Abstract
We present an Arabidopsis thaliana full-length transcription factor resource of 92% of root stele–expressed transcription factors and 74.5% of root-expressed transcription factors. We demonstrate its use with enhanced yeast one-hybrid (eY1H ) screening for rapid, systematic mapping of plant transcription factor–promoter interactions. We identified 158 interactions with 13 stele-expressed promoters, many of which occur physically or are regulatory in planta.
Transcriptional regulation has a key role in development and response to the environment. In plants, regulation of gene expression is complex with the majority of genes differently expressed in different tissues and cell types1,2. Gene regulatory networks in stele tissue, which contains vascular and associated cell types (Fig. 1a), are of great biological and biotechnological importance. Using high-spatial-resolution gene expression data and yeast one-hybrid (Y1H) assays, we have previously mapped 103 gene interactions between 64 transcription factors and eight microRNAs in Arabidopsis root stele tissue, 66 of which comprise transcription factor–promoter interactions, of which 49% we confirmed to be regulatory in planta. This validation rate is higher or on par with reports using chromatin immunoprecipitation–based assays3–5. Interactions had been identified by screening against a collection representing 24% of stele-expressed transcription factors6 and had been identified with only 25% of promoters, which is likely due to the limited size of the transcription factor collection. Furthermore, not all clones in the previous collection are full length, which may influence both false negatives and false positives. Here we report a comprehensive full-length collection of transcription factors expressed in the stele. We adapted the enhanced Y1H (eY1H) method7 to rapidly and systematically map stele and plant gene regulatory networks.
Figure 1.
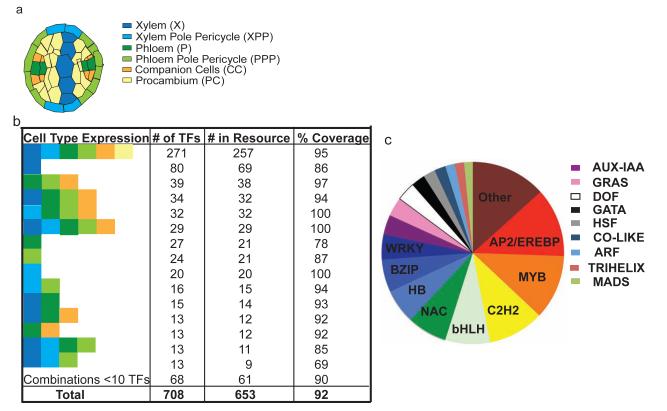
An Arabidopsis eY1H transcription factor resource. (a) Schematic of root stele cell types. (b) Stele cell-type expression profiles of the transcription factors present in our collection. Colors are as in a. (c) Family categorization of transcription factors within the resource. ‘Other’ are families with fewer than eight members (Supplementary Table 1). GRAS, gibberellic acid–insensitive, repressor of GA1 and scarecreow; DOF, DNA-binding with one finger; GATA, GATA DNA motif; HSF, heatshock factor; CO-like, Constans-like; ARF, auxin response factor; MADS, MCM1, Agamous, Deficiens and SRF box; HB, homeobox; and BZIP, basic leucine zipper.
We generated a sequence-verified collection of 653 Arabidopsis full-length transcription factor clones, representing 92% of all transcription factors reported to be expressed in the root stele (Fig. 1, Supplementary Notes and Supplementary Table 1) and 74.5% of all transcription factors expressed in the root. We obtained Gateway transcription factor Entry clones from public resources (484 clones), our previous studies (91 clones)6 or we performed de novo cloning (78 clones) (Supplementary Fig. 1). Gene models and transcription factor families associated with these clones are available in Supplementary Table 1. The library contains substantial representation (greater than 40 members) from the NAC (NAM, ATAF1, CUC2), bHLH (basic helix loop helix), C2H2, MYB and AP2/EREBP (APETALA2 and ethylene responsive element binding protein) families and has nearly comprehensive coverage of transcription factors expressed generally in the stele tissue and in specific cell types (Fig. 1b,c).
We had performed our previous screens using manual direct transformation–based assays with a low-copy activation domain vector6. In contrast, the eY1H configuration uses a mating-based assay along with a high-copy activation domain vector, an α mating type yeast bait strain, four technical replicates and automation7 (Online Methods). We tested both mating and direct-transformation assays with 94 previously screened transcription factors in both the low-copy and high-copy activation domain vector using a single Arabidopsis promoter (REVOLUTA, or REV) for which many interactors have been biologically validated6 (Supplementary Fig. 1). Mating-based Y1H assays in Caenorhabditis elegans using a low-copy activation domain vector detect about half as many interactions compared to haploid transformation assays8. We found similar results, and use of the high-copy eY1H activation domain vector largely rectified these inconsistencies (Supplementary Notes and Supplementary Fig. 2). We tested use of a hand replicator with this pipeline and obtained similar results (Supplementary Notes). We recombined the 653 transcription factor–encoding open reading frames with a high-copy GAL4-activation domain fusion Y1H prey vector (AD-2μ) (Fig. 1b and Online Methods) and fully sequence-verified all clones in the prey vector.
We detected transcription factor–promoter interactions in the eY1H assay using two promoter:reporter constructs: HIS3 and LacZ (Fig. 2a). We arranged transcription factors in 384-well plates with four technical replicates per transcription factor, one set of empty wells and one set with an empty vector as a negative control. At least two of the replicates must have displayed reporter activation with both reporters for an interaction to be recorded7.
Figure 2.
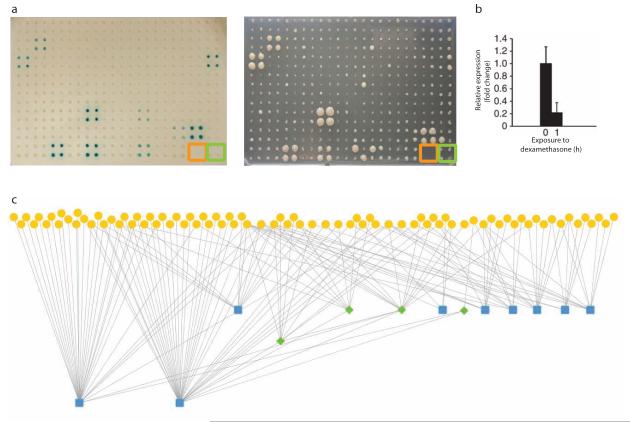
Using the transcription factor resource in an eY1H assay. (a) Reporter assay readout with the REV promoter and 94 transcription factors. Empty wells (orange) and negative control (green) are marked. Scale bar, 4 mm. (b) Relative expression of REV in the 35S-OBP2–glucocorticoid receptor line after induction with dexamethasone. Error bars, s.e.m. (n = 3; ~100 plants per biological replicate). (c) The stele gene regulatory network for the 13 screened promoters. Yellow circles are transcription factors, green diamonds are in the screen as both transcription factors and promoters, and blue squares are promoters.
We screened 13 previously assayed Arabidopsis stele-expressed gene promoters, including four for which we did not detect any interacting transcription factors previously. We had previously identified 36 interactions between 19 transcription factors and 9 promoters. With the enhanced transcription factor coverage we now identified 158 interactions between 85 transcription factors and all 13 promoters (Fig. 2b and Supplementary Tables 2 and 3). We observed 39% of previously identified interactions, which is similar to other comparisons of yeast assays (Supplementary Fig. 3a)8,9. Moreover, we identified 7 of 14 transcription factor–promoter interactions previously confirmed to occur physically or be regulatory in planta7 demonstrating that our approach can be used to identify biologically relevant interactions (Supplementary Fig. 3b). This false negative rate is comparable to that identified in yeast two-hybrid assays9,10. Finally, to validate a new interaction, we tested the interaction between the OBP2 transcription factor and the REV gene using a 35S-OBP2–glucocorticoid receptor translational fusion. OBP2 repressed expression of its target gene, REV, similar to its regulatory action on its target that is closely related to REV, PHABULOSA (PHB)6 (Fig. 2c).
Previously, we determined a significant positive correlation (P = 0.05) between genes bound by a relatively large number of transcription factors and their developmental importance, including PHB and REV6. We found that PHB and REV continue to have the greatest number of transcription factors binding to their promoters, with 30 transcription factors identified to bind to the PHB promoter and 37 to the REV promoter relative to the average number of 3.36 transcription factors per promoter. Furthermore, network complexity for these 13 promoters increased from three to four tiers of transcriptional regulation (Fig. 2c).
In summary, a nearly comprehensive root stele transcription factor resource combined with a high-throughput robotic approach to Y1H screens enabled rapid and systematic mapping of plant gene regulatory networks. These networks will increase our understanding of plant tissue complexity and development.
Supplementary Material
Supplementary Figure 1 Transcription factors in the resource were cloned de novo, obtained from our previous collection or from public sources, were fully sequenced, and then recombined with the activation domain vector. Clones were sequenced and then subsequently transformed in the Yα1867 yeast strain.
Supplementary Figure 2 Using the REV promoter, mating with the AD-2μ vector was the most sensitive assay. (a) Pictures of the reporter plates of screens resulting from mating of the REV promoter with transcription factor Plate 1 of the AD (low copy) and AD-2μ vectors. The black boxes indicate a positive interaction observed with both reporters (HIS3 and LacZ). (b) Venn diagram of interaction overlap between the direct transformation and mating assays using the AD and AD-2μ vectors. There was 100% overlap between the interactions obtained for the direct transformation assays of the AD and AD-2μ vectors. There was a 25% overlap between the AD and AD-2μ vectors in the mating assay. There was 50% overlap of interactions obtained from mating with the AD-2μ vector and the direct transformation assays. Moreover, two of the positives detected with mating the AD-2μ vector were obtained as weak positives with only one reporter, LacZ, in the direct transformation assays with both vectors.
Supplementary Figure 3 A comparison of direct transformation versus mating with the different activation domain vectors and the number of confirmed regulatory interactions identified. (a) Venn diagram showing the number of interactions obtained with the previous screen using direct transformation with the activation domain vector (left circle), mating with the AD-2μ vector (right circle) and their overlap. 14 out of 36 (39%) of the previously identified interactions were obtained. (b) Venn diagram showing the number of interactions from the previous screen which have been confirmed to be regulatory in planta (14), the interactions obtained from mating with the AD-2μ vector (7) and their overlap
Supplementary Table 1 The 653 transcription factors in the stele collection
Supplementary Table 2 Identified transcription factor-promoter interactions.
Supplementary Table 3 Interacting transcription factors.
Acknowledgments
This work was supported by University of California Davis startup funds to S.M.B., US National Institutes of Health grant GM082971 to A.J.M.W., US Department of Agriculture 1907-21000-030 to D.W., GM092412 and GM056006 to S.A.K., We thank K. Cavanaugh, D. Lavelle, L. Williams, P. Nittler, R. Michelmore, J. Pelletier, K. Vo, J. Foret, S. Winte and J. Harada for assistance.
Footnotes
Author Contributions S.M.B., D.W., A.J.M.W. and J.S.R.-H. conceived the project; J.S.R.-H. and A.J.M.W. provided the eY1H mating protocol; A.G. and D.K. performed the yeast experiments; A.G. and L.Z. cloned the de novo transcription factors; L.Z., M.T.-T., A.G., L.P. and Z.L. recombined the transcription factors; L.Z., G.B., J.L.P.-P. and S.A.K. accumulated and curated transcription factor entry clones; and S.M.B., A.G., D.W., L.Z., A.J.M.W. and J.S.R.-H. wrote the manuscript.
References
- 1.Brady SM, et al. Science. 2007;318:801–806. doi: 10.1126/science.1146265. [DOI] [PubMed] [Google Scholar]
- 2.Jiao Y, et al. Nat. Genet. 2009;41:258–263. doi: 10.1038/ng.282. [DOI] [PubMed] [Google Scholar]
- 3.Farnham PJ. Nat. Rev. Genet. 2009;10:605–616. doi: 10.1038/nrg2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moreno-Risueno MA, Busch W, Benfey PN. Curr. Opin. Plant Biol. 2010;13:126–131. doi: 10.1016/j.pbi.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaufmann K, et al. Science. 2010;328:85–89. doi: 10.1126/science.1185244. [DOI] [PubMed] [Google Scholar]
- 6.Brady SM, et al. Mol. Syst. Biol. 2011;7:459. doi: 10.1038/msb.2010.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reece-Hoyes JS, et al. Nat. Methods. 2011 Oct 30; doi:10.1038/nmeth.1748. [Google Scholar]
- 8.Vermeirssen V, et al. Nat. Methods. 2007;4:659–664. doi: 10.1038/nmeth1063. [DOI] [PubMed] [Google Scholar]
- 9.Chen YC, Rajagopala SV, Stellberger T, Uetz P. Nat. Methods. 2010;7:667–668. doi: 10.1038/nmeth0910-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braun P, et al. Nat. Methods. 2009;6:91–97. doi: 10.1038/nmeth.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1 Transcription factors in the resource were cloned de novo, obtained from our previous collection or from public sources, were fully sequenced, and then recombined with the activation domain vector. Clones were sequenced and then subsequently transformed in the Yα1867 yeast strain.
Supplementary Figure 2 Using the REV promoter, mating with the AD-2μ vector was the most sensitive assay. (a) Pictures of the reporter plates of screens resulting from mating of the REV promoter with transcription factor Plate 1 of the AD (low copy) and AD-2μ vectors. The black boxes indicate a positive interaction observed with both reporters (HIS3 and LacZ). (b) Venn diagram of interaction overlap between the direct transformation and mating assays using the AD and AD-2μ vectors. There was 100% overlap between the interactions obtained for the direct transformation assays of the AD and AD-2μ vectors. There was a 25% overlap between the AD and AD-2μ vectors in the mating assay. There was 50% overlap of interactions obtained from mating with the AD-2μ vector and the direct transformation assays. Moreover, two of the positives detected with mating the AD-2μ vector were obtained as weak positives with only one reporter, LacZ, in the direct transformation assays with both vectors.
Supplementary Figure 3 A comparison of direct transformation versus mating with the different activation domain vectors and the number of confirmed regulatory interactions identified. (a) Venn diagram showing the number of interactions obtained with the previous screen using direct transformation with the activation domain vector (left circle), mating with the AD-2μ vector (right circle) and their overlap. 14 out of 36 (39%) of the previously identified interactions were obtained. (b) Venn diagram showing the number of interactions from the previous screen which have been confirmed to be regulatory in planta (14), the interactions obtained from mating with the AD-2μ vector (7) and their overlap
Supplementary Table 1 The 653 transcription factors in the stele collection
Supplementary Table 2 Identified transcription factor-promoter interactions.
Supplementary Table 3 Interacting transcription factors.