Abstract
The long-term outcome of patients with mucoepidermoid carcinoma is poor. Limited availability of cell lines and lack of xenograft models is considered a major barrier to improved mechanistic understanding of this disease and development of effective therapies.
Objective
To generate and characterize human mucoepidermoid carcinoma cell lines and xenograft models suitable for mechanistic and translational studies.
Methods
Five human mucoepidermoid carcinoma specimens were available for generation of cell lines. Cell line tumorigenic potential was assessed by transplantation and serial in vivo passaging in immunodeficient mice, and cell line authenticity verified by short tandem repeat (STR) profiling.
Results
A unique pair of mucoepidermoid carcinoma cell lines was established from a local recurrence (UM-HMC-3A) and from the metastatic lymph node (UM-HMC-3B) of the same patient, 4 years after surgical removal of the primary tumor. These cell lines retained epithelial-like morphology through 100 passages in vitro, contain the Crtc1-Maml2 fusion oncogene (characteristic of mucoepidermoid carcinomas), and express the prototypic target of this fusion (NR4A2). Both cell lines generated xenograft tumors when transplanted into immunodeficient mice. Notably, the xenografts exhibited histological features and Periodic Acid Schiff (PAS) staining patterns that closely resembled those found in human tumors. STR profiling confirmed the origin and authenticity of these cell lines.
Conclusion
These data demonstrate the generation and characterization of a pair of tumorigenic salivary mucoepidermoid carcinoma cell lines representative of recurrence and lymph node metastasis. Such models are useful for mechanistic and translational studies that might contribute to the discovery of new therapies for mucoepidermoid carcinoma.
Keywords: Mouse models, Salivary gland cancer, Xenograft, Oral cancer, Crtc1-Maml2, Tumor recurrence, Metastasis
Introduction
Salivary gland cancers consist of a rare, yet rather diverse group of tumors that contribute to 2–6% of all head and neck cancers.1–3 They are divided into two main groups; those derived from the major salivary glands (parotid, submandibular, sublingual) and those originating from minor salivary glands lining the oral, nasopharyngeal cavity, and paranasal sinuses.1–3 There are more than 24 distinct morphological subtypes of salivary gland cancers recognized by the World Health Organization.1–4 Among the most common types of salivary gland tumors is the mucoepidermoid carcinoma, first described in 1895 by Volkmann.5 The standard of care for patients with mucoepidermoid carcinoma is surgery alone or in combination with radiation therapy, since chemotherapeutic drugs have shown modest effects.2,3 The search for safe and effective therapies for mucoepidermoid carcinomas is hindered by the relatively poor understanding of the pathobiology of this disease. Access to research tools, such as cell lines and xenograft models, is critical for a better understanding of the biology of salivary gland mucoepidermoid carcinomas and for the development of mechanism-based therapies. Notably, findings obtained with salivary gland mucoepidermoid carcinoma cells might be germane to the biology of mucoepidermoid carcinomas in other sites (e.g. lungs, breast, thyroid gland).
Mucoepidermoid carcinomas represent 30–35% of all salivary gland tumors and originate most often from major salivary glands.6 Mucoepidermoid tumors are graded (i.e. low, intermediate, high) based on several characteristics, such as mitotic rate, relative frequency of epidermoid and mucin-producing cells, cell differentiation, tumor size, cyst formation, perineural invasion, and metastatic disease.4,5,7,8 While patients with low or intermediate grade tumors have a 80–95% 5-year survival, patients with high grade mucoepidermoid carcinomas exhibit a 5-year survival of only up to 40%.4,6 Notably, metastatic spread to regional lymph nodes (30–70%) or to lungs and/or bone (10–20%) contributes to the poor outcome of patients.2 The mechanisms underlying the processes of salivary mucoepidermoid carcinoma migration and loco-regional invasion, as well as mechanisms involved in the homing of these cells to the lungs and bone, are largely unknown.
Importantly, a chromosomal translocation t(11;19) generating a fusion oncogene that consists of the mucoepidermoid carcinoma translocated gene (Mect)-1 (also called Crtc1, or Torc1) fused to the mastermind-like gene family (Maml)-2 is present in 38–81% of mucoepidermoid carcinomas.9–11 The resulting fusion protein is primarily found in low/intermediate grade mucoepidermoid carcinomas and has been correlated with the prognosis of patients.9,10,12,13 It has been shown that sustained expression of this fusion protein is necessary for the tumor cell growth of salivary gland cancers.14 A proposed model for the tumorigenic effect of the Crtc1-Maml2 fusion involves the activation of the Notch and/or cAMP-responsive element binding protein (CREB) signaling pathways leading to phenotypic changes that characterize the pathobiology of mucoepidermoid carcinomas.15
Very few mucoepidermoid carcinoma cell lines have been established to date. The Grénman laboratory generated the UT-MUC-1 cell line in the early nineties from a poorly differentiated mucoepidermoid carcinoma, and showed that it is highly resistant to radiation therapy.16 The NCI-Navy Branch in Bethesda, MD used tumor biopsies to generate 2 mucoepidermoid carcinoma cell lines (H292, H3118) and showed that both cell lines present a reciprocal t(11;19) translocation.9 And finally, Queimado and colleagues have used viral constructs containing the E6/E7 genes of HPV16 to stably transform the mucoepidermoid carcinoma cell line UTSW-MEC49.17 Here, we report the generation of 5 new mucoepidermoid carcinoma cell lines, and describe the detailed characterization of a highly tumorigenic pair of cell lines from the same patient who presented with a local recurrence (UM-HMC-3A) and lymph node metastases (UM-HMC-3B) four years after surgical removal of the primary tumor. This constitutes a unique pair of mucoepidermoid carcinoma cell lines that can be easily expanded in culture and that recapitulate the histology of the primary tumor when transplanted into immunodeficient mice.
Materials and Methods
Tumor specimens and generation of UM-HMC cell lines
Cell lines were generated from salivary mucoepidermoid carcinoma tumors that were surgically resected between March/2010 and August/2012, namely the University of Michigan-Human Mucoepidermoid Carcinoma (UM-HMC) series. As a control, we also established a cell line from a benign human pleomorphic adenoma (UM-HPA-1). Tumors were minced in small pieces, passed through a 25-ml pipette and centrifuged at 1,000 RPM, 4°C for 5 minutes. Minced tumor specimens were then placed in a sterile petri dish (Fisher Scientific, Pittsburgh, PA, USA) digested in 1× collagenase-hyaluronidase (Stem Cell Technologies, Vancouver, BC, Canada) at 37°C for 45–60 minutes. Tumors were disrupted manually every 15 minutes using a 25-ml pipette (1×) followed by a 10-ml pipette (2–3×) to facilitate dissociation. Single cell suspensions were prepared by passing the mixture through a 40-μm sieve (Fisher) placed in a Falcon tube containing 3–4 ml Fetal Bovine Serum (FBS, Invitrogen). Red blood cells were lysed for 1 minute using AKC lysis buffer (Invitrogen, Carlsbad, CA, USA) and spun at 1,000 RPM, 4°C for 5 minutes. Resulting cell populations were cultured in a salivary gland medium (SGM) consisting of high glucose Dulbecco’s Modified Eagle’s Medium (DMEM; Invitrogen) supplemented with 1% L-glutamine (Invitrogen), 1% antibiotic (AAA; Sigma-Aldrich, St. Louis, MO, USA), 10% FBS (Invitrogen), 20 ng/ml epidermal growth factor (Sigma-Aldrich), 400 ng/ml hydrocortisone (Sigma-Aldrich), 5 μg/ml insulin (Sigma-Aldrich), 50 ng/ml nystatin (Sigma-Aldrich), and 1% amphotericin B (Sigma-Aldrich), as suggested.17 After culture for 2–3 months, the antimicrobial regimen was changed to 5% Penicillin/Streptomicin (Invitrogen). Cell culture medium was changed every 2–3 days and cells were passed (1:3) using 0.05% trypsin (Invitrogen). Primary human dermal microvascular endothelial cells (HDMEC; Lonza, Walkersville, MD, USA) were cultured in endothelial growth medium (EGM2-MV; Lonza). This work was performed under approval from appropriate institutional review boards.
RT-PCR for CRTC1-MAML2 fusion oncogene
UM-HMC cells were plated at 2.0 ×105 cells per 100 mm3 dish and grown to 90% confluency in SGM. RNA was extracted using TRIZOL (Invitrogen) and one-step PCR (Invitrogen) was performed, according to manufacturer’s instructions. Primer sequences used to identify the CRTC1-MAML2 fusion were 5′-ATG GCG ACT TCG AAC AAT CCG CGG AA-3′ (forward) and 5′-CCA TTG GGT CGC TTG CTG TTG GCA GGA G-3′ (reverse) and result in a 196-base pair amplicon, as described.18 GAPDH was simultaneously run for each sample as loading controls.
Western blots
Whole cell lysates were generated with a NP-40 lysis buffer and resolved by PAGE. Membranes were probed with antibodies against human EGFR, E-Cadherin, Pan-cytokeratin and B-actin (Santa Cruz Biotechnology, Santa Cruz, CA, USA); Cytokeratin-7 and Vimentin (Cell Signaling, Beverly, MA, USA); CRTC1 (MECT1; Abcam, Cambridge, MA, USA); and NR4A2 (Sigma-Aldrich) overnight at 4°C.
UM-HMC-derived xenograft tumors
1×105 UM-HMC-3A (passage 14) or UM-HMC-3B (passage 27) cells and 9×105 HDMEC (Lonza) were seeded in biodegradable scaffolds and co-implanted subcutaneously in the dorsal region of severe combined immunodeficient (SCID) mice (CB-17 SCID; Charles River, Wilmington, MA, USA), as we described.19,20 First through fourth passage tumors were surgically retrieved when they reached 800–1,000 mm3. To maintain tumor propagation in vivo, xenografts were digested (as described above) and 1×105 tumor cells + HDMEC (9×105) were seeded in new biodegradable scaffolds and re-implanted into SCID mice. Tumor tissues were retrieved, fixed overnight in 10% buffered formalin (Fisher) at 4°C, and processed for immunohistochemistry. Human tumor specimens and cell line-derived xenografts were stained for Hematoxylin and Eosin and Periodic Acid Schiff (PAS) at University of Michigan core facilities.
UM-HMC cell line authentication
To verify the identity of cell lines and cell line-generated xenografts, genomic DNA from the patient’s tumor (reference), high passage UM-HMC-3A cells (passage 60) and UM-HMC-3B cells (passage 50), or xenograft tumors generated with UM-HMC-3A (passage 14) or UM-HMC-3B (passage 27) was purified using the Wizard Genomic DNA Purification Kit (Promega, Madison, WI, USA). DNA genotyping by short tandem repeat (STR) profiling was performed and analyzed independently by Biosynthesis Inc. (Lewisville, TX, USA).
Results
In vitro characterization of UM-HMC cells
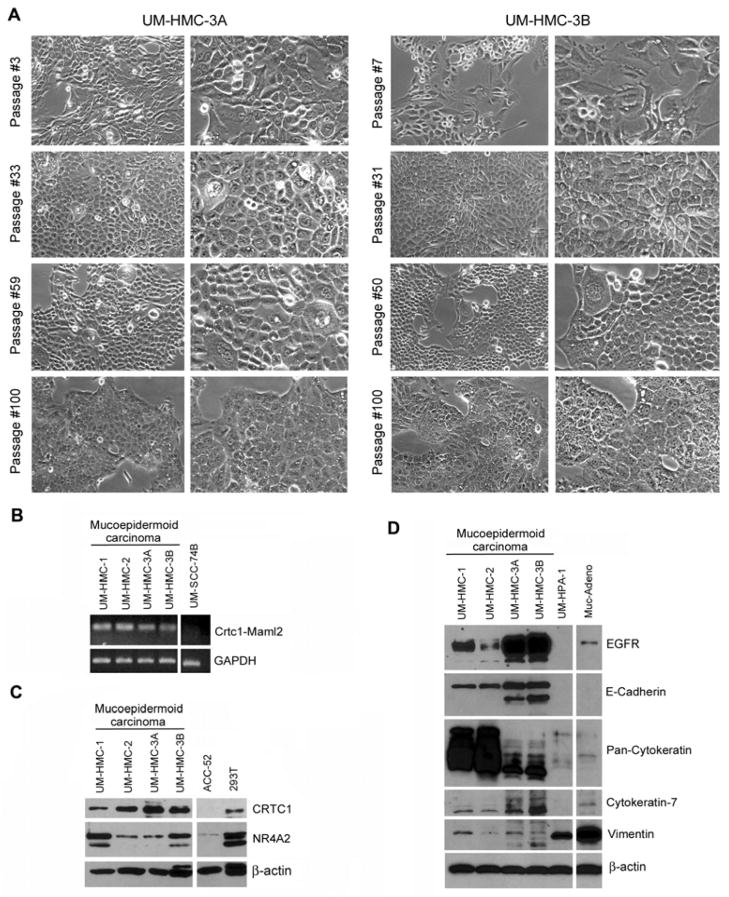
We had access to 4 specimens from surgical resections of human mucoepidermoid carcinomas to perform this work. The information about the patient age, gender, tumor site, grading and/or staging, and highest passage of the cell lines is provided in Table 1. We generated multiple UM-HMC cell lines and one benign pleomorphic adenoma that was used as control in selected experiments. Here, we will focus on the description and characterization of the UM-HMC-3A and UM-HMC-3B pair. Small colonies of attached cells were readily visible for UM-HMC-3A and UM-HMC-3B cells after 9 days of culture. We observed that these cells maintained a cobblestone epithelial-like morphology for at least 100 passages (Figure 1A).
Table 1.
Patient demographics and characteristics of the human tumors from which the UM-HMC and the UM-HPA-1 cell lines were generated.
| Cell Line | Highest passage | Patient demographics | Site | TNM | Stage | Grade | Perineural invasion | Angiolymphatic invasion | Prior treatment |
|---|---|---|---|---|---|---|---|---|---|
| UM-HMC-1 | 73 | 76 years old, African American male | Minor salivary gland of buccal mucosa | T4aN0M0 | IVa | Intermediate | Absent | Absent | None |
| UM-HMC-2 | 10 | 59 years old, Caucasian female | Parotid | T4bN0M0 | IVb | Intermediate | Present | NA | Partial excision |
|
UM-HMC-3A UM-HMC-3B |
100 100 |
73 years old, Caucasian female | Left hard palate (local recurrence) Lymph node (metastasis) |
T4bN | IVb | Intermediate | Present | Present | Complete excision Complete excision |
| UM-HPA-1 | 12 | 68 years old, Caucasian male | Parotid | NA | NA | NA | NA | NA | None |
Figure 1.
Characterization of human mucoepidermoid carcinoma cell lines derived from a local recurrence (UM-HMC-3A) and a metastatic lymph node (UM-HMC-3B). (A) Photomicrographs of UM-HMC3A and UM-HMC-3B cells cultured for 100 passages in vitro. Magnification at 100× (left columns) and 200× (right columns). (B) RT-PCR for screening of a panel of human mucoepidermoid carcinoma cell lines for the Crtc1-Maml2 fusion oncogene (196 bp). The head and neck squamous cell carcinoma cell line (UM-SCC-74B) was used as a negative control. (C) Western blot analysis of CRTC1 and its prototypic target NR4A2 in human mucoepidermoid carcinoma cell lines. The adenoid cystic carcinoma cell line (ACC-52) and the human embryonic kidney cell line (293T) were used as controls. (D) Western blot analysis of epithelial markers (EGFR, E-Cadherin, Pan-cytokeratin, Cytokeratin-7) and a mesenchymal marker (Vimentin) in human mucoepidermoid carcinoma cell lines. The benign human pleomorphic adenoma (UM-HPA-1) cell line was used as control for this experiment.
RT-PCR analysis revealed that all UM-HMC cell lines expressed the Crtc1-Maml2 fusion oncogene (Figure 1B). In contrast, human head and neck squamous cell carcinoma cells (UM-SCC-74B) used as negative control did not express this oncogene. Western blot analysis showed that all UM-HMC cell lines expressed wtCRTC1 and NR4A2, a member of NR4A orphan nuclear receptors and prototypic target for Crtc1-Maml2 (Figure 1C). CRTC1 and NR4A2 expression was low or undetectable in adenoid cystic carcinoma cells (ACC-52) used as controls.
In addition, we evaluated the expression of epithelial and mesenchymal markers in the mucoepidermoid carcinoma cells (Figure 1D). UM-HMC-1, UM-HMC-2, UM-HMC-3A and UM-HMC-3B exhibited high levels of several epithelial markers, i.e. epidermal growth factor receptor (EGFR), E-cadherin, pan-cytokeratin, and cytokeratin-7; and lower levels of the mesenchymal marker Vimentin. In contrast, the UM-HPA-1, expressed no EGFR, E-Cadherin, or cytokeratin 7; low levels of pan-cytokeratin and high levels of vimentin.
UM-HMC-3A, UM-HMC-3B cells generate xenografts that resemble the patient’s tumor
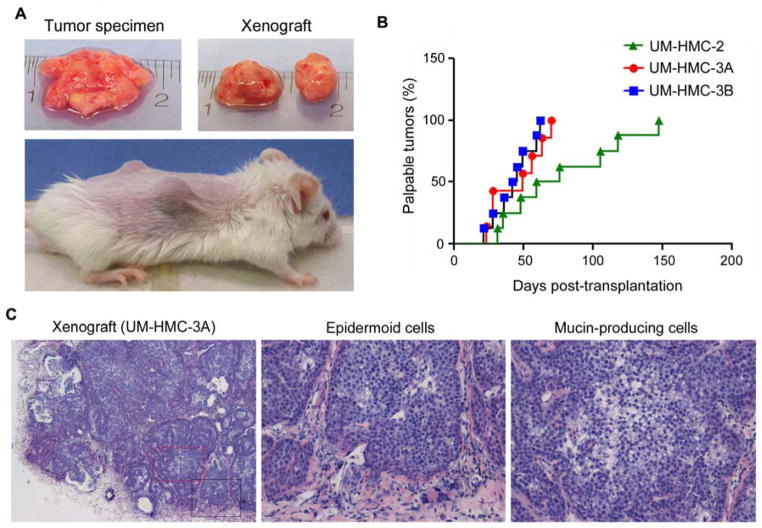
To verify the tumorigenic potential of the UM-HMC cells, we implanted all cell lines into immunodeficient mice. UM-HMC-3A and UM-HMC-3B cells consistently generated palpable xenograft tumors (Figure 2A, B). In ongoing experiments, transplantation of UM-HMC-1 (cells at passage 10 or passage 40) and control UM-HPA-1 (passage 4), have yet to grow into palpable tumors (Suppl. Figure 1). Histological analyses revealed that the xenograft tumors exhibit areas populated primarily by epidermoid-like cells while other areas are characterized by predominance of mucous-like cells, which is a typical feature of mucoepidermoid carcinomas (Figure 2C).
Figure 2.
UM-HMC cells generate xenograft tumors when implanted in immunodeficient mice. (A) Macroscopic view of a typical specimen of human mucoepidermoid carcinoma immediately after surgical removal (Top, left panel). Macroscopic view of xenografts generated upon transplantation of UM-HMC into immunodeficient mice (Top, right panel). And macroscopic view of xenograft tumors (bilateral) growing in the subcutaneous space of immunodeficient mice (bottom panel). (B) Graph depicting time to tumor palpability. Palpable tumors were defined as those surpassing the size of the biodegradable scaffold used to transplant UM-HMC-3A (n=8), UM-HMC-3B (n=6), or UM-HMC-2 (n=8). (C) Photomicrographs of histological sections (HE) obtained from xenograft tumors generated by the transplantation of UM-HMC-3A cells into immunodeficient mice. The boxes in the low magnification (40×) image depict the areas were the high magnification images (200×) were obtained from, i.e. red box (mucous-like cells) and black box (epidermoid-like cells).
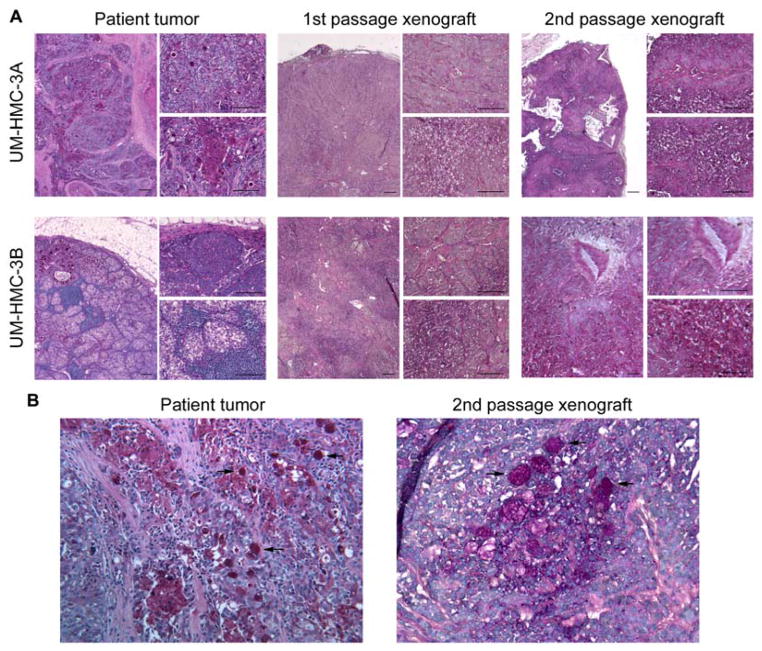
To determine if UM-HMC-3A and UM-HMC-3B consistently generated xenografts with the patient’s tumor characteristics, we transplanted these cells into SCID mice, waited until the 1st passage tumors reached approximately 1,000 mm3, digested them and transplanted the resulting cells into new mice to generate 2nd passage xenografts that were analyzed by an expert in oral pathology (Figure 3). The histology of the human tumors from which the cell lines were derived was used as control. The 1st and 2nd passage xenograft tumors closely resembled the human tumor morphology with areas of epidermoid cells (top inserts) and mucous generating cells (bottom inserts) clearly visible (Figure 3). Similarly, the patterns of Periodic acid Schiff (PAS) staining present in the human tumors are also shown in the 1st and 2nd passage UM-HMC-3A and UM-HMC-3B-derived xenografts (Figure 4A), suggestive of high levels of mucopolysaccharide secretion.21 PAS-positive globules are readily visible in both, the human tumor and the cell line-derived xenograft tumor (Figure 4B).
Figure 3.
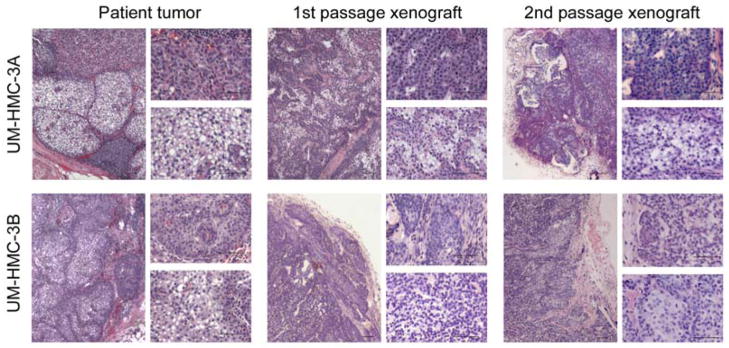
Xenografts generated with human mucoepidermoid carcinoma cell lines retain their histopathology over in vivo passages. UM-HMC-3A or UM-HMC-3B (100,000 cells/scaffold) were transplanted into immunodeficient mice. When tumors reached an average volume of 1,000 mm3, they were retrieved and digested, single cell suspensions were prepared, and 100,000 unsorted cells were seeded in new biodegradable scaffolds to passage them to new mice. Photomicrographs of histological sections (Hematoxilin/eosin) of the human tumor specimen from which the respective cell lines were generated, 1st and 2nd passages in mice. In each panel the left-side image is at low magnification (40×), the top right-side image depicts an area of predominance of epidermoid-like cells and the bottom right-side image depicts an area of mucous-like cells. Scale bars represent 50 μm.
Figure 4.
Human mucoepidermoid carcinomas and UM-HMC-derived xenografts secrete high levels of mucopolysaccharides. (A) Photomicrographs of histological sections stained with Periodic acid Schiff (PAS) of representative fields from the human tumors that generated the cell lines, as well as 1st and 2nd passage UM-HMC-3A and UM-HMC-3B-derived xenografts. In each panel the left-side image is at low magnification (40×), the top right-side image depicts an area of predominance of epidermoid-like cells and the bottom right-side image depicts an area of mucous-like cells. Scale bars represent 50 μm. (B) Photomicrographs depicting the similar PAS patterns in the human mucoepidermoid carcinoma surgical specimen and in the 2nd passage xenograft generated with the UM-HMC cell line derived from this specimen (200×). Black arrows point to PAS-positive globules in the human tumor and in the mouse xenograft.
To assess the specificity of the approach used for generation of tumorigenic mucoepidermoid carcinoma cell lines, we also transplanted UM-HMC-2 cells (passage 5) into SCID mice. The UM-HMC-2 cells consistently generated tumors, but the time to palpability was longer when compared to UM-HMC-3A or UM-HMC-3B (Figure 2B). Histological analysis of the xenograft tumors revealed similar morphological features as those exhibited by the patient’s tumor (Suppl. Figure 2).
Interestingly, we observed an overall trend for quicker tumor formation with increased in vivo passage of UM-HMC cell lines (Figure 5). The UM-HMC-3A cells generated 1st passage xenografts within 133–260 days, 2nd passage xenografts at 77–91 days, 3rd passage at around 55 days and 4th passage xenografts at around 42 days (Figure 5A). Notably, not all transplantations of UM-HMC-3A generated xenograft tumors in the 1st passage. However, the tumor take in subsequent passages of UM-HMC-3A was 100%. In contrast, we observed a 100% tumor take of the lymph node metastasis-derived cell line (UM-HMC-3B) since the 1st in vivo passage (Figure 5B).
Figure 5.
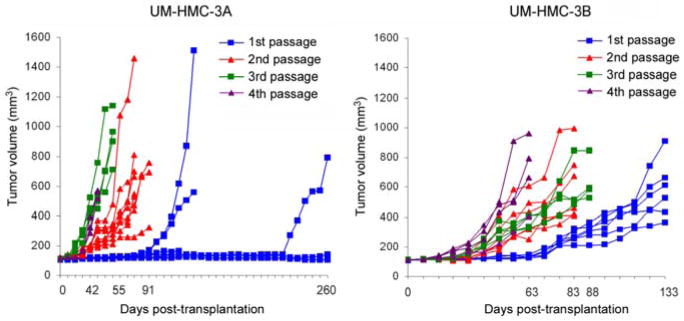
In vivo passage of xenografts generated with human mucoepidermoid carcinoma cell lines. UM-HMC-3A or UM-HMC-3B (100,000 cells/scaffold) were transplanted into immunodeficient mice. When tumors reached an average volume of 1,000 mm3, they were retrieved and digested, single cell suspensions were prepared, and 100,000 unsorted cells were seeded in new biodegradable scaffolds and passaged into new mice. (A,B) Graphs depicting growth of xenograft tumors generated with UM-HMC-3A (A) or UM-HMC-3B (B) cells over 4 in vivo passages.
And finally, to confirm that the cell lines and xenograft tumors were indeed derived from the patient’s specimens, we performed short tandem repeat (STR) profiling from the human tumor, xenograft tumors and high passage UM-HMC-3A and UM-HMC-3B cells. The genotypic analysis confirmed that cell lines were derived from the patient’s tumors and no contamination of other cell types was detected (Suppl. Figure 3). Likewise, the STR profiling confirmed that the xenograft tumors exhibit the same genotypic signature as the human specimen from which the tumor cells were isolated.
Discussion
The poor long-term survival of patients with malignant mucoepidermoid carcinomas demands new tools to better understand the pathobiology of these malignancies and to develop effective and safe therapies. Progress in this field has been hindered by the limited availability of tumorigenic mucoepidermoid carcinoma cell lines and xenograft mouse models. Here, we describe the process that led to the generation of several new mucoepidermoid carcinoma cell lines, and show the detailed characterization of a unique pair of cell lines derived from a patient with resistant disease. Notably, the features represented by these two cell lines, i.e. local recurrence (UM-HMC-3A) and metastatic spread (UM-HMC-3B) are the clinical features that ultimately determine the survival of patients with mucoepidermoid carcinoma. These cell lines and related xenograft models constitute a useful tool that can contribute to advances in the mechanistic understanding of this disease and in the development of new therapies.
A key step in the establishment of new cell lines is the optimization of culture conditions, particularly the selection of an appropriate cell culture medium. Using the cell culture medium previously reported by Queimado and collaborators as a basis,17 we’ve introduced a modification substituting RPMI for DMEM. The resulting medium appeared very appropriate for establishing and maintaining the UM-HMC cell lines in vitro. However, not all cell lines presented a robust growth for extended number of passages. While the UM-HMC-3A, UM-HMC-3B, and UM-HMC-1 have been expanded for 70–100 passages, other cell lines have proved to be more challenging in regards to in vitro expansion (e.g. UM-HMC-2). We thought initially that the difficulties associated with the continuous expansion of these cells in vitro were due to the high proportion of stromal fibroblasts in these cultures. However, the UM-HMC-2 cells present an epithelial expression profile (i.e. high EGFR, E-Cadherin, Pan-cytokeratin, Cytokeratin-7; and low Vimentin) but have been difficult to culture in vitro. Also, while robust in vitro growth correlates well with generation of xenograft tumors upon transplantation in mice for some cell lines (UM-HMC-3A, UM-HMC-3B), the UM-HMC-1 cells grow very well in culture but so far have not generated tumors in mice. On the other hand, UM-HMC-2 cells grow slowly in vitro, but are readily tumorigenic when transplanted into mice. We are currently performing experiments to understand possible reasons for these rather surprising observations.
An interesting observation is the noticeable improvement in tumor take and reduction in the time to tumor growth for the UM-HMC-3A cells transplanted into mice. We also noted that the time to tumor growth reduced considerably for UM-HMC-3B between the 1st passage and the 4th passage xenografts. These data suggest that passing mucoepidermoid carcinoma cells in vivo enables the selection of more tumorigenic cells. These cells may be more tumorigenic for several reasons that include, but are not limited to, increased cell survival, proliferation, improved adaptation to host (murine)-derived cytokines/growth factors, enhanced cancer stem cell (tumor-initiating cell) fraction. Alternatively, accelerated tumor growth upon in vivo passaging may be caused by the recruitment of host cells that support xenograft growth and that are being passed along with the tumor cells. Ongoing experiments are addressing such questions in the laboratory. Nevertheless, the fact that in vivo passaging greatly enhances the tumorigenic potential of the mucoepidermoid carcinoma cell lines has certainly enabled us to shorten the experiments and may also teach us important mechanistic lessons.
The xenograft tumors generated by the transplantation of mucoepidermoid carcinoma cell lines resembled very closely the histology of the human tumors from which they were derived. UM-HMC-3A tumors show a glandular pattern with both solid (epithelial-like) and mucous cells clearly visible and stable from passage to passage in mice. The positive PAS staining confirmed that the tumor cells are functional, mucous-secreting cells, a hallmark of mucoepidermoid carcinomas. The UM-HMC-3B tumors also show a glandular pattern and both solid and mucous cells. The first and second passage xenograft tumors show less of the mucous-secreting cells and more of a solid type pattern. This might explain the faster growth rates, and 100% tumor take for UM-HMC-3B cells compared to UM-HMC-3A cells. The finding that the cell lines UM-HMC-3A and UM-HMC-3B transplanted into mice reconstituted their parental tumors’ complex morphology with multiple cell types is somewhat puzzling. Perhaps one explanation for these findings is that these tumors contain cancer stem cells, which have also been found in head and neck squamous cell carcinomas (HNSCC)22 and other glandular malignancies (e.g. breast cancer).23 Notably, cancer stem cells have been reported in many cell lines, including HNSCC cells.24 It is possible that mucoepidermoid carcinomas follow the cancer stem cell hypothesis,25 and that these cell lines contain a sub-population of tumorigenic stem-like cells that retained multipotency and that can reconstitute the complex cellular heterogeneity of the parental tumors.
Of most concern is the issue of misidentification of cell lines.26 The short tandem repeat (STR) profiling is a relatively simple approach to determine human identity by examining sequences in DNA regions that are highly prone to variation that has been used in forensics for many years27, and has been proposed as a method to confirm the identity of cell lines.26 This approach was recently utilized to verify the identity of the cells that constitute the NCI-60 cell line panel.28 The authors showed that several cell lines have common origins. The problem of misidentification of cell lines is certainly relevant to salivary gland cancer research. It has been recently shown that several adenoid cystic carcinoma (ACC) cell lines are cross-contaminated with other cells.29 Genetic profiling revealed that ACC2, ACC3, and ACCM were HeLa cells, ACC5 contained T24 bladder cancer cells, ACNS were mouse cells and CAC2 were rat cells. These findings had devastating consequences for research in adenoid cystic carcinoma. Here, we used STR profiling to match the donor human tumor with the xenografts and cells lines derived from this tumor. The xenograft STR profiles shared 100% similarity with the STR profile of the donor human cancer. Interestingly, the UM-HMC-3A had one STR difference at the TH01 allele (changed from 9 to 8). The UM-HMC-3B also had one STR difference, but in this case, it was in the D13S317 allele (changed from 8 to 11). This difference constitutes a 93.3% match with the donor tissue, i.e. 31 out of 32 STRs evaluated here were identical. Notably, it has been proposed that 80% similarity or higher and/or a difference of up to one STR are considered within the definition of “identical” cell line origin.28 This is due to the observation that technical issues may lead to small variations in the STR profiling that could be considered normal. Alternatively, this minor difference might be attributable to genetic instability, which is a hallmark of cancer. Nevertheless, one concludes that the 2 cell lines described in detail here (UM-HMC-3A, UM-HMC-3B) are indeed derived from human mucoepidermoid carcinomas. Notably, the detection of the Crtc1-Maml2 translocation, characteristic of mucoepidermoid carcinomas9,30 further confirms the origin of these cell lines as true mucoepidermoid carcinoma cells.
In summary, we have described here the generation and characterization of mucoepidermoid carcinoma cell lines that are capable of generating xenograft tumors that closely resemble the histopathology of human cancers. While deeper characterization of these cells is necessary and indeed ongoing in the laboratory, the recognition of the potential usefulness of these cell lines to the scientific community interested in salivary gland cancer research, motivated us to present this work and make these cells immediately available. Also, we are currently using the knowledge gathered through this work to develop and characterize adenoid cystic carcinoma cell lines and related xenograft models. Well-characterized cell lines and mouse models are critically needed for the advancement of salivary gland cancer research and for the development of mechanism-based therapies that will improve the survival and quality of life of patients with these rare, yet devastating malignancies.
Supplementary Material
Acknowledgments
We thank the patients that provided us with the tumor specimens from which these cell lines were generated. We also thank Dr. Sarah Burgin for her insightful input to this work. Support for this work was provided by grant R01-DE21139 and R01-DE23220 from the NIH/NIDCR, and grant P50-CA97248 (University of Michigan Head Neck SPORE) from the NIH/NCI.
Footnotes
Conflict of interest statement: The authors have no conflict of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ellis GL, Auclair PL. In: Mucoepidermoid carcinoma. In Tumors of the Salivary Glands. Rosai J, Sobin LH, editors. Armed Forces Institute of Pathology; Washington, DC: 1996. pp. 155–172. [Google Scholar]
- 2.Chandana SR, Conley BA. Salivary gland cancers: current treatments, molecular characteristics and new therapies. Expert Rev. 2008;8:645–652. doi: 10.1586/14737140.8.4.645. [DOI] [PubMed] [Google Scholar]
- 3.Andry G, Hamoir M, Locati LD, Licitra L, Langendijk JA. Management of salivary gland tumors. Expert Rev. 2012;12:1161–1168. doi: 10.1586/era.12.92. [DOI] [PubMed] [Google Scholar]
- 4.Seethala RR. An update on grading of salivary gland carcinomas. Head Neck Pathol. 2009;3:69–77. doi: 10.1007/s12105-009-0102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Volkmann I. Über endotheliale Geschwülste zugleich ein Beitrag zu den Speicheldrüsen und Caumentumoren. Dtsch Z Chir. 1895;41:1. [Google Scholar]
- 6.McHugh CH, Roberts DB, El-Naggar AK, Hanna EY, Garden AS, Kies MS, Weber RS, Kupferman ME. Prognostic factors in mucoepidermoid carcinoma of the salivary glands. Cancer. 2012;118:3928–3936. doi: 10.1002/cncr.26697. [DOI] [PubMed] [Google Scholar]
- 7.Goode RK, Auclair PL, Ellis GL. Mucoepidermoid carcinoma of the major salivary glands: clinical and histopathologic analysis of 234 cases with evaluation of grading criteria. Cancer. 1998;82:1217–1224. doi: 10.1002/(sici)1097-0142(19980401)82:7<1217::aid-cncr2>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 8.Bai S, Clubwala R, Adler E, Sarta S, Schiff B, Smith RV, Gnepp DR, Brandwein-Gensler M. Salivary mucoepidermoid carcinoma: a multi-institutional review of 76 patients. Head Neck Pathol. 2012;7(19):1–8. doi: 10.1007/s12105-012-0405-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tonon G, Modi S, Wu L, Kubo A, Coxon AB, Komiya T, O’Neil K, Stover K, El-Naggar A, Griffin JD, Kirsch IR, Kaye FJ. t(11;19)(q21;p13) translocation in mucoepidermoid carcinoma creates a novel fusion product that disrupts a Notch signaling pathway. Nat Genet. 2003;33:208–213. doi: 10.1038/ng1083. [DOI] [PubMed] [Google Scholar]
- 10.Okabe M, Miyabe S, Nagatsuka H, Terada A, Hanai N, Yokoi M, Shimozato K, Eimoto T, Nakamura S, Nagai N, Hasegawa Y, Inagaki H. MECT-MAML2 fusion transcript defines a favorable subset of mucoepidermoid carcinoma. Clin Cancer Res. 2006;2:3902–3907. doi: 10.1158/1078-0432.CCR-05-2376. [DOI] [PubMed] [Google Scholar]
- 11.Tirado Y, Williams MD, Hanna EY, Kaye FJ, Batsakis JG, El-Naggar AK. CRTC1/MAML2 fusion transcript in high-grade mucoepidermoid carcinomas of salivary and thyroid glands and Warthin’s tumors: implications for histogenesis and biologic behavior. Genes Chromosomes Cancer. 2007;46:708–715. doi: 10.1002/gcc.20458. [DOI] [PubMed] [Google Scholar]
- 12.Behboudi A, Enlund F, Winnes M, Andrén Y, Nordkvist A, Leivo I, Flaberg E, Szekely L, Mäkitie A, Grenman R, Mark J, Stenman G. Molecular classification of mucoepidermoid carcinomas-prognostic significance of the MECT1-MAML2 fusion oncogene. Genes Chromosomes Cancer. 2006;45:470–481. doi: 10.1002/gcc.20306. [DOI] [PubMed] [Google Scholar]
- 13.Anzick SL, Chen WD, Park Y, Meltzer P, Bell D, El-Naggar AK, Kaye FJ. Unfavorable prognosis of CRTC1-MAML2 positive mucoepidermoid tumors with CDKN2A deletions. Genes Chromosomes Cancer. 2010;49:59–69. doi: 10.1002/gcc.20719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Komiya T, Park Y, Modi S, Coxon AB, Oh H, Kaye FJ. Sustained expression of Mect1-Maml2 is essential for tumor cell growth in salivary gland cancers carrying the t(11;19) translocation. Oncogene. 2006;25:6128–6132. doi: 10.1038/sj.onc.1209627. [DOI] [PubMed] [Google Scholar]
- 15.Kaye FJ. Emerging biology of malignant salivary gland tumors offers new insights into the classification and treatment of mucoepidermoid cancer. Clin Cancer Res. 2006;12:3878–3881. doi: 10.1158/1078-0432.CCR-06-0791. [DOI] [PubMed] [Google Scholar]
- 16.Grénman R, Pekkola-Heino K, Joensuu H, Aitasalo K, Klemi P, Lakkala T. UT-MUC-1, a new mucoepidermoid carcinoma cell line, and its radiosensitivity. Arch Otolaryngol Head Neck Surg. 1992;118:542–547. doi: 10.1001/archotol.1992.01880050096023. [DOI] [PubMed] [Google Scholar]
- 17.Queimado L, Lopes C, Du F, Martins C, Fonseca I, Bowcock AM, Soares J, Lovett M. In vitro transformation of cell lines from human salivary gland tumors. Int J Cancer. 1999;81:793–798. doi: 10.1002/(sici)1097-0215(19990531)81:5<793::aid-ijc21>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 18.Coxon A, Rozenblum E, Park YS, Joshi N, Tsurutani J, Dennis PA, Kirsch IR, Kaye FJ. Mect1-Maml2 fusion oncogene linked to aberrant activation of cyclic AMP/CREB regulated genes. Cancer Res. 2005;65:7137–7144. doi: 10.1158/0008-5472.CAN-05-1125. [DOI] [PubMed] [Google Scholar]
- 19.Nör JE, Peters MC, Christensen JB, Sutorik MM, Linn S, Khan MK, Addison CL, Mooney DJ, Polverini PJ. Engineering and characterization of functional human microvessels in immunodeficient mice. Lab Invest. 2001;81:453–463. doi: 10.1038/labinvest.3780253. [DOI] [PubMed] [Google Scholar]
- 20.Nör JE, Christensen J, Liu J, Peters M, Mooney DJ, Strieter RM, Polverini PJ. Up-Regulation of Bcl-2 in microvascular endothelial cells enhances intratumoral angiogenesis and accelerates tumor growth. Cancer Res. 2001;61:2183–2188. [PubMed] [Google Scholar]
- 21.Panicker N, Jariwala P, Buch A, Joshi M. The utility of periodic acid schiff with diastase and alcian blue stains on fine needle aspirates of breast and salivary gland neoplasms. J Cytol. 2012;29:221–225. doi: 10.4103/0970-9371.103938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Delerba P, et al. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci USA. 2007;104(3):973–978. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Locke M, Heywood M, Fawell S, Mackenzie IC. Retention of intrinsic stem cell hierarchies in carcinoma-derived cell lines. Cancer Res. 2005;65(19):8944–8950. doi: 10.1158/0008-5472.CAN-05-0931. [DOI] [PubMed] [Google Scholar]
- 24.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100(7):3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adams A, Warner K, Nör JE. Salivary gland cancer stem cells. Oral Oncol. 2013 Jun 27; doi: 10.1016/j.oraloncology.2013.05.013. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alston-Roberts C, Barallon R, Bauer SR, Butler J, Capes-Davis A, Dirks WG, Elmore E, Furtado M, Kerrigan L, Kline MC, Kohara A, Los GV, MacLeod RA, Masters JR, Nardone M, Nardone RM, Nims RW, Price PJ, Reid YA, Shewale J, Steuer AF, Storts DR, Sykes G, Taraporewala Z, Thomson J. Cell line misidentification: the beginning of the end. Nat Rev Cancer. 2010;10:441–448. doi: 10.1038/nrc2852. [DOI] [PubMed] [Google Scholar]
- 27.Butler JM. Genetics and genomics of core short tandem repeat loci used in human identity testing. J Forens Sci. 2006;51:253–65. doi: 10.1111/j.1556-4029.2006.00046.x. [DOI] [PubMed] [Google Scholar]
- 28.Lorenzi PL, Reinhold WC, Varma S, Hutchinson AA, Pommier Y, Chanock SJ, Weinstein JN. DNA fingerprinting of the NCI-60 cell line panel. Mol Cancer Ther. 2009;8:713–724. doi: 10.1158/1535-7163.MCT-08-0921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Phuchareon J, Ohta Y, Woo JM, Eisele DW, Tetsu O. Genetic profiling reveals cross-contamination and misidentification of 6 adenoid cystic carcinoma cell lines: ACC2, ACC3, ACCM, ACCNS, ACC5 and CAC2. PLoS One. 2009;4:1–7. doi: 10.1371/journal.pone.0006040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Enlund F, Behboudi A, Andrén Y, Oberg C, Lendahl U, Mark J, Stenman G. Altered Notch signaling resulting from expression of a WAMTP1-MAML2 gene fusion in mucoepidermoid carcinomas and benign Warthin’s tumors. Exp Cell Res. 2004;292:21–28. doi: 10.1016/j.yexcr.2003.09.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.