Abstract
Many orthosteric agonists differentially activate downstream effectors of GPCRs. Such defined induction of signaling has strongly supported the hypothesis termed ‘ligand-directed trafficking of receptor signaling’ (LDTRS). More recently, subtype-selective GPCR activators, such as allosteric agonists and positive allosteric modulators, have also exhibited the capacity to activate specific signaling pathways. Based on this finding, it may be possible to achieve ligand-specific receptor active states that optimize the biological responses specific to GPCRs. This review discusses recent studies in which both orthosteric and allosteric compounds have been demonstrated to induce LDTRS.
Keywords: Allosteric agonist, GPCR, LDTRS, ligand-directed trafficking of receptor signaling, orthosteric agonist, positive allosteric modulator, receptor trafficking
Introduction
GPCRs represent one of the largest classes of drug targets, with up to 40% of currently marketed drugs acting at these proteins [1,2]. GPCRs are active in almost every organ system and present a wide range of opportunities as therapeutic targets for many disorders, including cancer, cardiac dysfunction, CNS disorders and obesity [1]. Consequently, GPCRs have remained primary targets for intense research and drug development in many academic and industrial laboratories. These receptors have a common architecture at their transmembrane core, consisting of seven α-helices separated by intracellular and extracellular loops [3]. It is generally accepted that signaling through a GPCR involves neurotransmitter or ligand binding and subsequent modulation of a large variety of intracellular effectors, such as protein kinases and ion channels [4], via the activation of single or multiple G-protein families [5-8]. In addition, GPCRs can signal effectors independently of G-protein action to induce cellular behaviors, such as cellular proliferation and apoptosis, by induction of β-arrestin-dependent signaling [7, 9]. Given this multiplicity of potential interacting partners, it is clear that the measurement of a single response is insufficient to understand the complexities of signaling coupled to GPCR activation.
Ligand-directed trafficking of GPCR signaling: Inconsistent with a linear model of efficacy
Traditionally, detailed experimentation in tissues was used for the rigorous characterization and quantification of pharmacological responses [10,11]. These data led researchers to suggest that agonist-relative efficacy is a unique property of a drug, and that efficacy is independent of the effector pathway measured [10,11]. Consequently, to explain the complex behavior of many biologically active compounds, a model of linear efficacy was proposed, suggesting that the full complement of cellular responses coupled to a given GPCR was equally elicited. Although there have been accounts of many compounds adhering to this mode of action, it is becoming increasingly evident that some compounds differ in their ability to stimulate separate stimulus-response pathways coupled to GPCRs, suggesting effector pathway-dependent effects (ie, agonist-directed trafficking of receptor signaling [ADTRS]) [3,12-14]. For example, structurally unrelated orthosteric (competitive) agonists of serotonin 5-HT2A/2C receptors have demonstrated differential phospholipase C (PLC) and phospholipase A (PLA) activation, manifested as changes in the rank orders of potency or efficacy of compounds [15]. Similarly, some agonists of μ-opioid receptors have exhibited the ability to induce effector activation in the absence of receptor internalization, suggesting non-linear agonist-induced cellular behaviors [16-18]. This phenomenon is also not restricted to orthosteric agonists. Many reports have confirmed that allosteric ligands that interact with sites on the receptor that are distinct from the endogenous ligand (orthosteric) binding site can induce functionally specific cellular effects, suggesting allosteric ligand-induced differential signaling efficacies [19-22]. Based on these results, a working definition of efficacy can no longer be explained by a linear model, but will need to include differential efficacies for various signaling pathways downstream of GPCRs [14,23]. To accommodate this need, researchers have attempted to modify the conceptual framework underlying receptor theory. Many of these ideas can be classified using the general descriptive terms that include ADTRS, ‘agonist-directed signaling’, ‘ligand-bias’ and ‘functional selectivity’. For simplicity, this review will use the term ‘ligand-directed trafficking of receptor signaling’ (LDTRS).
Divergent GPCR signaling and multiplicity of effector activation
As noted, ligand-bound receptors can induce the activation of many signaling cascades. The dynamics of such responses are generally governed at multiple levels of the response process and, thus, differential activation of cellular responses downstream of ligand binding can diverge from multiple points. In many cases, signals can diverge at the level of the G-protein. Many datasets have confirmed that, once activated, Gα and Gβγ subunits can simultaneously, and synergistically, activate or inhibit downstream effectors (Figure 1A). The best-characterized example of such dual effector regulation is the simultaneous inhibition of adenylyl cyclase via Gα subunits and activation of PLC via Gβγ subunits. This phenomenon has been confirmed downstream of multiple GPCRs, including the 5-HT1A receptors in HeLa cells [24]and dopamine D2 receptors in LtK- fibroblasts [25]. Importantly, pertussis toxin (PTX) blocks these responses, suggesting that both pathways are affected by G-proteins belonging to the Gαi/o family (reviewed in reference [26]). Receptors have also been demonstrated to couple to multiple G-protein families, a finding that provides an additional level of signaling diversity downstream of GPCRs (Figure 1B). In some cases, up to four unrelated classes of G-proteins can be activated; for example, using human thyroid gland, Laugwitz et al demonstrated that the human thyrotropin receptor induced the incorporation of photoreactive [α-32P]guanosine triphosphate (GTP)-azidoanilide into immunopreciptated Gαi/o, Gαs, Gαq/11 and Gα12 proteins [27]. Furthermore, pretreatment with PTX caused a 35% increase in the accumulation of cAMP, suggesting functional coupling to both Gαi and Gαs G-proteins [27]. Similarly, receptor promiscuity for three G-protein families (Gαi/o, Gαs and Gαq/11) has been demonstrated for corticotropin-releasing hormone receptors [28], gonadotropin-releasing hormone receptor [29] and muscarinic M1 AChRs [6,30,31]. In each example, second messenger (eg, cAMP or inositol 1,4,5-trisphosphate [IP3]) production was induced, suggesting the functional interaction of receptors with multiple G-protein families. G-protein coupling promiscuity has also been demonstrated for neurotensin receptors [32]and for histamine H2 receptors [33]. Together, these results suggest that multiple effectors can be stimulated by agonist-induced receptor activation via an interaction with multiple G-proteins.
Figure 1. Multiple mechanisms of signaling divergence downstream of GPCRs.
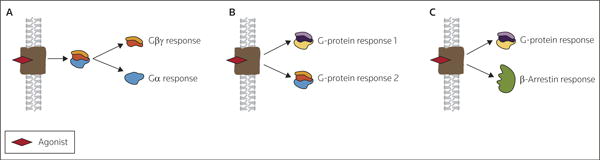
Signals can diverge downstream of agonist binding as a result of the dissociation of the Gβγ and Gα subunits. (B) Receptors can promiscuously couple to multiple G-protein families, providing an additional branch point for signaling. (C) Agonist binding can result in a multiplicity of effector coupling by the activation of either G-protein responses or β-arrestin responses.
(Adapted with permission from Elsevier Ltd and Hermans E: Biochemical and pharmacological control of the multiplicity of coupling at G-protein-coupled receptors. Pharmacol Ther (2003) 99(1):25-44. © 2003 Elsevier Ltd)
In addition to coupling to multiple G-protein families, several studies now confirm effector activation via β-arrestin-mediated protein scaffolding (Figure 1C). For example, stimulation of angiotensin II (AngII) type 1 (AT1) receptors in HEK293 cells increased the binding of both cRaf-1 and extracellular regulated kinase 2 (ERK2) to β-arrestin 2, an association thought to enhance cRaf-1 signaling and MEK-dependent activation of ERK2 [34]. Similarly, agonist-induced β-arrestin recruitment can act as a scaffold for other members of the MAPK family, including p38 protein kinases and c-Jun NH2 terminal kinases [35]. Interestingly, β-arrestin-dependent signaling to MAPKs can be dissociated from G-protein-dependent signaling. For example, full-length parathyroid hormone (PTH) induces a biphasic ERK response through type 1 PTH-related peptide receptors. The initial phase of this response is sensitive to PKA and PKC inhibitors, suggesting G-protein-mediated effects, while the delayed response is sensitive to siRNA-mediated β-arrestin depletion, suggesting signaling mediated exclusively by β-arrestins [36].
Thus, GPCRs can couple promiscuously to multiple effectors via interaction with G-proteins and/or β-arrestins. A functionally selective agonist could, theoretically, stimulate any combination of these responses, resulting in LDTRS (Figure 2A). To explain this behavior, a model with multiple active receptor states has been proposed in which distinct receptor conformations are coupled with distinct effectors [37-41]. In contrast, models of receptor behavior that include two active states better explain classical pharmacological predictions [42-44]. According to these models, ligands indifferently stabilize an intermediate active conformation, allowing simultaneous activation of all signaling pathways (Figure 2B).
Figure 2. Potential responses downstream of functionally selective GPCR agonists.

(A) Agonist 1 stimulates multiple responses downstream of a GPCR, whereas agonist 2 favors the induction of the G-protein response 1. (B) Agonist 3 acts as a non-biased ligand that has no preference for the response it elicits. (C) In the presence of a positive allosteric modulator (PAM), an agonist induces a receptor conformation favorable for G-protein response 2.
Orthosteric ligand-induced functional selectivity of GPCRs
Some of the first reports of LDTRS demonstrated that orthosteric agonists induce non-equivalent effects across multiple signaling pathways. For example, at the M1 AChR, the orthosteric agonist carbachol was demonstrated to stimulate both Gαq-mediated PLC activation and Gαs-mediated cAMP accumulation, whereas rigid analogs of ACh, including cevimeline (AF-102B), AF-150 and AF-151, did not stimulate cAMP production [45]. These findings have also been extended beyond M1 AChRs. For example, using a [35S]GTPγS binding/immunoprecipitation strategy in M2- or M4-expressing CHO cells, a later study demonstrated that the partial agonist pilocarpine was more effective in activating Gαi3 than Gαi1/2 G-proteins [30]. In addition, pilocarpine induced the activation of Gαi3 subunits, but not Gαq/11subunits, in CHO cells expressing M3 AChRs, suggesting differential activation of two families of G-proteins downstream of muscarinic (m)AChRs [30].
As noted, a study using a series of orthosteric agonists of 5-HT2A/2C subtypes demonstrated the differential induction of [3H] inositol phosphate (IP) accumulation and [14C]arachidonic acid (AA) release [15]. In this study, the 5-HT2C receptor agonist 3-trifuoromethylphenyl-piperazine (TFMPP) favored IP generation rather than AA release, whereas lysergic acid diethylaminde (LSD) favored AA release rather than IP generation [15]. Importantly, the study simultaneously measured AA release and IP accumulation, two mainly independent cellular responses, revealing a reversal in potency and efficacy for these two compounds and indicating actual LDTRS. Furthermore, these effects were observed in expression systems with little to no receptor reserves for either response, permitting the intrinsic activity of each agonist to be quantified by comparing the ratio of the maximal response of each drug to that of the full agonist 5-HT [15].
Allosteric ligand-induced functional selectivity of GPCRs
In addition to orthosteric ligand-induced effects, recent investigations have suggested that compounds that allosterically bind and modulate GPCRs can induce LDTRS. These compounds, typically referred to as allosteric activators or modulators, have exhibited selectivity for individual receptor subtypes and are providing exciting new approaches for the development of therapeutic agents. Allosteric activators include agonists, positive allosteric modulators (PAMs) and negative allosteric modulators (NAMs), and have been identified for multiple GPCR families, including metabotropic glutamate receptors (mGluRs), mAChRs, and adenosine and serotonin receptors [46-48]. PAMs and NAMs are two classes of allosteric modulator that have been demonstrated to modify receptor conformations, resulting in altered binding and/or functional properties of the orthosteric ligands. PAMs and NAMs are unlike agonists in that they are inactive in the absence of orthosteric ligands. It is also apparent that these modulators possess a rich repertoire of behaviors that extend beyond simple modification to orthosteric agonist affinity [37]. Specifically, these modulators may induce functionally selective receptor conformations more frequently than orthosteric ligands (Figure 2C). While this phenomenon is still poorly understood, a recent study demonstrated that two structurally diverse potentiators of M1 AChRs are distinct in their abilities to enhance the activation of downstream effectors [49]. In this study, the compounds VU-0029767 and VU-0090157 (both Figure 3) potentiated ACh-induced calcium mobilization, as is typically expected for an allosteric potentiator. However, VU-0090157 potentiated ACh-induced activation of phospholipase D (PLD) and phosphoinositide (PI) hydrolysis, while VU-0029767 had little or no effect on PLD activation or PI hydrolysis, suggesting allosteric modulator-induced functional selectivity. As M1-mediated signaling to PLD typically involves the activation of Gα12 or small G-proteins such as R-Ras [50,51], it was concluded that when bound simultaneously by ACh and VU-0029767, M1 AChRs adopt receptor conformations that are unable to form productive signaling complexes with non-Gαq signaling partners [49]. In a similar study, the differential activation of receptor responses coupled to mGluR5 were demonstrated upon binding of the allosteric potentiator N-(4-chloro-2-[(1,3-dioxo-1,3-dihydro-2H-isoindol2-yl) methyl]phenyl)-2-hydroxybenzamide (CPPHA; originating from Merck & Co Inc; Figure 3) [52]. Interestingly, CPPHA potentiated glutamate-induced ERK phosphorylation at low glutamateconcentrations, but decreased ERK responses at high glutamate concentrations. At the M4 AChR, the PAM LY-2033298 (Lilly Research Centre Ltd; Figure 3) was also demonstrated to stimulate pathway-specific allosteric modulation [53]. Using an operational model of allosterism and agonism, Leach et al demonstrated that the functional cooperativity between LY-2033298 and ACh was significantly greater for ERK1/2 and GSK-3β phosphorylation when compared with [35S]GTPγS binding [53]. Similarly, a NAM of the CRTH2R (chemoattractant-receptor-homologous molecule on T-helper 2 cells) has been described that is inactive with respect to coupling to G-protein-linked pathways, but is a potent antagonist of G-protein-independent β-arrestin coupling to the same receptor [54]. Together, these results provide multiple examples of allosteric modulator-induced receptor conformations that are non-equivalent across different signaling pathways.
Figure 3. Selected functionally selective positive allosteric modulators of GPCRs.

More recently, it has become evident that there is an additional class of allosteric ligand, referred to as allosteric agonists, that is capable of inducing the selective activation of receptor responses. These agonists bind GPCRs at topographically distinct sites and can induce cellular responses in the absence of endogenous ligand. Many cases of the differential activation of receptor responses by putative allosteric agonists have been observed at mAChRs. For example, a multiple assay approach that included [35S]GTPγS binding, as well as measurements of IP3 generation and adenylyl cyclase activity in M1-expressing CHO cells, was used to demonstrate that the allosteric agonists AC-42 (4-N-butyl-1-[4-(2-methylphenyl)-4-oxo-1-butyl] piperidine; ACADIA Pharmaceuticals Inc/Meiji Seika Kasisha Ltd; Figure 4) and 77-LH-28-1 (1-[3-(4-butyl-1-piperidinyl)propyl]-3,4-dihydro-2(1H)-quinolinone; GlaxoSmithKline plc; Figure 4) did not significantly induce [35S]GTPγS binding to Gαi1/2 when compared with the orthosteric agonists oxotremerine-M, pilocarpine and arecoline [19]. In contrast, Gαq/11 and Gαs-mediated signaling was induced by both types of agonists, suggesting that the allosteric agonists activated only distinct subsets of G-proteins [19]. A subsequent study by the same researchers demonstrated that the orthosteric agonists caused significant receptor internalization and downregulation, whereas prolonged exposure of M1-expressing CHO cells to AC-42 did not significantly alter either cell-surface or total cellular M1 AChR expression; 77-LH-28-1 caused some degree of receptor internalization, but did not induce receptor downregulation in these cells [22]. Similarly, the M1-selective allosteric agonists TBPB ((1-(1′-2-methylbenzyl)-1,4′-bipiperidin-4-yl)-1H -benzo [d] imidazol-2(3H)-one; Merck & Co Inc; Figure 4) and AC-260584 (4-[3-(4-butylpiperidin-1-yl)-propyl]-7-fuoro-4H-benzo[1,4] oxazin-3-one; ACADIA Pharmaceuticals/Meiji Seika Kaisha; Figure 4) were demonstrated to be functionally coupled to the ERK pathway and to induce calcium release; however, these compounds did not induce significant β-arrestin recruitment or internalization of the M1 AChR [20]. These effects are reminiscent of those of the prototypical analgesic opioid agonist morphine, which has been demonstrated to activate μ-opioid receptor-mediated effectors in the absence of receptor internalization in HEK293 cells [18]. However, in contrast to the reported lack of M1 AChR internalization following the addition of AC-260584, a recent report indicated that AC-260584 induced prolonged internalization in the absence of recycling of M1 AChRs in HEK293 cells [21].
Figure 4. Selected functionally selective allosteric agonists of GPCRs.
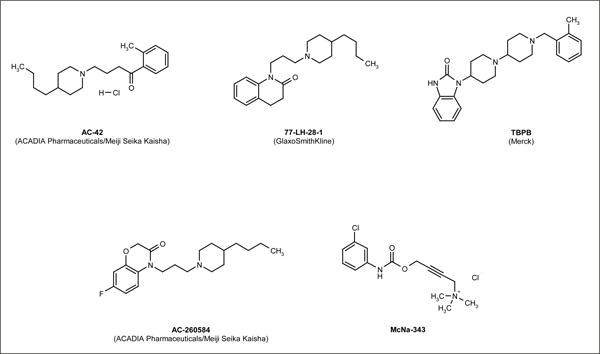
In addition to agonists of the M1 AChR, other putative allosteric agonists have been demonstrated to be functionally selective at different GPCRs. For example, in a study by Griffin et al, the partial agonist McNa-343 (4-[[[(3-chlorophenyl) amino]carbonyl] oxy]-N,N,N-trimethyl-2-butyn-1-aminium chloride; Figure 4) differentially activated effectors downstream of M2 AChRs [55]. In this study, concentration-response curve analysis was used to estimate the intrinsic relative activity of McN-A-343; this compound had 10-fold greater activity for stimulating M2-Gα15 responses compared with M2-Gαi responses [55]. A more recent study suggests that McNa-343 is a bitopic molecule composed of an orthosteric agonist moiety coupled to an allosteric modulator that induces the differential activation of M2 AChR responses by occupying both the orthosteric and allosteric binding sites [56]. This bitopic behavior can, theoretically, arise from either switching between two different binding modes or simultaneous occupation of both the orthosteric and allosteric sites.
Ligand-directed trafficking of receptor signaling at β-arrestins
It is important to reiterate that agonist-biased effects are not limited to cellular responses that are mediated by G-proteins; many orthosteric agents have demonstrated functional selectivity for β-arrestin-mediated scaffolding. For example, the β-adrenergic receptor antagonist carvedilol exhibited negative efficacy for Gαs-mediated signaling, but positive efficacy for β-arrestin-mediated ERK activation [57]. Similarly, the AngII analog Sar1Ile4Ile8-AngII, which acts as an agonist for the AT1 receptor, induced ERK activation in the absence of reported G-protein activation at AT1 receptors [58]. While there have been no reported examples of allosteric ligand-induced effector activation mediated exclusively by β-arrestin scaffolding, this pathway provides an additional arm of signaling that may aid the understanding of the potential functional effects that occur downstream of allosteric modulator-stabilized receptor states.
Conclusion
Given the examples of ligand-induced differential efficacies described in the preceding sections, it is clear that classifying compounds on the basis of their ability to modulate a single effector system does not provide a comprehensive description of the potential of a particular compound. Therefore, a multi-assay approach in a similar cellular background will be required to understand the pluridimensionality of GPCR agonists and modulators. Furthermore, estimates of the relative signaling efficacies will be required to compare functional selectivity between agonists. Such an approach was recently used to identify allosteric site mutations of the M2 AChR that provide ligand-selective signaling bias [59]. In terms of desensitization, the findings that allosteric and orthosteric agonists induce differential receptor endocytosis suggest that the intrinsic activity of an agonist and receptor endocytosis may not always be fundamentally linked. These findings are particularly interesting, given previous reports of a tight correlation between the intrinsic activity of an agonist and its propensity to induce receptor endocytosis [60].
The most parsimonious explanation for LDTRS is that the receptor conformation stabilized by a given ligand can be active toward one set of downstream effectors, while being inactive for another set of effectors [61]. A multi-state model in which distinct receptor conformations are involved in the coupling with distinct effectors has been proposed [37-41]. Based on this model, creative drug design could help to stabilize the most productive active receptor states for a particular pathway. In terms of drug discovery, the induction of LDTRS by orthosteric or allosteric ligands will change the current paradigm for proper target validation. Accordingly, investigators may find it helpful to generate as much information as possible on the most constructive and therapeutically relevant biological responses for a given disease, prior to beginning a search for a potential therapeutic ligand for a specific receptor subtype. Such findings could help prevent some of the undesired side effects commonly associated with therapeutic compounds by avoiding the activation of ancillary pathways unrelated to the targeted therapeutic process.
References
•• of outstanding interest
• of special interest
- 1.Filmore D. It's a GPCR World. Modern Drug Discov. 2004;7(11):24–28. [Google Scholar]
- 2.Hopkins AL, Groom CR. The druggable genome. Nat Rev Drug Discov. 2002;1(9):727–730. doi: 10.1038/nrd892. [DOI] [PubMed] [Google Scholar]
- 3.Hermans E. Biochemical and pharmacological control of the multiplicity of coupling at G-protein-coupled receptors. Pharmacol Ther. 2003;99(1):25–44. doi: 10.1016/s0163-7258(03)00051-2. [DOI] [PubMed] [Google Scholar]
- 4.Cabrera-Vera TM, Vanhauwe J, Thomas TO, Medkova M, Preininger A, Mazzoni MR, Hamm HE. Insights into G protein structure, function, and regulation. Endocr Rev. 2003;24(6):765–781. doi: 10.1210/er.2000-0026. [DOI] [PubMed] [Google Scholar]
- 5.Chalecka-Franaszek E, Weems HB, Crowder AT, Cox BM, Côté TE. Immunoprecipitation of high-affinity, guanine nucleotide-sensitive, solubilized μ-opioid receptors from rat brain: Coimmunoprecipitation of the G proteins Gα0, Gαi1, and Gαi3. J Neurochem. 2000;74(3):1068–1078. doi: 10.1046/j.1471-4159.2000.0741068.x. [DOI] [PubMed] [Google Scholar]
- 6.Offermanns S, Wieland T, Homann D, Sandmann J, Bombien E, Spicher K, Schultz G, Jakobs KH. Transfected muscarinic acetylcholine receptors selectively couple to Gi-type G proteins and Gq/11. Mol Pharmacol. 1994;45(5):890–898. [PubMed] [Google Scholar]
- 7••.Violin JD, Lefkowitz RJ. β-Arrestin-biased ligands at seven-transmembrane receptors. Trends Pharmacol Sci. 2007;28(8):416–422. doi: 10.1016/j.tips.2007.06.006. An outstanding review article detailing many of the β-arrestin-mediated signaling cascades. This paper also describes the propensity of ligands to induce signaling cascades via G-protein-dependent or G-protein-independent/β-arrestin-dependent signaling. [DOI] [PubMed] [Google Scholar]
- 8.Wise A, Sheehan M, Rees S, Lee M, Milligan G. Comparative analysis of the efficacy of A1 adenosine receptor activation of Gi/o α G proteins following coexpression of receptor and G protein and expression of A1 adenosine receptor-Gi/o α fusion proteins. Biochemistry. 1999;38(8):2272–2278. doi: 10.1021/bi982054f. [DOI] [PubMed] [Google Scholar]
- 9.DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. β-Arrestins and cell signaling. Annu Rev Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- 10.Stephenson RP. A modification of receptor theory. Br J Pharmacol Chemother. 1956;11(4):379–393. doi: 10.1111/j.1476-5381.1956.tb00006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furchgott RF. The use of β-haloalkylamines in the differentiation of receptors and in the determination of dissociation constants of receptor-agonist complexes. In: Harper NJ, Simmonds AB, editors. Advances in Drug Research. Vol. 3. Academic Press; London, UK: 1966. pp. 21–25. [Google Scholar]
- 12.Brink CB, Harvey BH, Bodenstein J, Venter DP, Oliver DW. Recent advances in drug action and therapeutics: Relevance of novel concepts in G-protein-coupled receptor and signal transduction pharmacology. Br J Clin Pharmacol. 2004;57(4):373–387. doi: 10.1111/j.1365-2125.2003.02046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kenakin T. On the definition of efficacy. Trends Pharmacol Sci. 1994;15(11):408–409. doi: 10.1016/0165-6147(94)90086-8. [DOI] [PubMed] [Google Scholar]
- 14.Kenakin T. Agonist-receptor efficacy. II. Agonist trafficking of receptor signals. Trends Pharmacol Sci. 1995;16(7):232–238. doi: 10.1016/s0165-6147(00)89032-x. [DOI] [PubMed] [Google Scholar]
- 15••.Berg KA, Maayani S, Goldfarb J, Scaramellini C, Leff P, Clarke WP. Effector pathway-dependent relative efficacy at serotonin type 2A and 2C receptors: Evidence for agonist-directed trafficking of receptor stimulus. Mol Pharmacol. 1998;54(1):94–104. In this seminal study, a reversal of agonist potency for orthosteric ligands of 5HT2A/2C receptors was demonstrated. Importantly, these experiments were conducted in the presence of low receptor reserves, and the data for AA accumulation and PI hydrolysis were collected simultaneously. [PubMed] [Google Scholar]
- 16.Groer CE, Tidgewell K, Moyer RA, Harding WW, Rothman RB, Prisinzano TE, Bohn LM. An opioid agonist that does not induce micro-opioid receptor–arrestin interactions or receptor internalization. Mol Pharmacol. 2007;71(2):549–557. doi: 10.1124/mol.106.028258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keith DE, Murray SR, Zaki PA, Chu PC, Lissin DV, Kang L, Evans CJ, von Zastrow M. Morphine activates opioid receptors without causing their rapid internalization. J Biol Chem. 1996;271(32):19021–19024. doi: 10.1074/jbc.271.32.19021. [DOI] [PubMed] [Google Scholar]
- 18.Whistler JL, von Zastrow M. Morphine-activated opioid receptors elude desensitization by β-arrestin. Proc Natl Acad Sci USA. 1998;95(17):9914–9919. doi: 10.1073/pnas.95.17.9914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19••.Thomas RL, Mistry R, Langmead CJ, Wood MD, Challiss RA. G protein coupling and signaling pathway activation by M1 muscarinic acetylcholine receptor orthosteric and allosteric agonists. J Pharmacol Exp Ther. 2008;327(2):365–374. doi: 10.1124/jpet.108.141788. An outstanding article detailing some of the first evidence that allosteric agonists differ compared with orthosteric agonists in their ability to induce the activation of signaling pathways. [DOI] [PubMed] [Google Scholar]
- 20•.Davis AA, Heilman CJ, Brady AE, Miller NR, Fuerstenau-Sharp M, Hanson BJ, Lindsley CW, Conn PJ, Lah JJ, Levey AI. Differential effects of allosteric M1 muscarinic acetylcholine receptor agonists on receptor activation, arrestin 3 recruitment, and receptor downregulation. ACS Chem Neurosci. 2010;1(8):542–551. doi: 10.1021/cn100011e. Demonstrated that M1 AChR allosteric agonists can induce effector activation, as indicated by the induction of intracellular calcium release and ERK phosphorylation However, these same agonists did not induce receptor internalization, suggesting a non-linear correlation between effector activation and receptor downregulation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davis CN, Bradley SR, Schiffer HH, Friberg M, Koch K, Tolf BR, Bonhaus DW, Lameh J. Differential regulation of muscarinic M1 receptors by orthosteric and allosteric ligands. BMC Pharmacol. 2009;9:14. doi: 10.1186/1471-2210-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22•.Thomas RL, Langmead CJ, Wood MD, Challiss RA. Contrasting effects of allosteric and orthosteric agonists on M1 muscarinic acetylcholine receptor internalization and down-regulation. J Pharmacol Exp Ther. 2009;331(3):1086–1095. doi: 10.1124/jpet.109.160242. A follow-up study to previous research on AC-42 and 77-LH-28-1 (reference [19]) that demonstrated the differential effects of these allosteric agonists on M1 AChR internalization and downregulation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H, Javitch JA, Roth BL, Christopoulos A, Sexton PM, Miller KJ, et al. Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther. 2007;320(1):1–13. doi: 10.1124/jpet.106.104463. [DOI] [PubMed] [Google Scholar]
- 24.Fargin A, Yamamoto K, Cotecchia S, Goldsmith PK, Spiegel AM, Lapetina EG, Caron MG, Lefkowitz RJ. Dual coupling of the cloned 5-HT1A receptor to both adenylyl cyclase and phospholipase C is mediated via the same Gi protein. Cell Signal. 1991;3(6):547–557. doi: 10.1016/0898-6568(91)90031-o. [DOI] [PubMed] [Google Scholar]
- 25.Vallar L, Muca C, Magni M, Albert P, Bunzow J, Meldolesi J, Civelli O. Differential coupling of dopaminergic D2 receptors expressed in different cell types Stimulation of phosphatidylinositol 4,5-bisphosphate hydrolysis in LtK- fibroblasts, hyperpolarization, and cytosolic-free Ca2+ concentration decrease in GH4C1 cells. J Biol Chem. 1990;265(18):10320–10326. [PubMed] [Google Scholar]
- 26.Gudermann T, Kalkbrenner F, Schultz G. Diversity and selectivity of receptor-G protein interaction. Annu Rev Pharmacol Toxicol. 1996;36:429–459. doi: 10.1146/annurev.pa.36.040196.002241. [DOI] [PubMed] [Google Scholar]
- 27.Laugwitz KL, Allgeier A, Offermanns S, Spicher K, Van Sande J, Dumont JE, Schultz G. The human thyrotropin receptor: A heptahelical receptor capable of stimulating members of all four G protein families. Proc Natl Acad Sci USA. 1996;93(1):116–120. doi: 10.1073/pnas.93.1.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grammatopoulos DK, Randeva HS, Levine MA, Kanellopoulou KA, Hillhouse EW. Rat cerebral cortex corticotropin-releasing hormone receptors: Evidence for receptor coupling to multiple G-proteins. J Neurochem. 2001;76(2):509–519. doi: 10.1046/j.1471-4159.2001.00067.x. [DOI] [PubMed] [Google Scholar]
- 29.Stanislaus D, Ponder S, Ji TH, Conn PM. Gonadotropin-releasing hormone receptor couples to multiple G proteins in rat gonadotrophs and in GGH3 cells: Evidence from palmitoylation and overexpression of G proteins. Biol Reprod. 1998;59(3):579–586. doi: 10.1095/biolreprod59.3.579. [DOI] [PubMed] [Google Scholar]
- 30.Akam EC, Challiss RA, Nahorski SR. Gq/11 and Gi/o activation profiles in CHO cells expressing human muscarinic acetylcholine receptors: Dependence on agonist as well as receptor-subtype. Br J Pharmacol. 2001;132(4):950–958. doi: 10.1038/sj.bjp.0703892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burford NT, Nahorski SR. Muscarinic M1 receptor-stimulated adenylate cyclase activity in Chinese hamster ovary cells is mediated by Gs α and is not a consequence of phosphoinositidase C activation. Biochem J. 1996;315(Pt 3):883–888. doi: 10.1042/bj3150883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grisshammer R, Hermans E. Functional coupling with Gαq and Gαi1 protein subunits promotes high-affinity agonist binding to the neurotensin receptor NTS-1 expressed in Escherichia coli. FEBS Lett. 2001;493(2-3):101–105. doi: 10.1016/s0014-5793(01)02281-5. [DOI] [PubMed] [Google Scholar]
- 33.Kuhn B, Schmid A, Harteneck C, Gudermann T, Schultz G. G proteins of the Gq family couple the H2 histamine receptor to phospholipase C. Mol Endocrinol. 1996;10(12):1697–1707. doi: 10.1210/mend.10.12.8961278. [DOI] [PubMed] [Google Scholar]
- 34.Luttrell LM, Roudabush FL, Choy EW, Miller WE, Field ME, Pierce KL, Lefkowitz RJ. Activation and targeting of extracellular signal-regulated kinases by β-arrestin scaffolds. Proc Natl Acad Sci USA. 2001;98(5):2449–2454. doi: 10.1073/pnas.041604898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by β-arrestins. Science. 2005;308(5721):512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- 36.Gesty-Palmer D, Chen M, Reiter E, Ahn S, Nelson CD, Wang S, Eckhardt AE, Cowan CL, Spurney RF, Luttrell LM, Lefkowitz RJ. Distinct β-arrestin- and G protein-dependent pathways for parathyroid hormone receptor-stimulated ERK1/2 activation. J Biol Chem. 2006;281(16):10856–10864. doi: 10.1074/jbc.M513380200. [DOI] [PubMed] [Google Scholar]
- 37.Christopoulos A, Kenakin T. G protein-coupled receptor allosterism and complexing. Pharmacol Rev. 2002;54(2):323–374. doi: 10.1124/pr.54.2.323. [DOI] [PubMed] [Google Scholar]
- 38.Kenakin T. Drug efficacy at G protein-coupled receptors. Annu Rev Pharmacol Toxicol. 2002;42:349–379. doi: 10.1146/annurev.pharmtox.42.091401.113012. [DOI] [PubMed] [Google Scholar]
- 39.Krumins AM, Barber R. The stability of the agonist β2-adrenergic receptor-Gs complex: Evidence for agonist-specific states. Mol Pharmacol. 1997;52(1):144–154. doi: 10.1124/mol.52.1.144. [DOI] [PubMed] [Google Scholar]
- 40.Leff P, Scaramellini C, Law C, McKechnie K. A three-state receptor model of agonist action. Trends Pharmacol Sci. 1997;18(10):355–362. doi: 10.1016/s0165-6147(97)01105-x. [DOI] [PubMed] [Google Scholar]
- 41.Scaramellini C, Leff P. A three-state receptor model: Predictions of multiple agonist pharmacology for the same receptor type. Ann N Y Acad Sci. 1998;861:97–103. doi: 10.1111/j.1749-6632.1998.tb10179.x. [DOI] [PubMed] [Google Scholar]
- 42.Changeux JP, Edelstein SJ. Allosteric receptors after 30 years. Neuron. 1998;21(5):959–980. doi: 10.1016/s0896-6273(00)80616-9. [DOI] [PubMed] [Google Scholar]
- 43.Karlin A. On the application of ‘a plausible model’ of allosteric proteins to the receptor for acetylcholine. J Theor Biol. 1967;16(2):306–320. doi: 10.1016/0022-5193(67)90011-2. [DOI] [PubMed] [Google Scholar]
- 44.Thron CD. On the analysis of pharmacological experiments in terms of an allosteric receptor model. Mol Pharmacol. 1973;9(1):1–9. [PubMed] [Google Scholar]
- 45•.Fisher A, Heldman E, Gurwitz D, Haring R, Barak D, Meshulam H, Marciano D, Brandeis R, Pittel Z, Segal M, et al. Selective signaling via unique M1 muscarinic agonists. Ann N Y Acad Sci. 1993;695:300–303. doi: 10.1111/j.1749-6632.1993.tb23070.x. Provides one of the first examples of LDTRS at M1 AChRs using rigid ACh analogs, indicating that these ligands differentially activate M1 signaling when compared with ACh. [DOI] [PubMed] [Google Scholar]
- 46.Conn PJ, Christopoulos A, Lindsley CW. Allosteric modulators of GPCRs: A novel approach for the treatment of CNS disorders. Nat Rev Drug Discov. 2009;8(1):41–54. doi: 10.1038/nrd2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Conn PJ, Jones CK, Lindsley CW. Subtype-selective allosteric modulators of muscarinic receptors for the treatment of CNS disorders. Trends Pharmacol Sci. 2009;30(3):148–155. doi: 10.1016/j.tips.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.May LT, Christopoulos A. Allosteric modulators of G-protein-coupled receptors. Curr Opin Pharmacol. 2003;3(5):551–556. doi: 10.1016/s1471-4892(03)00107-3. [DOI] [PubMed] [Google Scholar]
- 49••.Marlo JE, Niswender CM, Days EL, Bridges TM, Xiang Y, Rodriguez AL, Shirey JK, Brady AE, Nalywajko T, Luo Q, Austin CA, et al. Discovery and characterization of novel allosteric potentiators of M1 muscarinic receptors reveals multiple modes of activity. Mol Pharmacol. 2009;75(3):577–588. doi: 10.1124/mol.108.052886. Describes some of the first evidence suggesting that different allosteric modulators are unique in their abilities to induce receptor behavior. Interestingly, VU-0090157 potentiated ACh-induced activation of PLD and PI hydrolysis, whereas VU-0029767 had almost no effect on M1-mediated increases in PLD activity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.López De Jesús M, Stope MB, Oude Weernink PA, Mahlke Y, Börgermann C, Ananaba VN, Rimmbach C, Rosskopf D, Michel MC, Jakobs KH, Schmidt M. Cyclic AMP-dependent and Epac-mediated activation of R-Ras by G protein-coupled receptors leads to phospholipase D stimulation. J Biol Chem. 2006;281(31):21837–21847. doi: 10.1074/jbc.M604156200. [DOI] [PubMed] [Google Scholar]
- 51.Rümenapp U, Asmus M, Schablowski H, Woznicki M, Han L, Jakobs KH, Fahimi-Vahid M, Michalek C, Wieland T, Schmidt M. The M3 muscarinic acetylcholine receptor expressed in HEK-293 cells signals to phospholipase D via G12 but not Gq-type G proteins: Regulators of G proteins as tools to dissect pertussis toxin-resistant G proteins in receptor-effector coupling. J Biol Chem. 2001;276(4):2474–2479. doi: 10.1074/jbc.M004957200. [DOI] [PubMed] [Google Scholar]
- 52.Zhang Y, Rodriguez AL, Conn PJ. Allosteric potentiators of metabotropic glutamate receptor subtype 5 have differential effects on different signaling pathways in cortical astrocytes. J Pharmacol Exp Ther. 2005;315(3):1212–1219. doi: 10.1124/jpet.105.090308. [DOI] [PubMed] [Google Scholar]
- 53.Leach K, Loiacono RE, Felder CC, McKinzie DL, Mogg A, Shaw DB, Sexton PM, Christopoulos A. Molecular mechanisms of action and in vivo validation of an M4 muscarinic acetylcholine receptor allosteric modulator with potential antipsychotic properties. Neuropsychopharmacology. 35(4):855–869. doi: 10.1038/npp.2009.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mathiesen JM, Ulven T, Martini L, Gerlach LO, Heinemann A, Kostenis E. Identification of indole derivatives exclusively interfering with a G protein-independent signaling pathway of the prostaglandin D2 receptor CRTH2. Mol Pharmacol. 2005;68(2):393–402. doi: 10.1124/mol.104.010520. [DOI] [PubMed] [Google Scholar]
- 55.Griffin MT, Figueroa KW, Liller S, Ehlert FJ. Estimation of agonist activity at G protein-coupled receptors: Analysis of M2 muscarinic receptor signaling through Gi/o,Gs, and G15. J Pharmacol Exp Ther. 2007;321(3):1193–1207. doi: 10.1124/jpet.107.120857. [DOI] [PubMed] [Google Scholar]
- 56.Valant C, Gregory KJ, Hall NE, Scammells PJ, Lew MJ, Sexton PM, Christopoulos A. A novel mechanism of G protein-coupled receptor functional selectivity. Muscarinic partial agonist McN-A-343 as a bitopic orthosteric/allosteric ligand. J Biol Chem. 2008;283(43):29312–29321. doi: 10.1074/jbc.M803801200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57•.Wisler JW, DeWire SM, Whalen EJ, Violin JD, Drake MT, Ahn S, Shenoy SK, Lefkowitz RJ. A unique mechanism of β-blocker action: Carvedilol stimulates β-arrestin signaling. Proc Natl Acad Sci USA. 2007;104(42):16657–16662. doi: 10.1073/pnas.0707936104. An excellent study demonstrating that the β-blocker carvedilol stimulates ERK activity via β-arrestin, a finding that is absent with other adrenergic receptor antagonists. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Holloway AC, Qian H, Pipolo L, Ziogas J, Miura S, Karnik S, Southwell BR, Lew MJ, Thomas WG. Side-chain substitutions within angiotensin II reveal different requirements for signaling, internalization, and phosphorylation of type 1A angiotensin receptors. Mol Pharmacol. 2002;61(4):768–777. doi: 10.1124/mol.61.4.768. [DOI] [PubMed] [Google Scholar]
- 59•.Gregory KJ, Hall NE, Tobin AB, Sexton PM, Christopoulos A. Identification of orthosteric and allosteric site mutations in M2 muscarinic acetylcholine receptors that contribute to ligand-selective signaling bias. J Biol Chem. 285(10):7459–7474. doi: 10.1074/jbc.M109.094011. Quantified the coupling efficiency of a variety of M2 AChR mutants using an operational model of agonism and identified pathway-selective allosteric and orthosteric site mutations. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Szekeres PG, Koenig JA, Edwardson JM. The relationship between agonist intrinsic activity and the rate of endocytosis of muscarinic receptors in a human neuroblastoma cell line. Mol Pharmacol. 1998;53(4):759–765. doi: 10.1124/mol.53.4.759. [DOI] [PubMed] [Google Scholar]
- 61.Galandrin S, Oligny-Longpré G, Bouvier M. The evasive nature of drug efficacy: Implications for drug discovery. Trends Pharmacol Sci. 2007;28(8):423–430. doi: 10.1016/j.tips.2007.06.005. [DOI] [PubMed] [Google Scholar]


