Abstract
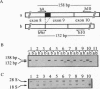
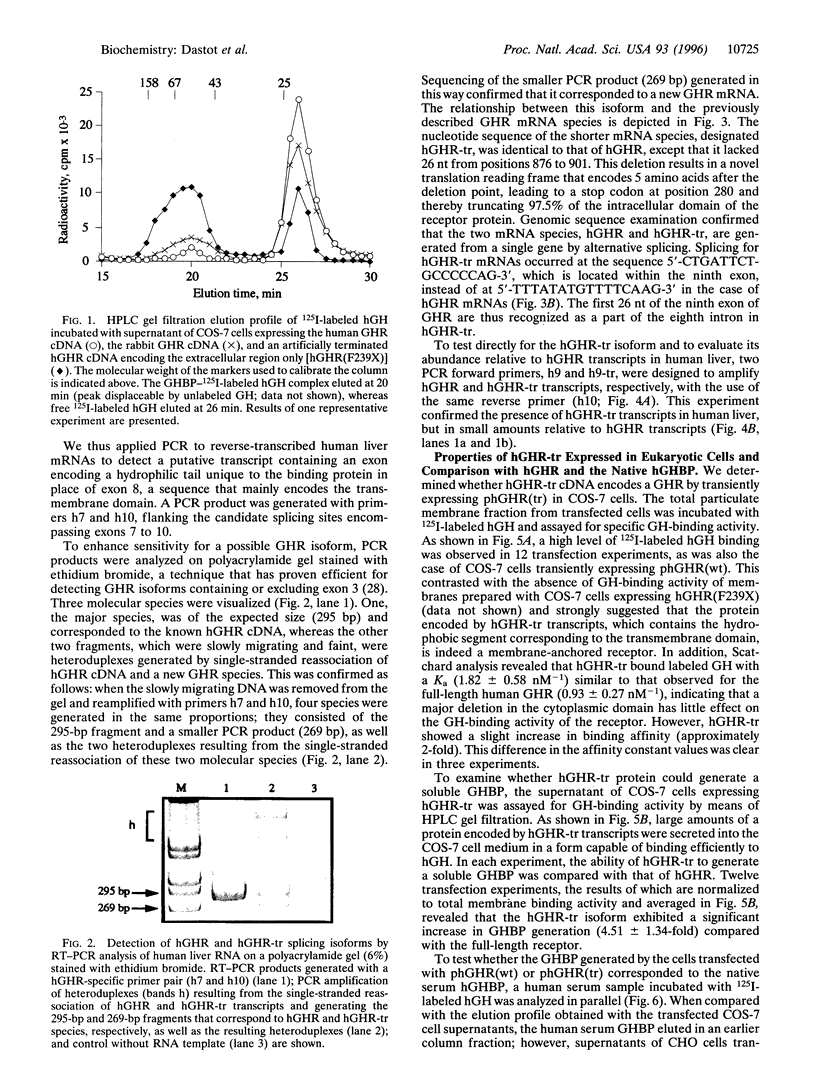
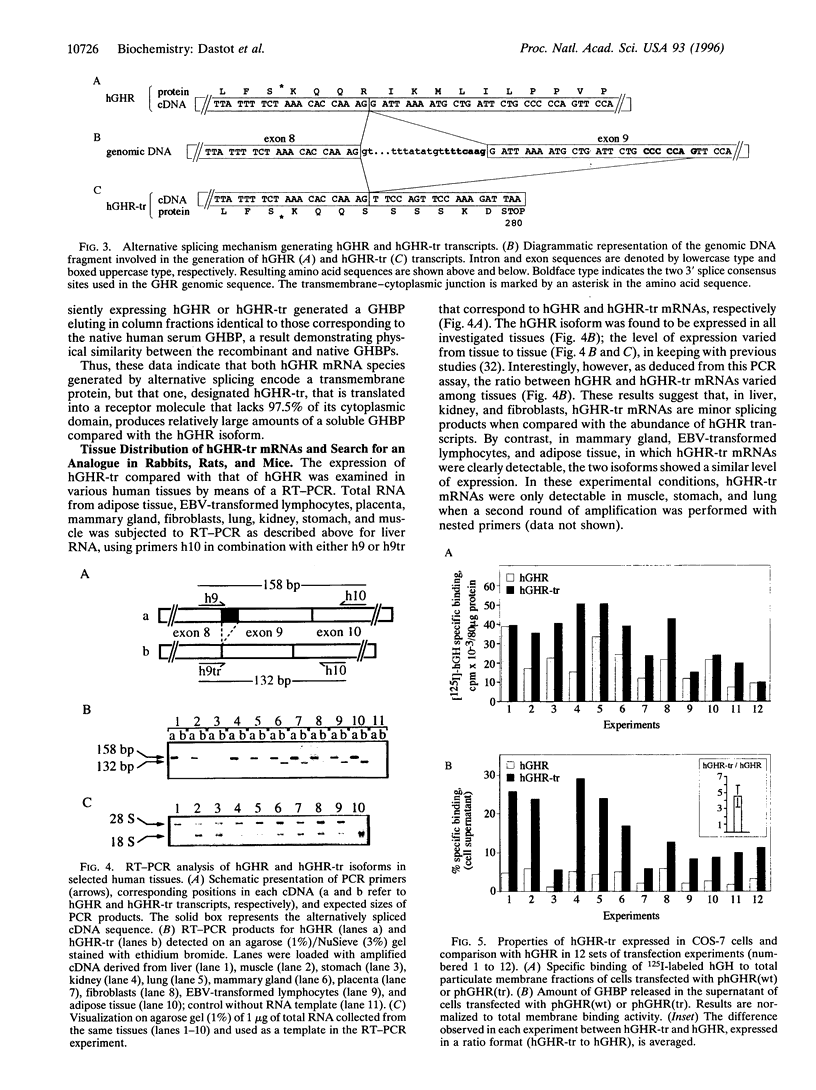
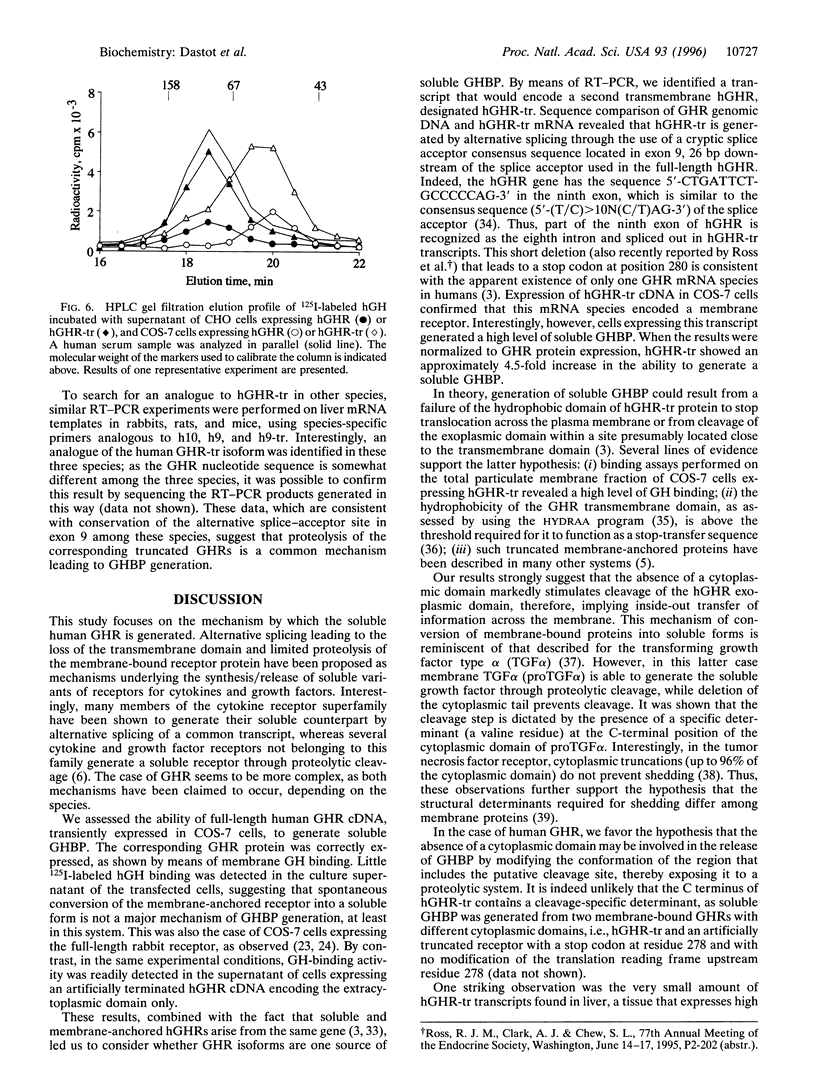
The mechanism underlying the generation of soluble growth hormone binding protein (GHBP) probably differs among species. In rats and mice, it involves an alternatively spliced mRNA, whereas in rabbits, it involves limited proteolysis of the membrane-bound growth hormone receptor (GHR). In humans, this latter mechanism is favored, as no transcript coding for a soluble GHR has been detected so far. To test this hypothesis, we analyzed COS-7 cells transiently expressing the full-length human (h) GHR and observed specific GH-binding activity in the cell supernatants. Concomitantly, an alternatively spliced form in the cytoplasmic domain of GHR, hGHR-tr, was isolated from several human tissues. hGHR-tr is identical in sequence to hGHR, except for a 26-bp deletion leading to a stop codon at position 280, thereby truncating 97.5% of the intracellular domain of the receptor protein. When compared with hGHR, hGHR-tr showed a significantly increased capacity to generate a soluble GHBP. Interestingly, this alternative transcript is also expressed in liver from rabbits, mice, and rats, suggesting that, in these four species, proteolysis of the corresponding truncated transmembrane GHR is a common mechanism leading to GHBP generation. These findings support the hypothesis that GHBP may at least partly result from alternative splicing of the region encoding the intracellular domain and that the absence of a cytoplasmic domain may be involved in increased release of GHBP.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amit T., Hacham H., Daily O., Hertz P., Barkey R. J., Hochberg Z. The Hep G2 cell line in the study of growth hormone receptor/binding protein. Mol Cell Endocrinol. 1994 May;101(1-2):29–36. doi: 10.1016/0303-7207(94)90216-x. [DOI] [PubMed] [Google Scholar]
- Baumbach W. R., Horner D. L., Logan J. S. The growth hormone-binding protein in rat serum is an alternatively spliced form of the rat growth hormone receptor. Genes Dev. 1989 Aug;3(8):1199–1205. doi: 10.1101/gad.3.8.1199. [DOI] [PubMed] [Google Scholar]
- Bazan J. F. Structural design and molecular evolution of a cytokine receptor superfamily. Proc Natl Acad Sci U S A. 1990 Sep;87(18):6934–6938. doi: 10.1073/pnas.87.18.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosenberg M. W., Pandiella A., Massagué J. The cytoplasmic carboxy-terminal amino acid specifies cleavage of membrane TGF alpha into soluble growth factor. Cell. 1992 Dec 24;71(7):1157–1165. doi: 10.1016/s0092-8674(05)80064-9. [DOI] [PubMed] [Google Scholar]
- Brakebusch C., Nophar Y., Kemper O., Engelmann H., Wallach D. Cytoplasmic truncation of the p55 tumour necrosis factor (TNF) receptor abolishes signalling, but not induced shedding of the receptor. EMBO J. 1992 Mar;11(3):943–950. doi: 10.1002/j.1460-2075.1992.tb05133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Kendall D. A. Artificial transmembrane segments. Requirements for stop transfer and polypeptide orientation. J Biol Chem. 1995 Jun 9;270(23):14115–14122. doi: 10.1074/jbc.270.23.14115. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Crosier K. E., Wong G. G., Mathey-Prevot B., Nathan D. G., Sieff C. A. A functional isoform of the human granulocyte/macrophage colony-stimulating factor receptor has an unusual cytoplasmic domain. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7744–7748. doi: 10.1073/pnas.88.17.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis S., Aldrich T. H., Ip N. Y., Stahl N., Scherer S., Farruggella T., DiStefano P. S., Curtis R., Panayotatos N., Gascan H. Released form of CNTF receptor alpha component as a soluble mediator of CNTF responses. Science. 1993 Mar 19;259(5102):1736–1739. doi: 10.1126/science.7681218. [DOI] [PubMed] [Google Scholar]
- Duquesnoy P., Sobrier M. L., Amselem S., Goossens M. Defective membrane expression of human growth hormone (GH) receptor causes Laron-type GH insensitivity syndrome. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10272–10276. doi: 10.1073/pnas.88.22.10272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duquesnoy P., Sobrier M. L., Duriez B., Dastot F., Buchanan C. R., Savage M. O., Preece M. A., Craescu C. T., Blouquit Y., Goossens M. A single amino acid substitution in the exoplasmic domain of the human growth hormone (GH) receptor confers familial GH resistance (Laron syndrome) with positive GH-binding activity by abolishing receptor homodimerization. EMBO J. 1994 Mar 15;13(6):1386–1395. doi: 10.1002/j.1460-2075.1994.tb06392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edens A., Southard J. N., Talamantes F. Mouse growth hormone-binding protein and growth hormone receptor transcripts are produced from a single gene by alternative splicing. Endocrinology. 1994 Dec;135(6):2802–2805. doi: 10.1210/endo.135.6.7988474. [DOI] [PubMed] [Google Scholar]
- Ehlers M. R., Riordan J. F. Membrane proteins with soluble counterparts: role of proteolysis in the release of transmembrane proteins. Biochemistry. 1991 Oct 22;30(42):10065–10074. doi: 10.1021/bi00106a001. [DOI] [PubMed] [Google Scholar]
- Eisenberg D., Schwarz E., Komaromy M., Wall R. Analysis of membrane and surface protein sequences with the hydrophobic moment plot. J Mol Biol. 1984 Oct 15;179(1):125–142. doi: 10.1016/0022-2836(84)90309-7. [DOI] [PubMed] [Google Scholar]
- Ellis L., Clauser E., Morgan D. O., Edery M., Roth R. A., Rutter W. J. Replacement of insulin receptor tyrosine residues 1162 and 1163 compromises insulin-stimulated kinase activity and uptake of 2-deoxyglucose. Cell. 1986 Jun 6;45(5):721–732. doi: 10.1016/0092-8674(86)90786-5. [DOI] [PubMed] [Google Scholar]
- Esposito N., Paterlini P., Kelly P. A., Postel-Vinay M. C., Finidori J. Expression of two isoforms of the human growth hormone receptor in normal liver and hepatocarcinoma. Mol Cell Endocrinol. 1994 Jul;103(1-2):13–20. doi: 10.1016/0303-7207(94)90064-7. [DOI] [PubMed] [Google Scholar]
- Fukunaga R., Ishizaka-Ikeda E., Seto Y., Nagata S. Expression cloning of a receptor for murine granulocyte colony-stimulating factor. Cell. 1990 Apr 20;61(2):341–350. doi: 10.1016/0092-8674(90)90814-u. [DOI] [PubMed] [Google Scholar]
- Fukunaga R., Seto Y., Mizushima S., Nagata S. Three different mRNAs encoding human granulocyte colony-stimulating factor receptor. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8702–8706. doi: 10.1073/pnas.87.22.8702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin R. G., Friend D., Ziegler S. F., Jerzy R., Falk B. A., Gimpel S., Cosman D., Dower S. K., March C. J., Namen A. E. Cloning of the human and murine interleukin-7 receptors: demonstration of a soluble form and homology to a new receptor superfamily. Cell. 1990 Mar 23;60(6):941–951. doi: 10.1016/0092-8674(90)90342-c. [DOI] [PubMed] [Google Scholar]
- Harrison S. M., Barnard R., Ho K. Y., Rajkovic I., Waters M. J. Control of growth hormone (GH) binding protein release from human hepatoma cells expressing full-length GH receptor. Endocrinology. 1995 Feb;136(2):651–659. doi: 10.1210/endo.136.2.7835299. [DOI] [PubMed] [Google Scholar]
- Hughes J. P., Friesen H. G. The nature and regulation of the receptors for pituitary growth hormone. Annu Rev Physiol. 1985;47:469–482. doi: 10.1146/annurev.ph.47.030185.002345. [DOI] [PubMed] [Google Scholar]
- Isaksson O. G., Edén S., Jansson J. O. Mode of action of pituitary growth hormone on target cells. Annu Rev Physiol. 1985;47:483–499. doi: 10.1146/annurev.ph.47.030185.002411. [DOI] [PubMed] [Google Scholar]
- Layton M. J., Cross B. A., Metcalf D., Ward L. D., Simpson R. J., Nicola N. A. A major binding protein for leukemia inhibitory factor in normal mouse serum: identification as a soluble form of the cellular receptor. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8616–8620. doi: 10.1073/pnas.89.18.8616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung D. W., Spencer S. A., Cachianes G., Hammonds R. G., Collins C., Henzel W. J., Barnard R., Waters M. J., Wood W. I. Growth hormone receptor and serum binding protein: purification, cloning and expression. Nature. 1987 Dec 10;330(6148):537–543. doi: 10.1038/330537a0. [DOI] [PubMed] [Google Scholar]
- Liebhaber S. A., Urbanek M., Ray J., Tuan R. S., Cooke N. E. Characterization and histologic localization of human growth hormone-variant gene expression in the placenta. J Clin Invest. 1989 Jun;83(6):1985–1991. doi: 10.1172/JCI114108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J., Pandiella A. Membrane-anchored growth factors. Annu Rev Biochem. 1993;62:515–541. doi: 10.1146/annurev.bi.62.070193.002503. [DOI] [PubMed] [Google Scholar]
- McPherson G. A. Analysis of radioligand binding experiments. A collection of computer programs for the IBM PC. J Pharmacol Methods. 1985 Nov;14(3):213–228. doi: 10.1016/0160-5402(85)90034-8. [DOI] [PubMed] [Google Scholar]
- Mercado M., DáVila N., McLeod J. F., Baumann G. Distribution of growth hormone receptor messenger ribonucleic acid containing and lacking exon 3 in human tissues. J Clin Endocrinol Metab. 1994 Mar;78(3):731–735. doi: 10.1210/jcem.78.3.8126150. [DOI] [PubMed] [Google Scholar]
- Mosley B., Beckmann M. P., March C. J., Idzerda R. L., Gimpel S. D., VandenBos T., Friend D., Alpert A., Anderson D., Jackson J. The murine interleukin-4 receptor: molecular cloning and characterization of secreted and membrane bound forms. Cell. 1989 Oct 20;59(2):335–348. doi: 10.1016/0092-8674(89)90295-x. [DOI] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullis P. E., Holl R. W., Lund T., Eblé A., Brickell P. M. Regulation of human growth hormone-binding protein production by human growth hormone in a hepatoma cell line. Mol Cell Endocrinol. 1995 Jun;111(2):181–190. doi: 10.1016/0303-7207(95)03567-q. [DOI] [PubMed] [Google Scholar]
- Müllberg J., Schooltink H., Stoyan T., Günther M., Graeve L., Buse G., Mackiewicz A., Heinrich P. C., Rose-John S. The soluble interleukin-6 receptor is generated by shedding. Eur J Immunol. 1993 Feb;23(2):473–480. doi: 10.1002/eji.1830230226. [DOI] [PubMed] [Google Scholar]
- Raines M. A., Liu L., Quan S. G., Joe V., DiPersio J. F., Golde D. W. Identification and molecular cloning of a soluble human granulocyte-macrophage colony-stimulating factor receptor. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8203–8207. doi: 10.1073/pnas.88.18.8203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renauld J. C., Druez C., Kermouni A., Houssiau F., Uyttenhove C., Van Roost E., Van Snick J. Expression cloning of the murine and human interleukin 9 receptor cDNAs. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5690–5694. doi: 10.1073/pnas.89.12.5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose-John S., Heinrich P. C. Soluble receptors for cytokines and growth factors: generation and biological function. Biochem J. 1994 Jun 1;300(Pt 2):281–290. doi: 10.1042/bj3000281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoda R. C., Seldin D. C., Chiang M. K., Peichel C. L., Vogt T. F., Leder P. Murine c-mpl: a member of the hematopoietic growth factor receptor superfamily that transduces a proliferative signal. EMBO J. 1993 Jul;12(7):2645–2653. doi: 10.1002/j.1460-2075.1993.tb05925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith W. C., Kuniyoshi J., Talamantes F. Mouse serum growth hormone (GH) binding protein has GH receptor extracellular and substituted transmembrane domains. Mol Endocrinol. 1989 Jun;3(6):984–990. doi: 10.1210/mend-3-6-984. [DOI] [PubMed] [Google Scholar]
- Sobrier M. L., Duquesnoy P., Duriez B., Amselem S., Goossens M. Expression and binding properties of two isoforms of the human growth hormone receptor. FEBS Lett. 1993 Mar 15;319(1-2):16–20. doi: 10.1016/0014-5793(93)80028-s. [DOI] [PubMed] [Google Scholar]
- Sotiropoulos A., Goujon L., Simonin G., Kelly P. A., Postel-Vinay M. C., Finidori J. Evidence for generation of the growth hormone-binding protein through proteolysis of the growth hormone membrane receptor. Endocrinology. 1993 Apr;132(4):1863–1865. doi: 10.1210/endo.132.4.8462483. [DOI] [PubMed] [Google Scholar]
- Takaki S., Tominaga A., Hitoshi Y., Mita S., Sonoda E., Yamaguchi N., Takatsu K. Molecular cloning and expression of the murine interleukin-5 receptor. EMBO J. 1990 Dec;9(13):4367–4374. doi: 10.1002/j.1460-2075.1990.tb07886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavernier J., Devos R., Cornelis S., Tuypens T., Van der Heyden J., Fiers W., Plaetinck G. A human high affinity interleukin-5 receptor (IL5R) is composed of an IL5-specific alpha chain and a beta chain shared with the receptor for GM-CSF. Cell. 1991 Sep 20;66(6):1175–1184. doi: 10.1016/0092-8674(91)90040-6. [DOI] [PubMed] [Google Scholar]
- Todokoro K., Kuramochi S., Nagasawa T., Abe T., Ikawa Y. Isolation of a cDNA encoding a potential soluble receptor for human erythropoietin. Gene. 1991 Oct 15;106(2):283–284. doi: 10.1016/0378-1119(91)90213-u. [DOI] [PubMed] [Google Scholar]
- Trivedi B., Daughaday W. H. Release of growth hormone binding protein from IM-9 lymphocytes by endopeptidase is dependent on sulfhydryl group inactivation. Endocrinology. 1988 Nov;123(5):2201–2206. doi: 10.1210/endo-123-5-2201. [DOI] [PubMed] [Google Scholar]
- Urbanek M., MacLeod J. N., Cooke N. E., Liebhaber S. A. Expression of a human growth hormone (hGH) receptor isoform is predicted by tissue-specific alternative splicing of exon 3 of the hGH receptor gene transcript. Mol Endocrinol. 1992 Feb;6(2):279–287. doi: 10.1210/mend.6.2.1569971. [DOI] [PubMed] [Google Scholar]
- Vigon I., Mornon J. P., Cocault L., Mitjavila M. T., Tambourin P., Gisselbrecht S., Souyri M. Molecular cloning and characterization of MPL, the human homolog of the v-mpl oncogene: identification of a member of the hematopoietic growth factor receptor superfamily. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5640–5644. doi: 10.1073/pnas.89.12.5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickelgren R. B., Landin K. L., Ohlsson C., Carlsson L. M. Expression of exon 3-retaining and exon 3-excluding isoforms of the human growth hormone-receptor is regulated in an interindividual, rather than a tissue-specific, manner. J Clin Endocrinol Metab. 1995 Jul;80(7):2154–2157. doi: 10.1210/jcem.80.7.7608270. [DOI] [PubMed] [Google Scholar]