Abstract
High-risk human papillomavirus (HPV) infections are necessary but insufficient causes of cervical cancers. Other risk factors for cervical cancer (e.g., pregnancy, smoking, infections causing inflammation) can lead to high and sustained nitric oxide (NO) concentrations in the cervix, and high NO levels are related to carcinogenesis through DNA damage and mutation. However, the effects of NO exposure in HPV-infected cells have not been investigated. In this study, we used the NO donor DETA-NO to model NO exposure to cervical epithelium. In cell culture media, 24-hour exposure to 0.25 to 0.5 mmol/L DETA-NO yielded a pathologically relevant NO concentration. Exposure of cells maintaining episomal high-risk HPV genomes to NO increased HPV early transcript levels 2- to 4-fold but did not increase viral DNA replication. Accompanying increased E6 and E7 mRNA levels were significant decreases in p53 and pRb protein levels, lower apoptotic indices, increased DNA double-strand breaks, and higher mutation frequencies when compared with HPV-negative cells. We propose that NO is a molecular cofactor with HPV infection in cervical carcinogenesis, and that modifying local NO cervical concentrations may constitute a strategy whereby HPV-related cancer can be reduced.
Introduction
Cervical cancer is the second most common cancer in women worldwide, killing >200,000 women annually (1). Infections with ≈15 types of human papillomaviruses (HPV) cause virtually all cases of cervical and other anogenital cancers (2). These carcinogenic or “high-risk” HPVs include types 16, 18, 31, 33, and 51 (reviewed in ref. 3). HPVs are small DNA viruses with circular genomes and have a strict tropism for squamous epithelial cells (i.e., human keratinocytes). High-risk HPV oncogenes E6 and E7 are main contributors to HPV-related cervical cancer, exerting their functions, in part, by functionally inactivating tumor suppressor proteins p53 and pRb, respectively. Yet, HPV infection in cervical epithelium is an insufficient cause of cervical cancer, and epidemiologic studies have revealed a number of risk factors. These include tobacco smoking, multiparity, long-term use of oral contraceptives, chronic inflammation, and other sexually transmitted infections [e.g., Chlamydia trachomatis and herpes simplex virus type 2 (HSV-2); ref. 3]. Interestingly, these cofactors have the common activity of increasing nitric oxide (NO) levels in the cervical microenvironment (4–6), and increased NO levels and markers of NO-mediated mutagenesis have recently been observed in the cervixes of women with cervical intraepithelial neoplasia (CIN; refs. 7, 8).
NO is a free radical generated from L-arginine by various NADPH-dependent enzymes called NO synthases (NOS; ref. 9). As a free radical, NO gives rise to various reactive nitrogen species that are important to its bioreactivity. High levels of NO and reactive nitrogen species produced at infectious or inflammatory sites can modify DNA bases in several ways, causing single- and double-strand breaks (DSB) and DNA cross-linking (10). Reactive nitrogen species can mediate G:C to T:A transversion by formation of 8-nitroguanine, a potential mutagen (11). NO-dependent DNA damage, mutagenic effects, and increased tumorigenesis have also been reported in vivo in inflammatory and infectious models wherein inducible NOS (iNOS) is induced or overexpressed (12–14).
In normal cells, high-level free radical damage can be genotoxic leading to cell death through apoptosis or necrosis (15). Consistent with this, high levels of NO are reported to activate the p53 pathway by inhibiting p53 nuclear export, hindering p53 degradation, and activating p53 by phosphorylation (16–18). In response to DNA damage from genotoxic insults, p53 plays an important role in maintaining genomic stability by initiating appropriate cell cycle arrest, DNA repair, senescence, or apoptosis (19). Although high NO concentrations can lead to apoptosis in cancer cell lines containing wild-type p53, cells with mutant p53 or lacking p53 are resistant to apoptosis on NO exposure (20). The majority of HPV-positive cervical cancer cells have wild-type p53, yet they show very low p53 activity due to E6-mediated degradation (21). The ability of p53 to maintain genomic stability in high-risk HPV–infected cells exposed to NO is unknown. NO has various effects on virus infections (reviewed in refs. 22, 23). It acts as an endogenous mutagen for RNA viruses. NO inhibits the replication of certain DNA and RNA viruses, yet others seem unaffected. However, the effect of NO on the HPV life cycle has not been described.
In this report, we investigated the effects of physiologically high NO doses on HPV-infected cells that normally maintain episomal viral genomes. We found that HPV early gene transcription, but not viral genome replication, increased in a NO-dose dependent manner. The NO-related up-regulation of early oncogene E6 resulted in decreased p53 protein levels and activity. Similarly, pRb levels were decreased coincident with HPV E7 transcript induction. As expected from the loss of p53 activity in NO-exposed HPV-infected cells, we detected significant DNA DSBs compared with HPV-negative cells. Apoptosis levels were increased in HPV-negative cells but not in HPV-infected cells. Furthermore, mutation frequencies were higher in surviving HPV-positive cells. These results strongly suggest that physiologic doses of NO could promote malignant progression of HPV-infected cells in vivo.
Materials and Methods
Cell culture, NO exposure, survival, and toxicity
W12E and CIN-612 9E cell lines were established from human CIN-1 biopsies. W12E cells express wild-type p53 and contain episomal HPV16 DNA (24). CIN-612 9E cells maintain episomal HPV31 genomes (25). W12E and CIN-612 9E cells were investigated at <20 passages (1:5 split) after cloning. C-33A is an HPV-negative human cervical carcinoma cell line that expresses mutant p53 (26). Primary human foreskin keratinocytes (HFK) and NIKS cells (Stratatech), a spontaneously immortalized HFK line, harbor wild-type p53 (27). W12E, CIN-612 9E, HFK, and NIKS cells were cocultivated with mitomycin C– treated J2/3T3 fibroblast feeders maintained in E medium containing 10% fetal bovine serum (FBS) and 100 units/mL Nystatin (Sigma-Aldrich; ref. 28); HFK and NIKS cells also required 10 ng/mL epithelial growth factor (Sigma-Aldrich). HaCaT, a spontaneously immortalized human epithelial cell line established from normal adult skin, expresses mutant p53 (29). HaCaT cells were maintained in DMEM/Ham's F-12 medium (Sigma-Aldrich) containing 10% FBS, 4× amino acids, 2 mmol/L L-glutamine, 100 units/mL penicillin, and 1 μg/mL streptomycin (Sigma-Aldrich). For NO donor treatment, cells were seeded without fibroblast feeder cells to be 50% to 70% confluent after 16 to 20 h and then were incubated in fresh medium containing the NO donor (Z)-1-[N-(2-aminoethyl)-N-(2-ammonioethyl)amino]diazen-1-ium-1,2-diolate (DETA-NO) or S-nitroso-N-acetylpenicillamine (SNAP; Cayman Chemicals). Cell culture supernatants were collected at indicated posttreatment time points. NO concentrations in supernatants were measured by two independent methods. Electrochemical analysis was accomplished with InNO Nitric Oxide Measuring System (Innovative Instruments, Inc.); the Griess method (Promega) assayed stable NO metabolites and . Trypan blue exclusion staining assessed cell numbers following 0, 12, and 24 h of DETA-NO donor treatment. Toxicity was measured as directed by assay of lactate dehydogenase (LDH) in cell-free supernatants following DETA-NO donor treatment (Sigma-Aldrich).
Nucleic acid extraction, reverse transcription, and quantitative PCR
Total cellular DNA was harvested as described (30). Total RNAs were extracted from cells using TRIzol (Invitrogen) and processed with the TURBO DNA-free Kit (Ambion) to remove copurifying viral and cellular DNA. Reverse transcription of total RNAs (0.2–0.5 μg) was done as reported (31). For quantitative PCR, 2 μL of each cDNA or total DNA were analyzed in triplicate using the SYBR Green I kit on the iCycler (Bio-Rad). The thermocycling profile was 1 min at 95°C, 40 cycles at 95°C for 30 s and 60°C for 30 s, 5 min at 72°C. Threshold cycle (Ct) numbers were determined using the iCycler IQ optical software (version 3.0a). PCR primers, product length, and amplification efficiency are presented in Supplementary Table S1. The efficiency of each amplification assay, using 10-fold serial pooled templates, was determined from respective templates versus CT efficiency plots in Q-Gene software (32). Triplicate quantitative PCR results from cDNA reactions were normalized to quantities of TATA-box binding protein (TBP) cDNA; DNA amplifications were normalized to GADPH gene copies using Q-Gene software (32). Final results are averages of three independent experiments, with Q test <0.94 and error bars representing SE.
Immunoblotting
Cells were lysed in standard radioimmunoprecipitation assay buffer. Proteins were quantified by Bradford assay, separated by 10% SDS-PAGE, and transferred onto polyvinylidene difluoride membranes (Millipore). Membranes were probed with primary monoclonal antibodies to p53 (Calbiochem) or pRb (Abcam), followed by secondary goat anti-mouse IgG conjugated to horseradish peroxidase (Sigma-Aldrich), and developed by chemiluminescence (Pierce). To control protein loading, membranes were stripped [62.5 mmol/L Tris-Cl (pH 6.8) 2% SDS, 100 mmol/L 2-mercaptoethanol] at 50°C for 20 min and reprobed with anti-β-tubulin antibody (Sigma-Aldrich).
p53 activity
Cells were transfected with p53-luc, a p53 responsive reporter (Stratagene), using the Keratinocyte Nucleofector Kit H (Amaxa). p53-luc contains 13 copies of the p53 consensus sequence driving expression of luciferase. At 24 h posttransfection, cells were refed with fresh medium containing various DETA-NO concentrations for another 24 h; cells were harvested and luciferase activity was detected using the Luciferase Assay System (Promega).
Neutral single-cell gel electrophoresis (comet assay)
Neutral comet assays were done according to Trevigen, Inc. instructions to assess NO-induced DNA DSBs. Briefly, cells were combined with molten agarose and lysed. Electrophoresis was done in 1× Tris-borate EDTA for 10 min at 1 V/cm and stained with SYBR Green I. Photography was done using a Zeiss fluorescence microscope and analyzed with Comet Assay Software Project (CASP). Olive tail moment (OTM), defined as the product of distance (in x direction) between the comet head center of gravity (CGH) and the tail center of gravity (CGT), accounting for the percent tail DNA (DNAT) wherein OTM = (CGTx − CGHx)/%DNAT, was determined for at least 80 cells per sample (33).
Apoptosis and necrosis
Apoptosis was detected by flow cytometry with Annexin V-phycoerythrin kit (BD Biosciences) according to the manufacturer's instructions. By fluorescence-activated cell sorting analysis, Annexin V-phycoerythrin fluorescence was recorded in FL-2 and 7-amino-actino-mycin D (7-AAD) in FL-3. Apoptotic cells were labeled with only Annexin V, necrotic cells with both Annexin V and 7-AAD, and living cells were negative for both.
Mutagenesis
NO−treated cells (24 h) were grown with feeder cells for 6 to 10 d without NO exposure to allow phenotypic expression and then plated in selective medium without feeder cells for 6-thioguanineR (6-TGR) selection to indicate mutation in the HPRT locus. Cells from each treatment were also plated in the absence of selective drug to determine plating efficiency (PE). Triplicates were investigated for each experimental group. After 2 wk, colonies were counted and the mutation frequency was calculated [mutation frequency = number of colonies/(number of seeded cells × PE) × 106 cells. Parallel control experiments assayed cellular mutation response to 4-nitroquinoline 1-oxide (4-NQO; 140 ng/mL for 1.5 h), a well-characterized mutagen.
Statistics
Statistical significance of differences in response was evaluated using the nonparametric Wilcoxon-Mann-Whitney test. Tests of linear trend were conducted using the Wald statistic by linear regression modeling the ordered DETA-NO concentrations as a continuous variable. Correlation between HPV E6 expression and p53 activity was calculated using Pearson's correlation. P ≤ 0.05 was considered significant. Statistical analyses for E6/E7 gene expression and p53 activity, as well as the respective correlations, were done using Stata version 10. All other statistical analyses were done using SigmaStat.
Results
Physiologically relevant, high concentrations of NO are nontoxic to cervical epithelial cells
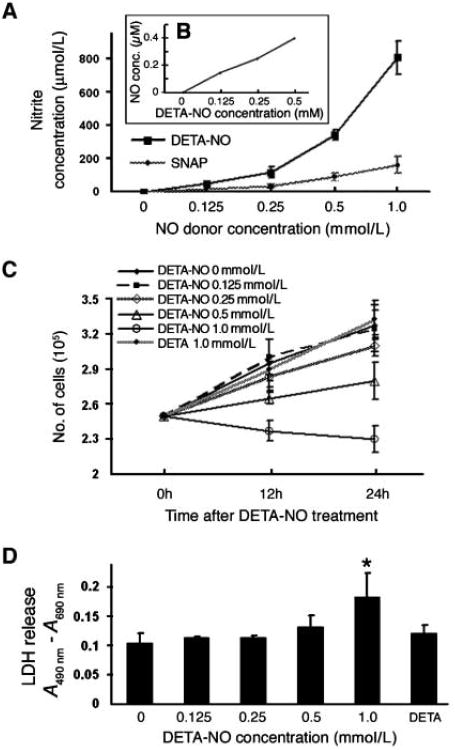
To study the effects of physiologically relevant cervical NO concentrations on HPV-infected cells, we quantified NO production from different concentrations of NO donors SNAP or DETA-NO in cell culture media after 24-hour exposure. NO production correlated with NO donor concentrations; dose-dependent increases in nitrite were detected from each donor and are consistent with other reports (4). At equivalent concentrations, DETA-NO generated more NO than did SNAP (Fig. 1A). Measurements of actual NO concentrations from 0.125 and 0.5 mmol/L of DETA-NO using an NO-specific electrode indicated relatively constant NO concentrations of 150 and 400 nmol/L (Fig. 1B), consistent with previous reports (34, 35). NO concentrations produced from 0.125 and 0.5 mmol/L of DETA-NO were comparable to those produced in vivo at sites of colonic crypt inflammation and airway inflammation (34, 36) and detected in cervical samplings during pregnancy (37).
Figure 1.
Effects of DETA-NO on cell proliferation and cytotoxicity. CIN-612 9E cells (HPV31 positive) were exposed to varying concentrations of SNAP, DETA-NO, or DETA for 24 h at 37°C. A, NO production determined by the Griess method analysis of nitrite production. Background values were subtracted from experimental average absorbance values. Points, average of four separate experiments; bars, SE. B, electrochemical analysis of NO production in cell culture media. C, cell proliferation monitored by quantifying live cells over time by trypan blue exclusion staining. Points, average of three separate experiments; bars, SE. D, cell cytotoxicity determined by detecting LDH release in cell-free culture supernatants. Columns, average of three separate experiments [values represent the absorbance (490 nm) minus the background (690 nm)]; bars, SE. *, P < 0.05.
On decomposition, DETA-NO releases NO and a parent amine, diethylenetriamine (DETA). Therefore, to control for potential biological effects in our assays, we tested the consequences of different DETA-NO concentrations as well as 1 mmol/L DETA on cell proliferation and cytotoxicity. DETA exposure did not affect cell proliferation, whereas DETA-NO treatment (0.25–1.0 mmol/L) resulted in a dose-dependent inhibition of proliferation (Fig. 1C). Neither DETA-NO nor DETA was cytotoxic to CIN-612 9E cells except at the highest DETA-NO dose (1 mmol/L; Fig. 1D). W12E, C-33A, and HFK cells responded similarly in cell proliferation assays to 0.125 to 0.5 mmol/L of DETA-NO, and no cytotoxicity was associated with these exposure doses (not shown).
NO exposure increases HPV early viral transcription but has no effect on viral genome levels
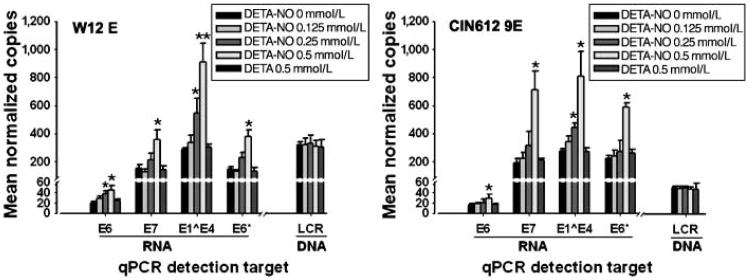
NO has inhibitory effects on replication of the DNA viruses HSV-2 (4) and hepatitis B virus (HBV; ref. 38). In high-risk HPVs, the E1, E2, E6, and E7 gene products have roles in viral genome replication and maintenance (30, 39, 40). Because NO also stimulates p53 accumulation (16), which could affect E6 protein functions, we hypothesized that NO exposure might inhibit HPV replication. We designed quantitative reverse transcription-PCR (RT-PCR) and quantitative PCR assays to assess the effect of elevated NO levels on viral transcription and replication in HPV-infected cells. HPV16-positive W12E and HPV31-positive CIN-612 9E cell lines maintain episomal viral genomes, and we used direct automated sequencing from cDNA to verify wild-type p53 coding regions in both cell lines (not shown). Cells were exposed to 0 to 0.5 mmol/L of DETA-NO or 0.5 mmol/L of DETA alone as a control. After 24 hours of NO exposure, total RNA and DNA were harvested for quantification of HPV early viral transcripts and genome copies. TBP cDNA and the GADPH gene were targeted as internal controls in quantitative RT-PCR and quantitative PCR assays, respectively. Exposure to increasing NO concentrations resulted in dose-dependent increases in E6, E7, E1ˆE4, and E6* RNAs in both HPV16- and HPV31-infected cells (Fig. 2). Compared with the unexposed control group, the increase in E1ˆE4 transcript levels was statistically significant as DETA-NO dose reached 0.25 mmol/L; at 0.5 mmol/L, HPV16 E1ˆE4 increased 2.9-fold and HPV31 E1ˆE4 increased 3.2-fold. The E6*, E6, and E7 transcript increases were statistically significant at 0.5 mmol/L DETA-NO exposure: HPV16 E6, 1.9-fold; E7, 2.4-fol; and E6*, 2.6-fold, respectively, and HPV31 E6, 2.2-fold; E7, 2.3-fold; and E6*, 2.6-fold, respectively. The E7 coding region is contained in all early transcripts, including those with the unspliced E6 open reading frame and RNAs with the spliced E6* form (see Supplementary Fig. S1). Therefore, the levels of E7 transcripts should generally represent the sum of E6 and E6* RNAs, and our results are consistent with this (Fig. 2). The DETA control had no effect on viral mRNA transcription. The increase in viral early transcription was not due to increased viral genome copy number because none of the treatments altered HPV genome levels in the cells (Fig. 2). These data show that exposure of HPV-infected cervical cells to physiologic levels of NO results in increased HPV early viral transcription without affecting viral genome replication.
Figure 2.
Viral transcript and genome copy numbers in HPV-infected cervical cells exposed to NO. W12E (HPV16) and CIN-612 9E (HPV31) cells were exposed to different concentrations of DETA-NO and to 0.5 mmol/L DETA for 24 h as indicated. DNase-treated total RNAs were subjected to reverse transcription. Triplicate reverse transcription reactions or DNA samples were analyzed by quantitative PCR (qPCR); the mean quantity of each target gene was normalized to the control gene (TBP for cDNA; GADPH for DNA). Columns, mean normalized quantities from three separate experiments; bars, SE. *, P < 0.05; **, P < 0.01, compared with the 0 mmol/L control in each group.
Early viral transcription increases coincide with p53 and pRb degradation
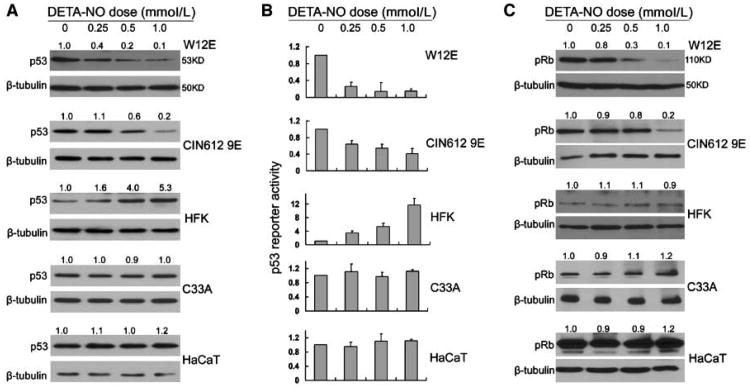
Low expression levels and lack of effective antibodies to HPV early proteins prompted us to evaluate downstream effects of increased viral early protein expression. High-risk HPV E6 oncoprotein induces proteasomal degradation of p53 (41). The E7 oncoprotein binds and degrades pRb and related family members p107 and p130 (42). Therefore, to investigate whether the increase in HPV E6 and E7 RNAs induced by NO exposure resulted in higher levels of viral oncoproteins, we assayed for p53 and pRb protein levels in treated HPV-infected cells by immunoblot. Results showed dose-dependent decreases in wild-type p53 levels in HPV-positive W12E and CIN-612 9E cells after DETA-NO treatment (Fig. 3A). However, consistent with previous reports, in low-passage HFK cells that carry wild-type p53 genes, DETA-NO treatment caused increased p53 levels. No obvious change in p53 levels was observed in HPV-negative cells expressing mutant p53 (C-33A and HaCaT cells; Fig. 3A). Similarly, pRb levels were reduced in HPV-infected cells, but were unaffected in keratinocytes lacking HPV (Fig. 3C).
Figure 3.
p53 and pRb levels after DETA-NO treatment in HPV-positive and HPV-negative human keratinocytes. All NO exposures were for 24 h. Immunoblot analysis (A and C) to determine relative total p53 and pRb protein levels in cells. Each sample was normalized to β-tubulin protein levels. Values directly above each lane represent the normalized protein levels determined by densitometry compared with the untreated control in each group. A, the amount of total protein analyzed for p53 varied: W12E and CIN-612 9E cells, 10 μg; HFK, 30 μg; C-33A and HaCaT cells, 0.5 μg. B, transcriptional activity of p53 in cells following p53-luc transfection and NO exposure. Each experimental sample was normalized to the untreated control. Columns, average of three independent experiments; bars, SE. C, for pRb immunoblots, 50 μg of total soluble protein were analyzed. The autoradiographs were cropped around the regions of interest.
Because p53 protein levels do not always correlate with p53 activity, we assayed for p53 transcriptional activity by transfecting a p53-reponsive reporter into cells before NO exposure. Wild-type p53 activity was reduced in HPV-infected cells following NO exposure in a manner consistent with increased HPV E6 expression and the corresponding decrease in p53 levels (Fig. 3B). A significant negative correlation was observed between HPV E6 transcript level and p53 activity in both W12E (P = 0.02) and CIN-612 9E cells (P = 0.005). In HPV-negative HFKs harboring wild-type p53, NO exposure increased p53 transcriptional activity concomitant with increased p53 protein levels as expected. The activity of mutant p53 was unaffected in NO-exposed C-33A and HaCaT cells.
NO induces DNA DSBs in HPV-infected cells
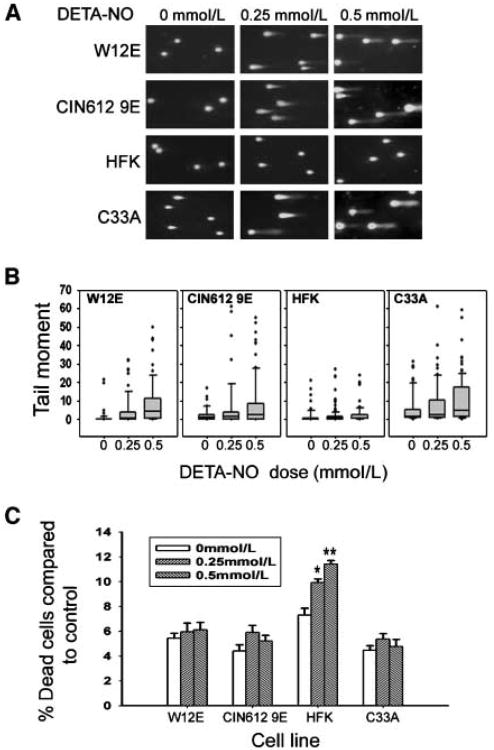
NO exposure causes DNA DSBs, the most cytotoxic lesions, in mammalian cells (43, 44). Given that p53 functions are inactivated in HPV-infected cells exposed to NO, we hypothesized that DSBs would be more pronounced in HPV-positive cells compared with uninfected normal HFK after NO exposure. DNA DSBs were evaluated in W12E, CIN-612 9E, HFK, and C-33A cells using the neutral comet assay after 24-hour exposure to increasing concentrations of DETA-NO. DNA tail moments were greater in each of the four groups of cells after NO exposure; however, NO-induced tail moments in W12E and CIN-612 9E cells were significantly higher than those in normal HFK cells (Fig. 4 A and B). As expected, the tail moments were the greatest in C-33A cells, expressing mutant p53, with even the untreated cells showing comet tail moments longer than those in other untreated cells.
Figure 4.
DETA-NO effects on DSBs and cell death. All NO exposures were for 24 h. A, neutral comet assay. B, box-and-whisker plots quantifying data from ≥80 cells per group from A to illustrate the distribution and statistics of the data set. The box bottom and top are 25th and 75th percentiles of the data set, respectively; the central band is 50th percentile (median). Vertical lines extend to 10th and 90th percentiles. Closed dots indicate outlying data points. C, Annexin V staining and quantification by flow cytometry for apoptotic death. Columns, average of three separate experiments; bars, SE. *, P < 0.05; **, P < 0.01, compared with 0 mmol/L control group.
In normal mammalian cells, extreme cellular stress and genotoxicity induce p53 expression to trigger either DNA repair or apoptotic cell death as a protective mechanism. To determine whether apoptosis was involved in the NO-related DNA damage in HPV-infected cells, Annexin V and 7-AAD staining was used. There were significant increases in the percentage of apoptotic HFK cells in response to DNA DSB induced after treatment with 0.25 mmol/L (P < 0.05) and 0.5 mmol/L (P < 0.01) of DETA-NO (Fig. 4C). Although there was slightly higher cell death in HPV-infected cells after DETA-NO treatment, the increase was not statistically significant. Likewise, NO-induced DSBs were not associated with greater cell death in C-33A cancer cells containing mutant p53. Therefore, NO exposure caused more DSBs in HPV-infected cells; yet, the suppressed p53 activity was unable to trigger the normal apoptotic cell death response pathways.
DNA mutations accumulate in HPV-infected cells exposed to NO
DSB-DNA damage repair is error-prone and increases the likelihood of DNA mutations. Because p53 is thought to be involved in the repair process and our data show decreased normal apoptosis functions, we assayed mutation accumulation in NO-exposed HPV-infected cells. Because it is difficult to sustain low-passage HFK cells for more than 10 passages, we used a spontaneously immortalized clone of HFKs, NIKS cells, as our uninfected cell line. NIKS cells displayed similar characteristics as HFKs in p53 activity and DNA damage following NO exposure (data not shown). The cell lines were differentially sensitive to 6-TG: Whereas spontaneous (0 mmol/L DETA-NO) 6-TGR mutation frequencies in W12E and CIN-612 9E were similar to those in NIKS cells, the C-33A carcinoma cells showed 2.4-fold higher (P < 0.01) spontaneous mutation frequency than NIKS cells (Table 1). After 24-hour treatment with 0.25 and 0.5 mmol/L of DETA-NO, we detected 2.6-fold (P < 0.01) and 8.8-fold (P < 0.01) increases of 6-TGR mutation frequencies in W12E cells and 2.2-fold (P < 0.05) and 7.0-fold (P < 0.01) increases in CIN-612 9E cells as compared with their untreated (0 mmol/L) control groups. However, HPV-negative NIKS cells treated with 0.25 and 0.5 mmol/L of DETA-NO had smaller increases in mutation frequencies (1.8-fold, P > 0.05 and 2.4-fold, P < 0.05). C-33A carcinoma cells had significantly higher mutation frequencies at 3.3-fold (P < 0.01) and 9.5-fold (P < 0.01), respectively. As a positive control, mutagen 4-NQO induced 6-TGR mutations at frequencies significantly higher in W12E, CIN-612 9E, and C-33A cells than in NIKS cells when compared at the same dose levels. Therefore, NO exposure results in higher DNA mutation frequencies in HPV-infected cells, a phenotype related to the lower p53 activity in these cells.
Table 1. DETA-NO–induced HPRT gene mutation frequency after 24 h of exposure (n = 3).
| Cell line | DETA-NO (mmol/L) | HPRT mutants/106 cells |
|---|---|---|
| W12E | 0 | 7.2 ± 1.3 |
| 0.25 | 18.6 ± 1.6* | |
| 0.5 | 63.1 ± 6.0* | |
| 4NQO, 140 ng/mL | 18.4 ± 2.5† | |
| CIN-612 9E | 0 | 8.0 ± 1.5 |
| 0.25 | 17.4 ± 3.7† | |
| 0.5 | 55.9 ± 10.0* | |
| 4NQO, 140 ng/mL | 26.1 ± 8.4† | |
| NIKS | 0 | 4.9 ± 1.5 |
| 0.25 | 7.3 ± 0.8 | |
| 0.5 | 11.8 ± 3.4† | |
| 4NQO, 140 ng/mL | 10.1 ± 1.1† | |
| C-33A | 0 | 11.3 ± 2.5 |
| 0.25 | 37.5 ± 9.6* | |
| 0.5 | 107.3 ± 22.5* | |
| 4NQO, 140 ng/mL | 37.4 ± 3.8* |
P < 0.01, compared with 0 mmol/L donor controls.
P < 0.05, compared with 0 mmol/L donor controls.
Discussion
Natural history studies indicate that nearly every sexually active woman will acquire at least one cervical high-risk HPV infection over the course of their lives, yet relatively few will progress to cancer. Expression of high-risk HPV E6 and E7 oncogenes predispose infected cells to cancer, in part, by functional inactivation of two critical tumor suppressor proteins, p53 and pRb, respectively. Whereas E6/E7 expression is sufficient for immortalization, it is insufficient for transformation in vitro, suggesting that exogenous factors are required for further progression toward tumorigenesis (reviewed in ref. 3).
Many putative cofactors for HPV-induced cervical cancer have been identified through epidemiologic case-control studies, including tobacco smoke, high parity, inflammation, and coinfection with Chlamydia or HSV-2. We noted that all of these exposures could lead to increased cervical NO levels either directly (e.g., tobacco smoke metabolites) or indirectly through the local induction of iNOS in the cervical epithelium or resident macrophages and other innate immune cells. We reasoned that sustained NO in the microenvironment of high-risk HPV-infected cells could promote tumorigenesis resulting from E6-mediated loss of p53 response to NO-induced genotoxic stress. Whereas there is some evidence for increased NO levels or NO-associated mutagenesis (e.g., formation of 8-nitroguanine) in the cervixes of women with CIN relative to controls (7, 8), the in vitro effect of physiologically relevant doses of NO on HPV-infected cells maintaining episomal viral genomes has not been determined.
We did not observe any NO effect on HPV episomal genome replication; however, we did see a statistically significant dose-dependent increase in HPV early E6, E7, E6*, and E1ˆE4 mRNA expression in both HPV16- and HPV31-containing cells. This contrasts with antiviral NO effects on other DNA viruses, such as HSV-2 and HBV, and some RNA viruses (22, 23). The mechanism underlying stimulation of HPV gene expression in response to NO exposures is under investigation. NO can affect the activities of activator protein 1, Sp-1, and nuclear factor-κB transcription factors (45) known to regulate HPV gene expression (46, 47). Interestingly, HPV16 E6/E7–expressing keratinocytes show substantial iNOS down-regulation (48). Although this may have evolved primarily as a means of immune escape, it supports the notion that a microenvironment enriched in diffusible NO could be detrimental to a normal, nonpathogenic HPV life cycle.
Regardless of the mechanism by which NO affects HPV gene expression, the functional consequence of up-regulation of HPV E6 and E7 oncoproteins is decreased levels of p53 and pRb. Although cells infected with HPV16 and HPV31 show residual p53 activity and detectable p53 and pRb proteins under basal conditions, increased E6 and E7 expression following NO exposure positively correlated with significant reductions in pRb levels and both p53 protein levels and activity. Conversely, uninfected HFKs expressing wild-type p53 showed marked p53 protein accumulation and increased p53 activity following NO exposure, consistent with a normal NO-induced genotoxic stress response.
We directly examined the effect of NO on genome integrity in HPV-infected and uninfected cells because many studies have found increases in DSBs and mutation in response to NO exposure in cells lacking functional tumor suppressor pathways (17, 18). As expected, we observed a dose-dependent increase in DNA DSBs and mutation frequency in HPV-positive cells exhibiting decreased p53 levels and activity after NO exposure. More importantly, we saw minimal apoptosis in response to the NO-induced genotoxicity in the HPV-infected cells, in contrast to the NO dose–dependent increase in apoptosis observed in the uninfected HFKs. Together, our observations suggest that physiologically relevant, high NO exposure results in survival of HPV-infected cells harboring serious DNA damage.
Cervical cancer and its precursors are characterized by significant genetic damage (3). However, this is a rare outcome of typical HPV infections, and mechanisms leading to accumulated genomic instability in HPV-infected cells have remained elusive. Our approach to identify possible sources of genomic instability was to look for pathways common to epidemiologically identified cervical cancer cofactors. One such common pathway is increased NO exposure in the cervix. Whereas there are no reports describing cervical NO measurements over serial times or extended periods in patients, except during pregnancy and delivery (49), NO levels are high at various time points in vivo, as discussed above. NO levels modeled here are primarily produced in vivo by iNOS, whose expression is tightly regulated under normal conditions. Toxicity is related to NO concentration and exposure dosages as a function of time in vitro(50, 51). Effects are insignificant if neither or only one threshold is exceeded; when both are exceeded, toxicity directly correlates with dosage increase. In studying physiologically relevant, high NO concentrations (150–400 nmol/L), we found significant toxicity with 24-hour exposure to ≥200 nmol/L NO, suggesting that the concentration and exposure time exceeded both thresholds. Presumably, longer NO exposures in vivo would increase toxicity risk (DNA damage and mutation), possibly due to dysregulated iNOS expression. Yet, it is unknown whether procarcinogenic responses in vivo result from transiently high NO concentrations or constitutive iNOS activation.
We confirm that cells infected with carcinogenic HPV types respond to high NO exposures with decreased p53 and pRb levels, DNA damage and mutation, and inappropriate survival, suggesting that NO exposure is a biologically plausible molecular cofactor in HPV-mediated carcinogenesis. Thus, we propose a cervical cancer model (Fig. 5) wherein cofactor exposure can result in increases in cervical NO levels precipitating a chain of procarcinogenic events. This experimental model provides the impetus for expanded epidemiologic studies of cervical NO expression to better understand the in vivo exposure variability as it relates to HPV infection and risk of CIN. Support for this model by future studies would advocate potential therapeutic effects of pharmacologic decreases in cervical NO levels.
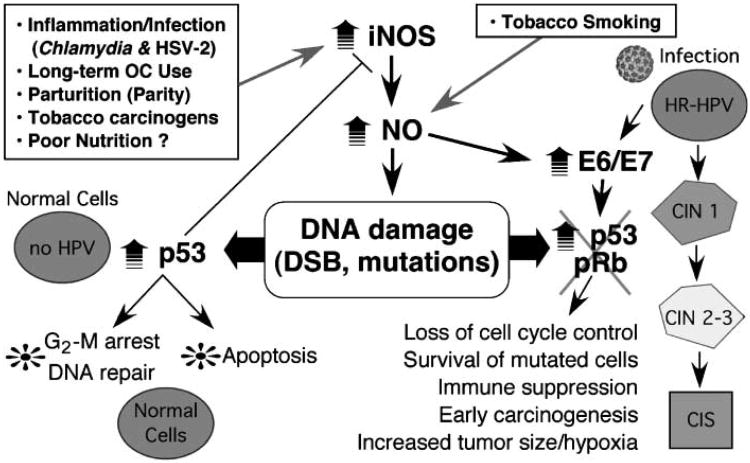
Figure 5.
Model for HPV and NO interaction in cervical cancer progression. Infection with high-risk (HR) HPVs is a necessary but insufficient cause of cervical cancer (right). Epidemiologically defined cofactors (boxed), including C. trachomatis and HSV-2 infections, chronic inflammation, long-term oral contraceptive (OC) use, multiparity, and tobacco smoking, increase NO levels in the cervical microenvironment. Most of these factors cause increased iNOS expression in cervical epithelium or tissue macrophages, whereas some (e.g., tobacco smoke) contain NO. Similar to the work of others, we find that in normal cells, NO induces high p53 levels leading to DNA repair or apoptosis wherein mutant cells generally fail to survive. However, in high-risk HPV–infected cells, NO causes increased early mRNA expression, decreased pRb and p53 levels, low p53 activity and apoptotic indices, and increased survival of mutant cells. These data are consistent with NO as a molecular cofactor with HPV infection in cervical carcinogenesis.
Supplementary Material
Acknowledgments
Grant support: American Cancer Society grant RSG-05-149-01-MBC (M.A. Ozbun), NIH Career Development Award P50 CA098252 (P.E. Gravitt), Oxnard Foundation (M.A. Ozbun), and NIH grant CO6 RR012511.
We thank Profs. N. Fusenig (Department of Experimental Oncology, DKFZ, Heidelberg, Germany) for HaCaT cells, C. Wheeler (Department of Molecular Genetics & Microbiology, University of New Mexico, Albuquerque, NM) for HFKs, L. Laimins (Department of Microbiology-Immunology, Northwestern University, Chicago, IL) for CIN 612 9E cells, P. Lambert (McArdle Laboratory of Cancer Research, University of Wisconsin, Madison, WI) and M. Stanley (Department of Pathology, University of Cambridge, Cambridge, United Kingdom) for W12E cells, and Profs. G. Timmins (College of Pharmacy, University of New Mexico, Albuquerque, NM) and R. H. Glew (Department of Biochemistry, University of New Mexico, Albuquerque, NM) for critical reading of the manuscript.
Footnotes
Note: Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Walboomers JMM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–9. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 3.zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002;2:342–50. doi: 10.1038/nrc798. [DOI] [PubMed] [Google Scholar]
- 4.Benencia F, Gamba G, Cavalieri H, et al. Nitric oxide and HSV vaginal infection in BALB/c mice. Virology. 2003;309:75–84. doi: 10.1016/s0042-6822(02)00057-0. [DOI] [PubMed] [Google Scholar]
- 5.Carratelli CR, Rizzo A, Paolillo R, Catania MR, Catalanotti P, Rossano F. Effect of nitric oxide on the growth of Chlamydophila pneumoniae. Can J Microbiol. 2005;51:941–7. doi: 10.1139/w05-080. [DOI] [PubMed] [Google Scholar]
- 6.Chang K, Lubo Z. Review article: steroid hormones and uterine vascular adaptation to pregnancy. Reprod Sci. 2008;15:336–48. doi: 10.1177/1933719108317975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hiraku Y, Tabata T, Ma N, Murata M, Ding X, Kawanishi S. Nitrative and oxidative DNA damage in cervical intraepithelial neoplasia associated with human papilloma virus infection. Cancer Sci. 2007;98:964–72. doi: 10.1111/j.1349-7006.2007.00497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tavares-Murta BM, de Resende AD, Cunha FQ, Murta EFC. Local profile of cytokines and nitric oxide in patients with bacterial vaginosis and cervical intraepithelial neoplasia. Eur J Obstet Gynecol Repro Biol. 2008;138:93–9. doi: 10.1016/j.ejogrb.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 9.Lala PK, Chakraborty C. Role of nitric oxide in carcinogenesis and tumour progression. Lancet Oncol. 2001;2:149–56. doi: 10.1016/S1470-2045(00)00256-4. [DOI] [PubMed] [Google Scholar]
- 10.Ohshima H. Genetic and epigenetic damage induced by reactive nitrogen species: implications in carcinogenesis. Toxicol Lett. 2003:140–141. 99–104. doi: 10.1016/s0378-4274(02)00506-4. [DOI] [PubMed] [Google Scholar]
- 11.Kawanishi S, Hiraku Y, Pinlaor S, Ma N. Oxidative and nitrative DNA damage in animals and patients with inflammatory diseases in relation to inflammation-related carcinogenesis. Biol Chem. 2006;387:365–72. doi: 10.1515/BC.2006.049. [DOI] [PubMed] [Google Scholar]
- 12.Ding X, Hiraku Y, Ma N, et al. Inducible nitric oxide synthase-dependent DNA damage in mouse model of inflammatory bowel disease. Cancer Sci. 2005;96:157–63. doi: 10.1111/j.1349-7006.2005.00024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoki Y, Hiraku Y, Ma N, et al. iNOS-dependent DNA damage in patients with malignant fibrous histiocytoma in relation to prognosis. Cancer Sci. 2007;98:163–8. doi: 10.1111/j.1349-7006.2006.00376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hussain SP, He P, Subleski J, et al. Nitric oxide is a key component in inflammation-accelerated tumorigenesis. Cancer Res. 2008;68:7130–6. doi: 10.1158/0008-5472.CAN-08-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marnett LJ, Riggins JN, West JD. Endogenous generation of reactive oxidants and electrophiles and their reactions with DNA and protein. J Clin Invest. 2003;111:583–93. doi: 10.1172/JCI18022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glockzin S, von Knethen A, Scheffner M, Brune B. Activation of the cell death program by nitric oxide involves inhibition of the proteasome. J Biol Chem. 1999;274:19581–6. doi: 10.1074/jbc.274.28.19581. [DOI] [PubMed] [Google Scholar]
- 17.Schneiderhan N, Budde A, Zhang Y, Brune B. Nitric oxide induces phosphorylation of p53 and impairs nuclear export. Oncogene. 2003;22:2857–68. doi: 10.1038/sj.onc.1206431. [DOI] [PubMed] [Google Scholar]
- 18.Kim SJ, Hwang SG, Shin DY, Kang SS, Chun JS. p38 kinase regulates nitric oxide-induced apoptosis of articular chondrocytes by accumulating p53 via NFκB-dependent transcription and stabilization by serine 15 phosphorylation. J Biol Chem. 2002;277:33501–8. doi: 10.1074/jbc.M202862200. [DOI] [PubMed] [Google Scholar]
- 19.Harris SL, Levine AJ. The p53 pathway: positive and negative feedback loops. Oncogene. 2005;24:2899–908. doi: 10.1038/sj.onc.1208615. [DOI] [PubMed] [Google Scholar]
- 20.Ho YS, Wang YJ, Lin JK. Induction of p53 and p21/WAF1/CIP1 expression by nitric oxide and their association with apoptosis in human cancer cells. Mol Carcinog. 1996;16:20–31. doi: 10.1002/(SICI)1098-2744(199605)16:1<20::AID-MC4>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 21.Tommasino M, Accardi R, Caldeira S, et al. The role of TP53 in cervical carcinogenesis. Hum Mutat. 2003;21:307–12. doi: 10.1002/humu.10178. [DOI] [PubMed] [Google Scholar]
- 22.Reiss CS, Komatsu T. Does nitric oxide play a critical role in viral infections? J Virol. 1998;72:4547–51. doi: 10.1128/jvi.72.6.4547-4551.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akaike T, Maeda H. Nitric oxide and virus infection. Immunology. 2000;101:300–8. doi: 10.1046/j.1365-2567.2000.00142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stanley MA, Browne HM, Appleby M, Minson AC. Properties of a non-tumorigenic human cervical keratinocyte cell line. Int J Cancer. 1989;43:672–6. doi: 10.1002/ijc.2910430422. [DOI] [PubMed] [Google Scholar]
- 25.Hummel M, Hudson JB, Laimins LA. Differentiation-induced and constitutive transcription of human papillomavirus type 31b in cell lines containing viral episomes. J Virol. 1992;66:6070–80. doi: 10.1128/jvi.66.10.6070-6080.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Auersperg N. Long-term cultivation of hypodiploid human tumor cells. J Natl Cancer Inst. 1964;32:135–63. [PubMed] [Google Scholar]
- 27.Lambert PF, Ozbun MA, Collins A, Holmgren S, Lee D, Nakahara T. Using an immortalized cell line to study the HPV life cycle in organotypic “raft” cultures. In: Davy C, Doorbar J, editors. Human papilloma viruses: methods and protocols Totowa (NJ) Humana Press Inc.; pp. 2005pp. 141–55. [DOI] [PubMed] [Google Scholar]
- 28.Meyers C. Organotypic (raft) epithelial tissue culture system for the differentiation-dependent replication of papillomavirus. Methods Cell Sci. 1996;18:201–10. [Google Scholar]
- 29.Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988;106:761–71. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ozbun MA, Meyers C. Human papillomavirus type 31b E1 and E2 transcript expression correlates with vegetative viral genome amplification. Virology. 1998;248:218–30. doi: 10.1006/viro.1998.9285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ozbun MA. Infectious human papillomavirus type 31b: purification and infection of an immortalized human keratinocyte cell line. J Gen Virol. 2002;83:2753–63. doi: 10.1099/0022-1317-83-11-2753. [DOI] [PubMed] [Google Scholar]
- 32.Muller PY, Janovjak H, Miserez AR, Dobbie Z. Processing of gene expression data generated by quantitative realtime RT-PCR. Biotechniques. 2002;32(6):1372–4. 8–9. [PubMed] [Google Scholar]
- 33.Konca K, Lankoff A, Banasik A, et al. A cross-platform public domain PC image-analysis program for the comet assay. Mutat Res. 2003;534:15–20. doi: 10.1016/s1383-5718(02)00251-6. [DOI] [PubMed] [Google Scholar]
- 34.Bove PF, Hristova M, Wesley UV, Olson N, Lounsbury KM, van der Vliet A. Inflammatory levels of nitric oxide inhibit airway epithelial cell migration by inhibition of the kinase ERK1/2 and activation of hypoxia-inducible factor-1α. J Biol Chem. 2008;283:17919–28. doi: 10.1074/jbc.M709914200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thomas DD, Espey MG, Ridnour LA, et al. Hypoxic inducible factor 1α, extracellular signal-regulated kinase, and p53 are regulated by distinct threshold concentrations of nitric oxide. Proc Natl Acad Sci U S A. 2004;101:8894–9. doi: 10.1073/pnas.0400453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chin MP, Schauer DB, Deen WM. Prediction of nitric oxide concentrations in colonic crypts during inflammation. Nitric Oxide. 2008;19:266–75. doi: 10.1016/j.niox.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 37.Vaisanen-Tommiska M, Nuutila M, Aittomaki K, Hiilesmaa V, Ylikorkala O. Nitric oxide metabolites in cervical fluid during pregnancy: further evidence for the role of cervical nitric oxide in cervical ripening. Am J Obstet Gynecol. 2003;188:779–85. doi: 10.1067/mob.2003.161. [DOI] [PubMed] [Google Scholar]
- 38.Chang WW, Su IJ, Chang WT, Huang W, Lei HY. Suppression of p38 mitogen-activated protein kinase inhibits hepatitis B virus replication in human hepatoma cell: the antiviral role of nitric oxide. J Viral Hepatitis. 2008;15:490–7. doi: 10.1111/j.1365-2893.2007.00968.x. [DOI] [PubMed] [Google Scholar]
- 39.Thomas JT, Hubert WG, Ruesch MN, Laimins LA. Human papillomavirus type 31 oncoproteins E6 and E7 are required for the maintenance of episomes during the viral life cycle in normal keratinocytes. Proc Natl Acad Sci U S A. 1999;96:8449–54. doi: 10.1073/pnas.96.15.8449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sverdrup F, Khan SA. Replication of human papillomavirus (HPV) DNAs supported by the HPV type 18 E1 and E2 proteins. J Virol. 1994;68:505–9. doi: 10.1128/jvi.68.1.505-509.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scheffner M, Huibregtse JM, Vierstra RD, Howley PM. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell. 1993;75:495–505. doi: 10.1016/0092-8674(93)90384-3. [DOI] [PubMed] [Google Scholar]
- 42.Munger K, Baldwin A, Edwards KM, et al. Mechanisms of human papillomavirus-induced oncogenesis. J Virol. 2004;78:11451–60. doi: 10.1128/JVI.78.21.11451-11460.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bentz BG, Hammer ND, Radosevich JA, Haines GK., III Nitrosative stress induces DNA strand breaks but not caspase mediated apoptosis in a lung cancer cell line. J Carcinog. 2004;3:16. doi: 10.1186/1477-3163-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kiziltepe T, Hideshima T, Ishitsuka K, et al. JS-K, a GST-activated nitric oxide generator, induces DNA double-strand breaks, activates DNA damage response pathways, and induces apoptosis in vitro and in vivo in human multiple myeloma cells. Blood. 2007;110:709–18. doi: 10.1182/blood-2006-10-052845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bodgan C. Nitric oxide and the regulation of gene expression. Trends Cell Biol. 2001;11:66–75. doi: 10.1016/s0962-8924(00)01900-0. [DOI] [PubMed] [Google Scholar]
- 46.Fontaine V, Van der Meijden E, De GJ, Ter SJ, Struyk L. A functional NF-κB binding site in the human papillomavirus type 16 long control region. Virology. 2000;272:40–9. doi: 10.1006/viro.2000.0363. [DOI] [PubMed] [Google Scholar]
- 47.Bernard HU. Gene expression of genital human papillomaviruses and considerations on potential antiviral approaches. Antivir Ther. 2002;7:219–37. [PubMed] [Google Scholar]
- 48.Guess JC, McCance DJ. Decreased migration of Langerhans precursor-like cells in response to human keratinocytes expressing human papillomavirus type 16 E6/E7 is related to reduced macrophage inflammatory protein-3α production. J Virol. 2005;79:14852–62. doi: 10.1128/JVI.79.23.14852-14862.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Väisänen-Tommiska MRH. Nitric oxide in the human uterine cervix: endogenous ripening factor. Ann Med. 2008;40:45–55. doi: 10.1080/07853890701716802. [DOI] [PubMed] [Google Scholar]
- 50.Li CQ, Pang B, Kiziltepe T, et al. Threshold effects of nitric oxide-induced toxicity and cellular responses in wild-type and p53-null human lymphoblastoid cells. Chem Res Toxicol. 2006;19:399–406. doi: 10.1021/tx050283e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang C, Trudel LJ, Wogan GN, Deen WM. Thresholds of nitric oxide-mediated toxicity in human lymphoblastoid cells. Chem Res Toxicol. 2003;16:1004–13. doi: 10.1021/tx0340448. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.