff E. Epstein-barr virus nuclear antigen 1 does not cause lymphoma in C57BL/6J mice. Journal of Virology. 2008;82(8):4180–4183. doi: 10.1128/JVI.02596-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Malik-Soni N, Frappier L. Proteomic profiling of EBNA1-host protein interactions in latent and lytic Epstein-Barr virus infections. Journal of Virology. 2012;86:6999–7002. doi: 10.1128/JVI.00194-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Everett RD, Meredith M, Orr A, Cross A, Kathoria M, Parkinson J. A novel ubiquitin-specific protease is dynamically associated with the PML nuclear domain and binds to a herpesvirus regulatory protein. EMBO Journal. 1997;16(7):1519–1530. doi: 10.1093/emboj/16.7.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Salsman J, Jagannathan M, Paladino P, Chan PK, Dellaire G, et al. Proteomic profiling of the human cytomegalovirus UL35 gene products reveals a role for UL35 in the DNA repair response. Journal of Virology. 2012;86:806–820. doi: 10.1128/JVI.05442-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lee HR, Choi WC, Lee S, Hwang J, Hwang E. Bilateral inhibition of HAUSP deubiquitinase by a viral interferon regulatory factor protein. Nature Structural & Molecular Biology. 2011;18:1336–1344. doi: 10.1038/nsmb.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jager W, Santag S, Weidner-Glunde M, Gellermann E, Kati S, et al. The ubiquitin-specific protease USP7 modulates the replication of Kaposi's sarcoma-associated herpesvirus latent episomal DNA. Journal of Virology. 2012;86:6745–6757. doi: 10.1128/JVI.06840-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Li M, Chen D, Shiloh A, et al. Deubiquitination of p53 by HAUSP is an important pathway for p53 stabilization. Nature. 2002;416(6881):648–653. doi: 10.1038/nature737. [DOI] [PubMed] [Google Scholar]
- 148.Li M, Brooks CL, Kon N, Gu W. A dynamic role of HAUSP in the p53-Mdm2 pathway. Molecular Cell. 2004;13(6):879–886. doi: 10.1016/s1097-2765(04)00157-1. [DOI] [PubMed] [Google Scholar]
- 149.Cummings JM, Rago C, Kohli M, Kinzler KW, Lengauer C, Vogelstein B. Tumour suppression: disruption of HAUSP gene stabilizes p53. Nature. 2004;428(6982):486–487. doi: 10.1038/nature02501. [DOI] [PubMed] [Google Scholar]
- 150.Nicholson B, Suresh Kumar KG. The multifaceted roles of USP7: new therapeutic opportunities. Cell Biochemistry and Biophysics. 2011;60(1-2):61–68. doi: 10.1007/s12013-011-9185-5. [DOI] [PubMed] [Google Scholar]
- 151.Frappier L, Verrijzer CP. Gene expression control by protein deubiquitinases. Current Opinion in Genetics and Development. 2011;21(2):207–213. doi: 10.1016/j.gde.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 152.Holowaty MN, Sheng Y, Nguyen T, Arrowsmith C, Frappier L. Protein interaction domains of the ubiquitin-specific protease, USP7/HAUSP. Journal of Biological Chemistry. 2003;278(48):47753–47761. doi: 10.1074/jbc.M307200200. [DOI] [PubMed] [Google Scholar]
- 153.Saridakis V, Sheng Y, Sarkari F, et al. Structure of the p53 binding domain of HAUSP/USP7 bound to epstein-barr nuclear antigen 1: implications for EBV-mediated immortalization. Molecular Cell. 2005;18(1):25–36. doi: 10.1016/j.molcel.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 154.Sheng Y, Saridakis V, Sarkari F, et al. Molecular recognition of p53 and MDM2 by USP7/HAUSP. Nature Structural and Molecular Biology. 2006;13(3):285–291. doi: 10.1038/nsmb1067. [DOI] [PubMed] [Google Scholar]
- 155.Hu M, Gu L, Li M, Jeffrey PD, Gu W, Shi Y. Structural basis of competitive recognition of p53 and MDM2 by HAUSP/USP7: implications for the regulation of the p53-MDM2 pathway. PLoS Biology. 2006;4(2, article e27) doi: 10.1371/journal.pbio.0040027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sivachandran N, Sarkari F, Frappier L. Epstein-Barr nuclear antigen 1 contributes to nasopharyngeal carcinoma through disruption of PML nuclear bodies. PLoS Pathogens. 2008;4(10, article e1000170) doi: 10.1371/journal.ppat.1000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sivachandran N, Dawson CW, Young LS, Liu FF, Middeldorp J, et al. Contributions of the Epstein-Barr virus EBNA1 protein to gastric carcinoma. Journal of Virology. 2012;86:60–68. doi: 10.1128/JVI.05623-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Salomoni P, Ferguson BJ, Wyllie AH, Rich T. New insights into the role of PML in tumour suppression. Cell Research. 2008;18(6):622–640. doi: 10.1038/cr.2008.58. [DOI] [PubMed] [Google Scholar]
- 159.Bernardi R, Pandolfi PP. Structure, dynamics and functions of promyelocytic leukaemia nuclear bodies. Nature Reviews Molecular Cell Biology. 2007;8(12):1006–1016. doi: 10.1038/nrm2277. [DOI] [PubMed] [Google Scholar]
- 160.Takahashi Y, Lallemand-Breitenbach V, Zhu J, De Thé H. PML nuclear bodies and apoptosis. Oncogene. 2004;23(16):2819–2824. doi: 10.1038/sj.onc.1207533. [DOI] [PubMed] [Google Scholar]
- 161.Guo A, Salomoni P, Luo J, et al. The function of PML in p53-dependent apoptosis. Nature Cell Biology. 2000;2(10):730–736. doi: 10.1038/35036365. [DOI] [PubMed] [Google Scholar]
- 162.Wang ZG, Ruggero D, Ronchetti S, et al. Pml is essential for multiple apoptotic pathways. Nature Genetics. 1998;20(3):266–272. doi: 10.1038/3073. [DOI] [PubMed] [Google Scholar]
- 163.Pearson M, Carbone R, Sebastiani C, et al. PML regulates p53 acetylation and premature senescence induced by oncogenic Ras. Nature. 2000;406(6792):207–210. doi: 10.1038/35018127. [DOI] [PubMed] [Google Scholar]
- 164.Gurrieri C, Capodieci P, Bernardi R, et al. Loss of the tumor suppressor PML in human cancers of multiple histologic origins. Journal of the National Cancer Institute. 2004;96(4):269–279. doi: 10.1093/jnci/djh043. [DOI] [PubMed] [Google Scholar]
- 165.Geoffroy MC, Chelbi-Alix MK. Role of promyelocytic leukemia protein in host antiviral defense. Journal of Interferon and Cytokine Research. 2011;31(1):145–158. doi: 10.1089/jir.2010.0111. [DOI] [PubMed] [Google Scholar]
- 166.Everett RD, Chelbi-Alix MK. PML and PML nuclear bodies: implications in antiviral defence. Biochimie. 2007;89(6-7):819–830. doi: 10.1016/j.biochi.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 167.Reichelt M, Wang L, Sommer M, et al. Entrapment of viral capsids in nuclear PML cages is an intrinsic antiviral host defense against Varicella-zoster virus. PLoS Pathogens. 2011;7(2, article e1001266) doi: 10.1371/journal.ppat.1001266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Everett RD. DNA viruses and viral proteins that interact with PML nuclear bodies. Oncogene. 2001;20(49):7266–7273. doi: 10.1038/sj.onc.1204759. [DOI] [PubMed] [Google Scholar]
- 169.Sivachandran N, Cao JY, Frappier L. Epstein-Barr virus nuclear antigen 1 hijacks the host kinase CK2 to disrupt PML nuclear bodies. Journal of Virology. 2010;84(21):11113–11123. doi: 10.1128/JVI.01183-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sivachandran N, Wang X, Frappier L. Functions of the Epstein-Barr virus EBNA1 protein in viral reactivation and lytic infection. Journal of Virology. 2012;86:6146–6158. doi: 10.1128/JVI.00013-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sarkari F, Wang X, Nguyen T, Frappier L. The herpesvirus associated ubiquitin specific protease, USP7, is a negative regulator of PML proteins and PML nuclear bodies. PLoS ONE. 2011;6(1, article e16598) doi: 10.1371/journal.pone.0016598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Scaglioni PP, Yung TM, Cai LF, et al. A CK2-Dependent mechanism for degradation of the PML tumor suppressor. Cell. 2006;126(2):269–283. doi: 10.1016/j.cell.2006.05.041. [DOI] [PubMed] [Google Scholar]
- 173.Scaglioni PP, Yung TM, Choi SC, Baldini C, Konstantinidou G, Pandolfi PP. CK2 mediates phosphorylation and ubiquitin-mediated degradation of the PML tumor suppressor. Molecular and Cellular Biochemistry. 2008;316(1-2):149–154. doi: 10.1007/s11010-008-9812-7. [DOI] [PubMed] [Google Scholar]
- 174.Wood VHJ, O'Neil JD, Wei W, Stewart SE, Dawson CW, Young LS. Epstein-Barr virus-encoded EBNA1 regulates cellular gene transcription and modulates the STAT1 and TGFβ signaling pathways. Oncogene. 2007;26(28):4135–4147. doi: 10.1038/sj.onc.1210496. [DOI] [PubMed] [Google Scholar]
- 175.Kim HS, Lee MS. STAT1 as a key modulator of cell death. Cellular Signalling. 2007;19(3):454–465. doi: 10.1016/j.cellsig.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 176.Flavell JR, Baumforth KRN, Wood VHJ, et al. Down-regulation of the TGF-beta target gene, PTPRK, by the Epstein-Barr virus-encoded EBNA1 contributes to the growth and survival of Hodgkin lymphoma cells. Blood. 2008;111(1):292–301. doi: 10.1182/blood-2006-11-059881. [DOI] [PubMed] [Google Scholar]
- 177.Valentine R, Dawson CW, Hu C, et al. Epstein-Barr virus-encoded EBNA1 inhibits the canonical NF-κB pathway in carcinoma cells by inhibiting IKK phosphorylation. Molecular Cancer. 2010;9, article 1 doi: 10.1186/1476-4598-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lassoued S, Ameur RB, Ayadi W, Gargouri B, Mansour RB, Attia H. Epstein-Barr virus induces an oxidative stress during the early stages of infection in B lymphocytes, epithelial, and lymphoblastoid cell lines. Molecular and Cellular Biochemistry. 2008;313(1-2):179–186. doi: 10.1007/s11010-008-9755-z. [DOI] [PubMed] [Google Scholar]
- 179.Cerimele F, Battle T, Lynch R, et al. Reactive oxygen signaling and MAPK activation distinguish Epstein-Barr Virus (EBV)-positive versus EBV-negative Burkitt’s lymphoma. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(1):175–179. doi: 10.1073/pnas.0408381102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Gruhne B, Sompallae R, Marescotti D, Kamranvar SA, Gastaldello S, Masucci MG. The Epstein-Barr virus nuclear antigen-1 promotes genomic instability via induction of reactive oxygen species. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(7):2313–2318. doi: 10.1073/pnas.0810619106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kamranvar SA, Masucci MG. The Epstein-Barr virus nuclear antigen-1 promotes telomere dysfunction via induction of oxidative stress. Leukemia. 2011;25(6):1017–1025. doi: 10.1038/leu.2011.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Cao JY, Mansouri S, Frappier L. Changes in the nasopharyngeal carcinoma nuclear proteome induced by the EBNA1 protein of Epstein-Barr virus reveal potential roles for EBNA1 in metastasis and oxidative stress responses. Journal of Virology. 2012;86(1):382–394. doi: 10.1128/JVI.05648-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Murakami M, Lan K, Subramanian C, Robertson ES. Epstein-Barr virus nuclear antigen 1 interacts with Nm23-H1 in lymphoblastoid cell lines and inhibits its ability to suppress cell migration. Journal of Virology. 2005;79(3):1559–1568. doi: 10.1128/JVI.79.3.1559-1568.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Choudhuri T, Murakami M, Kaul R, et al. Nm23-H1 can induce cell cycle arrest and apoptosis in B cells. Cancer Biology and Therapy. 2010;9(12):1065–1078. doi: 10.4161/cbt.9.12.11995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Babcock GJ, Hochberg D, Thorley-Lawson DA. The expression pattern of Epstein-Barr virus latent genes in vivo is dependent upon the differentiation stage of the infected B cell. Immunity. 2000;13(4):497–506. doi: 10.1016/s1074-7613(00)00049-2. [DOI] [PubMed] [Google Scholar]
- 186.Münz C. Epstein-Barr virus nuclear antigen 1: from immunologically invisible to a promising T cell target. Journal of Experimental Medicine. 2004;199(10):1301–1304. doi: 10.1084/jem.20040730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Khanna R, Burrows SR, Moss DJ. Immune regulation in Epstein-Barr virus-associated diseases. Microbiological Reviews. 1995;59(3):387–405. doi: 10.1128/mr.59.3.387-405.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Blake N, Lee S, Redchenko I, et al. Human CD8+ T cell responses to EBV EBNA1: HLA class I presentation of the (Gly-Ala)-containing protein requires exogenous processing. Immunity. 1997;7(6):791–802. doi: 10.1016/s1074-7613(00)80397-0. [DOI] [PubMed] [Google Scholar]
- 189.Levitskaya J, Coram M, Levitsky V, et al. Inhibition of antigen processing by the internal repeat region of the Epstein-Barr virus nuclear antigen-1. Nature. 1995;375(6533):685–688. doi: 10.1038/375685a0. [DOI] [PubMed] [Google Scholar]
- 190.Levitskaya J, Sharipo A, Leonchiks A, Ciechanover A, Masucci MG. Inhibition of ubiquitin/proteasome-dependent protein degradation by the Gly-Ala repeat domain of the Epstein-Barr virus nuclear antigen 1. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(23):12616–12621. doi: 10.1073/pnas.94.23.12616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Sharipo A, Imreh M, Leonchiks A, Imreh S, Masucci MG. A minimal glycine-alanine repeat prevents the interaction of ubiquitinated IκBα with the proteasome: a new mechanism for selective inhibition of proteolysis. Nature Medicine. 1998;4(8):939–944. doi: 10.1038/nm0898-939. [DOI] [PubMed] [Google Scholar]
- 192.Heessen S, Leonchiks A, Issaeva N, et al. Functional p53 chimeras containing the Epstein-Barr virus Gly-Ala repeat are protected from Mdm2- and HPV-E6-induced proteolysis. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(3):1532–1537. doi: 10.1073/pnas.022306499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Dantuma NP, Heessen S, Lindsten K, Jellne M, Masucci MG. Inhibition of proteasomal degradation by the Gly-Ala repeat of Epstein-Barr virus is influenced by the length of the repeat and the strength of the degradation signal. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(15):8381–8385. doi: 10.1073/pnas.140217397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Zhang M, Coffino P. Repeat sequence of Epstein-Barr virus-encoded nuclear antigen 1 protein interrupts proteasome substrate processing. Journal of Biological Chemistry. 2004;279(10):8635–8641. doi: 10.1074/jbc.M310449200. [DOI] [PubMed] [Google Scholar]
- 195.Daskalogianni C, Apcher S, Candeias MM, Naski N, Calvo F, Fåhraeus R. Gly-Ala repeats induce position- and substrate-specific regulation of 26 S proteasome-dependent partial processing. Journal of Biological Chemistry. 2008;283(44):30090–30100. doi: 10.1074/jbc.M803290200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Tellam J, Connolly G, Green KJ, et al. Endogenous presentation of CD8+ T cell epitopes from Epstein-Barr virus-encoded nuclear antigen 1. Journal of Experimental Medicine. 2004;199(10):1421–1431. doi: 10.1084/jem.20040191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Yin Y, Manoury B, Fåhraeus R. Self-inhibition of synthesis and antigen presentation by Epstein-Barr virus-encoded EBNA1. Science. 2003;301(5638):1371–1374. doi: 10.1126/science.1088902. [DOI] [PubMed] [Google Scholar]
- 198.Fahraeus R. Do peptides control their own birth and death? Nature Reviews Molecular Cell Biology. 2005;6:263–267. doi: 10.1038/nrm1590. [DOI] [PubMed] [Google Scholar]
- 199.Tellam J, Fogg MH, Rist M, et al. Influence of translation efficiency of homologous viral proteins on the endogenous presentation of CD8+ T cell epitopes. Journal of Experimental Medicine. 2007;204(3):525–532. doi: 10.1084/jem.20062508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Apcher S, Daskalogianni C, Manoury B, Fåhraeus R. Epstein barr virus-encoded EBNA1 interference with MHC class I antigen presentation reveals a close correlation between mRNA translation initiation and antigen presentation. PLoS Pathogens. 2010;6(10, article e1001151) doi: 10.1371/journal.ppat.1001151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Apcher S, Komarova A, Daskalogianni C, Yin Y, Malbert-Colas L, Fåhraeus R. mRNA translation regulation by the gly-ala repeat of Epstein-Barr virus nuclear antigen 1. Journal of Virology. 2009;83(3):1289–1298. doi: 10.1128/JVI.01369-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Tellam J, Smith C, Rist M, et al. Regulation of protein translation through mRNA structure influences MHC class I loading and T cell recognition. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(27):9319–9324. doi: 10.1073/pnas.0801968105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Sun X, Barlow EA, Ma S, et al. Hsp90 inhibitors block outgrowth of EBV-infected malignant cells in vitro and in vivo through an EBNA1-dependent mechanism. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(7):3146–3151. doi: 10.1073/pnas.0910717107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Brink AATP, Meijer CJLM, Nicholls JM, Middeldorp JM, Van den Brule AJC. Activity of the EBNA1 promoter associated with lytic replication (Fp) in Epstein-Barr virus associated disorders. Journal of Clinical Pathology. 2001;54(2):98–102. doi: 10.1136/mp.54.2.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Lear AL, Rowe M, Kurilla MG, et al. The Epstein-Barr virus (EBV) nuclear antigen 1 BamHI F promoter is activated on entry of EBV-transformed B cells into the lytic cycle. Journal of Virology. 1992;66(12):7461–7468. doi: 10.1128/jvi.66.12.7461-7468.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Schaefer BC, Strominger JL, Speck SH. The Epstein-Barr virus BamHI F promoter is an early lytic promoter: Lack of correlation with EBNA 1 gene transcription in group 1 Burkitt’s lymphoma cell lines. Journal of Virology. 1995;69(8):5039–5047. doi: 10.1128/jvi.69.8.5039-5047.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Sides MD, Block GJ, Shan B, et al. Arsenic mediated disruption of promyelocytic leukemia protein nuclear bodies induces ganciclovir susceptibility in Epstein-Barr positive epithelial cells. Virology. 2011;416(1-2):86–97. doi: 10.1016/j.virol.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]


